EDAN SE-1 User Manual

EDAN Agile PLM Electronic Signature Information
--Signatures related to this document and performed in EDAN Agile PLM.
文件名称(Document Name):SE-1说明书_英文
文件编号(Number):01.54.019929
版本(Version):1.6
产品型号(Product Model):SE-1
项目编码(Project Code):2080C000
签批信息(Signature):
作者(Originator) : 肖 丽军 (xiaolijun) 2016-10-20 15:31:58
审核人(Reviewers) : 董 宁 (dongning) 2016-10-20 15:53:43
审核人(Reviewers) : 肖 玉华 (xiaoyuhua) 2016-10-20 16:30:19
批准人(Approvers) : 王 力维 (wangliwei) 2016-10-24 10:36:04
批准人(Approvers) : 杨 洁 (yangjie) 2016-10-24 11:04:30
批准人(Approvers) : 陈 浩杰 (chenhaojie) 2016-10-24 09:21:25
版权©深圳市理邦精密仪器股份有限公司 (Copyright©Edan Instrument,Inc.)
理邦保密文件
EDAN CONFIDENTIAL


About this Manual
P/N: 01.54.019929
MPN: 01.54.019929016
Release Date: Oct. 2016
© Copyright EDAN INSTRUMENTS, INC. 2005-2016. All rights reserved.
Statement
This manual will help you understand the operation and maintenance of the product better. It is
reminded that the product shall be used strictly complying with this manual. User’s operation
failing to comply with this manual may result in malfunction or accident for which EDAN
INSTRUMENTS, INC. (hereinafter called EDAN) can not be held liable.
EDAN owns the copyrights of this manual. Without prior written consent of EDAN, any
materials contained in this manual shall not be photocopied, reproduced or translated into other
languages.
Materials protected by the copyright law, including but not limited to confidential information
such as technical information and patent information are contained in this manual, the user shall
not disclose such information to any irrelevant third party.
The user shall understand that nothing in this manual grants him, expressly or implicitly, any
right or license to use any of the intellectual properties of EDAN.
EDAN holds the rights to modify, update, and ultimately explain this manual.
Responsibility of the Manufacturer
EDAN only considers itself responsible for any effect on safety, reliability and performance of
the equipment if:
Assembly operations, extensions, re-adjustments, modifications or repairs are carried out by
persons authorized by EDAN, and
The electrical installation of the relevant room complies with national standards, and
The instrument is used in accordance with the instructions for use.
I

Terms Used in this Manual
This guide is designed to give key concepts on safety precautions.
WARNING
A WARNING label advises against certain actions or situations that could result in personal
injury or death.
CAUTION
A CAUTION label advises against actions or situations that could damage equipment, produce
inaccurate data, or invalidate a procedure.
NOTE
A NOTE provides useful information regarding a function or a procedure.
II

Table of Contents
Chapter 1 Safety Guidance ........................................................................................................... 1
1.1 Indications for Use/Intended Use ........................................................................................ 1
1.2 Warnings and Cautions ........................................................................................................ 1
1.2.1 Safety Warnings ........................................................................................................ 1
1.2.2 Lithium Battery Care Warnings ................................................................................ 5
1.2.3 General Cautions ....................................................................................................... 6
1.2.4 Cleaning & Disinfection Cautions ............................................................................ 7
1.3 List of Symbols ................................................................................................................... 7
Chapter 2 Introduction ................................................................................................................ 10
2.1 Top Panel ........................................................................................................................... 10
2.2 Key Panel and Keys .......................................................................................................... 11
2.3 Patient Cable Socket and Signal Interface ........................................................................ 14
2.4 Mains Connection and Switch .......................................................................................... 16
2.5 Bottom Panel ..................................................................................................................... 17
2.6 Function Features .............................................................................................................. 18
Chapter 3 About SE-1 Application Interface ............................................................................. 19
3.1 About the Main Interface .................................................................................................. 19
3.2 About the System Setup Interface ..................................................................................... 20
Chapter 4 Operation Preparations ............................................................................................. 21
4.1 Power and Earthling .......................................................................................................... 21
4.2 Loading/Replacing Recorder Paper .................................................................................. 22
4.3 Preparing the Patient ......................................................................................................... 23
4.3.1 Instructing the Patient ............................................................................................. 23
4.3.2 Preparing the Skin ................................................................................................... 23
4.4 Connecting the Patient Cable to the Electrocardiograph and Electrodes .......................... 24
4.5 Attaching Electrodes to the Patient ................................................................................... 25
4.6 Inspection Before Power-On ............................................................................................. 28
Chapter 5 Switching On the Electrocardiograph ...................................................................... 30
Chapter 6 Entering Patient Information ................................................................................... 31
Chapter 7 Printing ECG Reports ............................................................................................... 32
7.1 Auto Mode ......................................................................................................................... 32
7.2 Manual Mode .................................................................................................................... 33
III

7.3 ECG Report ....................................................................................................................... 34
Chapter 8 Settings ........................................................................................................................ 35
8.1 Filter Settings .................................................................................................................... 36
8.2 Recording Settings ............................................................................................................ 36
8.3 Date and Time Settings ..................................................................................................... 37
8.4 Print Head Test .................................................................................................................. 37
8.5 External Input/Output Settings .......................................................................................... 38
8.6 Key Beep and QRS Beep Settings .................................................................................... 38
8.7 Rhythm Lead Settings ....................................................................................................... 38
Chapter 9 Switching Off the Electrocardiograph ..................................................................... 39
