Datrend Infutest 2000 E User manual

Infutest 2000
Series E
Dual Channel Infusion Pump Analyzer
Operating Manual


Infutest 2000 Series E
Dual Channel Infusion Pump Analyzer
Operating Manual
© 2007 Datrend Systems Inc.
Unit #1 - 3531 Jacombs Road
Richmond, BC • CANADA. • V6V 1Z8
Tel 604.291.7747 or 800.667.6557 • Fax 604.294.2355
e-mail customerservice@datrend.com


To order this manual, use Part Number 6100-109
Revision
C Add Standard Cable 18-Jul-2008
Revision History
Description Date
Copyright
Datrend Systems Inc. (“DSI”) agrees to a limited copyright release that allows you to reproduce manuals and other
printed materials for use in service training programs and other technical publications. If you would like other
reproductions or distributions, submit a written request to Datrend Systems Inc.
Unpacking and Inspection
Follow standard receiving practices upon receipt of the instrument. Check the shipping carton for damage. If
damage is found, stop unpacking the instrum ent. Notify the freight carrier and ask for an agent to be present while
the instrument is unpacked. There are no special unpacking instructions, but be careful not to damage the
instrument when unpacking it. Inspect the instrument for physical damage such as bent or broken parts, dents, or
scratches.
Claims
Our routine method of shipment is via common carrier. Upon delivery, if physical damage is found, retain all packing
materials in their original condition and conta ct the carrier immediately to file a claim.
If the instrument is delivered in good physical condition but does not operate within specifications, or if there are any
other problem s not caused by shipping dam age, please contact your local sales representative or DSI immediately.
Standard Terms and Conditions
Refunds & Credits
Please note only serialized products (products labelled with a distinct serial number) and accessories are eligible for
partial refund and/or credit. Non-serialized parts and accessory items (cables, carrying cases, auxiliary modules,
etc.) are not eligible for return or refund. In order to receive a partial refund/credit, the product must not have been
damaged, and must be returned complete (meaning all manuals, cables, accessories, etc.) within 90 days of original
purchase and in “as new” and resalable condition. The Return Procedure must be followed.
Return Procedure
Every product returned for refund/credit must be accom panied by a Return Material Authorization (RMA) num ber,
obtained from Datrend Customer Service. All items being returned must be sent prepaid (freight, duty, brokerage,
and taxes ) to our factory location.
Restocking Charges
Products returned within 30 days of original purchase are subject to a minimum restocking fee of 15%. Products
returned in excess of 30 days after purchase, but prior to 90 days, are subject to a minimum restocking fee of 20%.
Additional charges for damage and/or missing parts and accessories will be applied to all returns. Products which
are not in “as new” and resalable condition, are not eligible for credit return and will be returned to the customer at
their expense.
Certification
This instrument was thoroughly tested and inspected and found to m eet DSI’s m anufacturing specifications when it
was shipped from the factory. Calibration measurements are traceable to the National Research Council of Canada
(NRC) and/or the National Institute of Standards and Technology (NIST). Devices for which there are no NRC/NIST
calibration standards are measured against in-house performance standards using accepted test procedures.
Page i

Warranty
Warranty and Product Support
Datrend Systems Inc. ("DSI") warrants this instrument to be free from defects in materials and workmanship under
normal use and service for two (2) years from the date of original purchase, providing the instrum ent is calibrated in
accordance with Appendix C of this manual. During the warranty period DSI will, at our option, either repair or
replace a product at no charge that proves to be defective; provided you return the product (shipping, duty,
brokerage and taxes prepaid) to DSI. Any and all transportation charges incurred are the responsibility of the
purchaser and are not included within this warranty. This warranty extends only to the original purchaser and does
not cover damage from abuse, neglect, accident or misuse or as the result of service or modification by other than
DSI. IN NO EVENT SHALL DATREND SYSTEMS INC. BE LIABLE FOR CONSEQUENTIAL DAMAGES.
No warranty shall apply when damage is caused by any of the following:
!Introduction of fluids not specified in the instruction manual,
!Application of excessive pressure or vacuum/suction to the device inputs,
!Failure to rinse or clear the fluid flow path as instructed in the manual,
!Power failure, surges, or spikes,
!Dam age in transit or when m oving the instrum ent,
!Improper power supply such as low voltage, incorrect voltage, defective wiring or inadequate fuses,
!Accident, alteration, abuse or misuse of the instrum ent,
!Fire, water damage, theft, war, riot, hostility, acts of God, such as hurricanes, floods, etc.
Only serialized products (those items bearing a distinct serial number tag) and their accessory items are covered
under this warranty. PHYSICAL DAMAGE CAUSED BY MISUSE OR PHYSICAL ABUSE IS NOT COVERED
UNDER THE W A RRANTY. Item s such as cables and non-serialized m odules are not covered under this warranty.
This warranty gives you specific legal rights and you may have other rights, which vary from province to province,
state to state, or country to country. This warranty is limited to repairing the instrument to DSI's specifications.
When you return an instrument to DSI for service, repair or calibration, we recommend shipment using the original
shipping foam and container. If the original packing materials are not available, we recommend the following guide
for repackaging:
!Use a double-walled carton of sufficient strength for the weight being shipped.
!Use heavy paper or cardboard to protect all instrument surfaces. Use nonabrasive material around all projecting
parts.
!Use at least four inches of tightly packed, industrial-approved, shock-absorbent material all around the
instrum ent.
DSI will not be responsible for lost shipments or instruments received in damaged condition due to improper
packaging or handling. All warranty claim shipments must be made on a prepaid basis (freight, duty, brokerage, and
taxes). No returns will be accepted without a Return Materials Authorization ("RMA”) number. Please contact
Datrend at 1-800-667-6557 to obtain an RMA number and receive help with shipping/customs documentation.
Recalibration of instruments, which have a recommended annual calibration frequency, is not covered under the
warranty.
Warranty Disclaimer
Should you elect to have your instrument serviced and/or calibrated by someone other than Datrend Systems,
please be advised that the original warranty covering your product becomes void when the tamper-resistant Quality
Seal is removed or broken without proper factory authorization. We strongly recommend, therefore, that you send
your instrument to Datrend Systems for service and calibration, especially during the original warranty period.
In all cases, breaking the tamper-resistant Quality Seal should be avoided at all cost, as this seal is the key to your
original instrument warranty. In the event that the seal must be broken to gain internal access to the instrument (e.g.,
in the case of a customer-installed firmware upgrade), you must first contact Datrend Systems at 1-800-667-6557.
You will be required to provide us with the serial number for your instrument as well as a valid reason for breaking
the Quality Seal. You should break this seal only after you have received factory authorization. Do not break the
Quality Seal before you have contacted us! Following these steps will help ensure that you will retain the original
warranty on your instrument without interruption.
Page ii
WARNING
Unauthorized user modifications or application beyond the published specifications may result in electrical shock
hazards or im proper operation. Datrend System s will not be responsible for any injuries sustained due to
unauthorized equipment modifications.
DSI DISCLAIMS ALL OTHER WARRANTIES, EXPRESSED OR IMPLIED, INCLUDING ANY WARRANTY OF
MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE OR APPLICATION.
THIS PRODUCT CONTAINS NO USER-SERVICEABLE COMPONENTS.
UNAUTHO RIZED REMOVAL OF THE INSTRUMENT COVER SHALL VO ID
THIS AND ALL OTHER EXPRESSED OR IMPLIED WARRANTIES.
®
Fluke is a registered trademark of Fluke Corporation
MS-DOS, QBasic, Visual Basic, and Visual C++, Microsoft, and Windows are registered trademarks of Microsoft Corp.
®
Brita is a registered trademark of BRITA Worldwide.
Jet Dry is a registered tradem ark of Reckitt Benckiser plc
®
Page iii

