
Operator’s Manual
TM
E L E C T R O S U R G I C A L U N I T

LIMITED WARRANTY
For a period of two years following the date of delivery,
CONMED Corporation warrants the CONMED System
5000™ Electrosurgical Generator against any defects in
material or workmanship and will repair or replace (at
CONMED’s option) the same without charge, provided
that routine maintenance as specified in this manual has
been performed using replacement parts approved by
CONMED. This warranty is void if the product is used in
a manner or for purposes other than intended.
© 2007 CONMED Corporation
525 French Road
Utica, New York 13502 U.S.A.
U.S. Patent Nos. 4,961,739 - 5,152,762 - 5,626,575-
6,830,569 - 6,835,082 - 6,875,210 - 6,939,347 -
D477,082 - D477,408.
For Technical Service or Return Authorization Phone:
303-699-7600 / 1-800-552-0138 Extension 5274
Fax 303-699-1628
For Customer Service or to order parts phone:
1-800-448-6506 / 315-797-8375 / Fax 315-735-6235
or contact your CONMED Representative.
European Authorized Representative
MDSS GmbH
Schiffgraben 41
D - 30175 Hannover
Germany
The revision level of this manual is specified by the
highest revision letter found on either the inside front cover
or enclosed errata pages (if any).
Manual Number 60-8016-ENG Rev. N 11/07
Unit Serial Number_________________________________
TM
Table of Contents
& List of Illustrations
Section Title Page
1.0 General Information .........................................................................1-1
1.1 Cautions ...................................................................................................... 1-1
1.1.1 Cautions For Equipment Preparation ................................................................................1-2
1.1.2 Cautions For Patient Preparation .......................................................................................
1.1.3 Cautions For Use ..............................................................................................................
1.1.4 Cautions For Testing or Servicing .....................................................................................1-5
1.1.5 Electromagnetic Compatibility ..........................................................................................
1.1.5.1 EN/IEC 60601-1-2 Table 201 ...........................................................................................
1.1.5.2 EN/IEC 60601-1-2 Table 202 ...........................................................................................
1.1.5.3 EN/IEC 60601-1-2 Table 204 ...........................................................................................
1.1.5.4 EN/IEC 60601-1-2 Table 206 ...........................................................................................
1.2 Specifications .............................................................................................. 1-9
1.2.1 Mains Overcurrent Protection ...........................................................................................1-9
1.2.2 Mains Frequency Leakage .................................................................................................1-9
1.2.3 Regulatory Compliance ...................................................................................................
1.2.4 Operation ........................................................................................................................
1.2.5 Power Display Accuracy ..................................................................................................
1.2.6 Line Regulation ..............................................................................................................1-10
1.2.7 Environmental .................................................................................................................
1.2.8 Contact Quality Monitor .................................................................................................
1.2.9 Audio Specifications ........................................................................................................
1.2.10 Other Specifications ........................................................................................................
1.2.11 Operating Modes and Nominal Output Parameters .........................................................1-11
1.3 Explanation of Symbols ............................................................................. 1-12
1.3.1 Control Panel ..................................................................................................................1-12
1.3.2 Interior ............................................................................................................................
1.3.3 Output/Control Panel ......................................................................................................1-12
1.3.4 Rear Panel .......................................................................................................................1-13
1.4 Output Characteristic Curves .................................................................... 1-14
2.0 Installation and Operation ................................................................ 2-1
2.1 Initial Inspection ......................................................................................... 2-1
2.2 Installation .................................................................................................. 2-1
2.2.1 Installation Of Fuses .........................................................................................................2-1
2.3 Preliminary Checks .....................................................................................2-1
2.3.1 Preliminary Functional Testing ..........................................................................................2-1
2.3.2 Preliminary Performance Testing ......................................................................................
2.4 Controls, Displays and Connectors .............................................................2-3
2.4.1 Control Panel ....................................................................................................................2-3
2.4.2 Output Panel .....................................................................................................................
2.4.3 Rear Panel .........................................................................................................................
2.5 Set Up For Use ........................................................................................... 2-7
2.6 Oper
2.6.1 General ...........................................................................................................................2-10
2.6.2 Monopolar Pulse Cut ......................................................................................................2-10
ation .................................................................................................. 2-10
1-2
1-4
1-6
1-6
1-7
1-8
1-9
1-10
1-10
1-10
1-10
1-10
1-10
1-11
1-12
2-3
2-5
2-6

Section Title Page
2.6.3 Monopolar Pulse Coag ....................................................................................................2-11
2.6.4 Fluids Specialty Mode .....................................................................................................
2.6.5 Lap Specialty Mode .........................................................................................................
2.6.6 Programming ..................................................................................................................
2.6.6.1 Storing Programs ............................................................................................................
2.6.6.2 Using Programs ..............................................................................................................
