Cheetah Medical Starling SV User manual

Starling™SV
UserGuide
R-IFU-05/Revision3

STARLING SV SYSTEM
User Guide
In the US:
CheetahMedical, Inc. 600 SE Maritime Ave Suite 220
Vancouver, WA 98661
USA
Toll free:(+1) 866-751-9097 Telephone: (+1)360-828-8685 Fax: (+1)360-718-8154
In Europe and Asia
Cheetah Medical (UK) Limited
1, Irmar House
59, Cookham Road
Maidenhead
Berkshire SL6 7EP
United Kingdom
Telephone: (+44) 1628 636806
Fax: (+44) 1628 788802
E-mail: cheetah@cheetah-medical.com Web Site:www.cheetah-medical.com
Cheetah Medical 2016

Table of Contents
Conventions used in this manual......................................... |
4 |
||
1.0 |
Introduction..................................................................... |
6 |
|
1.1 |
Introduction..................................................................... |
6 |
|
1.2 |
General Description........................................................ |
7 |
|
1.3 |
Front and Buttons........................................................... |
7 |
|
1.4 |
Rear and cables................................................................ |
8 |
|
1.5 |
Specifications................................................................. |
11 |
|
1.6 |
NIBP............................................................................... |
12 |
|
1.7 |
Bedside Care.................................................................. |
22 |
|
1.8Label................................................................................. |
22 |
||
1.9 |
Battery Mode................................................................. |
23 |
|
1.10 |
Warnings and Precautions.......................................... |
23 |
|
Warnings............................................................................... |
23 |
||
Precautions........................................................................... |
24 |
||
1.11 |
Cleaning and Disinfecting the Starling SV Device..25 |
||
1.12 |
Special Patient Populations........................................ |
25 |
|
1.13 |
Indications for Use...................................................... |
26 |
|
1.14 |
Limitations of Use....................................................... |
27 |
|
1.15 |
SpO2............................................................................. |
28 |
|
2.0 |
Screens Overview.......................................................... |
33 |
|
2.1 |
Start Up Screen.............................................................. |
33 |
|
2.2 |
Menu Screen.................................................................. |
34 |
|
2.3 |
New Patient Screen....................................................... |
35 |
|
2.4 |
Existing patient.............................................................. |
36 |
|
2.5 |
Run Screen..................................................................... |
37 |
|
2.6 |
Trend Display Screen.................................................... |
43 |
|
2.7 |
Tabular Display Screen................................................. |
44 |
|
2.8 |
Protocol Dashboard Display screen............................ |
46 |
|
2.9 |
Hemodynamic Dashboard Screen............................... |
46 |
|
2.10 |
Settings Screen............................................................. |
47 |
|
2.11 |
Set Option.................................................................... |
50 |
|
2.12 |
General Options.......................................................... |
51 |
|
2.13 |
Test Options................................................................ |
52 |
|
2.14 |
NIBP Options............................................................. |
53 |
|
2.15 |
SpO2 Options.............................................................. |
54 |
|
2.16 |
Art Options.................................................................. |
55 |
|
2.17 |
Connectivity................................................................. |
56 |
|
2.18 |
Network Configuration............................................. |
57 |
|
2.19 |
Date and Time............................................................. |
58 |
|
2.20 |
Device Mode................................................................ |
59 |
|
2.21 |
Service Mode Screen................................................... |
60 |
|
2.22 |
Live Demo and Demo Screens.................................. |
61 |
|
2.23 |
Shut down Screen........................................................ |
62 |
|
2.24 |
WI-FI options.............................................................. |
63 |
|
3.0B Basic Functions........................................................... |
64 |
||
3.1 |
Saving a New Patient.................................................... |
64 |
|
3.2 |
Performing a Session.................................................... |
64 |
|
3.3 |
Viewing Test Results..................................................... |
67 |
|
3.4 |
Entering Events............................................................. |
69 |
|
3.5 |
Calibrating the Touch Screen....................................... |
71 |
|
4.0 |
Settings & Advanced Functions.................................. |
72 |
|
4.1 |
Height Units................................................................... |
73 |
|
4.2 |
Weight Units.................................................................. |
73 |
|
4.3 |
Electric Grid.................................................................. |
73 |
|
4.4 |
Audible Alarm................................................................ |
73 |
|
4.5 |
Date Format................................................................... |
73 |
|
4.6 |
Time Format.................................................................. |
73 |
|
4.7 |
Test Type........................................................................ |
74 |
|
4.8 |
Sample Interval.............................................................. |
74 |
|
4.9 |
TFCd............................................................................... |
75 |
|
4.10 |
NIBP............................................................................. |
75 |
|
4.11 |
NIBP Operation Mode............................................... |
76 |
|
4.12 |
NIBP Time Interval.................................................... |
76 |
|
4.13 |
NIBP Initial Inflation Pressure.................................. |
76 |
|
4.14 |
Date and Time Setup.................................................. |
78 |
|
4.15 |
Connectivity Wizard.................................................... |
78 |
|
4.16 |
Networking Wizard..................................................... |
79 |
|
4.17 |
Hgb Posting Period..................................................... |
80 |
|
4.18 |
SpO2 Operation Mode............................................... |
80 |
|
4.19 |
Art Operation Mode................................................... |
81 |
|
6.0 |
Measurements Short Description................................ |
82 |
|
6.1 CO................................................................................... |
82 |
||
6.2 |
CI..................................................................................... |
82 |
|
6.3 |
HR................................................................................... |
82 |
|
6.4 VET................................................................................ |
82 |
||
6.5 dX/dt.............................................................................. |
82 |
||
6.6 |
SV.................................................................................... |
82 |
|
6.7 |
SVI.................................................................................. |
83 |
|
6.8 |
SVV................................................................................. |
83 |
|
6.9 |
TFC................................................................................. |
83 |
|
6.10 |
TFCd............................................................................. |
83 |
|

