Page 1

123
Page 2

Page 3

TABLE OF CONTENTS
Introduction....................................................................................................................................................................4
Indications for Use........................................................................................................................................................4
Safety Warning..............................................................................................................................................................4
Contraindications...........................................................................................................................................................4
Warnings..........................................................................................................................................................................4
Precautions.....................................................................................................................................................................5
Adverse Reactions.........................................................................................................................................................7
Symbol and Title............................................................................................................................................................8
Environmental Condition for Transport and Storage..................................................................................................9
Electromagnetic Compatibility....................................................................................................................................9
How the Device Works................................................................................................................................................14
Setup..............................................................................................................................................................................19
Operating Instruction..................................................................................................................................................20
Performance Specifications......................................................................................................................................20
Cleaning and Maintenance.........................................................................................................................................21
Trouble Shooting..........................................................................................................................................................21
Recommended Use Positions......................................................................................................................................23
Contact Information....................................................................................................................................................24
Page 4

INTRODUCTION
Compex LT, a transcutaneous electrical nerve stimulation (TENS) pain management device delivers
electric pulses to the user’s body through electrodes to block and relieve pain. The portable and compact
device features 12 modes with differing frequencies to target fatigued and sore muscles and helps in
relieving aches and pains in various parts of the body such as the waist, shoulders, joints, hands and feet.
INDICATIONS FOR USE
To be used for temporary relief of pain associated with sore and aching muscles in the shoulder, waist,
back, arm, and leg, due to strain from exercise or normal household and work activities
SAFETY WARNING
CONTRAINDICATIONS
» Do not use this device on patients who have a cardiac pacemaker, implanted defibrillator, or other
implanted metallic or electronic device, because this may cause electric shock, burns, electrical
interference, or death.
» Do not use this device on patients whose pain syndromes are undiagnosed.
WARNINGS
» Do not apply stimulation over the patient’s neck because this could cause severe muscle spasms
resulting in closure of the airway, difficulty in breathing, or adverse effects on heart rhythm or blood
pressure.
» Do not apply stimulation across the patient’s chest, because the introduction of electrical current into
4
Page 5

the chest may cause rhythm disturbances to the patient’s heart, which could be lethal.
» Do not apply stimulation over, or in proximity to, cancerous lesions.
» Do not apply stimulation when the patient is in the bath or shower.
» If you have one of the following conditions, please consult with your physician before purchasing or
using this device.
» Acute disease, malignant tumor, infective disease, pregnant, heart disease, high fever, abnormal
blood pressure, lack of skin sensation or an abnormal skin condition, any condition requiring the active
supervision of a physician
PRECAUTIONS
» Do not use this device while driving.
» Do not use this device while sleeping.
» Do not use this device in high humidity areas such as a bathroom.
» Keep the device away from wet, high temperature and direct-sunlight.
» Keep this device out of reach of children.
» Stop using this device at once if you feel pain, discomfort, dizziness or nausea and consult your
physician.
» Do not attempt to move the electrode pads while the device is operating.
» Do not use the device around the heart, on the head, mouth, pudendum or blemished skin areas.
» Do not apply stimulation of this device in the following conditions:
» (1) Across the chest because the introduction of electrical current into the chest may cause rhythm
disturbances to the heart, which could be lethal.
» (2) Over painful areas. Please consult with your physician before using this device if you have
painful areas.
» (3) Over open wounds or rashes, or over swollen, red, infected, or inflamed areas or skin eruptions
(e.g., phlebitis, thrombophlebitis, varicose veins). Apply stimulation only to normal, intact, clean, healthy
skin.
5
Page 6

» (4) In the presence of electronic monitoring equipment (e.g., cardiac monitors, ECG alarms).
The electronic stimulator may not operate properly when the electrical stimulation device is in use.
» (5) While operating machinery, or during any activity in which electrical stimulation can put
you at risk of injury.
» (6) On children
BE AWARE OF THE FOLLOWING.
» (1) Consult with your physician before using this device. The stimulation of the device may:
» i. cause lethal rhythm disturbances to the heart in susceptible individuals
» ii. disrupt the healing process after a recent surgical procedure
» (2) The device is not effective for pain of central origin, including headache.
» (3) The device is not a substitute for pain medications and other pain management therapies.
» (4) The device has no curative value.
» (5) The device is a symptomatic treatment and, as such, suppresses the sensation of pain that
would otherwise serve as a protective mechanism.
» (6) The long-term effects of electrical stimulation are unknown.
» (7) The user may experience skin irritation, burns or hypersensitivity due to the electrical stim-
ulation or electrical conductive medium (gel).
» (8) The user has suspected or diagnosed epilepsy, the user should follow precautions recom-
mended by his or her physician.
» (9) Use caution if the user has a tendency to bleed internally, such as following an injury or
fracture.
» (10) Use caution if stimulation is applied over the menstruating or pregnant uterus.
» (11) Use caution if stimulation is applied over areas of skin that lack normal sensation.
» (12) Stop using the device if the device does not provide pain relief.
» (13) Use this device only with the leads, electrodes, and accessories that the manufacturer
recommends.
6
Page 7

