Chattanooga Intelect Neo User Manual
Intelect® Neo Clinical
Therapy System
User Manual
Operator and
Installation
Instructions


Intelect® Neo Clinical Therapy System
TABLE OF CONTENTS
FOREWORD . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
PRECAUTIONARY INSTRUCTIONS . . . . . . . . . . . . . . . . 2
SETUP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
COMPONENTS. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
HEAD TO CART ASSEMBLY . . . . . . . . . . . . . . . . . . . . . . . . 5
NEO LEG TO CART ASSEMBLY/ADJUSTMENT . . . . . 6
MODULE INSTALLATION . . . . . . . . . . . . . . . . . . . . . . . . . . 7
MODULE-SPECIFIC INFORMATION . . . . . . . . . . . . . . . . 8
MODULE KIT CONTENTS . . . . . . . . . . . . . . . . . . . . . . . . . 9
THERAPY SYSTEM START-UP . . . . . . . . . . . . . . . . . . . . . 10
NOMENCLATURE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
OPERATOR INTERFACE. . . . . . . . . . . . . . . . . . . . . . . . . . . 11
MODULE SLOTS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
ULTRASOUND APPLICATOR. . . . . . . . . . . . . . . . . . . . . . 12
LASER APPLICATOR. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
PATIENT REMOTE/LASER INTERRUPT SWITCH . . . . 14
PATIENT REMOTE/LASER INTERRUPT SWITCH
INSTALLATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
INSTALLING THE LASER INTERLOCK . . . . . . . . . . . . . 15
GENERAL TERMINOLOGY . . . . . . . . . . . . . . . . . . . . . . . . 16
SYSTEM SOFTWARE SYMBOLS . . . . . . . . . . . . . . . . . . 16
SCREEN DESCRIPTION . . . . . . . . . . . . . . . . . . . . . . . . . . 17
PRECAUTIONARY INSTRUCTIONS . . . . . . . . . . . . . . . 18
INDICATIONS. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
ELECTROTHERAPY INDICATIONS. . . . . . . . . . . . . . . . . 21
SEMG & STIM INDICATIONS . . . . . . . . . . . . . . . . . . . . . . 23
ULTRASOUND INDICATIONS . . . . . . . . . . . . . . . . . . . . . 24
LASER INDICATIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
SPECIFICATIONS. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
SYSTEM SPECIFICATIONS AND DIMENSIONS . . . . 26
POWER COMBINATION AND ELECTROTHERAPY. . 26
VACUUM . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
ULTRASOUND SPECIFICATIONS. . . . . . . . . . . . . . . . . . 27
LASER SPECIFICATIONS . . . . . . . . . . . . . . . . . . . . . . . . . . 27
LASER APPLICATOR TECHNICAL
SPECIFICATIONS. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
LASER PROTECTIVE EYEWEAR SPECIFICATIONS . . 31
LASER LABELS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32
WAVEFORMS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
UTILITIES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
UTILITIES AND OPTIONS . . . . . . . . . . . . . . . . . . . . . . . . . 39
PATIENT PREPARATION . . . . . . . . . . . . . . . . . . . . . . . . . 41
ELECTRODE PLACEMENT . . . . . . . . . . . . . . . . . . . . . . . . 41
DURA-STICK™ ELECTRODES . . . . . . . . . . . . . . . . . . . . . 41
ELECTROTHERAPY PATIENT PREPARATION . . . . . . . 41
IONTOPHORESIS PATIENT PREPARATION. . . . . . . . . 42
VACUUM ELECTRODE PATIENT PREPARATION. . . . 43
SEMG & STIM PATIENT PREPARATION . . . . . . . . . . . . 45
LASER PATIENT PREPARATION . . . . . . . . . . . . . . . . . . . 46
ULTRASOUND PATIENT PREPARATION . . . . . . . . . . . 47
OPERATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48
HOME SCREEN . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48
TREATMENT SCREENS . . . . . . . . . . . . . . . . . . . . . . . . . . . 49
CPS (CLINICAL PROTOCOL SETUP) . . . . . . . . . . . . . . . 50
ELECTROTHERAPY. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 50
VACUUM OPERATION. . . . . . . . . . . . . . . . . . . . . . . . . . . . 52
SEQUENCING . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 53
STRENGTH/DURATION (S/D) CURVE. . . . . . . . . . . . . . 54
IONTOPHORESIS OPERATION . . . . . . . . . . . . . . . . . . . . 55
ULTRASOUND OPERATION. . . . . . . . . . . . . . . . . . . . . . . 56
COMBINATION OPERATION . . . . . . . . . . . . . . . . . . . . . . 57
SEMG OPERATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 59
LASER OPERATION. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61
DATA. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 63
SAVING TO USB FLASH DRIVE/PATIENT DATA. . . . . 63
CUSTOM PROTOCOLS . . . . . . . . . . . . . . . . . . . . . . . . . . . 63
ANATOMICAL LIBRARY . . . . . . . . . . . . . . . . . . . . . . . . . . 66
TROUBLESHOOTING . . . . . . . . . . . . . . . . . . . . . . . . . . . . 67
