EDAN SONOTRAX, SONOTRAX Basic A, SONOTRAX Lite, SONOTRAX Pro, SONOTRAX II Basic User Manual
...

I
About this Manual
P/N: 01.54.36708-17
Release Date: Aug. 2010
© Copyright EDAN INSTRUMENTS, INC. 2007-2010. All rights reserved.
Statement
This manual will help you understand the operation and maintenance of the product better. It
is reminded that the product shall be used strictly complying with this manual. User’s
operation failing to comply with this manual may result in malfunction or accident for which
Edan Instruments, Inc. (hereinafter called EDAN) can not be held liable.
EDAN owns the copyrights of this manual. Without prior written consent of EDAN, any
materials contained in this manual shall not be photocopied, reproduced or translated into
other languages.
Materials protected by the copyright law, including but not limited to confidential information
such as technical information and patent information are contained in this manual, the user
shall not disclose such information to any irrelevant third party.
The user shall understand that nothing in this manual grants him, expressly or implicitly, any
right or license to use any of the intellectual properties of EDAN.
EDAN holds the rights to modify, update, and ultimately explain this manual.
Responsibility of the Manufacturer
EDAN only considers itself responsible for any effect on safety, reliability and performance
of the equipment if:
Assembly operations, extensions, re-adjustments, modifications or repairs are carried out by
persons authorized by EDAN, and
The electrical installation of the relevant room complies with national standards, and
The instrument is used in accordance with the instructions for use.
Upon request, EDAN may provide, with compensation, necessary circuit diagrams, and other
information to help qualified technician to maintain and repair some parts, which EDAN may
define as user serviceable.
Product Information
Product Name: Ultrasonic Pocket Doppler
Model: SONOTRAX Lite, SONOTRAX Basic, SONOTRAX Basic A, SONOTRAX Pro,
SONOTRAX II, SONOTRAX II Pro

II
Terms Used in this Manual
This guide is designed to give key concepts on safety precautions.
WARNING
A WARNING label advises against certain actions or situations that could result in personal
injury or death.
CAUTION
A CAUTION label advises against actions or situations that could damage equipment,
produce inaccurate data, or invalidate a procedure.
NOTE
A NOTE provides useful information regarding a function or a procedure.

III
Table of Contents
Chapter 1 Safety Guide..............................................................................................................1
1.1 Intended Use....................................................................................................................1
1.2 Safety Precautions ........................................................................................................... 1
1.3 Symbols...........................................................................................................................3
Chapter 2 Doppler and Accessories..........................................................................................4
2.1 Features ...........................................................................................................................4
2.2 Appearance......................................................................................................................5
2.3 Display Panel...................................................................................................................6
2.4 Buttons ............................................................................................................................7
2.5 Socket .............................................................................................................................. 8
2.6 Probes .............................................................................................................................. 9
2.6.1 Waterproof Obstetrical Probes.............................................................................. 9
2.6.2 Waterproof Vascular Probes .................................................................................9
2.6.3 Probe Socket........................................................................................................10
2.7 Batteries.........................................................................................................................11
Chapter 3 Operation ................................................................................................................12
3.1 Opening the Package and Checking..............................................................................12
3.2 Installing/Replacing Battery..........................................................................................12
3.3 Probe Operation.............................................................................................................14
3.4 Switching on the Doppler..............................................................................................15
3.5 Modes Setting................................................................................................................15
3.5.1 Real-time FHR Display Mode (Mode 1).............................................................16
3.5.2 Averaged FHR Display Mode (Mode 2).............................................................16
3.5.3 Manual Mode (Mode 3) ......................................................................................16
3.5.4 Backlight Brightness Setting Mode (Mode 4).....................................................16
3.5.5 Record Sampling Frequency Setting Mode (Mode 5) ........................................16
3.6 Fetal Heart (FH) Examining..........................................................................................17
3.7 FH Sound Recording and Playing by Build-in Recorder..............................................18
3.8 FH Sound Recording by PC .......................................................................................... 18
3.8.1 Recording Sounds ...............................................................................................18
3.8.2 Playing Sound Files.............................................................................................20
3.8.3 Burning CD or Sending in Email ........................................................................20
3.8.4 Record Troubleshooting......................................................................................20
3.9 Vascular Examining (Optional) ....................................................................................23
3.10 Switching Off the Doppler ..........................................................................................25
3.11 Replacing/Charging the Battery .................................................................................. 25
Chapter 4 Product Specifications ...........................................................................................27
Chapter 5 Maintenance ...........................................................................................................31
5.1 Maintenance .................................................................................................................. 31

IV
5.2 Cleaning ........................................................................................................................31
5.3 Disinfection of the Probe...............................................................................................31
Chapter 6 Warranty and After-Sales Service........................................................................32
6.1 Warranty........................................................................................................................ 32
6.2 After-Sales Service........................................................................................................32
Appendix 1 Ordering Information .........................................................................................33
Appendix 2 EMC Information-Guidance and Manufacture’s Declaration .......................34
A2.1 Electromagnetic Emissions - for all Equipment and Systems....................................34
A2.2 Electromagnetic Immunity - for all Equipment and Systems ....................................34
A2.3 Electromagnetic Immunity - for all Equipment and Systems that are not
Life-supporting....................................................................................................................35
A2.4 Recommended Separation Distances .........................................................................36
Appendix 3 Overall Sensitivity................................................................................................37
SONOTRAX Series Ultrasonic Pocket Doppler User Manual Safety Guide
- 1 -
Chapter 1 Safety Guide
NOTE:
This user manual is written to cover the maximum configuration. Therefore, your model
may or may not have some of the parameters and functions described, depending on
what you have ordered.
