Page 1

Index 2MF
SpO2 Simulator
PN 3341210
September 2008, Rev. 1
© 2008 Fluke Corporation, All rights reserved. Printed in USA. Specifications subject to change without notice.
All product names are trademarks of their respective companies.
Users Manual
Page 2

Fluke Biomedical warrants this instrument against defects in materials and workmanship
for one full year from the date of original purchase. During the warranty period, we will
repair or, at our option, replace at no charge a product that proves to be defective,
provided you return the product, shipping prepaid, to Fluke Biomedical. This warranty
does not apply if the product has been damaged by accident or misuse or as the result of
service or modification by other than Fluke Biomedical. IN NO EVENT SHALL FLUKE
BIOMEDICAL BE LIABLE FOR CONSEQUENTIAL DAMAGES.
Only serialized products and their accessory items (those products and items bearing a
distinct serial number tag) are covered under this one-year warranty. PHYSICAL
DAMAGE CAUSED BY MISUSE OR PHYSICAL ABUSE IS NOT COVERED
UNDER THE WARRANTY. Items such as cables and nonserialized modules are not
covered under this warranty.
Recalibration of instruments is not covered under the warranty.
This warranty gives you specific legal rights, and you may also have other rights which
vary from state to state, province to province, or country to country. This warranty is
limited to repairing the instrument to Fluke Biomedical’s specifications.
Warranty Disclaimer
Should you elect to have your instrument serviced and/or calibrated by someone other
than Fluke Biomedical, please be advised that the original warranty covering your
product becomes void when the tamper-resistant Quality Seal is removed or broken
without proper factory authorization. We strongly recommend, therefore, that you send
your instrument to Fluke Biomedical for factory service and calibration, especially during
the original warranty period.
Warranty and Product Support
Page 3

Notices
All Rights Reserved
© Copyright 2008, Fluke Biomedical. No part of this publication may be reproduced, transmitted, transcribed, st ored in a retrieval
system, or translated into any language without the written permission of Fluke Biomedical.
Copyright Release
Fluke Biomedical agrees to a limited copyright release that allows you to reproduce manuals and ot her printed ma terials for use in
service training programs and other technical publications. If you woul d like other reproductions or distribution s, submit a wr itten
request to Fluke Biomedical.
Unpacking and Inspection
Follow standard receiving practices upon receipt of the instrument. Check the shipping carton for damage. If damage is found, stop
unpacking the instrument. Notify the carrier and ask for an agent to be present while the i nstrument is unpacked. There are no special
unpacking instructions, but be careful not to dam age the instrum ent when unpacking it. Inspect the i nstrument for physical dam age such
as bent or broken parts, dents, or scratche s.
Technical Support
For application support or answers to technical questions, either email techservices@flukebiomedical.com or call 1-800- 85 0-4608 or 1440-248-9300.
Claims
Our routine method of shipment is via comm on carrier, FOB origin. Upo n delivery, if physical dam age is found, re tain all packing
materials in their original condition and contact the carrier immediately to file a claim. If the instrument is delivered in good physical
condition but does not operate within specifications, or if there are any other problems not caused by shipping damage, please contact
Fluke Biomedical or your local sales representative.
Standard Terms and Conditions
Refunds and Credits
Please note that only serialized products and their accessory items (i.e., products and items bearing a distinct serial number
tag) are eligible for partial refund and/or credit. Nonserialized parts and accessory items (e.g., cables, carrying cases,
auxiliary modules, etc.) are not eligible for return or refund. Only prod u c ts r e t ur n e d w i t h i n 9 0 d a y s f r om th e d a t e o f orig i nal
purchase are eligible for refund/credit. In order to receive a partial refund/credit of a product purchase price on a serialized product, the
product must not have been damaged by the custom er or by the carrier chosen by the cust omer to return the goods, an d the product
must be returned complete (meaning with all manuals, cables, accessories, etc.) and in “as new” and resalable condition. Products not
returned within 90 days of purchase, or products which are n ot in “as new” and resalable co ndition, are not eligible for credit return and
will be returned to the customer. The Return Procedure (see below) must be followed to assure prompt refund/credit.
Restocking Charges
Products returned within 30 days of original purchase are su bject to a minim um restocking fee of 15 %. Products returne d in excess of
30 days after purchase, but prior to 90 days, ar e subject to a minim um restocking fee of 20 %. Addit ional charges for da mage and/or
missing parts and accessories will be applied to all returns.
Page 4
Return Procedure
All items being returned (including all warranty- claim shipments) m ust be sent freight-pre paid to our factory locati on. When yo u return
an instrument to Fluke Biomedical, we recommend using United Parcel Service, Federal Express, or Air Parcel Post. We also
recommend that you insure your shipment for its actual replacement cost. Fluke Biomedical will not be responsible for lost shipments
or instruments that are received in damaged condition due to improper packaging or handling.
Use the original carton and packaging material for shipment. If they are not available, we recommend the following guide for
repackaging:
Use a double-walled carton of sufficient strength for the weight being ship ped.
Use heavy paper or cardboard to protect all instrument surfaces. Use nonabrasive material around all projecting parts.
Use at least four inches of tightly packed, industry-approved, shock-absorbent material around the instrument.
Returns for partial refund/credit:
Every product returned for refund/credit must be accompanied by a Return Material Authorization (RMA) number, obtained from our
Order Entry Group at 1-800- 850-4608 or 1-44 0-248-930 0.
Repair and calibration:
To find the nearest service center, goto www.flukebiomedical.com/service
In the U.S.A.:
Cleveland Calibration Lab
Tel: 1-800-850-4606
Email: globalcal@flukebiomedical.com
Everett Calibration Lab
Tel: 1-888-993-5853
Email: service.status@fluke.com
In Europe, Middle East, and Africa:
Eindhoven Calibration Lab
Tel: +31-402-675300
Email: ServiceDesk@fluke.com
In Asia:
Everett Calibration Lab
Tel: +425-446-6945
Email: service.international@fluke.com
, or
Certification
This instrument was thoroughly tested and inspected. It was found to meet Fluke Biomedical’s manufacturing specifications w hen it
was shipped from the factory. Calibration measurements are traceable to the National Institute of Standards and Technology (NIST).
Devices for which there are no NIST calibration standards are measured against in-house performance standards using accepted test
procedures.
WARNING
Unauthorized user modifications or application beyond the published specifications may result in electrical shock hazards or
improper operation. Fluke Biomedical will not be responsible for any injuries sustained due to unauthorized equipment
modifications.
Restrictions and Liabilities
Information in this document is subject to change and does not represent a commitment by Fluke Biomedical. Changes made
to the information in this document will be incorporated in new editions of the publication. No responsibility is assumed by
Fluke Biomedical for the use or reliability of software or equipment that is not supplied by Fluke Biomedical, or by its
affiliated dealers.
Manufacturing Location
The Index 2MF Pulse Oximeter Analyzer is manufactured in Everett, Washington by Fluke Biomedical, 6920 Seaway Blvd.,
Everett, WA, U.S.A.
Page 5

Table of Contents
Chapter Title Page
1 Introducing the Index 2MF.................................................................. 1-1
Capabilities ........................................................................................................ 1-1
Index 2MF Compatibility .................................................................................. 1-1
Index 2MF Features........................................................................................... 1-2
Package Contents............................................................................................... 1-2
F Version....................................................................................................... 1-2
FE Version..................................................................................................... 1-2
Registration Card........................................................................................... 1-2
Precautions......................................................................................................... 1-2
Electromagnetic Interference and Susceptibility ............................................... 1-3
EC EMC Directive 89/336/EEC.................................................................... 1-3
EN 50081-1, CLASS A-Emissions ............................................................... 1-3
EN 50082-1 Immunity................................................................................... 1-3
USA FCC CLASS A..................................................................................... 1-3
Canadian Department of Communications Class A...................................... 1-4
Safety............................................................................................................. 1-4
EC Directive 73/23/EEC, Low-Voltage Directive ........................................ 1-4
Getting Started................................................................................................... 1-4
Starting the Simulator and Using Menus....................................................... 1-4
Using the Optical Finger Probe (All Versions) ............................................. 1-5
2 Blood Oxygen and Pulse Oximeters.................................................. 2-1
Blood Pressure................................................................................................... 2-1
Gases in Blood................................................................................................... 2-1
Pulse Oximeters................................................................................................. 2-2
How Pulse Oximeters Work.......................................................................... 2-2
Spectrophotometry ........................................................................................ 2-2
References.......................................................................................................... 2-4
3 Index 2MF Overview............................................................................ 3-1
The Index 2MF "Finger".................................................................................... 3-1
Simulator Electrical Testing (MFE Version only)............................................. 3-1
Simulation Settings............................................................................................ 3-1
Running Tests.................................................................................................... 3-1
i
Page 6

Index 2MF
Users Guide
Evaluating Test Results ..................................................................................... 3-2
Simulator Functionality ..................................................................................... 3-2
4 The Main Menus .................................................................................. 4-1
Introduction........................................................................................................ 4-1
The Main Menu 1 .............................................................................................. 4-1
The Main Menu 2 .............................................................................................. 4-3
5 Configuring the Simulator.................................................................. 5-1
Configuring for Specific Oximeter Make.......................................................... 5-1
Accessing the Make Menu................................................................................. 5-1
Verified Oximeter Makes .................................................................................. 5-2
R-Curve Specifications...................................................................................... 5-2
Customizing a Make Not Included Within Index 2MF ..................................... 5-3
Operation ........................................................................................................... 5-4
6 Setting and Changing Simulation Factors........................................ 6-1
About Simulations and Presets .......................................................................... 6-1
Setting Simulations............................................................................................ 6-1
The Simulations Menu .................................................................................. 6-1
Changing the Default SpO2 Setting .............................................................. 6-3
Setting the O2 Saturation Level..................................................................... 6-3
Raising or Lowering the Pulse Rate .............................................................. 6-4
Returning to Main Menu 1............................................................................ 6-4
Using Preset Patient Conditions......................................................................... 6-4
Setting the Light Artifact................................................................................... 6-7
Setting 02 and Pulse Rate Step Size .................................................................. 6-8
7 Testing the Pulse Oximeter Limits..................................................... 7-1
Introduction........................................................................................................ 7-1
Testing Pulse Oximeter Limits .......................................................................... 7-1
Simulating Oxygen Conditions.......................................................................... 7-1
Simulating the Pulse Rate.................................................................................. 7-3
Simulating Pulse Amplitude.............................................................................. 7-5
Simulating Asystole or No Pulse....................................................................... 7-7
8 Using Test Programs .......................................................................... 8-1
The AUTO Menus ............................................................................................. 8-1
Navigating Through the Programming Process............................................. 8-1
The Program Definition Cycle ...................................................................... 8-2
The Automatic Test Parameters .................................................................... 8-2
Accessing the Autosequences Menu (AUTO)............................................... 8-3
Creating a Custom Test Program (Autosequence)............................................. 8-4
Selecting a Test Program............................................................................... 8-5
Optionally Renaming the Test Program........................................................ 8-6
Configuring the Program............................................................................... 8-7
Selecting Print Settings............................................................................. 8-8
Setting the 02 Level .................................................................................. 8-9
Setting the Pulse Rate................................................................................ 8-9
Setting the Simulation Cycle..................................................................... 8-10
Selecting the Make.................................................................................... 8-10
Choosing Your Tests................................................................................. 8-11
ii
Page 7

Contents (continued)
Selecting Pulse Amplitude........................................................................ 8-12
Selecting Motion....................................................................................... 8-12
Selecting Presets........................................................................................ 8-13
Saving Your Program.................................................................................... 8-13
Running an Automatic Test Program ................................................................ 8-14
Returning an Automatic Test Program to Default State.................................... 8-15
9 Electrical Probe Test........................................................................... 9-1
Electrical Probe Testing..................................................................................... 9-1
Probe Test Selection .......................................................................................... 9-2
LED Testing .................................................................................................. 9-2
Photodiode Test............................................................................................. 9-3
Resistance Testing......................................................................................... 9-4
10 Adjusting the LCD Screen.................................................................. 10-1
Introduction........................................................................................................ 10-1
LCD Contrast Settings....................................................................................... 10-1
11 Manufacturers Mode ........................................................................... 11-1
Introduction........................................................................................................ 11-1
Automated Testing............................................................................................. 11-1
Manufacturer Parameters Available For Simulations........................................ 11-1
Mathematical Background................................................................................. 11-2
Notes on the Formulas................................................................................... 11-3
The R-Value Equation................................................................................... 11-3
Accessing Manufacturers' Tests......................................................................... 11-4
Setting the Signal Source............................................................................... 11-4
Setting the R-Value ....................................................................................... 11-5
Adjusting the Step Amount........................................................................... 11-6
12 Creating Your Own R-Curve............................................................... 12-1
Introduction........................................................................................................ 12-1
Generating an R-Curve from a Pulse Oximeter................................................. 12-2
Downloading an R-Curve into the Simulator .................................................... 12-2
Appendices
A Printing and Data Transfer.......................................................................... A-1
B Error Messag es and Corrective Measures ................................................... B-1
C Accessories List........................................................................................... C-1
D Specifications .............................................................................................. D-1
E Computer Control........................................................................................ E-1
F Typical Questions and Answers.................................................................. F-1
G Custom Probe Test Cables and Electrical Simulation Cables..................... G-1
H Glossary....................................................................................................... H-1
iii
Page 8

Index 2MF
Users Guide
iv
Page 9

List of Tables
Table Title Page
5-1. Oximeters with Technology Options ..................................................................... 5-4
5-2. Oximeters with R-Curve Parameters...................................................................... 5-4
6-1. Simulations Menu Selections................................................................................. 6-2
6-2. Level 0 Presets ....................................................................................................... 6-5
6-3. Light Artifacts........................................................................................................ 6-7
8-1. Automatic Test Parameters .................................................................................... 8-2
v
Page 10

Index 2MF
Users Guide
vi
Page 11

List of Figures
Figure Title Page
2-1. Sphygmomanometer .............................................................................................. 2-1
2-2. Formula for Determining Saturated Oxygen Level................................................ 2-2
2-3. Diagram of Sa mple Finger Probe for a Typical Pulse Oximeter............................ 2-3
2-4. Diagram of Light Absorbers in Tissue................................................................... 2-3
2-5. AC/DC Infrared and Red Absorption Ratio........................................................... 2-3
3-1. Basic Simulator Functions ..................................................................................... 3-2
8-1. The Program Creation Sequence............................................................................ 8-2
vii
Page 12