(14) DO NOT SHARE THE USE OF THE ELECTRODE PADS WITH OTHERS.
(15) DO NOT USE THE DEVICE WHI LE IT’S CHARGING.
(16) THE DEVICE CONTAINS THE LITHIUM BATTERY. IF OVERHEATING OF THE D EVICE OCCURRED DURING THE CHARGING, STOP THE CHARGING OR OPERATION IMMEDIATELY AND
REPORT TO THE SELLER.
(17) DISPOSE OF THE BATTERY-CONTAINING DEVICE ACCORDING TO THE LOCAL, STATE, OR
FEDERAL LAWS.
THE LONG-TERM EFFECTS OF ELECTRICAL STIMULATION ARE UNKNOWN.
SINCE THE EFFECTS OF STIMULATION OF THE BRAIN ARE UNKNOWN, STIMULATION SHOULD
NOT BE APPLIED ACROSS THE HEAD, AND ELECTRODES SHOULD NOT BE PLACED ON OPPOSITE
SIDES OF THE HEAD.
THE SAFETY OF ELECTRICAL STIMULATION DURING PREGNANCY HAS NOT BEEN ESTABLISHED.
ADVERSE REACTIONS
» Patients may experience skin irritation and burns beneath the electrodes applied to the skin;
» Patients may experience headache and other painful sensations during or following the application
of electrical stimulation near the eyes and to the head and face.
» Patients should stop using the device and should consult with their physicians if they experience
adverse reactions from the device
THE ENVIRONMENTAL CONDITION FOR NORMAL WORKING, TRANSPORT, AND
STORAGE
» - Normal working ambient temperature: 5°C ~40°C
» - Normal working ambient humidity: 15% RH ~90% RH
» - Store and transport ambient temperature: -25°C ~70°C
» - Store and transport ambient humidity: 0% RH ~90% RH
7
Page 8
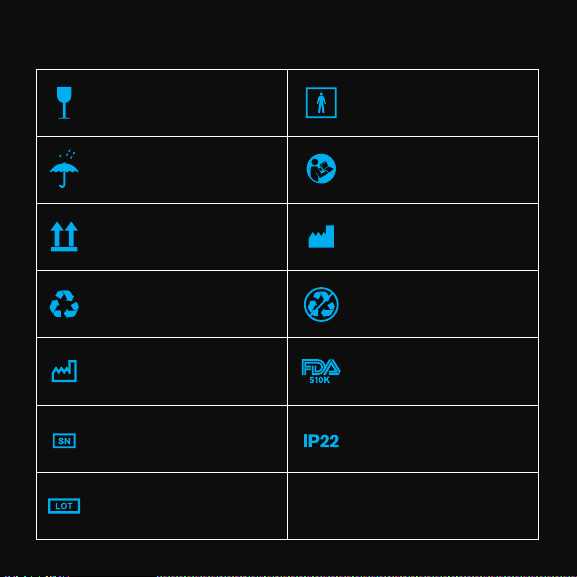
SYMBOL AND TITLE
Fragile; handle with care Type BF applied part
Keep away from water and rain
This way up Manufacturer
Product package recyclable Non-recyclable
Date of manufacture FDA 510(k) cleared
Serial number IP code of the device
Batch code
8
CAUTION, Read manual before
operating this product
Page 9