1.1 Intended Use
The SONOTRAX Series Ultrasonic Pocket Dopplers are intended to be used by health care
professionals including registered nurses, practical nurses, midwives, ultrasound technicians, and
physicians assistants, by prescription from licensed physicians in hospitals, clinics and private
offices.
The 2 MHz and/or 3 MHz waterproof probes are indicated for the detection of fetal heart rate
from early gestation thru delivery and as a general indication of fetal well being. They can also be
used to verify fetal heart viability following patient trauma.
The 4 MHz, 5 MHz and/or 8 MHz waterproof vascular probes are indicated for the detection of
blood flow in veins and arteries for assisting in the detection of peripheral vascular disease.
1.2 Safety Precautions
This unit is internally powered equipment, and it is an IEC/EN 60601-1 Type B applied
part. Type B protection means that the connection between the equipment and personnel
complies with permitted leakage currents and dielectric strength of IEC/EN 60601-1.
WARNING and CAUTION messages must be observed. To avoid the possibility of injury,
observe the following precautions during the operation of the device.
WARNING
1 This device is not intended for treatment.
2 This device is not explosion-proof and can not be used in the presence of flammable
anaesthetics.
3 Do not touch the signal input/output connector and the patient simultaneously.
4 We recommend that exposure to ultrasound should be kept as low as reasonably
achievable. This is considered to be good practice and should be observed at all
time.
5 Only use probes provided by the manufacturer.
6 The stretching length of probe cable should be less than two meters in order to avoid
the cable breaking away from the probe socket.
SONOTRAX Series Ultrasonic Pocket Doppler User Manual Safety Guide
- 2 -
WARNING
7 Check if the equipment has visible evidence of damage that may affect the
personnel’s safety or the device’s capability before use. If the damage is evident,
replace the defective part(s) before use.
8 Accessory equipment connected to the analog and digital interfaces must be
certified according to the respective IEC/EN standards (e.g. IEC/EN 60950 for data
processing equipment and IEC/EN 60601-1 for medical equipment). Furthermore all
configurations shall comply with the valid version of the system standard IEC/EN
60601-1-1. Anybody who connects additional equipment to the signal input
connector or signal output connector to configure a medical system must ensure that
the system complies with the requirements of the valid version of the system
standard IEC/EN 60601-1-1. If in doubt, consult our technical service department or
your local distributor.
9 The device shall only be used when the battery cover is closed.
10 Do not throw batteries in fire as this may cause explosion.
11 Do not attempt to charge normal alkaline batteries. They may leak, catch fire or even
explode.
12 Replacement of the battery shall be done at least 1.5 meters away from patients.
13 Charge the lithium-ion polymer battery with the special charger supplied by the
manufacturer.
CAUTION
1 Federal (U.S.) law restricts this device to sale by or on the order of a physician.
2 Refer servicing to qualified personnel.
3 The main unit is designed for continuous operation and is ‘ordinary’. Do not immerse
it in any liquid (i.e. not drip or splash-proof).
4 Electromagnetic Interference - Ensure that the environment in which the device is
operated is not subject to any source of strong electromagnetic interference, such as
radio transmitters, mobile telephones, etc.
5 SONOTRAX Series Ultrasonic Pocket Doppler is a tool to aid the healthcare
professional and should not be used in place of normal fetal monitoring.
6 Keep the device in a clean environment and avoid vibration during storage.
7 The device and accessories are to be disposed of according to local regulations after
their useful lives. Alternatively, they can be returned to the dealer or the
manufacturer for recycling or proper disposal. Batteries are hazardous waste. Do
NOT dispose them together with house-hold garbage. At the end of their life hand
the batteries over to the applicable collection points for the recycling of waste
batteries. For more detailed information about recycling of this product or battery,
please contact your local Civic Office, or the shop where you purchased the product.