Page iv
INFUTEST 2000E OPERATING MANUAL
Table of Contents
1.PERFORMANCE SPECIFICATIONS.....................................1
1.1Single Rate Test.................................................. 1
1.1.1 Continuous Flow Conditions.................................... 1
1.1.2 Pulsatile Flow Conditions. .....................................2
1.2Dual Rate Test................................................... 3
1.2.1 Continuous Flow Conditions ONLY.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
1.3PCA Pump Test. .................................................4
1.3.1 Continuous Flow Conditions ONLY.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
1.4Occlusion Pressure Test............................................ 5
1.4.1 Pressure Measurement........................................5
1.4.2 Nurse Call Detection..........................................5
1.5Data Logger. ....................................................5
1.6Interface........................................................ 6
1.7Accessories. ....................................................8
2.OVERVIEW OF INSTRUMENT. ........................................9
2.1General Description. .............................................. 9
2.2Description of Test Procedures......................................1 3
2.2.1 Single Rate Test............................................ 1 3
2.2.2 Dual Rate Test.............................................1 4
2.2.3 PCA Pump Test. ...........................................1 4
2.2.4 Occlusion Pressure Test......................................1 4
2.3Test Fluids. ....................................................1 5
3.INITIAL SET-UP.................................................... 1 7
3.1Connections.................................................... 1 7
3.2Priming........................................................ 1 8
3.3Optional Connections............................................. 2 2
4.OPERATION AND RELATED DISPLAYS. ............................... 2 5
4.1Powering Up and Setting the Clock................................... 2 5
4.2Setting Up a Test. ...............................................2 9
4.3Test Measurement Concepts. ...................................... 3 0
4.3.1 SynchroStart............................................... 3 0
4.3.2 End-of-Infusion Analysis...................................... 3 0
4.3.3 Flow Pattern Classification.................................... 3 0
4.4Starting a Test. .................................................3 1
4.5Running a Test..................................................3 1
Table of Contents # Page v

INFUTEST 2000E OPERATING MANUAL
4.6Viewing the Test Summary......................................... 3 4
4.6.1 SUMMARY Screen for Single-Rate Test.. . . . . . . . . . . . . . . . . . . . . . . . . 3 4
4.6.2 SUMMARY Screen for Dual-Rate Test. . . . . . . . . . . . . . . . . . . . . . . . . . . 3 5
4.6.3 SUMMARY Screen for PCA Pump Test. . . . . . . . . . . . . . . . . . . . . . . . . . 3 5
4.6.4 SUMMARY Screen for Occlusion Pressure Test. . . . . . . . . . . . . . . . . . . 3 6
4.6.5 SUMMARY Screen Usage. ................................... 3 7
4.7Viewing the Data Log.............................................3 8
4.7.1 Data Log Description. ....................................... 3 8
4.7.2 DATA Screen Usage. ....................................... 3 8
4.8Output of Summary and Data Logs................................... 4 1
4.9Alarms. .......................................................4 5
4.9.1 Nonrecoverable Alarms. .....................................4 5
4.9.2 Recoverable Alarms......................................... 4 5
4.10 AutoSequences............................................... 4 7
5.TEST USAGE GUIDELINES. ......................................... 5 1
5.1General Rules...................................................5 1
5.1.1 Test Fluids. ...............................................5 1
5.1.2 Pump IV Sets and Syringes. ..................................5 3
5.1.3 Cleaning..................................................5 4
5.1.4 Input and Output Connections. ................................5 5
5.1.5 Flushing Prior to Test Startup.................................. 5 6
5.2Single Rate Test................................................. 5 8
5.2.1 Test Description............................................5 8
5.2.2 Test Usage................................................ 6 0
5.3Dual-Rate Test..................................................6 2
5.3.1 Testing Piggyback Operation.................................. 6 2
5.3.2 Testing Titrated Rates. ...................................... 6 3
5.3.3 Testing KVO Operation....................................... 6 4
5.3.4 Testing Bolus-Plus-Basal Mode Operation. . . . . . . . . . . . . . . . . . . . . . . . 6 4
5.3.5 Dual-Rate “PULSATILE” Warning.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 5
5.4PCA Pump Test. ................................................6 6
5.4.1 Test Description............................................6 6
5.4.2 Test Usage................................................ 6 7
5.4.3 PCA Pump “PULSATILE” Warning. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 8
5.5Occlusion Pressure Test...........................................6 9
5.5.1 Measuring Occlusion Pressure................................. 6 9
5.5.2 Checking the Nurse Call Alarm.................................6 9
5.6AutoSequences. ................................................7 1
5.6.1 Running Default Test Sequences. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 1
5.6.2 Reloading Default Test Sequences.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 3
5.6.3 Adding the Occlusion Test to Sequences. . . . . . . . . . . . . . . . . . . . . . . . . 7 3
5.7Understanding Your Test Results.................................... 7 5
Table of Contents # Page vi

