Fedegari FVA User manual

OPERATING MANUAL
STERILIZER MOD. FVA
Document Code: #68181 Rev.1
0123
AUTOCLAVE
Model: FVA2 -- FVA3
THIS MANUAL’S INFORMATION REFERS TO THE MACHINE, COMPLETE WITH ALL OPTIONAL
FITTINGS. THE MACHINE CONFIGURATION DESCRIBED BELOW DEFINES THE OPTIONS
AVAILABLE DURING FINAL TESTS, BEFORE DELIVERY.
MACHINE CONFIGURATION:
F BASIC MACHINE
f
K10 STEAM GENERATOR
f K20 VACUUM PUMP
f K30 EFFLUENT COOLER
f K40 INTERNAL AIR ELECTROCOMPRESSOR
f K43 EXTERNAL AIR ELECTROCOMPRESSOR
f K50 AIR STERILIZING FILTER
f K51 NETWORK COMPRESSED AIR SUPPLY UNIT
f K52 QUICK COOLING UNIT
f K53 SPONTANEOUS COOLING
f K61 CHAMBER PRESSURE LEAK TEST
f K62 DECONTAMINATION CYCLE
f K70 STEAM SUPPLY DEFLECTION UNIT
f K80 ADDITIONAL HEAT PROBE
f K81 PROCESS PRINTER
f K82 KIT EN285--DATALOGGER
f HW1 SECONDARY RS232 SERIAL OUTPUT
f SW1 CONTROL FUNCTION FO
f T30 DEIONIZER
f SW2 CYCLE REPETITION
FEDEGARI Autoclavi S.p.A.
S.S. 235 km 8 -- 27010 Albuzzano (PV) -- Italia
+39 0382 434111 +39 0382 434150 http://www.fedegari.com
WARNING
The information contained in this document may be modified without prior notice.FEDEGARI does not
provide any kind of guarantee on this manual.FEDEGARI is not liable for any errors contained herein
and/or for any incidental/ consequential damageregarding the supply , performance or use of this manual,
This document’s information are our property. None of its parts may be photocopied, reproduced disclosed
in another language without FEDEGARI’s prior written consent.
-- I --
May 2001
#68181 Rev.1

Model: FVA--HT
MACHINE CONFIGURATION:
F K10 STEAM GENERATOR
F K30 EFFLUENT COOLER
F K50 AIR STERILIZ ING FILTER
F K51 NETWORK COMPRESSED AIR SUPPLY UNIT
F K52 QUICK COOLING UNIT
F K61 CHAMBER PRESSURE LEAK TEST
F K62 DECONTAMINATION CYCLE
F K80 ADDITIONAL HEAT PROBE
F K81 PROCESS PRINTER
F HW2 HIGH PATHOGEN
F SW2 CYCLE REPETITION
This sterilizer is designed in such a way as to prevent any contamination hazard to the environment and the operators.
For this reason, the sterilizer operates as follows:
1. It does not extract the air from the chamber, since all filters, even high--retention ones, are useless in the presence
of small agents, such as prions.
2. It keeps in the chamber the condensate that forms during the process, until the sterilization has been completed;
however, the first condensate which may be produced is not sterile.
The steam + air mixture forming in the sterilization chamber is kept homogeneous by a fan positioned inside the
chamber. As a matter of fact, the air density (at the same temperature and pressure) is approximately 1.7 times higher
than the water steam density, and without this measure the air would stratify on the bottom of the chamber, resulting in
intolerable temperature gradients.
The temperature of the steam + water mixture forming in the chamber will depend on the selected sterilization
temperature.
The mixture pressure will consist of the addition of two factors:
1. The water steam pressure at the selected sterilization temperature.
2. The air pressure at the selected sterilization temperature.
If, for example, the sterilization process is carried out at 140_C:
-- factor 1 is 3,61 absolute bar (water steam pressure 140_C);
-- factor 2 can be calculated as follows: when the sterilizer is closed at room temperature (approx. 20_C), the
pressure of the air closed in the chamber is about the room pressure, i.e. 1,0 absolute bar. The air, however, heats
up at 140_C, increasing its pressure in the ratio of initial to final heating temperatures, and accordingly pressure
becomes:
1,0 x
140 + 273
20 + 273
=1,0x
413
= 1,41 absolute bar
293
-- The total pressure of the steam + air mixture in the chamber will be 138_C: 3,61 + 1,41 = 5,02 absolute bar.
The sterilizer is therefore designed to operate up to 4.70 rel. or manometric bar (i.e. above the atmospheric pressure),
up to 5.70 absolute bar.
-- II --
#68181 Rev.1
May 2001

ADDITION AND CHANGE REGISTRATION
Name
function
PREPARED BY: IDO FAP 23/05/01
CHECKED AND
APPROVED BY:
Review Date Description
F.I.DT.00089.A.0.E.06.97 15/06/97 First issue, software version VC0001.0
F.I.DT.00089.B.0.E.01.98 19/01/98 Modifications in and additions to all Chapters
F.I.DT.00089.B.1.E.06.98 15/06/98
ING FAF 23/05/01
Name
acronym
Modifications in and additions to Paragraphs: 2.4.1. -- 3.3.1.
-- 3.3.2.
5.5.1. -- 5.5.2. -- 6.2.9. -- 8.3.3. 8.3.4.1. -- 8.4.1. -- 8.4.4.
Date
(dd/mm/yy)
Signature
Addition of Paragraph 8.4.5.
F.I.DT.00089.C.1.E.02.99 25/02/99 New version - - software VC0002.0 and VC0002.1
New version -- software VC0002.4
F.I.DT.00089.D.1.E.12.99 13/12/99
#68181 Rev.1 23/05/01 New software version: VC0002.9
May 2001
Upgrade software VC0002.5 (allow to imput new serial
number. -- es. NA0123ED)
-- II I --
#68181 Rev.1

TABLE OF CONTENTS
1. FOREWORD 1.............................................................
1.1. PUBLICATION IDENTIFICATION 1..................................................
1.1.1. Allied documentation 1.............................................................
1.2. PUBLICATION OBJECTIVE 1......................................................
1.3. REFERENCES TO RULES AND STANDARDS 1.....................................
1.4. USING THE MANUAL 1...........................................................
1.5. UPDATES 2......................................................................
2. INTRODUCTION 3..........................................................
2.1. IDENTIFICATION 3...............................................................
2.2. SERVICING 3....................................................................
2.3. RISKS DERIVING FROM USING THE SOFTWARE 4.................................
2.4. SAFETY WARNINGS 4............................................................
2.4.1. General warnings 4...............................................................
2.4.2. Machine state 8...................................................................
2.4.3. Operators 9......................................................................
2.4.3.1. Operators’ qualification 9...........................................................
2.4.3.2. Personal protection means 10......................................................
2.4.4. Recommendations and precautionary measures to be adopted by the user 11............
2.4.5. Emergencies 11...................................................................
3. GENERAL INFORMATION 13................................................
3.1. GENERAL DESCRIPTION 13......................................................
3.1.1. Foreword 13......................................................................
3.1.2. Main components 13..............................................................
3.1.2.1. Sterilization chamber 15...........................................................
3.1.2.2. Cover and Sealing system 15.......................................................
3.1.2.3. Technical cabinet 16...............................................................
3.1.2.4. Electric system 16.................................................................
3.1.2.5. Electric board 16..................................................................
3.1.2.6. Plumbing and pneumatic plant 16...................................................
3.1.2.7. Operator panel 16.................................................................
3.1.2.8. Electronic process controller 17.....................................................
3.1.2.9. Main additional components (optional) 18............................................
-- IV --
#68181 Rev.1
May 2001

