
DEXIS® DICOM Conformance Statement 3.0
DEXIS
DICOM Conformance Statement
®
3.0
1

DEXIS® DICOM Conformance Statement 3.0
The software described in this document has been validated in accordance
with the governing DICOM standard at the time of this document's release. Pro
vision Dental Systems shall not be liable for errors contained herein or consequential damages in connection with the furnishing, performance, or use of
this document.
Provision Dental Systems reserves the right to revise this publication and to
make changes to its content at any time, without obligation to notify any per
son or entity of such revisions and changes.
Many of the designations used by manufacturers and sellers to distinguish
their products are claimed as trademarks. Where those designations appear in
this document, and Provision Dental Systems, Inc. was aware of a trademark
claim, the designations have been printed in caps or initial caps.
Provision Dental Systems, Inc.
460 Seaport Court, Suite 101
Redwood City, CA 94063
(888) 883-3947
-
-
Version 3.0, 30-Apr-2004
Copyright © 2000-2004 Provision Dental Systems, Inc. All Rights Reserved
2

DEXIS® DICOM Conformance Statement 3.0
1Introduction
This chapter provides general information about the purpose, scope and contents of this Conformance Statement.
1.1 Scope and Field of Application
The scope of this DICOM Conformance Statement is to facilitate data exchange
with the DEXIS
specifies the compliance to the DICOM 3.0 standard. It contains a short de
scription of the applications involved and provides technical information
about the data exchange capabilities of the equipment. The main elements de
scribing these capabilities are: the supported DICOM Service Object Pair (SOP)
Classes, Roles, Information Object Definitions (IOD) and Transfer Syntaxes.
The field of application is the integration of the DEXIS equipment into an environment of other medical devices and practice management software. This
Conformance Statement should be read in conjunction with the DICOM 3.0
standard and it’s addenda.
1.2 Intended Audience
®
X-ray system and the DEXIS Software suite. This document
-
-
This Conformance Statement is intended for:
• system integrators of medical equipment,
• software designers implementing DICOM interfaces.
It is assumed that the reader is familiar with the DICOM 3.0 standard.
Readers wishing to obtain more familiarity with the content and terminology of
DICOM 3.0 standard are encouraged to obtain and review the standard prior to
reading this Conformance Statement. More information on acquiring this doc
ument and its updates on the DICOM standard may be found on the website of
the National Electrical Manufacturer's Association (NEMA) at http://www.ne
ma.org.
-
-
3

DEXIS® DICOM Conformance Statement 3.0
1.3 Contents and Structure
The DICOM Conformance Statement is contained in chapters 2 through 8 and
follows the contents and structuring requirements of DICOM PS 3.2-2003.
1.4 Revision History
Revision Date Description Person
1.0 5 Nov. 2000 Initial Draft M. Pfeiffer
2.0 1 Nov. 2002 Adaptation to CD-R Image Interchange Profile
3.0 30 April 2004 Addition of Networking Features H. Feuerhahn
H. Feuerhahn
1.5 Definitions, Terms and Abbreviations Used
AE DICOM Application Entity
CR Computed Radiography
DICOM Digital Imaging and Communications in Medicine
FSC File Set Creator
FSR File Set Reader
FSU File Set Updater
IOD DICOM Information Object Definition
RWA Real-World Activity
SCP Service Class Provider
SCU Service Class User
SOP Service-Object Pair
Tag A 32 bit integer consisting of a group/element pair
UID Unique Identifier
VM Value Multiplicity
VR Value Representation
1.6 References
DICOM standard:
4