INFUTEST 2000E OPERATING MANUAL
6.REMOTE CONTROL. ...............................................7 9
6.1Overview.......................................................7 9
6.2Connections.................................................... 8 0
6.3Command Syntax................................................8 0
6.4Command Types and Return Values. ................................ 8 1
6.5Command List...................................................8 2
6.6Command Descriptions............................................8 3
6.6.1 Test Control Commands - RUN TEST. . . . . . . . . . . . . . . . . . . . . . . . . . . 8 3
6.6.2 Test Control Commands - RUN AUTOSEQUENCE. . . . . . . . . . . . . . . . . 8 4
6.6.3 Test Control Commands - STOP TEST/ABORT AUTOSEQUENCE. . . . 8 4
6.6.4 Report Output Control - PRINT REPORT. . . . . . . . . . . . . . . . . . . . . . . . . 8 5
6.6.5 Report Output Control - DOWNLOAD REPORT.. . . . . . . . . . . . . . . . . . . 8 5
6.6.6 Get Data Commands - GET TEST SUMMARY.. . . . . . . . . . . . . . . . . . . . 8 6
6.6.7 Get Data Commands - GET REAL-TIME DATA. . . . . . . . . . . . . . . . . . . . 8 7
6.7Programming Examples...........................................9 0
6.7.1 QBasic Programming........................................ 9 0
6.7.2 medTester Programming. ....................................9 8
APPENDIX A. BACK PRESSURE TESTING................................9 9
APPENDIX B. OPERATIONAL OVERVIEW...............................103
B.1General Description. ............................................ 103
B.2Flow Measurement System........................................ 104
B.3Pressure Measurement System....................................106
APPENDIX C. CALIBRATION.......................................... 107
C.1Annual Calibration...............................................107
C.2Calibration Verification. .......................................... 107
C.2.1 Pressure Accuracy Verification - Field Test. . . . . . . . . . . . . . . . . . . . . . 107
C.2.2 Flow Accuracy Verification - Field Test. . . . . . . . . . . . . . . . . . . . . . . . . . 107
Table of Contents # Page vii

INFUTEST 2000E OPERATING MANUAL
Table of Contents # Page viii

INFUTEST 2000E OPERATING MANUAL
Introduction
This chapter provides the performance specifications of the Infutest
2000E (Infutest) Infusion Device Analyzer.
1. PERFORMANCE SPECIFICATIONS
The following specifications apply to both channels of the Infutest 2000 Series E Dual
Channel Infusion Device Analyzer.
1.1 Single Rate Test
1.1.1 Continuous Flow Conditions
I. Flow Measurement
a. Nominal range: 0.1 - 1200 ml/hr
b. Maximum flow measurable and displayed: 1700 ml/hr
c. Minimum flow measurable and displayed: 0.04 ml/hr
d. Maximum display resolution: 0.001 ml/hr
e. Measuring time: 10 min. at 0.1 ml/hr
f. Accuracy
Average flow: +/- 1%, 0.1-1200ml/hr
g. Ranges: LOW - 0.1 to 170 ml/h
h. Range selection: Automatic
i. Internal nominal effective
collection volume: LOW range: 0.014 ml
20 sec. max. above 6 ml/hr
HIGH - 170 to 1200 ml/h
HIGH range: 1.1 ml
Introduction/Chapter 1 # Page 1

INFUTEST 2000E OPERATING MANUAL
II. Volume Measurement
a. Range: 0 - 9999 ml
b. Maximum display resolution: 0.001 ml
c. Accuracy: +/- 1%
III. Back Pressure Measurement
a. Range: 0 - 300 mmHg
b. Zero offset error: +/- 5 mmHg
c. Display resolution: 1 mmHg
d. Accuracy: +/- 1% FS,
IV. Elapsed Time Measurement
a. Range: 0 - 100 hours
b. Display format: hh:mm:ss
c. Accuracy: +0, -1second
1.1.2 Pulsatile Flow Conditions
+/- zero offset error
I. Flow Measurement
a. Nominal range: 5 - 1200 ml/hr
b. Maximum flow measurable and displayed: 1700 ml/hr
c. Minimum flow measurable and displayed: 2.75 ml/hr
d. Maximum display resolution: 0.001 ml/hr
e. Measuring time: 14 min. at 5 ml/hr
20 sec. max. above 200 ml/hr
f. Accuracy:
Average flow +/- 1%
g. Ranges: HIGH range only
(internally selected)
h. Internal nominal effective
collection volume: 1.1 ml
II. Volume Measurement
a. Range: 0 - 9999 ml
b. Maximum display resolution: 0.001 ml
c. Accuracy: +/- 1%
Introduction/Chapter 1 # Page 2