3.1.2.10. Loading/unloading devices 22......................................................
3.2. SAFETY SYSTEMS 23............................................................
3.2.1. Opening/closing system 23.........................................................
3.2.2. Safety valves 25..................................................................
3.3. DATA SHEET 26..................................................................
3.3.1. Main dimensions and weights 26....................................................
3.3.2. Operational data and main performance 27...........................................
3.4. RECOMMENDED USE 28.........................................................
3.5. FORBIDDEN USE 28..............................................................
4. TRANSPORT, HANDLING, STORAGE 29.....................................
4.1. GENERAL WARNINGS 29.........................................................
4.2. TRANSPORT 29..................................................................
4.2.1. General information 29.............................................................
4.2.2. Transport instructions 29...........................................................
4.2.2.1. Packaging 29.....................................................................
4.2.3. Specific procedures 29.............................................................
4.2.3.1. Warnings 29......................................................................
4.2.3.2. Lifting the machine packaging 30....................................................
4.2.3.3. Handling on the installation site 30..................................................
4.3. STORAGE 31....................................................................
4.3.1. Short shut--downs 31..............................................................
4.3.2. Prolonged shut downs 31..........................................................
5. INSTALLATION AND DISMISSION 32........................................
5.1. ENVIRONMENTAL CONDITIONS FOR USE 32.......................................
5.2. SPACE REQUIRED FOR USE AND MAINTENANCE 32...............................
5.3. PACKING REMOVAL 32...........................................................
5.4. POSITIONING THE MACHINE 33...................................................
5.5. CONNECTIONS TO POWER SOURCES AND SERVICE NETWORKS 34...............
5.5.1. Hydraulic connections of the users 34...............................................
5.5.1.1. DEIONIZER options (T30) 35.......................................................
5.5.2. Wiring 36.........................................................................
5.6. INSTALLATION MONITORING 37...................................................
5.7. DISMISSION 37..................................................................
-- V --
May 2001
#68181 Rev.1

6. USING THE STERILIZER 38.................................................
6.1. OPERATION DESCRIPTION 38....................................................
6.1.1. General information 38.............................................................
6.2. CONTROLS 39...................................................................
6.2.1. General information 39.............................................................
6.2.2. Control desks 39..................................................................
6.2.3. Display 40........................................................................
6.2.4. Keyboard 41......................................................................
6.2.5. Main switch 44....................................................................
6.2.6. Printer (optional) 44...............................................................
6.2.7. Datalogger -- Kit EN285 (optional K82) 45...........................................
6.2.8. Emergency button 51..............................................................
6.2.9. Control pressure--vacuum gauges 51................................................
6.2.10. Manual pressure relief valve of the chamber (not available on FVA--HT model) 52.........
6.3. ACCESS LEVELS AND OPERATING MODES 53.....................................
6.3.1. Access levels 53..................................................................
6.3.2. Operating modes 54...............................................................
6.4. OPERATING PROCEDURES 55....................................................
6.4.1. Warnings 55......................................................................
6.4.2. General information 55.............................................................
6.4.3. Sterilization programs 55...........................................................
6.4.3.1. Working parameters 56............................................................
6.4.3.2. Program parameters 61............................................................
6.4.3.3. General system parameters 67.....................................................
6.4.4. Operating phases 69..............................................................
6.4.4.1. Menu page access logic 69.........................................................
6.4.4.2. ”Step--by--step” and ”Cycle stop” functions 69.........................................
6.4.4.3. F0 70............................................................................
6.4.4.4. Opening/closing logic of the cover 71................................................
6.4.4.5. Printing function (if a printer is available) 71..........................................
6.4.5. Operating procedures and warnings 72..............................................
6.4.5.1. Switching on and initial checks 73...................................................
6.4.5.2. Panel functions and operation enabling 74...........................................
6.4.5.3. Program start 76..................................................................
6.4.5.4. Batch loading 78..................................................................
6.4.5.5. Process control 79................................................................
6.4.5.6. Batch unloading 83................................................................
6.4.5.7. Deactivation or switching off 84.....................................................
6.5. CONFIGURATION 85..............................................................
-- VI --
#68181 Rev.1
May 2001

6.5.1. General Information 85............................................................
6.5.2. Program parameter configuration 85.................................................
6.5.3. Changing general parameters 87....................................................
6.6. CONTROL SYSTEM MAINTENANCE 88.............................................
6.6.1. General information 88.............................................................
6.6.2. Changing date and time 89.........................................................
6.6.3. Monitoring the operator panel 89....................................................
6.6.4. Autodiagnose 90..................................................................
6.6.5. Alarm List 91.....................................................................
6.6.6. Sterilizer data 91..................................................................
6.7. AUXILIARY FUNCTIONS 92........................................................
6.7.1. Transferring system regulation data 93...............................................
6.7.2. Managing the stored alarm list 93...................................................
6.7.3. Language select 94...............................................................
6.7.4. Modify password 94...............................................................
6.7.5. Delayed start 95..................................................................
7. ALARMS AND FAULT FINDING 98...........................................
7.1. ALARMS 98......................................................................
7.2. FAULT FINDING 101..............................................................
7.3. FAULT DETECTION PROCEDURES 102............................................
7.3.1. Autodiagnose functions 105........................................................
8. MAINTENANCE AND SERVICING 109........................................
8.1. GENERAL WARNINGS 109........................................................
8.2. FOREWORD 109.................................................................
8.3. PREVENTIVE MAINTENANCE 109.................................................
8.3.1. General information and warnings 109...............................................
8.3.2. Visual inspection 110..............................................................
8.3.2.1. Preliminary operations 110.........................................................
8.3.2.2. Inspection 110....................................................................
8.3.3. Cleaning 112.....................................................................
8.3.3.1. Cleaning the chamber 112..........................................................
8.3.3.2. Cleaning the chamber filter 113.....................................................
8.3.3.3. Cleaning the heat probe 114........................................................
8.3.3.4. Cleaning the operator interface devices 114..........................................
8.3.3.5. Cleaning the cover and the external cabinet 114......................................
8.3.3.6. Cleaning the batch transport baskets 114............................................
May 2001
-- VI I --
#68181 Rev.1