Index 2MF
Users Guide
viii
Page 13

Capabilities
The Index 2MF SpO2 Simulator (hereafter the Simulator) allows accurate verification of
pulse oximeters by allowing you to test them in a variety of ways. The Simulator
provides simulations that allow thorough testing of the complete pulse oximeter,
including the optical sensors.
For the first time, there is now a reliable way to gauge the condition and performance of
standard pulse oximeters. Fluke Biomedical provided the Simulator to the medical and
health industry to allow measuring pulse oximeters currently on the market against
widely accepted performance standards.
Using the Simulator as a virtual patient's index finger, you set the the Simulator to
simulate a patient with virtually any combination of blood oxygen conditions. When the
pulse oximeter under test connects to the Simulator, measure its responses against a set of
predetermined readings for a patient with the preset saturation levels and pulse
conditions. This procedure matches the pulse oximeter's results against the simulations.
Electrically, the Simulator can verify probe diodes, wire continuity, LEDs, and oximeter
accuracy.
Chapter 1
Introducing the Index 2MF
Like all of Fluke Biomedical's hardware and software systems, the Simulator is backed
by Fluke Biomedical's superior support system. If the Simulator ever fails to work
perfectly, please refer to the phone, fax, and Internet numbers at front of this book to
contact Fluke Biomedical's Technical Support Staff.
Index 2MF Compatibility
The Simulator can quickly establish the state of any given pulse oximeter and determine
the performance qualities of the device. The Simulator can test and evaluate most
oximeters in the market today.
Because each pulse oximeter manufacturer uses a slightly different technology and
algorithm to measure SpO2 (Saturation of Peripheral Oxygen), Fluke Biomedical has preprogrammed into the Simulator a number of different R-values versus SpO2 curves for
specific manufacturers. This ensures that the Simulator provides the closest possible
simulation. It is also possible to download additional R-value curves directly into the
Simulator for non-volatile storage and use. Additionally, the MFE version of the
Simulator has adapter cables to test most popular oximeters and probes through electrical
simulations.
1-1
Page 14

Index 2MF
Users Guide
Note
The Simulator is not intended to be a pulse oximeter calibrator.
Index 2MF Features
• Conduct saturated peripheral oxygen (SPO2) simulations with saturation levels
between 35% and 100% (in 1% increments)
• Complete probe and electronics assembly testing either optically or electrically (FE
version only)
• Probe continuity check (MFE version)
• Variable heart rate settings from 30 beats per minute to 250
• Preset simulations reproduce several patient conditions
• Alarm tests for response time, recovery time, and pulse amplitude
• Portable, weighing in at under 3 pounds, with a 10" x 10" footprint
• Programmable auto sequences
• Computer controllable
• Menu driven with softkey interface and 2-line by 24-character LCD (liquid crystal
display) supertwist, alphanumeric display
• Rechargeable lead acid battery, with 8 hours of continuous operation and built-in low
battery indicator
Package Contents
The contents of the Simulator package as shipped include:
F Version
• Index 2MF Pulse Oximeter Tester
• Battery Charger
• User's Guide and Registration Card
FE Version
• Index 2MF Pulse Oximeter Tester
• Battery Charger
• Users Guide and Registration Card
• Ohmeda and Nellcor Probe and Oximeter Adapter (electrical cables)
Registration Card
Once the the Simulator is successfully up and running, complete the postage-paid
registration card and mail it to Fluke Biomedical.
Precautions
Observe these precautions when using the Simulator; like any electronic equipment, it
should be adequately protected both when moving and in storage.
1-2
W Caution
When connecting the Simulator to a peripheral, such as a PC’s
RS232 serial port or printer, power OFF both the Simulator and
the peripheral device during connection and disconnection.
Failure to follow this precaution may result in damage to the
equipment.
Page 15

Introducing the Index 2MF
The Simulator battery life can be seriously shortened by leaving
the instrument turned on for many hours after the low battery
alarm sounds. To avoid damage:
• Package and handle the Simulator to ensure that the power
switch cannot accidentally turn on during shipment.
• Always connect the Simulator to its charger when not in
use. The Simulator allows continuous charging, a practice
that ensures full power charge whenever needed. The
Simulator may also be used while charging.
• Always turn off the Simulator and connect it to its charger
when the low battery alarm sounds. The Simulator can be
used within about 1 minute of commencing charge. The
Simulator picks up two or more hours of battery run-time
for each hour of charger connect time, even when running
with the charger connected.
• Avoid placing the Simulator in contact with, or in close
proximity to, Electrosurgery units (ESUs), MRIs, and
defibrillators.
Electromagnetic Interference and Susceptibility 1
• Only use an appropriately rated battery charger to avoid
damage to the Simulator’s battery. Based on the testing
below, the Simulator bears the CE mark.
Electromagnetic Interference and Susceptibility
EC EMC Directive 89/336/EEC
EN 50081-1, CLASS A-Emissions
The Simulator has been type tested by an independent testing laboratory and found to
meet the requirements of EC Directive 89/336/EEC for Radiated Emissions and Line
Conducted Emissions. Verification was to the limits and methods of EN 55011. The
device is classified as EN 55011, Group 1, Class A.
EN 50082-1 Immunity
The Simulator was also tested and found to meet requirements for Electrostatic Discharge
Susceptibility, Radiated Susceptibility, and Electrical Fast Transient/Burst Susceptibility.
Verification of compliance was conducted to the limits and methods of EN 500821:1992, IEC 801-2, IEC 801-3, and IEC 801-4.
Note
If using a battery charger while the Simulator is in the Electrical
Simulation Mode, the user may observe a spike in the voltage on the line to
the battery charger, thereby producing erratic results. If you suspect this
has occurred, retest the oximeter with the battery charger disconnected.
USA FCC CLASS A
This equipment has been tested and found to comply with the limits for a Class A digital
device, pursuant to Part 15 of the FCC Rules.
These limits provide reasonable protection against harmful interference when operating
the equipment in a commercial environment. Like similar medical equipment, this
1-3
Page 16

Index 2MF
Users Guide
equipment generates, uses, and can radiate radio frequency energy and, if not installed
and used in accordance with the instruction manual, may cause harmful interference to
radio communications. Operation of this equipment in a residential area could cause
interference, in which case the user will be required to correct the interference.
Canadian Department of Communications Class A
This digital apparatus does not exceed Class A limits for radio emissions from digital
apparatus set out in the Radio Interference Regulations of the Canadian Department of
Communications.
Le present appareil numerique n'met pas du bruits radioelectriques depassant les limites
applicables aux appareils numerique de la Class A prescrites dans le Reglement sur le
brouillage radioelectrique edicte par le ministere des Communications du Canada.
Note
The Simulator, like many pulse oximeters, may have its operation affected
by strong electromagnetic sources, such as electrosurgery equipment. It
may also be affected by imaging equipment, such as Magnetic Resonance
Imaging (MRI). It is the user’s responsibility to verify performance of the
Simulator prior to use in these kinds of environments.
Safety
The Simulator is a battery-powered device which operates at voltages that are considered
intrinsically safe. Independent laboratory approval to a test standard is thus not required.
The battery charger used must meet the safety requirements for your country.
EC Directive 73/23/EEC, Low-Voltage Directive
The Simulator operates below 75 VDC, thus EN 61010-1 is not applicable.
Getting Started
Unpack the Simulator from the shipping carton. Check that the shipment is complete and
all parts are intact.
Starting the Simulator and Using Menus
Slide the power switch to the "I" (on) position to power up the system. The LCD
sequentially displays the following screens for about two seconds each before ending on
the Main Menu 1 screen:
• Bio-Tek Instruments Inc. with model number and version
• Software version number and copyright years
• Make of last selected R-curve
• Main Menu 1 screen
1-4
esl001.eps
Page 17

Introducing the Index 2MF
Getting Started 1
When the Main Menu 1 displays in the LCD, the following options are available:
esl002.eps
The Main Menu 1 consists of the primary test functions available to the Simulator, with
the softkeys SIM, LMTS, PRBE, CUST, and more. Press the up arrow below the
menu item to select a menu option, change a setting, or to advance to additional menus.
Note
If you see a message warning you that your battery is low, you need to
attach the battery charger. Call Fluke Biomedical for assistance if needed.
Toggle between Main Menu 1 and Main Menu 2 with the more softkey.
For example, you have these options at the Main Menu 1 screen:
Press the softkey SIM, and then MAN to open the Simulations menu. The menu
“Simulations:” appears in the Simulator LCD. This menu allows you to set oxygen
saturation levels and heart rate in beats-per-minute (BPM).
Press the softkey MAN to set the Sp02 levels from 35 to 100%, and the heart rate from 25
to 250 BPM.
1. The LCD indicates current Sp02 level and BPM. Additional softkey options now
available allow you to set the SpO2 levels (02+ and 02-), as well as increase and
decrease the BPM (BPM+ and BPM-).
Return to the previous menu, Main Menu 1, by pressing the esc softkey. For detailed
information on using the test features, refer to the relevant chapter in this users guide.
Using the Optical Finger Probe (All Versions)
Connect the finger probe of the pulse oximeter under test to the Simulator finger probe
attachment. Position the pulse oximeter LEDs on the bottom of the Simulator finger
probe attachment.
From the Main Menu 1, select the SpO2 and Heart Rate settings by pressing the
appropriate + or - (plus or minus) keys, and viewing the settings displayed on the the
Simulator LCD.
Note
Oximeters take from 5 to 20 pulses to respond to a change in simulated
SpO2 or BPM.
1-5
Page 18