INFUTEST 2000E OPERATING MANUAL
III. Back Pressure Measurement
- Same as for Continuous Flow Measurement
IV. Elapsed Time Measurement
-Same as for Continuous Flow Measurement
1.2 Dual Rate Test
1.2.1 Continuous Flow Conditions ONLY
I. Flow Measurement
a. Nominal range: 0.1 - 170 ml/hr
b. Maximum flow measurable and displayed: 200 ml/hr
c. Minimum flow measurable and displayed: 0.04 ml/hr
d. Maximum display resolution: 0.001 ml/hr
e. Measuring time: 10 min. at 0.1 ml/hr
f. Accuracy:
Average flow: +/- 1%
g. Ranges: LOW range only
h. Delivery period determination: Automatic
i. Internal nominal effective collection volume: 0.014 ml
20 sec. max. above 6 ml/hr
II. Volume Measurement
- See 1.1 SINGLE RATE TEST, Continuous Flow Measurement
III. Back Pressure
- See 1.1 SINGLE RATE TEST, Continuous Flow Measurement
IV. Elapsed Time Measurement
- See 1.1 SINGLE RATE TEST, Continuous Flow Measurement
Introduction/Chapter 1 # Page 3

INFUTEST 2000E OPERATING MANUAL
1.3 PCA Pump Test
1.3.1 Continuous Flow Conditions ONLY
I. Flow Measurement, Bolus Delivery period
a. Nominal range: 0.1 - 170 ml/hr
b. Maximum flow measurable and displayed: 200 ml/hr
c. Minimum flow measurable and displayed: 0.04 ml/hr
d. Maximum display resolution: 0.001 ml/hr
e. Measuring time: 10 min. at 0.1 ml/hr
f. Accuracy:
Average flow: +/- 1%
g. Ranges: LOW range only
h. Lockout detection: Automatic
i. Internal nominal effective collection volume: 0.014 ml
II. Volume Measurement
20 sec. max. above 6 ml/hr
- See 1.1 SINGLE RATE TEST, Continuous Flow Measurement
III. Back Pressure Measurement
- See 1.1 SINGLE RATE TEST, Continuous Flow Measurement
IV. Elapsed Time Measurement
- See 1.1 SINGLE RATE TEST, Continuous Flow Measurement
V. Lockout Time Measurement
a. Range: 0 - 100 minutes
b. Display format: mm:ss
c. Accuracy: +/- 2 seconds
Introduction/Chapter 1 # Page 4

INFUTEST 2000E OPERATING MANUAL
1.4 Occlusion Pressure Test
1.4.1 Pressure Measurement
a. Display Units: mmHg, psi
b. Range: 0 - 2586 mmHg(0 - 50 psi)
c. Display resolution: 1 mmHg(0.1 psi)
d. Measuring time: 2 seconds
e. Zero offset error: +/- 5 mmHg (0.1 psi)
f. Accuracy: +/- 1% +/- zero offset error
1.4.2 Nurse Call Detection
a. Nurse Call signal from pump sampled once per second.
b. Nurse Call signal can be produced by any of: relay contacts, open
collector output, TTL output, RS-232 level, or 20 mA current loop.
c. Nurse Call signal may be in either logical state at start of Occlusion
Pressure Test.
1.5 Data Logger
I. Memory Type:
II. Capacity:
2 channel 900 flow measurements per channel
4 channel 450 flow measurements per channel
III. Data Output:
NVRAM (battery-backed)
700 pressure measurements per channel
350 pressure measurements per channel
Directable to printer or serial port following
completion of a test or AutoSequence.
Introduction/Chapter 1 # Page 5

INFUTEST 2000E OPERATING MANUAL
1.6 Interface
I. User Interface:
II. Fluid interface:
Inputs A and B: Delrin twistlock, self-sealing
Common output A&B: Delrin twistlock
III. PCA Pump Trigger Outputs:
Mechanical - One 1/4" female stereo phono
Electrical - Relay contacts rated at 120 VAC, 1 A;
40-character by 8-line backlit LCD
4 front panel soft-keys
Internal beeper
LCD contrast control (rear panel)
jack per channel
normally open and normally
closed contacts available.
IV. Nurse Call Inputs:
Mechanical - One 1/4" female stereo phono
jack per channel
Electrical - 50K-ohm differential input impedance.
V. Parallel Port:
Centronix standard printer interface,
DB-25 female connector
VI. Printer Driver:
Epson MX/FX Series, 80 cps
80 columns and 66 lines per page
VII. Serial Port:
Mechanical - DB-25 male connector
Electrical - RS-232C; bidirectional; CTS handshaking;
9600 baud, 8 data bits, no parity bit, 1 stop bit
VIII. Power Supply:
120 VAC 60 Hz @ 12 W
(230/240 VAC 50 Hz Europe/UK)
(100 VAC 50-60 Hz Japan)
Introduction/Chapter 1 # Page 6

INFUTEST 2000E OPERATING MANUAL
IX. Fuse:
X. Environment:
XI. Dimensions:
XII. Weight:
AGC 500mA, 250V @ 100-120 VAC Input
AGC 300mA, 250V @ 220-240 VAC Input
1 5 E C to 40EC
10% to 90% RH
Indoor Use Only
Category II
Pollution Degree 2
12" W x 12" D x 6" H
30.5 cm W x 30.5 cm D x 15.2 cm H
10 lbs.
4.5 kg
Introduction/Chapter 1 # Page 7

INFUTEST 2000E OPERATING MANUAL
1.7 Accessories
I. Standard Accessories:
AC line cord
I/O tubing set, comprising two IEC recommended 21 gauge flow restrictors,
1
two three-way stop cocks and two Luer-lock to Delrin twistlock extension sets,
P/N 7300-005
Flushing syringe (60cc)
Infutest 2000 companion CD, including Infutest 2000 Operating Manual in
Adobe PDF format, and Data Transfer and Graphing Program (DTP) for
Windows, P/N 6950-001 (also available for download from the Datrend web
site - www.datrend.com)
RS-232 Serial Port cable, DB25F - DB9F, P/N 3140-040
II. Optional Accessories:
6' RS-232 Serial Port cable, DB25 - DB25 (SSC -1), P/N 7100-062
6' PCA Pump trigger cable, unterminated (PCA -1), P/N 7100-061
6' Nurse Call Alarm cable, unterminated (NCA -1), P/N 7100-060
6' Parallel Printer Cable (PPC-1), P/N 7100-064
Remote Sensor Module (RSM):
- North America, 120 VAC/60 Hz, P/N: 8000-024
- United Kingdom, 240 VAC/50Hz, P/N: 8000-025
- Europe, 220 VAC/50 Hz, P/N:8000-026
- Japan, 100 VAC/50-60Hz, P/N: 8000-030
RSM Connector Cable (AUX-1), P/N 7100-279
(comes standard with RSM)
1
IEC 601-2-24, Part 2, Particular requirements for safety of infusion pumps and controllers
Introduction/Chapter 1 # Page 8

