Chattanooga Intelect TENS Instruction Manual

®
Intelect TENS
Chapter Page
1 INTRODUCTION
1.1 General information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
1.2 Cautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
1.3 Indications for use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
1.4 Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
1.5 Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2
1.6 Adverse Reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
2 PRODUCT DESCRIPTIONS
2.1 Front and Rear Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-6
3 STIMULATION MODES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-9
4 INSTRUCTIONS FOR USE
4.1 Check Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10
4.2 Connect electrodes to lead wires . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11
4.3 Connect lead wires to unit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
4.4 Place electrodes on skin . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
4.5 Adjust Output . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
4.6 Select the mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
Contents

Chapter Page
4.7 Adjust the Pulse Rate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
4.8 Adjust the Pulse Width . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
4.9 Adjust Timer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
4.10 Adjust Channel Amplitude . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
4.11 Turn Unit Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .17
4.12 Patient Compliance Timer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .17
4.13 Portability . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .20
4.14 " Low Battery " indicator . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .20
4.15 Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .21
4.16 Care of Electrodes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .21
4.17 Care of Electrode cords . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .21
5 HANDLING AND STORAGE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .22
6 SPECIFICATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .22
7 ACCESSORIES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .23
8 TROUBLESHOOTING . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .24
9 WARRANTY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .25
1
1.1 General information:
This TENS is a lightweight and portable medical device which can help to reduce pain and discomfort. It utilizes low
electric-current to stimulate muscle nerves to achieve the symptomatic relief of chronic intractable pain, posttraumatic and post-surgical pain.
1.2. Cautions
Federal law (USA) restricts this device to sale by or on the order of practitioners licensed by the State in which they
practice to use or order the use of the device.
1.3. Indications for use:
This device is used in symptomatic relief of chronic intractable pain, post-traumatic and post-surgical pain.
1.4 Warnings:
1.4.1 The long-term effects of chronic electrical stimulation are unknown.
1.4.2 Stimulation should not be applied over the carotid sinus nerves, particularly in patients with a known
sensitivity to the carotid sinus reflex.
1.4.3 Stimulation should not be applied over the neck or mouth. Severe spasm of the laryngeal and pharyngeal
muscles may occur and the contractions may be strong enough to close the airway or cause difficulty in
breathing.
1.4.4 Stimulation should not be applied transthoracically in that the introduction of electrical current into the
heart may cause cardiac arrhythmias.
1. INTRODUCTION