DEXIS® DICOM Conformance Statement 3.0
PS 3.2-2003 Conformance
PS 3.3-2003 Information Object Definitions
PS 3.4-2003 Service Class Specifications
PS 3.5-2003 Data Structures and Encoding
PS 3.6-2003 Data Dictionary
PS 3.7-2003 Message Exchange
PS 3.8-2003 Network Communication Support for Message Exchange
PS 3.10-2003 Media Storage and File Format for Media Interchange
PS 3.11-2003 Media Storage Application Profiles
1.7 Trademarks
DEXIS is a registered trademark in the USA, UK, France, Germany, Belgium,
Switzerland, Netherlands, Luxemburg, Sweden, Spain, Italy.
1.8 Connectivity and Interoperability
This Conformance Statement by itself does not guarantee successful interoperability of DEXIS equipment with non-DEXIS equipment or non-DEXIS software. The user (or the user’s agent) should be aware of the following issues:
The implementation of the DEXIS DICOM interface has been carefully tested to
assure correspondence with this Conformance Statement. But the Conform
ance Statement by itself and the DICOM standard does not guarantee interoperability of DEXIS modalities and modalities of other vendors. The user (or the
user’s agent) must compare the relevant Conformance Statements and if a suc
cessful interconnection should be possible, the user is responsible to specify
an appropriate test suite and to validate the interoperability, which is required.
A network environment may need additional functions out of the scope of DI
COM.
-
-
-
2Implementation Model
The DEXIS Software Release 6 is a comprehensive range of software modules
that allow for tailored solutions in the dental imaging field. The software appli
cations are categorized in packages, for instance the DEXwrite package for referral letters. The Application Entities responsable for exchanging data through
the DICOM interface are:
-
5