INFUTEST 2000E OPERATING MANUAL
Overview
This chapter gives an overview of the capabilities of the Infutest, the tests
performed and test fluid recommendations.
2. OVERVIEW OF INSTRUMENT
2.1 General Description
The Infutest is an automated, self-contained system for performing flow rate, effused
volume, and occlusion pressure tests on medical infusion devices. In addition to the
Single Rate test, Infutest is also capable of automatically checking Patient-Controlled
Analgesia (PCA) pumps for correct lockout operation, and for testing pumps capable of
delivering dual rates - two preset volumes at differing flow rates (i.e. primary/secondary
delivery or delivery "piggybacking").
The Infutest has two independently-functioning channels, designated A and B, for
simultaneously performing any of four basic, selectable test procedures on up to two
infusion devices. Tests performed simultaneously may be different for each infusion
device being evaluated. For example, a Single Rate Test may be performed on a syringe
pump connected to Channel A while a PCA pump is undergoing a lockout time
measurement on Channel B. In addition to the manual selection of the four basic tests
(Single Rate, Dual-Rate, PCA and Occlusion Pressure), Infutest has the ability to create
pump test protocols, termed AutoSequences. The nine (9) test sequences are user
configurable to automate testing and data collection based on manufacturer or user test
protocols. AutoSequences may greatly improve on productivity by standardizing on
test protocols, and minimizing user setup time.
In addition to the two channel Infutest, a Remote Sensor Module (RSM) may be added
to expand the Infutest to a four channel system. Throughout the manual, wherever
reference is made to the capabilities of Channel A or B, or switching from Channel A
to B, this may be extended to include channels C and D if an RSM is connected. All
actions of the RSM are controlled by the Infutest base unit and all results are presented
on the Infutest LCD display.
Overview/Chapter 2 # Page 9
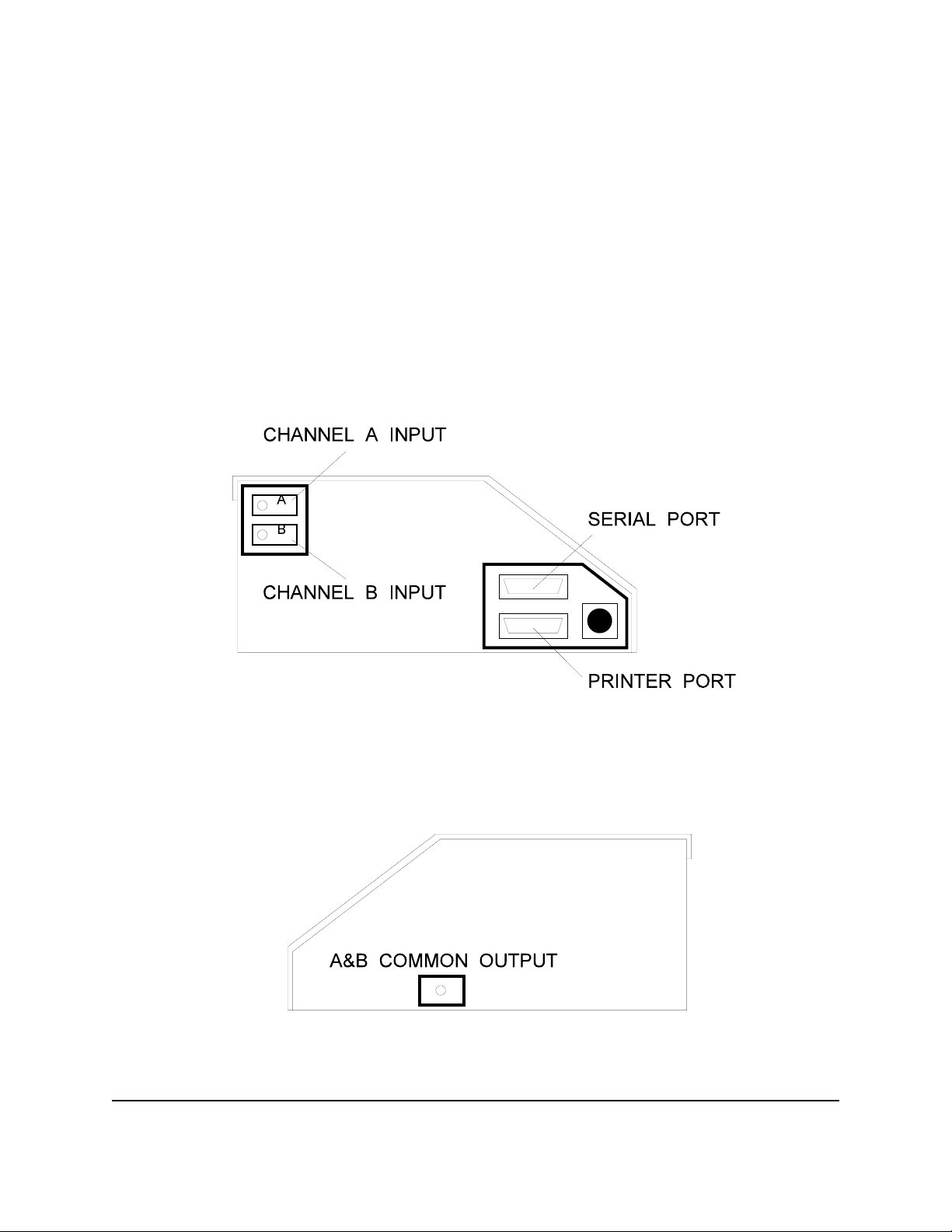
INFUTEST 2000E OPERATING MANUAL
On its front panel, the Infutest provides a menu-driven user interface which is
implemented with a liquid crystal display (LCD) and four multi-function, software-
