Eutech Instruments AMMONIUM EPOXY User Manual

Instruction Manual Ammonium Electrode
EUTECH INSTRUMENTS PTE LTD.
AMMONIUM ION ELECTRODE
INSTRUCTION MANUAL
GENERAL INSTRUCTIONS
Eutech Instruments Ammonium Ion Electrode is used to measure ammonium ions in aqueous
solutions in a more simple, quick, accurate, and economical manner.
Required Equipment
1. A pH/mV meter or an ion meter, either line operated or portable.
2. Semi-logarithmic 4-cycle graph paper for preparing calibration curves when using the
meter in the mV mode.
3. A magnetic stirrer.
4. Eutech Ammonium Ion Combination Epoxy-body Electrode, Code no. EC-NH4-03.
Required Solutions
1. Deionized or distilled water for solution and standard preparation.
2. Eutech Ionic Strength Adjuster (ISA), 5M NaCl, Code no. EC-ISA-AM1-BT to keep a
constant background ionic strength present in the solution. To prepare the 5M NaCl ISA
from your own laboratory stock, add 292 grams of reagent-grade sodium chloride (NaCl)
to a 1,000 ml volumetric flask about half full of distilled water. Swirl the flask to dissolve
the solid and fill to the mark with distilled water. Cap the flask and invert several times to
mix the solution. Add 2 ml of ISA to every 100 ml of sample or standard solution for a
background ionic strength of 0.10M.
3. Eutech Ammonium Standard, 0.1M NH4Cl, Code no. EC-SCS-AM1-BT. To prepare this
standard, add 5.34 grams of reagent-grade ammonium chloride to a 1 liter volumetric flask
about half full of distilled water. Swirl the flask to dissolve the solid and fill to the mark
with distilled water. Cap the flask and invert several times to mix the solution.
4. Eutech Ammonium Standard, 1,000 ppm NH
this standard, add 2.97 grams of reagent-grade ammonium chloride to a 1 liter volumetric
flask about half full of distilled water. Swirl the flask to dissolve the solid and fill to the
mark with distilled water. Cap the flask and invert several times to mix the solution.
+1
, Code no. EC-SCS-AM2-BT. To prepare
4
1

Ammonium Electrode Instruction Manual
GENERAL PREPARATION
Electrode Preparation
Remove any rubber cap(s) covering the electrode tip(s) and the rubber inserts covering the filling
holes of the reference electrode. Fill the combination electrode or the reference electrode with the
filling solution shipped with the electrode to a level just below the fill hole. No preparation is
required for a sealed reference electrode. Gently shake the electrode downward in the same manner
as a clinical thermometer to remove any air bubbles that might be trapped behind the ammonium
membrane. Prior to first usage, or after long term storage, immerse the ammonium electrode in
ammonium standard for thirty minutes. The electrode is now ready for use. Connect the electrode(s)
to the proper terminal(s) as recommended by the meter manufacturer.
Electrode Slope Check (with standard pH/mV meter)
(Check electrodes each day)
To a 150 ml beaker, add 100 ml of distilled water and 2 ml of ISA. Place the beaker on a magnetic
stirrer and begin stirring at a constant rate. After assuring that the meter is in the mV mode, lower
the electrode tip(s) into the solution.
Using a pipet, add 1 ml of 0.1M or 1,000 ppm ammonium standard to the beaker. When the reading
is stable, record the millivolt reading. Using a pipet, add 10 ml of the standard used above to the
beaker. When the reading has stabilized, record the millivolt reading.
Determine the difference between the two readings. A difference of 56±2 mV indicates correct
electrode operation, assuming the solution temperature is between 20o and 25oC. See the
TROUBLESHOOTING section if the potential change is not within this range.
Slope is defined as the change in potential observed when the concentration changes by a factor of
10.
Electrode Slope Check (with ion meter)
(Check electrodes each day)
Prepare standard ammonium solutions whose concentrations vary by tenfold. Use either the 0.1M
+1
NH
or the 1,000 ppm NH
4
+1
standard stock solutions. Use the serial dilution method for this
4
preparation.
To a 150 ml beaker, add 100 ml of the lower value standard and 2 ml of ISA. Place the beaker on
the magnetic stirrer and begin stirring at a constant rate. Lower the electrode tip(s) into the solution.
Assure that the meter is in the concentration mode. Adjust the meter to the concentration of the
standard and fix the value in the memory according to the meter manufacturer's instructions.
Rinse the electrode(s) with distilled water and blot dry. To a 150 ml beaker, add 100 ml of the
higher value standard and 2 ml of ISA. Place the beaker on the magnetic stirrer and begin stirring at
a constant rate. Lower the electrode tip(s) into the solution.
Adjust the meter to the concentration of the standard and fix the value in the memory. Read the
electrode slope according to the meter manufacturer's instructions. Correct electrode operation is
2

Instruction Manual Ammonium Electrode
indicated by a slope of 90-100%. See the TROUBLESHOOTING section if the slope is not within this
range.