8.3.3.7. Cleaning line filters 114............................................................
8.3.4. Scheduled performance tests 115...................................................
8.3.4.1. Checking the chamber safety valves and the steam generator, if any 115................
8.3.4.2. Checking the chamber manual pressure relief valve 115...............................
8.3.4.3. Checking chamber sealing (if vacuum pump is available) 116...........................
8.3.4.4. Checking chamber pressure (if pressurization with compressed air is available) 116.......
8.3.4.5. Checking the cover grommet 117....................................................
8.3.4.6. Checking compressed air plant tightness 117.........................................
8.3.4.7. Checking heat probe (Pt100) 117...................................................
8.3.4.8. Temperature check of the cover opening safety system 118............................
8.3.4.9. Checking the compressed air reducer (if the solenoid valve for compressed
air inlet is available) 118............................................................
8.3.4.10. Calibrating the heat probe 118......................................................
8.3.4.11. Calibrating the pressure transducer 120..............................................
8.3.4.12. Checking instruments with a control pressure gauge 123...............................
8.3.4.13. Checking the chamber internal temperature with control sensors 124....................
8.3.4.14. Checking print quality, paper amount and feed (if a printer is available) 124...............
8.4. TROUBLESHOOTING 125.........................................................
8.4.1. Replacing the operator panel battery 125.............................................
8.4.2. Clearing paper jams (if a printer is available) 125......................................
8.4.3. Replacing the sterile filter 126.......................................................
8.4.4. Replacing the PLC battery 126......................................................
8.4.5. Replacing the power cable 128.....................................................
8.4.6. Replacing fuses 129...............................................................
ENCLOSURES 132.................................................................
-- VI I I --
#68181 Rev.1
May 2001
1. FOREWORD
1.1. PUBLICA TION IDENTIFICAT ION
The ”OPERATING MANUAL” is an official document issued by FEDEGARI AUTOCLAVI S.p.A. (hereinafter referred
to as FEDEGARI) and forms and integral part of the machine. It is identified by a document code printed on the cover,
to allow for its identification, traceability and reference.
FEDEGARI reserves the right to change this manual at any time and without notice.
1.1.1. Allied documentation
Main manuals and documents
-- Technical reference documents, including:
* Installation drawing;
* P&ID drawing;
* Instrument legend;
* Wiring diagrams.
1.2. PUBLICATION OBJECTIVE
This manual is addressed to the users of sterilizers belonging to the series mentioned in the heading. It includes the
information required to use the machine properly. Good performance and durability of this machine, as well as
operators’ safety and processed product protection, depend on compliance with this manual’s rules and instructions.
Read it carefully and comply with its requirements and instructions.
Its information is updated to the printing date.
1.3. REFERENCES TO RULES AND STANDARDS
This manual has been prepared in conformity with:
-- Enclosure ”I” to directive 89/392/CEE and amendments thereof.
-- UNI EN 292/2 -- 1992.
1.4. USING THE MANUAL
NOTE
Keep this manual as long as the machine is used.
Make sure that all updates are promptly included in the manual.
As it forms an integral part of the machine, it should always follow it, inside the firm where the
machine is used or elsewhere.
This manual consists of two sections. The first section, the pages of which are identified by Roman numerals (e.g. I, II,
III, IV...etc.), is composed by:
-- 1 --
May 2001
#68181 Rev.1
-- the Title Page;
-- the addition and change registration;
-- the Table of Contents.
Through this section, the user can identify the publication and its update level. The table of contents allows to find the
desired topic in the manual.
The second section, the pages of which are identified by Arabic numerals (e.g. 1,2,3,...n), provides the sterilizer
operating and maintenance instructions. Read all this manual, page after page. Learn and remember all warnings.
As the operator should be qualified for using sterilizers, all general information and instructions they are acquainted
with have been omitted.
All operations require great care and caution. Throughout this manual, the following ”warnings” inform the user about
difficult and dangerous operations.
NOTE
Indicates important information pointed out outside the text.
WARNING
Indicates a condition in which lack of caution or wrong procedures are likely t o damage -- also
irreparably -- the sterilizer.
CAUTION!
Indicates a dangerous situation for people.
In addition to these general warnings, this manual includes other special symbols, printed throughout its pages,
similar to the symbols applied to the machines by means of plates and/or decalcomania in order to indicate dangerous
areas and behavior.
Before approaching the machine to start any operations and maintenance jobs, carefully read the above mentioned
warnings and instructions and understand them.
Carefully keep this manual. Handle it with care and do not damage its content, even partially.
The operator must promptly replace the manual or any lost, damaged or completely/partially illegible plates, stickers
etc. applied to the machine.
NOTE
Do not remove, tear or re--write this manual or its sections. In case of operations or situations other
than those described in this manual, do not hesitate to contact the manufacturer for any updates.
Keep the manual in a dry and cool place.
1.5. UPDATES
Any updates may be communicated to the machine owner using single sheets, complete with all instructions required
to add them to the manual.
Should the sterilizer be sold, its owner must notify FEDEGARI about all related references, so that the manufacturer
will be able to deliver any additions/updates to the new owner.
-- 2 --
#68181 Rev.1
May 2001
2. INTRODUCTION
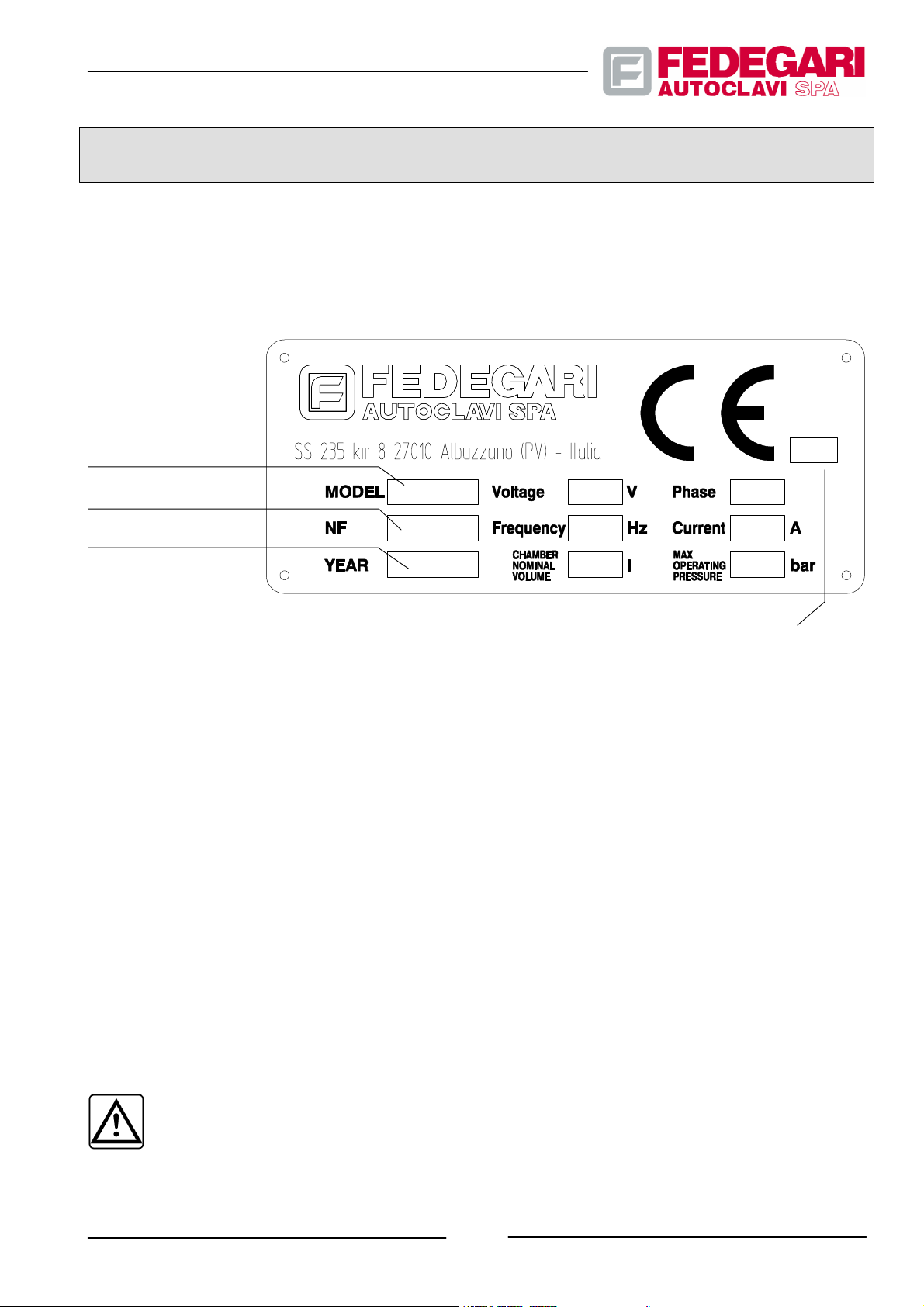
2.1. IDENTIFICATION
Figure 2.1 shows the facsimile of the sterilizer CE identification plate.
STERILIZER
MODEL
SERIAL NUMBER
YEAR OF FABRICATION
NOTIFIED BODY’S INITIAL
(MARKED ONLY IF THE MACHINE
IS A MEDICAL DEVICE)
Figure 2.1 -- CE identification plate
0123
2.2. SERVICING
FEDEGARI is always at its customer’s disposal, whether directly or indirectly through its agents/local dealers, for any
operations required. Suitable equipment and specialized personnel are available for overhaul and servicing. Please
ask FEDEGARI AUTOCLAVI S.p.A SS.235, km 8 -- 27010 Albuzzano (PAVIA) -- Italy, for the names and address of
FEDEGARI SERVICE CENTERS in Italy and abroad.
This machine is guaranteed according to the Sales Agreement General Conditions.
The guarantee will no longer be valid:
-- if the machine is repaired without the consent of the manufacturer or of FEDEGARI Service Centers ;
-- when using spare parts other than the original ones;
-- should the machine be used for any operations other than those recommended;
-- in case of non--compliance with this manual’s instructions.
WARNING
FEDEGARI cannot be held liable for any failures and operational faults due to non--compliance with
preventive maintenance suggested by the manufacturer and indicated in this manual.
-- 3 --
May 2001
#68181 Rev.1