2.6.7 Remote Power Control
2.6.7.1 Changing Monopolar Power Remotely ...........................................................................
..................................................................................................2-11
2-11
2-11
2-11
2-11
2-11
2-12
2.7 User Maintenance ..................................................................................... 2-12
2.7.1 General Maintenance Information ................................................................................... 2-12
2.7.2 Cleaning ..........................................................................................................................
2.7.3 Periodic Inspection ..........................................................................................................
2.7.4 Periodic Performance Testing ..........................................................................................
2-12
2-12
2-12
2.8 In Case of Difficulty .................................................................................. 2-12
2.8.1 Dispersive Electrode Alarm .............................................................................................2-12
2.8.1.1 Single Dispersive Electrode Alarm ...................................................................................
2.8.1.2 Dual Dispersive Electrode Alarm ....................................................................................
2.8.2 Acc Codes .......................................................................................................................
2.8.3 Err Codes ........................................................................................................................
2.8.4 If All Else Fails ................................................................................................................
2-12
2-13
2-13
2-13
2-13
2.9 Environmental Protection .........................................................................2-13
Figure/Title Page
Figure 1.1 Output Power vs. Power Setting ........................................................................................1-14
Figure 1.2 Display vs. Open Circuit Peak Voltage ...............................................................................
Figure 1.3 Load Regulation, Monopolar Pure Cut .............................................................................
Figure 1.4 Load Regulation, Monopolar Blend 1 ...............................................................................
Figure 1.5 Load Regulation, Monopolar Blend 2 ...............................................................................
Figure 1.6 Load Regulation, Monopolar Blend 3 ...............................................................................
Figure 1.7 Load Regulation, Monopolar Pinpoint Coag .....................................................................
Figure 1.8 Load Regulation, Monopolar Standard Coag ....................................................................
Figure 1.9 Load Regulation, Monopolar Spray Coag .........................................................................
Figure 1.10 Load Regulation, Bipolar Micro ......................................................................................
Figure 1.11 Load Regulation, Bipolar Macro .....................................................................................
Figure 1.12 Load Regulation, Lap Spray ............................................................................................
Figure 1.13 Load Regulation, Lap Standard .......................................................................................
Figure 2.1 Control Panel .......................................................................................................................
Figure 2.2 Output Panel .......................................................................................................................
Figure 2.3 Rear Panel ...........................................................................................................................
Figure 2.4 Accessory Schematics .........................................................................................................
Figure 2.5 Accessory Connections ......................................................................................................
1-14
1-15
1-15
1-16
1-16
1-17
1-17
1-18
1-18
1-19
1-19
1-20
2-4
2-6
2-7
2-14
2-14
TM
General Information
Section 1.0
This manual provides the set up and operating
instructions for the System 5000™ Electrosurgical
Unit (ESU). Electrosurgery can be dangerous
to patients, staff and other equipment if mis
used. Please understand and follow the warnings
and cautions that are included in this manual.
Technical specifications, performance characteristic
curves and user maintenance instructions are also
included.
The System 5000™ provides a broad range
of capabilities in a single, general-purpose
electrosurgical generator. This rugged ESU fulfills
the operational and safety needs of the modern
operating room by providing:
• Four monopolar cutting modes: Pure, Blend
1, Blend 2 and Blend 3.
• Three monopolar coagulation modes: Spray,
Standard and Pinpoint.
• Two bipolar modes: Micro and Macro
• Two specialty modes and a general surgery
mode:
• General Mode provides full power perfor
mance for open surgical procedures.
• Fluids Specialty Mode provides immediate
energy delivery for procedures performed
in a fluid medium.
• Laparoscopic Specialty Mode provides
optimal safety by limiting output voltage
and minimizing the potential harmful
effects of capacitive coupling.
• Pulse Cut Mode provides precise modulated
energy delivery for critical dissection.
• Pulse Coagulation Mode provides a modulated
waveform for unsurpassed precision and con
trol.
• Radio Frequency (RF) isolated and indepen
dent outputs.
• The proven Automatic Return Monitor
(A.R.M.™) contact quality monitoring system.
• Continuous microprocessor safety monitoring.
-
-
-
-
Features include:
• Dynamic Response Technology delivers opti
mal clinical effects in all operational modes
through the continuous synchronization of
current and voltage.
• Bipolar Output Meter provides visual and
audible feedback to surgeon during tubal liga
tions, vasectomies, and other procedures.
• ReadiPlug™ universal accessory receptacle
eliminates the need for foot-controlled adapters.
• Nine programmable memory settings provide
set-up convenience.
• Automatic programming restores the ESU to
the last settings used.
• Remote Power Control (PC) allows power set
ting changes using standard hand-controlled
pencils.
• Independent power setting available for all
modes.
• Ability to change power settings from the con
trol panel while the ESU is activated.
• Two handswitched receptacles and a separate
footswitched receptacle enable multiple acces
sory connections.
• Simultaneous activation in non-contact
monopolar coagulation modes.