6.11 |
TFCd0........................................................................... |
83 |
|
6.12 |
BP.................................................................................. |
83 |
|
6.13 MAP.............................................................................. |
83 |
||
6.14 |
TPR............................................................................... |
84 |
|
6.15 |
TPRI............................................................................. |
84 |
|
6.16 |
CP.................................................................................. |
84 |
|
6.17 |
CPI................................................................................ |
84 |
|
6.18 |
Z0.................................................................................. |
84 |
|
6.19 |
DO2I............................................................................ |
84 |
|
6.20 |
SpO2............................................................................. |
84 |
|
6.21 |
Hgb............................................................................... |
84 |
|
6.22 |
SBP............................................................................. |
84 |
|
6.23 |
DBP............................................................................. |
85 |
|
7.0 |
Troubleshooting............................................................ |
86 |
|
7.1 |
|
Error Messages............................................................ |
86 |
7.2 |
|
Warning Messages....................................................... |
90 |
7.3 |
|
Other Problems........................................................... |
90 |
8..0SERVICE AND MAINTENANCE......................... |
93 |
||
8.1 |
Cleaning.......................................................................... |
93 |
|
8.2 |
Fuse Replacement......................................................... |
94 |
|
8.3 |
Chargeable Batteries...................................................... |
94 |
|
8.4Electromagnetic Compatibility – Manufacturer Declaration
............................................................................................... |
95 |
9.2 Equipment and Accessories Inventory..................... |
102 |
Returning Parts under Warranty...................................... |
102 |
Shipping Parts.................................................................... |
103 |
Conventions used in this manual
WARNINGIndicatesconditionsorpracticesthatcouldleadtopatientinjury, illness,ordeath
CAUTIONIndicatesconditionsorpracticesthatcoulddamagetheequipmentor otherproperty.
NoteProvidesadditionalimportantinformation.
 Consultoperatinginstructions
Consultoperatinginstructions
Manufacturer
 Temperaturerange
Temperaturerange
 Relativehumidityrange
Relativehumidityrange
 AtmosphericPressurerange
AtmosphericPressurerange
Useby
Donotreuse
LatexFree
Europeanapprovalmark
 AuthorizedrepresentativeintheEuropeanCommunity
AuthorizedrepresentativeintheEuropeanCommunity
 SerialNumber
SerialNumber
 TypeBFAppliedPart
TypeBFAppliedPart
 Storeinacoolandkeptawayfromdirectsunlightplace
Storeinacoolandkeptawayfromdirectsunlightplace
Storeinadryplace
Donotopen
Donotdisposeof,contactforrecycling
 FCCSymbol
FCCSymbol
Non-ionizing electromagnetic symbol
5
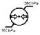
PatientInformationBar-AppearsonallRunscreens.Thisbarholdsallofthe patient'sinfoasitwasinsertedintothesystem,suchas:ID,Name,Gender,Age, Weight,HeightandBSA.ThePatientInformationBaralsoholdstheTestTypeIcon ('R'forresttestingor'S'forstresstesting).
RunBar-AppearsonallRunscreens.ThisbarholdstheStudyRefreshClock, Alarmstatusicon,Teststatusicons(play,pauseorstop)andtheMessageDisplay area.
MenuBar–AppearsonalloftheSTARLINGSV'sscreens.Thisbarholdsthe
ScreenName,OperationHelpline,Date,TimeandBatterystatus.
NoteInordertoreachthesubmenu–firstenterthescreen'spop-upmenuby clickingtheSTARLINGSV'sBackbutton.Then,eitherclicktheSTARLINGSV's OKbuttonoruseitsRightarrowkeytoenterthesubmenu.*Alliconsdescribedaccording
toorderofappearance-fromlefttoright
5

Chapter
1
1.0Introduction
1.1Introduction
The Starling™ SV System is a portable, non-invasive cardiac output (CO) monitoring device based on BIOREACTANCE® technology. It also measuresHeart Rate (HR), Stroke Volume (SV), Stroke Volume Variation (SVV), Ventricular Ejection Time (VET), Mean Arterial Pressure (MAP), Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Blood Oxygenation (SpO2) and Thoracic Fluid Content (TFC). In addition the system calculates clinical parameters such as Cardiac Index (CI), Stroke Volume Index (SVI), Total Peripheral Resistance (TPR), Total Peripheral Resistance Index (TPRI), Cardiac Power (CP), Cardiac Power Index (CPI), Oxygen Delivery Index (DO2I), Thoracic Fluid Content delta (TFCd) and Thoracic Fluid Content delta from baseline(TFCd0) based on the above measured parameters or based on a manually entered parameters such as manually entered Hemoglobin (Hgb), manually entered blood oxygenation (SpO2) or manually entered Mean Arterial Pressure (MAP). The NIBP functionality measures and displays systolic, diastolic, and mean arterial pressures. The SpO2 functionality measures and displays SpO2.
The Starling SV® Operator’s Manual contains instructions and information for the operation and maintenance of the Starling SV System.
A thorough understanding of the system controls and connections is vital for instrument operation. It is therefore essential to read this manual carefully before attempting to use the Starling SV System, in order to ensure optimal performance and trouble free service life. This manual should be retained for future reference.
6
1.2 General Description
TheStarlingSVSystemdimensionsare:
Height: 220mm
Width:290mm
Depth:190mm
TotalWeight:4.3Kg
1.3 Front and Buttons
On the front there is an 10.4" LCD screen, knoband three buttons. . In order to turn the unit on, press continuously on the power button  until it turnsGreen or Orange and the Cheetah Logo is prompted on the screen; in order to turn the unit off, use the Shut Down command on the mainmenu (section 2.22).
until it turnsGreen or Orange and the Cheetah Logo is prompted on the screen; in order to turn the unit off, use the Shut Down command on the mainmenu (section 2.22).
Alternatively, pressing the power button continuously for several seconds will also turn the unit off, however this shut down method is discouraged because some test information may not be saved. Use the STARLING SV'sknob in order to choose commandsfrom the menu, to change measurements' display during a test. In addition, the knob can be used to browse and look up values within fields, to move the cursor between fields, to browse test segments when reviewing patient test results. The STARLING SV'sBack button servesto operate the pop-up menu and to return the cursor to previous placement. The menu
button servesto operate the pop-up menu and to return the cursor to previous placement. The menu  button opensand closes the menu display when pressed.
button opensand closes the menu display when pressed.
Figure1.1 StarlingSVSystemFrontPanel
7