2.3. RISKS DERIVING FROM USING THE SOFTWARE
The use of the software supplied by Fedegari together with its products is licensed to the customer, who will be fully
responsible for it (see also the General T erms of Sale).
CAUTION!
Fedegari or its representatives cannot be held liable for direct/indirect damage deriving from using
the software, from its failures or the above mentioned license.
2.4. SAFETY WARNINGS
2.4.1. General warnings
CAUTION!
Most labor accidents are due to non--compliance with safety rules.
All operators using the sterilizer must know and comply with the rules mentioned in this manual and
on warning plates.
Always observe the rules recommended below.
CAUTION!
Some electrical circuits of the machine remain supplied also when the master switch is off (in the ”0”
position). These circuits are located inside the electrical board an d are adequately indicated.
CAUTION!
Do not inhibit the machine safety devices. When safety is not assured, all operations are allowed only
to FEDEGARI’s personnel, or, with the manufacturer’s authorization, to the user’s trained staff.
CAUTION!
Before using the sterilizer, ALWAYS check that the materials to be processed are compatible with the
cycles and steps of the sterilization program to be started.
Make sure that the mate rials are free of molecular instability risks which, during product handling or
processing, are likely to cause explosions.
CAUTION!
Do not perform any kind of jobs and changes on the machine and its fittings.
Do not modify the sterilizer parts in order to fit other devices.
In case of malfunctioning/accidents due to non--compliance with the above mentioned instructions,
#68181 Rev.1
-- 4 --
May 2001

FEDEGARI shall not be liable for the related consequences.
Do not hesitate to contact FEDEGARI whenever changes to the machine are required.
CAUTION!
CAREFULLY position the heat probe used also as a safety device for chamber opening temperature,
according to the following instructions.
Pay attention to the temperature displayed as:
* when the heat probe is placed between -- not inside -- the bottles of one of the lowest batch layers,
the heat probe can detect the sterilization chamber temperature.
Therefore, always TAKE INTO ACCOUNT that the time required by bottle liquid to reach the
temperature displayed by the heat probe is directly proportional to the bottle volume.
For 50 cc bottles, the safety margin is 30° C (for larger bottles, this margin is to be increased) and
the chamber must be cooled at a temperature not below 40°C, so that the bottle internal
temperature is 70°C, which is an acceptable value.
* When placing the heat probe inside a bottle, plunged in its liquid, safety limits are not needed,
although the sample bottle could be broken during its processing.
In this case, the heat probe will indicate the chamber temperature instead of the bottle
temperature. Therefore, before opening the sterilizer, the operator MUST check that the heat
probe placed inside the chamber is indicating the temperature based on the safety margin
mentioned in the previous section (about 40°C).
CAUTION!
When extracted from the machine, bottles having an internal temperature of more than 70°C could
explode, thus jeopardizing the operators.
The maximum safety te mperature allowing to open the cover must be set at <70°C and parameter
”CHECK FINAL T” must be enabled by setting value ”1”.
CAUTION!
Thermal sterilization in the presence of low--boiling liquids (i.e. with steam tension higher than the
water tension at the same temperature) and/or liquids flammable in the air (i.e. characterized by a
ceiling of concentration in the air above which a fire may propagate) involves two kinds of hazard.
* At the temperatures typical of thermal sterilization (i.e. at least 105°C) a low--boiling liquid can
develop such a high pressure that the safety valve opens, letting any gas and/or steam out of the
sterilizer, or preventing the correct performance of the sterilization process. For example, the
presence of alcohol in a closed room causes a partial pressure, in the aeriform phase, of approx.
4.5 absolute bar at the traditional temperature of 121°C. This pressure depends only on
temperature and is not affected by the quantity of alcohol. When the regulation of a s aturated
steam ste rilization occurs ”in temperature”, at 121°C, the pressure tends to reach approx. 6.5
absolute bar, and the safety valve opens well before reaching this value. When the sterilization is
regulated ”in pressure”, the pressure developed by the low--boiling liquid simulates the
May 2001
-- 5 --
#68181 Rev.1
reaching of the regulation pressure, preventing the supply of steam to the chamber; the
temperature reached at the regulation pressure (3.50 absolute bar in case of decontamination
cycles with Fedegari FOB and FVA sterilizers) is not 121°C, being just above 100°C. The process
may indefinitely stop in the heating phase. Therefore, the presence of any low--boiling liquid may
result in the hazardous release of contaminated material from the chamber or in the impossibility
to carry out the sterilization.
* The eventual flammability of the liquid involves, of course, a fire hazard. The concentration of
flammable vapors in the air, as necessary for ignition, decreases based on temperature, while
the true concentration increases according to it. In most cases, a fire may break out due to the
level (even negligible) of static electricity entering with the vapor or, most frequently, with the
cooling air. Also in those cases that, at present, are well--known, the damage has been limited to
the interior of the sterilizer (that, of course, is shut--down), though it cannot be denied that the
possible opening of the safety valve may propagate fire also outside it. A similar hazard is
present when vacuum is applied at the end of the sterilization process. The flammable mixture is
sucked by a machine not meant for it and any cause of firing may have serious consequences,
that cannot be calculated.
CAUTION!
Before starting a sterilization cycle, make sure that the manual pressure relief valve is perfectly
closed.
CAUTION!
C02 fire--fighting means are recommended in order to provide preventive protection.
WARNING
Always assure that:
* the supply voltage to the sterilizer is constantly kept within ±10% of the nominal value required.
* The compressed air pressure to the sterilizer pneumatic circuits is stable, within ±10% of the
nominal value required.
* the saturated steam pressure oscillation for the sterilization chamber is kept within ±10% of the
nominal values required.
* the pressure of water feed line to the sterilizer is kept within ±10% of the nominal values required.
Failure to comply with these recommendations may cause malfunctions during the sterilization
cycles and damage the machine.
WARNING
Do not use alcohol, petrol, other solvents or acid reaction substances for cleaning or washing the
chamber or the sterilizer components, as they are likely to attack stainless steel surfaces and
#68181 Rev.1
-- 6 --
May 2001

elastomer parts.
Use water, also slightly diluted with neutral detergents, according to this manual’s maintenance
instructions.
CAUTION
To avoid cuts during maintenance of the air circulation fan in the sterilization chamber, do not
disassemble the fan and motor protections unless the plant is completely isolated from power
supply sources.
WARNING
For any kind of processes, es pecially in case of manual operations or dangerous processes (e.g.
decontamination), Fedegari guarantees the sterilization carried out by its machines provided the
process manager authorizes proper performance of all cycle phases envisaged for the product, by
signing the document printed by the sterilizer control system for approval.
CAUTION!
Do not use the sterilizer and its fittings to perform jobs other than those permitted and specified in
this manual.
CAUTION!
These sterilisers are not electromedical devices.
If they are installed near medical or surgical operation areas, they must never be placed at less than
2.0 metres from the patient for the entire period o f the operation.
May 2001
-- 7 --
#68181 Rev.1
Figure 2.2 shows the warning plate position on the machine, pointing out any potential dangers.
Bleu
Yellow
Symbol/Plate name
General danger
Yellow
heck safety valve
on a periodical basis
WARNING
CHECK EVERY MONTH
THE SAFETY VALVES
EFFICIENCY
Hot surfaces
HOT
SURFACES
Plate position
1
USERS
SAFETY VALVE(S) VENT PIPE
2
COVER OPENING/CLOSING KNOB --
3
MASTER SWITCH
4
MANUAL PRESSURE RELIEF VALVE OF
5
THE CHAMBER
CHAMBER’S PRESSURE RELIEF PIPE
6
1
INSIDE THE TECHNICAL CABINET PANEL
2
AT THE REAR OF THE ELECTRICAL
BOARD
3
REAR PANEL NEAR THE SAFETY
VALVE(S) VENT PIPE
MACHINE CASING
1
AUTONOMOUS STEAM GENERATOR
2
(with option K10)
Electrical hazard
Yellow
Red
WARNING
1
DO NOT OPERATE ON LIVE
ELECTRIC EQUIPMENT
HOWEVER SPECIFIC AUTHORISATION MUST BE
OBTAINED FROM THE PERTAININGAUTHORITY
NOT LESS THAN TWO PERSONS MUST OPERATE IN
DANGEROUS C ONDITIONS
DO NOT START WORK WITHOUT HAVING
PERFORMED THE NECESSARY PRECAUTIONS
ELECTRICAL BOARD
Figure 2.2 -- Plates indicating potential dangers
2.4.2. Machine state
This machine allows for three different operating modes.
The first one (USE) allows to give the machine simple orders, while checking it in full safety, from its starting to process
completion, as well as to obtain the reports and/or messages confirming successful process performance.
The second operating mode (CONFIGURATION) allows to reconfigure the machine applications (sterilization
programs), while defining the general parameters controlling the activities and functions common to all sterilization
processes.
-- 8 --
#68181 Rev.1
May 2001

