
TWIN STIM® III
INSTRUCTION MANUAL

This manual is valid for the InTENSityTM Twin Stim III
TENS/EMS Combo Stimulator
This user manual is published by Current Solutions™, LLC
Current Solutions™, LLC does not guarantee its contents and
reserves the right to improve and amend it at any time without
prior notice. Amendments may however be published in new
editions of this manual.
All Rights Reserved.Rev.V1.1 © 2010
:
United States Federal Law restricts this device to sale by
or on the order of a physician or licensed practitioner
Declaration of conformity:
Current Solutions™, LLC declares that the device complies
with following normative documents:
IEC60601-1, IEC60601-1-2, I EC60601-2-
ISO10993-5,
ISO10993-10, ISO10993-1
10, IEC60601-1-4,

Table of Contents
3
1. SAFETY INFORMATION…………………………………………………………………....
noitpircsed lareneG 1.1
1.2 Medical background
1.3 Indication for use
1.4 Contraindications
1.5 Warnings, Cautions, Adverse Reactions
2.1 Front and Rear panel
2.2 LCD display
3. SPECIFICATION……………………………………………………………..…………………....
3.1 Accessories
3.2 Technical information
3.3 The waveforms of the stimulation programs
4. INSTRUCTIONS FOR USE
4.1 Battery
4.2 Connect electrodes to lead wires
4.3 Connect lead wires to device
4.4 Electrodes
4.5 Turn ON
4.6 Select the Therapeutic Mode
4.7 Steps to set a new program
4.8 Adjust Channel Intensity
4.9 Safty lock feature
4.10 Stop the treatment
4.11 Turn OFF
4.12 Low battery indicator
NOITATNESERP .2 ………………………………………………………………..………………...
4
12
15
.........………………………..………………………………
19
5. PROGRAM...........................................................................................................................…
6. CLEANING AND CARE…………………………………………………….………………..
6.1 Tips for skin care
6.2 Cleaning the device
6.3 Electrodes
6.4 Cleaning the Electrode cords
6.5 Maintenance
8. STORAGE…………………………………………………………………………………….............
9. DISPOSAL…………………………………………………………………………………................
10. ELECTROMAGNETIC COMPATIBITY (EMC)
11.
GLOSSARY OF SYMBOLS
WARRANTY
12.
GNITOOHSELBUORT .7 ....……………………………………………………………………
TABLES
……
28
29
32
33
33
33
35
46
4
1.Safety information
1.1 General
InTENSityTM Twin Stim III
two
therapeutic modes: Transcutaneous Electrical Nerve Stimulator
(TENS) and Electrical Muscle Stimulation (EMS), which are used
for pain relief and electrical muscle stimulation. The stimulator
sends gentle electrical current to underlying nerves and muscle
group via electrodes applied on the skin. The parameters of device
are controlled by the buttons on the front panel. The intensity level
is adjustable according to the needs of patients.
1.2 Medical background
EXPLANATION OF PAIN
Pain is a warning system and the body’s method of telling us that
something is wrong. Pain is important; without it abnormal conditions
may go undetected, causing damage or injury to vital parts of our
bodies. Even though pain is a necessary warning signal of trauma or
malfunction in the body, nature may have gone too far in its design.
Aside from its value in diagnosis, long-lasting persistent pain serves
no useful purpose. Pain does not begin until the coded message
travels to the brain where it is decoded, analyzed, and then reacted to.
The pain message travels from the injured area along the small
nerves leading to the spinal cord. Here the message is switched to
different nerves that travel up the spinal
is a portable electrotherapy device featuring
cord to the brain. The pain
message is then interpreted, referred back and the pain is felt.
EXPLANATION OF TENS
Transcutaneous Electrical Nerve Stimulation (TENS) is a noninvasive, drug free method of controlling pain. TENS uses tiny
electrical impulses sent through the skin to nerves to modify your
pain perception. TENS does not cure any physiological problem;
it only helps control the pain. TENS does not work for everyone;
however, in most patients it is effective in reducing or eliminating
5
the pain, allowing for a return to normal activity.
HOW TENS WORKS
There is nothing “magic” about Transcutaneous Electrical Nerve
Stimulation (TENS). TENS is intended to be used to relieve pain.
The TENS unit sends comfortable impulses through the skin that
stimulate the nerve (or nerves) in the treatment area. In many
cases, this stimulation will greatly reduce or eliminate the pain
sensation the patient feels. Pain relief varies by individual patient,
mode selected for therapy, and the type of pain. In many patients,
the reduction or elimination of pain lasts longer than the actual
period of stimulation (sometimes as much as three to four times
longer). In others, pain is only modified while stimulation actually
occurs. You may discuss this with your physician or therapist.
EXPLANATION OF EMS
Electrical Muscle Stimulation (EMS) is an internationally accepted
and proven way of treating muscular injuries. It works by sending
electronic pulses to the muscle needing treatment; this causes the
muscle to exercise passively. It is a product derived from the
square waveform, originally invented by John Faraday in 1831.
Through the square wave pattern it is able to work directly on
muscle motor neurons. This device has low frequency and this in
conjunction with the square wave pattern allows direct work on
muscle groupings. This is being widely used in hospitals and
sports clinics for the treatment of muscular injuries and for the re-
education of paralyzed muscles, to prevent atrophy in affected
muscles and improving muscle tone and blood circulation.
HOW EMS WORKS
The EMS units send comfortable impulses through the skin that
stimulate the nerves in the treatment area. When the muscle
receives this signal it contracts as if the brain has sent the signal
itself. As the signal strength increases, the muscle flexes as in