ELECTROMAGNETIC COMPATIBILITY
AND FCC COMPLIANCE STATEMENT
1) This product needs special precautions regarding electromagnetic compatibility (EMC) and needs to
be installed and put into service according to the EMC information provided, and this unit can be affected
by portable and mobile radio frequency (RF) communications equipment.
2) Do not use a mobile phone or other devices that emit electromagnetic fields, near the unit. This may
result in incorrect operation of the unit.
3) Caution: This unit has been thoroughly tested and inspected to assure proper performance and
operation.
4) Caution: This machine should not be used in conjunction with other equipment.
GUIDANCE AND MANUFACTURER’S DECLARATION – ELECTROMAGNETIC EMISSION
The device is intended for use in the electromagnetic environment specified below. The user should assure that it is used in such
an environment.
EMISSION TEST COMPLIANCE ELECTROMAGNETIC ENVIRONMENT – GUIDANCE
RF emissions
CISPR 11
Group 1 The device uses RF energy only for its internal function. Therefore, its
RF emissions are very low and are not likely to cause any interference
in nearby electronic equipment
9
Page 10

RF emission
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Class B The device is suitable for use in all establishments, including domestic
Class A
Complies
establishments and those directly connected to the public low-voltage
power supply network that supplies buildings used for domestic
purposes.
GUIDANCE AND MANUFACTURE’S DECLARATION – ELECTROMAGNETIC IMMUNITY
The device is intended for use in the electromagnetic environment specified below. The user should assure that it is used in such an
environment.
IMMUNITY TEST IEC 60601 TEST LEVEL COMPLIANCE LEVEL ELECTROMAGNETIC
ENVIRONMENT GUIDANCE
Electrostatic discharge (ESD)
IEC 61000-4-2
±6 kV contact
±8 kV air
±6 kV contact
±8 kV air
Floors should be wood, concrete or ceramic tile. If floors
are covered with synthetic
material, the relative humidity
should be at least 30%.
Electrical fast transient/burst ±2 kV for power supply lines
±1 kV for
10
Not applicable
(internal batte
Main power quality should be
that of a typical commercial or
hospital environment.
Page 11

IEC 61000-4-4 input/output lines powered) X
Surge
IEC 61000-4-5
Voltage dips, short interruptions and voltage variations on
power supply input lines
IEC 61000-4-11
± 1 kV line(s) to line(s)
± 2 kV line(s) to earth
<5% UT
(>95% dip in UT)
for 0.5 cycle
40% UT
(60% dip in UT)
for 5 cycles
70% UT
(30% dip in UT)
for 25 cycles
<5% UT
(>95% dip in UT)
for 5 sec
Not applicable
(internal battery powered)
Not applicable
(internal battery powered)
Main power quality should be
that of a typical commercial or
hospital environment.
Main power quality should be
that of a typical commercial
or hospital environment. If
the user requires continued
operation during power main
interruptions, it is recommended that the device is powered
by an uninterruptible power
supply or a battery.
11
Page 12

Power frequency (50Hz/60Hz)
magnetic field IEC 61000-4-8
NOTE UT is the a.c. mains voltage prior to application of the test level.
3 A/m 3 A/m Power frequency magnetic fields should
be at levels
characteristic of a typical location in a typical
commercial or hospital environment.
GUIDANCE AND MANUFACTURE’S DECLARATION – ELECTROMAGNETIC IMMUNITY
The device is intended for use in the electromagnetic environment specified below. The user should assure that it is used in such
an environment.
IMMUNITY TEST IEC 60601 TEST
LEVEL
COMPLIANCE
LEVEL
3 Vrms
ELECTROMAGNETIC ENVIRONMENT –
GUIDANCE
Portable and mobile RF communications equipment
should be used no closer to any part of the device,
including cables than the recommended separation
distance calculated from the equation applicable to
the frequency of the transmitter.
Recommended separation distance
12
Conducted RF
IEC 61000-4-6
3 Vrms
150 kHz to 80 MHz
Page 13

d = 1,2√P 80 MHz to 800 MHz
Radiated RF
IEC 61000-4-3
3 V/m
80 MHz to 2.5 GHz
3 V/m
d = 2,3√P 80 MHz to 2,5 MHz
Where P is the maximum output power rating of the transmitter
in watts (W) according to the transmitter manufacturer and d
is the recommended separation distance in meters (m).
Field strengths from fixed RF transmitters, as determined
by an electromagnetic site survey,a should be less than the
compliance level in each frequency range.
Interference may occur in the vicinity of equipment marked
with the following symbol:
NOTE 1 At 80 MHz and 8 00 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects, and people.
b
13
Page 14

