Comen S8 Service Manual

Defibrillation Monitor S Series
Service Manual
Shenzhen Comen Medical InstrumentsCo., Ltd.

Defibrillator Monitor S Series Service Manual
Copyright
Shenzhen Comen Medical Instruments Co.,Ltd Version: 1.0
Product Name: Defibrillation Monitor
Model: S8
Statement
Shenzhen Comen Medical Instruments Co., Ltd. (belowComen is the copyright of this
non-publicly published maintenance manual and has the right to treat it as confidential
information. This Service Manual is a reference for the maintenance of Comen products.
Others have no right to disclose the contents of this manual to others.
This Service Manual contains proprietary information protected by copyright law.
Copyright, no part of this Service Manual may be photocopied, copied or translated into
another language without the prior written consent ofComen.
Comen is not liable for errors in the service manual or for incidental or
consequential damages resulting from the actual performance and use of this service
manual.Comen does not provide other parties with a franchise under the patent law.Comen
is not liable for legal consequences resulting from violations of patent law and any
third party rights.
The contents of the maintenance manual can be changed without notice.
Contact
Name: Shenzhen Comen Medical InstrumentsCo., Ltd. After-sales Service Department
Address:10-11F, Building 1A, 1A Building, Feiyada Clock Building, South Ring Road,
Matian Street, Guangming District, Shenzhen, China
Tel:0755-26431236
2

Defibrillator Monitor S Series Service Manual
Fax:0755-26431232
Customer Service Phone:4007009488
Zip Code:518052
3

Defibrillator Monitor S Series Service Manual
Directory
目录
Chapter 1 Safety ................................................................................................................................................ 6
1.1 Safety Information ............................................................................................................................. 6
1.2 Symbol Description ........................................................................................................................ 11
Chapter2 Warranty ......................................................................................................................................... 12
2.1 Warranty Terms .............................................................................................................................. 12
2.2 Exclusion of warranty ....................................................................................................................... 12
2.3 Maintenance procedures ................................................................................................................. 13
2.3.1 Fill in the Customer Complaint Form (SCF) ............................................................................ 14
2.3.2 Submit a customer complaint form and choose a solution ................................................... 14
2.3.3 RMA Form............................................................................................................................... 15
2.3.4 Return ..................................................................................................................................... 15
Chapter 3 Introduction to Principles ............................................................................................................... 17
................................................................................................................................................................. 17
3.1 Overview ......................................................................................................................................... 17
3.1.1 Introduction to Defibrillator ................................................................................................... 17
3.1.2 System Composition ............................................................................................................... 17
3.1.3 system structure diagram....................................................................................................... 19
3.2 Module Introduction ....................................................................................................................... 19
3.2.1 Main control board................................................................................................................. 19
3.2.2 AC power board ...................................................................................................................... 22
3.2.3 Keyboard ................................................................................................................................
22
3.2.4 DC power board .................................................................................................................. 24
3.2.5 Treatment module ................................................................................................................. 26
3.2.6 Side panel ............................................................................................................................... 32
3.2.7 NIBP module ........................................................................................................................... 33
3.2.8 IBP module ............................................................................................................................. 36
3.2.9 ECG Module ............................................................................................................................ 36
3.2.10 SPO2 Module ........................................................................................................................ 37
Chapter 4 Troubleshooting ............................................................................................................................. 41
................................................................................................................................................................. 41
4.1 Introduction ...................................................................................................................................... 41
4.2 Replaceable parts ........................................................................................................................... 41
4.3 Checking the monitor ....................................................................................................................... 41
4.4 Checking the software ...................................................................................................................... 41
4.5 Check the error message prompt ................................................................................................ 42
4.6 Troubleshooting.............................................................................................................................. 42
4.6.1 Electricity failure ..................................................................................................................... 42
4

Defibrillator Monitor S Series Service Manual
4.6.2 Display failure ......................................................................................................................... 43
4.6.3 Battery failure ......................................................................................................................... 44
4.6.4 SPO2 failure ........................................................................................................................ 45
4.6.5 NIBP failure ........................................................................................................................ 46
4.6.6 TEMP Module failure ....................................................................................................... 47
4.6.7 Key failure ........................................................................................................................... 48
4.6.8 Software upgrade failure................................................................................................ 48
4.6.9 Technical alarm information ......................................................................................... 49
4.6.10 Electrode piece cable connection failure ................................................................ 49
4.6.11 Treatment Module failure ............................................................................................ 50
Chapter 5 software upgrade ........................................................................................................................... 52
................................................................................................................................................................. 52
5.1 Upgrade Preparation ..................................................................................................................... 52
5.2 System software upgrade .............................................................................................................. 52
Chapter 6 Performance Verification ............................................................................................................... 54
................................................................................................................................................................. 54
6.1 NIBP test ......................................................................................................................................... 54
6.1.1 NIBP Air leak test .................................................................................................................... 54
6.1.2 NIBP Pressure calibration ....................................................................................................... 55
6.2 IBP test ............................................................................................................................................ 56
6.3 ECG test .......................................................................................................................................... 57
6.4 SpO
test ......................................................................................................................................... 57
2
6.5 TEMP test........................................................................................................................................ 58
5
Defibrillator Monitor S Series Service Manual
Chapter 1 Safety
1.1 Safety Information
WARNING
● You may be prompted serious consequences, endanger the safety or
adverse events Case. Failure to follow the warning will result in death or
serious personal injury or property damage to the user or patient.