• Channeled accessory receptacles direct plugs
into position, making attachments less cum
bersome.
• Illuminated receptacles for greater visibility.
• Integrated operating room control system
capability.
• Integrated interface for activation of smoke
evacuators and similar devices.
-
-
1.1 Cautions
This equipment, in conjunction with connected
accessories, is intended to produce high-frequency
electrical energy for the controlled destruction of
tissue.
-
-
-
-
1-1

Safe and effective electrosurgery is dependent not
only on equipment design, but also on factors
under the control of the operator. It is important
that the instructions supplied with this equipment
be read, understood and followed in order to
ensure safe and effective use of the equipment.
1.1.1 Cautions For Equipment Preparation
• Use only accessories that comply with the rel
evant regulatory standards for your location
and meet the requirements of Section 1.2,
Section 1.4, and Figure 2.4. Use of other
accessories may result in increased emissions
or decreased immunity of the ESU.
• Reusable accessory cables should be periodically function and safety tested in accordance
with the original manufacturer’s instructions.
• Visually inspect all accessories before each use
to verify the integrity of insulation and the
absence of obvious defects. In particular, elec
trode cables and endoscopic accessories should
be checked for damage to the insulation.
• The System 5000™ is equipped to connect
three monopolar accessories at one time for
the convenience of the surgical staff. Unused
accessories should be stowed in a safe, electri
cally insulated place such as a non-conductive
holster, isolated from the patient. CONMED
recommends accessories not be connected
unless needed.
• Never connect more than one accessory at
a time to any one receptacle, not including
the dispersive electrode receptacle when the
appropriate CONMED adapter is used.
• Use only a hospital grade, 3-prong, power
cord rated to meet the specifications in
Section 1.2 and all of the requirements for
safe grounding of the ESU. The user should
verify that the power receptacle with which
this ESU is used is properly grounded, cor
rectly polarized and of the proper frequency
per Section 1.2. Do not use ground cheater
plugs or extension cords.
• Do not place liquid containers on top of
the ESU. Wipe spilled liquids off the ESU
immediately. To preclude inadvertent entry of
liquids, do not operate this ESU except in its
normal position.
• Do not stack other devices or equipment on
top of or adjacent to the System 5000™.
The CONMED Stacking Adapter (Cat. No.
-
-
60-8030-001) allows two System 2500™ or
System 5000™ units to be stacked in a safe
manner.
• Confirm all accessories are properly connected
to the appropriate receptacles before powering
the ESU.
• Potentially hazardous conditions may exist
when accessories of similar connector types
are combined. Be certain accessories are
appropriate for the type of generator output
used. Use only CONMED Electrosurgery
footswitches. Confirm bipolar leads are
connected only to the bipolar receptacles.
Connecting bipolar accessories to monopolar
outputs may result in patient injury.
• Do not reuse disposable (single use) accesso
ries.
• Do not use cords as handles as damage to the
-
-
insulation and increased risk of burns or other
injury may result.
• A failure in the ESU could cause an unintend
ed increase in output power. Verify that the
ESU is functioning correctly prior to use.
• Prior to use, verify that devices connected
to the Activation Relay Connector function
properly in a manner that is synchronized
with ESU power delivery.
• Equipment connected to the Serial Interface
Connector must be approved by CONMED
and must be connected in accordance with
instructions accompanying the equipment.
Verify proper operation prior to ESU use.
1.1.2 Cautions For Patient Preparation
• Electrosurgery should NEVER be performed
in the presence of flammable anesthetics,
flammable prep solutions or drapes, oxidiz
ing gases such as Nitrous Oxide (N
oxygen-enriched environments. The risk of
igniting flammable gases or other materials is
inherent in electrosurgery and cannot be elim
inated by device design. Precautions must
be taken to restrict flammable materials and
substances from the electrosurgical site. They
may be present in the form of an anesthetic,
life support, skin preparation agent, produced
by natural processes within body cavities or
originate in surgical drapes, tracheal tubes or
other materials. There is a risk of pooling
of flammable solutions in body depressions
such as the umbilicus and in body cavities,
O) or in
2
-
-
-
-
1-2

such as the vagina. Any fluid pooled in these
areas should be removed before the high fre
quency surgical equipment is used. Due to
the danger of ignition of endogenous gases,
the bowel should be purged and filled with
nonflammable gas prior to abdominal surgery.
To avoid the risk of tracheal fires, never use
electrosurgery to enter the trachea during tra
cheotomy procedures.
• The System 5000™ mobile pedestal is made of
nonconductive plastic, that can hold a static
charge. It should not be used in a flammable
environment, as described above.
• Only non-flammable agents should be used for
cleaning and disinfection wherever possible.
• Exercise care when relocating the ESU to
avoid electrostatic charge buildup in the pres
ence of flammable materials, as there is a risk
of igniting these materials if a spark should
occur.