Index 2MF
Users Guide
1-6 2-1
Page 19
Blood Oxygen and Pulse Oximeters
Blood Pressure
Blood pressure readings provide valuable information about the condition of our bodies,
indicating health or the lack of it. As the heart contracts (systole) and relaxes (diastole),
the volume of freshly-oxygenated blood increases and decreases measurably within the
artery walls. This action causes the artery walls to expand and contract in rhythm with the
heart. The force of the blood exerted upon the artery walls is what is called blood
pressure. Contraction produces the highest pressure, and relaxation the lowest.
A sphygmomanometer (shown in Figure 2-1) is one tool for measuring blood pressure.
When our blood pressure is taken, it is measured at the brachial artery in the forearm in
millimeters of mercury (mmHg). If our blood pressure reading is at or near 120 mmHg
(systolic) over 80 mmHg (diastolic), we are considered to be in peak health, all else being
normal.
Chapter 2
Figure 2-1. Sphygmomanometer
esl003.eps
Gases in Blood
Blood pressure is not the whole story, however, since the exact concentration of gases
such as carbon dioxide and especially oxygen in your blood cannot be determined by a
simple blood pressure test.
To determine gas concentrations accurately, specifically saturated oxygen, a blood-gas
sensing device must be used, and must be capable of detecting the wide range of nominal
values for these gases. Gas concentrations in blood, specifically oxygen (O2) and carbon
Page 20
Index 2MF
Users Guide
dioxide (CO2), can be expressed as milliliters of gas per liter of blood, and can be
indicated by the partial pressure that the gases exert in your blood at a given temperature.
Pulse Oximeters
Because of their ease of use in many hospital- and critical-care situations, pulse oximeters
have greatly increased in popularity since their introduction. Today, pulse oximeters are
virtually required equipment in situations where the monitoring of arterial oxygen
saturation (SaO2) is essential, such as when anesthesia is in use, both during an operation
and in post-operative recovery, intensive care, transport, and patient home care.
Pulse oximeters have proven to be capable and reliable, being highly accurate in
measuring blood SaO2 in the range of 80-100%, while at the same time needing little, if
any, calibration. No patient preparation is required before using the pulse oximeter; in
addition, the devices are so simple to operate that specialized training is unnecessary.
How Pulse Oximeters Work
Pulse oximeters are defined as non-invasive, arterial, oxygen-saturation monitors which
measure the ratio of two principal forms of hemoglobin in the blood: saturated arterial
hemoglobin (also called oxyhemoglobin), HbO2/SAT, to unsaturated (or reduced)
hemoglobin, Hb.
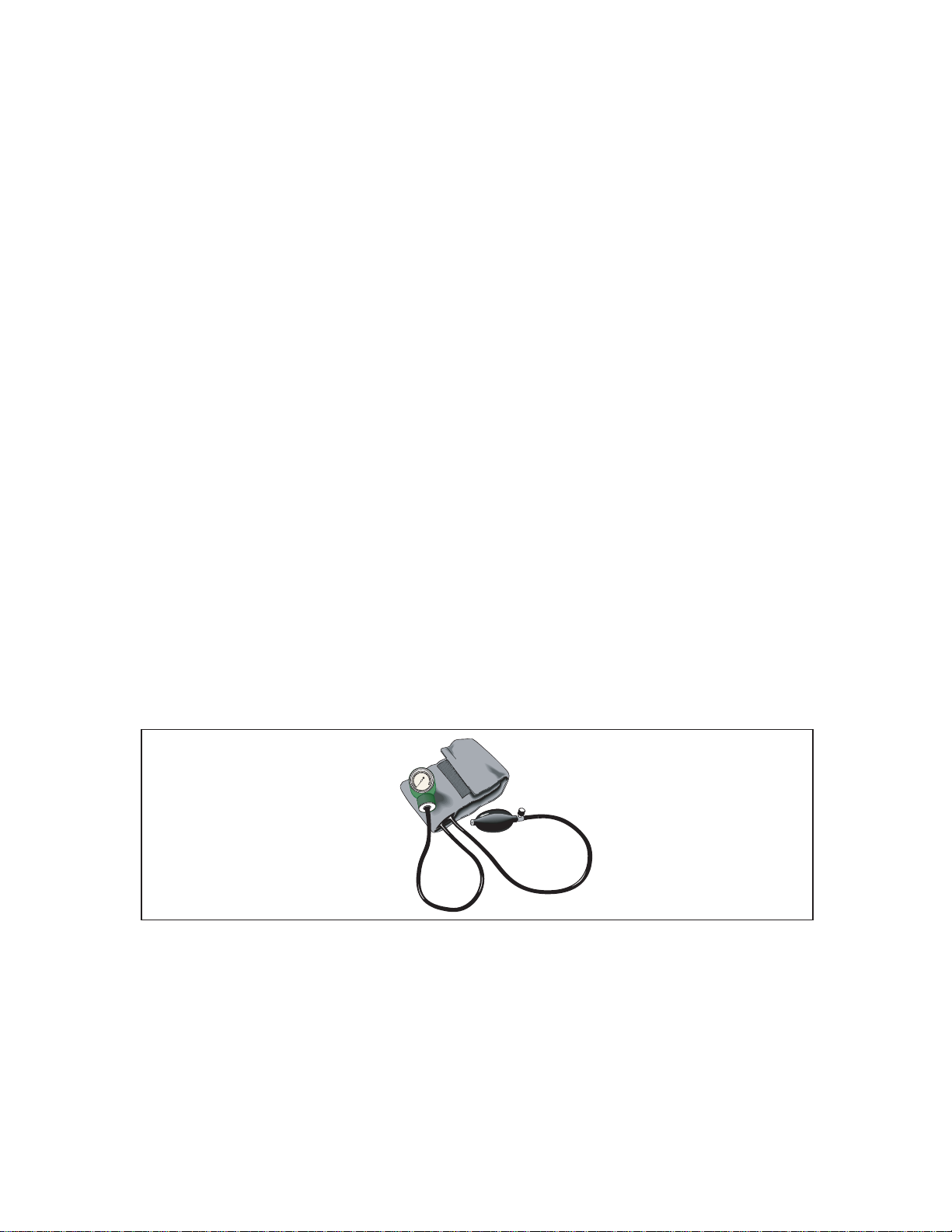
The arterial oxygen saturation, SaO2, is defined as the ratio of the concentration of
oxyhemoglobin (cHbO2) to the concentration of HbO2 + Hb (cHbO2 + cHb). Oxygen
saturation is commonly expressed as a percentage and is calculated according to the
formula in Figure 2-2.
Figure 2-2. Formula for Determining Saturated Oxygen Level
Using this information, a correctly calibrated and operating pulse oximeter can accurately
predict the level of oxygen in the blood, which in turn provides valuable data about the
health of a patient, and in the case of anesthesia and post-operative recovery, the status of
the patient.
Spectrophotometry
Pulse oximeters operate on the principle known as spectrophotometry, using wavelengths
of light to determine the concentration of oxygen in the blood. Because we already know
the wavelengths for the light absorption of blood hemoglobin, we can mathematically
determine the arterial oxygen saturation in a patient's blood.
The light emitting diodes (LED's) of a pulse oximeters shine two types of light—near
infrared light (at 940 nanometers) and red light (at 660 nanometers)—wavelengths that
pass through the skin and which are absorbed by both the oxyhemoglobin and the
reduced hemoglobin. These light beams pass through the index finger of a patient to
photo detectors on the opposite side of the pulse oximeter.
esl004.eps
2-2
Page 21
Blood Oxygen and Pulse Oximeters
Pulse Oximeters 2
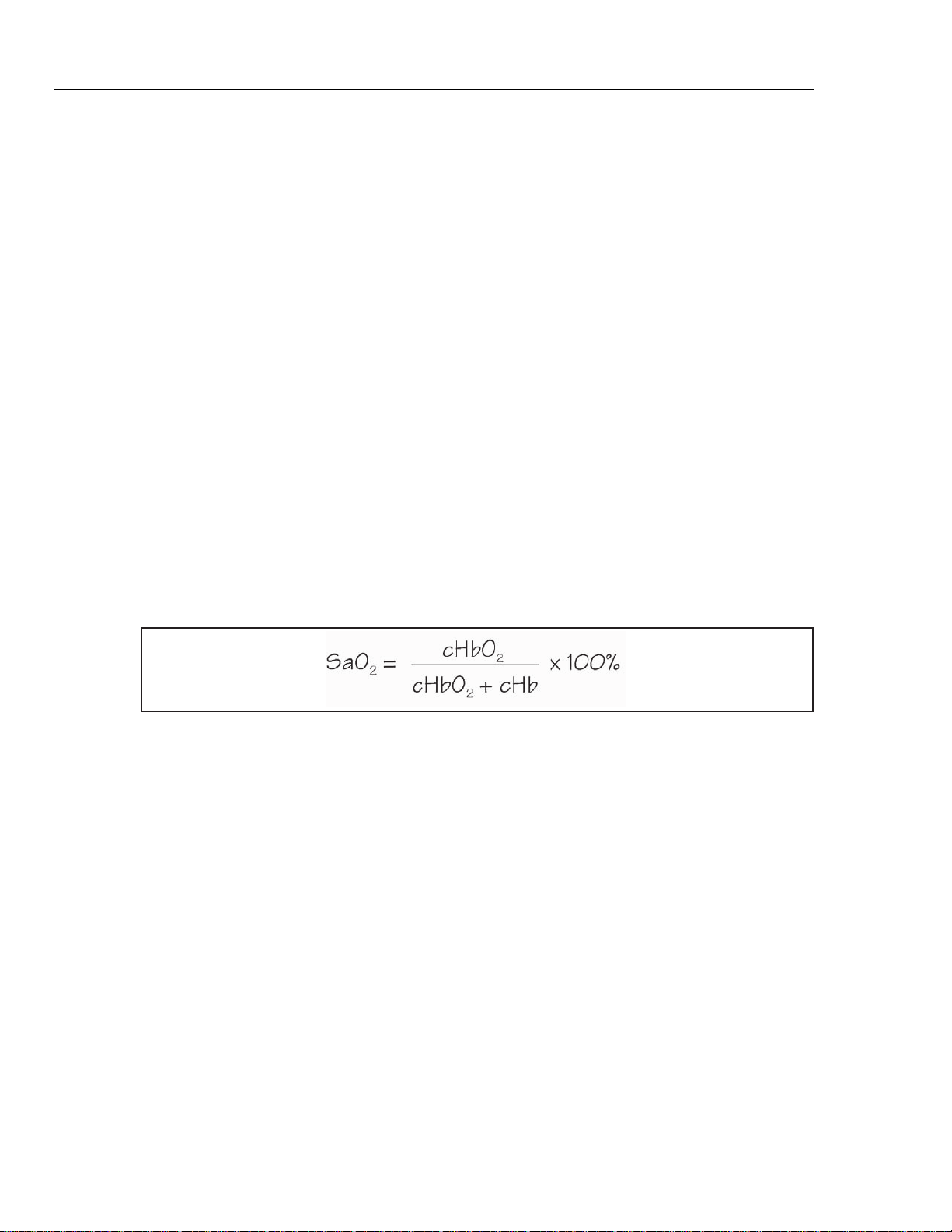
Figure 2-3 shows a typical pulse oximeter configuration, noting the location of the red
and infrared LED's.
Figure 2-3. Diagram of Sample Finger Probe for a Typical Pulse Oximeter
940nm Infrared LED
660nm Red LED
Photosensor
esl005.eps
Using this dual light emitting and sensing technology, the pulse oximeter determines the
amount of light absorbed by the blood and calculates the percent of oxygen saturation
(SaO2).
However, it is not quite that simple. Pulse oximeters must also calculate out the effect of
absorption caused by the presence of venous and capillary blood and soft tissue in order
to obtain the true SaO2 value. To do so, pulse oximeters use a system that distinguishes
between “AC" components (the pulsating arterial blood) and “DC" components (the non-
pulsating components mentioned just above).
Figure 2-4 shows the different AC and DC components graphically.
AC
Light
Absorption
DC
Figure 2-4. Diagram of Light Absorbers in Tissue
Absorption due to pulse
Capillary blood
esl006.eps
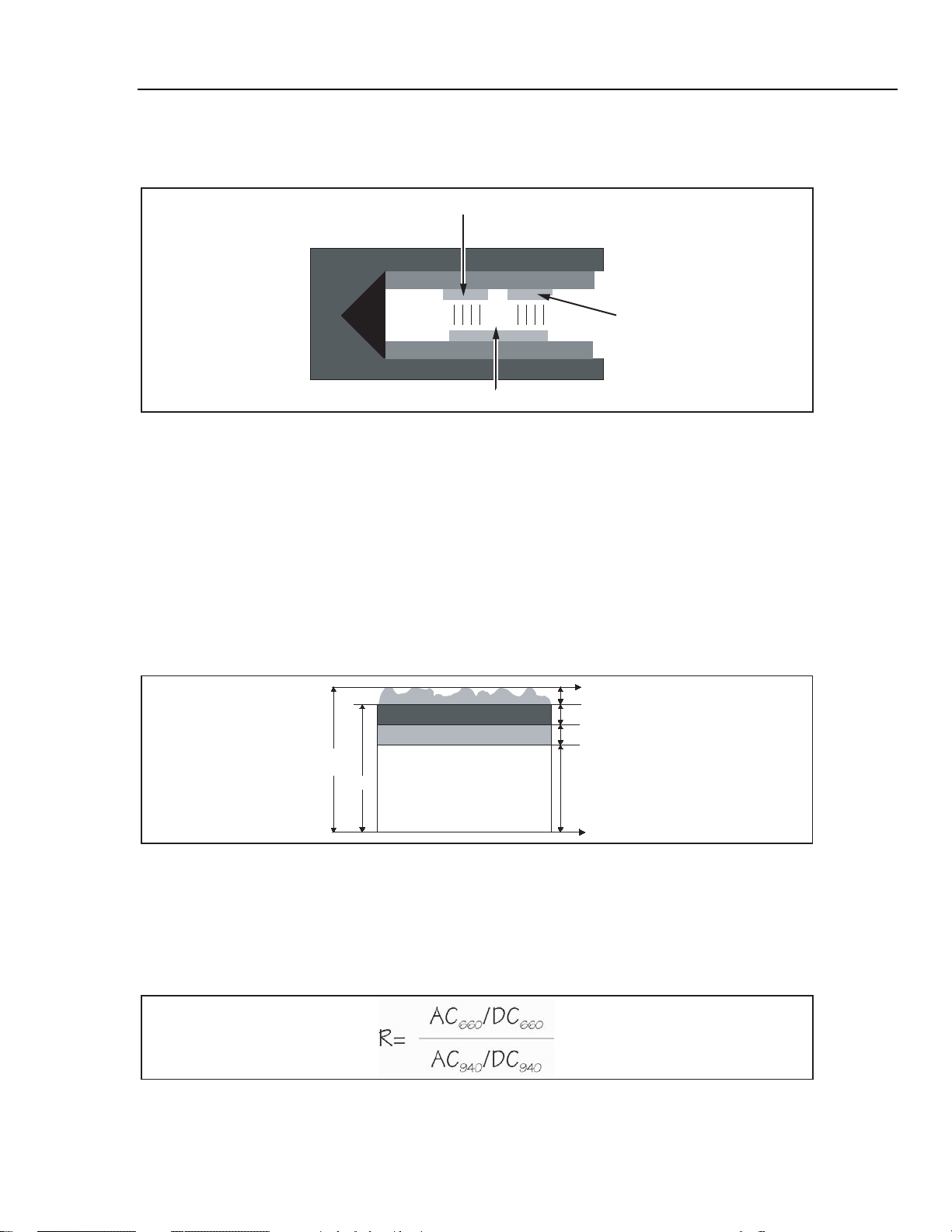
The pulse oximeter determines the AC component of absorbency at each wavelength and
divides this by the corresponding DC (amplitude) component. This results in a "pulseadded" absorbency that is independent of the light intensity. The ratio (R) of these pulseadded absorbances is calculated using the formula shown in Figure 2-5.
Figure 2-5. AC/DC Infrared and Red Absorption Ratio
esl007.eps
When the ratio of red-to-infrared absorbance equals 1.00, the saturation is approximately 81%.
2-3
Page 22

Index 2MF
Users Guide
References
• Accuracy and Precision of Fourteen Pulse Oximeters, B. Hannhart, et al, Neonatal Intensive Care,
Nov./Dec. 1991.
• Clinical Pulse Oximetry, Thomas L. Petty, MD, Anesthesiology, v. 70, no. 1, Jan. 1989.
• Oximetry/Blood Gas, --- , Medical Electronics, Oct. 1989.
• The Concise Columbia Encyclopedia, (Columbia University Press, 1991), Microsoft Bookshelf, 1992.
2-4
Page 23

Index 2MF Overview
The Index 2MF "Finger"
The Simulator system has a patented optical “finger” that takes the place of an actual
patient's index finger. This finger works with any pulse oximeter that detects SpO2
(Saturation of Peripheral Oxygen) through the index finger (as opposed to the earlobe or
toe) tissue.
Inserting the Index finger into the pulse oximeter probe effectively connects the two
devices for testing purposes.
Note
When connecting a finger sensor, onto the Simulator's “finger,” make sure
that the red LEDs (light emitting diodes) are on the bottom and that the
pulse oximeter fingertip sensor is on as far as possible.
Simulator Electrical Testing (MFE Version only)
Electrical testing of oximeters is similar to optical testing. However, all electrical testing
simulations are output through the electrical port on the back of the Simulator, thus
eliminating the probe from the circuit. FBC makes specific cables to connect the
electrical port on the Simulator to oximeter port for electrical testing. Check FBC website
or price list for details about these specific cables. A separate probe check is performed
via the probe port on the back of the unit that analyzes LEDs, photodiodes, and wire
resistance for potential problems.
Chapter 3
Simulation Settings
Once connected, the Simulator can simulate virtually any patient condition, with
programmable SpO2 ranges from 35% to 100%, pulse rates from 30 BPM to 250 BPM,
and an amplitude factor (non-pulsatile components such as soft tissue and venous blood,
also called the DC component) of 0% to 20%.
Running Tests
The Simulator runs through a series of simulations to test the sensitivity and performance
limits of the pulse oximeter in question. You can run one test or a series of tests, with
3-1
Page 24
Index 2MF
Users Guide
stable or fluctuating oxygen and pulse levels. You can even connect the Simulator to a
computer to control the Simulator and run all tests.
Evaluating Test Results
The Simulator is what may be called a "Transfer Standard.” Such a standard lets you take
measurements on an unknown device, and see how closely it compares to a known device
measured with the same transfer standard (or an identical one).
Fluke Biomedical has measured the operational characteristics of different makes of
oximeters, all believed to be properly calibrated and operating correctly. Users access
these measurements when selecting an oximeter make for testing.
For a Transfer Standard such as the Simulator, simulating devices which have readout
resolutions of 1 % (oximeters) or repeatability of simulations better than 1 % is
mandatory. The Simulator repeatability is conservatively specified at better than +
standard deviation.
In Fluke Biomedical's experience with oximeters characterized by make, oximeters track
the SpO2 simulations typically within 1 % to 2 % from 100 % - 60 %. Below 60 %, many
pulse oximeters have unspecified accuracies; results can vary widely. That means, for
example, that if you have set up a simulation of 92 %, you can expect your oximeter to
read 92 %, ±1 %.
1
Print results for study and archiving purposes directly from the Simulator via the built-in
serial RS-232 port or the Centronics parallel printer port. Results can also be sent over a
serial cable directly to a computer, for onscreen evaluation or storage in a database.
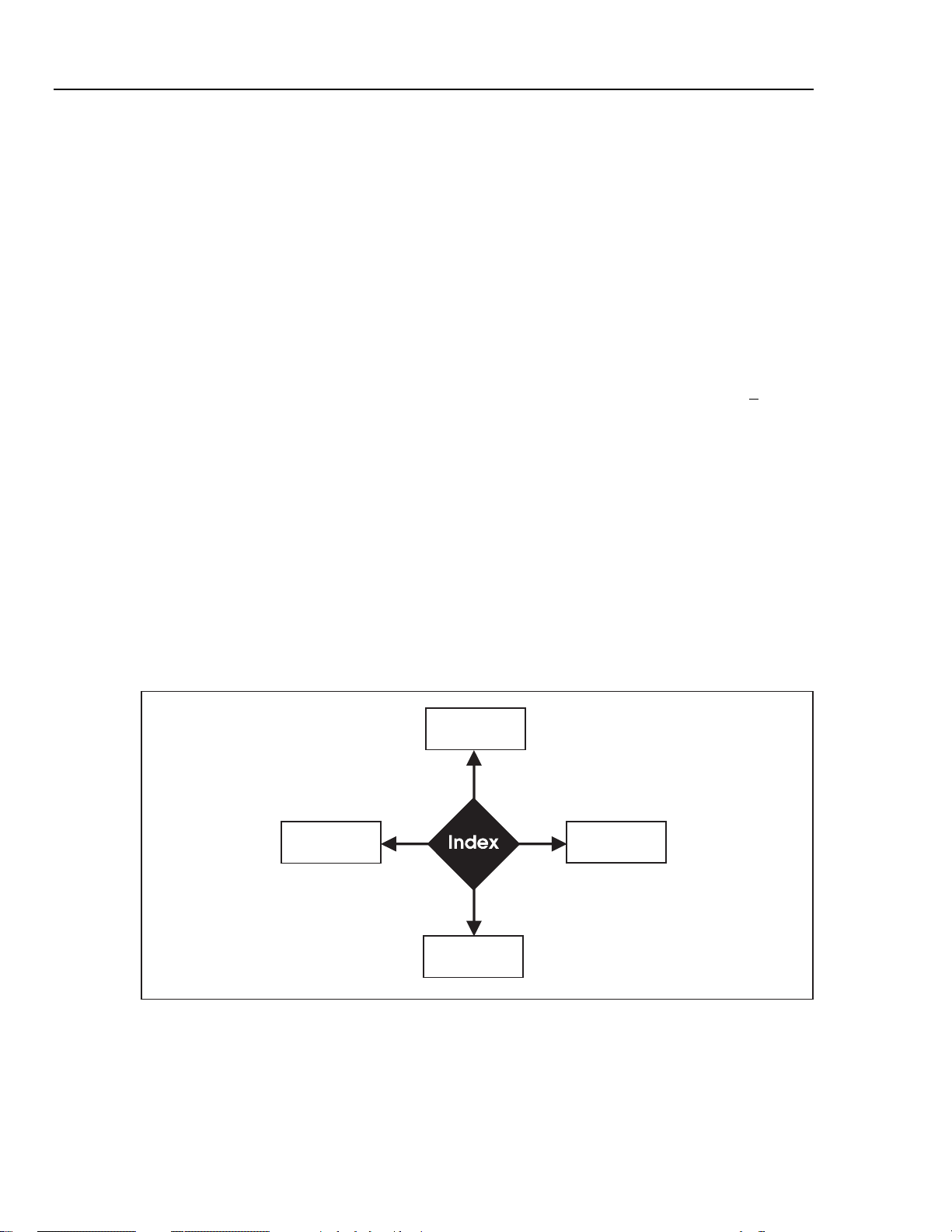
Simulator Functionality
Figure 3-1 illustrates the basic functions of the Simulator system.
Control by
Computer
Manual
Testing
Automatic
Testing
Print
Result
3-2
Figure 3-1. Basic Simulator Functions
esl008.eps
Page 25