DEXIS® DICOM Conformance Statement 3.0
• The DEXIS Import/Export Application Entity is integrated into the DEXray
and DEXimage packages. It deals with the exchange of images through removable media.
• The DEXcom Application Entity is integrated into the DEXIS Administration
package. It deals with the exchange of images through computer networks
using the DICOM standard.
Through these two Application Entities the DEXIS Software provides the following DICOM data exchange features:
• DEXIS can export DICOM File Sets to removable media; it thus serves as DI-
COM File Set Creator (FSC). If the images are not internally stored in DICOM
file format, they are converted when exported.
• DEXIS can browse through DICOM File Sets on removable media and can im-
port images from these file sets; it thus serves as DICOM File Set Reader
(FSR).
• DEXIS can verify communication with a DICOM server; it is thus a Service
Class User (SCU) of the Verification Service Class.
• DEXIS can store images on a DICOM server; it is thus a Service Class User
(SCU) of the Storage Service Class.
• DEXIS can query DICOM servers for patients, studies, series, and images; it
is thus a Service Class User (SCU) of the Query/Retrieve Service Class.
• DEXIS can receive images from a DICOM server and store them in its local da-
tabase; during this operation it serves thus as a Service Class Provider
(SCP) of the Storage Service Class.
• DEXIS can query Modality Worklist Servers for entries; it is thus a Service
Class User (SCU) of the Modality Worklist Service Class.
2.1 Application Data Flow Diagrams
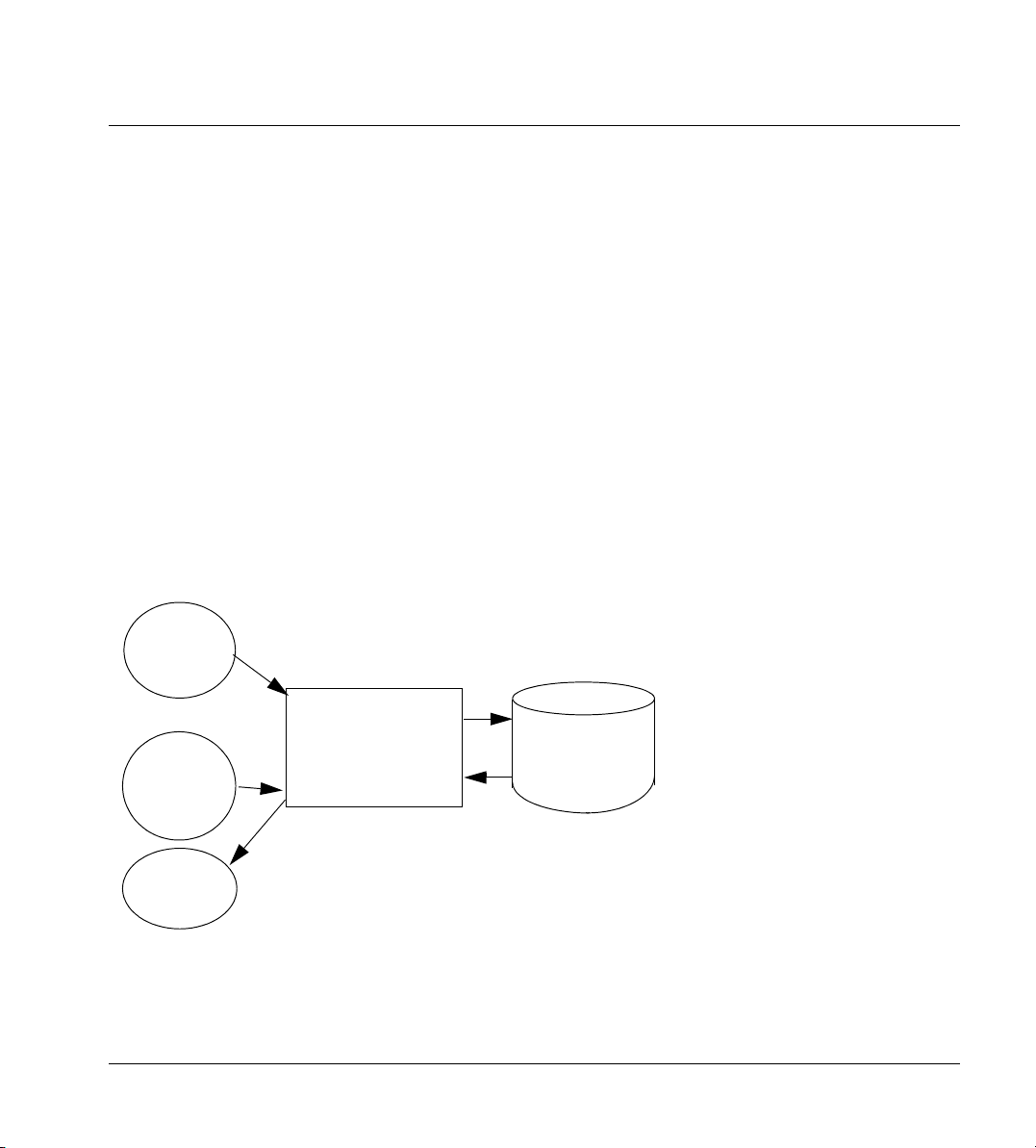
2.1.1 Removable Media
The DICOM import and export functionalities for the CD-R device are handled
by the DEXIS Import/Export Application Entity (AE). The DEXIS Import/Export
Application Entity is commanded by the user to perform DICOM services oper
-
6
DEXIS® DICOM Conformance Statement 3.0
ating on the DICOM media through the use of buttons and menu selections on
the graphical user interface of the station.
The user requests the creation of a DICOM file set by selecting the images in
the list displayed by the export function and by selecting DICOM as the file for
mat. By selecting a drive and optional folder the file set can either be created
directly on a removable media (CD-R) or in some temporary folder on the hard
disk and written to CD-R by selecting images in the local Browser, and by a drag
and drop of those images on the CD-R icon.
The user can request the reading of a DICOM file set written on a CD-R by selecting the CD-R drive in the browser of the import function, and browsing the
archive using the displayed folders and files. He can then import the selected
items by double-clicking on them or by selecting them and clicking the “Im
port” button.
For the purpose of this Conformance Statement import and export is restricted
to CD-R media, but the same methods can be applied to other removable me
dia.
User
requests
export to
CD-R
FSC
FSR
CD-R
User
requests
import
from CD-R
DEXIS
Import/Export
AE
-
-
-
Extract,
store
images
7

DEXIS® DICOM Conformance Statement 3.0
2.1.2 Networking
The DICOM networking functions are handled by the DEXcom Application Entity
(AE). The DEXcom Application Entity is commanded by the user to perform DI
COM services through the use of buttons and menu selections on the graphical
user interface of the station. Some of the functions can be set to work automat
ically.
When new images are acquired, they are stored in the local database. If the
preferences of the DEXcom component are set so, all newly acquired images
are copied automatically to one or more DICOM Storage Servers. Storage of im
ages to specific servers can also be invoked by the user through menus.
Through a user interface dialog the user can enter queries for patients, studies,
series, or images present on a DICOM Server. He can then - based upon the dis
played query result - select images, studies, or patient files to be retrieved from
the the server. The retrieved items are then entered into the local image data
base.
If a Worklist server is present, the user can enter queries to it to either obtain
the whole Modality Worklist, or specified parts of it.
-
-
-
-
-
8