defined push buttons ("soft keys"). The left panel of the Infutest (Figure 1) provides
both a Centronix-standard parallel port (DB25 female) for connection to an Epson FX
Series or compatible printer, and a RS-232 serial port (DB25 male) for data output to a
personal computer or other data collection instrument. Also located on the left panel
are two Delrin twistlock, self -sealing connectors which serve as a fluid inputs for
Channels A and B. Fluid exits the Infutest via a Delrin twistlock connector which
provides the Channel A & B common output (Figure 2). Inputs for pump Nurse Call
signals and outputs for triggering PCA pumps are provided on the Infutest rear panel
(Figure 3).
Figure 1 - Infutest left panel
Figure 2 - Infutest right panel
Overview/Chapter 2 # Page 10
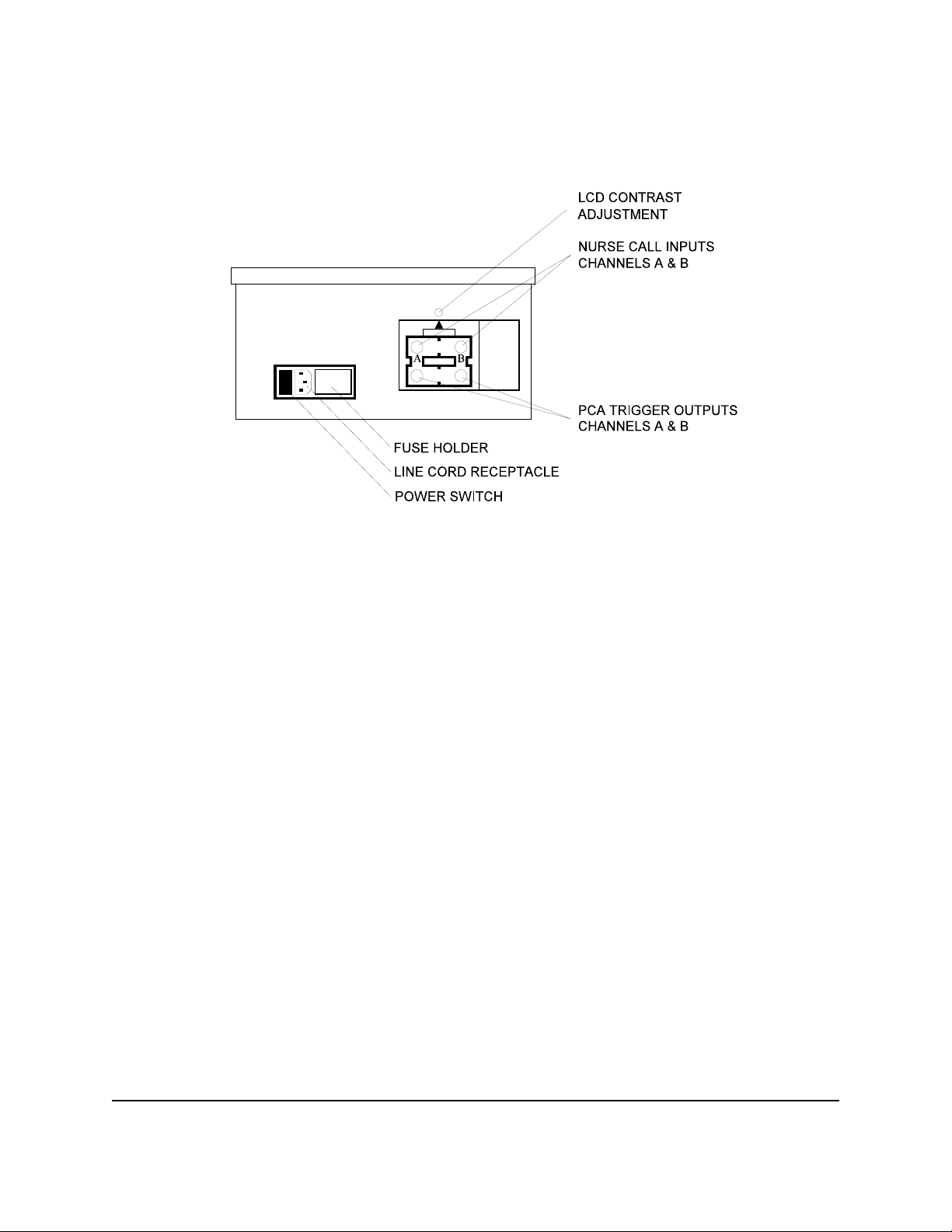
INFUTEST 2000E OPERATING MANUAL
Figure 3 - Infutest rear panel
The Infutest incorporates a time/date clock and a data logger capable of storing up to
900 flow measurements and 700 occlusion pressure measurements per channel. A "flow
measurement" comprises elapsed time (in hours, minutes and seconds), flow rate,
average flow, total effused volume and back pressure for the Single Rate, Dual-Rate and
PCA Pump Tests. A “pressure measurement” comprises elapsed time, occlusion
pressure and Nurse Call Alarm status for the Occlusion Pressure Test.
When an RSM is connected to the Infutest, the data log is initialized to 450 flow and 350
pressure measurements for each of the four channels.
The Infutest also provides a Summary for each channel. A Summary condenses the raw
data obtained from a test into a few important values, such as the average flow measured
during the test, and the total volume of fluid that was effused. An Occlusion Pressure
Summary is also included to provide a synopsis of Occlusion Pressure Test results.
Upon completion of a test, contents of the channel Data Log, the channel Summary, or
both may be output to a printer or downloaded to a personal computer via the serial
port. Multiple copies of the test results may be output in succession, and each copy may
be directed to either the printer or the serial port at the user's option.
Overview/Chapter 2 # Page 11