The third operating mode (MAINTENANCE) includes all operating modes allowing to service and/or control (also ” ON
LINE ”) several software functions and the functional operating logics controlling the sterilizer.
2.4.3. Operators
When analyzing the works described in this manual, concerning the machine life cycle steps, FEDEGARI has taken
into account the operator’s qualities.
2.4.3.1. Operators’ qualification
Please find below a profile about the professional qualities required to each operator using the sterilizer.
The operator:
-- Must know the physical and chemical principles concerning gas, fluid and liquid dynamics during sterilization
processes;
-- Must be acquainted with environmental and behavioral problems, to protect the environment and other products
from microbiological and particulate contamination;
-- Must thoroughly know the operating principles of the machine, its structure, main blocks as well as the allocation of
the control units allowing to disconnect the machine from its main supply sources;
-- Must be properly trained for all operations required to start and control the plant and able to record the sterilization
data, also referring to the workmanlike manufacture rules in force in the country where the machine is used;
-- Must be properly trained to evaluate all events occurring during the sterilization cycle;
-- Must be properly trained to evaluate any alarms activated during the cycle;
-- Must be properly trained for stopping the machine in case of an emergency (also concerning the batch);
-- Must be properly trained and able to perform the preventive and corrective maintenance required for operator
interface performance.
CAUTION!
This recommended profile is to be integrated with other professional skills required by the rules in
force in the country where the machine is used.
May 2001
-- 9 --
#68181 Rev.1
2.4.3.2. Personal protection means
Based on the batch to be processed, the operator must protect his body against the direct or indirect influence of
chemicals (whether organic or not) or microbiological elements (e.g. viruses, bacteria), forming the batch to be
sterilized or aggregated to it.
These substances can be noxious by contact, inhalation or contamination.
Always wear suitable protection means, such as masks, suits, gaberdines, gloves, protective glasses, footwear, boots
etc...
CAUTION!
The operator MUST be allowed to check the batch before its delivery, to verify the type of material
included.
CAUTION!
The operator must wear suitable work clothing, to be protected against chemical, physical and/or
microbiological risks, deriving from contact with a wide range of materials, including gases and
liquids.
The work clothing material and structure must be fit for protecting the operator against any
contact/contamination dangers.
The buyer is responsible for the use of highly protective means suitable for the type of product to be
processed, in compliance with the rules in force in the country where the machine is used.
WARNING
All personal protection means must permit the operator t o move freely in order to carry out the
necessary operations and to properly see with the best angulation and limited sight distortion.
Use only certified protection means. All protection means must be carefully used and kept (e.g. wear
them correctly, tighten the related closing systems, replace mask filters etc.).
WARNING
Personal protection means are sometimes recommended in order to protect the processed product
against contamination (e.g., during unloading operations).
To treat products free of chemical or microbiological risks:
-- Wear a waterproof cotton one--piece suit or other work clothing, allowing for good transpiration and covering the
whole body, except for your face;
-- Wear suitable, comfortable shoes, for proper mechanical protection from spilled liquids;
-- Wear light, protective glasses against sprays. They should not be made of glass, without fragmentation danger,
transparent with the best refractive index, visual angulation and low distortion.
#68181 Rev.1
-- 10 --
May 2001

2.4.4. Recommendations and precautionary measures to be adopted by the user
The user must fit the installation area with suitable lighting devices, complying with safety and accident prevention
rules.
The user must assure that the area before the sterilizer is provided with an antislip flooring, in aggregate or polymeric
materials, easy to wash and fit for chemical and thermal disinfection.
The floor must be fit for any processing, according to the products to be sterilized.
Clear and legible warnings must be installed near the work station, to prevent unauthorized personnel from entering
the work area.
Before delivery, the user must train its operators and make sure that they are acquainted with the machine and able to
carry out the necessary operations, according to the skill profile mentioned in section
2.4.3.1.
2.4.5. Emergencies
The user must fit the area with proper fire--fighting stations.
CAUTION!
C02 fire--fighting means are recommended in order to provide preventive protection.
In an emergency, the operator must be free to reach the main switch and the primary feed shut--off valves.
Electric system black--out
In case of black--out, the main switch is activated to isolate all downstream elements.
All processes started before the black--out will be stopped.
When the electric power is restored, after resetting the main switch, the sterilizer enters an emergency state, waiting to
receive a manual control which brings the sterilization process to the cycle end phase or to the
Emergency--Decontamination phase, after an electrical blackout has occurred, during the ”Heating” or ”Sterilization”
phases, in the Decontamination Program.
The sterilization chamber features a pneumatic cover seal and the related devices, which assures chamber tightness
also in case of pneumatic or power failure.
Depressurizing the door pneumatic seal takes more than depressurizing the chamber.
The atmospheric pressure inside the chamber, caused by sealing failure due to a black--out, is reached after at least
20 hours, based on the machine maintenance level.
Pneumatic, steam and water supply failure
All supply line failures are detected by proper sensors and could considerably increase the time required to complete
the steps of the process in progress.
The sterilizer enters an emergency state, waiting for the reset of suitable parameters.
May 2001
-- 11 --
#68181 Rev.1

Emergency stop
Press the ”EMERGENCY” mushroom--head push--button to stop power supply to the machine by means of the main
switch. Refer to the instructions of item ”Electric system black--out”.
#68181 Rev.1
-- 12 --
May 2001