SONOTRAX Series Ultrasonic Pocket Doppler User Manual Safety Guide
- 3 -
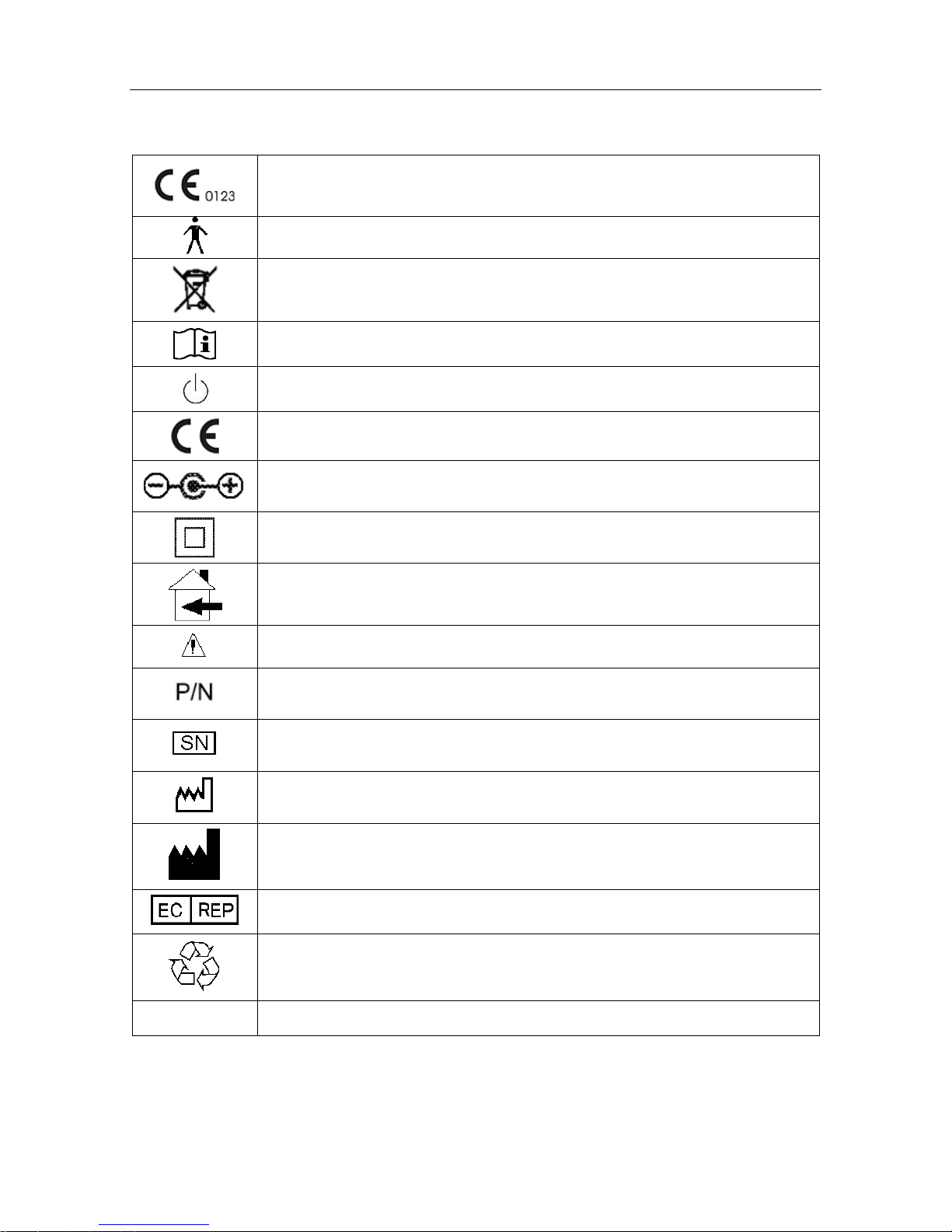
1.3 Symbols
The symbol indicates that the device complies with the European Council
Directive 93/42/EEC concerning medical devices.
Type B applied part.
The symbol indicates that the device should be sent to the special agencies
according to local regulation for separate collection after its useful life.
Consult Instructions for Use
Stand-by
CE mark
Polarity of d.c. power connector
Class II equipment
Can be used in residential areas
Attention. Refer to accompanying documents.