CAUTION
● Indicates a potentially hazardous or unsafe operation that, if not avoided,
could result in minor personal injury, product malfunction, damage, or
property damage, and may result in more serious injury in the future.
PAY ATTENTION TO
● important precautions, provide instructions or explanations to make
better use of this product.
WARNING
6
Defibrillator Monitor S Series Service Manual
● This defibrillator is intended for use in clinical patient care and only allows
trained physicians and nurses to use the defibrillator.
● Before use, the user should check that the instrument and its accessories
are working properly and safely.
● The alarm volume and alarm upper and lower limits should be set for
different patients. When monitoring a patient, do not rely solely on the
audible alarm system to monitor the patient. If the alarm volume is set too
small or completely turned off, the alarm may be invalidated and the
patient's safety may be compromised. The most reliable method of patient
monitoring should be to pay close attention to the actual clinical condition
of the patient.
● This device can only be connected to a power outlet with protective
grounding. If the power socket is not connected to a grounding wire, do
not use the socket and use a rechargeable battery device to supply power.
● Do not open the case of the instrument to avoid possible electric shock
hazards. Any repairs and upgrades to the monitor must be performed by a
service person trained and authorized by Comen.
● When handling packaging materials, they should be disposed of in
accordance with local laws and regulations or the hospital's waste disposal
regulations. Packaging materials must be placed out of reach of children.
7
Defibrillator Monitor S Series Service Manual
● Do not use the instrument in a location where flammable materials such as
anesthesia are placed to prevent an explosion or fire.
● Carefully install the power cord and various accessory cables to avoid
patient entanglement or suffocation, cable entanglement, or electrical
interference.
● Do not use a mobile phone near the monitor. The mobile phone generates
an excessive radiation field and interferes with the monitor's function.
● For patients with pacemakers, the heart rate monitor may pulse
pacemakers during cardiac arrest or arrhythmia. Do not rely solely on the
heart rate monitor alarm. Patients with pacemakers should be closely
monitored. Refer to the monitor's instruction manual for the ability of the
device to suppress the pacemaker.
● During defibrillation, the operator should not touch the patient, the table
and the instrument. Check that the function is normal before using these
cables again.
● The interconnecting device with the monitor should form an equipotential
body (the protective ground is effectively connected).
● When the monitor is shared with the electrosurgical device, the user
(doctor or nurse) should ensure the patient's safety.
8
Defibrillator Monitor S Series Service Manual
● The physiological waveforms, physiological parameters and alarm
information displayed by the monitor are for medical reference only and
cannot be directly used as clinical treatment basis.
● Electromagnetic fields can affect the performance of the instrument, so
the use of other Instrumentsin the vicinity of the instrument must meet
the appropriate EMC requirements. For example, mobile phones, X-rays, or
MRI devices can all be sources of interference because they emit high-
intensity electromagnetic radiation.
● This is not a treatment device.
● After defibrillation, the recovery time of ECG is not more than 10s, and the
recovery time of other parameters is not more than 5s.
● To prevent burns to the patient when the monitor is used with a high
frequency surgical device, avoid any conductive connections between the
sensor and cable and the high frequency surgical device.
CAUTION
● To install or carry the instrument properly to prevent the instrument from
falling, colliding, being subjected to strong oscillations or other
mechanical external forces.