TouchScreen:
Inadditiontothethreebuttonsandknob,theSTARLINGSVSystemisequipped withatouchscreen.
NavigationinMenus
Touchamenuoptiontoimmediatelyselectit.ToopentheSideMenuwiththe
TouchScreen,touchtheMenuNameonthesideofthescreen.
BrowsingThroughTrendsandTableDisplay
Tobrowsethroughthetrendsandtablespresentedbythemonitor,touchthearrows ofthededicatedslider.ForfasterbrowsingdragtheSliderbody.Itisalsopossibleto sweepthefingeroverthetrendstothedirectionrequired.
BrowsingThroughEvents
IntheNumericalandTrendsdisplaysskipbetweeneventsbytouchingtheEvents
BrowsingToolonthescreen
Changingthetrendsscale
Inordertochangethescaleofthetrendsmovetwofingersapartortowardseach
other. ItisalsopossibletochangethetrendsscalebychoosingTimeScale fromtheSideMenuViewOptionandthenthetimescalerequired.
ItisalsopossibletochangethetrendsscalebychoosingTimeScale fromtheSideMenuViewOptionandthenthetimescalerequired.
1.4 Rear and cables
Observetherearsideandtherightsideofthemonitorbox:
8

Figure1.2 BackpaneloftheStarlingSV
Figure 1.3 Front and side of theStarling SV
9

Inthesideofthedevicetherearefivecablesockets:
1.StarlingSVPatientCableconnector–theconnectorisintendedfortheStarling SVPatientCable.Theotherendofthepatientcableshouldbeconnectedtothe StarlingSVSensorswhichareappliedtothepatient’sbodyasdescribedinthe section'PerformingaTest'(sectionnumber3.2).
NoteTheStarlingSVPatientCableisconnectedtotheStarlingSVusingan ODUtypeconnector.Inordertoconnectitpushtheconnectorandturnit clockwiseuntilit’slockedinordertopreventdisconnectionthatwouldleadtoloss ofmonitoringfunction.
WARNINGItisadvisedtocleantheStarlingSVPatientCablebetween patientsinordertopreventthetransferofinfectiousdiseases(seeinSection8.2. ServiceandMaintenance,Cleaning)
2.NIBPcuffconnector–ConnecttheNIBPcufftothemonitorusingtheNIBP connector(pleaserefertofigure1.2).
3.SpO2connector–ConnecttheSpO2SensortothemonitorusingtheSpO2 connector(pleaserefertofigure1.2).
Intherearofthemonitorthefollowingconnectorarepreset:
1.IBPconnector–Forfuturefunctionality.
2.Powersocket–standardelectricpowersocket. Thedevicecanalsofunctiononits internalchargeablebatteries.Connectingthiscabletoanelectricsourcewillsupply themonitorfromthemaingridandchargethebatteries.
3.USBport–connectingastandardUSBcableallowstheusertodownloadresults ofprevioustestsstoredintheStarlingSVtotheStarlingSV EMRPCapplication. InordertoperformtheseactionsitisnecessarytoinstalltheStarlingSVEMRPC softwaretoaPC.ThesoftwareCDcomeswitheverynewmonitorandcanalso beaccessedbycontactingaCheetahMedicalServicerepresentative. TheUSB portalsoenablesvarioustechnicaloperationssuchasuploadingofsoftware upgrades.
4.Serialport–ToconnectthemonitortoanexternalEMRserialconnection.
5.Ethernet–ToconnectthemonitortoanexternalEMREthernetconnection.
10

1.5 Specifications
|
|
SkinVoltageTolerance: |
±300mV |
FrequencyResponse: |
8Hz |
TimeConstant: |
0.125Sec |
CMRR: |
70db |
Filter: |
75KHz |
SafetyRequirements: |
AccordingtoIEC/EN60601-1standard.ClassI |
|
EquipmentBFtypeappliedpart. |
RatedVoltage/Current: |
Powerswitchsupply100Vto240V |
Ratedfrequency |
50/60Hz. |
Fuses: |
2XT2AL250V |
A/D |
converterat12bit(2.5mVLSB). |
Samplerate |
500persec. |
Features |
Hemodynamicdisplayoptions |
|
Height/Weightunits |
|
DedicatedStarlingSVElectronicMedicalRecords |
|
interface(XlSPDF&XMLfileformats,Password |
|
Protection) |
|
Hemodynamicstatusreportwithvisit-to-visitdata |
|
ClinicalProtocolWizards |
|
EventManagement |
|
TouchScreencontrol |
|
SerialandEthernetconnections |
AmbientTemperatureandHumidity: |
|
|
|
Operationcondition: |
10-40°C/50-104°F,50-75%RH |
Atmosphericpressure: |
700hPato1060hPa |
Storagecondition: |
0-50°C/32-122°F,50-75%RH |
Deliverycondition: |
0-50°C/32-122°F,50-75%RH |
TechnicalCharacteristicsofAcquisitionModule
IsolatedpreamplifierinaccordancewithIEC60601-1standard.
NoteTheStarlingSVisnotdefibrillatorproof.
11

1.6 NIBP
Terminology:
Oscillometry
Theoscillometricmethodofbloodpressuremeasurementisanon-invasivemethod thatmonitorstheamplitudeofcuffpressurechangesduringcuffdeflationto determinearterialbloodpressure.Thecuffpressureisfirstelevatedabovethepatient systolicbloodpressurelevelandthecuffbeginstodeflateatacertainrate.Theinitial riseinamplitudeofthesepressurefluctuationsduringcuffdeflationcorresponds closelytothesystolicbloodpressure.Asthecuffisfurtherdeflated,thesepressure fluctuationsincreaseinamplitudeuntilapeakisreachedwhichisusuallyreferredtoas themeanarterialpressure(MAP).Ascuffdeflationcontinues,thediastolicpressure canbedeterminedbasedupontherapidlydiminishingamplitudeofthepressure fluctuations.Thussystolic,MAPanddiastolicbloodpressurescanbeaccurately obtainedbysupervisingthepressurefluctuationswhilecontrollingthecuffdeflation rate.
mmHg
MillimetersofMercury,whichisthemostcommonunitofmeasureforpressurein non-invasivebloodpressure.
bpm
Beatsperminute,whichisthemostcommonunitofmeasureforpulserate.
Introduction:
TheNIBPmoduleoftheSTARLINGSVdeviceisanoscillometricbloodpressure system. Themoduleisdesignedtotakebloodpressuremeasurementsondemand. Aftereachbloodpressuremeasurement,theModulewilldiscardthepreviousblood pressureresults.AllModuleoperatingparameterswillresettodefaultvaluesatpowerup.AllModulebloodpressurevaluesarediscardedwhentheModuleisreset
PopulationsthatmayusetheNIBPfunction:
Forpediatricandadultpatients,bloodpressuremeasurementsmadewiththe AdvantageOEMBPModuleSeriesareequivalenttothoseobtainedbytrained observersusingthecuff/stethoscopeauscultatorymethodwithinthelimitsprescribed byANSI/AAMISP10(meanerrordifferenceof±5mmHgorless,standard deviationof8mmHgorless).
Bloodpressuremeasurementsdeterminedwiththisdeviceareequivalenttothose obtainedbyatrainedobserverusingthecuff/stethoscopeauscultationmethod, withinthelimitsprescribedbytheAmericanNationalStandard,Electronicor automatedsphygmomanometers.TheNIBPModuleperformancewithcommon arrhythmias,suchasatrialorventricularprematurebeatsoratrialfibrillation,hasbeen verifiedbyuseofapatientsimulator.
12