A: Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and mobile radios, amateur
radio, AM, and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic
environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in
the location in which the device is used exceeds the applicable RF compliance level above, the device should be observed to verify
normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating
the device.
B: Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
RECOMMENDED SEPARATION DISTANCES BETWEEN
PORTABLE AND MOBILE RF COMMUNICATIONS EQUIPMENT AND THE DEVICE.
The device is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The user can
help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications
equipment (transmitters) and the device as recommended below, according to the maximum output power of the communications
equipment.
RATED MAXIMUM
SEPARATION DISTANCE ACCORDING TO FREQUENCY OF TRANSMITTER(M)
OUTPUT POWER OF
TRANSMITTER
(W)
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
14
150 KHZ TO 80 MHZ 80 MHZ TO 800 MHZ 800 MHZ TO 2.5 GHZ
Page 15

For transmitters rated at a maximum output power not listed above, the recommended separation distance in meters (m) can be
estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 8 00 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects, and people.
15
Page 16

This device complies with Part 15 of the FCC Rules. Operation is subject to the following two conditions:
(1)This device may not cause harmful interference, and
(2)This device must accept any interference received, including interference that may cause undersired operation.
The subject device has been tested and found to comply with the limits for a Class B digital device, pursuant to part 15 of the FCC
rules. These limits are designed to provide reasonable protection against harmful interference in a residential installation.
The product generates, uses, and can radiate radio frequency energy and, if not installed and used accordance with the instructions,
may cause harmful interference to radio communications.
However, there is no guarantee that the interference will not occur in a particular installation. If the product does cause harmful
interference to radio or television reception, which can be determined by turning the product on or off, the user is encouraged to try
to correct the interference by one or more of the following measures:
a) Reorient or relocate the receiving antenna;
b) Increase the separation between the product and the receiver;
c) Consult the dealer or an experienced radio / TV technician for help.
d) Connect the equipment into an outlet on a circuit different from that to which the receiver is connected.
Changes or modifications to this product not expressly approved by the party responsible for compliance could void the user’s
authority to operate the equipment.
16
Page 17

PRODUCT SPECIFICATIONS
TECHNICAL INFORMATION
MOD EL/TYPE Compex LT WEIGHT 40g
POWER SUPPLY Internal 3.7V Li-ion battery A UTOMATIC SHUTOFF 20 minutes
WAVEFORM AND WAVE
SHAPE
PULSE DURATION 100us (Microseconds) TYPE OF PR OTECTION
PULSE FREQUENCY 1-100Hz (Hz=vibration per
OUTPUT VO LTAGE Max. 70Vpp ±20%(at 500ohm
TREATMENT TIM E 10 min, 20 min, 30 min, 40
OUTPUT INTE NSITY 0 to 20 levels, adjustable MODE OF OPERATION Continuous operation
MODES 12 auto modes SOFTWARE VERSION A0
TYPICAL OPERATION TIME
OF BATTERY
Biphasic rectangular wave
pulse
second)
load)
min, 50 min, 60 min
Use of both channels at level
10, the battery can be used
570 mins after fully charged.
At level 20, the battery can be
used for 180 mins after fully
charged.
Note: Not intended to be sterilized.
Not for use in an OXYGEN RI CH ENVIRON MENT
AGAINST ELECTRIC SHOCK
AGAINST ELECTRIC SHOCK
LIFETIME FOR ELECTRODE Storage for 2 year(no use(,
DEGREE OF PROTECTION
GRADE OF WATERPROOF IP22
PRODUCT LIFE 1 year
LIFE O F BATTERY 300 uses
Adapter for charging DC5V/output current 0.3-2.0A
Type BF applied part
Internally powered equipment
Times of reusable: 30 times
17
Page 18

PRODUCT SPECIFICATIONS
PROGRAM NAME TIME MIN. FREQUEN CY (HZ) PULSE WI DTH
MODE 1 10,20,30,40,50,60 5 100
MODE 2 10,20,30,40,50,60 12.5-55.5 100
MODE 3 10,20,30,40,50,60 1.2 100
MODE 4 10,20,30,40,50,60 100 10 0
MODE 5 10,20,30,40,50,60 100 10 0
MODE 6 10,20,30,40,50,60 12.5-55.5,1.25,100,100,
MODE 7 10,20,30,40,50,60 20 100
MODE 8 10,20,30,40,50,60 55.5 100
MODE 9 10,20,30,40,50,60 55.5 100
MODE 10 10,20,30,40,50,60 69 100
MODE 11 10,20,30,40,50,6 0 69 100
Mode 12 10,20,30,40,50,60 69 10 0
18
12.5-55.5...
Accessories included
((S)
100
in the package.
(1). Control Unit (1)
(2). Electrodes (4)
(3). Output wire (2)
(4). Micro USB Charger (1)
(5). Quick Start Guide (1)
Page 19