physical exercise. Then when the pulse ceases, the muscle
relaxes and the cycle is repeated. The goal of electrical muscle
stimulation is to achieve contractions or vibrations in the muscles.
Normal muscular activity is controlled by the central and
peripheral nervous systems, which transmit electrical signals to
the muscles. EMS works similarly but uses an external source
(the stimulator)with electrodes attached to the skin for transmitting
electrical impulses into the body. The impulses stimulate the
nerves to send signals to a specifically targeted muscle, which
reacts by contracting, just as it does with normal muscular activity.
1.3 Indication for use
InTENSityTM Twin Stim III
For Transcutaneous Electrical Nerve Stimulator therapeutic
modes (TENS):
Stimulator may be used for
1) Symptomatic relief of chronic intractable pain.
2) Post traumatic pain.
3) Post surgical pain.
For Electrical Muscle Stimulation / Neuromuscular Stimulation
therapeutic mode (EMS):
1) Relaxation of muscle spasm.
2) Increase of blood flow circulation.
3) Prevention of disuse atrophy.
4) Muscle re-education.
5) Maintaining or increasing range of motion.
imulation of lower leg muscles to
prevent venous thrombosis.
IMPORTANT SAFETY INFORMATION!
Read the instruction manual before operation. Be sure to comply
with all “Contraindications”, “Warnings”, “Cautions” and “Adverse
reactions” in the manual. Failure to follow instructions can cause
harm to user or device.

1.4 Contraindications
1.5 Warnings, Cautions and Adverse Reactions
7

8

CAUTIONS:
out of reach of children.
you are in any doubt whatsoever.

13) Some patients may experience skin irritation or
hypersensitivity due to the electrical stimulation or silicone
rubber. If rash develops or pain persists, discontinue use and
consult a doctor.
14) Electrode placement and stimulation settings should be based
on the guidance of prescribing practitioner.
15) Effectiveness is highly dependent upon patient selection by a
person qualified in the management of pain afflicted patients.
16) Isolated cases of skin irritation may occur at the site of the
electrode placement following long-term application. If this
occurs, discontinue use and consult your physician.
17) The electrodes are only to be placed on healthy skin. Avoid
skin irritation by ensuring that good contact is achieved
between electrodes and skin.
18) If the stimulation levels are uncomfortable or become
uncomfortable, reduce the stimulation Intensity to a
comfortable level and contact your physician if problems
persist.
19) This device should not be used while driving, operating
machinery, close to water, or during any activity in which
involuntary muscle contractions may put the user at undue risk
of injury.
20) Never use the device in rooms where aerosols (sprays) are
used or pure oxygen is being administered.
21) Do not use it near any highly flammable substances, gases or
explosives.
22) Do not use this device at the same time as other equipment
which sends electrical pulses to your body.
23) Do not confuse the electrode cables and contacts with your
headphones or other devices, and do not connect the
electrodes to other devices.
24) Do not use sharp objects such as pencil point or ballpoint pen
to operate the buttons on the control panel.
25) Inspect Applicator cables and associated connectors before
each use.
26) Turn the device off before applying or removing electrodes.
27) Electrical stimulators should be used only with the leads and
electrodes recommended for use by the manufacturer.
28) This device has no AP/APG protection. Do not use it in the
presence of explosive atmosphere and flammable mixture.