2
1.4.5 Stimulation should not be applied over swollen, infected, or inflamed areas or skin eruptions, e.g., phlebitis,
thrombophlebitis, varicose veins, etc.
1.4.6 Stimulation should not be applied over, or in proximity to, cancerous lesions.
1.4.7 For external use only.
1.4.8 Do not use TENS on the eye area.
1.4.9 This device should be used only under the continued supervision of a licensed medical practitioner.
1.4.10 Safety of TENS devices for use during pregnancy or delivery has not been established.
1.4.11 Electronic equipment such as ECG monitors and ECG alarms may not operate properly when TENS is in use.
1.4.12 Apply the electrodes to clean, dry, and unbroken skin only.
1.4.13 This device should not be used while driving, operating machinery, or during any activity in which
involuntary muscle contractions may put the user at undue risk of injury.
1.4.14 This device should be kept out of the reach of children.
1.4.15 Keep electrodes separate during treatment. Electrodes in contact with each other could result in improper
stimulation or skin burns.
1.5 Precautions:
1.5.1 Caution should be used for patients with suspected or diagnosed heart problems.
1.5.2 Caution should be used for patients with suspected or diagnosed epilepsy.
1.5.3 Caution should be used in the presence of the following:
(a)When there is a tendency to hemorrhage following acute trauma or fracture
(b)Following recent surgical procedures when muscle contraction may disrupt the healing process.
3
(c)Over the menstruating or pregnant uterus
(d)Over areas of the skin which lack normal sensation.
1.5.4 Some patients may experience skin irritation or hypersensitivity due to the electrical stimulation or electrical
conductive medium. The irritation can usually be reduced by using an alternate conductive medium, or
alternate electrode placement.
1.5.5 Electrode placement and stimulation settings should be based on the guidance of the prescribing
practitioner.
1.5.6 This device should be used only with the leads and electrodes recommended for use by the manufacturer.
1.5.7 Isolated cases of skin irritation may occur at the site of the electrode placement following long-term
application.
1.5.8 Effectiveness is highly dependent upon patient selection by a person qualified in the management of painafflicted patients.
1.5.9 If the stimulation levels are uncomfortable or become uncomfortable, reduce the stimulation amplitude to a
comfortable level and contact your physician if problems persist.
1.6 Adverse Reactions:
1.6.1 Possible skin irritation or electrode burn under the electrodes may occur.
1.6.2 Possible allergic skin reaction to tape or gel may occur.
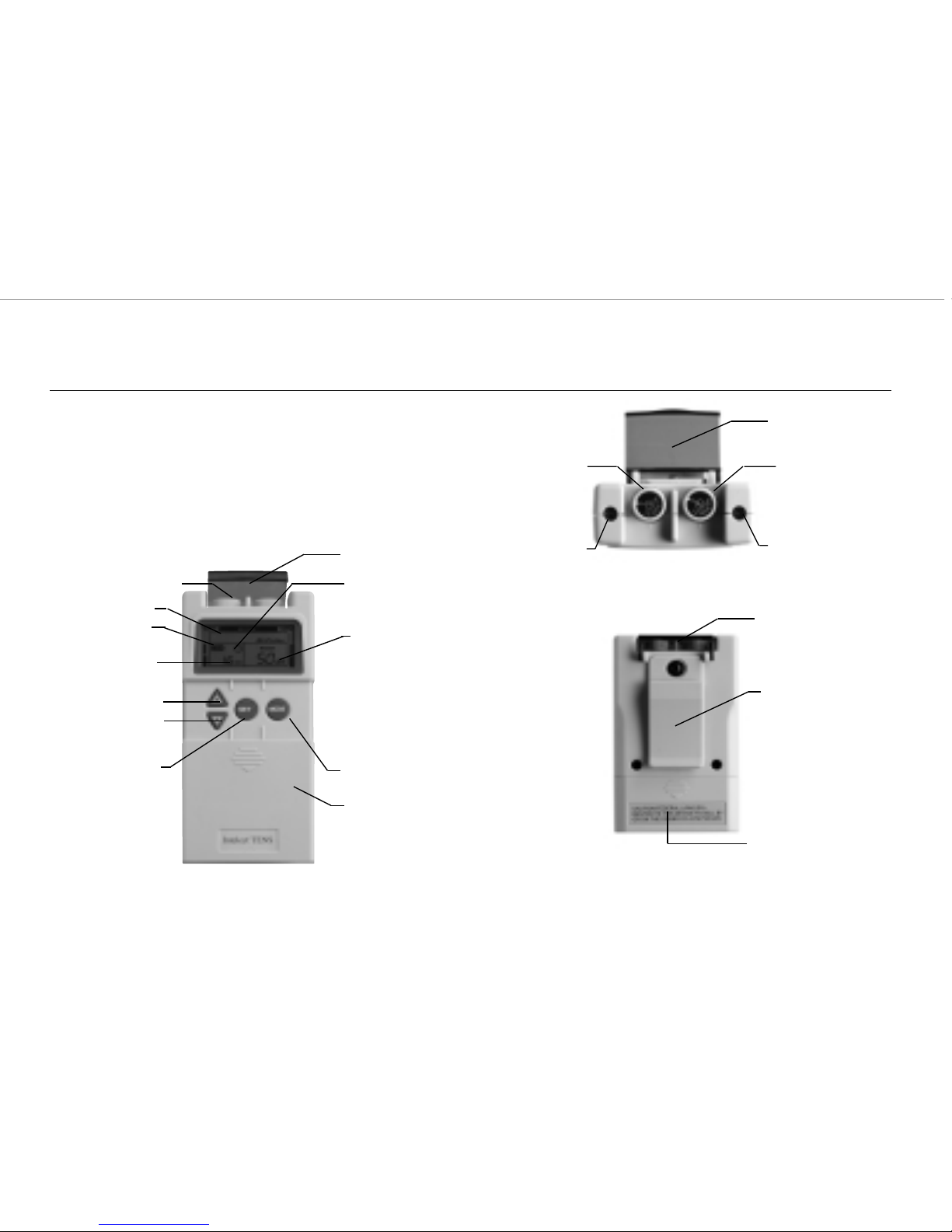
2.1 Front and Rear panel:
2. PRODUCT DESCRIPTIONS
4
Front View
Back View
Top View
Channel 1 On/Off and
Amplitude Control
Channel 1 Output
Receptacle
Channel 2 Output
Receptacle
Channel 2 On/Off and
Amplitude Control
Amplitude Knob
Pulse Rate
Pulse Width
Battery Cover
Clip
Timer
Lid Cover
Mode Button
Mode
Battery Indicator
Set Button
Increase Button
Decrease Button
Channel Output
Knob Cover
Knob Cover
Knob Cover

5
Knob Cover:
An acrylic knob cover protects Amplitude Controls from accidental user touch when
the unit is being used. After adjusting the output, remember to have the cover closed.
Lid Cover:
A panel covers the controls for Mode, Set, INCREASE and DECREASE adjustments. Your medical professional may
ask to set these controls for you and request you leave the cover in place.
Amplitude Controls:
It controls the "INTENSITY" level of stimulating pulses. These controls (located at the top of the unit) regulate the
amplitude, or intensity, of the stimulation and are the ON/OFF CONTROL. The ON indicator signal will stay lit as
long as the unit is working, and mimics the output of the electrical pulse.
Caution: If the stimulation levels are uncomfortable or become uncomfortable, reduce the stimulation
intensity to a comfortable level and contact your physician if problems persist.
"INCREASE" control button (triangle-button)
This button increases the pulse width from 50~300µs, increases the pulse rate from 2 to 150Hz, and increases the
timer from 5 to 90 mins in continuous mode.

6
"DECREASE" control button (inverted triangle-button)
This button decreases the pulse width from 300~50µs, decreases the pulse rate from 150 to 2Hz, and decreases the
timer in continuous mode to from 90 to 5 min.
"MODE" button (round-button on the right side of the control panel)
This button selects a Stimulation-Mode. It offers the mode status from five types of stimulation modes which are
Burst, Normal, MRW (Modulated Rate and Width), SD (Strength Duration) and Bi-Pulse.
"SET" button (round-button on the middle of the control panel)
This button sets the rate, width and timer. Press the 'SET' button to enter a parameter setting mode including rate,
width and timer.
"LCD screen"
This LCD will be utilized to display stimulating mode/ pulse width/ pulse rate and to display the timer. The channel
output is indicated on the left side (Channel 1) and right side (Channel 2) of the LCD screen. The modes show on
the top of the LCD panel. The pulse rate and width show on the middle-right of the screen. The timer and clock
symbol show on the middle-left of the screen; the clock symbol will flash in final 5 min.
Battery compartment
9 Voltage battery- 1 pc
 Loading...
Loading...