Part Number
Serial Number
Date Of Manufacture
Manufacturer
Authorized Representative in the European Community
General symbol for recovery / recyclable
Rx only (U.S.) Federal (U.S.) Law restricts this device to sale by or on the order of a physician
SONOTRAX Series Ultrasonic Pocket Doppler User Manual Doppler and Accessories
- 4 -
Chapter 2 Doppler and Accessories
2.1 Features
There are six different models available: SONOTRAX Lite, SONOTRAX Basic, SONOTRAX
Basic A, SONOTRAX Pro, SONOTRAX II and SONOTRAX II Pro.
SONOTRAX Lite is for simple auscultation (intermittent listening). SONOTRAX Basic,
SONOTRAX Basic A, SONOTRAX Pro, SONOTRAX II and SONOTRAX II Pro not only
detect fetal heart sound; they also display the fetal heart rate on a LCD screen.
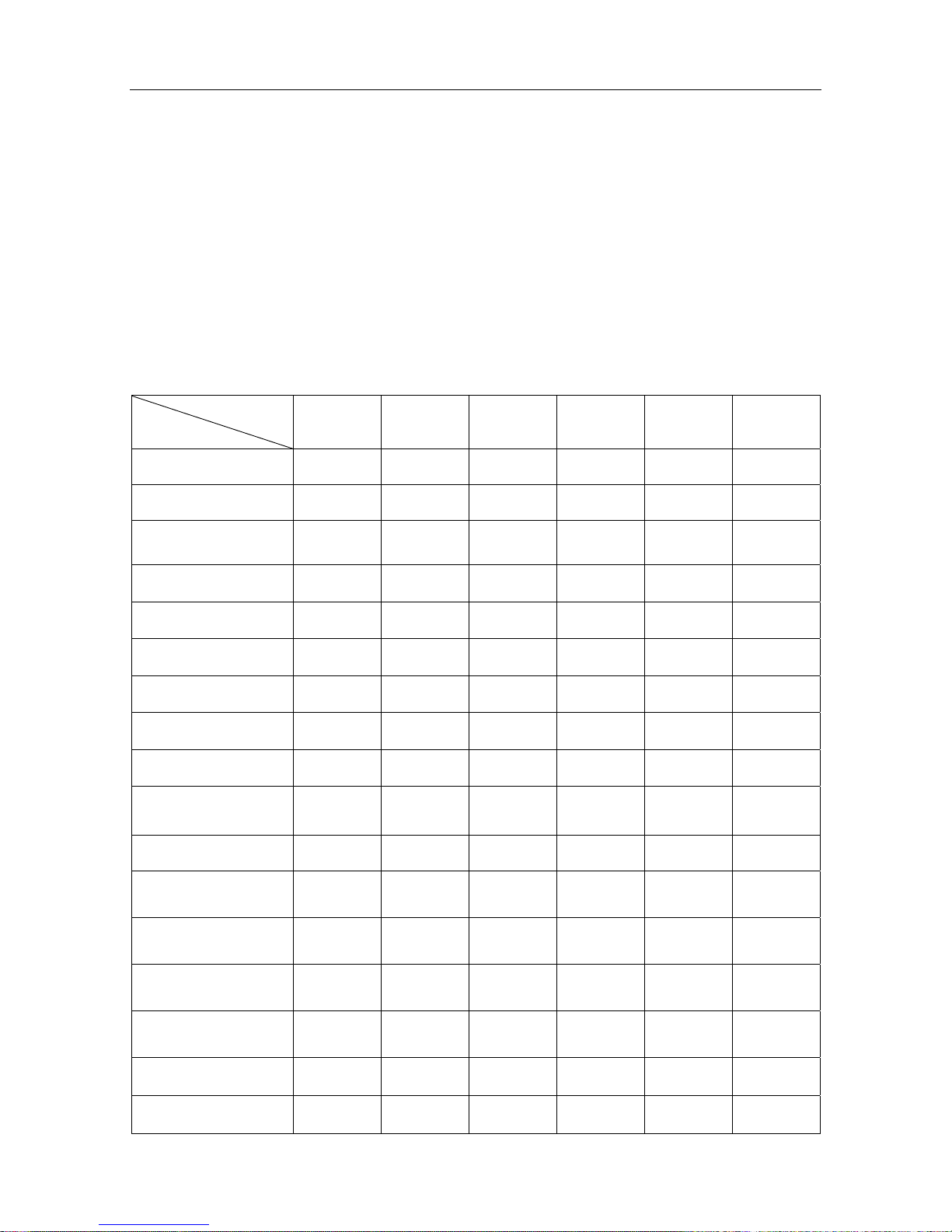
The features of the Dopplers are listed in the following chart:
Model
Function
SONOTRAX
Lite
SONOTRAX
Basic
SONOTRAX
Basic A
SONOTRAX
Pro
SONOTRAX
II
SONOTRAX
II Pro
LCD Display
√ √ √ √ √
LCD Backlight
√ √ √ √
Mini USB Probe
Socket
√ √ √ √ √ √
Probe Detecting
√ √ √ √ √ √
Probe Identifying
√ √ √ √ √
Built-in Speaker
√ √ √ √ √ √
Attached Earphone
√ √ √ √ √ √
Volume Adjustable
√ √ √ √ √ √
Modes Switching
√ √ √ √ √
Audio Recording and
Playing
√ √
Alkaline Battery (9V)
√ √ √ √
Rechargeable NI-MH
Battery (8.4V)
√ √ √ √
Lithium-ion Polymer
Battery
√ √
Battery Charged in
Machine
√ √
Low Battery Detecting
& indicating
√ √ √ √ √ √
Auto Shutdown
√ √ √ √ √
Vascular Examining
√ √ √ √ √ √
SONOTRAX Series Ultrasonic Pocket Doppler User Manual Doppler and Accessories
- 5 -
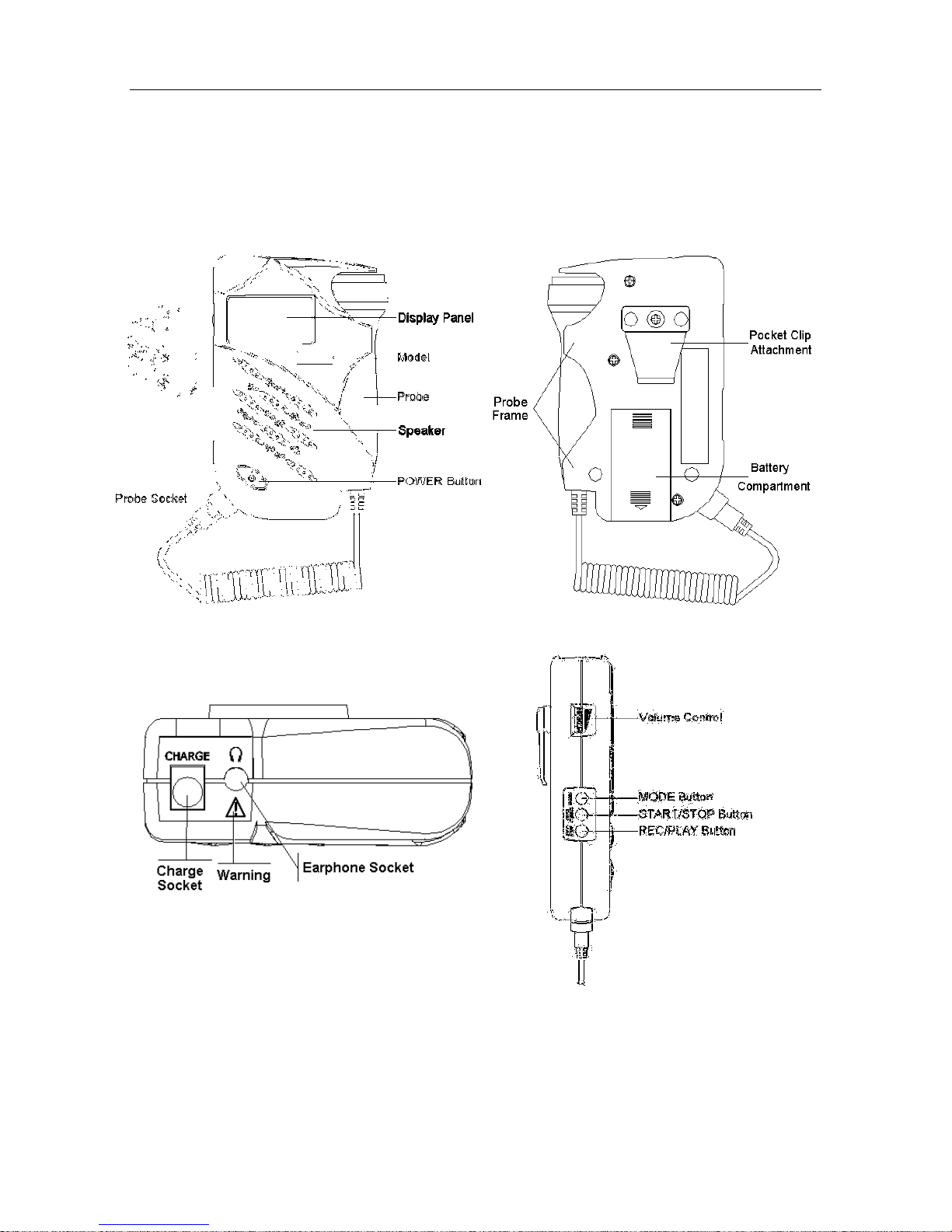
2.2 Appearance
NOTE:
The pictures and interfaces in this manual are for reference only.