Introduction
When you first turn on the Simulator, it sequentially displays the following screens for
about one second each before ending with the Main Menu 1 screen:
• Bio-Tek Instruments Inc. and model number and version
• Software version number and copyright years
• Make of last selected R-curve
Chapter 4
The Main Menus
Note
If at power-up, the batteries are low and in need of recharging, the message
“
WARNING! BATTERY LOW!” appears on the LCD.
esl009.eps
The Main Menu 1
The Main Menu 1 choices give you access to the full Simulator system. This section
explains each softkey and its associated menu choices.
Note
Pressing the esc softkey (visible in many menu screens) returns you to a
previous menu. Pressing more, when visible, toggles between menus.
SIM lets you set oxygen saturation levels and heart rates (BPM.)
4-1
Page 26

Index 2MF
Users Guide
fgg010.eps
Selecting SIM opens the menu displaying the following:
• MAN to run manual simulations
• PRESET to run factory-defined simulations
• AMB to set ambient light levels
• CUST to create custom makes
• esc softkey to return to Main Menu 1
LMTS lets you set the alarm limits for oxygen, beats per minute, pulse amplitude, and
lets you set up a timer for asystole.
fgg011.eps
Selecting LMTS opens the menu displaying the following:
• O2 to set up Sp02 alarm limits level and response time
• BPM to set up rate alarm limits and response time
• AMP to select pulse amplitude limits
• ASYS to set up asystole
4-2
PRBE offers the electrical probe test menu to analyze oximeter probe integrity. Tests
include LED voltages, photo diode results, and pin-to-pin resistance.
fgg012.eps
Selecting PRBE tests attached probes and displays the following options:
• LEDS to view the red and infrared LEDs
• PHTO to view photo diode results
• RES to view pin-to-pin resistance
• REDO (requires an attached probe)
• esc softkey to return to Main Menu 1
Page 27

The Main Menus
The Main Menu 2 4
The CUST menu options are for creating custom makes by choosing a light technology
and R-Curve. This custom make can be stored for later use. Custom makes are used when
the pulse oximeter being tested is not in the factory preset makes.
The Main Menu 2
The softkey more toggles between Main Menu 1 and Main Menu 2. Main Menu 2
allows you to select a pulse oximeter type by make, configure the Simulator serial
(RS232) port, and adjust the contrast of the LCD screen.
The choices available at the Main Menu 2 include:
• AUTO to create and run tailored test procedures
• UTIL to change the display and configure the RS232
• OPTS to open the Advanced Options menu
• MAKE to select the pulse oximeter being tested
• more to toggle Main Menu 1
fgg013.eps
fgg161.eps
Each of these Main Menu choices is discussed separately in the next few chapters.
Note
Pressing
esc at any point within the Simulator will always return you to
any previous menu, until you have returned to Main Menu 1 or 2.
4-3
Page 28

Index 2MF
Users Guide
4-4 5-1
Page 29

Configuring the Simulator
Configuring for Specific Oximeter Make
Light technology and R-Curve data must be known in order to test a pulse oximeter,
optically via finger or electrically. You can configure the Simulator to match the make of
pulse oximeter(s) you will be testing. The Simulator stores the definitions for different
makes of pulse oximeters. You can change variables for each of the pulse oximeter types
to match the devices you will be testing.
Accessing the Make Menu
Change the make of the pulse oximeter stored in the Simulator's memory using the Make
menu.
1. To get to the Make menu, press more from Main Menu 1.
Chapter 5
2. You will then see the screen for the Main Menu 2. Press the softkey MAKE.
fgg014.eps
fgg169.eps
Page 30

Index 2MF
Users Manual
You will see the following appear in the Simulator's LCD display:
3. Press the softkey for the + or - to scroll through the Simulator's pre-programmed
pulse oximeter makes and models. In the screen that follows, the selected make is the
Datascope.
4. To return to Main Menu 2, press esc.
5. Then, to return to Main Menu 1, press the softkey more.
Verified Oximeter Makes
The following makes of oximeters have been verified as working properly, both
electronically and with their probes, and are pre-programmed into the Simulator:
• BCI
• Criticare
• Datascope
• Datex
• HP
• Masimo
• Nellcor
• Nihon-Kohden
• Ohmeda
• Respironics
®
(3101)
®
(504)
®
(Passport)
®
(CardioCap, Ultima, Satellite Trans, AS/3, 251)
®
(Merlin)
®
®
(N-100, 200)
®
(Lifescope)
®
(3700) and Nova
®
esl016.eps
esl017.eps
5-2
R-Curve Specifications
Fluke Biomedical, in cooperation with the manufacturers, developed the R-Curves for the
devices listed previously.
Page 31

Configuring the Simulator
Customizing a Make Not Included Within Index 2MF 5
Note
The cooperative venture does not represent an endorsement of Index 2MF
by these manufacturers. Fluke Biomedical assumes the responsibility for
the R-Curves, and any results obtained from the use of Index 2MF.
Note
Some oximeters utilize technology and probes licensed from other
manufacturers. The R-Curve selected must correspond to the technology
used to ensure accurate results. Please consult oximeter manufacturer for
the correct technology and corresponding R-Curve within the Simluator.
W Caution
Other manufacturers’ oximeters can be electronically tested by
the Simulator, but will require special care in preparation of an
adapter cable and interpretation of the results. Damage could
occur to the Simulator or the oximeter if the adapter cable is not
correct. See the “Customizing a Make Not Included Within Index
2MF” section in this chapter for more information.
Note
Six additional manufacturer's profiles are incorporated in the Simulator.
These six profiles may be changed at the Download Make menu by
selecting Make at Main Menu 2. Additionally, five CUSTOM R-Curves can
be stored in the Simulator. Refer to Appendix E, Computer Control, for
information on how to download a manufacturer's profile into the
Simulator.
• Sat-trak®
• Nonin Onyx®
• N-10
®
®
®
*
only.
2
• Palco 300
• Invivo
• Invivo
* The R-Curves for these devices are specified from 100 to 80% O
Customizing a Make Not Included Within Index 2MF
The MF version of the simulator has incorporated many popular oximeter makes into its
data base. Unfortunately, there are many more manufacturers that cannot be incorporated
due to Simulator resource limitations and oximeter availability.
The Simulator has a “custom make” feature that allows you to create a make using just
the oximeter. The Simulator requires two elements to perform a simulation on a given
oximeter: Technology of light or light intensity and R-Curve. The Simulator will work
with most oximeters with excellent results once these choices are entered. These two
elements can be obtained from the manufacturer directly, or by trial and error. Table 5-1
lists typical oximeters that have the various technology options and Table 5-2 lists
oximeters with their R-Curve parameters.
5-3
Page 32

Index 2MF
Users Manual
Note
The Simulator accuracy specifications are not valid for user defined custom
makes. The Simulator can provide specified repeatability of
±
1 standard
deviation.
Table 5-1. Oximeters with Technology Options
Technology Options Typical Oximeters
Normal Intensity Nellcor, BCI
Low Intensity Hewlett-Packard, Ohmeda, Invivo
High Intensity Datex
Very High Intensity None known
Table 5-2. Oximeters with R-Curve Parameters
Curve No. Brand HiOx Level
1 BCI 93 82/83
2 Criticare 94 80/79
3 Datascope 94 85
4 Datex 95 84
5 Hewlett-Packard 96 85
6 Nellcor 93 80
7 Hihon Kohden 94 84
8 Novametrix 96 87/88
9 Ohmeda 95 86/85
10 Respironics 93 80
Once a technology and a curve have been determined, save the custom make for future
use.
Operation
To access the custom menu, press CUST from the main menu.
Oxy @ R = 0.7
LoOx Level
Oxy @ R = 1.0
5-4
Chose the slot to customize by pressing +CST to cycle through the available names.
Custom #1 through #5 are the default factory names. To change the name press NAME.
There are five available slots for saving custom makes. Press + or – to change the
alphabet. Press → to move the character. Press SAVE to save the name and the results.
Press TEC to test for technology type.
Select the appropriate technology type #1 - #4. Technology types offer various light level
thresholds. Refer to Table 5-1 for a list of technology light levels. The technology to use
may already be known based on the manufacturer’s information. If not, continue for
process of elimination testing.
Press HiOx to test oximeter stabuility at the high oxygen setting.
Page 33

Configuring the Simulator
Operation 5
The beeper sounds and the menu changes to HiOx. With the oximeter probe connected to
the Simulator, look for a steady reading on the oximeter. The number may be in the low
90s, but not necessarily. If the oximeter is not displaying a stable number, change
technology types again to find a stable reading.
Once a stable reading has been obtained, press LoOx to verify that the pulse oximeter is
stable at low oxygen levels. The number should be near 80%, but may be further away.
Change technology types to find a stable reading.
Press HiOx once more after a stable reading is achieved at low oxygen levels to verify
that high oxygen is still stable. Once a technology is found that locks on and gives stable
readings at low and high oxygen levels, note these readings as seen on the oximeter and
refer to the R-Curve list in Table 5-2.
Find a high level and low level that are similar to your readings and move to the left of
the chart for a curve number to use in the next step. Press CRV to advance to the next and
final step.
Choose an R-Curve that best fits this oximeter.
To do this, press +CRV to advance through the list of installed MFG R-Curves by
number. See Table 5-2. This number was determined in the previous step.
Use the +O2 and –O2 keys to verify that the oximeter tracks the curve up and down the
O
scale (70 to 100 % should be sufficient). Once a best curve fit is determined, press
2
SAVE to save the custom make.
The custom make appears in the make list in Main Menu 2.
Note
“Custom Make” percent O2 accuracy is not guaranteed. The Simulator can
only guarantee accuracy of repeatability in this mode of operation.
5-5
Page 34

Index 2MF
Users Manual
5-6 6-1
Page 35

Chapter 6
Setting and Changing Simulation Factors
About Simulations and Presets
One of the main functions of the Simulator is to test the performance of a pulse oximeter
for any possible patient condition. The Simulator allows you to set any combination of
blood-oxygen levels, heart rates, and pulse amplitude, as well as simulate a virtual range
of medical physical conditions and set an ambient light artifact.
Setting this group of default factors allows you to test an oximeter and determine its
weaknesses, if any. The Simulator will display and optionally print areas in your pulse
oximeter performance that may be unacceptable, and document correct performance.
The simulations set here are used as the defaults when testing the pulse oximeter.
Setting Simulations
To access the Simulations Menu, press the softkey SIM from the Main Menu.
The Simulations Menu
The Simulations menu appears in the LCD with these choices:
• MAN to manually set the O2 and BPM
• PRESET to set a patient’s health condition
• AMB to set an ambient light artifact
• STEP to change step size for saturation and BPM
• esc to return to previous menu (Main Menu 1)
fgg010.eps
Page 36

Index 2MF
Users Manual
Note
Do not connect the MFE version to two pulse oximeters simultaneously.
The simulator must synchronize with Red and IR signals from only one
oximeter.
fgg030.eps
Additional settings become available in the Simulations menu, as detailed in Table 6-1.
Table 6-1. Simulations Menu Selections
Selection Description
MAN Allows you to set the SpO2 levels, from 35% to 100%, and the pulse rate from 30
BPM to 250 BPM. To set the SpO2 level other than the default of 96%, or the BPM
default of 75, use MAN.
PRESET Allows you to select from the presets for patient conditions, including:
• A normal patient
• A patient with a weak pulse
• An obese patient
• A geriatric patient
• A bradycardic patient (one with an abnormally slow pulse below 60 BPM)
• A tachycardic patient (one with an excessively rapid pulse)
• Motion
AMB Allows you to set an ambient light artifact for sunlight or two types of artificial light.
To set an ambient light condition other than sunlight default, use AMB.
6-2
STEP Allows you to set a step size for both SpO2 and BPM settings. The default setting
for the Simulator is to increase or decrease SpO2 settings by 2 % per button push
and to increase or decrease BPM settings by 5 BPM per button push. For finer or
coarser level change, use STEP.
Note
To set a patient condition other than that of the Simulator default of
“normal,” use PRESET.
Page 37