Chapter 10 Hint Information ...................................................................................................... 40
Chapter 11 Cleaning, Care and Maintenance ........................................................................... 41
11.1 General Points ................................................................................................................. 41
11.2 Cleaning .......................................................................................................................... 41
11.2.1 Cleaning the Main Unit ......................................................................................... 42
11.2.2 Cleaning the Patient Cable .................................................................................... 42
11.2.3 Cleaning the Reusable Electrodes ......................................................................... 42
11.3 Disinfection ..................................................................................................................... 43
11.3.1 Disinfecting the Main Unit .................................................................................... 43
11.3.2 Disinfecting the Patient Cable ............................................................................... 44
11.3.3 Disinfecting the Reusable Electrodes .................................................................... 44
11.4 Care and Maintenance ..................................................................................................... 44
11.4.1 Recharge and Replacement of Battery .................................................................. 44
11.4.2 Recorder Paper ...................................................................................................... 45
11.4.3 Maintenance of Main Unit, Patient Cable and Electrodes .................................... 46
Chapter 12 Accessories and Ordering Information .................................................................. 49
Chapter 13 Warranty and Service .............................................................................................. 51
13.1 Warranty .......................................................................................................................... 51
13.2 Contact information ........................................................................................................ 51
Appendix 1 Technical Specifications .......................................................................................... 52
A1.1 Safety Specifications ...................................................................................................... 52
A1.2 Environment Specifications ........................................................................................... 53
A1.3 Physical Specifications ................................................................................................... 53
A1.4 Power Supply Specifications .......................................................................................... 53
A1.5 Performance Specifications ............................................................................................ 54
IV

Appendix 2 EMC Information .................................................................................................... 56
Appendix 3 Abbreviation ............................................................................................................. 60
V
SE-1 Electrocardiograph User Manual Safety Guidance
Chapter 1 Safety Guidance
This chapter provides important safety information related to the use of the single channel
e
lectrocardiograph.
1.1 Indications for Use/Intended Use
The intended use of the single channel electrocardiograph is to acquire ECG signals from adult
and pediatric patients through body surface ECG electrodes. The electrocardiograph is intended
to be used only in hospitals or healthcare facilities by doctors and trained healthcare professionals.
The cardiogram recorded by the single channel electrocardiograph can help users to analyze and
diagnose heart disease. However the ECG is offered to clinicians on an advisory basis only.
1.2 Warnings and Cautions
In order to use the electrocardiograph safely and effectively, and avoid possible dangers caused
by improper operation, please read through the user manual and be sure to be familiar with all
functions of the equipment and proper operation procedures before use.
Please pay more attention to the following warning and caution information.
Note:
1. This device is not intended for home use.
2. The pictures and interfaces in this manual are for reference only.
1.2.1 Safety Warnings
WARNING
1. The electrocardiograph is intended to be used by qualified physicians or personnel
professionally trained. They should be familiar with the contents of this user manual
before operation.
2. Only qualified service engineers can install this equipment, and only service
engineers authorized by the manufacturer can open the shell. Otherwise, safety
hazards may happen.
3. Only qualified installation or service engineers can shift the mains supply shift switch
(100V-115V~/220V-240V~) according to local mains supply specifications.
- 1 -

SE-1 Electrocardiograph User Manual Safety Guidance
WARNING
4. The results given by the equipment should be examined based on the overall clinical
condition of the patient, and they can not substitute for regular checking.
5. This device is not intended for treatment.
6. EXPLOSION HAZARD - Do not use the electrocardiograph in the presence of
flammable anesthetic mixtures with oxygen or other flammable agents.
7. SHOCK HAZARD - The power receptacle must be a hospital grade grounded outlet.
Never try to adapt the three-prong plug to fit a two-slot outlet.
8. If the integrity of the external protective conductor is in doubt, the equipment should
be powered by a built-in rechargeable battery.
9. Do not use this equipment in the presence of high static electricity or high voltage
equipment which may generate sparks.
10. This equipment is not designed for direct cardiac application.
11. Only the patient cable and other accessories supplied by the manufacturer can be
used. Or else, the performance and electric shock protection cannot be guaranteed.
12. The use of patient cable and other accessories not supplied by the manufacturer may
result in increased emissions or decreased immunity of the equipment.
13. Make sure that all electrodes are connected to the patient correctly before operation.
14. Ensure that the conductive parts of electrodes and associated connectors, including
neutral electrodes, do not come in contact with earth or any other conducting objects.
15. Electrodes with defibrillator protection should be used while defibrillating. To avoid a
polarization or DC offset voltage, use non-polarizing electrodes (which will not form a
DC offset voltage when subjected to a DC current) such as silver/silver-chloride types
if there is a situation where there is a likelihood that a defibrillation procedure will be
necessary.
16. There is no danger for patients to use pacemakers. However, if a pacemaker is used,
the results given by the equipment may be invalid, or lose the clinical significance.
17. Do not touch the patient, bed, table or the equipment while using the ECG together
with a defibrillator.
18. Do not touch accessible parts of electrical equipment and the patient simultaneously.
19. In order to avoid being burnt, please keep the electrodes far away from the radio knife
while using electrosurgical equipment.
- 2 -

SE-1 Electrocardiograph User Manual Safety Guidance
WARNING
20. Disposable electrodes must be used during defibrillation
21. Accessory equipment connected to the analog and digital interfaces must be certified
according to the respective IEC/EN standards (e.g. IEC/EN 60950 for data processing
equipment and IEC/EN 60601-1 for medical equipment). Furthermore all configuration
shall comply with the valid version of the standard IEC/EN 60601-1-1. Therefore
anybody, who connects additional equipment to the signal input or output connector to
configure a medical system, must make sure that it complies with the requirements of
the valid version of the system standard IEC/EN 60601-1-1. If in doubt, consult our
technical service department or your local distributor.