MEASUREMENT
Measuring Hints
The sensing membrane is normally subject to water uptake and might appear milky. This has no
effect on performance. All samples and standards should be at the same temperature for precise
measurement, preferably ambient temperature.
Constant, but not violent, stirring is necessary for accurate measurement. Magnetic stirrers can
generate sufficient heat to change the solution temperature. To counteract this effect, place a piece
of insulating material, such as styrofoam sheet or asbestos sheet, between the stirrer and beaker.
Always rinse the electrode tip(s) with distilled water and blot dry with a fresh tissue between
readings to prevent solution carryover.
Check the electrode for air bubbles adhering to the membrane surface after immersion in solution.
Agitate the electrode gently to remove the air bubbles.
A slow or sluggish electrode response may indicate surface contamination of the ammonium
electrode membrane. Soak the electrode tip in distilled water for about 5 minutes to clean the
membrane. Rinse the membrane and soak in diluted standard solution for about 5 minutes to restore
performance.
When measuring samples with high ionic strength, prepare standards with compositions similar to
that of the sample.
Dilute concentrated samples (over 0.1M) before measurement. Recalibrate every few hours for
routine measurement.
Sample Requirements
Make sure that the samples and standards are at the same temperature. About a 2% error will be
introduced for a 1oC difference in temperature. Temperature should normally be less than 40oC
with intermittent measurements allowed to 50oC.
All samples and standards must be aqueous. They must not contain organic solvents.
Interference found in Table 3 should be absent.
Units of Measurement
Ammonium concentrations are measured in units of parts per million as ammonium, parts per
million as N, moles per liter, or any other convenient concentration unit. Table 1 indicates some of
the concentration units.
3
Ammonium Electrode Instruction Manual
TABLE 1: Concentration Unit Conversion Factors
ppm NH
+
ppm N moles/liter NH
4
+
4
1.80 1.40 1x10-4
18.00 14.00 1x10-3
180.00 140.00 1x10-2
Measurement Procedure
Direct Measurement
A simple procedure for measuring a large number of samples. A single meter reading is all that is
required for each sample. The ionic strength of samples and standards should be made the same by
adjustment with ISA for all ammonium samples. The temperature of both sample solution and of
standard solutions should be the same.
Direct Measurement of Ammonium (using a standard pH/mV meter)
1. Prepare 10
-
, 10
3
-
, and 10
4
-
M or 100, 10, and 1 ppm standards by serial dilution of the
2
0.1M or 1,000 ppm standard. Add 2 ml of ISA per 100 ml of standard.
4
2. Place the most dilute solution (10
-
M or 1 ppm) on the magnetic stirrer and begin stirring
at a constant rate. After assuring that the meter is in the mV mode, lower the electrode
tip(s) into the solution. When the reading has stabilized, record the mV reading.
3
3. Place the midrange solution (10
-
M or 10 ppm) on the magnetic stirrer and begin stirring.