3. GENERAL INFORMATION
3.1. GENERAL DESCRIPTION
3.1.1. Foreword
FV A vertical steam sterilizers are flexible and high--performance small--sized semiautomatic machines for
sterilization processes to be carried out in laboratories and hospitals (where they are not use as production
equipment).
3.1.2. Main components
The sterilizer (see Figure 3.1) consists of the following main components:
-- Sterilization chamber;
-- Cover and related sealing system;
-- Technical cabinet complete with a shutter covering the electrical board;
-- Electric system;
-- Hydraulic and pneumatic installations;
-- Operator panel;
-- PLC electronic process controller console;
-- Additional components (optional);
-- Fittings for product loading/unloading;
May 2001
-- 13 --
#68181 Rev.1
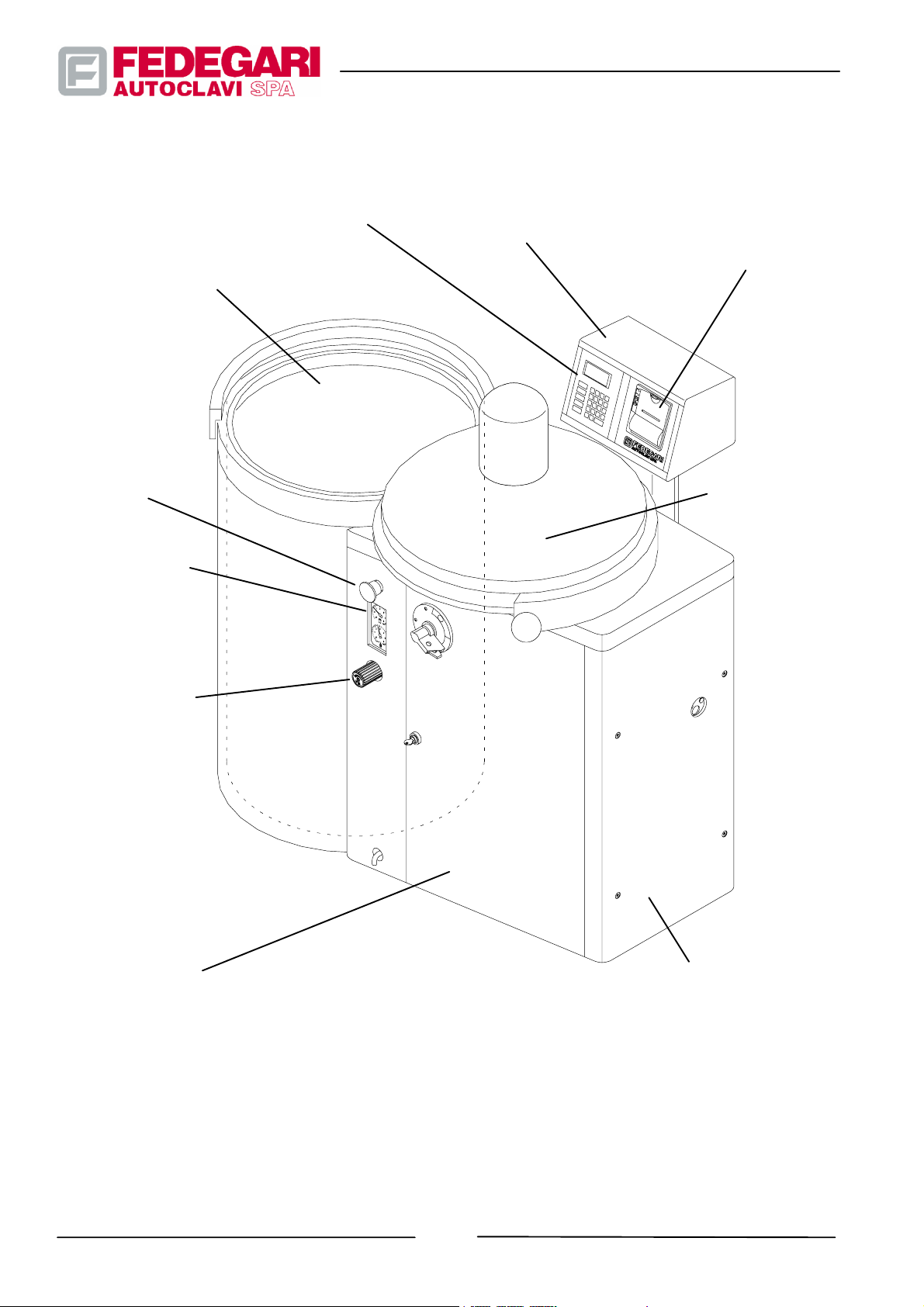
Sterilization chamber
Operator panel
Electronic process
controller
Printer (K81)
or
Kit EN285 Datalogger (K82)
Emergency
pushbutton
Vacuum--pressure
gauges
Manual Valve for
pressure discharge
in the chamber
(not present for
model FVA--HT)
Cover and
relevant sealing
system
Switchboard panel
#68181 Rev.1
Technical cabinet
Figure 3.1 -- Main components of the sterilizer
-- 14 --
May 2001
3.1.2.1. Sterilization chamber
The pressurized sterilization chamber features a cylindrical section and a vertical axis, and houses the material to be
processed. The purge system on its bottom collects the condensate to be drained. The chamber is in AISI 316Ti gloss
finished steel with Ra < 0,19 mm surface roughness
3.1.2.2. Cover and Sealing system
Cover
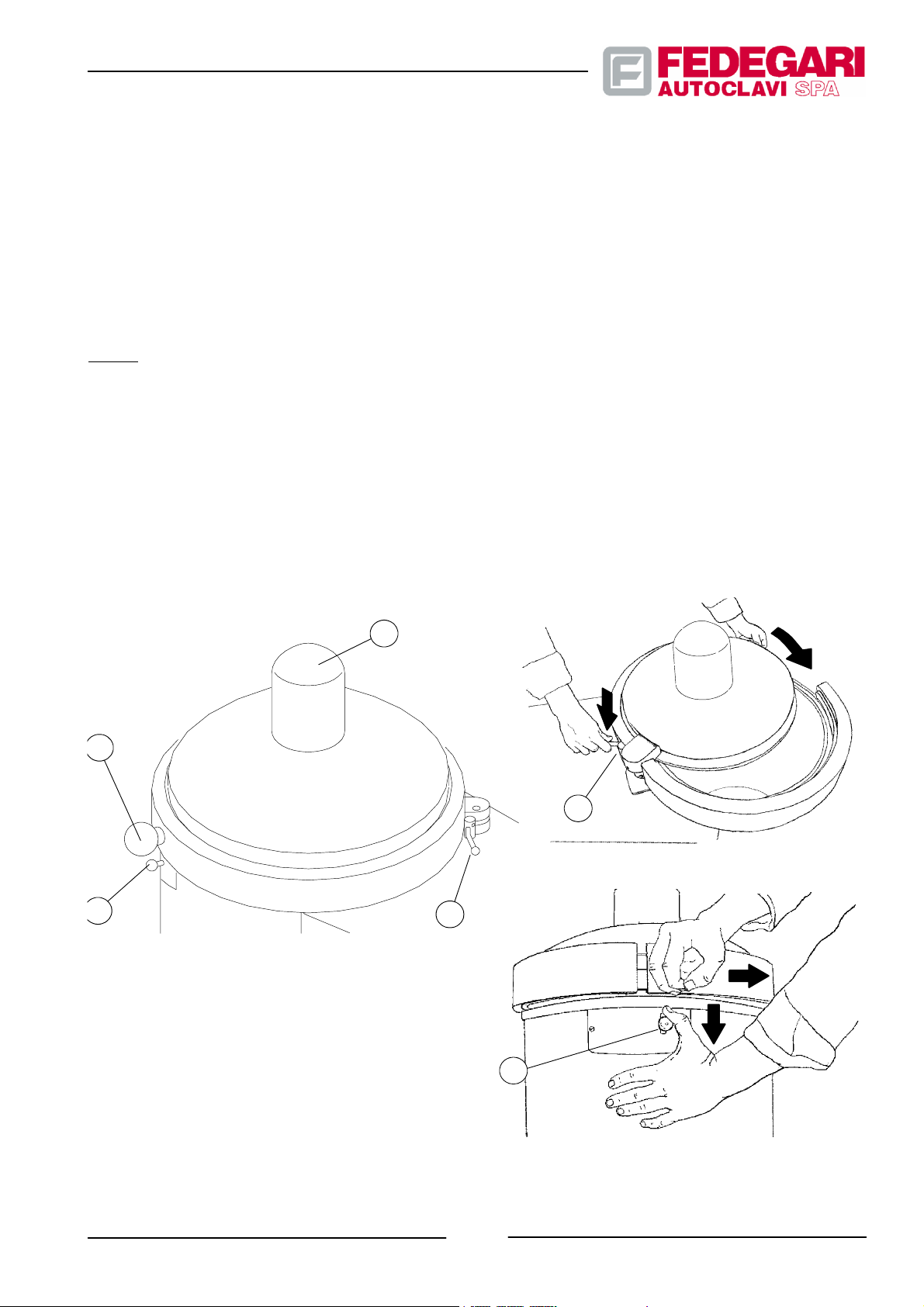
The horizontally rotating cover (see Figure 3.2), in position ”Closed door” is coupled with the chamber through a ”C”
band which, even under minimum pressure inside the chamber prevents the cover from rotating and opening.
The chamber cover is fitted with a horizontally rotating knob (pos. 1) for easy opening and closing,. When closing the
cover, a safety lever (pos. 2) positioned on its top, is moved by the operator with both hands, to prevent damaging his
fingers. Once closed, the cover can be opened by lowering the lever (pos.3), which releases a safety device.
The particular profile of the cover allows you to install a special (optional)fan, with magnetic joint (pos. 4), to be used for
specific phases of batch cooling.
1
3
LEGEND
1. Knob
2. Safety lever
3. Cover release lever
4. Fan (optional)
4
2
2
3
May 2001
Figure 3.2-- Cover closing system
-- 15 --
#68181 Rev.1