9
Defibrillator Monitor S Series Service Manual
● Before the device is powered on, please confirm that the power supply
used meets the requirements of the instrument's nameplate label or the
power supply voltage and frequency specified in the instruction manual.
● When the instrument and accessories are about to expire, they must be
disposed of in accordance with relevant local laws and regulations or the
hospital's rules and regulations.
● Disposable accessories can only be used once, and repeated use can result
in performance degradation or cross-contamination.
● If the monitor will not be used for a long time, remove the battery and
keep it in a safe place.
● Defibrillation recovery time of less than 10s
PAY ATTENTION TO
● Install the instrument in a location that is easy to observe, operate, and
maintain.
● This instrument cannot be used at home.
● The instrument is limited to one patient at a time.
● The life of this monitor is 5 years.
10
Defibrillator Monitor S Series Service Manual
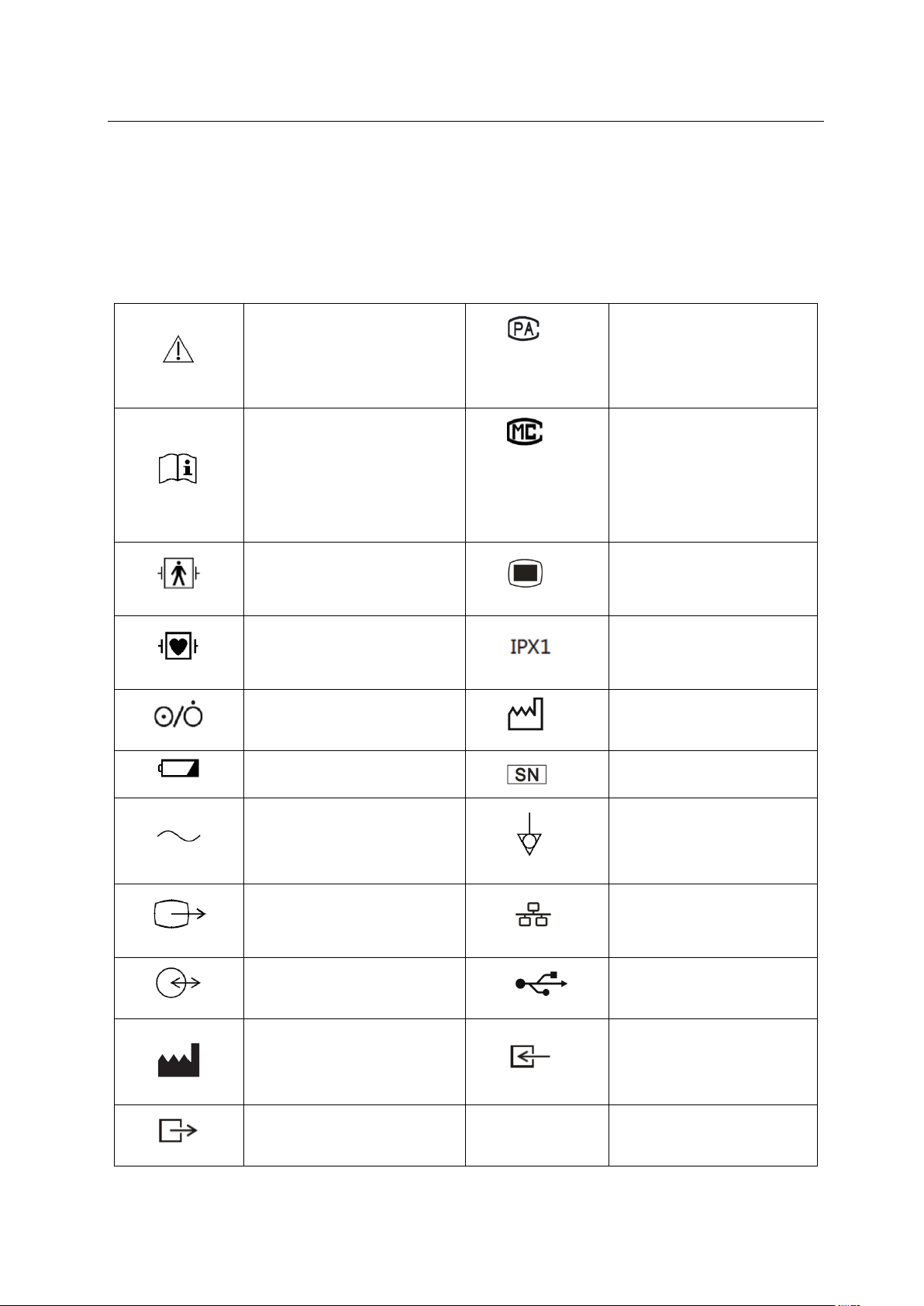
measuring instrument type
manufacturing measuring
network connection
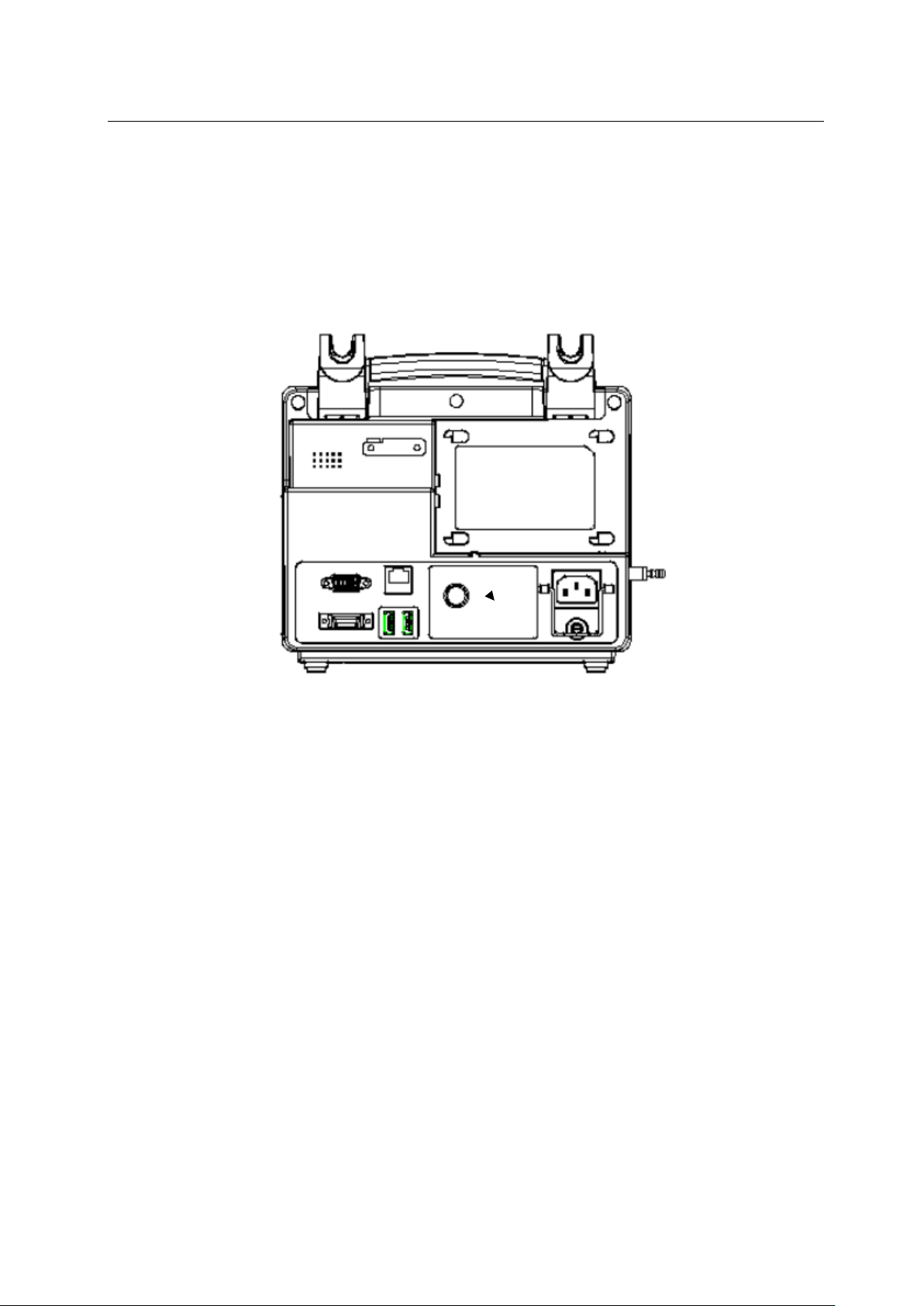
1.2 Symbol Description
(1) Instrument Symbol
Note!