• This ESU is equipped with the Automatic
Return Monitor (A.R.M.™), which monitors
the quality of the dispersive electrode con
nection. When a correctly functioning single
dispersive electrode is connected to the ESU,
A.R.M.™ verifies the connections between the
ESU, the dispersive electrode cable and the
dispersive electrode. It DOES NOT verify
that a single dispersive electrode is in contact
with the patient. When using a dual disper
sive electrode, A.R.M.™ confirms the total
resistance is within the preset safety range.
Proper application and visual inspection of the
dispersive electrode is required for safe opera
tion.
• The use and proper placement of a dispersive
electrode is a key element in safe and effec
tive electrosurgery. Follow manufacturer’s
directions and recommended practices for the
preparation, placement, use, surveillance and
removal of any dispersive electrode supplied
for use with this electrosurgical unit.
• Apply the dispersive electrode over a well-vas
cularized muscle mass that is thoroughly clean
and dry. Clean and clip site, as necessary, to
provide adequate electrical connection and per
hospital policy. Avoid placement over scar tis
sue, bony prominences or other areas where
pressure points on small areas might develop.
• Because of the risk of burns, needles should
never be used as a dispersive electrode for
-
-
-
-
-
-
-
-
electrosurgery. The entire area of the disper
sive electrode should be placed so that the
entire conductive area is in firm contact with
an area of the patient’s body that has a good
blood supply and is as close to the operative
site as possible. In general, electrosurgical
current paths should be as short as possible
and should run either longitudinally or in a
diagonal direction to the body, not laterally
and under no circumstances lateral to the tho
rax.
• Dispersive electrodes and probes of monitor
ing, stimulating and imaging devices can
provide paths for high frequency currents
even if they are battery powered, insulated
or isolated at 50/60 Hz. The risk of burns
can be reduced but not eliminated by placing
the probes as far away as possible from the
electrosurgical site and the dispersive elec
trode. Protective impedances incorporated
in the monitoring leads may further reduce
the risk of these burns. Needles should not
be used as monitoring electrodes during
electrosurgical procedures.
• When high frequency surgical equipment
and physiological monitoring equipment are
used simultaneously on the same patient, all
monitoring electrodes should be placed as far
as possible from the surgical site and disper
sive electrode. Needle monitoring electrodes
are not recommended. Monitoring systems
incorporating high frequency current limiting
devices are recommended whenever possible.
• The active electrode should not be used in the
vicinity of electrocardiograph electrodes.
• Heat applied by thermal blankets or other
sources is cumulative with the heat pro
duced at the dispersive electrode (caused by
electrosurgical currents). Choosing a disper
sive electrode site that is remote from other
heat sources may minimize risk of a patient
injury.
• Electrosurgery, by its nature produces signifi
cant levels of electromagnetic interference
(EMI) when the ESU is activated. This EMI
may damage or impair the function of other
-
electronic equipment in the operating room,
especially equipment that makes contact
with the patient. Adverse effects can only be
mitigated by use of equipment specifically
designed to tolerate electrosurgical interfer
-
-
-
-
-
-
-
-
-
1-3

ence. Cables subject to flexing should be
inspected frequently for shielding integrity.
• Other equipment in the operating room,
including portable or mobile communica
tions equipment, may produce EMI, which
can affect the function of the ESU. Adverse
effects can only be mitigated by use of equip
ment with EMI characteristics proven below
recognized limits. In the event of suspected
interference from other equipment, discon
tinue use of the ESU until the problem can be
remedied.
• The patient should not be allowed to come
into contact with metal items that are ground
ed or have an appreciable capacitance to earth.
Examples of this would be operating tables,
supports, etc.
• Jewelry and other metallic items can cause
localized burns if they make contact with
grounded items and should be removed from
the patient prior to use of electrosurgery.
• Skin to skin contacts, such as between the arm
and body of a patient or between the legs and
thighs, should be avoided by the insertion of
dry gauze.
• The use of electrosurgery on patients with
cardiac pacemakers, AICDs, neurostimulators
or other active implants is potentially hazard
ous. The implant may be irreparably dam
aged and/or the high frequency energy of the
electrosurgical output may interfere with the
function of the implant. Ventricular fibrilla
tion may occur. Precautions should be taken
to ensure the patient’s well-being is main
tained in the event of such interaction. The
manufacturers of the implants should be con
sulted for advice before operating on a patient
with an implant. These precautions also apply
to operating room personnel with similar
implants.
• To minimize the possibility of cardiac pace
maker interference, place the dispersive elec
trode such that the electrosurgical current
path does not intersect the path of the pace
maker or leads.
1.1.3 Cautions For Use
• Safe and effective electrosurgery is dependent
not only on equipment design, but also on
factors under the control of the operator. It
is important that the instructions supplied
-
-
with this equipment be read, understood and
followed in order to ensure safe and effective
use of the equipment. Only properly quali
fied and trained operators should perform
electrosurgery. The operator and their sup
port personnel must be diligent in assuring
-
-
-
-
-
-
-
-
-
-
that the ESU is properly configured and that
proper settings are used. The ESU must be
located to assure the operator or their support
personnel can readily verify the settings.