Take 2.0MHz obstetrical probe for example.
Figure 2-1 Front Panel Figure 2-2 Rear Panel
Figure 2-3 Top Panel Figure 2-4 Left Panel
SONOTRAX Series Ultrasonic Pocket Doppler User Manual Doppler and Accessories
- 6 -
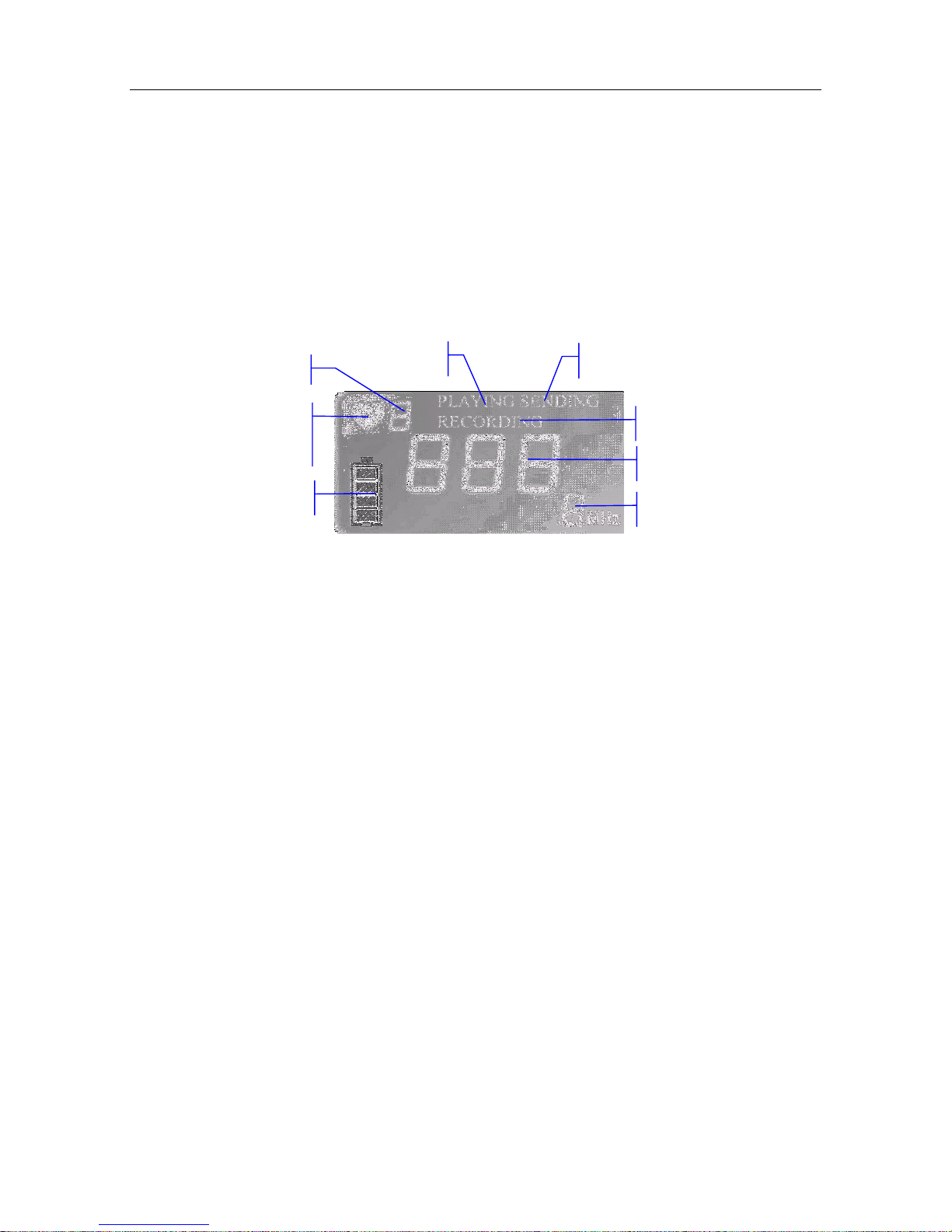
2.3 Display Panel
SONOTRAX Lite has a LED in the bottom left corner of its display panel area. When powered
on, the LED turns green. If the LED flashes in green, it indicates that the probe is disconnected or
poorly connected. If the LED flashes in orange, it indicates that the battery is too low to support
working. Change for a new battery or charge the rechargeable battery in time.