approval mark and number
2015R124-44
refer to the operation manual
Guangdong
00000700
BF type application part, with
anti-defibrillation function
CF type application part, with
anti-defibrillation function
open, shutdown Key
use battery work light
instrument license mark and
number
main menu
waterproof level
production date mark
sequence number mark
AC power indicator light
VGA interface
multi-function interface
manufacturer
output port
table 1
11
equipotential symbol
symbol
USB interface
input port
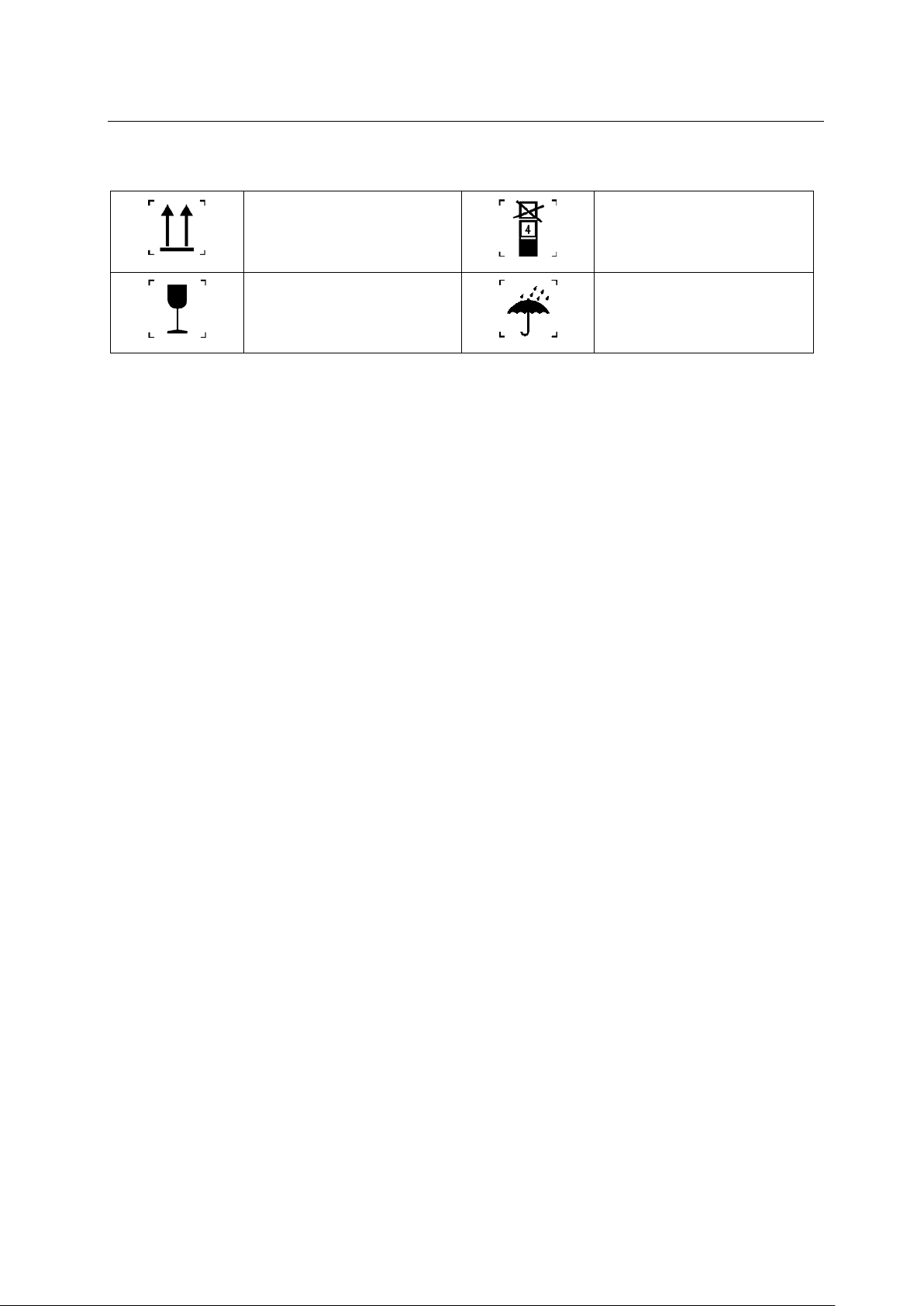
(2) packaging symbol
Defibrillator Monitor S Series Service Manual
up
fragile goods
stacking layer limit
rainproof
gauge2
Chapter2 Warranty
2.1 Warranty Terms
Comen provides a 24 month (host) warranty or a 6 month (accessory) warranty for all licensed products,
starting with the customer's purchase date. If your product is determined to be defective and promptly
notified to us during the warranty period, Comen will repair the product or replace it with a new product or
accessory.