• PLEASE NOTE: Federal law (U.S.A.)
requires that all health care facilities must
report to the manufacturer of a medical
device, any death or serious injury or illness
to a patient related to the use of a medical
device. Serious injuries or illness involving
the use of a medical device must be reported
to the manufacturer of the device (or to the
FDA if the manufacturer of the device is not
known) within 10 working days of the inci
dent. Summary reports of such injuries must
also be submitted directly to the FDA twice
a year. Patient deaths related to the use of a
medical device must be reported to the manu
facturer and the FDA. For further informa
tion, please contact the Regulatory Affairs
Department of CONMED Electrosurgery at
800-552-0138, 303-699-7600 or FAX 303699-9854.
• Do not use monopolar electrosurgery on small
appendages, as in circumcision or finger sur
gery, as it can cause thrombosis and other
unintended injury to tissue proximal to the
surgical site. Should the surgeon decide that
the bipolar electrosurgical technique is accept
able for circumcision, do not apply the bipolar
electrosurgical current directly to circumcision
clamps.
• Apparent low power output or failure of the
electrosurgical equipment to provide the
expected effect at otherwise normal settings
may indicate faulty application of the disper
sive electrode, failure of an electrical lead or
excessive accumulation of tissue on the active
electrode. Do not increase power output
before checking for obvious defects or misap
plication of the dispersive electrode. Check
for effective contact of the dispersive electrode
to the patient anytime the patient is moved
after initial application of the dispersive elec
trode.
-
-
-
-
-
-
-
-
-
-
1-4

• Studies have shown that smoke generated during electrosurgical procedures may be harmful
to surgical personnel. These studies recom
mend using a surgical mask and adequate ven
tilation of the smoke using a surgical smoke
evacuator or other means.
• In the event that the system resets due to a
power interruption or low voltage, check the
contact of the dispersive electrode prior to
resuming electrosurgery.
• If a dispersive electrode or A.R.M.™ alarm is
sounded intraoperatively, physically confirm
proper dispersive electrode attachment to the
patient and confirm that the display falls with
in the set range. Smooth the dispersive elec
trode surface with hand to ensure electrode
contact to patient skin. Replace the dispersive
electrode if necessary.
• Simultaneous activation can be used in both
Standard and Spray monopolar coagulation
modes. Caution should be used as the output
from either active electrode may change as a
result of activation of a second output or end
ing activation of an output. Power sharing is
unlikely to be equal because of differences in
electrode to tissue distance and other factors.
This unequal power sharing can be enough
to stop power delivery to one electrode if the
second electrode is close to tissue and the first
electrode is somewhat above the tissue. The
motion or deactivation of one electrode can
cause the other electrode to start delivering
power when it had been too far away from
tissue to arc before the first electrode change.
Simultaneous activation can also increase
leakage currents, which can be hazardous to
the patient. It is recommended that a second
electrosurgical generator be used when it is
necessary to perform simultaneous operation.
• The cables to the surgical electrodes (active,
bipolar or dispersive electrodes) should be
positioned in such a way that contact with the
patient or other leads is avoided.
• Confirm the desired specialty mode is selected
prior to use to ensure output characteristics
are suitable for the intended procedure.
• Confirm the desired bipolar mode is selected
prior to use to ensure output characteristics
are suitable for the intended procedure.
-
-
• The output power selected should be as low
as possible and activation times should be as
short as possible for the intended purpose.
-
-
-
• The clinical use of electrosurgery is intermit
tent in nature. This ESU should not be acti
vated continuously for extended periods of
time.
• When uncertain of the proper setting for the
power level in a given procedure, start with a
low setting and increase as required.
• Confirm that Pulse Cut Mode is properly
selected prior to activation to ensure that
improper application does not result in
patient injury. Set the monopolar coag power
to 0 when using Pulse Cut to ensure that an
accidental activation of coag does not cause
patient injury. Listen for the distinct Pulse
Cut Activation Tone during activation to con
firm that Pulse Cut is indeed active.
• Observe all caution and warning symbols
printed on the ESU.
• The operating room staff should never contact
electrosurgical electrodes (either active or dis
persive) while the RF output of the ESU is
energized.
• The electrodes of recently activated accessories may be hot enough to burn the patient
or ignite surgical drapes or other flammable
material.
• Do not ignore unexpected tones. Check to
determine the cause of the tone, otherwise
injury can occur.
• Temporarily unused active electrodes should
be stored in an electrically insulated holster.
The unused active electrode should never
be placed on the patient. This is especially
important for laparoscopic procedures.
• Ensure that the footswitches are not inadver
tently depressed in order to prevent acces
sories from being unintentionally activated.