22. Connecting any accessory (such as external printer) or other device (such as the
computer) to this electrocardiograph makes a medical system. In that case, additional
safety measures should be taken during installation of the system, and the system
shall provide:
a) Within the patient environment, a level of safety comparable to that provided by
medical electrical equipment complying with IEC/EN 60601-1, and
b) Outside the patient environment, the level of safety appropriate for non-medical
electrical equipment complying with other IEC or ISO safety standards.
23. All the accessories connected to system must be installed outside the patient vicinity,
if they do not meet the requirement of IEC/EN 60601-1.
24. SHOCK HAZARD - Don't connect non-medical electrical equipment, which has been
supplied as a part of the system, directly to the wall outlet when the non-medical
equipment is intended to be supplied by a multiple portable socket-outlet with an
isolation transformer.
25. SHOCK HAZARD - Don't connect electrical equipment, which has not been supplied
as a part of the system, to the multiple portable socket-outlet supplying the system.
26. Do not connect any equipment or accessories that are not approved by the
manufacturer or that are not IEC/EN 60601-1 approved to the electrocardiograph. The
operation or use of non-approved equipment or accessories with the
electrocardiograph is not tested or supported, and electrocardiograph operation and
safety are not guaranteed.
- 3 -

SE-1 Electrocardiograph User Manual Safety Guidance
WARNING
27. Do not use the additional multiple portable socket-outlet or extension cord in the
medical electrical system, unless it's specified as part of the system by manufacturer.
And the multiple portable socket-outlets provided with the system shall only be used
for supplying power to equipment which is intended to form part of the system.
28. The summation of leakage current should never exceed leakage current limits while
several other units are used at the same time.
29. The potential equalization conductor can be connected to that of other equipment
when necessary, to make sure that all these devices are connected to the potential
equalization bus bar of the electrical installation.
30. The EQUIPMENT is protected against malfunctions caused by electrosurgery
according to the clause 36.202.101 in the standard IEC 60601-2-25.
31. The electrocardiograph shall not be serviced or maintained while in use with a patient.
32. The appliance coupler or mains plug is used as isolation means from supply mains.
Position the electrocardiograph in a location where the operator can easily access the
disconnection device.
33. The medical electrical equipment needs to be installed and put into service according
to Appendix 2 EMC information.
34. The equipment should not be used adjacent to or stacked with other equipment, refer
to the recommended separation distances provided in Appendix 2 EMC Information.
35. Portable and mobile RF communications equipment can affect medical electrical
equipment, refer to the recommended separation distances provided in Appendix 2
EMC Information.
36. Assembly of the electrocardiograph and modifications during actual service life shall
be evaluated based on the requirements of IEC60601-1.
- 4 -

SE-1 Electrocardiograph User Manual Safety Guidance
1.2.2 Lithium Battery Care Warnings
WARNING
1. Improper operation may cause the lithium battery (hereinafter called battery) to be hot,
ignited or exploded, and it may lead to the decrease of the battery capacity. It is
necessary to read the user manual carefully and pay more attention to warning
messages.
2. Only qualified service engineers authorized by the manufacturer can open the battery
compartment and replace the battery, and batteries of the same model and
specification should be used.
3. Danger of explosion -- Do not reverse the anode and the cathode when installing the
battery.
4. Do not heat or splash the battery or throw it into fire or water.
5. When leakage or foul smell is found, stop using the battery immediately. If your skin or
cloth comes into contact with the leakage liquid, cleanse it with clean water at once. If
the leakage liquid splashes into your eyes, do not wipe them. Irrigate them with clean
water first and go to see a doctor immediately.
6. The device and accessories are to be disposed of according to local regulations after
their useful lives. Alternatively, they can be returned to the dealer or the manufacturer
for recycling or proper disposal. Batteries are hazardous waste. Do NOT dispose of
them together with house-hold garbage. At the end of their lives hand the batteries
over to the applicable collection points for the recycling of waste batteries. For more
detailed information about recycling of this product or the battery, please contact your
local Civic Office, or the shop where you purchased the product.
7. Only when the device is off can the battery be installed or removed.
8. Remove the battery from the electrocardiograph when the electrocardiograph is not
used for a long time.
9. If the battery is stored alone and not used for a long time, we recommend that the
battery should be charged at least once every 6 months to prevent overdischarge.
- 5 -

SE-1 Electrocardiograph User Manual Safety Guidance
1.2.3 General Cautions
CAUTION
1. Avoid liquid splash and excessive temperature. The temperature must be kept
between 5 ºC and 40 ºC during operation, and it should be kept between -20 ºC and
55 ºC during transportation and storage.
2. Do not use the equipment in a dusty environment with bad ventilation or in the
presence of corrosive.
3. Make sure that there is no intense electromagnetic interference source around the
equipment, such as radio transmitters or mobile phones etc. Attention: large medical
electrical equipment such as electrosurgical equipment, radiological equipment and
magnetic resonance imaging equipment etc. is likely to bring electromagnetic
interference.
4. Before use, the equipment, patient cable and electrodes etc. should be checked.
Replacement should be taken if there is any evident defectiveness or aging symptom
which may impair the safety or the performance.