Setting and Changing Simulation Factors
Setting Simulations 6
Changing the Default SpO2 Setting
The default settings (for the current session from power-up, when no prior changes have
been made) are for a simulated patient with 96% saturated oxygen levels and with a pulse
of 75 beats per minute.
To change either of these settings, press MAN on the Simulations Menu.
The manual settings screen appears on the LCD, displaying the current SpO2 and BPM:
Setting the O2 Saturation Level
You can raise or lower, as needed, the simulated degree of oxygen saturation in the
virtual patient.
1. To raise the O2 level two steps at a time, press O2+.
fgg031.eps
esl032.eps
esl033.eps
2. To lower the O2 level two steps at a time, press O2-.
6-3
esl034.eps
Page 38

Index 2MF
Users Manual
3. Press the softkeys to raise (O2+) or lower (O2-) as many times as needed to adjust
the SpO2 level.
Raising or Lowering the Pulse Rate
The Simulator also lets you adjust the pulse, for a customized virtual patient.
1. To raise the pulse rate five beats at a time, press the softkey BPM+.
2. To lower the pulse rate five beats at a time, press the softkey BPM-.
3. Press the appropriate softkey(s) as many times as needed to adjust the BPM.
Returning to Main Menu 1
To return to the Main Menu, press esc twice.
Using Preset Patient Conditions
The Simulator preset feature combines SpO2 levels, heart rates, signal strength, motion,
and pulse amplitude into 24 preset conditions, which simulate a broad range of normal
and abnormal patient conditions. The intent of this feature is to challenge the pulse
oximeter under test with a variety of patient conditions to show operation over a complete
range. The preset values are not meant for use as "clinical calls" and are generic in scope.
esl035.eps
esl036.eps
6-4
Page 39

Setting and Changing Simulation Factors
Table 6-2. Level 0 Presets
Using Preset Patient Conditions 6
Preset Name Saturation
Normal 98 % 55 BPM 5.0 % - - 0 % 00
Normal/ Tap 98 % 55 BPM 5.0 % 78 % 2.5 Hz 7 % 01
Normal/ Shiver 98 % 55 BPM 5.0 % 78 % 6.0 Hz 15 % 02
Weak Pulse 90 % 95 BPM 0.65 % - - 0 % 03
Weak Pulse/
Tap
Weak Pulse/
Shiver
Bradycardia 88 % 45 BPM 5.0 % - - 0 % 06
Brachycardia/
Shiver
Hypoxic 70 % 95 BPM 2.0 % - - 0 % 08
Hypoxic/ Tap 70 % 95 BPM 2.0 % 50 % 4.3 Hz 3 % 09
Hypoxic/
Shiver
90 % 95 BPM 0.65 % 60 % 4.3 Hz 1 % 04
90 % 95 BPM 0.65 % 60 % 6.0 Hz 3 % 05
88 % 45 BPM 5.0 % 68 % 6.0 Hz 10 % 07
70 % 95 BPM 2.0 % 50 % 6.0 Hz 8 % 10
Pulse
Rate
Signal
Strength
Saturation Frequency Amplitude
Motion
[presets#]
Neonate 90 % 180 BPM 1.0 % - - 0 % 11
Neonate
/Shiver
Tachycardia 85 % 130 BPM 1.2 % - - 0% 13
Geriatric 92 % 95 BPM 2.4 % - - 0 % 14
Obese 93 % 90 BPM 3.0 % - - 0 % 15
Brady Tap #2 88 % 45 BPM 5.0 % 96/100 % 3.9 Hz 0/4 % 16
Hypox Tap #2 70 % 95 BPM 2.0 % 96/100 % 4.3 Hz 0/3 % 17
Weak Tap #2 80 % 95 BPM 0.9 % 96/100 % 3.6 Hz 0/1 % 18
Normal Tap #2 93 % 55 BPM 5.0 % 96/100 % 2.5 Hz 0/3 % 19
Asystole 91 % 90 BPM 2.0 % 96/98 % 1.1 Hz 0/1.1 % 20
LowFreq1 80 % 75 BPM 1.0 % 93/97 % 0.5 Hz 0/4.2 % 21
LowFreq2 70 % 75 BPM 1.0 % 96/100 % 0.5 Hz 0/4.2 % 22
Slow Tap 80 % 75 BPM 1.0 % 96/100 % 2.0 Hz 0/3.0 % 23
90 % 180 BPM 1.0 % 70 % 6.0 Hz 5 % 12
Note
When presets with tap or shiver are selected, there is a 10-second delay
before the motion simulation starts.This delay will prevent an oximeter
from "locking on" to patient parameters from a previous simulation.
The Simulator is preset to simulate the SpO2 and pulse rate ratios.
1. Access the Simulations menu by pressing the softkey SIM in Main Menu 1.
6-5
Page 40

Index 2MF
Users Manual
The Simulations menu appears in the LCD screen:
2. To view the preset patient condition, press the softkey PRESET.
The values preset at the factory appear on the LCD screen:
fgg010.eps
fgg038.eps
esl039.eps
6-6
3. Press the softkey under the + or - to scroll through and select from the available
presets.
4. Return to the Main Menu 1 at this time, press esc again.
Note
Upon leaving the Presets menu, the Simulator returns to its default
(normal) preset patient condition simulation.
esl040.eps
Page 41

Setting and Changing Simulation Factors
Setting the Light Artifact 6
Setting the Light Artifact
You can set a light artifact with the Simulator to test oximeters under different simulated
ambient (surrounding) light conditions. The available simulations are as shown in Table
6-3.
Table 6-3. Light Artifacts
Light Type Frequency Description
Sunlight n/a Simulates sunlight by controlling the output from an LED so that a
light level is added to the simulation. This light level is present
both between, and during, red and infrared pulses.
Artificial 50 Hz Hz (Hertz) is the frequency of the light measured in cycles per
second. Artificial light is simulated by superimposing 50 Hz noise
on the pleth wave.
Artificial 60 Hz Simulated by superimposing 60 Hz noise on the pleth wave.
1. To select an ambient light condition, press AMB from the Simulations menu.
The AMBIENT setting displays the last setting used, in this example, NORMAL,
which is changed using the softkeys shown here:
Note
The O2 and BPM simulation used is the one last set using the SIM menu, or
the default as shipped, if no changes have yet been made.
2. Press a softkey to change the preferred ambient light presets from those used in a
previous test:
fgg041.eps
esl042.eps
esl043.eps
6-7
Page 42

Index 2MF
Users Manual
3. Once you have selected an ambient light setting, you can return to the Simulations
menu by pressing esc. Press esc again to return to Main Menu 1.
Setting 02 and Pulse Rate Step Size
You can vary the size of the steps for O2 and pulse rates used when setting up tests and
testing oximeters with the Simulator.
If you select an O2 step size of 5, for example, then when you increase or decrease SpO2,
the SpO2 will increase or decrease by 5%.
Note
Step amounts come in to play in three places from the Main Menus:
• With the Simulation option MAN (manual) submenu
• With the LMTS (limits) option 02 | 02 Alarm and BPM | Rate
Alarm submenu
• With the AUTO (Autosequence) menu’s PROG submenu for setting
SpO2 level and pulse rate
Set the step amount for SpO2 levels using the following steps:
1. To choose a step amount for the SpO2 level and the pulse rate, press STEP from the
simulations menu.
esl170.eps
2. To increase or decrease the O2 step amount, press O2+ or O2-, respectively, as
needed.
esl046.eps
3. To increase or decrease the pulse rate step amount, press BPM+ or BPM- as needed.
6-8
4. Once you have finished setting the O2 and pulse rate step amount, press esc to
return to the Simulations menu. Press esc again to return to Main Menu 1.
esl047.eps
Page 43

Testing the Pulse Oximeter Limits
Introduction
Once you have determined the range in which you want to test a pulse oximeter for SpO2
and rate, you can test the pulse oximeter for upper and lower limits for response time.
Testing Pulse Oximeter Limits
You can set your oximeter to sound an alarm whenever any pre-determined limits are
reached during the testing of your pulse oximeter.
From the Main Menu 1, press the LMTS softkey.
Chapter 7
The following menu appears in the LCD:
Note
Exit this menu with the softkey esc, which always returns you to the
previous menu, in this case the Main Menu 1.
Simulating Oxygen Conditions
You can test the sensitivity of your pulse oximeter by setting the simulated oxygen levels.
1. To set the oxygen limits, press
there. Then, press the softkey O2 in the Limits menu.
LMTS at the Main Menu 1 if you are not already
fgg011.eps
esl049.eps
7-1
Page 44

Index 2MF
Users Manual
The display changes to present these options:
When setting new oxygen limits, the Simulator displays the last setting used (or 96%
if not previously changed) and resets the time to 00.0 seconds.
2. Press the softkeys +
or - to start the timer, and the screen displays the “Timing!”
message.
esl049.eps
esl051.eps
esl052.eps
• Pressing the softkey + (plus) will increase by the step amount set previously
using the Simulations menu without affecting the timing function.
• Pressing the softkey –
(minus) will decrease by the step amount without
affecting the timing function.
Note
Continue pressing + or - until the O2 percent you want to test for is
displayed. Each press pf the softkey + or
– resets the timer internally and
restarts the timing.
3. If the O2 setting is outside the oximeter's alarm limit, the alarm will sound on the
pulse oximeter being tested after a period of time. Press STOP immediately. This
stops the timer, and displays the “elapsed time to alarm” for the specified simulation
level.
4. To print the results of the alarm, press PRT.
esl053.eps
7-2
Page 45

Testing the Pulse Oximeter Limits
Simulating the Pulse Rate 7
esl054.eps
The results will be sent out the RS232 and the Centronics ports to an attached printer.
Note
You can connect the Simulator to any printer with a serial or parallel
Centronics-type port or to any IBM-compatible PC, including laptops and
notebooks. For information on how to connect the Simulator to other
systems or peripherals, see “Appendix A: Printing & Data Transfer”.
If a printer or computer is attached, your results should look like this example:
O2 Alarm Response Time:
-------------------------
13.0 sec
5. When you are finished testing the pulse oximeter for SpO2 readings, press esc to
return to the Limits menu.
Simulating the Pulse Rate
The Simulator simulates a variety of patient conditions, while you observe the effect of a
changing pulse rate with a static SpO2 on the pulse oximeter, as well as the effects of
using a changing pulse rate with a changing SpO2 percentage.
You can vary the pulse rate (beats per minute) of the simulated patient index finger or
simulate the pulse rate electrically.
1. To access the pulse rate alarm menu, press the softkey LMTS from Main Menu 1,
and then press BPM to access the Rate Alarm menu.
You will see this pulse Rate Alarm menu displayed on the LCD:
esl055.eps
esl056.eps
7-3
Page 46

Index 2MF
Users Manual
Note
Notice, as with most menus, the last softkey, esc, will always return you to
the previous menu, in this case the Limits menu.
When setting a new BPM limit, the Simulator always starts with the last pulse rate
setting.
• Press
+ and the simulator will incrementally increase the pulse rate from the value
shown on the display.
Note
Pressing the softkey + (plus) will increase by the step amount set previously
using the Simulations menu without affecting the timing function. Pressing
the softkey – (minus) will decrease by the step amount without affecting the
timing function
• The Simulator displays the message “Timing!” and maintains an internal timer
until you press STOP or the + or – softkeys.
esl057.eps
esl058.eps
• Press - and the simulator will decrease the pulse rate from the displayed value
with each softkey press.
2. If the selected simulation rate exceeds the oximeter alarm rate, the alarm will sound
on the pulse oximeter being tested after a period of time. You should then press the
STOP softkey immediately.
esl059.eps
The timer stops, and displays the elapsed seconds.
Note
Pressing the softkeys + or – resets the Rate Alarm timer.
3. Press softkey PRT to send the testing results to the printer.
7-4
Page 47

Testing the Pulse Oximeter Limits
Simulating Pulse Amplitude 7
The results will be sent to the RS232 and Centronics port.
Note
Connect the Simulator to any printer with a serial port or parallel
Centronics port, or to any IBM-compatible PC, including laptops and
notebooks. For information on how to connect to other systems or
peripherals, see “Appendix A: Printing & Data Transfer”.
If a printer or computer is attached, you will see results that look something like this
example:
Rate Alarm Response Time
-------------------------
10.0 sec
Press esc to return to the Limits menu.
esl060.eps
esl061.eps
Simulating Pulse Amplitude
The peak-to-peak amplitude of the simulated blood pressure wave can be increased or
decreased. Decreasing amplitude corresponds to a weakening pulse. You can decrease
amplitude to find where the oximeter fails to find the pulse.
You can quickly set a pulse amplitude limit.
1. To access the pulse amplitude alarm menu, press LMTS from Main Menu 1, and then
AMP from the Limits menu:
2. The Pulse Amplitude menu displays in the LCD screen:
7-5
esl062.eps
Page 48

Index 2MF
Users Manual
esl063.eps
3. To raise the Pulse Amp percentage (AC factors) in the simulated blood condition,
press the softkey +:
esl064.eps
The amplitude will increase in increments of 1% with every softkey press.
4. To lower the percentage of AC factors in the simulated blood condition, press the –
softkey:
esl065.eps
The amplitude will decrease by 1% with every softkey press down to 1 %. From 1 %
to 0.1 % the value will decrease by 0.1 %. Below 0.1 %, the value will decrease by
0.025 % per button press. Inputs below 6 % are rounded internally to the nearest
0.024 %, and above 6 % are rounded to the nearest 0.072 %. The displayed value is
the rounded value nearest the value displayed per the preceding increment/decrement
rules. If the pulse amplitude rate exceeds the oximeter alarm rate, the alarm will
sound on the pulse oximeter being tested after a period of time.
5. To print the results of the alarm, press the PRT softkey.
The results will be sent to the RS232 or Centronics port.
esl067.eps
7-6
Page 49

Testing the Pulse Oximeter Limits
Simulating Asystole or No Pulse 7
Note
You can connect the Simulator to any printer with a serial port or parallel
Centronics-style port or to any IBM-compatible PC, including laptops and
notebooks. For information on how to connect to other systems or
peripherals, see “Appendix A: Printing & Data Transfer”.
An attached computer or printer produces results that look something like this example:
Pulse Amplitude Test
------------------------At 8% the signal is lost
6. When you are done, press the softkey esc to return to the Limits menu.
Simulating Asystole or No Pulse
In a clinical setting, a no-pulse condition is life threatening. Most pulse oximeters sound
alarms in response to this condition. The Simulator can measure the response time of
these alarms.
The Simulator can simulate asystole (lack of cardiac electrical activity) to further test an
oximeter. Set up the asystole test with these steps:
1. To set up asystole condition for testing a pulse oximeter's sensitivity, first press
LMTS from Main Menu 1.
2. Then, press ASYS at the Limits menu to access the Asystole menu:
You will see the Asystole menu display in the LCD screen:
3. Press the START softkey to begin the asystole test.
esl068.eps
esl069.eps
esl070.eps
4. The asystole simulation starts and the LCD shows the “Timing!” message.
7-7
Page 50