Place footswitches in locations that necessi
tate deliberate action in order to activate the
footswitch. Use caution when selecting the
correct footswitch to activate.
1.1.4 Cautions For Testing or Servicing
• Service should not be attempted without refer
ence to the System 5000™ service manual
(Catalog Number 60-8017-ENG).
-
-
-
-
-
-
-
-
1-5
• This electrosurgical unit should be tested by
a Hospital Qualified Biomedical Technician
on a periodic basis to ensure proper and safe
operation. It is recommended that examina
-
tion of the ESU be performed at least yearly.
• Refer all servicing to a Hospital Qualified
Biomedical Technician. Your CONMED sales
representative will be happy to assist you in
getting your equipment serviced.
• High voltages are developed within the ESU
that are accessible when the top cover is
removed. These voltages are potentially dan
gerous and should be treated with extreme
caution.
• The high voltage DC power supply in the
System 5000™ is equipped with a bleeder
resistor to dissipate the charge on the filter
capacitor. However, it takes several seconds
after power is removed to bleed that charge
down to a safe level. It is recommended that
at least thirty (30) seconds be allowed to
elapse before touching or attempting to per
form any maintenance involving the power
supply or power amplifier.
• Never remove or install any parts with the
power cord connected to AC mains.
• Avoid contact with the output leads when the
ESU is activated. Periodically inspect the
test leads used for the output connections for
obvious defects.
• Although this ESU will withstand momentary
short circuits on the output, prolonged short
circuits may damage the ESU. Short-circuit
ing the output should be avoided since it is
neither necessary nor desirable.
• Since the clinical use of electrosurgical units
is intermittent in nature with duty cycles on
the order of 10%, this ESU is not designed
to operate for extended periods of continu
ous output. When testing, it is recommended
that duty cycles be limited to 10 seconds
-
activation with delays of 30 seconds between
activations.
• Activating the System 5000™ in other than its
normal operating position impairs the heat
dissipation capability of the heat sink.
• Ensure that the two top cover screws are tight
ened and always perform a power-up check to
confirm a normal power-up sequence before
returning the ESU to service.
• Improperly connecting test equipment can
cause electric shock and destruction of equip
-
ment.
• Turn unit off and wait until storage capacitors
have discharged before connecting test equip
-
ment.
• Loss of power supply isolation can cause elec