5. The following safety checks should be performed at least every 24 months by a
qualified person who has adequate training, knowledge, and practical experience to
perform these tests.
a) Inspect the equipment and accessories for mechanical and functional damage.
b) Inspect the safety relevant labels for legibility.
c) Inspect the fuse to verify compliance with the rated current and circuit-breaking
characteristics.
d) Verify that the device functions properly as described in the instructions for use.
e) Test the protection earth resistance according to IEC/EN 60601-1: Limit 0.1 ohm.
f) Test the earth leakage current according to IEC/EN 60601-1: Limit: NC 500μA, SFC
1000μA.
g) Test the enclosure leakage current according to IEC/EN 60601-1: Limit: NC 100μA,
SFC 500μA.
h) Test the patient leakage current according to IEC/EN 60601-1: Limit: NC a.c. 10μA,
d.c. 10μA; SFC a.c. 50μA, d.c. 50μA.
i) Test the patient auxiliary current according to IEC/EN 60601-1: Limit: NC a.c. 10μA,
d.c. 10μA; SFC a.c. 50μA, d.c. 50μA.
- 6 -
SE-1 Electrocardiograph User Manual Safety Guidance
CAUTION
j) Test the patient leakage current under single fault condition with mains voltage on the
applied part according to IEC/EN 60601-1: Limit: 50μA (CF)
The data should be recorded in an equipment log. If the device is not functioning properly