Adverse Reactions:
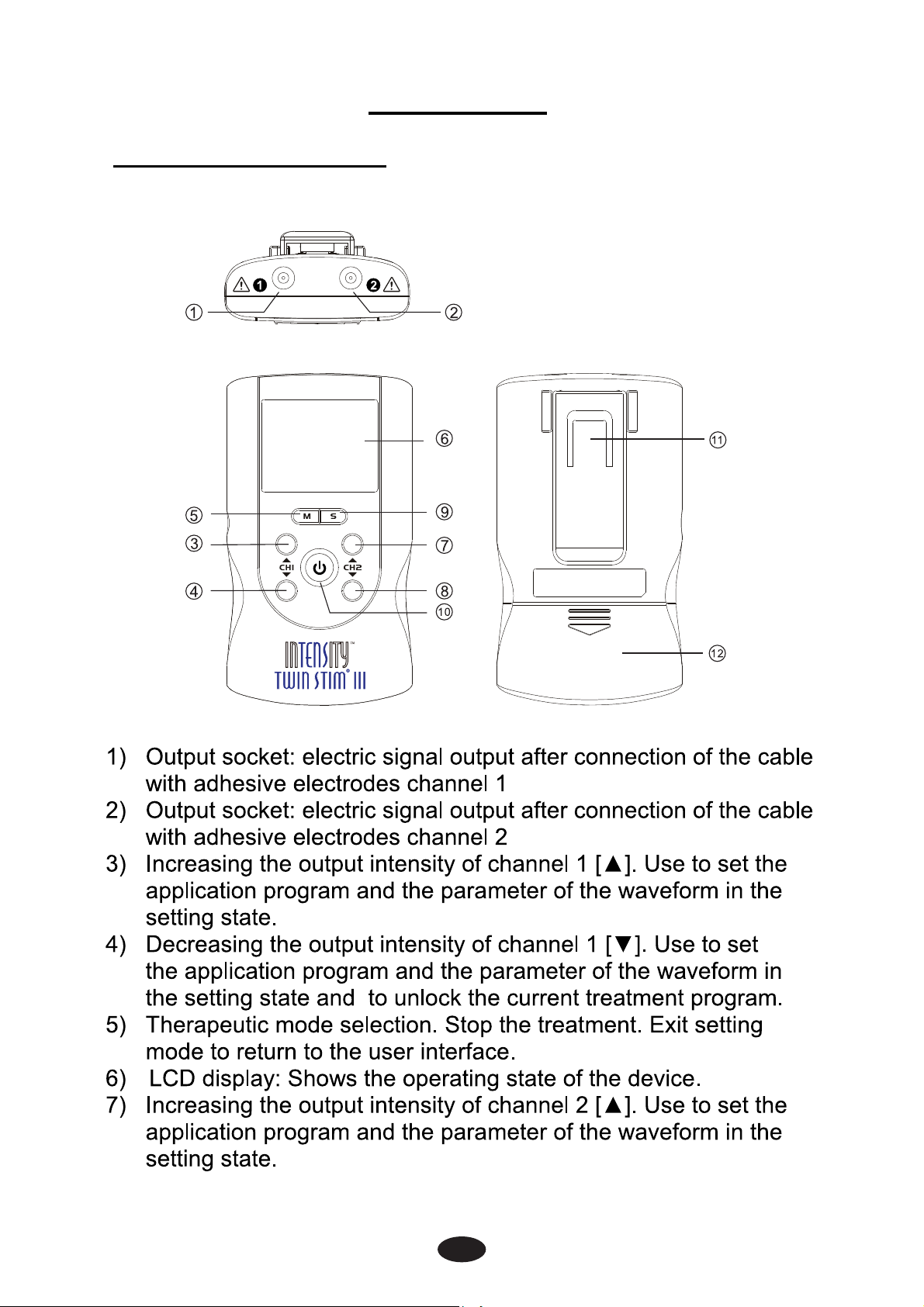
2. Presentation
12
2.1 Front and Rear Panel

intensity of channel 2 [▼]. Use to set
13
rameter of the waveform in
2.2 LCD display
the current treatment program.
ss the button to enter setting
▲] and [▼].
Press [ ] button and hold for
cover for user access.
or Displays the cycle time for
y for channel 1 (CH1); Display of
S waveform of contraction
raction (working) time

12) Display of the output intensity for channel 2 (CH2); Display
of waveform pulse rate or EMS waveform of relaxation time
in setting state.
13) Displays channel 2.
14) EMS waveform of relaxation time.
15) Displays the treatment time or EMS waveform of ramp up and
ramp down time.
16) Low-battery indicator.

3. Specification
3.1 Accessories
No DESCRIPTION Q’TY
1 Electrical stimulator device 1 piece
2 Electrode Leads 2 pieces
3 1.5”x 1.5” Adhesive Electrodes 4 pieces
4 9V Alkaline Battery, type 6LR61 1 piece
5 Instruction Manual 1 piece
6 Carrying case 1 piece
3.2 Technical information
Channel Dual, isolated between channels
Power
Operating
conditions
Storage
conditions
Dimensions 4.5×2.55×0.9 inches(L*W*H)
Weight 0.28 lbs(With battery)
Tolerance
Timer
9.0 V DC-1 Alkaline *6LR61 battery(optional AC Adaptor)
5°C to 40°C (41 to 104℉ ℉)with a relative humidity of
30%-75%,atmospheric pressure from 700 to 1060 Hpa
-10°C to 50°C (14℉ to 122℉)with a relative humidity of
10%-90%,atmospheric pressure from 700 to 1060 Hpa
There may be a ±5% tolerance of all setting and ±10%
tolerance of output of intensity.
Adjustable, from 1 to 60 minutes or continuous, Adjustable
in 1minutes each step. Treatment time countdown
automatically.
Electrode
Detection
Function
The amplitude level will be reset to 0mA when the
amplitude level is 12mA or greater and an open circuit at
either channel is detected.