trical shock. When servicing the high voltage
power supply, assume internal isolation is
compromised until verified otherwise.
1.1.5 Electromagnetic Compatibility
Following are guidance and manufacturer’s declarations regarding electromagnetic compatibility for the
System 5000™.
1.1.5.1 EN/IEC 60601-1-2 Table 201
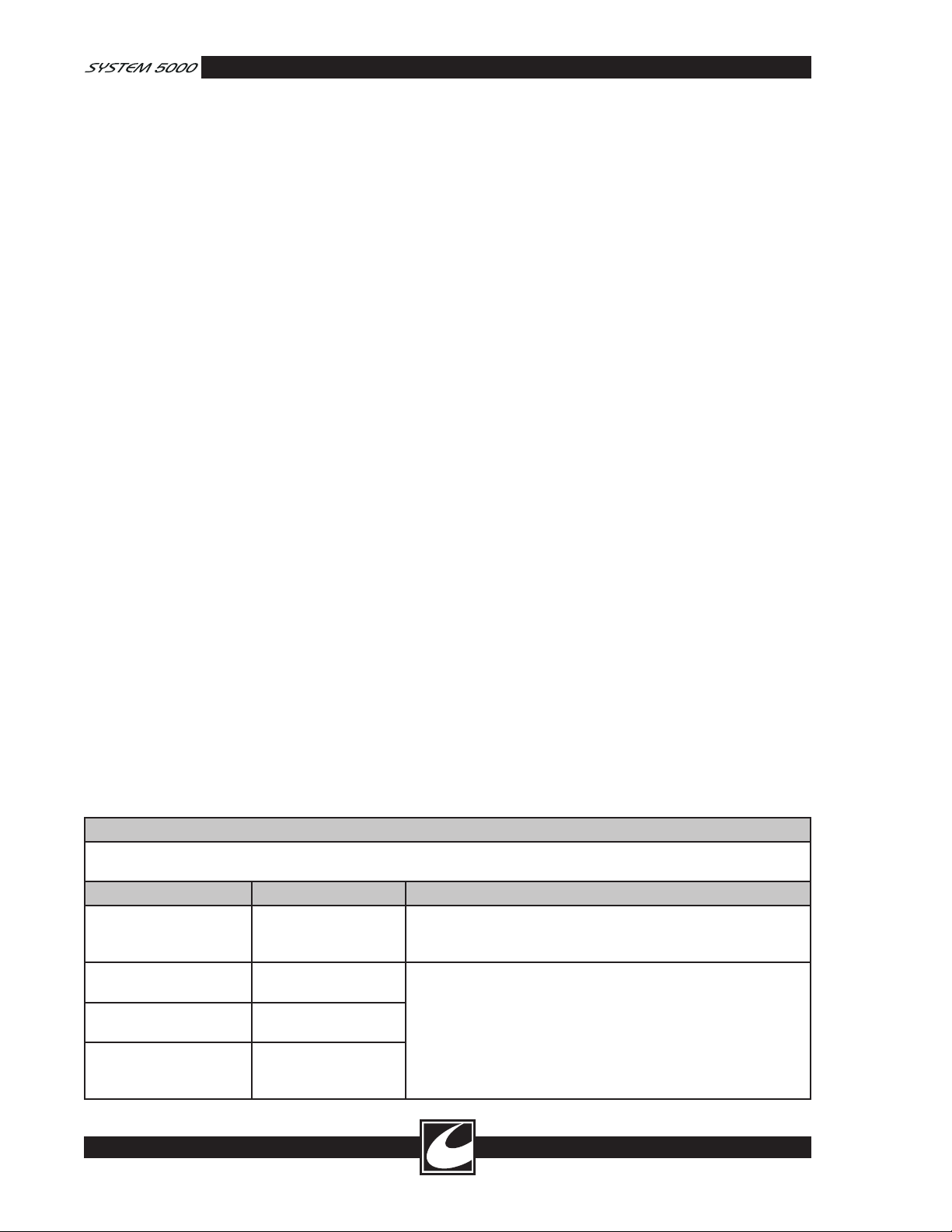
Guidance and Manufacturer’s Declaration – Electromagnetic Emissions
The System 5000™ Electrosurgical Unit is intended for use in the electromagnetic environment specified below. The
customer or the end user of the System 5000 Electrosurgical Unit should assure that it is used in such an environment.
Emissions test Compliance Electromagnetic environment - guidance
RF emissions
CISPR 11
RF emissions
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/
Flicker emissions
IEC 61000-3-3
Group 2 The System 5000 Electrosurgical Unit must emit electromagnetic
energy in order to perform its intended function. Nearby elec
tronic equipment may be affected.
Class A The System 5000 Electrosurgical Unit is suitable for use in all
establishments, other than domestic establishments and those
Class A
Complies
directly connected to the public low-voltage power supply net
work that supplies buildings used for domestic purposes.
-
-
1-6
1.1.5.2 EN/IEC 60601-1-2 Table 202
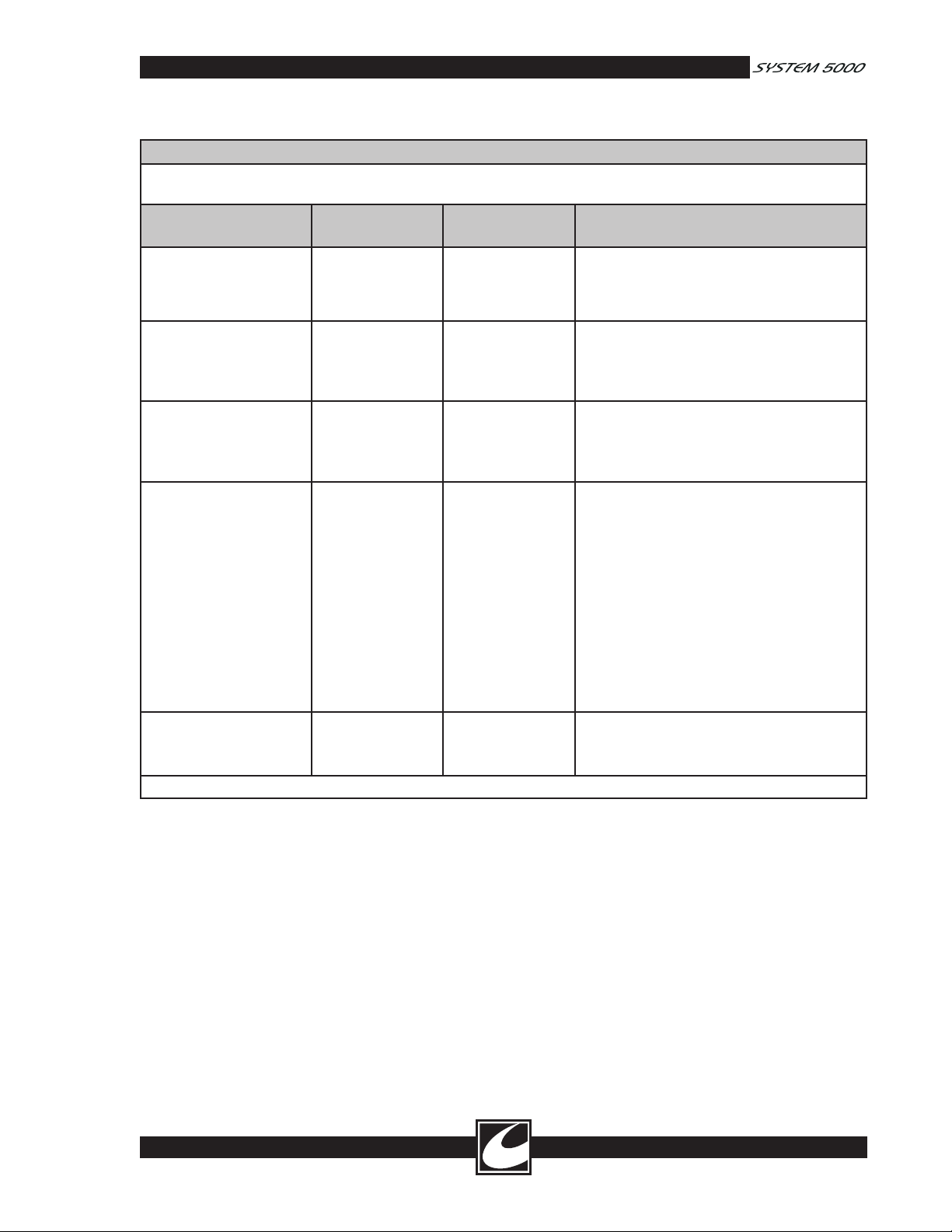
Guidance and Manufacturer’s Declaration – Electromagnetic Immunity
The System 5000™ Electrosurgical Unit is intended for use in the electromagnetic environment specified below. The
customer or the end user of the System 5000 Electrosurgical Unit should assure that it is used in such an environment.
Immunity Test IEC60601 test
level
Electromagnetic discharge
(ESD)
IEC 61000-4-2
Electrical fast transient/
burst
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage dips, short inter
ruptions and voltage
variations on power sup
ply input lines
IEC 61000-4-11
Power frequency (50/60
Hz) magnetic field
IEC 61000-4-8
NOTE UT is the a.c. mains voltage prior to application of the test level.
±6 kV contact
±8 kV air
±2 kV for power
supply lines
±1 kV for input/
output lines
±1 kV differential
mode
±2 kV common
mode
-
<5 % UT
(>95 % dip in UT)
-
for 0.5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
<5 % UT
(>95 % dip in UT)
for 5 sec
3 A/m 3 A/m Power frequency magnetic fields should be at
Compliance
level
±6 kV contact
±8 kV air
±2 kV for power
supply lines
±1 kV for input/
output lines
±1 kV differential
mode
±2 kV common
mode
<5 % UT
(>95 % dip in UT)
for 0.5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
<5 % UT
(>95 % dip in UT)
for 5 sec
Electromagnetic environment -
guidance