Sealing system
The sealing system consists of a silicone rubber seal. To seal the sterilization chamber when the cover is closed,
supply the seal housing with compressed air. The seal is pushed outwards and pressed against the cover.
3.1.2.3. Technical cabinet
The external cabinet, in polyurethane resin and featuring an AISI 304 stainless steel frame, includes the hydraulic,
electric and pneumatic installations, the process electronic controller components and, according to the machine
configuration, the autonomous steam generator, the vacuum pump and other optionals.
3.1.2.4. Electric system
The sterilizer electric system complies with EN61010--1, EN61010--2--041, EN60204--1 European standards.
The electric circuit is fitted into a suitably ventilated box, which can be easily reached from the machine front.
The plant includes all control lines of electrohydraulic or electropneumatic actuators of the motors, of the electronic
process controller,of the autonomous steam generator,if any. The plant connects the process controller to all sensors
and transducers (e.g. pressure switches, heat probes etc.), as well as to the operator interface devices. It also
provides for the primary feed line and its protection plant.
3.1.2.5. Electric board
The electric board is located in front of the machine cabinet and lodges the main electric and electronic
feeding/disconnecting devices, as well as the distribution equipment, including the main switch. This switch features a
release coil allowing to open it, should the protection devices be activated, and to disconnect power supply to the
machine.
3.1.2.6. Plumbing and pneumatic plant
The plumbing and pneumatic plant (see the P&ID scheme) consist of pipes, solenoid valves, pneumatic and
electropneumatic valves. The hydraulic plant is in AISI 316L stainless steel and is fitted with small sanitary flanges,
with the related closing clamps for connecting each element and suitable elastomer seals.
3.1.2.7. Operator panel
The Operator Panel, located on the top of the cabinet, consists of a LCD 4 lines, 20 columns display and of a
membrane keyboard, through which the operator manages the interactive dialogue with the machine. It also features
RAM , with emergency power supply and FLASHEPROM for the user program, a serial port for the printer interface
and a RS 232 serial port for PLC interfacing.
#68181 Rev.1
-- 16 --
May 2001

3.1.2.8. Electronic process controller
The electronic process controller consists of a programmable logic controller (PLC) which, together with specific
hardware blocks and other electronic equipment, controls the machine as well as all operations regarding the
interactive dialog with the operator.
It features 16 digital inputs/outputs, RS232 serial port for interfacing with the operator’s panel, RAM with emergency
power supply. The controller can use EPROM or EEPROM and features another RS232 serial port to transmit the
cycle data to an outer system (master), with a well--defined ASCII protocol.
The control software is certified according to IEC1131--3 standards.
The controller:
-- manages the dialogue with the operator through the interface devices;
-- controls the hydraulic/pneumatic plant actuators;
-- detects the system logics from the field (electric and hydraulic/pneumatic plant), based on the confirmation of set
activations;
-- detects the analog values of pressure and temperature measurements carried out, through specific modules
converting the analog signal from temperature and pressure sensors into a PLC--readable digital signal.
The process controller consists of the following units:
A PLC Matsushita FP--M--C32TC 5K--DC24V;
B Operator panel EXOR R&D T--line TCP01R--04--0245;
C Printer CUSTOM FT190SP;
D Module TECNA ADPLC/F32, converting analog signals into digital signals, with a minimum of 2 digital input and
output lines, for the direct reading of a 420 mA sensor and of two 4--wire heat probes Pt100.
E Expansion card provided with 12 inputs and 8 digital outputs;
F Datalogger CUSTOM FT190DL (K82).
Figure 3.3 shows the functional connections between the main blocks forming the process controller.
May 2001
-- 17 --
#68181 Rev.1
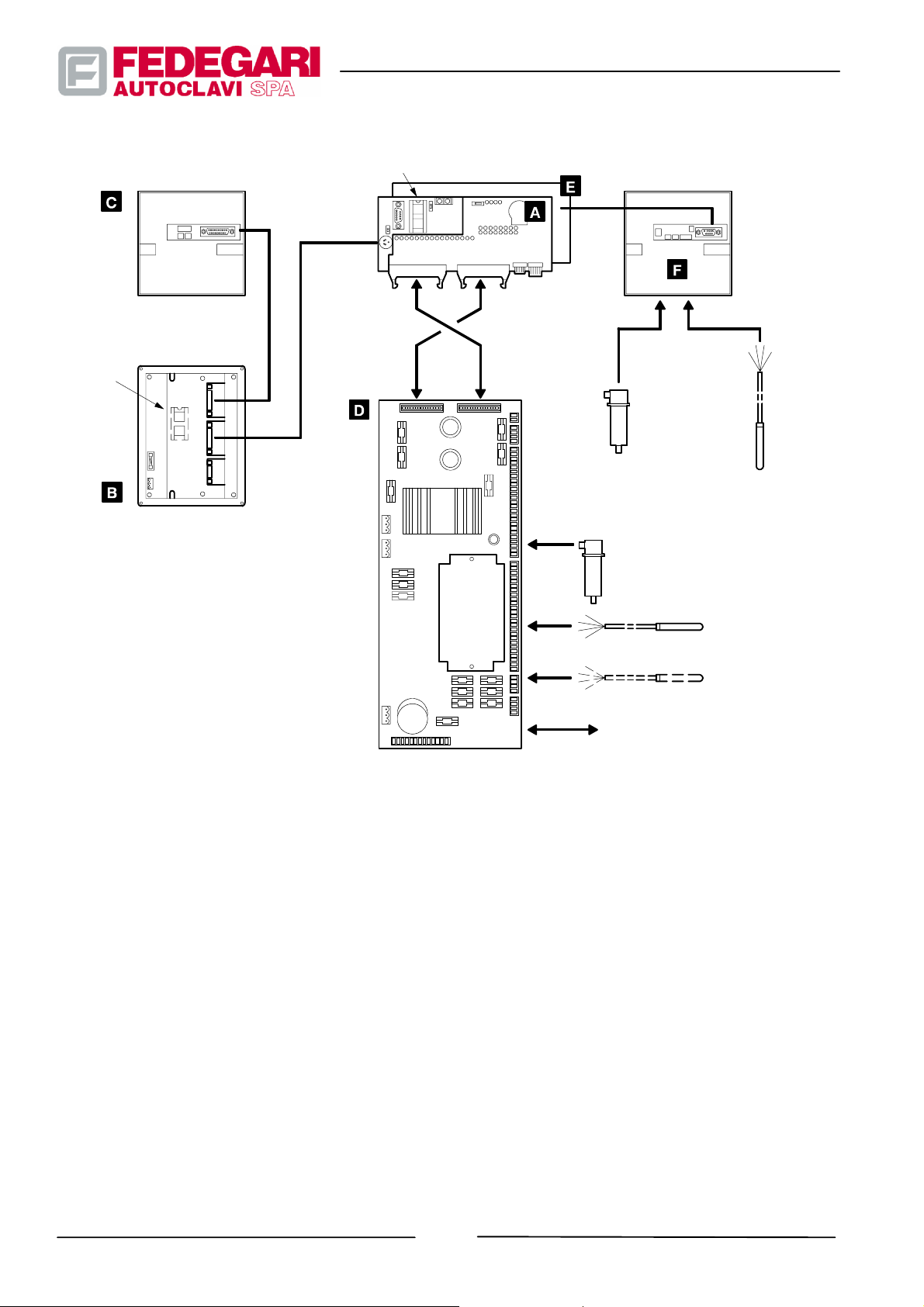
PRINTER
(optional K81)
RS232
EPROM
EXPANSION
(optional K82)
DATALOGGER
(optional K82)
EPROM
OPERATOR
PANEL
RS232
16 16
IN OUT
OUT IN
MASTER
BOARD
PLC
PRESSURE
TRANSDUCER
4- -20 mA
(optional K82)
PRESSURE
TRANSDUCER
4--20 mA
4--WIRES PT100 HEAT
PROBES
(optional K80)
ANALOG/DIGITAL SIGNALS
FROM/TO
THE FIELDS
Pt100 4 WIRES
HEAT PROBE
(optional K82)
Figure 3.3 -- Process electronic controller--functionals connections
3.1.2.9. Main additional components (optional)
Main optional components:
-- Steam generator;
-- Va cu um pu mp ;
-- Effluent cooler;
-- Air electrocompressor;
-- Air sterilising filter;
-- Network compressed air supply unit;
-- Quick cooling unit;
-- Spontaneous cooling;
#68181 Rev.1
-- 18 --
May 2001