For SONOTRAX Basic, SONOTRAX Basic A, SONOTRAX Pro, SONOTRAX II and
SONOTRAX II Pro, while self-testing, the LCD is shown as follows:
Figure 2-5 LCD display status while self-testing
Working Mode:
It indicates the mode in which the Doppler is working.
FHR Refresh Frequency:
When the examination starts, the heart shape symbol flashes, and its frequency indicates the FHR
refreshing frequency.
Battery Indicator:
For SONOTRAX Basic, a battery symbol appears on the LCD after it’s powered on. When the
battery is low, this symbol flashes; the Doppler will shut down automatically after 7 seconds.
For SONOTRAX Basic A, SONOTRAX Pro, SONOTRAX II and SONOTRAX II Pro, there
is a battery symbol on the LCD. The green panes in it indicate the battery electric energy. The
green panes disappear gradually with the energy consumption. When the electric energy is low,
the empty battery symbol flashes. Approximately five minutes later, the Doppler shuts down
automatically.
PLAYING:
PLAYING is lit when the Doppler is playing the recorded fetal heart sound.
RECORDING:
RECORDING is lit when the Doppler is recording fetal heart sound.
FHR Refresh
Frequency
Battery Indicator
Recording
FHR Display
Probe Type
Playing
Communication
Working Mode

SONOTRAX Series Ultrasonic Pocket Doppler User Manual Doppler and Accessories
- 7 -
FHR Displaying:
This area displays the fetal heart rate value, and its unit is bpm (beat per minute).
Probe Type:
The Doppler can detect and identify the probe: when the probe is not connected or poorly
connected, the “---” symbol and “MHz” symbol on the LCD screen flash; when the probe is well
connected, the flashing stops, and the probe type is shown on the LCD screen.
Communication
The Doppler can communicate with PC. This function is reserved.
2.4 Buttons
At most there are four push buttons (Power, MODE, START/STOP and REC/PLAY) and a
volume control button on the main unit of the Doppler. Their primary functions are as follows:
(1) Power Button
Function: Switch on or off the Doppler.
(2) MODE Button
(Only for SONOTRAX Basic / SONOTRAX Basic A / SONOTRAX Pro / SONOTRAX II /
SONOTRAX II Pro)
Function: Select the working mode.
(3)
START/STOP Button
(Only for SONOTRAX Basic / SONOTRAX Basic A / SONOTRAX Pro / SONOTRAX II /
SONOTRAX II Pro)
Function: Start/ stop examining (Mode 3)/ setting (Mode 4 and Mode 5).
(4) REC/PLAY
(Only for SONOTRAX Pro/ SONOTRAX II Pro)
Function: Start/ stop recording or playing fetal heart sound.
(5) Volume Control Indicator
Function: Adjust volume. Rotate the volume gear clockwise to turn up the volume, while
rotate it anti-clockwise to turn down the volume.
SONOTRAX Series Ultrasonic Pocket Doppler User Manual Doppler and Accessories
- 8 -
2.5 Socket
The two sockets are located on the top panel of the Doppler.
(1) Earphone socket
: the earphone or line-in cable connects to the Doppler via this socket.
Figure 2-6 Line-in Cable
Signal Interface
1 GND 2 Signal
3 Signal 4 Signal
5 Signal
(2) Charge socket
: the special lithium-ion polymer battery charger connects to the
Doppler via this socket. (For SONOTRAX II and SONOTRAX II Pro
only)
NOTE:
Accessory equipment connected to the analog and digital interfaces must be certified
according to the respective IEC/EN standards (e.g. IEC/EN 60950 for data processing
equipment and IEC/EN 60601-1 for medical equipment). Furthermore all configurations
shall comply with the valid version of the system standard IEC/EN 60601-1-1. Anybody
who connects additional equipment to the signal input connector or signal output
connector to configure a medical system must ensure that the system complies with the
requirements of the valid version of the system standard IEC/EN 60601-1-1. If in doubt,
consult our technical service department or your local distributor.