Technical specifications for Transcutaneous Electrical Nerve
p
Stimulator (TENS) mode
Waveform Mono-phase square pulse wave
Pulse amplitude
Pulse Width Adjustable, from 50 to 300us microseconds, 10μS/step
Pulse Rate Adjustable, from 1 to 150 Hz, 1 Hz/step
Burst (B)
Normal (N)
Pulse Width Modulation (M)
Pulse Rate Modulation (M1)
Adjustable, 0~105mA peak at 1000 ohm Load each
channel, 1mA/Ste
Burst rate: Adjustable, 0.5 ~ 5Hz
Pulse width adjustable,50-300uS
Frequency fixed=10Hz
The pulse rate and pulse width are adjustable. It
generates continuous stimulation based on the setting
value.
The pulse width is automatically varied in a cycle time.
The pulse width is decreased from its original setting to
60% in setting cycle time, and then increased from
60% to its original setting in nest setting cycle time. In
this program, pulse rate (1 to 150Hz), pulse width (50
to 300us) and cycle time (5 to 30 sec) are fully
adjustable.
The pulse rate is automatically varied in a cycle time.
The pulse rate is decreased from its original setting to
60% in setting cycle time, and then increased from
60% to its original setting in nest setting cycle time. In
this program, pulse rate (1 to 150Hz), pulse width (50
to 300us) and cycle time (5 to 30 sec) are fully
adjustable.
.

Technical specifications for Electrical Muscle Stimulation
(EMS) mode
Waveform: Mono-phase square pulse wave
Pulse amplitude
Pulse Width
Pulse Rate Adjustable, from 1 to 150 Hz, 1 Hz/step
Contraction time Adjustable, 1~60 seconds , 1 Sec./ step
Relaxation (OFF) time Adjustable, 0~60 seconds , 1 Sec./ step
Adjustable, 0~105mA peak at 1000 ohm Load each
channel, 1mA/Step.
Adjustable, from 50 to 300μS microseconds,
Ramp time
Synchronous (S)
Alternate (A)
Delay (D) The Stimulation of the CH2 will occur after CH1 is
Adjustable, 1~6 seconds, 1 Sec. / step, The “On” time
will increase and decrease in the setting value.
Stimulation of both channels occurs synchronously.
The “ON” time including “Contraction”, “Ramp Up” and
“Ramp Down” time. ON TIME=Contraction + Ramp up
+ Ramp down
The Stimulation of the CH2 will occur after the 1st
operation of CH1 is completed. In this program, The
“ON” time including “Contraction”, “Ramp Up” and
“Ramp Down” time. The OFF Time should be equal or
more than the ON Time
ON TIME=Contraction + Ramp up + Ramp down
OFF TIME ≥ ON TIME
started + Delay Time. In this program, The “ON” time
includes “Contraction”, “Ramp Up” and “Ramp Down”
time. ON TIME = Contraction + Ramp up + Ramp
down time. The OFF Time should be equal or more
than the ON Time + Delay Time. Time Delay time is
adjustable from 1 to 10 seconds in this program.

3.3 The waveforms of the stimulation programs
Burst (B)
Normal (N)
Pulse Width Modulation
Pulse Rate Modulation
cycle time

19
Synchronous(S)
Alternate (A)
Delay (D)
4 .1 Battery
4.1.1 Check/Replace the 9V ALKALINE battery
Over time, in order to ensure the
functional safety of device, the battery
must be periodically changed.
4. Instruction for use

4.1.2 Disposal of battery
20
Spent batteries do not belong in household waste.
Dispose of the battery according to the current federal,
state and local regulations.
Caution:
1) Battery may be fatal if swallowed. Therefore, keep the
battery and the product out of the range of children, if a
battery was swallowed, consult a physician immediately.
2) If a battery has leaked, avoid contact with skin, eyes and
mucus membranes, Rinse the affected spots with lots of
clear water immediately and contact a physician right
away.
3) Battery may not be charged, dismantled, thrown into fire
or short-circuited.
4) Protect battery from excess heat; Take the battery out of
the product if they are spent or in case you no longer use
the article. This prevents damage caused by leaking battery.
5) Always replace the same type battery.
4.2 Connect electrodes to lead wires
Insert the lead wire connector into
electrode connector (standard 0.08 inch
female connection). Make sure there are
no bare metal pins exposed.

Caution:
21
Always use the electrodes with the requirements of the
IEC/EN60601-1
as with CE mark, or which are legally marketed in the US
under 510(K) procedure.
4.3 Connect lead wires to device
1) Before proceeding to this step, be sure the device is completely
turned OFF.
2) The wires provided with the system insert
located on top of the device.
3) Holding the insulated portion of the connector, push the plug
end of the wire into one of the jacks (see drawing); one or two
sets of wires may be used.
4) This device has two output receptacles controlled by Channel 1
and Channel 2 at the top of the unit. You may choose to use
one channel with one pair of lead wires or both channels with
ISO10993-1/-5/-10 and IEC/ EN60601-1-2, such
into the jack sockets
two pairs of lead wires. Using both channels gives the user the
advantage of stimulating two different areas at the same time.
Caution:
Do not insert the plug of the
patient lead wire into any AC power supply socket.