Floors should be wood, concrete or ceramic
tile. If floors are covered with synthetic mate
rial, the relative humidity should be at least
30%.
Mains power quality should be that of a typi
cal commercial or hospital environment.
Mains power quality should be that of a typical commercial or hospital environment.
Mains power quality should be that of a typi
cal commercial or hospital environment. If
the user of the System 5000 Electrosurgical
Unit requires continued operation during
power mains interruptions, it is recommended
that the System 5000 Electrosurgical Unit be
powered from an uninterruptible power sup
ply or a battery.
levels characteristic of a typical location in a
typical commercial or hospital environment.
-
-
-
-
1-7
1.1.5.3 EN/IEC 60601-1-2 Table 204
Guidance and Manufacturer’s Declaration – Electromagnetic Immunity
The System 5000™ Electrosurgical Unit is intended for use in the electromagnetic environment specified below. The
customer or the end user of the System 5000 Electrosurgical Unit should assure that it is used in such an environment.
Immunity Test IEC60601 test
level
Compliance
level
Electromagnetic environment - guidance
Portable and mobile RF communications equipment
should be used no closer to any part of the System
5000™ Electrosurgical Unit, including cables, than the
recommended separation distance calculated from the
equation applicable to the frequency of the transmitter.
Recommended separation distance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
a
b
3 Vrms
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) tele
phones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast
cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due
to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured
field strength in the location in which the System 5000™ Electrosurgical Unit is used exceeds the
applicable RF compliance level above, the System 5000™ Electrosurgical Unit should be observed
to verify normal operation. If abnormal performance is observed, additional measures may be
necessary, such as reorienting or relocating the System 5000™ Electrosurgical Unit.
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than [v1] V/m.
3 Vrms
3 V/m
d = 1.2√P
d = 1.2√P 80 MHz to 800 MHz
d = 2.3√P 800 MHz to 2.5 GHz
where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and
tance in meters (m).
Field strengths from fixed RF transmitters, as determined
by an electromagnetic site survey,
compliance level in each frequency range.
Interference may occur in the vicinity of equipment
marked with the following symbol:
d is the recommended separation dis-
a
should be less than the
b
-
1-8
 Loading...
Loading...