or fails any of the above tests, the device has to be repaired.
6. Ruptured fuse must only be replaced with that of the same type and rating as the
original.
1.2.4 Cleaning & Disinfection Cautions
CAUTION
1. Turn off the power before cleaning and disinfection. If the mains supply is used, the
power cord should be dragged out of the outlet. Prevent the detergent from seeping
into the equipment.
2. Do not immerse the unit or the patient cable into liquid under any circumstances.
3. Do not clean the unit and accessories with abrasive fabric and avoid scratching the
electrodes.
4. Any remainder of detergent should be removed from the unit and the patient cable
after cleaning.
5. Do not use chloric disinfectant such as chloride, sodium hypochlorite etc.
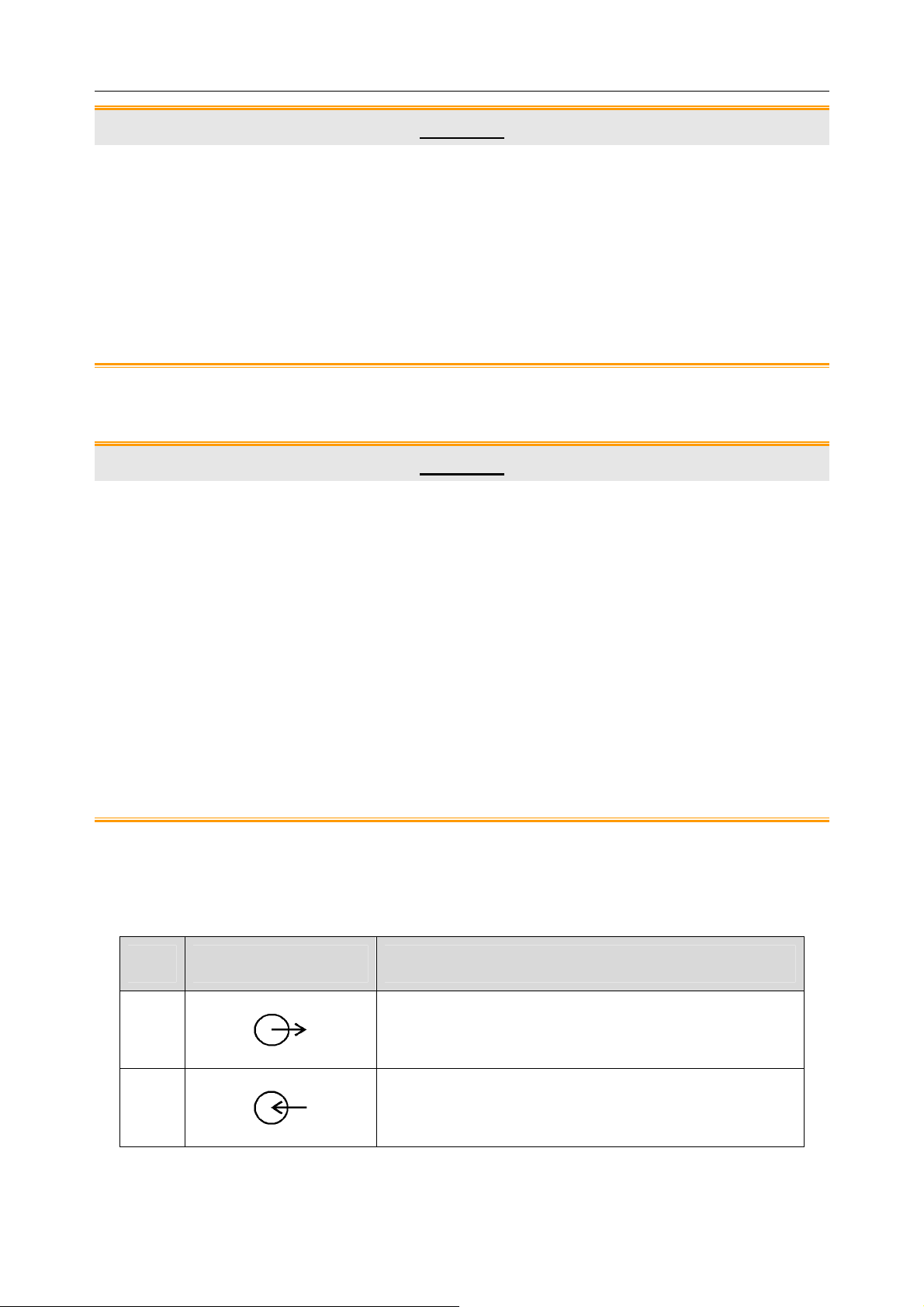
1.3 List of Symbols
No. Symbol Description
1
2
Output
Input
- 7 -
SE-1 Electrocardiograph User Manual Safety Guidance
3
4
5
6
7
8
DEFIBRILLATION-PROOF TYPE CF APPLIED
PART
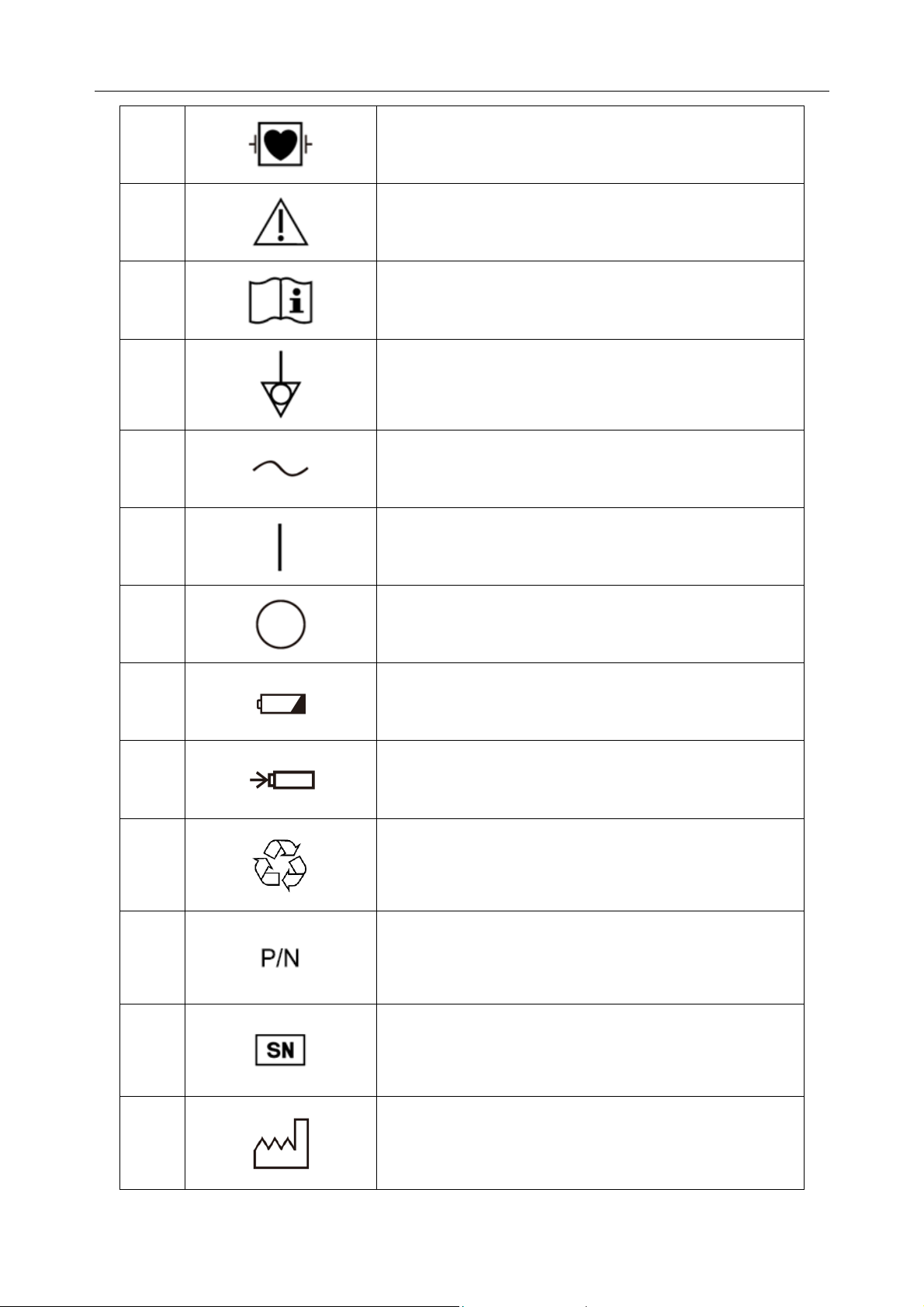
Attention, consult ACCOMPANYING DOCUMENTS
Operating instructions
Equipotential grounding
Alternating Current
"ON" (power)
9
10
11
12
13
14
"OFF" (power)
Battery check
Battery recharging indicator
General symbol for recovery/recyclable
Part Number
SERIAL NUMBER
15
Date of manufacture
- 8 -