Forneonatalpatients,bloodpressuremeasurementsmadewiththeAdvantageOEM BPModuleSeriesareequivalenttothoseobtainedbyintra-arterialbloodpressure deviceswithinthelimitsprescribedbyANSI/AAMISP10:1992&2002(meanerror differenceof±5mmHgorless,standarddeviationof8mmHgorless).
Theautomaticselectionbetweenthe3NIBPpopulationmodesisperformedwhen theenteringthepatientdemographicinformationasfollows:
Adult–Ageabove12years(Automatic,default)
Pediatric–Weightabove7Kgandage12andbelow(Automatic) Neonate–Weight2.5Kgandbelow(Automatic)
NOTETheusershallbegiventheoptiontoselectNeonateorPediatricNIBP populationmodeifthepatientweightisbetween2.6Kgand7Kgs.
NOTEinNeonatemodethefollowingrangesapply:
Systolic |
40-130mmHg |
MAP |
26-110mmHg |
Diastolic |
20-100mmHg |
InitialInflationPressure |
90mmHg(default) |
|
Variablefrom60to140mmHg |
Warnings&Precautions
Thisdeviceshouldnotbeusedwhenoscillometricpulsesmaybealteredbyother devicesortechniquessuchasExternalCounterpulsation(ECP)orIntraAortic BalloonPumpCounterpulsation.
DONOTusetheNIBPmoduleforanypurposeotherthanspecifiedinthis manualwithoutwrittenconsentandapprovalfromCheetahMedical.
DONOTusethedeviceinthepresenceofflammablegaseousanesthesiaagents becauseofflamehazard.
DONOTattachthecufftoalimbbeingusedforIVinfusionsoranyother intravascularaccess,therapyoranarterio-venous(A-V)shunt.Thecuffinflationcan temporarilyblockbloodflow,potentiallycausingharmtothepatient.
DONOTapplytheBPcuffoverawoundasthiscancausefurtherinjury.
DONOTForpatientsthathavehadamastectomy,cuffshouldbeappliedto oppositearm.
CAUTIONSubstitutionofacomponentdifferentfromthatsuppliedmayresultin measurementerror.Repairsshouldbeundertakenonlybypersonneltrainedor authorizedbyCheetahMedical.Donotmodifythisequipmentwithoutauthorization fromCheetah-Medical.
13

CAUTIONAccuracyofanybloodpressuremeasurementmaybeaffectedbythe positionofthesubject,hisorherphysicalconditionanduseoutsideoftheoperating instructionsdetailedinthismanual.Interpretationofbloodpressuremeasurements shouldbemadeonlybyaphysicianortrainedmedicalstaff.
CAUTIONAllairhosesandcuffsusedtoconnectthepatienttothemodulemust beapprovedbyCheetahMedical.Hosesofacertainmaterialand/ordurometermay causethemoduletoperforminanimproperfashion.Also,cuffsthatareextremely smallorlargeinvolumemaycauseerrorstooccurdependingontheBPmode selection.AirhosesaswellascuffsareofferedasaccessoriesfromCheetahMedical.
CAUTIONIfthebloodpressurecuffisonthesamelimbasmonitoring equipment(i.e.,pulseoximeterprobe),thepressurizationwithinthecuffcancause temporarylossoffunctionofthemonitoringequipment.
CAUTIONIfthebloodpressurecuffisonthesamelimbasapulseoximeter probe,theoxygensaturationresultswillbealteredwhenthecuffoccludesthebrachial artery.
CAUTIONToobtainaccuratebloodpressurereadings,thecuffmustbethe correctsize,andalsobecorrectlyfittedtothepatient.Incorrectsizeorincorrect fittingmayresultinincorrectreadings.
CAUTIONWhenacuffisusedonapatientforanextendedlengthoftime,be suretooccasionallycheckthelimbforpropercirculation.
CAUTIONIntra-armdifferencesvarybetweenpeople.Donotassumethat measurementsfrombotharmsaresame.
CAUTIONThemodulemaynotoperatecorrectlyifusedorstoredoutsidethe relevanttemperatureorhumidityrangesdescribedinthePerformancespecifications.
CAUTIONIntendedpatientpopulationincludeadult,pediatricandneonate patients.Safetyandeffectivenessonpregnantwomenandneonateshavenotbeen tested.
CAUTIONForneonatepopulations,theclinicaleffectivenessofthisdevicehas notbeenestablishedinthepresenceofdysrhythmias.Arterialreferencesitesincluded femoral,umbilicalandradialarteries.
NOTEForadultandpediatricpopulations,K5wasusedtoclinicallyvalidate diastolicpressure.
CAUTIONCheckthatoperationoftheunitdoesnotresultinprolonged impairmentofthecirculationofthepatient.
14