DEXIS® DICOM Conformance Statement 3.0
Acquire new
images
Select
images for
storage
Enter query
Process
response
Ask for
retrieval
Extract, store
images
Enter query
DEXcom
AE
Local
C-Store
C-Find
C-Move
C-Store
C-Find
Remote
Storage
SCP
Query/
Retrieve
SCP
Worklist
SCP
Process
response
2.2 Functional Definition of Application Entities
The DEXIS Import/Export Application Entity supports the following functions:
• Can write a DICOM File Set (FSC) on a CD-R.
• Can read a DICOM File Set (FSR) on a CD-R.
9

DEXIS® DICOM Conformance Statement 3.0
The DEXcom Application Entity acts as a service class user (SCU) in the following roles:
• SCU of the Verification Service Class (C-Echo operations)
• SCU of the Storage Service Class (C-Store operations)
• SCU of the Query / Retrieve Service Class (C-Find and C-Move operations)
• SCU of the Worklist Management Service Class (C-Find operations)
The DEXcom Application Entity acts as a service class provider (SCP) in the following role:
• SCP of the Storage Service Class (during C-Move operations only)
2.3 Sequencing Requirements
Non applicable.
2.4 General Meta Information Options (see PS 3.10)
Implementation Class UID (0002,0012)=’1.2.840.114059.1.1.6.1.50.1’.
Implementation Version Name (0002,0013)=’DEXIS20021101’.
3 Application Entity Specifications
3.1 DEXIS Import/Export AE Specification
The DEXIS Import/Export Application Entity provides conformance to the Media Storage Service Class according to the following table
10
DEXIS® DICOM Conformance Statement 3.0
3.1.1 File Meta Information for the DEXIS Import/Export AE
Supported
Application
Profiles
STD-GEN-CD Export to CD-R FSC Interchange
STD-GEN-CD Import from CD FSR Interchange
Real World Activity Role Service
Class
Option
3.1.2 Real-World Activities for this Application Entity
3.1.2.1 Real-World Activity: Export to CD-R
The DEXIS Software acts as an FSC using the interchange option when requested to copy SOP Instances from the local data base to the CD-R.
3.1.2.1.1 DICOM Directory
The DEXIS Software writes directory records Patient, Study, Series and Image.
Images are referenced via the Image Directory Entry attribute Referenced File ID
(0004,1500). DEXIS does not employ Multi-Referenced Directory Entries.
3.1.2.1.2 SOP Specific Conformance
The file sets created by the DEXIS Software can contain information objects of
the following SOP Classes:
Information Object Definition
Media Storage Directory
Storage
Digital X-ray Image Storage
- For Presentation
SOP Class UID Transfer Syntax Transfer Syntax UID
1.2.840.10008.1.3.10
1.2.840.10008.5.1.4.1.1.1.1 Explicit VR Little Endian 1.2.840.10008.1.2.1
Explicit VR Little Endian 1.2.840.10008.1.2.1
11

DEXIS® DICOM Conformance Statement 3.0
Information Object Definition
Digital Intra-oral X-ray
Image Storage - For Pres
entation
VL Photographic Image
Storage
SOP Class UID Transfer Syntax Transfer Syntax UID
1.2.840.10008.5.1.4.1.1.1.3 Explicit VR Little Endian 1.2.840.10008.1.2.1
-
1.2.840.10008.5.1.4.1.1.77.1.4
Explicit VR Little Endian 1.2.840.10008.1.2.1
3.1.2.2 Real-World Activity: Import from CD-R
The DEXIS Software acts as an FSR using the interchange option when requested to copy SOP Instances from the CD-R to the local data base.
3.1.2.2.1 DICOM Directory
The Import function of the DEXIS Software serves to import image file in all formats known to the software. If inside this function a location is selected that
contains a valid DICOM data set, the DICOMDIR file is listed. When the user
clicks on the DICOMDIR entry, all image files and descriptive information (pa
tient name, image date, anatomic region) are listed, and the user can select the
image files to import. If the patient names do not match, the user is asked for
confirmation.
There are no requirements or restrictions on the contents of the DICOMDIR. If
the DICOMDIR is missing, the media can be browsed and the individual image
files can be imported anyhow.
-
3.1.2.2.2 SOP Specific Conformance
The Import function of the DEXIS Software accepts information objects of the
following SOP Classes:
SOP Class Name SOP Class UID Transfer Syntax Transfer Syntax UID
Digital X-ray Image Storage
- For Presentation
1.2.840.10008.5.1.4.1.1.1.1 Explicit VR Little Endian
Implicit VR Little Endian
1.2.840.10008.1.2.1
1.2.840.10008.1.2
12