SE-1 Electrocardiograph User Manual Safety Guidance
16
17
18
19
20
21
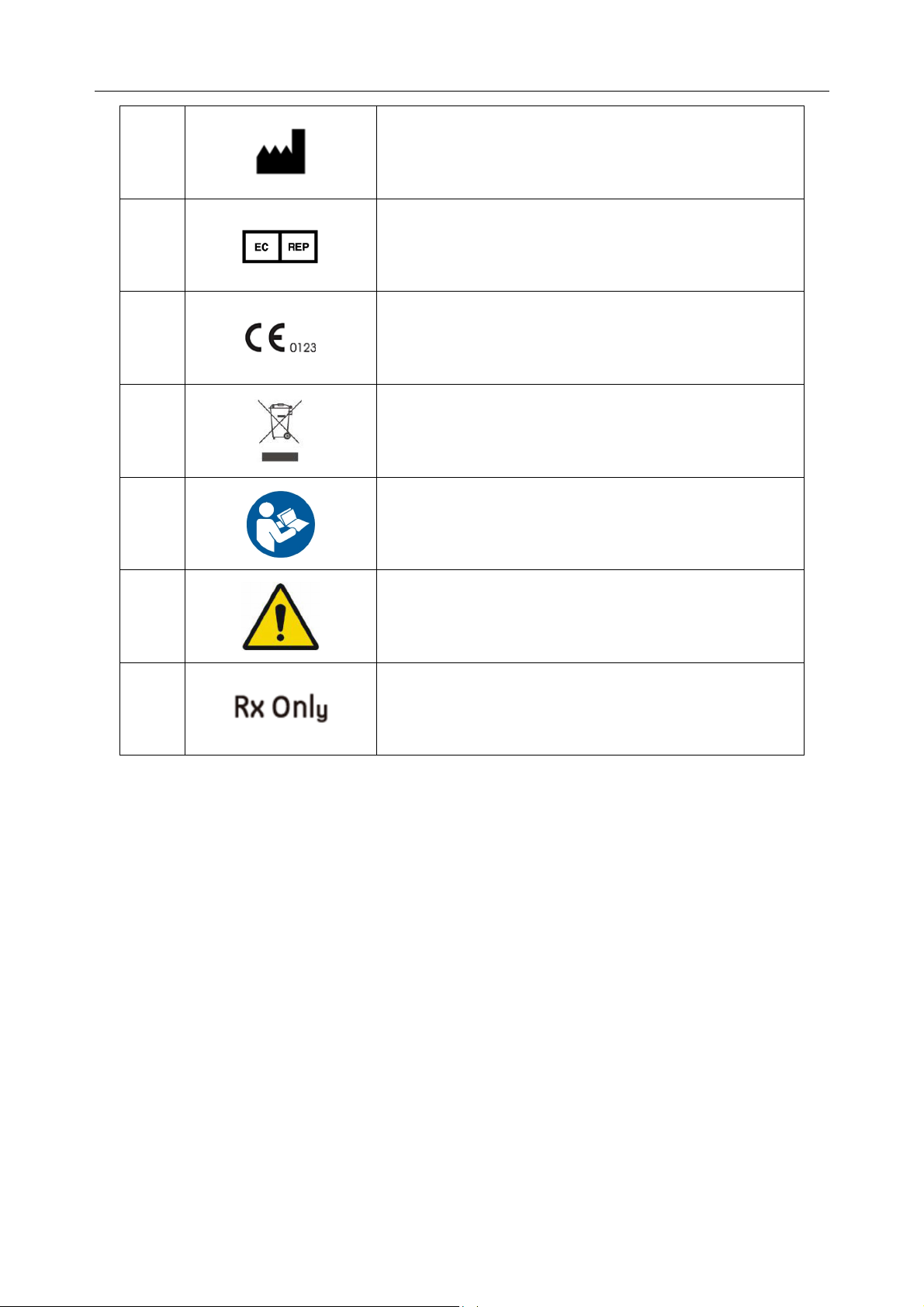
MANUFACTURER
AUTHORISED REPRESENTATIVE IN THE
EUROPEAN COMMUNITY
CE marking
Disposal method
Refer to User Manual
(Background: Blue; Symbol: White)
Warning
(Background: Yellow; Symbol&Outline: Black)
22
Caution: Federal (U.S.) law restricts this device to sale
by or on the order of a physician.
NOTE: The user manual is printed in black and white.
- 9 -

SE-1 Electrocardiograph User Manual Introduction
Chapter 2 Introduction
SE-1 electrocardiograph is a high-performance single channel digital electrocardiograph.
1-channel ECG wave and the real-time heart rate can be viewed on the LCD screen, and printed
out by using a high resolution thermal recorder. The auto and manual modes can be chosen freely.
SE-1 can be powered by the mains supply or a built-in rechargeable lithium battery.
Configuration: main unit and accessories, including patient cable, chest electrodes, limb
electrodes, thermal recorder paper, power cord etc.
WARNING
1. This equipment is intended for use on adult and pediatric patients only.
2. This equipment is not designed for direct cardiac application.
3. The results given by the equipment should be examined based on the overall clinical
condition of the patient, and they can not substitute for regular checking.