After rinsing the electrode(s) with distilled water and blotting dry, immerse the electrode
tip(s) in the solution. When the reading has stabilized, record the mV reading.
2
4. Place the most concentrated solution (10
-
M or 100 ppm) on the magnetic stirrer and begin
stirring. After rinsing the electrode(s) with distilled water and blotting dry, immerse the
electrode tip(s) in the solution. When the reading has stabilized, record the mV reading.
5. Using the semi-logarithmic graph paper, plot the mV reading (linear axis) against the
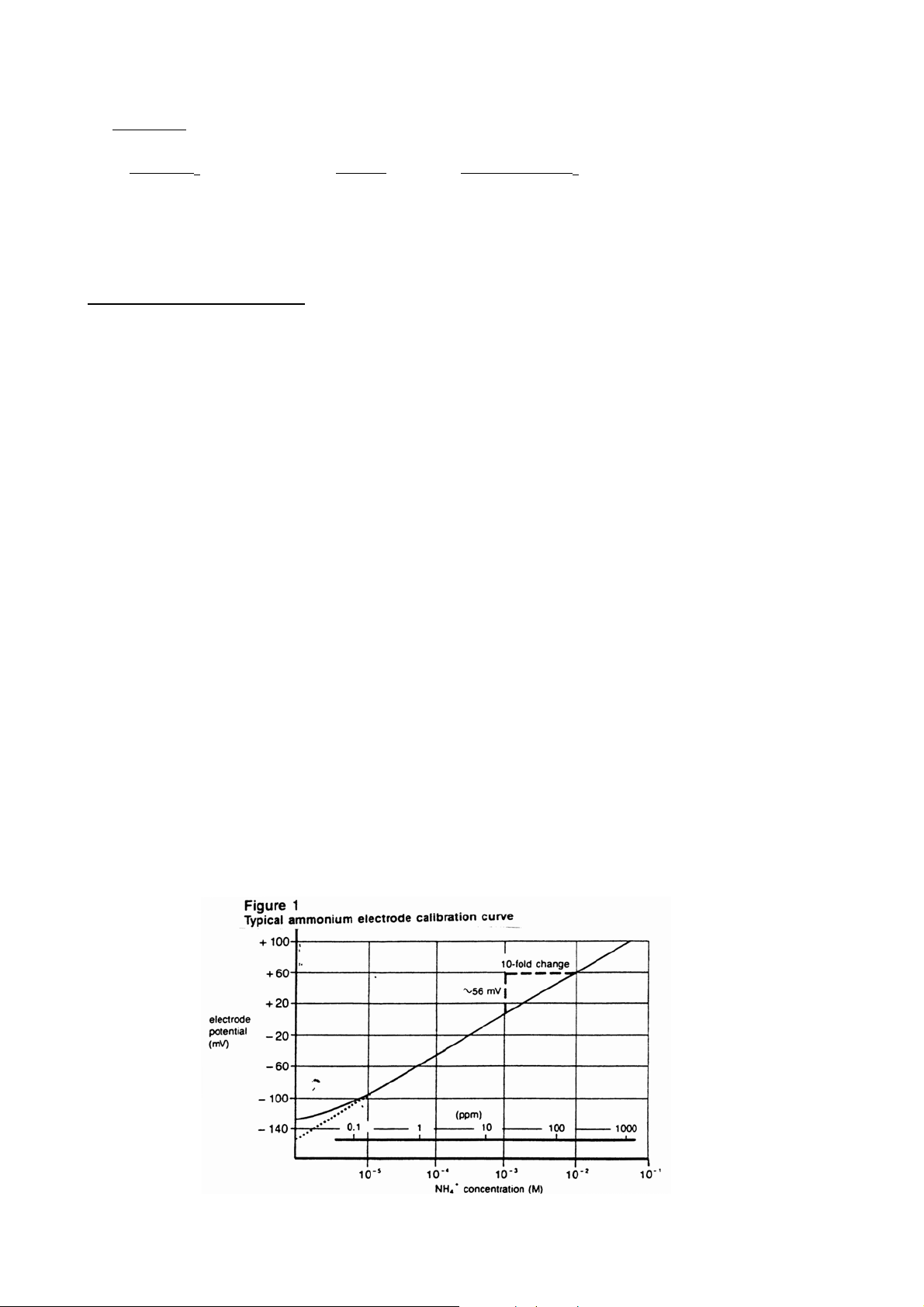
concentration (log axis). A typical calibration curve can be found in Figure 1.
4

Instruction Manual Ammonium Electrode
A calibration curve is constructed on semi-logarithmic paper when using a
pH/mV meter in the direct measurement procedure. The measured electrode
potential in mV (linear axis) is plotted against the standard concentration (log
axis). In the linear region of the curve, only three standards are necessary to
determine a calibration curve. In the non-linear region, additional points must be
measured. The direct measurement procedures given are for the linear portion of
the curve. The non-linear portion of the curve requires the use of low level
5
procedures. Extrapolate the curve down to about 1x10
-
M or 0.2 ppm.
6. To a clean, dry 150 ml beaker, add 100 ml of sample and 2 ml of ISA. Place the beaker on
the magnetic stirrer and begin stirring. Place the electrode tip(s) in the solution. When the
reading has stabilized, record the millivolt reading. Determine the concentration directly
from the calibration curve.
7. The electrode(s) should be re-calibrated every 1-2 hours. Simply repeat Steps 2-5 above.
Direct Measurement of Ammonium (using an ion meter)
1. By serial dilution of the 0.1M or 1,000 ppm ammonium standard, prepare two ammonium
standards whose concentration is near the expected sample concentration. Measure out
100 ml of each standard into individual 150 ml beakers and add 2 ml of ISA to each.
2. Place the more dilute solution on the magnetic stirrer and begin stirring at a constant rate.
Assure that the meter is in the concentration mode.
3. Lower the electrode tip(s) into the solution.
4. Adjust the meter to the concentration of the ammonium standard and fix the value in the
memory according to the meter manufacturer's instructions after stabilization of the
reading.
5. Rinse the electrode tip(s) with distilled water and blot dry.
6. Place the more concentrated solution on the magnetic stirrer and begin stirring at a
constant rate.
7. Lower the electrode tip(s) into the solution.
8. Adjust the meter to the concentration of the ammonium standard and fix the value in the
memory according to the meter manufacturer's instructions after stabilization of the
reading.
9. For low level measurements, place the rinsed, dried electrode(s) into a solution containing
100 ml of distilled water and 2 ml of ISA. After stabilization, fix the blank value in the
meter according to the meter manufacturer's instructions.
10. Place 100 ml of the sample and 2 ml of ISA in a 150 ml beaker, place it on the magnetic
stirrer, and begin stirring.
5
 Loading...
Loading...