4.4 Electrode
22
4.4.1 Electrode options
The electrodes are disposable and should be routinely replaced
when they start to lose their adhesive nature. If you are unsure
of your electrodes adhesive properties, order new replacement
electrodes. Replacement electrodes should be re-ordered
through or on the advice of your physician to ensure proper
quality. Follow application procedures outlined in electrode
packing, to maintain optimal stimulation and to prevent skin
irritation.
4.4.2 Place electrodes on skin
Apply electrodes to the exact site indicated by your physician or
therapist. Be sure the skin surface over which electrodes are
placed is thoroughly cleaned and dried. Make sure the electrodes
are placed firmly to the skin and make good contact between the
skin and the electrodes. Place the electrodes over the skin; attach
them properly, firmly, and evenly.
Caution:
1) Before applying the selfadhesive electrodes, it is
recommended to
wash and degrease the skin, and then dry it.
2) Do not turn on the device when the self-adhesive
electrodes are not positioned on the body.
3) Never remove the self-adhesive electrodes from the skin
while the device is still turned on.
4) It is recommended that, at minimum, 1.5” x 1.5” selfadhering electrodes are used at the treatment area

4.4.3 Electrode placement
The placement of electrodes can be one of the most important
parameters in achieving success with therapy. Of utmost
importance is the willingness of the physician to try the various
styles of electrode placement to find which method best fits the
needs of the individual patient.
Every patient responds to electrical stimulation differently and
their needs may vary from the conventional settings suggested
here. If the initial results are not positive, speak to your
physician about alternative stimulation settings and/or
electrode placements. Once an acceptable location has been
achieved, mark down the electrode sites and the device
settings, so the patient can easily continue treatment.
4.5 Turn on
Before using the device for the first time, you are strongly advised
to take careful note of the contraindications and safety measures
detailed at the beginning of this manual (Safety information), as
this powerful equipment is neither a toy nor a gadget! In order to
turn on the device, PRESS and RELEASE the [ ] button.The
operation page appears on the
screen.
4.6 Select the Therapeutic Mode
There are 2 therapeutic modes
available –TENS and EMS. The
therapeutic mode can be selected
by pressing the [M] control.

Caution:
24
Consult your physician for your suitable therapeutic mode
4.7 Steps to Set a New Program
4.7.1 TENS Setting
Press the [S] button cycle to enter the setting state. The settings
can be adjusted according to the following steps:
1) Set the Therapeutic Program
There are 4 programs in TENS therapeutic
mode available –Burst (B), Normal (N), Pulse
Width Modulation (M), and Pulse Rate
Modulation (M1). The therapeutic program can
be selected by pressing the [▲] and [▼]
button. When you choose to “B” program,
program [B] outside of the box will be flashing.
2) Set Cycle Time (Optional)
Cycle time is adjustable from 5 to 30 seconds. Only modulation
has this
and
3) Set Timer
Press [S] button cycle to enter this setting. The treatment time is
adjustable from 1 to 60 minutes or Continuous. Press [▲
button control to adjust setting. You can set the timer to
“Continuous” mode by pressing the [▲] control when it shows 60
minutes. The output will be shut off when time is up.
4) Set Pulse Width
Pulse Width is adjustable from 50 uS to 300 uS. Press [S] button to
enter this menu, then press [▲] or [▼]button to adjust the setting.
parameter setting. Press [S] button cycle to enter this menu,
then
press
the [▲] and [▼] button to adjusting the setting.
] or [
▼]

25
5) Set Pulse Rate
Pulse rate is adjustable from 1 Hz to 150 Hz (0, 5 Hz to 5 Hz for
Burst). Press [S] button cycle to enter this menu, and then press
[▲] or [▼]button to adjust the setting.
4.7.2 EMS Setting
Press the [S] button cycle to enter the setting
state. The settings can be adjusted
according to the following steps:
1) Set the Therapeutic Program
There are 3 programs in EMS therapeutic
mode available –Synchronous, Alternate and Delay,. The
therapeutic program can be selected by pressing the [▲] and
[▼]button. When you choose to [S] program, program [S] outside of
the box will be flashing.
2) Set Timer
Press [S] button cycle to enter this setting. The treatment time is
adjustable from 1 to 60 minutes or Continuous. Press [▲] or [▼]
control to adjust setting. You can set the timer to “Continuous” mode
by pressing the [▲] button when it shows 60 minutes. The output
will be shut off when time is up.
3) Set Pulse Width
The pulse width determines the length of time. Each electrical
signal is applied through the skin, which controls the strength and
sensation of the stimulation. Press [S] button cycle to enter this
setting. The pulse width is adjustable from 50 to 300 uS.
Press [▲] or [▼]button to adjust the setting.
4) Set Pulse Rate
The pulse rate determines how many electrical impulses are
applied through the skin each second. Press [S] button cycle to
enter this menu. By pressing the [▲] or [▼]button to adjusting the
setting. The pulse rate is adjustable from 1 Hz to 150 Hz.
5) Set Delay Time (Optional)
Delay time is adjustable from 1 to 10 seconds.