Index 2MF
Users Manual
When the alarm sounds, press the softkey STOP.
The timer ceases counting and the elapsed time (in seconds) displays on the screen.
Note
You can connect the Simulator to any printer with a serial or parallel
Centronics-style port or to any IBM-compatible PC, including laptops and
notebooks.
5. To print the results of the test for review or archiving, press PRT.
Selecting PRT sends results to the RS232 and Centronics ports. With a printer or
computer attached, the results should look something like this example:
Asystole Response Time
---------------------
24.0 sec
For information on how to connect to other systems or peripherals, see “Appendix A:
Printing & Data Transfer”.
esl071.eps
esl072.eps
7-8
6. When you are done, press the softkey esc to return to the Limits menu.
Page 51

The AUTO Menus
The Simulator provides several pre-defined test programs to automatically sequence tests.
You would ordinarily have to manually set each test parameter every time you start the
Simulator and connected it to a pulse oximeter for testing. Pre-defined test programs save
you time, allowing you to avoid resetting test parameters for every pulse oximeter testing
session.
You can use fourteen pre-defined programs (also called autosequences), or you can
customize any or all of them and save them for repeated use. This allows you to run tests
on specific equipment or for specific patient conditions as often as needed, without
having to re-enter the settings every time.
Using a pre-defined program, the Simulator will run through the tests one by one, at the
settings you determine and for the time for each test that you specify. You are only
prompted to press a button to begin each test in sequence.
You can even tailor the test reports to contain only the information you want to see; for
example, excluding or including the device information header (which contains serial
numbers, device names and so on).
Chapter 8
Using Test Programs
Navigating Through the Programming Process
Define a set of parameters starting at the Main Menu 2 by selecting AUTO. Set the
variables for autosequencing, after you have selected to program, run, or change
programs, in one of two ways:
• Pressing
• Pressing the key labeled SEL (to accept the values shown in display)
Move to the next screen in the series as you define your automatic program by pressing
ADV when available (you can also use ADV to bypass any screen, as needed).
8-1
+ or - to increase or decrease a value
Page 52

Index 2MF
Users Guide
The Program Definition Cycle
Figure 8-1 shows in a simple, graphic way, the steps involved in creating a custom
program.
1. Select and name
the program.
6. Save the
program.
5. Select tests.
Figure 8-1. The Program Creation Sequence
The Automatic Test Parameters
Parameters listed in Table 8-1 are different for each program, viewable or changeable in
the LCD screen.
Table 8-1. Automatic Test Parameters
Name Description
2. Set oxygen
and pulse rate
levels
3. Set simulation
cycles.
4. Set the
R-type.
esl073.eps
8-2
Program Name Enter a name for this program. You can use defaults of PROG0
through PROG13 for the name, or you can enter any name of up
to eight-characters in length. The name can include letters (A-Z),
numbers (0-9), and spaces.
Print Heading Set whether you want the heading page to print.
Print Actuals Set whether you want the actuals to print. Actuals are the test
results for each of the tests. The default printout returns summary
information only.
SpO2 Setting #1 Set the percent oxygen level for the first test.
SpO2 Setting 2-9 Set the percent oxygen level for any/all subsequent tests.
Pulse Rate Setting #1 Set the BPM (beats per minute) for the first test.
Pulse Rate Setting 2-9 Set the BPM (beats per minute) for any or all subsequent tests.
Cycle Number of Pleth
Waves
Set a cycle time by selecting number of pleth waves. The
selected cycle time applies to each test in the sequence.
Page 53

Using Test Programs
Table 8-1. Auto Test Parameters (cont.)
Name Description
Make Set the make of machine being tested, such as Nellcor, HP,
Criticare, Ohmeda, Invivo Research, and so on.
O2 Alarm Test Set the Simulator to either test or bypass the SpO2 limits test.
BPM Alarm Test Set the Simulator to either test or bypass the BPM units test. If
Yes, an alarm will sound at the conclusion of testing the pulse
rate.
Pulse Amplitude Test Set the Simulator to either test or bypass the amplitude test.
Motion Test Set the Simulator to test the oximeter for patient motion.
Use Presets Set the Simulator to allow for the selection of preset patient
conditions (geriatric, obese, normal, etc.) at test time.
The AUTO Menus 8
Accessing the Autosequences Menu (AUTO)
To access the Autosequences menu, press more on Main Menu 1 and then press AUTO
on Main Menu 2. See the section “Creating a Custom Test Program” for the additional
parameters available under each option.
After you press AUTO, the following options become available:
The Autosequences menu provides the following options:
fgg181.eps
esl075.eps
• PROG to define and edit programs
• RUN to start an autosequence
• DEFS to restore defaults
• esc to return to prior menus
8-3
Page 54

Index 2MF
Users Guide
PROG lets you define or edit a set of program parameters that you can have run
automatically when needed.
esl076.eps
Press the softkey RUN to run any of the up to fourteen autosequences (pre-defined test
programs).
esl077.eps
Pressing the softkey DEFS lets you restore any or all of the programs to their default
factory settings.
esl078.eps
8-4
Creating a Custom Test Program (Autosequence)
Setting up a custom program in requires a few simple steps:
• access the Autosequences menu
• enter or select a program name
• define program parameters (enter test levels, for example)
• save the program
The program can then be run at any time after saving.
Since the program remains in non-volatile memory, it is unaffected if the instrument's
power is turned off. The program will only be changed if you modify it or return to its
default (factory-shipped) state.
Page 55

Using Test Programs
Creating a Custom Test Program (Autosequence) 8
Selecting a Test Program
Start by accessing the Autosequences menu:
1. Press the softkey AUTO on Main Menu 2.
The Autosequences menu displays the following:
2. Select PROG to define a custom autosequence program.
This Autosequences screen appears on the LCD, with the Sequence: indicating the
current program (in this instance, Program 0).
fgg132.eps
esl080.eps
esl076.eps
esl082.eps
You can then modify one of the pre-programmed test programs, from PROG0 through
PROG13. The screen displays the last program selected, for example, if you were last
using Program 5, the Simulator displays PROG 5 when you select PROG.
PROG0 through PROG13 are identical as shipped from Fluke Biomedical.
• To scroll through the list of programs (in ascending alphanumeric order), press
softkey + (plus sign).
8-5
Page 56

Index 2MF
Users Guide
• To scroll through the list of programs (in descending alphanumeric order), press
softkey - (minus sign).
Optionally Renaming the Test Program
You can rename any of the test programs. The name can be from one to eight characters
in length, containing letters, numbers, and spaces; for example “TEST1”, “TEST22A”
or “DS TEST”.
1. To edit the program name, press NAME.
esl083.eps
esl084.eps
8-6
esl085.eps
The LCD screen shows the program name for the last program used (or PROG 0 the
first time the Simulator is powered up):
esl086.eps
2. The cursor blinks over the first selected character. To edit the program name, move
between positions using the (arrow) softkey →
.
3. To edit the characters in the name, use + or – softkeys to increment or decrement the
alphabetic character.
• Since this option opens with position one selected, you do not need to press
• To select the position two, press
• To select the position three, press
→ once
→ twice
→
Page 57

Using Test Programs
Creating a Custom Test Program (Autosequence) 8
4. The name is limited to eight characters maximum. Continuing to press the softkey →
eventually returns the cursor to the first character.
5. In the example below, the second position was changed (the letter “R” in PROG 1).
Press - to decrease the letter or number as in the example below until you reach the
letter L (notice that the R is now an L).
esl087.eps
6. Or, press + to increase the letter or number in sequence; as shown below, notice that
the L is now an O.
esl088.eps
7. When you have finished editing the program name, press esc. You will be asked if
you want to save the changes.
8. Press Yes to save or No to not save.
9. Press esc to return to the Autosequence menu.
esl089.eps
The Sequence screen appears on the LCD screen. The program name, if altered,
will follow the message “Sequence:” and display the new name.
8-7
esl090.eps
Page 58

Index 2MF
Users Guide
Configuring the Program
From the Sequence menu, re-configure the parameters and values in a program, which
may include everything from BPM to oximeter model being tested; this section details
resetting those program’s values and parameters.
1. Press ADV to begin defining your program.
At any time during the program-definition process, you can press esc and
return to this initial Sequence screen. (Pressing esc at the Sequence
screen returns you to the Autosequences menu and will prompt you to save
or not save your program; from there, pressing esc returns you to the
Main menu.)
Selecting Print Settings
You can customize a program to include optional heading and actuals information at print
time with the following procedure:
1. Press SEL to toggle the Print Heading? from No to Yes.
Note
esl091.eps
esl092.eps
8-8
2. Move to the next step in the process by pressing ADV:
You can then set whether or not you want to print the actuals. Actuals refer to the
observed test data. If you select not to have actuals printed, you will see summary
information only.
Note
For information on a test printout, headings, and actuals, refer to
“Appendix A: Data Transfer Notes”.
esl093.eps
Page 59

Using Test Programs
Creating a Custom Test Program (Autosequence) 8
3. Press SEL to change the Include Actuals: response from YES to NO as needed.
4. Move to the next step in the process by pressing the softkey ADV.
Setting the 02 Level
You can set up to ten different oxygen levels and up to ten different pulse rates for the
simulation autosequences.
In each screen, the softkey ADV moves ahead to the next settable option.
Use the NEXT softkey to simply advance through the O2 and rate level
settings for each test.
1. After selecting a test, set the oxygen level for test number one:
Note
esl094.eps
esl095.eps
2. Press + or - to adjust the simulated oxygen level up or down for the first O
(1% O2 per button push). You can set up to ten O2 tests. Pressing NEXT at Rate
Setting #10 brings you back to O2 Setting #1.
3. Press NEXT to set the simulated pulse rate.
If you do not plan to test for O2, press ADV to bypass these settings.
Setting the Pulse Rate
You can set up to ten different pulse rates for the Simulator to use during a single
autosequence program.
1. Again, use
+ and/or - to adjust the BPM up or down for the first pulse rate test.
Note
test
2
esl096.eps
8-9
Page 60

Index 2MF
Users Guide
2. Press NEXT to set the pulse rate for each additional rate test, if required. You can set
up to ten rate tests. Pressing NEXT
Setting #1.
• Use + and/or - to adjust the pulse rate up or down.
• Press NEXT as many times as needed to enter up to ten different test values.
When you are done setting a time in cycles (or to bypass this screen), press ADV
to continue.
Setting the Simulation Cycle
Oximeters require a fixed number of cycles to evaluate and respond to a change in input.
You can set the expected response time (in waveform cycles), after which the Simulator
will beep twice. The double beep alerts you that the oximeter response to the simulation
should be complete.
esl097.eps
at Rate Setting # 10 brings you back to O2
esl098.eps
8-10
You can set the time, in pleth wave cycles, that each simulation in the autosequence
program will run before a double beep, using the following procedures:
1. Use
+ and/or - to adjust the time in cycles, up or down (by five pleth cycles per
button push).
esl099.eps
2. When you are done setting a time in cycles (or to bypass this screen), press ADV to
continue.
Page 61

Using Test Programs
Creating a Custom Test Program (Autosequence) 8
Selecting the Make
Next, select the make of the pulse oximeter that you will be testing.
• Press
+ or - to scroll up and down the list of pre-programmed oximeter types. For
more information on configuring the Simulator for a specific make of oximeter, refer
to Chapter 5.
• Once you have selected the oximeter make, press the softkey ADV to continue.
esl100.eps
esl101.eps
Choosing Your Tests
Your next step in creating a custom program is to choose the tests that you want the
Simulator to run. Use the following procedure:
1. Press SEL to toggle the O2 Alarm Response: response between NO and YES, as
needed.
2. When you are done (or to simply bypass this screen), press ADV to continue.
esl102.eps
esl103.eps
8-11
Page 62

Index 2MF
Users Guide
3. Next, press SEL to change the Rate Alarm Test response from NO to YES, or
YES to NO, as needed.
4. When you are done (or to simply bypass this screen), press ADV to continue.
esl104.eps
esl105.eps
esl106.eps
8-12
Selecting Pulse Amplitude
Press SEL to toggle the Pulse Amplitude response from NO to YES, as needed.
When you are done (or to simply bypass this screen), press ADV to continue.
Selecting Motion
the Simulator can also test an oximeter's sensitivity to patient motion.
1. Press SEL
to turn the motion test option on or off (YES or NO):
esl107.eps
esl108.eps
Page 63

Using Test Programs
Creating a Custom Test Program (Autosequence) 8
2. When you are done (or to simply bypass this screen), press ADV to continue.
Selecting Presets
Next, select if the pre-programmed patient types will be used..
1. Press SEL to use pre-programmed patient types (normal, weak heart, obese, or
geriatric, for example).
2. When you are done (or to simply bypass this screen), press ADV to continue, which
returns you to the Sequence menu.
esl109.eps
esl110.eps
esl111.eps
Saving Your Program
Saving the program values and parameters is the final step in creating a custom program.
1. Press the esc softkey at any time (press esc twice or ADV from the Include
Presets: screen) to return to the Sequence menu:
esl112.eps
2. Pressing esc prompts you to save your program.
8-13
Page 64

Index 2MF
Users Guide
3. Press YES to save your program, or NO to skip.
• If you press YES, you will see a message indicating your program is being
saved:
• If you press NO, you will be returned to the Autosequences menu without saving
the new program (or without saving modifications to an existing program,
whichever applies):
esl113.eps
esl114.eps
esl115.eps
8-14
Running an Automatic Test Program
You can run a saved program at any time with the following procedure:
1. Start at the Autosequences menu.
• Press RUN to display the “RUN:” screen.
esl116.eps
esl117.eps
Page 65

Using Test Programs
Returning an Automatic Test Program to Default State 8
• At the RUN screen, use + or - to display the program to be run, if it is not the
first program automatically displayed.
2. To run the displayed program, press the softkey START.
The program will prompt you as needed to press ADV to move from test to test.
3. When finished, the LCD screen displays the message “Program 2 Complete!” and
presents the option of printing the results (as well as the number of copies to print).
4. Press esc to return to the Run screen. Press esc again to return to the
Autosequences menu.
Returning an Automatic Test Program to Default State
You can return a modified (and saved) autosequence program to its default state, that is,
the way it was programmed at the factory, at any time.
1. From the Autosequences menu, press the DEFS softkey.
esl118.eps
esl119.eps
esl120.eps
A screen similar to the following displays the question “restore?” and the program
number:
8-15
esl121.eps
Page 66