CAUTIONNeonatesmeasurementsmustalwaysusea3meterspatienthose inordertoavoideoverpressureerrorscausedbyalackofairvolumewithinthe overallpneumaticsystem.
CAUTIONAcompressedorkinkedconnectionhosemaycausecontinuouscuff pressureresultinginbloodflowinterferenceandpotentiallyharmfulinjurytothe patient.
CAUTIONToofrequentBPmeasurementscancauseinjurytothepatientdueto bloodflowinterference.
CAUTIONPressurizationoftheBPcuffcantemporarilycauselossoffunctionof simultaneouslyusedmonitoringequipmentonthesamelimb
WARNINGRegardingtheeffectofbloodflowinterferenceandresultingharmful injurytothePATIENTcausedbycontinuousCUFFpressureduetoconnection tubingkinking.
WARNINGRegardingtheneedtocheck(forexample,byobservationofthelimb concerned)thatoperationoftheAUTOMATEDSPHYGMOMANOMETERdoes notresultinprolongedimpairmentofthecirculationofthebloodofthepatient.
AdverseReactions
Allergicexanthema(symptomaticeruption)intheareaofthecuffmayresult, includingtheformationofurticaria(allergicreactionincludingraisededematous patchesofskinormucousmembranesandintenseitching)causedbythefabric materialofthecuff.
Petechia(aminutereddishorpurplishspotcontainingbloodthatappearsontheskin surface)formationorRumple-Leedephenomenon(multiplepetechia)ontheforearm followingtheapplicationofthecuff,whichmayleadtoIdiopathicthrombocytopenia (spontaneouspersistentdecreaseinthenumberofplateletsassociatedwith hemorrhagicconditions)orphlebitis(inflammationofavein)maybeobserved.
CuffSelection&Placement
Itisimportanttoselectthecuffsizethatisappropriatetothediameterofthepatient's upperarm.UsetheRangeLinesontheinsideofthecufftodeterminethecorrectsize cufftouse.
WrapthecuffaroundthearmmakingsurethattheArteryMarkerisalignedoverthe brachialartery(asshowninFigure1.3).Ifpossible,donotwrapthecuffoverthe patient'sclothing.Thecuffshouldfitsnugtothepatient'sarmformaximum oscillometricsignalquality.Anappropriatesizedcuffshouldbeplacedonthenon-
15

dominatearmwheretheloweredgeofthecuffislocated2cmabovetheantecubital fossa(interiorbendoftheelbow).
Figure 1.3NIBPCuffPlacement
Ensurethattheairhosefromthemonitortothecuffisnotcompressed,crimpedor damaged.
The midpoint of the subject's upper arm should be supported at heart level for proper measurement accuracy. When the cuff is below heart level, measurement results may be higher and when the cuff is above heart level, measurement results maybelowerthancomparativeresultsobtainedatheartlevel.
Pleaserememberthatusingacuffthatisthewrongsizemaygivefalseand misleadingresults.
NoteTheNIBPmoduleisdesignedtoworkwithSunTechcuffsandhoses. TheuseofcuffsandhosesnotsuppliedbyCheetah-MedicalorSunTechmay compromiseperformanceandaccuracy.
Note The initial inflation pressure will be selected via the "Initial Inflation" option that is located on the NIBP Settings' screen unless automatically specified bytheNIBPpatientpopulationmode(Neonate,Pediatric).
CuffsizesavailableforusewiththeStarlingSV:
|
|
|
Range |
Bladder |
Part # |
Description/Size |
|
(cm) |
(cm) |
98-0080-04 |
All-Purp, Small Adult |
|
17-25 |
9.8x20.3 |
98-0080-05 |
All-Purp, Small Adult Long |
|
17-25 |
9.8x20.4 |
98-0080-06 |
All-Purp, Adult |
|
23-33 |
13x27.5 |
98-0080-07 |
All-Purp, Adult Long |
|
23-33 |
13x27.6 |
98-0080-08 |
All-Purp, Large Adult |
|
31-40 |
16.3x33 |
98-0080-09 |
All-Purp, Large Adult Long |
|
31-40 |
16.3x33 |
98-0080-10 |
All-Purp, Thigh |
|
38-50 |
20x40 |
16

NIBPModulePerformance
Method of Measurement
Oscillometric. Diastolic values correspond to Phase 5
Korotkoff sounds.
Blood Pressure Range: |
Systolic: |
ADULT |
40-260 mmHg |
|
|
PEDIATRIC |
40-230 mmHg |
|
|
NEONATE |
40-130 mmHg |
|
MAP: |
ADULT |
26-220 mmHg |
|
|
PEDIATRIC |
26-183 mmHg |
|
|
NEONATE |
26-110 mmHg |
|
Diastolic: |
ADULT |
20-200 mmHg |
|
|
PEDIATRIC |
20-160 mmHg |
|
|
NEONATE |
20-100 mmHg |
Pulse Rate Range: |
30 to 220 BPM (Beats Per Minute) |
|
|
Pulse Rate Accuracy: |
± 2% or ± 3 BPM, whichever is greater |
|
|
Cuff Deflate Rate: |
Deflation step size varies with heart rate, cuff pressure |
||
|
and cuff volume. |
|
|
Initial Inflation Pressure: |
ADULT: |
160 mmHg (Default) |
|
|
|
Variable from 120 to 280 mmHg |
|
|
PEDIATRIC: |
140 mmHg (Default) |
|
|
|
Variable from 80 to 280 mmHg |
|
|
NEONATE: |
90 mmHg (Default) |
|
|
|
Variable from 60 to 140 mmHg |
|
Clinical Accuracy: |
Meets accuracy requirements of ANSI/AAMI SP10, |
||
|
EN1060-4 and ISO 81060-2. |
|
|
Pressure Transducer Accuracy: |
±3mmHg between 0 mmHg and 300 mmHg for |
||
|
operating conditions between 0°C and 50°C |
||
Recommended Frequency of |
The pressure transducer calibration should be verified on |
||
Pressure Transducer Calibration: |
a yearly interval |
|
|
Operating Conditions: |
0°C to 50°C, 15% to 95% non-condensing humidity |
||
Storage Conditions: |
-20°C to 65°C, 15% to 90% non-condensing humidity |
||
Altitude: |
Measurement accuracy is not affected by altitude |
||
Startup Initialization Period: |
7 seconds |
|
|
Minimum time between |
50 milliseconds |
|
|
commands: |
|
|
|
Patient Safety:
Internal operating software ensures that:
Maximum initial inflation time is limited to 75 seconds
Duration of blood pressure reading is limited to 130 seconds (Adult/Peds mode)
120 seconds (Adult/Peds Motion Tolerant mode)
75 seconds (Neonate mode)
Additional redundant safety circuitry oversees normal operation and will override to abort a reading if:
cuff pressure exceeds 300 mmHg (Adult & Pediatric modes) or 150mmHg (Neonate mode) at any time
the cuff has been inflated for 180 seconds (Adult & Pediatric modes) or 90 seconds (Neonate mode)
17