Only Delay therapeutic program has this parameter setting. Press [S]
button cycle to enter this menu, and then press the [▲] and [▼]
button to adjusting the setting.
6) Set Ramp Time
The ramp time controls the time of output current that increase from
0 to the
7) Set Contract Time
The contract Time controls the time of stimulation. The contraction
time can be adjusted. Press [S] button cycle to enter this menu, and
then press the [▲] and [▼] button to adjusting the setting. Both
channels’ stimulation is cycled on and off by the contraction and
relaxation settings. The range is adjustable from 1 to 60 seconds.
Caution:
Contract time does not include the ramp up and ramp down
time; ON time=Ramp up + Contract time + Ramp down.
8) Set Relaxation (OFF) time
The Off Time controls the time of relaxation. The relaxation time can
be adjusted. Press [S] button cycle to enter this menu, and then
press the [▲] and [▼] button to adjusting the setting. Both channels'
stimulation is cycled on and off by the contraction and relaxation
settings. The range is adjustable from 0 to 60 seconds.
In Alternate program, the OFF Time should be equal or more than
the ON Time. (OFF TIME ≥ON TIME)
4.8 Adjust Channel Intensity
Press the intensity control button ([▲] and [▼]) to control the
intensity output. Slowly press the intensity button control until you
reach the setting recommended by your physician or therapist.
Repeat for the other channel, if both channels are to be used.
Caution:
1) If the stimulation levels are uncomfortable or become
uncomfortable, reduce the stimulation intensity to a
comfortable level and contact your medical practitioner if
problems persist.
2) If the electrodes are not placed firmly on the skin or the

device is not connected to the electrodes, and the
27
stimulator’s output intensity surpasses 12mA, the amplitude
level will be reset to 0 mA automatically.
4.9. Safety Lock Feature
The Safety Lock Feature automatically activates after there is no
operation in the panel for 30 seconds by locking out the ability to
press the buttons.This is a safety feature to prevent accidental
changes to your settings and to prevent accidental increases to the
intensity levels.You can press either one of the button to unlock
the device.
4.10. Stop the treatment
When you have activated the treatment timer, you can press the [M]
button or the [
Caution:
Default state, if the button is locked, you can press only one
of the [ ] buttons to unlock, and then press the [M] button or
] button to control or stop the treatment.
the [ ] button to control stop the treatment.
4.11. Turn OFF
PRESS [ ] button and HOLD for approximately 3 seconds to
turn
Caution:
1) If there is no operation in the panel for 2 minutes in the
2) In shutdown state, keep pressing the channel 2[ ]first, and
OFF the device.
waiting state, the device will be turned off automatically.
then press [ ]button at the same to restore factory
parameter settings

4.12. Low battery indicator
28
When the low power indicator flashes, the device will be turned off
automatically, the battery should be replaced with a new one as
soon as possible. However, the unit may continue to operate for an
extended period depending on the setting intensity level.
5. Program
Mode Program Modulation
Method
TENS
EMS
B
N
M
M1
S
A
D
Burst
Continuous
Pulse width
modulation
Frequency
modulation
Synchronous
mode
Asynchronous
mode
Delay mode
Frequency Pulse
Width
0.5-5Hz
1-150Hz 50-300us 1-60min,continuous
1-150Hz 50-300us 1-60min,continuous
1-150Hz 50-300us 1-60min,continuous
1-150Hz 50-300us 1-60min,continuous
1-150Hz 50-300us 1-60min,continuous
1-150Hz 50-300us 1-60min,continuous
50-300us 1-60min,continuous
Treatment time

6. Cleaning and Care
6.1 Tips for skin care
To avoid skin irritation, especially if you have sensitive skin, follow
these suggestions:
ou will be placing the electrodes,
using mild soap and water before applying electrodes, and
after taking them off. Be sure to rinse soap off thoroughly and
dry skin well.
2) Excess hair may be clipped with scissors; do not shave
stimulation area.
3) Wipe the area with the skin preparation your clinician has
recommended. Let this dry. Apply electrodes as directed.
4) Many skin problems arise from the “pulling stress” from
adhesive patches that are excessively stretched across the
skin during application. To prevent this, apply electrodes from
center outward; avoid stretching over the skin.
5) To minimize “pulling stress”, tape extra lengths of lead wires
to the skin in a loop to prevent tugging on electrodes.
6) When removing electrodes, always remove by pulling in the
direction of hair growth.
6.2 Cleaning the device
1) Remove the battery from the device every time when you clean.
2) Clean the device after use with a soft, slight moistened cloth.
In case of more extreme soiling you can also moisten the cloth
with mild soapy water.
3) Do not use any chemical cleaners or abrasive agents for
cleaning.
6.3 Electrodes
1) Use the device only with the leads and electrodes provided by

the manufacturer. Use only the electrode placements and
stimulation settings prescribed by your physician or therapist.
2) It is recommended that, at minimum, 1.5” x 1.5” self-adhering
electrodes should be used at the treatment area.
3) Inspect your electrodes before every use. Replace electrodes
as needed. Reusable electrodes may cause slight skin irritation,
lose adhesion and deliver less stimulation if overused.
To use these electrodes:
To remove your electrodes:

Caution:
31
1)
Do not pull on the electrode wire. Doing so may damage
the wire and electrode.
2)
Do not apply to broken skin.
3)
The electrodes should be discarded when they are no
longer adhering.
The electrodes are intended for single patient use only.
4)
If irritation occurs, discontinue use and consult your
5)
clinician.
6)
Read the instructions for use of self-adhesive electrodes
before application.
7)
Always use the electrodes with the requirements of the
IEC/EN60601-1, ISO10993-1/-5/-10 and IEC/ EN60601-1-2,
such as with CE mark, or are legally marketed in the US
under 510(K) procedure.
6.4 Cleaning the Electrode's cords
Clean the electrode cords by wiping them with a damp cloth.
Coating them lightly with talcum powder will reduce tangles and
prolong the life.
6.5 Maintenance