Index 2MF
Users Guide
2. Use + to locate the program you want to reset, if it is not the first program
automatically displayed. Or, use ALL to restore defaults for all autosequence
programs.
esl122.eps
3. Press OK to reset the defaults for the listed program.
esl123.eps
• If you selected (ALL), the LCD flashes the message “Loading defaults - Please
Wait…” until restoration is complete. the Simulator returns to the Restore program
screen. Exit this by pressing esc.
• If you selected a single program (by pressing OK in the restore screen), the LCD
indicates the restoring of the program selected, and then returns to the Restore
program screen. Exit this by pressing esc.
8-16
Page 67

Electrical Probe Testing
Fluke Biomedical has validated that the following oximeter probes can be functionally
tested using the Simulator (MFE Version only) when connected via the correct probe
cable adapter:
• BCI®(3101)
• Criticare® (504)
• Datascope® (Passport)
• Nellcor® (N-100)
• Nihon-Kohden® (Lifescope)
• Novametrix®
• Ohmeda® (3700)
Chapter 9
Electrical Probe Test
• Respironics®
W Caution
Other manufacturers’ oximeter probes can be tested by the
Simulator, but will require special care in preparation of an
adapter cable and interpretation of the results. Damage could
occur to the Simulator or the oximeter probe if the adapter
cable is not correct. Do not plug other brands of oximeter
probes into an adapter cable unless you know that the pinouts
and impedances are the same.
The Simulator allows you to verify the electrical continuity and integrity of most
oximeter probes using the following steps:
1. Connect the probe under test to the back of the the Simulator using the appropriate
adapter cable.
9-1
Page 68

Index 2MF
Users Guide
Note
The MFE version ships with Nellcor and Ohmeda probe and oximeter
adapters. Please contact Fluke Biomedical regarding the availability of
additional adapter cables.
W Caution
Attempting to create adapter cables without all details of the
oximeter probe-operating scheme could result in damage to the
oximeter, the Simulator, or both.
2. Press the PRBE softkey at Main Menu 1:
The PRBE softkey initiates a testing sequence that includes:
• Verifying that a Probe Test Board is installed in the Simulator
• Checking whether or not a probe adapter cable is connected to the probe port
• Performing all measurements for LED, photo detector, and resistance tests
Probe Test Selection
The user can then choose the specific probe test.
• LED and photodiode electrical test
• Pin-to-pin resistances
• Photodiode detector optical test
Fgg012.eps
esl125.eps
9-2
Note
During the photodiode test, the finger probe being tested should not be
attached to the Index finger.
LED Testing
The Probe test should end with the following menu displayed. Press the LEDS softkey:
Page 69

Electrical Probe Test
Probe Test Selection 9
esl126.eps
The Simulator applies 1.0 mA current AC signal source to the red and infrared LEDs.
The Simulator performs the electrical test separately on each diode to confirm they are
functioning properly. The voltage drop across each element is measured and displayed.
Values can range from 0.0 to 4.0:
• 0.0 Volts = LED shorted
• 1.4 +/- Volts = LED OK
• Any value of 4.0 or greater will be displayed as “> 4.0.”
esl127.eps
The LEDS test should return a PHTO reading of approximately 0.6 volts if electrically
good. Use the resulting numbers as a baseline for similar probes. For example, 1.6 may
be a typical red LED and 1.1 a typical IR LED result for Nellcor probes. A 0.0 results
would indicate a shorted diode, while >4.0 V would indicate either an open diode or that
the voltage drop across the diodes has exceeded the Simulator’s forward-voltage test
range.
Photodiode Test
This test looks at the probe as a functioning entity. Numbers close to zero support a faulty
probe diagnosis.
Test the photodiode probe by pressing the PHTO key.
esl172.eps
The red LED lights up and the Simulator measures the resulting photodiode output. The
red LED then goes out and again measures the output. The difference indicates the
response to a 1 mA LED illuminating current.
The same test measures the infrared LED.
Both tests are repeated multiple times to average out ambient light, with the results
displayed as a pair of numbers; one for red, and one for infrared, on a fixed scale giving
an indication of photodiode response to each color. A higher number yields more
response. Numbers can range from 0-20,000 or more. It is a nominal value only.
9-3
Page 70

Index 2MF
Users Guide
Develop baseline values for comparison purposes when testing marginal, defective, or
suspect probes by logging voltage readings from operational probes.
These values update continually during testing.
During the photodiode test, the finger probe being tested should not be
attached to the Index 2MF finger.
Displayed numbers will vary due to both the general probe condition and
as a function of the distance and angle between the two LEDs and the photo
detector.
Take care to maintain alignment when inspecting the SpO2 sensor.
Reusable SpO2 sensors with built-in spring clips typically maintain
consistent alignment between the LEDs and the photo detector, therefore
yielding consistent results.
However, test results when inspecting a flexible disposable “tape style”
probe will fluctuate unless you take care to maintain the optimal
mechanical position of the probe. As a suggestion, wrap the disposable
probe on a small white plastic cylinder or tube (not your finger) to maintain
the mechanical alignment of the LEDs to the photo detector.
These values update continually.
Resistance Testing
Press RES key
Note
at the "DONE. SELECT RESULT" screen.
esl128.eps
9-4
esl173.eps
The Simulator checks for resistances between all wires (separately testing each one,
every pin to every other pin, except for those wire pair combinations where a LED would
draw normal current).
The Simulator displays resistances under 150,000 Ohms.
Pressing the MORE softkey displays all pin combinations that have resistances. Results
must be interpreted in context of the probe schematic; some probes contain resistors,
some do not. A resistance where none should exist indicates a faulty probe. For example,
a resistance between a resistor shown on the probe schematic as floating and one of the
LED or photodiode leads would certainly be faulty.
Page 71

Electrical Probe Test
Probe Test Selection 9
Note
These values do not update continually. Use REDO when checking for
intermittent connections.
esl129.eps
9-5
Page 72

Index 2MF
Users Guide
9-6 10-1
Page 73

Introduction
Users may adjust the liquid crystal display (LCD) screen, increasing or decreasing the
contrast to accommodate different ambient lighting situations.
This function is not the same as setting an ambient light artifact
(sunlight/artificial light) for oximeter testing under the Simulations menu.
The steps outlined here allow adjusting the screen contrast on the LCD
itself.
LCD Contrast Settings
To adjust the LCD contrast, access the Utilities menu directly from the Main Menu 2. To
access the Utilities menu, proceed as follows:
1. Press more on Main Menu 1.
Chapter 10
Adjusting the LCD Screen
Note
The Main Menu 2 displays in LCD:
fgg015.eps
fgg161.eps
Page 74

Index 2MF
Users Guide
Note
Pressing more returns you to Main Menu 1.
Pressing esc in any of the following screens returns you to Main Menu 2.
2. Press UTIL to access the Utilities Menus:
The Utilities menu allows you to choose from these options:
Note
For information on RS232, CKSM, and PTST functions, refer to
“Appendix A, Printing and Data Transfer”. Pressing esc will return you to
Main Menu 2.
3. Press DISP to access the LCD controls:
fgg143.eps
fgg133.eps
10-2
fgg134.eps
The LCD shows the current contrast level setting, which may or may not be the
default value (5).
esl135.eps
4. Adjust the contrast of the LCD by pressing + to increase the contrast of the LCD.
Continue to press softkey
+ to change the contrast until it suits you and the lighting
conditions.
Page 75

Adjusting the LCD Screen
LCD Contrast Settings10
Note
The maximum level that the contrast can be set to is 7 (the minimum setting
is 0).
5. Press softkey
- to decrease the LCD contrast.
Note
The third and fourth keys are not activated for LCD contrast adjustment.
6. When the LCD contrast is acceptable, press esc to return to the Utilities menu.
7. Press esc again to return to Main Menu 2.
esl136.eps
esl137.eps
fgg139.eps
8. From the Main Menu 2, press the softkey more to return to Main Menu 1.
10-3
Page 76

Index 2MF
Users Guide
10-4 11-1
Page 77

Introduction
Fluke Biomedical has included several features in the Simulator specifically designed to
aid manufacturers of pulse oximeters. Those features ease the following manufacturingspecific needs:
• The automatic production testing of pulse oximeters
• The automatic production testing of oximeter probes
• The design and development of new pulse oximeters
Automated Testing
With the Simulator, manufacturers of pulse oximeters can test their products using a
standard PC. The computer must have a serial communications (COM) port and a
communications program, such as the Microsoft Windows
Chapter 11
Manufacturers Mode
TM
“HyperTerminal” program.
To test a pulse oximeter using a PC, connect the Simulator to the computer via one of the
communications ports, with the unit under test connected via a second communications
port.
You send special commands to the Simulator using the computer; the Simulator then acts
on the commands to test an oximeter. You can additionally use the computer to monitor
responses of the unit under test (UUT). With the computer, you can do any of the
following:
• Log the oximeter's responses for a production test record
• Compare the oximeter's responses to preset limits to determine whether the
system passes or fails quality standards
You can complete these procedures by testing oximeters against a reference probe.
Alternatively, you can test the probes against a reference oximeter.
Manufacturer Parameters Available For Simulations
Although percent oxygen and beats per minute are sufficient for a basic check of an
oximeter, these simulations cannot by themselves probe the limits of an oximeter's
performance in order to evaluate the its design.
Page 78

Index 2MF
Users Guide
Fluke Biomedical has equipped the Simulator to allow you the widest array of simulation,
with the capability for direct control of the following the Simulator functionality:
• Bulk red light attenuation
• Bulk infrared light attenuation
• Peak-to-peak red pleth wave amplitude control
• Peak-to-peak infrared pleth wave amplitude control
• Shape of the pleth wave
• Simultaneous attenuation of both red and infrared by selected percent
• Generation of simulated ambient light
Note
See “Appendix E: Computer Control” for more information on the the
Simulator computer control command. Refer to “Appendix A: Printing &
Data Transfer”, for information on the RS232 port and how to set
communications parameters (baud rate and parity settings).
Mathematical Background
The Simulator assumes that any oximeter under test uses the “ratio of ratios” formula,
where a quantity “R” correlates with SpO2, the dc terms are fixed attenuations, and the
ac terms are peak-to-peak measures of additional attenuation due to the pleth wave (see
the next section for help interpreting the formulas). The “ratio of ratios” formula is:
Oximeters perceive the Simulator to be using a variant of the above formula, which
causes the dc terms to “drop out”.
In the following formula, “REDUUT” means RED-light, Unit Under Test
and “IREDUUT” is for InfraRed light, Unit Under Test.
R=redac/reddc
iredac/ireddc
math_bg_formula.eps
Note
11-2
Page 79

Manufacturers Mode
Mathematical Background11
The the Simulator calculates the R-value (as far as the oximeter is concerned) with this
formula:
(0<=ac <=255) (0<=rdc <=4095) REDUUT
255
__________________________________________________________________
(0<=ac <=255)
255
Notes on the Formulas
The ac term above attenuates both red and infrared pleth waves identically, to control
simulated pleth strength. The default value is 255. In addition, red-light dc (rdc) and
infrared light dc (irdc) modulate REDUUT and IREDUUT, which are transmissivity
numbers. Zero would mean that no UUT (Unit Under Test) pulse would be allowed to
pass through the Simulator. Attenuation would be 4,095 minus the value set. The default
value here is 1,000.
(0<=rac <=4095)
**
4095
4095
(0<=rdc <=4095) REDUUT
4095
(0<=irdc <=4095) IREDUUT
**
(0<=irdc <=4095) IREDUUT
4095
4095
(0<=irac <=4095)
4095
esl140.eps
esl141.eps
Red-light ac (rac) and infrared light ac (irac) are true attenuations. A value of 1,000, for
example, means that the pleth wave will attenuate the UUT pulse by approximately 25%
of the maximum pleth amplitude. The default value for irac is 1,000. Values for rac
range from 400 to 4,000, corresponding to .400 to 4.000 R-values; this covers the
required range.
By having the pleth attenuation operate on the dc attenuated UUT flash, the Simulator
becomes immune to fluctuations in oximeter flash amplitude, which can vary
considerably.
To reduce the corresponding dc term only, you increase the pleth amplitude
term; conversely, to increase only the pleth amplitude term, you reduce the
corresponding dc term. Keep this in mind when setting up performance
limits tests.
The R-Value Equation
By dropping out terms that equate to one, and by accounting for the dc terms being
transmissivities, we can reduce the previous equation to this:
Note
rac / (4095 - rdc)
R =
irac / (4095 - irdc)
esl142.eps
11-3
Page 80

Index 2MF
Users Guide
This is the equation used to obtain the R-values used in the Simulator, and presented on
the LCD screen.
Although the equation eliminates the effect of changes in the UUT flash amplitude on the
R-value, bulk attenuation of an actual finger is an important characteristic. By varying
rdc or irdc values and determining how large a range of variation is “not seen” by the
oximeter being tested, you can get a good idea of the oximeter's electronics' dynamic
range and its processing capability. This is independent of finger thickness and
pigmentation.
Note
By varying the ac term, you can determine the oximeter's ability to track
and hold a pleth wave as it weakens to zero, and then re-acquire the wave
as it strengthens.
Accessing Manufacturers' Tests
Access the manufacturers' test settings with the LCD screen.
Press the softkey OPTS on Main Menu 2.
The Advanced Options menu displays in the LCD with two menu options in addition to
the escape softkey.
The following options become available on the Advanced Options menu:
• SRC Sets the pulse signal source
• RVAL Sets the R-value
• esc Returns you to the Options Menu
Setting the Signal Source
1. Press the SRC softkey on the Advanced Options menu:
fgg181.eps
esl144.eps
11-4
esl145.eps
Page 81

Manufacturers Mode
Accessing Manufacturers' Tests11
The Waveform Source screen provides two options:
esl146.eps
2. Select the oximeter by pressing UUT (Unit Under Test) as the source for the pulse
amplitude signal.
esl147.eps
3. Alternatively, press DC. The Simulator will ignore the oximeter's pulse amplitude
and will use instead a fixed dc level for infrared and red pulse simulations.
esl148.eps
The signals will pass through the Simulator based on their original amplitude.
4. To return to the Advanced Options menu, press esc.
Setting the R-Value
You can also enter a custom R-value using the following procedure:
1. Access the Advanced Options menu (if you have not already done so) by pressing
OPTS on Main Menu 2.
2. Press RVAL on the Advanced Options menu:
esl149.eps
You will see a screen appear similar to the following:
11-5
Page 82