INFUTEST 2000E OPERATING MANUAL
To accommodate long-term testing or special testing needs, the Infutest may be
controlled remotely via RS-232C using the Infutest Remote Control (IRC) command set
(see Section 6). This command set allows the Infutest to be setup and controlled by a
user designed program. Data may be collected in real-time, allowing long-term testing
and data collection.
The Infutest incorporates a non-volatile memory which allows the instrument to be
disconnected from the line and transported without losing previously acquired test
results. At power-up, the user may select to view or output the contents of a channel’s
Data Log or Summary, which are both retained in the non-volatile memory. Initial test
settings are also preserved when the AC supply is disconnected.
A Windows based software accessory, available from Datrend Systems (the Infutest Data
Transfer Program - DTP), facilitates transfer of test results from the Infutest to a
personal computer, and conversion of Infutest Data Log and Test Summary Report files
to numeric data files suitable for importing into most spreadsheet or database programs.
DTP also has the ability to graphically display and print flow versus time graphs and
trumpet (error bounds) graphs.
Overview/Chapter 2 # Page 12

INFUTEST 2000E OPERATING MANUAL
2.2 Description of Test Procedures
Each channel of the Infutest is capable of making the following measurements:
i Flow Rate ranging from 0.1 up to 1200 milliliters per hour;
ii Effused Volume up to 9999 milliliters;
iii Pulsatile Flow ranging from 5 up to 1200 milliliters per hour;
iv Keep-Vein-Open (KVO) Rate following primary delivery;
v PCA pump Delivery Rate during bolus infusion, ranging from 0.1 up
to 170 milliliters per hour;
vi Bolus Volume delivered by PCA pump;
vii Number of Boluses delivered from PCA pump, up to 255;
viii Lockout Time between PCA pump bolus deliveries, in minutes and
seconds;
ix PCA pump Basal Rate following bolus delivery (using Dual-Rate Test);
x Back Pressure applied to infusion device, up to 300 mmHg;
xi Occlusion Pressure produced by infusion device, up to 2586 mmHg
(50 psi); and
xii Status of Nurse Call Alarm from infusion device (ON or OFF).
The above measurements are organized into four basic test protocols. These
test protocols are:
2.2.1 Single Rate Test
Use the Single Rate Test to evaluate the basic performance of all medical
infusion devices. The Single Rate Test measures Flow Rate (instantaneous and
average), Effused Volume, and Back Pressure.
In addition to accommodating continuous flow infusion devices, the Single Rate
test also incorporates automated detection and averaging of pulsatile flow for
increased measurement accuracy of bolus-discharge and other non-continuous
pumping devices.
Overview/Chapter 2 # Page 13

INFUTEST 2000E OPERATING MANUAL
2.2.2 Dual Rate Test
Use the Dual-Rate Test to evaluate infusion devices capable of
primary/secondary delivery or delivery "piggybacking", that is, delivery of a first
preset volume at a first rate followed by delivery of a second preset volume at a
second rate. The Dual-Rate Test measures Flow Rate (instantaneous and
average), Effused Volume, and Back Pressure for each delivery programmed
into the Dual-Rate pump.
Also use the Dual-Rate Test to evaluate Keep-Vein-Open (KVO) operation and
PCA Bolus-Plus-Basal mode delivery with infusion devices which incorporate
these features.
2.2.3 PCA Pump Test
Use the PCA Pump Test to evaluate the basic performance of Patient-Controlled
infusion devices. The PCA Pump Test measures Flow Rate (instantaneous and
average) and Bolus Volume for each bolus delivery, the Lockout Time between
bolus deliveries, and the Number of Boluses delivered during the test. Back
Pressure is also measured.
2.2.4 Occlusion Pressure Test
Use the Occlusion Pressure Test to measure the maximum pressure generated
by an infusion device when its output is occluded. Also use the Occlusion
Pressure Test to check the operation of the Nurse Call Alarm on pumps which
incorporate this feature.
Overview/Chapter 2 # Page 14
INFUTEST 2000E OPERATING MANUAL
2.3 Test Fluids
The following test fluids are acceptable for use with the Infutest:
1. DISTILLED WATER
2. CLEAN DOMESTIC WATER (i.e. "tap water")
Common distilled water is the preferred test fluid. If domestic water is employed,
occasional flushing with distilled water may be required following use, depending on
hardness and quality of the local water. The test fluid should be colourless and should
not contain visible particulate matter. An agent for reducing surface tension of the test
fluid is normally not required for routine use. However a wetting agent, such as Jet Dry,
may be added if required. In the case of Jet Dry, a concentration of 1.0 ml per litre of
test fluid is recommended as a starting point. Wetting agent concentration may have to
be varied depending the purity of the test fluid.
IMPORTANT:
THE USE OF SALINE SOLUTION AS A TEST FLUID IS STRONGLY DISCOURAGED,
AND WILL VOID THE W ARRANTY. IN THE EVENT SALINE SOLUTION IS
ACCIDENTALLY USED, THE INFUTEST SHOULD BE FLUSHED WITH DISTILLED
WATER.
DO NOT USE DEXTROSE IN WATER (eg. D5W, D25W) OR OTHER VISCOUS TEST
FLUIDS WITH THE INFUTEST. USE OF SUCH FLUIDS WILL VOID THE WARRANTY.
If the instrument is in daily use, both channels should be kept primed between tests
provided that distilled water or low hardness domestic water is employed. If the
instrument is to be stored for several months or transported, fluid should be drained
from the unit by forcing air into each channel input with a large syringe while the
alternate input is blocked. The instrument should then be carefully blown out from the
A and B inputs to the common output using clean, dry compressed air.
For the remainder of this manual, the test fluid is assumed to be distilled water or lowhardness domestic water, and is referred to generically as "water".
Overview/Chapter 2 # Page 15
INFUTEST 2000E OPERATING MANUAL
IMPORTANT:
IV sets which have or may have come in contact with
saline or dextrose or other IV fluids should not be used on
the Infutest. If an administration set must be re-used (i.e.
as part of an incident investigation), ensure the set has
been flushed out thoroughly with clean water before
connecting the set to the Infutest. Most IV fluids contain
salts and sugars which can degrade and potentially ruin
the high-precision flow sensors inside the Infutest.
It is always best to use a new administration set when
testing a pump. However, the same administration set may
be re-used to test several pumps provided the set is primed
only with distilled or “sterile” water. Change IV set per
the manufacturer’s recommendations to ensure that test
results reflect clinical use of the infusion device.
Overview/Chapter 2 # Page 16