2.1 Top Panel
LCD Screen
USB Interface
Recorder Paper
Recorder
Key Panel
(Reserved)
External Input/Output
Socket
RS232 Socket
Patient Cable Socket
Figure 2-1 Main Unit
- 10 -
SE-1 Electrocardiograph User Manual Introduction
2.2 Key Panel and Keys
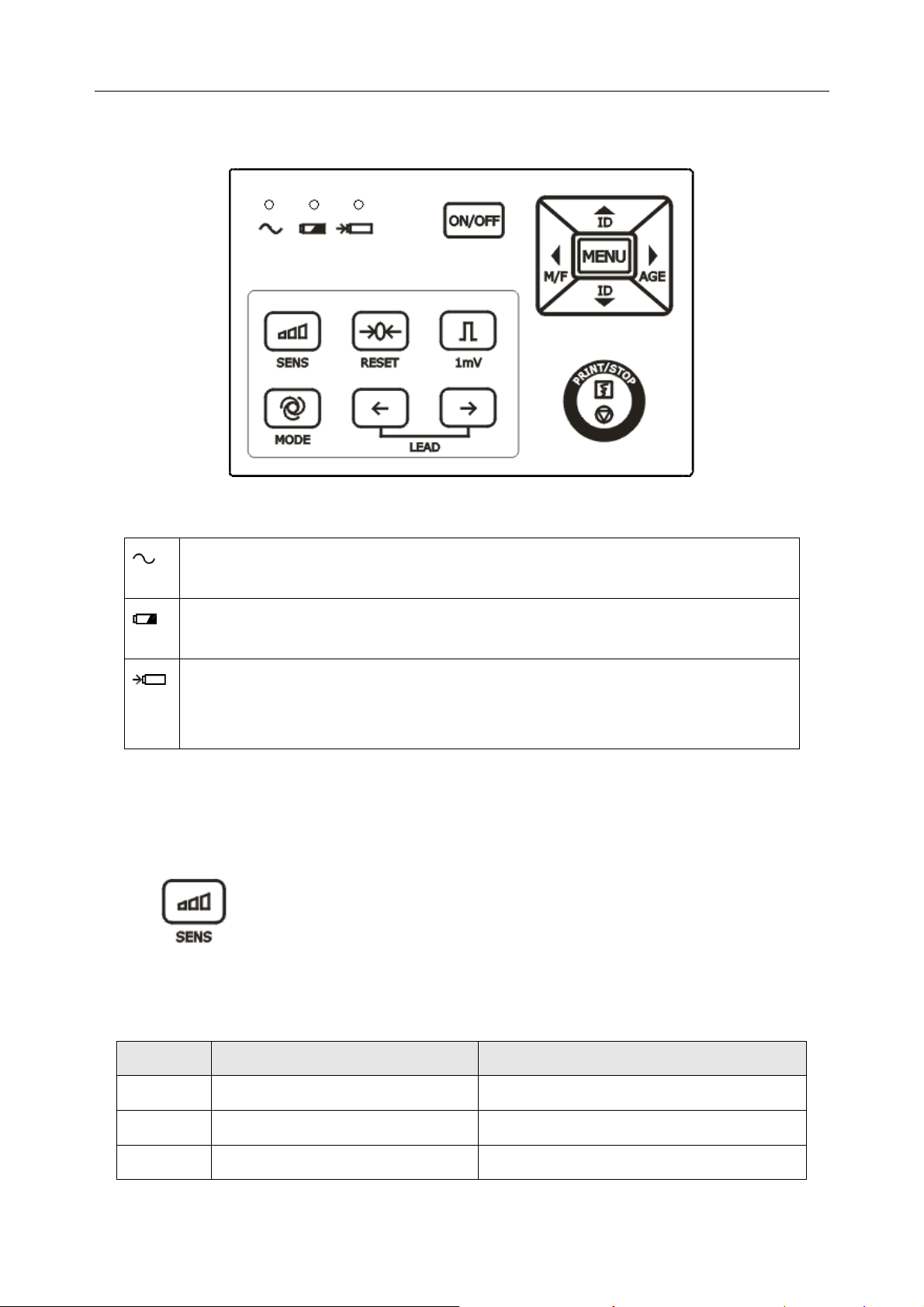
1) Indicator
Mains supply indicator: when the device is powered by the mains supply, the
indicator will be lit.
Battery indicator: when the device is powered by a built-in rechargeable lithium
battery, the indicator will be lit.
Battery recharging indicator: after you connect the power cord to the outlet and
press the mains switch, both the battery recharging indicator and the mains
supply indicator will be lit.
Note: When the device is powered off, the recharging indicator is still lit after t
he battery is fully charged.
2) SENS (Sensitivity Switch Key)
The sensitivity switch order: ×1 → ×2 → AGC → · 25 → · 5.
The measurable and recordable ECG signal range varies with the sensitivity, as the
following list shows.
Option Sensitivity Signal Range
×1 10mm/mV -2.5mV ~ +2.5mV
×2 20mm/mV -1.25mV ~ +1.25mV
AGC Adjust sensitivity automatically Vary with the adjusted sensitivity
- 11 -
SE-1 Electrocardiograph User Manual Introduction
· 25 2.5mm/mV -10mV ~ +10mV
· 5 5mm/mV -5mV ~ +5mV
If the fluctuating range of the ECG signal is great, it’s better to choose AGC because the
sensitivity can be adjusted automatically in this mode.
Note: This key is ineffective during the printing course in the auto mode.
3) RESET (Lead Locking Key)
In the manual mode, during the printing course, pressing this key can lock the lead, and
then the corresponding ECG wave shows a straight line. The lead will be unlocked
automatically after 1 second. So in the case of baseline drift, press this key to draw the
baseline to zero quickly, and the ECG wave resumes after 1 second.
4) 1mV Calibration Key
In the manual mode, press this key to print a 1mV calibration mark during the printing
course.
5) MODE (Mode Switch Key)
Press this key to select a working mode among four auto modes and a manual mode. The
switch order of leads in each mode is listed in Table 2-1.
Table 2-1 Lead Switch Order in Different Modes
Mode Switch Order (from Left to Right)
MANU І II III aVR aVL aVF V1 V2 V3 V4 V5 V6
AUTO1 І II III aVR aVL aVF V1 V2 V3 V4 V5 V6
AUTO2 aVL І aVR II aVF III V1 V2 V3 V4 V5 V6
AUTO3 І aVR V1 V4 II aVL V2 V5 III aVF V3 V6
AUTO4 2-channel auto mode (AUTO1 + Rhythm Lead)
- 12 -
SE-1 Electrocardiograph User Manual Introduction
6) LEAD (Lead Switch Key)
In the manual mode, press this key to switch the leads in order.