DEXIS® DICOM Conformance Statement 3.0
SOP Class Name SOP Class UID Transfer Syntax Transfer Syntax UID
Digital X-ray Image Storage
- For Processing
Digital Intra-oral X-ray
Image Storage - For Pres
entation
Digital Intra-oral X-ray
Image Storage - For
Processing
Secondary Capture Image 1.2.840.10008.5.1.4.1.1.7 Explicit VR Little Endian
Computed Radiography
Image
VL Photographic Image
Storage
VL Endoscopic Image Storage
VL Photographic Image
Storage
1.2.840.10008.5.1.4.1.1.1.1.1 Explicit VR Little Endian
Implicit VR Little Endian
1.2.840.10008.5.1.4.1.1.1.3 Explicit VR Little Endian
-
1.2.840.10008.5.1.4.1.1.1.3.1 Explicit VR Little Endian
1.2.840.10008.5.1.4.1.1.1 Explicit VR Little Endian
1.2.840.10008.5.1.4.1.1.77.1.4
1.2.840.10008.5.1.4.1.1.77.1.1
1.2.840.10008.5.1.4.1.1.77.1.2
Implicit VR Little Endian
Implicit VR Little Endian
Implicit VR Little Endian
Implicit VR Little Endian
Explicit VR Little Endian
Implicit VR Little Endian
Explicit VR Little Endian
Implicit VR Little Endian
Explicit VR Little Endian
Implicit VR Little Endian
3.2 DEXcom AE Specification
1.2.840.10008.1.2.1
1.2.840.10008.1.2
1.2.840.10008.1.2.1
1.2.840.10008.1.2
1.2.840.10008.1.2.1
1.2.840.10008.1.2
1.2.840.10008.1.2.1
1.2.840.10008.1.2
1.2.840.10008.1.2.1
1.2.840.10008.1.2
1.2.840.10008.1.2.1
1.2.840.10008.1.2
1.2.840.10008.1.2.1
1.2.840.10008.1.2
1.2.840.10008.1.2.1
1.2.840.10008.1.2
The DEXcom AE provides standard conformance to the following Service Object
Pair (SOP) Classes.
SOP Class Name SOP Class UID
Digital X-ray Image Storage - For Presentation 1.2.840.10008.5.1.4.1.1.1.1
Digital X-ray Image Storage - For Processing 1.2.840.10008.5.1.4.1.1.1.1.1
Digital Intra-oral X-ray Image Storage - For Presentation 1.2.840.10008.5.1.4.1.1.1.3
Digital Intra-oral X-ray Image Storage - For Processing 1.2.840.10008.5.1.4.1.1.1.3.1
13

DEXIS® DICOM Conformance Statement 3.0
SOP Class Name SOP Class UID
Secondary Capture Image Storage 1.2.840.10008.5.1.4.1.1.7
Computed Radiography Image Storage 1.2.840.10008.5.1.4.1.1.1
VL Photographic Image Storage 1.2.840.10008.5.1.4.1.1.77.1.4
VL Endoscopic Image Storage 1.2.840.10008.5.1.4.1.1.77.1.1
VL Microscopic Image Storage 1.2.840.10008.5.1.4.1.1.77.1.2
Patient Root Query/Retrieve Information Model - Find 1.2.840.10008.5.1.4.1.2.1.1
Patient Root Query/Retrieve Information Model - Move 1.2.840.10008.5.1.4.1.2.1.2
Modality Worklist 1.2.840.10008.5.1.4.31
Verification 1.2.840.10008.1.1
3.2.1 Association Establishment Policies
3.2.1.1 General
All associations with DEXcom are established using the DICOM 3.0 Standard
application context. The maximum length PDU that DEXcom will support is
16,384 bytes.
3.2.1.2 Number of Associations
DEXcom opens one association at a time. Multiple simultaneous associations
are not supported.
3.2.1.3 Asynchronous Nature
Asynchronous operations are not supported.
14