LCD DISPLAY
SETUP
ON / OFF
A CHANNEL
MODE SELECTION
INTENSITY DECREASE
OUTPUT A CH ANNEL OUTPUT B CH ANNEL
USB PORT
INTENSITY INCREASE
TIMER
B CHANNEL
PAUSE / START
19
Page 20

OPERATING INSTRUCTION
• Electronic Pulse Stimulator (TENS)needs to be charged for up to 8 hours before the first use.
• Connect a pair of electrode pads to one connecting wire by snapping them on; the other end of the connecting wire is connected
to the left output of the device. Similarly, the other pair of electrode pads is connected to the remaining connecting wire and the
right output of the device.
• Attach one pair or two pair of the electrode pads to the treatment area, such as shoulder and legs.
• Turn the unit switch on. When being on, the unit will automatically start at Mode 1.
• When being on, if the pad doesn’t stick to the skin, the word “PAD” on LCD will flash and remind you to stick pads well. When
“PAD” is flashing, you will not be able to increase the intensity.
• To change the mode press the M button, and gradually increase the intensity of the A (left) output by pressing the + button;
decrease the intensity by pressing the - button.
• When pressing the ( button, the unit will stop.
• When pressing T, one of the six timers could be selected.
• When pressing the B button once, switch from the control of the A(left) output to that of the B (right) output:
only the intensity of the B output could increase or decrease by pressing the + or - button. When pressing the A button again, switch
back to the A output; only the intensity of the A output could increase or decrease.
RECOMMENDED PRACTICE:
•Duration suggested for each skin area is 20 min and 2 times per day. Consult with your physician for longer and more frequent
uses.
•Start from the lowest intensity and gradually adjust the intensity to a comfortable level at a scale from 1 to 20.
•Good skin care is important for a comfortable use of device. Be sure the treatment site is clean of dirt and body lotion.
•Keeping the electrode in the storage bag after use will extend its lifespan. The electrode is disposable and should be replaced when
it loses the adhesiveness.
•When there is no bar inside battery icon and the battery icon keeps flashing, the power is low and the device will power off after 15
seconds. Recharge before using.
20
Page 21

CLEANING AND MAINTENANCE
To clean use a damp cloth to wipe the device. The electrodes are disposable and should be replaced when they lose their adhesiveness. Avoid touching the gel side of the pad as this will cause the electrode to lose its adhesiveness.
TROUBLE SHOOTING
If your device is not operating properly, please check below for common problems and suggested solutions. If the recommended
action does not solve the problem, please contact customer care.
PROBLEM POSSIBLE CAUSE SOLUTION
One pad feels stronger than
the other
The intensity is not felt with a
very weak intensity level
This is normal. Different
areas of your body will react
differently.
Pads are not attached to the
body firmly.
Nothing needs to be done. Make sure the pads are moist and
making good contact.
Attach both pads firmly to the skin.
The transparent films are still
stuck to the pads.
The pads stack together
or overlap.
The cord is not properly
connected to the unit.
The intensity setting is
getting weak.
Peel off film on the adhesive surface of pads.
Do not stack pads together or overlap pads.
Connect cord correctly into the jack.
Increase the intensity level.
21
Page 22

The battery capacity is low. Charge the device.
The skin turns red or the skin
feels irritated
No power source; no display
on LCD.
Power cuts off during use The batter y is weak. Charge the device.
It is difficult to attach the pad
to the skin
22
The adhesive surface of the
pads is dirty or dry.
The therapy time is too
long or the intensity is set
too high.
The electrode pad surface is
worn out.
The battery capacity is
depleted.
The cord is broken. Replace the cord.
Have you removed the transparent film from the pad?
Wipe the adhesive surface of pads gently with a wet fingertip.
Reduce the application time or reduce the intensity.
Replace electrode pad.
Charge the device.
Peel off film on the adhesive surface of pads.
Page 23

RECOMMENDED USE POSITIONS
SHOULDERS BACK BUT TOCKS ABDOMEN
ARMS LEGS FEET
23
Page 24

CONTACT INFORMATION
Distributed by DJO, LLC
JKH Health Co., Ltd.
Address: 4-5F, Building 12, Hengmingzhu Industrial Park, Xinqiao Tongfuyu Industrial Area,
Shajing, Baoan District, Shenzhen, China
Tel: +86-755-27926589
Fax: +86-755-29970323
E-mail: info@JKHhealth.com
24
Page 25

PL-029GA VERSION 1.1
25
 Loading...
Loading...