2.2 Exclusion of warranty
The Comen warranty will not apply to the following:
Unreasonable use or damage caused by man
Damage caused by unstable voltage or super normal range voltage
Damage caused by irresistible factors, such as fires and earthquakes
Damage caused by unreasonable operation or repair by an unauthorized maintenance organization or
individual
12
Defibrillator Monitor S Series Service Manual
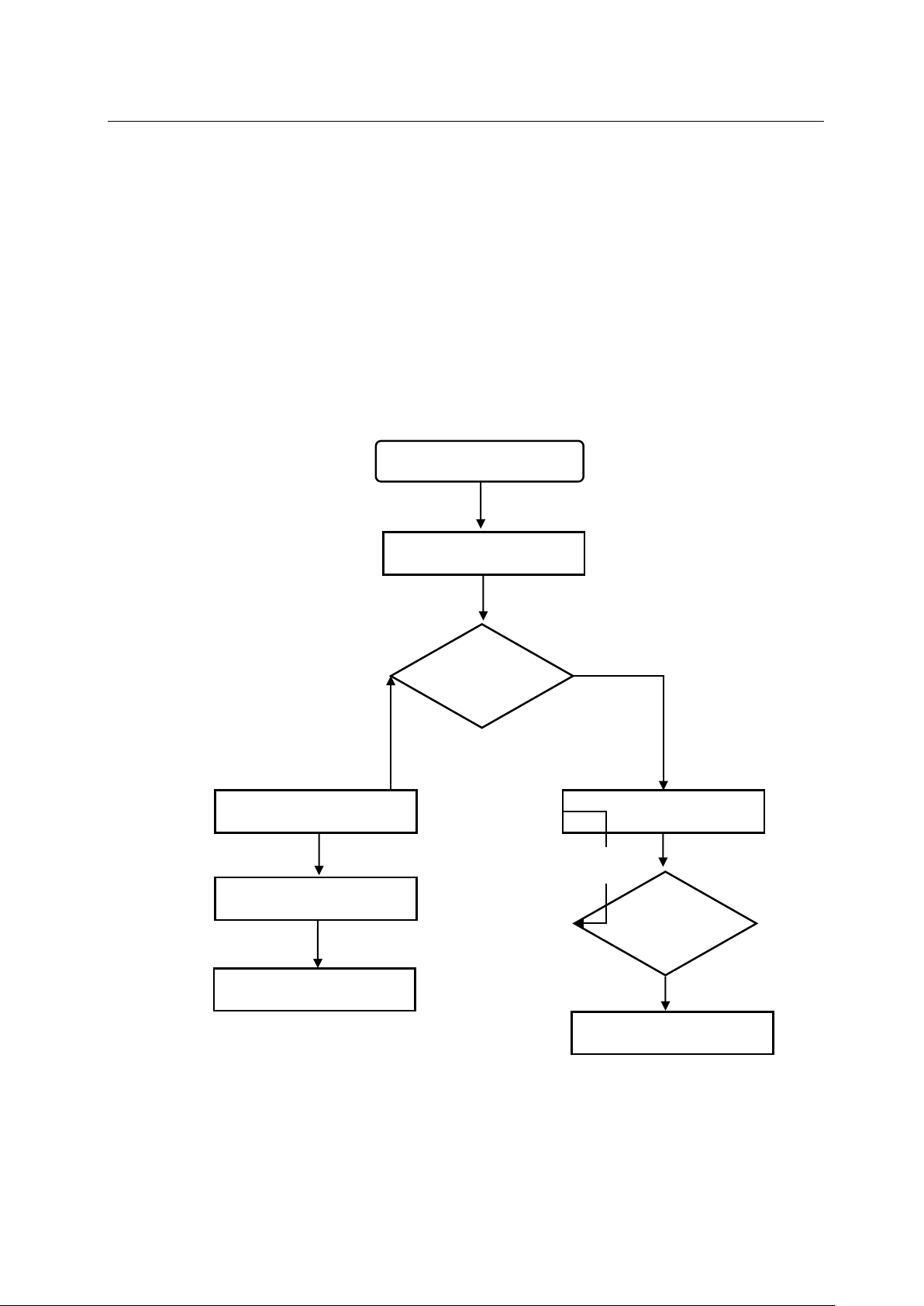
Repair request
Instrument collection
Instrument
status
Return to customer
Inspection / repair
testing
Failed
qualified
Damage caused by attachments that are not approved by Comen.