-- Pressurized chamber leak test;
-- Decontamination cycle;
-- Steam supply deflection unit;
-- Additional heat probe;
-- Process printer;
-- Control function F
-- Secondary RS232 serial output;
-- Datalogger;
-- Deionizer.
;
0
Steam generator (K10)
The steam generator is installed when no saturated steam is available to feed the sterilizer, or when the quality of the
available steam is poor. The steam generator consists of pressurized reservoir D001, in AISI 316 stainless steel,
supplied with deionized water evaporating by means of three electric heating elements RX02 fitted on the top of the
reservoir. The loading of deionized water, supplied by the feed network and by a proper container through an electric
pump PA 0 1 , is controlled by solenoid valve E009, which opens pneumatic valve S009. Check valve R004 prevents
water from flowing to the pump. Pressure switch P004 detects pressure inside the generator, checks the heating
element power supply and warns the process controller when the pressure reached allows to supply the chamber.
Sensors L001 and L002 detect the water level inside the pressurized body.
The steam generator is also fitted with the following devices, for the pressurized reservoir operational safety:
-- Manual discharge valve V004;
-- Safety valve Y002;
-- Pressure gauge M003 detecting reservoir D001 pressure, clearly visible to the operator;
-- The rmo sta t T002, controlling the heating element temperature and signaling dry operation;
-- The rmo sta t T003, if required, checking the generator maximum temperature (TÜV).
Vacuum pump unit (K20)
It consists of a liquid ring pump PV01, fed by the network water. Water enters the pump through solenoid valve E011
and is regulated by valve V003.ValveV007 adjusts the air ballast necessary to the vacuum pump through the air
intake. Solenoid valve E011 opens when the motor is energized.
The pump is used to create continuous as well as discontinuous (impulse) vacuum in the sterilizer chamber.
This pump is required whenever air cushions in the batch could prevent its sterilization, as well as for product drying.
Effluent cooler (K30)
Effluent cooler W001 consists of a small mixing reservoir, fed by network water.The drain line condensate and gases
are sent to the reservoir before being discharged outside the machine. The cooling system is regulated by thermostat
May 2001
-- 19 --
#68181 Rev.1

T004 and solenoid valve E001, located on the network water feeding line. The cooler is used in order to lower the
effluent temperature (up to 60_C) before letting it outside the sterilizer.
The cooling unit must be fitted whenever low temperature effluent is required, e.g. when the regulations of the
installation site do not allow for high--temperature effluent, or when the drain lines connected to the sterilizer are in
plastic material, which is likely to be damaged by high temperature.
Internal air electrocompressor (K40)
It can be fitted into the machine cabinet, to be used when no network compressed air is available.
The electrocompressor model and the related plenum chamber must be chosen based on the machine configuration.
If the sterilizer is not used to process liquids, compressed air is only needed to feed the cover seal and the pneumatic
valves. A 2 liters plenum chamber is sufficient to guarantee proper operation.
External Air electrocompressor (K43)
When the machine is used to carry out sterilization processes requiring compressed air, an external compressor must
be installed featuring a plenum chamber with more than 10 liters capacity.
Sterilizing filter for air (K50)
The air filtering unit consists of a filter F007 which can be sterilized in the sterilizer, with 0,22 mm retention, fitted in a
plastic container.
Network compressed air supply unit (K51)
This unit consists of solenoid valve E010 and pressure reducer VR02, which supplies the chamber with sterile
compressed air. This unit must be installed on the sterilizer when the batch to be sterilized requires an air
counter--pressure on completion of the sterilization process.
Quick cooling unit (K52)
The quick cooling unit basically consists of a magnetic fan CV12 and a heat exchanger located in the cover concavity.
They are installed so that the sterilization chamber volume is not reduced. The fan coupled to the motor ME12 through
a suitable magnetic joint must assure air circulation inside the chamber. Air is cooled when coming into contact with
the surface of the heat exchanger, supplied by network water. This procedure allows to reduce the liquid cooling time
and to increase the sterilizer performance. The quick cooling unit is recommended whenever the sterilizer is used to
process heat--sensitive liquids, in particular when they are stored in large bottles.
Spontaneous cooling (K53)
This option allows the ”Slow spontaneous cooling” of the load.
It requires the use of load interception electro--valve E007, that during the ”Spontaneous cooling” phase remains
closed until the pressure inside the chamber is lower than/equal to the average of maximum and minimum balancing
pressures.
The opening of the valve allows the discharge of the condensate produced during the cooling phase. The phase ends
-- 20 --
#68181 Rev.1
May 2001

as soon as the target Temperature is reached.
During the ”Spontaneous cooling” phase, the pressure inside the chamber reaches balancing pressure values
spontaneously, without creating a pressure drop that is likely to damage the batch, if consisting of liquids contained in
reservoirs.
NOTE
The presence of option K51 ”Newtork compressed air supply unit” or option K52 ”Quick cooling
unit” (including K51), disables the effect of option K53 ”Spontaneous cooling”, that in these cases is
not enabled.
Pressurized chamber leak test (K61)
This option is used to perform the pressurized chamber leak test. It consists in shutting--off the discharge, usually
connected to the chamber through a calibrated orifice. This test can be performed as an alternative to vacuum leak
test in sterilizers lacking the necessary equipment. To carry out the pressure leak test, the machine must be fitted with
the air supply unit in counterpressure (K51).
Decontamination cycle (K62)
This cycle is installed on the machine in order to sterilize products involving microbiological pollution risks. During the
sterilization phase, start solenoid valve E071 to let saturated steam in, either through the chamber purge system or
directly through S008. The chamber and the purge system must have the same temperature. Solenoid valve E007 is
closed until completion of the sterilization process. Therefore, the sterilization chamber batch must not be removed
until the process is completed, i.e. when the chamber content has been sterilized. The maximum allowed temperature
is 121°C, equal to a 3.4 bar pressure with air/steam mixture.
Steam supply deflection unit (K70)
This device allows to selectively feed a sterilizer featuring an autonomous steam generator (K10), also with network
steam. The operator enters a process controller parameter for manually deviating a three--way valve V009 inside the
sterilizer technical cabinet.
Additional heat probe (K80)
This Pt 100 heat probe T005 is identical to that fitted on the sterilizer chamber. It can be connected to the process
controller, which will provide for the second temperature measurement, to be printed with the first one, or to an
optional external recorder.
Process printer (K81)
It is an alphanumeric printer installed next to the operator interface, in order to print detailed process documents. The
printer is provided with a 200 dpi thermal printing mechanism, uses 57.7mm wide paper and is connected to the
controller through a RS232 serial output.
Secondary R S232 serial output (HW1)
This option consists of a special software version loaded on the process controller, which enables the second PLC
RS232 serial output. It can be used to download in real time all data of the process in progress to a remote PC, in ASCII
format, or to connect a remote PC featuring the Fedegari software CVB- -LINK.
May 2001
-- 21 --
#68181 Rev.1
 Loading...
Loading...