2
1
3
5
SONOTRAX Series Ultrasonic Pocket Doppler User Manual Doppler and Accessories
- 9 -
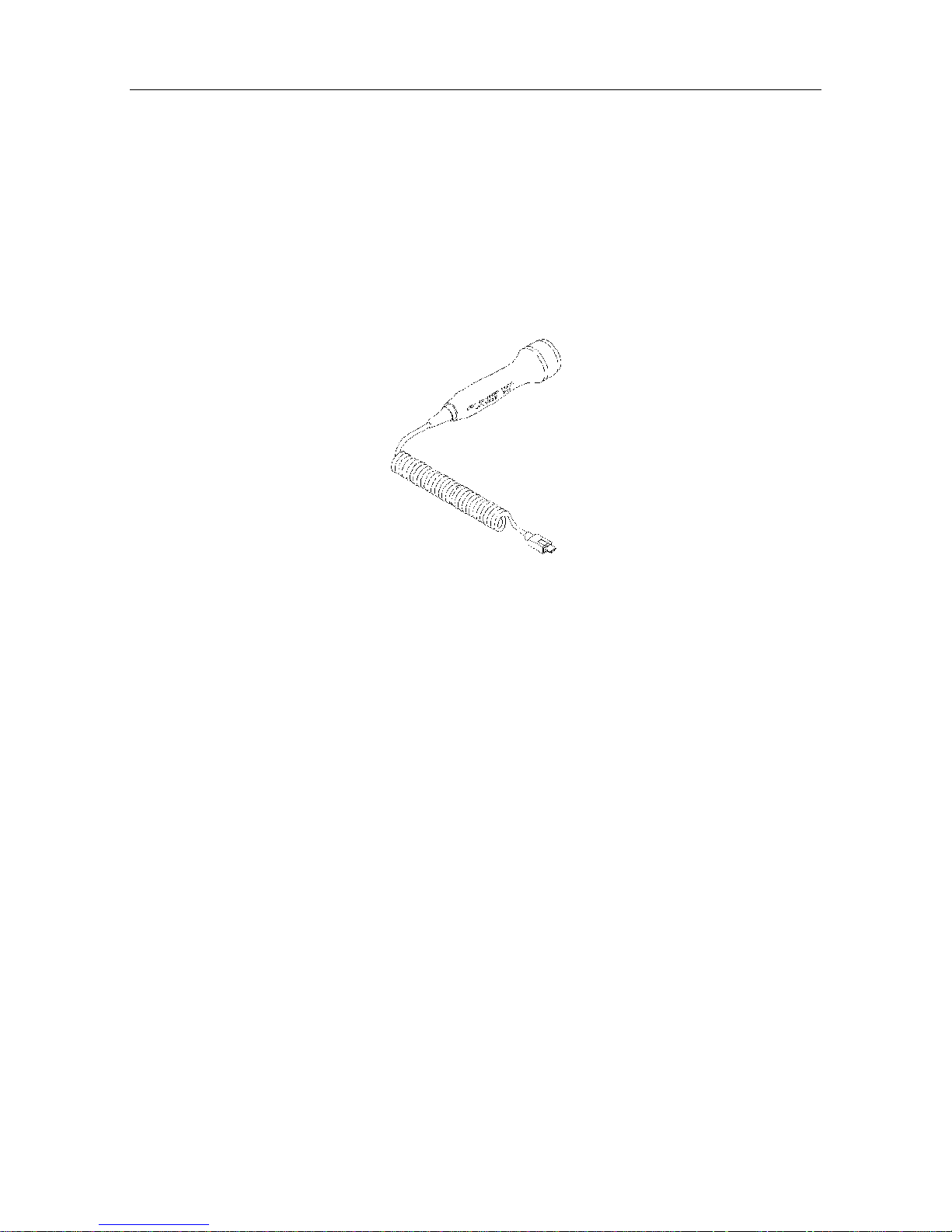
2.6 Probes
2.6.1 Waterproof Obstetrical Probes
2.0MHz/3.0MHz waterproof obstetrical probes can be connected to the main unit for fetal heart
examining.
The 2MHz obstetrical probe features in deep penetration and is designed for use during the third
trimester pregnancy. The 3MHz obstetrical probe features in high sensitivity and is designed for
use during the entire pregnancy.
Figure 2-7 2.0/3.0MHz obstetrical probe
The main information on the probe is as follows:
CD2.0/3.0: CD stands for continuous wave Doppler, 2.0/3.0 means central frequency is 2.0
MHz/3.0MHz.
MS3-14320: Part number of the 2.0MHz waterproof probe.
MS3-14321: Part number of the 3.0MHz waterproof probe.
A: Version number of the probe.
SNxxxxx: Serial number of the probe.
Waterproof: The probe is waterproof.
IPX8: Water Ingress Protection Code. It indicates that this probe can work continuously for five
hours when being immersed into water within one meter deep.
2.6.2 Waterproof Vascular Probes
The 4.0MHz/5.0MHz/8.0MHz waterproof vascular probes can be connected to the main unit for
artery and vein blood flow examining.
 Loading...
Loading...