INFUTEST 2000E OPERATING MANUAL
Set-up
This chapter describes the initial set-up of the Infutest, describes the basic
fluid connections, fluid priming, and serial and parallel output
connections.
3. INITIAL SET-UP
3.1 Connections
a. Place the instrument on a stable, horizontally level surface. Connect electrical
power to the unit by inserting the power cable into the rear panel receptacle
(Figure 3) and connecting the cable to a grounded outlet.
b. Connect the input tubing set (consisting of the IEC recommended 21 gauge
flow restrictor, three-way stop cock, and extension set) to the Channel A fluid
input (Figure 1, Figure 4). Similarly, connect the second input tubing set to
Channel B. This arrangement is recommended for proper instrument
operation.
c. Connect the output extension set to the Channel A & B common output
(Figure 2, Figure 7). The A & B Output should drain into a collection vessel
of appropriate volume ideally located on the bench near the Infutest. A small
(250 to 500 ml) beaker will suffice for most test situations.
The collection vessel should not be located more than 36" below the level of
the A & B Output. This will prevent a vacuum from being applied to the
Infutest which could damage the internal pressure sensors, or cause a
pressure zero error when the Infutest is powered up.
Refer to the instructions in Appendix A if a Back Pressure is to be applied to the Infutest to oppose
the flow from the infusion device.
Set-up/Chapter 3 # Page 17
INFUTEST 2000E OPERATING MANUAL
3.2 Priming
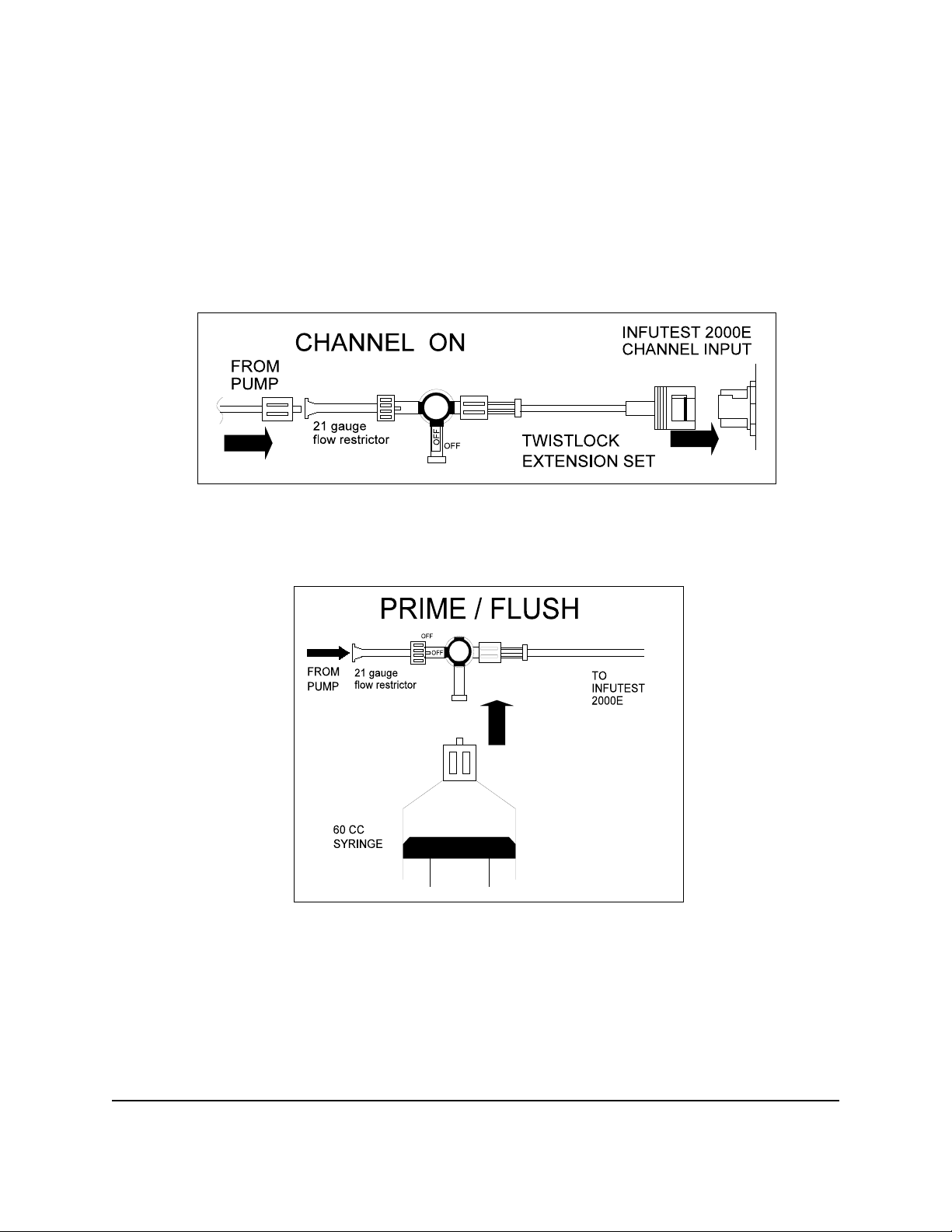
Figure 4, Figure 5 and Figure 6 on the following page show the three possible
positions of the stop cock connected to a channel input. As illustrated, the three
positions are referred to as ON, PRIME or FLUSH, and OFF.
Figure 4 - Connection of stop cock to channel input, channel ON.
Figure 5 - Stop cock position for priming / flushing
Set-up/Chapter 3 # Page 18
 Loading...
Loading...