7) PRINT/STOP Key
Press this key to begin or stop printing ECG reports.
8) ON/OFF Key
After you connect the power cord to the outlet and press the mains switch, press this key
to turn on or off the device.
9) MENU Key
Press this key to open the system setup interface.
10) ID Setting Key
Press these two keys to set the patient ID. Press the up arrow key to increase the ID
number and press the down arrow key to decrease the ID number.
11) M/F
Press the M/F key to set the sex of the patient to Male or Female.
- 13 -
SE-1 Electrocardiograph User Manual Introduction
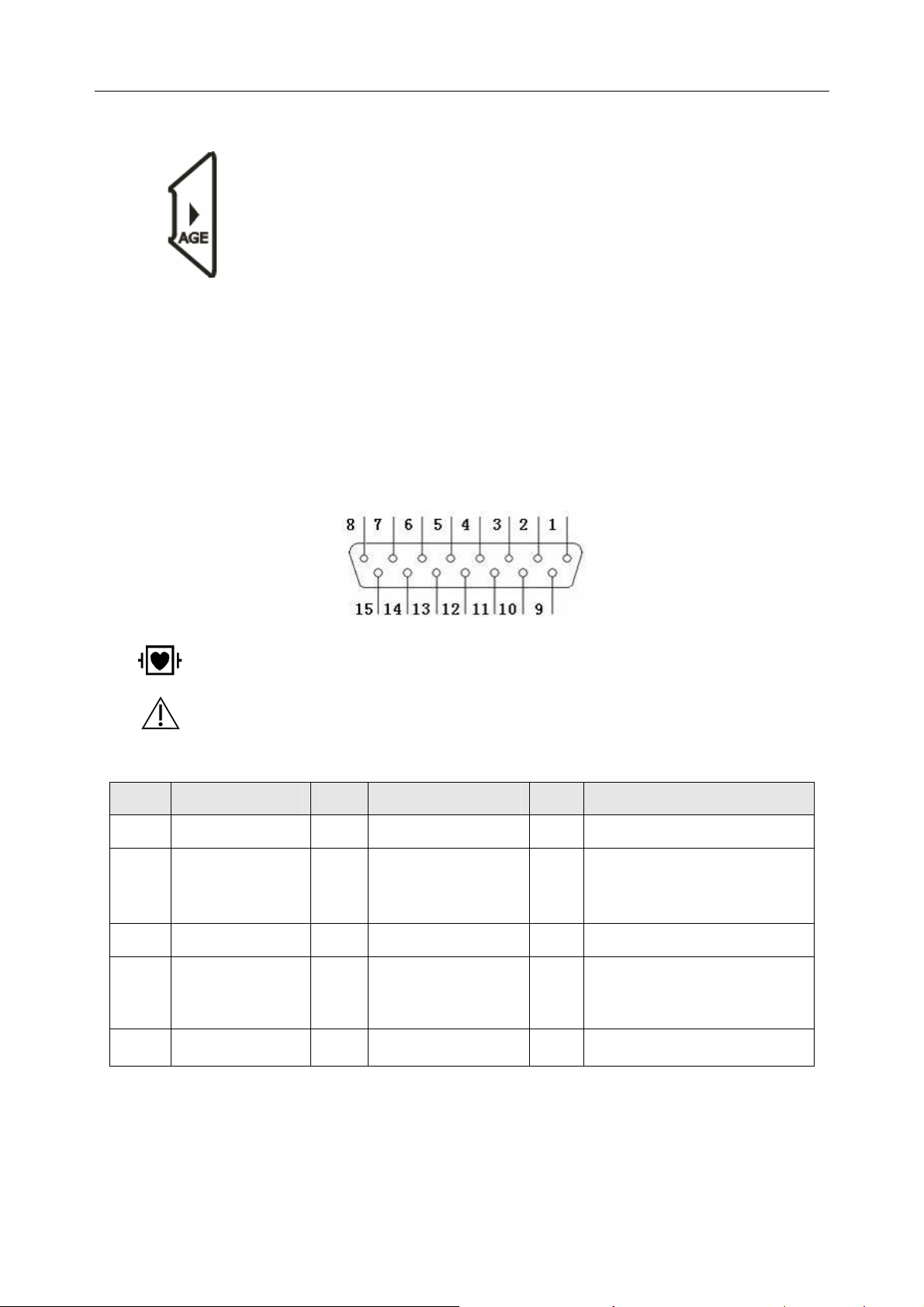
12) AGE
Press the AGE key to set the patient’s age group to CHILD, ADULT or OLD.
2.3 Patient Cable Socket and Signal Interface
As Figure 2-1 shows, on the right side of the main unit are the patient cable socket, RS232
socket (reserved), external input/output socket and USB interface (reserved).
1) Patient Cable Socket
: DEFIBRILLATION-PROOF TYPE CF APPLIED PART
: Attention, consult ACCOMPANYING DOCUMENTS
Definitions of corresponding pins:
Pin Signal Pin Signal Pin Signal
1
2
3
4
5
C2 / V2
C3 / V3
C4 / V4
C5 / V5
C6 / V6
6
7
8
9
10
SH
NC
NC
R / RA
L / LA
11
12
13
14
15
RF (N) / RL
F / LL
C1 / V1
or NC
C1 / V1
RF (N) /RL
or NC
Note: The left side of “/” is European standard; and the right side is American standard.
- 14 -
 Loading...
Loading...