DEXIS® DICOM Conformance Statement 3.0
3.2.1.4 Implementation Identifying Information
The Implementation Class Unique Identifier (UID) for the DEXcom Application
Entity (AE) is:
2.16.840.1.114059.1.1.6.1.50.1
The Implementation Version Name for the DEXcom AE is:
DEXIS20040430
3.2.2 Association Initiation by Real-World Activity
The DEXcom Application Entity attempts to initiate an association for the appropriate DICOM Service Class.
The association is closed when one of the following has occurred:
• all the images have been sent to the remote application (Storage)
• all the images have been received from the remote application (Retrieve)
• the Query results have been received from the remote application (Query
and Worklist)
The client is also able to abort the association when a timeout or an error occurs.
3.2.2.1 Real-World Activity for Send Image Operations
DEXcom initiates associations for the transfer of images to a DICOM Image
Storage Server.
3.2.2.1.1 Associated Real-World Activity for Send Image Operations
Once the Storage association has been established, a C-STORE message is
sent by DEXcom.
15
DEXIS® DICOM Conformance Statement 3.0
3.2.2.1.2 Proposed Presentation Contexts for Send Image Operations
The presentation contexts that are proposed by DEXcom for the Send Image
operation are specified in the following table::
SOP Class Name SOP Class UID Transfer Syntax Transfer Syntax UID
Digital X-ray Image Storage
- For Presentation
Digital X-ray Image Storage
- For Processing
Digital Intra-oral X-ray
Image Storage - For Pres
entation
Digital Intra-oral X-ray
Image Storage - For
Processing
Secondary Capture Image 1.2.840.10008.5.1.4.1.1.7 Explicit VR Little Endian
Computed Radiography
Image
VL Photographic Image
Storage
VL Endoscopic Image Storage
VL Photographic Image
Storage
1.2.840.10008.5.1.4.1.1.1.1 Explicit VR Little Endian
Implicit VR Little Endian
1.2.840.10008.5.1.4.1.1.1.1.1 Explicit VR Little Endian
Implicit VR Little Endian
1.2.840.10008.5.1.4.1.1.1.3 Explicit VR Little Endian
-
1.2.840.10008.5.1.4.1.1.1.3.1 Explicit VR Little Endian
1.2.840.10008.5.1.4.1.1.1 Explicit VR Little Endian
1.2.840.10008.5.1.4.1.1.77.1.4
1.2.840.10008.5.1.4.1.1.77.1.1
1.2.840.10008.5.1.4.1.1.77.1.2
Implicit VR Little Endian
Implicit VR Little Endian
Implicit VR Little Endian
Implicit VR Little Endian
Explicit VR Little Endian
Implicit VR Little Endian
Explicit VR Little Endian
Implicit VR Little Endian
Explicit VR Little Endian
Implicit VR Little Endian
1.2.840.10008.1.2.1
1.2.840.10008.1.2
1.2.840.10008.1.2.1
1.2.840.10008.1.2
1.2.840.10008.1.2.1
1.2.840.10008.1.2
1.2.840.10008.1.2.1
1.2.840.10008.1.2
1.2.840.10008.1.2.1
1.2.840.10008.1.2
1.2.840.10008.1.2.1
1.2.840.10008.1.2
1.2.840.10008.1.2.1
1.2.840.10008.1.2
1.2.840.10008.1.2.1
1.2.840.10008.1.2
1.2.840.10008.1.2.1
1.2.840.10008.1.2
All these SOP classes conform to the standard Storage Services as specified in
the DICOM 3.0 Standard.
3.2.2.2 Real-World Activity for Query Operations
DEXcom initiates associations for the transfer of query information from a DICOM Image Storage Server.
16
DEXIS® DICOM Conformance Statement 3.0
DEXcom also inititiates associations for getting the Modality Worklist or part of
it from a DICOM Worklist Server.
3.2.2.2.1 Associated Real-World Activity for Query Operations
Once the Query association has been established, a C-FIND message is sent
and the responses from the remote application are waited for by DEXcom.
3.2.2.2.2 Presentation Contexts for Query Operations.
SOP Class Name SOP Class UID
Patient Root Query/Retrieve Information Model - Find 1.2.840.10008.5.1.4.1.2.1.1
Modality Worklist 1.2.840.10008.5.1.4.31
3.2.2.3 Real-World Activity for Retrieve Operations
DEXcom initiates associations for the transfer of images from a DICOM Image
Storage Server.
3.2.2.3.1 Associated Real-World Activity for Retrieve Operations
Once the Retrieve association has been established, a C-MOVE message is
sent and the reponses waited for by DEXcom. If a C-STORE request is received
from the remote application, DEXcom acts as a Service Class Provider (SCP) of
the Storage Service Class.
3.2.2.3.2 Presentation Contexts for Retrieve Operations.
SOP Class Name SOP Class UID
Patient Root Query/Retrieve Information Model - Move 1.2.840.10008.5.1.4.1.2.1.2
17
DEXIS® DICOM Conformance Statement 3.0
3.2.2.4 Real-World Activity for Verify Operations
DEXcom initiates associations for verifying communication with a DICOM Server.
3.2.2.4.1 Associated Real-World Activity for Verify Operations
Once the Verify association has been established, a C-ECHO message is sent
and the responses from the remote application are waited for by DEXcom.
3.2.2.4.2 Presentation Contexts for Verify Operations.
SOP Class Name SOP Class UID
Verification 1.2.840.10008.1.1
4 Communication Profiles
4.1 Supported Communication Stacks
DEXIS provides TCP/IP Network Communication Support as defined in PS 3.8
of the DICOM 3.0 Standard.
4.2 TCP/IP Stack
DEXIS communicates over the TCP/IP protocol stack on any physical interconnection supporting the TCP/IP stack. DEXIS inherits the stack from the operating system on which it executes.
4.3 Physical Media Support
DEXIS is indifferent to the physical medium over which the TCP/IP executes.
18