The product serial number is not clear.
Other damage caused by the instrument or the component itself.
2.3 Maintenance procedures
No need to
Need to be
Apply for accessories
Office maintenance
Product
Return to office/client
figure 1
13
Defibrillator Monitor S Series Service Manual
Serial number
(SN) see this
2.3.1 Fill in the Customer Complaint Form (SCF)
Fill in the customer complaint form in detail: model number, serial number (SN) and problem description.
Without this information, Comen will not be obliged to accept this complaint. Customers can apply for a
complaint form from Comen's after-sales department if needed.
nameplate
Figure 1
2.3.2
Submit a customer complaint form and choose a solution
Once the after-sales department receives the customer complaint form, the engineer will provide the
customer solution within three days. And deal with it according to the following two situations:
Warranty period:
There are two options:
1) After receiving the Return Authorization Form (RMA) from the After-sales Department, the customer
can return the defective part and inform the delivery number. We will send a new part with the loading
invoice to your address as soon as possible.
2) You can also send the signed statement to us by mail or fax. The statement has the legal effect to ensure
that the customer or the end customer will send the faulty part to Comen in time. In this case, we will
send the replacement parts and loading invoices in a timely manner.
14
Defibrillator Monitor S Series Service Manual
Caution
Once the customer complaint form has been confirmed, Comen engineers will provide you with
a return authorization and declaration form.
The customer is responsible for shipping, insurance and custom s fees for the shipment of the
product to Comen.
The warranty period has expired:
After receiving the return authorization form, the customer will send the faulty part to Comen. Our
engineers will analyze the faulty parts and negotiate with the customer to repair or replace the faulty parts.
After the maintenance fee has been paid, we will send out new replacement parts as soon as possible.
Caution
The customer is responsible for all costs (including freight, insurance and customs fees) during
the transportation process.
2.3.3 RMA Form
Before returning the goods, the customer will receive an RMA Form (Return Authorization Form)
provided by our after-sales department, which includes the RMA number, a brief description of the returned
parts and shipping instructions. When shipping, please indicate the RMA number on the box.
Caution
In the absence of a notice to the Comen after-sales department, we will not be obligated to process
returns from the terminal or customer. And the shipper is responsible for all possible expenses.
2.3.4 Return
Please follow the steps below
When disassembling the instrument, use an anti-static requirements. Do not touch the instrument
directly with your hands.
Pack the parts carefully before shipping.
15

Defibrillator Monitor S Series Service Manual
Please indicate the RMA number on the package.
When describing the product, please use the “*** sample” format to reflect the total value on the
invoice and mark “sample, no commercial value”. .
Before returning, please check with Comen (for example: total amount, address and other necessary
information on the invoice).
After receiving confirmation from Comen, please return the faulty part.
16

Chapter 3 Introduction to Principles
3.1 Overview
Defibrillator Monitor S Series Service Manual
3.1.1 Introduction to Defibrillator
S-Series defibrillation monitors can monitor ECG, RESP, SpO2, NIBP, Invasive Blood Pressure (IBP), Carbon
Dioxide (CO2), Body Temperature (TEMP), etc. Vital sign parameters. This product is suitable for manual
defibrillation, AED defibrillation, pacing and vital signs monitoring.
3.1.2 System Composition
The defibrillator monitor consists of a host, a battery, a defibrillation electrode plate (defibrillation
external electrode plate, and a defibrillation internal electrode plate) and corresponding functional
accessories. Mainly achieve the following functions:
1. Defibrillation monitor defibrillation release energy accuracy;
2. Pace frequency accuracy;
3. Pace current accuracy;
17

Defibrillator Monitor S Series Service Manual
4. Heart rate detection accuracy;
5. Respiratory measurement accuracy:
6. Body temperature measurement error;
7. Non-invasive blood pressure measurement accuracy:
8. Invasive blood pressure measurement accuracy;
9. Blood oxygen saturation measurement accuracy;
10. Pulse rate measurement accuracy.
CO2 measurement accuracy:
18
 Loading...
Loading...