7. Troubleshooting
f
r
r
32
If your device does not seem to be operating correctly, refer to
the chart below to determine what may be wrong. Should none
of these measures correct the problem, the device should be
serviced.
Proble
Display fails to light up Battery contact failure 1. Try fresh batteries.
m
Possible Cause Solutio
2. Ensure batteries are
inserted correctly. Check
the following:
• All contacts are in place
n
Stimulation weak
Stimulation is uncomfortable
Intermittent output Lead wires
Electrodes or Lead Wires
1. Dried out or contaminated
2. Placement of lead wires
3.Old/worn/damaged
electrodes or lead wires
Replace and re-connect
Replace
1.Decrease intensity.
2.Reposition the electrodes.
3.Replace.
4.Replace electrodes with
ones that have an active
area no less than 1.5” X 1.5”
1. Veri
2. Turn down the intensity.
3. If still intermittent after replacing
y connection is secure and
firmly seated.
Rotate lead wires in socket 90°.
If still intermittent, replace lead
wire.
Stimulation is ineffective. Improperelectrode and
Program option in use
applicator placement
Unknown
lead wire, a component may have
failed. Call the repair department.
Some programs will seem
intermittent. This is expected.
to the Program Option
Refe
Reposition electrode and applicato
Contact clinician.

8. Storage
33
1) For a prolonged pause in treatment, store the device in a
dry room and protect it against heat, sunshine and
moisture and remove the battery.
2) Store the device in a cool, well-ventilated place
3) Never place any heavy objects on the device.
9. Disposal
Used fully discharged batteries must be disposed of in a
specially labeled collection container, at toxic waste
collection points or through an electrical retailer.
Please dispose of the device in accordance with the legal
obligation.
10. Electromagnetic Compatibility (EMC) Tables
Guidance and manufacturer’s declaration - electromagnetic emissions
The device is intended for use in the electromagnetic environment specified below.
The customer or the user assures that it is used in such an environment.
Emissions test Compliance Electromagnetic environment – guidance
RF emissions
CISPR 11
RF emissions
CISPR11
Harmonic
emissions
lEC 61000-3-2
Voltage fluctuations
/ flicker emissions
lEC 61000-3-3
Guidance and manufacturer’s declaration — electromagnetic immunity
Group 1
Class B
Not applicable
Not applicable
The device uses RF energy only for its internal
function. Therefore, its RF emissions are very low
and are not likely to cause any interference in
nearby electronic equipment.
The device is suitable for use in all establishments
other than domestic and those directly connected to
the public low-voltage power supply network that
supplies buildings used for domestic purposes.
The device is intended for use in the electromagnetic environment specified below. The
customer or the user should assure that it is used in such an environment.
Immunity test IEC 60601
test level
Electrostatic
discharge (ESD)
lEC 61000-4-2
±6 kV contact
±8 kV air
Compliance
Level
±6 kV contact
±8 kV air
Electromagnetic environment -Guidance
Floors should be wood, concrete or
ceramic tile. If floors are covered with
synthetic material, the relative humidity
should be at least 30 %.

The de
v
s
y
c
0
d
4
I
2
d
h
d
o
o
a
s
t
a
a
d
t
r
a
t
f
e
c
m
M
y
a
r
e
h
a
n
0
r
t
s
p
n
s
t
a
s
a
n
o
s
v
c
r
upar
m
c
d
r
m
q
e
u
m
o
o
e
A
c
o
h
v
v
v
s
m
n
c
m
o
i
t
d
M
0
e
d
v
g
r
c
p
m
o
r
i
e
n
h
m
o
d
b
n
M
M
o
(
u
R
r
e
o
c
h
c
e
s
c
o
o
e
e
n
s
t
s
o
s
s
e
n
p
V
t
s
34
or theu
Guid
ice is inten
er should
nce and-
ded for use
ssure that i
anufacture
in. the elec
t is used in
’s declarati
romagnetic
uch an en
n. Electro
environme
ironment.
agnetic im
t specified
unity
below. The
customer
Immunit
Condu
lEC 61
Radiate
61000-
test
ted RF
00-4-6
RF lEC
-3
EC 60501 t
I
l
evel
Vrms 150
3
t
o 80 MHz
3
V/m80 MH
5 GHz
2
est Com
level
kHz
3 Vr
to
z
3 V/
liance
m
s
m
Ele
tromagneti
table and
Po
ipment sh
eq
t of the dev
rec
ommended
fro
the equat
of t
he transmit
Re
ommende
ere P is th
W
of t
he transmit
the
. Transmitt
rec
ommended
)
(m
Fie
ld strength
as
etermined
su
vey, should
co
pliance le
fre
uency ran
Int
rference m
eq
ipment ma
sy
bol:
uld be use
ion applica
, 80
, 80
maximum
ay occur In
environme
obile RF c
ce, includin
separation
er.
separatio
Hz to 800
MHz to 2,5
er In watts
r manufact
separation
from fixed
by an elect
be less tha
el in each
e.
ked with th
nt - guidan
mmunicati
no closer t
g cables, th
distance ca
le to the fr
distance
Hz
Hz
utput pow
W) accordi
rer and d I
distance in
F transmit
omagnetic
n the
the vicinity
following
e
ns
any
an the
lculated
quency
r rating
g to
the
meters
ers,
ite
f
NOTE
NOTE
affecte
1. Field
2. Over
telep
broa
envir
If the
RF c
norm
nece
At 80 MHz
These gui
by absorp
strengths f
ones and l
cast canno
nment due
measured
mpliance l
l operation
sary, such
he frequen
ends 800
elines ma
ion and refl
om fixed tr
nd mobile
be predict
to fixed RF
ield strengt
vel above,
. If abnorm
as reorienti
y range 15
Hz. the hig
not apply i
ection from
nsmitters,
adios, ama
d theoretic
transmitter
in the loc
should be o
l performa
g or reloca
kHz to 80
her frequen
all situati
structures,
uch as bas
eur radio,
lly with ac
, an electr
tion in whic
bserved to
ce is obser
ting the de
MHz, field
y range a
ns. Electro
bjects and
stations f
M and FM
uracy. To a
magnetic s
the devic
erify
ed, additio
ice.
trengths s
plies.
agnetic pr
people.
r radio (cell
adio broad
ssess the e
te survey s
is used ex
al measur
ould be les
pagation i
ular/cordle
ast and TV
lectromagn
ould be co
eeds the a
s may be
than [Vi]
s)
tic
sidered.
plicable
/m.