The module meets all relevant parts of the following Safety
Standards:
IEC60601-1:1997 2nd Edition
IEC60601-1:2005 3rd Edition
IEC/EN60601-2-30:1999/2000
IEC 80601-2-30:2009
AAMI SP10:2002(R)2008
ISO 81060-2:2009
EN1060-1:1996+A2:2009
EN1060-3:1997+A2:
ErrorCodeList&Definitions
CAUTIONWenanNIBPerroroccurs,measurementvalueswillstillbereported.
Thesemeasurementvaluesshouldbedisregardedwhenanerrorcodeisreported.
Artifact/ErraticOscillometricSignal
CorrectiveAction:
•Thepatientmayhavebeenmovingtoomuch.
•Checkthatthecuffisinthecorrectpositions.
•Checkthatthecorrectsizecuffisbeingapplied.
Exceededretrycount
CorrectiveAction:
•Thepatientmayhavebeenmovingtoomuch.
•Checkthatthecuffisproperlytightened.
•Checkthatthecuffisinthecorrectposition.
•Checkthatthecorrectsizecuffisbeingapplied.
•Checkthatthereisnoexcessiveclothingbetweenthearmandthecuff.
Exceededmeasurementtimelimit
CorrectiveAction:
•Thepatientmayhavebeenmovingtoomuch.
•Checkthatthecuffisproperlytightened.
•Checkthatthecuffisinthecorrectposition.
•Checkthatthecorrectsizecuffisbeingapplied.
•Checkthatthereisnoexcessiveclothingbetweenthearmandthecuff.
18

PneumaticBlockage
CorrectiveAction:
•Checkthatthehosehasnosharpbendsorispinched.
•Checkthatthepatientisnotlyingonthecuff.
•Checkthatthecuffisinthecorrectposition.
InflateTimeout,AirLeakorLooseCuff
CorrectiveAction:
•Checkthatthehoseisconnectedtothesystemandthecuff.
•Checkthatthecuffisproperlytightened.
•Checkthatthecuffisinthecorrectposition.
•Checkthatthecorrectsizecuffisbeingapplied.
•Checkthatthecuffisnotleakingair.
•Checkthatthehoseconnectionsarenotdamagedorloose.
SafetyTimeout
CorrectiveAction:
•Checkthepatient.
•Checkthatthecuffisinthecorrectposition.
•Thepatientmayhavebeenmovingtoomuch.
•TakeanotherBPreading.
CuffOverpressure
CorrectiveAction:
•Checkthatthecorrectsizecuffisbeingapplied.
•Checkthatthehosehasnosharpbendsorispinched.
•Checkthatthecuffisinthecorrectposition.
•Checkthatthepatientisnotlyingonthecuff.
Powersupplyoutofrangeorotherhardwareproblem
CorrectiveAction:
Servicemayberequired.CallaCheetahMedicalrepresentative.
19

Permissionproblem
CorrectiveAction:
Servicemayberequired.CallaCheetahMedicalrepresentative.
Transduceroutofrange
CorrectiveAction:
Ifpossible,performcalibration.
Servicemayberequired.CallaCheetahMedicalrepresentative.
ADCoutofrange
CorrectiveAction:Servicemayberequired.
CallaCheetahMedicalrepresentative.
20
EEPROMcalibrationdatafailure
CorrectiveAction:
Ifpossible,performcalibration.
Servicemayberequired.CallaCheetahMedical representative
ErrorMeasuringNIBP–CriticalNIBPmoduleerror.
CorrectiveAction:
Servicemayberequired.CallaCheetahMedical representative
DeviceBusy–NIBPdeviceisnotrespondingasitisinthe middleofanactivity.
CorrectiveAction:
•RetrytoobtainanNIBPreading.
•Ifthiserrorpersists,restarttheunitandtryagaintoobtainan NIBPreading.
Ifyoustillencountertheerrormessageafterrestartingtheunit, servicemayberequired.CallaCheetahMedicalrepresentative
21

1.7 Bedside Care
ToensurethattheStarlingSVsignalswillberecordedtoanadequate standard,itisimportanttofollowthebelowguidelines:
StarlingSVSensorPlacement
1)Shavebodyhairandwipethepatient’sskinwithalcohol(70%)ifnecessary. Ifalcoholisusedtopreptheskin,itisadvisedtolettheskindrypriorto placementoftheSTARLINGSVsensorsinordertopreserveoptimalgel performance.Finally,usetheskin-prep“sandpaper”providedinthesensor settoroughentheskin.
PlacetheSTARLINGSVSensorsonthepatient’sskinasseenbellow(Figure1.4)
Figure1.4StarlingSVPrewiredSensorPlacement
NoteWhenthesensorsaremisplacedorbecomedetachedthescreenwilldisplaya warningthatwillnotifytheuserthatthereislossofsignalorpoorsignalquality.
CAUTIONDonotuseStarlingSVsensorsaftertheexpirationdate.
CAUTIONIntheunlikelyeventthatthepatienthasanallergicreactiontothe STARLINGSVsensors,detachthesensorsimmediatelyfromthepatientandavoid anyfurtherplacementofStarlingSVsensorsonthispatient.
1.8 Label
ThefollowinglabelisattachedtotheCheetahSTARLINGSVdevice:
22

1.9 Battery Mode
TheStarlingSVmonitorcanfunctionwithoutexternalpowerconnection.Inthis modeofoperation,whenfullycharged,thebatteriescanprovideupto6hoursof power.Tofullychargethebatterywhenitisdepleted,itisnecessarytoconnectthe devicetotheexternalpowersupplyforapproximately4hours.
Whenthemonitorisdisconnectedfromtheexternalpowersupplythebatteries automaticallypowertheunitsothattheusershouldnotexperienceanyinterruption
inthetest.Thebatterychargeindicator reflectsthatthesystemcurrently operatesinbatterymodeandisdependentonbatterycapacity.Whenconnectedto theexternalpowersourcethebatterychargeindicatorwillindicatethatthebatteryis
reflectsthatthesystemcurrently operatesinbatterymodeandisdependentonbatterycapacity.Whenconnectedto theexternalpowersourcethebatterychargeindicatorwillindicatethatthebatteryis
beingcharged andtheamountofbatterychargeremaininginagraphicway.
andtheamountofbatterychargeremaininginagraphicway.
Ifthebatterylevelfallsto10%orlower, awarningmessagewillappear.
awarningmessagewillappear.
Toprotectthemonitorandthemonitordata,thesystemwillshutdownautomatically withoutuserpromptifthechargelevelfallsunder5%.
CAUTIONIn order to prevent loss of data or study interruption, working in battery mode is only recommended for short periods of time, for example during transport and not for routine testing.
1.10 Warnings and Precautions
Warnings
WARNINGUSFederalLawrestrictsthesaleofthisinstrumentto,oronthe orderofaphysician.
WARNINGWhentheUSBconnectionisnotused,thesocketshouldbecovered withtheUSBcapatalltimes.
23