DEXIS® DICOM Conformance Statement 3.0
5 Extensions/Specializations/Privatizations
The following restriction applies to SOP instances of the VL Photographic Image Storage Class:
The tag “Photometric Interpretation” (0028,0004) shall have one of the values:
RGB or MONOCHROME2.
There is no restriction on SOP instances of the Digital X-ray Image Storage
Class and on the SOP instances of the Digital Intra-oral X-ray Image Storage
Class.
The Application Entity uses private tags according to the following description:
Name of Private Tag Tag Description
Private Tag Range (0029,0029) Reserves the (0029,29xx) Range
Description (0029,2920) Environmental Description
Orientation (0029,2921) Computed Type and Orientation
Parameter 1 (0029,2922) Various Parameters
Parameter 2 (0029,2923) More Parameters
Teeth (0029,2924) ISO Tooth Descr.
Jaw (0029,2925) ISO Jaw Descr.
Quadrant (0029,2926) ISO Quadrant Descr.
CRC (0029,2927) CRC Check Sum
6Configuration
The DEXIS Software can be set to store all images internally in DICOM image file
format; if this setting is on, and all images are stored in DICOM file format, no
conversion is done when importing or exporting images in DICOM format. Im
ages not stored internally in DICOM format are converted when exported; these
images also contain all the necessary information.
-
19

DEXIS® DICOM Conformance Statement 3.0
7 Support of Extended Character Sets
The DEXIS Software will only create SOP instances containing the DICOM default character set as defined in PS 3.5.
8 Codes and Controlled Terminology
The SOP Classes supported by this implementation require the Codes and Controlled Terminology for the Anatomy Imaged according to PS 3.3 C.8.11.2 which
are fully supported.
20
 Loading...
Loading...