port
able and mo
Reco
ommended s
obile RF com
separation d
mmunication
distances be
ns equipmen
etween
nt and the de
evice
The dev
disturba
electrom
RF com
maximu
For tran
separat
frequen
watts (W
NOTE I
applies
propaga
absorpt
ances are co
magnetic int
mmunication
um output po
Ra
ated maximu
o
output powe
nsmitters rat
tion distance
ncy of the tra
W) accordab
I At 80 MHz
tion and refle
vice is inten
er
of
transmitter
W
0,01
0,1
1
10
100
. NOTE 2 Th
ation is affec
ded for use
ontrolled. Th
terference by
s equipmen
ower of the c
um
ted at a max
e d in meters
ansmitter, w
ble to the tra
and 800 MH
hese guideli
cted by
ection from
he customer
150 kHz to 8
ximum outpu
ansmitter ma
in an electro
y maintainin
t (transmitte
communicat
Se
80 80
0.12
0.38
1.2
3.8
12
s (m) can be
here P is the
Hz. the sepa
ines may no
structures, o
omagnetic e
r or the user
ng a minimu
ers) and the
tions equipm
eparation dis
MHz
M
ut power not
e estimated
e maximum
anufacturer.
aration dista
ot apply in al
objects and
environment
r of the devic
m distance
as recomme
ment.
stance acco
transmit
MHz to 800
t listed abov
using the eq
output pow
ance for the
ll situations.
people.
0
0.12
0.38
1.2
3.8
12
t in which ra
ce can help
between po
ended below
ording to freq
tter
Hz
MH
ve, the recom
quation app
wer rating of t
higher frequ
Electromag
800
diated RF
prevent
rtable and m
w, according
quency of
MHz to 2,5
0.23
0.73
2.3
7.3
23
mmended
plicable to th
the transmit
uency range
gnetic
mobile
g to the
GHz
e
tter in
11. G
lossary
of Sym
Batch code…
Serial numbe
Attention: Re
Meaning of t
electric device
w
waste after thei
an
nd take this de
which is resp
Degree of El
bols
……..0100000
er……50000001
ead the operat
he symbols on
es are recycla
ir useful life! H
evice to the ap
onsible for wa
ectrical Protec
1
ting instruction
n the product,
able material a
Help us to prot
ppropriate colle
aste disposal i
ction BF
n for use!
the packagin
and should not
tect the enviro
ection points.
n your area if
g or in the ope
t be disposed
onment and sa
Please contac
you have any
erating
of with
ave
ct the
y
questions.
instructions:
household
resources
organization
35

36
12. Warranty
Please contact your dealer in case of a claim under the
warranty. If you have to send the unit back to your provider,
enclose a copy of your receipt and state what the defect is.
The following warranty terms apply:
1) The warranty period for device is one year from date of
purchase. In case of a warranty claim, the date of purchase
has to be proven by means of the sales receipt or invoice.
2) Repairs under warranty do not extend the warranty period
either for the device or for the replacement parts.
3) The following is excluded under the warranty:
All damage which has arisen due to improper treatment, e.g.
nonobservance of the user instruction.
All damage which is due to repairs or tampering by the customer
or unauthorized third parities.
Damage which has arisen during transport from the
manufacturer to the consumer or during transport to the
service centre.
Accessories which are subject to normal wear and tear.
4) Liability for direct or indirect consequential losses caused by
the unit is excluded even if the damage to the unit is
accepted as a warranty claim.




Manufactured for:
Current SolutionsTM LLC
3814 Woodbury Drive
Austin,TX 78704
Ph:(800)871-7858
www.currentsolutionsnow.com
 Loading...
Loading...