Precautions
CAUTIONStrictlyfollowthewarninginstructionsinthismanual.
CAUTIONTheinstrumentisfragile;topreventdamagepleasehandlewithcare andavoiddroppingit.Becarefulwhileunpacking.Forsystemrepackinginstructions pleaserefertopage75onthisguide.
CAUTIONEnsurethatthesystemisonlyusedbyatrainedpersonfamiliarwithall systemoperatingprocedures. Hospitalpersonnelshouldcompleteatrainingprogram priortooperatingtheStarlingSV.
CAUTIONToavoiddamagetotheinstrumentorsensors,becarefulofliquid spillagewhilecleaning.
CAUTIONDonotexposetheinstrumentorsensorstosprays,liquidsoranyother typeofsolvent.
CAUTIONToavoiddamagetothesurfaceoftheinstrumentorsensors:
Avoidexcessivepressurewhencleaningthelettering.
Donotusesolventsordetergentsforcleaning.
Donotcleanthesensorswithanabrasive.
NoteScratchesonthesurfaceofthesensorswillcauseinaccuratedatarecording.
CAUTIONToavoiddamagetotheLCDscreen-donotexposetheinstrumentto directsunlightforlongperiodsoftime.
CAUTION Userisprohibitedfromchanging,adding,removingordisassembling anysystemparts. Warrantyshallnotapplytoanydefects,failureordamagecausedby improperuseand/orimproperorinadequatemaintenanceandcare.
CAUTION TheunitisclassifiedasClassIIb,continuouslyoperated,ordinary equipmentwithappliedpartandwithsignalinputoutputparts.Thedeviceisnot intendedforuseinthepresenceofflammablemixtures.
NoteThecontentsofthismanualaresubjecttochangewithoutpriornoticefor improvementofanalysisprecision.
NoteUsedsensorsshouldnotbeplacedwithcommonhouseholdwasteproducts. Contactlocalauthoritiesforthelocationofachemicalwastecollectionprogram nearestyou.
24

1.11Cleaning and Disinfecting the Starling SV Device
CAUTION The Starling SV system is classified as non-waterproof. To avoid damage to the STARLING SV monitor and its accessories (including the STARLING SV patient cable, the NIBP hose and cuff), avoid entrance of liquids during use and cleaning.
CAUTION Be sure to turn the power off and disconnect the AC power cord from the power source before performing cleaning procedures.
CAUTION To avoid damage to the surface of the instrument:
Avoid excessive pressure when cleaning the printed labeling on the monitor frame in the front and rear panel, as well as the adhesive labels on the rear panel
Take care not to scratch the front display while cleaning
CAUTIONStarling SVsensorsare single-use disposable products and should never be reused, cleaned or exposed to liquids. If necessary, replace the sensorsif they become wet or soiled. Reuse of the sensors can cause infection, allergic reaction and loss of hemodynamic parameters.
To clean or disinfect the monitor perform the following:
This instrument requires routine cleaning, which includes removal of any soil or dirt from the external surfaces. A soft cloth dampened lightly with water may be used.
To disinfect external monitor surfaces, and reusable accessories such as the Starling SV patient cable, clean with 70% medical grade alcohol using a dampened soft towel or wipe.
Do not expose the instrument, patient cable orsensorsto sprays, or any other type of solvents.
ForStarling SV accessories labeled as manufactured by third-party suppliers, follow the respective manufacturer’s instructions for recommended care and maintenance.
1.12 Special Patient Populations
Therearepopulationsofspecificinterestinwhichthismonitorhasnotbeen tested,including.
Patientswithcongenitalheartdiseaseassociatedwithcomplexintra-cardiacshunts.
PatientswithContinuousFlowLVADs: TheStarlingSVtechnologyworksby detectingpulsatilechangesinaorticbloodvolume. WithacontinuousflowLVAD, bydefinition,therearelittleifanypulsationsunlesstheheartitselfejects.Therefore,
25

useoftheStarlingSVdeviceinthesepatientscouldbeusefulindetectingthe contributionofthenativehearttothecardiacoutput,butwouldnotdetectthe contributionoftheLVAD. Theresults,therefore,shouldbeinterpretedwiththis inmind.
1.13 Indications for Use
The Starling SV with NIBP and SpO2 functionalities is a portable, hemodynamic monitoring non-invasive Cardiac Output monitoring device that monitors and displays a patient’sCardiac Output (CO) in Ltr/Min with a Non Invasive Blood Pressure (NIBP) function that non-invasively measures and displays blood pressure (diastolic, systolic and mean arterial pressure) and heart rate and with a SpO2 function that non-invasively measures and displays blood oxygen saturation (SpO2). In addition, by slaving off the Philip’s patient monitor the STARLING SV can display continuous blood pressure measurements taken from the arterial line. For slaving the Philip patient monitor contact a Cheetah Medical Service representative The device displays associated hemodynamic parameters based on measurements or calculations of measurements already incorporated into the Starling SV. These parameters are:
Cardiac Index (CI),
Stroke Volume (SV),
Stroke Volume Index (SVI),
Stroke Volume Variation (SVV),
Heart Rate (HR),
Ventricular Ejection Time (VET),
Total Peripheral Resistance (TPR),
Total Peripheral Resistance Index (TPRI),
Cardiac Power (CP),
Cardiac Power Index (CPI),
Blood Oxygenation (SPO2)
Oxygen Delivery Index (DO2I),
Electrical impedance of the chest cavity (Z0),
26