Index 2MF
Users Guide
3. To raise or lower the R-value one step-amount at a time, use the softkeys +R or -R.
In the sample below, the step amount is 0.020, and thus pressing the softkey +R
raises the previous R-value from 0.610 to 0.630:
4. Continue pressing the softkeys +R or -R until the required R-value displays on the
LCD.
Adjusting the Step Amount
You can adjust the step to change the value that increases or decreases the R-value with
each button push.
1. First, use the arrow softkey to set the cursor under the step digit that you want to
adjust. The cursor (flashing in the digit field) highlights first “0” in the sample above
of 0.020. Advance to the next digit position by pressing -->.
esl150.eps
esl151.eps
11-6
esl152.eps
2. Continue to press --> until the cursor is where you want it. Each time you press -->,
the cursor moves one position to the right.
3. With the cursor highlighting the digit you want to change, press +STP to increase the
numeric value. In the sample that follows, the step is increasing in the second
position by one to 0.120:
esl153.eps
4. You can now continue to use the softkeys +R or -R to adjust the R-value.
5. When you are done, press esc to return to the Advanced Options menu, then press
esc again to return to Main Menu 2.
Page 83

Introduction
The Simulator will store six download R-Curves into download slots according to
specific light levels. You may trade a preprogrammed make for one that you use more
regularly. This chapter explains how to create an R-Curve and download it to one of the
“download slots” for future use.
The six slots are organized by light level technology as follows:
• Download Slot 1 = Medium Light
• Download Slot 2 = Medium Light
Chapter 12
Creating Your Own R-Curve
• Download Slot 3 = Medium Light
• Download Slot 4 = Medium Light
• Download Slot 5 = Low Light
• Download Slot 6 = Low Light
It is important that you save the R-Curve for the manufacturer in the correct light level
slot. You can verify this by checking with the manufacturer or by trial and error. For
comparison purposes, Nellcor and BCI use medium light level technology while HewlettPackard, Ohmeda and Invivo use low light level technology and Datex uses high light
level technology.
You will need a computer, an RS-232 cable with a null modem, the oximeter to be
characterized, a pencil, and the sample Index R-Curve Data Sheet (found on page 4 of
this chapter).
12-1
Page 84

Index 2MF
Users Guide
Generating an R-Curve from a Pulse Oximeter
1. Set up the Pulse Oximeter and attach it to the Simulator.
2. From MAIN MENU 1 select SIM | MAN, set:
SpO2 = 100%
BPM = 100
3. From MAIN MENU 2 select OPTS | RVAL, set:
Step = 0.010
Note
TheR-value displayed on the Simulator will automatically be set to a value
that corresponds to the SpO
4. Step the R-value up by 0.010 and record the SpO2 reading from the Pulse Oximeter.
Make sure not to record an R-value until you can determine an “average” R-value, as
more than one R- can correspond to the same SpO2 percentage. For example:
SpO2 R-Val
99 .48
99 .49
99 .50
All three R-value reading have same SpO2 value (Avg. = .49)
value displayed on the Pulse Oximeter.
2
5. Continue recording R-values until SpO2 = 50%
Downloading an R-Curve into the Simulator
1. Create an ASCII file with the numbers obtained on the data sheet. The file
should look like this:
[rcurve:!2 :N-10
:250:245:152,151,149,147,146,145,143,141,139,137,136,134,133,132,131,127,124,123,121,119,118,116
,114,112,111,110,108,106,104,102,100,098,096,094,092,090,088,086,084,081,078,076,073,070,067,064
,061,058,055,051,046,]iiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiii ii
Where: [rcurve sets the Simulator up to receive an R-Curve:
2 places the curve in download slot 3 (0-5)
N-10 is the name to appear (must be no more than 9 characters)
250 is the infrared DC Value
245 is the Red DC Value
152 is the start of the R-Value numbers (152 is a three-digit number for 50% SPO2)
2. Use the LD.BAT file as described on page E-5 to download a curve (Filename).
Basically, at the DOS prompt, set the following:
Mode COM1: 96, N, 8, 1
Copy [Fil ename] COM1:
12-2
Page 85

Creating Your Own R-Curve
Downloading an R-Curve into the Simulator12
Short Form Instructions
PN 2227040 Rev. A
INDEX R-CURVE DATA SHEET
Date: Recorded by:
Pulse Oximeter Model:
Pulse Oximeter Serial Number:
Pulse Oximeter Verified On:
Probe Used:
Heart Rate Used: R-Curve Downloaded in Position Number:
Notes: ___
________________________________
______________________________________________________________________
Avg. R-Value SpO2 Avg. R-Value SpO2 Avg. R-Value SpO2
100 83 66
99 82 65
98 81 64
97 80 63
96 79 62
95 78 61
94 77 60
93 76 59
92 75 58
91 74 57
90 73 56
89 72 55
88 71 54
87 70 53
86 69 52
85 68 51
84 67 50
12-3
Page 86

Index 2MF
Users Guide
12-4
Page 87

Appendices
Appendix Title Page
A Printing and Data Transfer .................................................................................. A-1
B Error Messages and Corrective Measures........................................................... B-1
C Accessories List .................................................................................................. C-1
D Specifications...................................................................................................... D-1
E Computer Control................................................................................................ E-1
F Typical Questions and Answers.......................................................................... F-1
G Custom Probe Test Cables and Electrical Simulation Cables............................. G-1
H Glossary............................................................................................................... H-1
Page 88

Index 2MF
Users Guide
Page 89

Introduction
This Appendix provides information on the Simulator’s RS232 and printer ports,
including how to configure the port on your printer or computer.
Appendix A
Printing and Data Transfer
The Simulator provides a male D (9-pin) bi-directional serial port located at the back side
of the unit, for the transfer of data to a computer or printer, and for computer control of
the Simulator. The Data Computer Equipment (DCE) wiring configuration is shown
Figure A-1.
Index 2MF RS232 Configuration
Fluke Biomedical offers a bi-directional serial null modem cable for connect the
Simulator to your computer (IBM compatible) or serial printer. Please specify Fluke
Biomedical Part Number 2238626 when ordering.
Figure A-1. Male D Cable End
1- C-Lock
2- TX
3- RX
4- DTR/DSR
5- Ground
6- DTR/DSR
7- RTS/CTS
8- RTS/CTS
9- Unused
esl165.eps
C-Lock
Pin 1 of the serial connector provides the Simulator’s synchronized pulse. This pulse is
normally in phase with the start of each 64-point pleth wave. The phase of the sync pulse
with regard to the wave can be varied using the [CPHASE nn] command, where nn is a
number in the range of 0 to 63. The pulse is a negative going attenuated logic level. For
more information, refer to “Appendix E: Computer Control”.
A-1
Page 90

Index 2MF
Users Guide
Printer Port
The Simulator also has a Centronics parallel printer port. Information to be printed is sent
out both the serial and parallel ports. Figure A-2 shows the pin-out for the parallel port.
1
AUX0
2
SENSRC
3
DAC 8 SEL
4
RECV
5
TRANS
6
D8
7
D9
8
D10
9
D11
10
No connection
11
Busy
12, 13, 14, 15, 16, 17, 18, 19, 20
No connection
21, 22, 23, 24, 25
Ground
esl154.eps
Figure A-2. Index 2MF Parallel Port D-25
A Standard D-25 pin to Centronics printer cable works with the Simulator. Please specify
Fluke Biomedical Part Number 2238072 when ordering.
Configuring the Serial (RS232) Port
Configure the Simulator to match your printer's or computer's communications needs by
setting baud rate and parity. Once configured, you can then send the Simulator's test
results directly from the Simulator to a printer through a serial connection.
If you have installed communications software on your computer system, you can also
send data to your computer or control the Simulator through a computer with the the
Simulator 's built-in RS232 serial port.
When communicating with the Simulator through a computer, you can choose from the
following to match your system requirements.
Bits per Second:
• 300 bps
• 600 bps
• 1200 bps
A-2
Page 91

Appendices
• 2400 bps
• 9600 bps
Printing and Data Transfer A
Parity:
• None
• Even
• Odd
Stop and data bits:
• 1 or 2 stop bits
• 7 or 8 data bits
Accessing the Utilities Menu
1. Press UTIL on the the Simulator Main menu 2 (first press more if you are viewing
Main Menu 1).
You will see the following menu appear in the Simulator 's LCD display:
2. Press UTIL to access the Utilities menu.
3. The following Utilities screen offers four configurable options:
fgg014.eps
fgg161.eps
fgg133.eps
A-3
Page 92

Index 2MF
Users Guide
4. Press R232 to set the RS232 port parameters:
esl167.eps
5. Press the softkey under the baud rate, parity type, and data and stop bit sizes to set the
correct communications parameters for your printer or other device.
Table A-1 describes parameters used for PC to peripheral communication.
Table A-1. Communication Parameters
Parameter Description
BAUD Each press of the button decreases the baud rate from 9600 to 2400, 1200, 600,
and 300 bps. When 300 is displayed, a button push then shows 9600.
PAR Pressing this button toggles the parity from No parity (N) to Even parity (E) to Odd
parity (O) and back.
DATA Pressing this button toggles the data bits back and forth from 7 to 8.
STOP Pressing this button toggles the stop bit back and forth from 1 to 2.
When you finish setting the communication control parameters:
• Press esc to return to the Utilities menu.
• Press esc
again to return to the Main menu 2.
• Press more to return to the Main menu 1.
A-4
Page 93

Appendices
Printing and Data Transfer A
Printing
Sample Index 2MF Test Results Output
The Simulator Autosequence printout shown below requires using the previous settings
to establish communication between the the Simulator and a printer.
• The optional header is all information from the “Control#” field down to the
“Technician” field.
• Actuals are all information under the “Settings:” heading. A normal report includes
only the fields Alarm Response Time, Rate Alarm Response Time, and Pulse
Amplitude Test.
Fluke Biomedical
SPO2 Simulator Ver: 2.00
-------------------------------------Control#: __________________________
Serial #: __________________________
Model: _____________________________
Mfr: _______________________________
Location: __________________________
Technician: ________________________
Date: ______________________________
Simulations: NELLCOR
--------------------------------------Settings: Actual:
# O2 RATE O2 RATE
1 100% 60 BPM 99 59
2 95% 65 BPM 95 64
3 90% 70 BPM 90 70
4 85% 75 BPM 85 75
5 84% 80 BPM 84 80
6 83% 85 BPM 83 85
7 82% 90 BPM 82 90
8 81% 95 BPM 81 95
9 80% 100 BPM 80 100
10 79% 105 BPM 79 105
--------------------------------------Alarm Response Time:12 Seconds at 95% O2
--------------------------------------Hi Rate Alarm Response Time:10 secs at 80
BPM
Lo Rate Alarm Response Time:10 secs at 80
BPM
--------------------------------------Pulse Amplitude Test
At 85%, the signal is lost.
--------------------------------------Motion Response Time: 10 Seconds
---------------------------------------
End of test.
esl155.eps
A-5
Page 94

Index 2MF
Users Guide
A-6
Page 95

Appendix B
Error Messages and Corrective
Measures
Introduction
This Appendix lists all of the error messages or beeps that may appear on the Simulator's
LCD screen during normal operation. Solutions are given for each message.
Messages
If the battery has run down, the following message appears:
Warning! Low Battery
Although the battery included with the Simulator can run continuously for up to 8 hours,
it does need regular recharging, especially after long periods of disuse. In addition, the
battery can only be recharged a certain number of times, after which it needs to be
replaced.
To recharge the battery, plug the battery charger that came with the Simulator into the
Simulator charger jack and plug the battery charger into a wall socket, using the proper
line cord.
To fully recharge the battery, allow the battery charging at least 12 hours.
Disconnecting the battery charger and using the battery prior to the full 12
hour charge time will result in an incomplete charge. The Simulator may
only work for a very short time under such circumstances.
Note
Beep Indications
Three beeps
You have pressed a button that is not activated for the particular screen or function. Try
again.
Continuous beeps
Continuous beeps warn of a low battery. See the recharging instructions above.
B-1
Page 96

Index 2MF
Users Guide
Other Error Indications
Pulse Oximeter Not Reading SpO2 or Heart Rate
The display reads: "NO RED LED NO IRED LED Check probe or press esc"
The LEDs on the Pulse Oximeter must be positioned on the bottom of the Simulator 's
finger probe attachment. Make sure the Pulse Oximeter's finger probe is centered and
pushed as far forward as possible on Simulator's finger probe attachment.
B-2
Page 97

Introduction
Table C-1 presents a list of accessories available for the Simulator. The User's Guide and
a battery charger (U.S. or European) are standard equipment.
Appendix C
Accessories List
Table C-1. Accessories
Description Part Number
Index 2MF User's Guide 3341210
Carrying Case 2204282
Charger (USA) 2521465
RS232 Cable 2238626
Printer Cable 2238072
Electrical Probe Test Cables See price list
Electrical Simulation Cables See price list
C-1
Page 98

Index 2MF
Users Guide
C-2
Page 99

Appendix D
Specifications
Equipment Specifications
The following specifications apply to the Index 2MF and 2MFE.
%O2
Range .................................................................35 to 100 %
Resolution...........................................................1 %
Accuracy.............................................................100 to 75 %: +
Repeatability....................................................... +
Rate
Range .................................................................30 to 250 BPM
Resolution...........................................................1 BPM
Accuracy............................................................. 1 % +
Pulse Amplitude
Range .................................................................0 to 20 %
Resolution...........................................................1 %
Battery Life............................................................At least 4 hours of continuous use
Battery Charger
Type....................................................................Lead-Acid battery charger only
Input....................................................................100 – 250 V, 60 – 60 Hz, 0.3 A
Output................................................................. 12 V dc, 0.5 A
Probe Test ............................................................. Index 2MFE version only
Continuity/Resistance Test
Test Matrix.......................................................... Measures all combinations of possible interconnections in an XX point
Range .................................................................250 Ω to 150 kΩ
Accuracy............................................................. 5 % of reading
LED/Detector Voltage Test
Test Format ........................................................Measures the voltage drop across Red LED, infrared LED, and the
Test Signal..........................................................Constant current source at 1.0 mA
Open Circuit........................................................2.5 V max
Measurement/Display Range .............................0.0 to 4 V
Accuracy............................................................. 5 % of reading, 0.4 to 4 V
74 to 50 %: +
<50 %: unspecified
1 standard deviation
1 BPM
matrix.
photo detector when the internally generated test signal is applied.
1 % ± accuracy of the pulse oximeter under test.
3 % ± the accuracy of the pulse oximeter under test.
D-1
Page 100

Index 2MF
Users Guide
Dynamic Test
Checksum.............................................................. The sum of all locations in the program chip. For service only.
Test Format...........................................................Photo detector/diode response to both the red and infrared light
generated by the probe when pulsed by an internal test signal.
Test Signal..........................................................Pulsed between the two LEDs, constant current level at 1.0 mA
Test Results........................................................Nominal range of 0 to 20,000
Note
The above specifications are for pre-programmed makes only, 20 % pulse
amplitude SpO2. Results for Presets, custom makes, and various pulse
amplitudes are unspecified and depend on the algorithm of the specific
pulse oximeter being tested.
D-2
 Loading...
Loading...