Thoracic Fluid Content (TFC),
Thoracic Fluid Content change from preset time period (TFCd) and
Thoracic Fluid Content from baseline (TFCd0).
Changes in SV, CO and other hemodynamic parameters which are derived by Bioreactance®as a result of posture
The Starling SV with NIBP and SpO2 functionalities is intended for use within hospitals and other healthcare facilities (e.g., outpatient clinics) that provide patient care.
1.14 Limitations of Use
ClinicalSituationsPotentiallyAffectingSTARLINGSVAlgorithm
AccuracyorPerformance
NoteMostoftheclinicalsituationsandpatientconditionslistedbelowmayimpact absolutevalues,butshouldnotaffectdeviceresponsivenessandsensitivity/specificity toassessdirectionalhemodynamicchanges.Theexceptionisincircumstancesof severeexternalpacemakerinterference.
ExamplesofconditionsthatcaninfluencereportedCO,influencemonitor accuracy,orresultinsuboptimalsignalquality.
1.Severeaorticinsufficiency
Theregurgitationfractionassociatedwithseverecasesofaorticinsufficiencymay resultinoverestimationofthenetforwardCO.ThatisbecauseStarlingSV measurestheejectionbutdoesnotsubtractthebackwardregurgitationthattakes placeduringdiastole.
2.Severeanatomicabnormalitiesofthethoracicaorta
Severeanatomicabnormalitiesofthethoracicaorta,suchasalargesyntheticaortic graft,largeaorticaneurysmorlargeaorticdissectioncanimpacttheaccuracyor performanceofhemodynamicparameters.Theabnormalityhastobelargein ordertohaveameaningfulimpactonmonitoraccuracy.
3.Externalpacemakersandinternalpacemakerswithunipolarelectrodes
Usecautioninmonitoringpatientswithexternalpacemakers,andpatientswith relativelyoldermodelsofinternalpacemakerswhichutilizeunipolarelectrodes. Someexternalpacemakersandunipolarinternalpacemakerscanaddelectrical artifacttotheStarlingSVBIOREACTANCEsignal.
27

NoteItispossibletocompletelymitigateorsignificantlyalleviatetheproblemby placingtheStarlingSVsensor2.5inches(approximately6cm)ormoreawayfrom theexternalpacemakerpercutaneouslead. Unipolarinternalpacemakersarerarely usednowadays.
1.15 SpO2 Introduction:
Pulseoximetryisanon-invasivemethodallowingthemonitoringoftheoxygen contentofapatient'shemoglobin.
Asensorisplacedonathinpartofthepatient'sbody,usuallyafingertipor earlobe,orinthecaseofaninfant,acrossafoot.Lightwithredwavelengthsand lightwithinfraredwavelengthsissequentiallypassedfromonesidetoaphoto detectorontheotherside.Changingabsorbanceofeachofthetwowavelengths ismeasured,allowingdeterminationoftheabsorbancesduetothepulsing arterialbloodalone,excludingvenousblood,skin,bone,muscle,fat,and(in mostcases)fingernailpolish.Basedupontheratioofchangingabsorbanceofthe redandinfraredlightcausedbythedifferenceincolorbetweenoxygen-bound (brightred)andoxygen-unbound(darkredorblue,inseverecases)blood hemoglobin,ameasureofoxygenation(SpO2-thepercentofhemoglobin moleculesboundwithoxygenmolecules)canbemade.
PopulationsthatmayusetheSpO2function:
TheStarlingSVSpO2moduleisintendedforusewithneonatal,pediatric,and adultpatientsduringbothnomotionandmotionconditionsandforpatients whoareeitherwellorpoorlyperfused,dependentontheSpO2sensorused.
SpO2SensorsfortheNELL-1SpO2Module:
TheNELLCORDS100SPO2sensorcomessuppliedwiththeSTARLINGSV withtheSpO2functionality. Inaddition,theStarlingSVwithNIBPandSpO2 functionalityisalsocompatiblewithanyofthefollowingNELLCORSpO2 sensorsthathavethefollowingaccuracyspecifications(*Arms):
|
Sensor |
Adults/Pediatrics |
Neonates |
|
|
Accuracy |
Accuracy |
REUSABLE: |
D-YS (Infant to Adult) |
± 3 digits |
± 4 digits |
|
D-YS with D-YSE Ear |
± 3.5 digits |
N/A |
|
Clip |
|
|
|
D-YS with D-YSPD |
± 3.5 digits |
N/A |
|
SpotClip |
|
|
|
DS-100A |
± 3 digits |
N/A |
|
OXI-A/N |
± 3 digits |
± 4 digits |
DISPOSABLE: |
OXI-P/I |
± 3 digits |
N/A |
OxiCliq A: |
± 2.5 digits |
N/A |
|
|
OxiCliq P: |
± 2.5 digits |
N/A |
|
OxiCliq N (adult): |
± 2.5 digits |
±3.5digits |
28

OxiCliq I: |
± 2.5 digits |
N/A |
*Arms:Allaccuraciesareexpressedas±“X”digits.Pulseoximeterequipment measurementsarestatisticallydistributed;abouttwothirdsofpulseoximeter measurementscanbeexpectedtofallinthisaccuracy(ARMS)range.
"Neonatal Accuracy: When sensors are used on neonatal subjects as recommended, the specified accuracy range is increased by + 1 digit, as compared to adult usage, to account for the theoretical effect on oximeter measurements of fetal hemoglobin in neonatal blood. For example, OxiCliqN accuracy on neonates is + 3.5 digits, rather than + 2.5."
CleaningandmaintenanceinstructionfortheSpO2sensoraredescribedinthe specificSpO2sensorusermanualwhichissuppliedwiththesensor.
TheSpO2sensorisconnectedtothemonitorviathededicatedSpO2cablethat connectstothededicatedconnectiononthebackofthemonitor(SeeFigure
1.2).
Placethesensoronathinpartofthepatient’sbody(e.g.,fingertiporearlobe,or inthecaseofaninfant,acrossafoot). TostartSpO2monitoringwhilerunning atest,selectStartSpO2fromthemainmenuifSpO2automaticmodeisnotthe defaultmode. WhenthisisdoneSpO2monitoringshallcommence automatically.
NELL-1SpO2ModuleSpecifications:
ThefollowingaretheSpO2modulespecifications:
Patient Range Neonate through adult
Operating Temperature 0 °C to +60°C
Relative Humidity 15% to 95% non-condensing
Altitude 1,000 feet below sea level to 10,000 feet above sea level
Operating Mechanical Shock Per IEC 600068-2-27, 100G, 6 ms half sine
Operating Sinusoidal Vibration Per IEC 600068-2-6, 10 Hz to 500 Hz, 1 G peak, 10 sweeps/axis
Operating Random Vibration Per IEC 600068-2-34, 20 Hz to 500 Hz, 0.02 g2/Hz
Storage Temperature -40 °C to +70°C
Relative Humidity 15% to 95% non-condensing
Storage Altitude 1,000 feet below sea level to 20,000 feet above sea level
Vibration Per NSTA Project 1A
Drop Per NSTA Project 1A
Saturation 1% to 100% SpO2
SpO2SensorsfortheXPODSpO2Device:
29
 Loading...
Loading...