TROUBLESHOOTING CODES . . . . . . . . . . . . . . . . . . . . . 67
ACCESSORIES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 70
REPLACEMENT ACCESSORIES . . . . . . . . . . . . . . . . . . . . 70
MAINTENANCE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 73
FUSE INFORMATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . 73
INSTRUCTION FOR SOFTWARE UPGRADE . . . . . . . 73
COPY OF MANUAL . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 73
CLEANING THE INTELECT® NEO CLINICAL
THERAPY SYSTEM . . . . . . . . . . . . . . . . . . . . . . . . . . . . 74
VACUUM MAINTENANCE . . . . . . . . . . . . . . . . . . . . . . . . 74
CALIBRATION REQUIREMENTS . . . . . . . . . . . . . . . . . . . 74
SERVICE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 75
WARRANTY REPAIR/OUT OF WARRANTY REPAIR . 75
WARRANTY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 76
APPENDIX. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 77
OVERVIEW OF LASER THERAPY . . . . . . . . . . . . . . . . . . 77
TREATMENT TIPS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 77
COMMON TERMS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 78
ELECTROMAGNETIC COMPATABILITY TABLES . . . . 79
1
Intelect® Neo Clinical Therapy System
FOREWORD
This manual is intended for users of Intelect® Neo Clinical
Therapy System. It contains general information on
operation, precautionary practices, and maintenance.
In order to maximize use, e ciency, and the life of the
system, please read this manual thoroughly and become
familiar with the controls, as well as the accessories before
operating the system.
In addition to the above information, this manual
contains care and installation instructions for the optional
Cart, Channel 1/2 Electrotherapy module, Channel 1/2
Electrotherapy module + sEMG module, Channel 3/4
Electrotherapy module, Vacuum module, Laser module,
and Ultrasound module for the users of the Intelect® Neo
Clinical Therapy System.
Speci cations put forth in this manual were in e ect at
the time of publication. However, owing to DJO’s policy of
continual improvement, changes to these speci cations
may be made at any time without noti cation on the part
of DJO.
Before administering any treatment to a patient, the
users of this equipment should read, understand and
follow the information contained in this manual for each
mode of treatment available, as well as the indications,
contra indications, warnings and precautions. Consult
other resources for additional information regarding the
application of electrotherapy, ultrasound, and laser.
Product Description
The Intelect® Neo Clinical Therapy System is a modular
system used with or without an optional Cart, allowing for
the inclusion of Channel 1/2 Electrotherapy module with
or without sEMG, Channel 3/4 Electrotherapy module,
Vacuum module, Laser module, and Ultrasound module.
PRECAUTIONARY INSTRUCTIONS
The precautionary instructions found in this section and
throughout this manual are indicated by speci c symbols.
Understand these symbols and their de nitions before
operating this equipment. The de nition of these symbols
are as follows:
!"#$%&'
Text with a “CAUTION” indicator explains possible
safety infractions that have potential to cause minor or
moderate injury or damage to the equipment.
(")'%'*
Text with a “WARNING” indicator explains possible safety
infractions that will potentially cause serious injury and
equipment damage.
+"'*,)
Text with a “DANGER” indicator will explain possible safety
infractions that are imminently hazardous situations that
would result in death or serious injury.
Text with a “DANGEROUS VOLTAGE” indicator
serves to inform the user of possible hazards
resulting in the electrical charge delivered to
the patient in certain treatment con gurations
of TENS waveforms.
Warning; Corrosive substance
Warning; Laser beam
To maximize functionality and life of Intelect® Neo, be sure
to:
• Stay current with the latest clinical developments in
the field of electrotherapy, ultrasound, laser therapy,
sEMG and sEMG + electrotherapy.
• Observe all applicable precautionary measures for
treatment.
• Keep informed of appropriate indications and
contraindications for the use of the Intelect® Neo
Clinical Therapy System.
NOTE: This equipment is to be used only under the
prescription and supervision of a licensed medical
practitioner.
Explosion Hazard - Text with an “Explosion
Hazard” indicator will explain possible safety
infractions if this equipment is used in the
presence of ammable anesthetics, mixture
with air, oxygen, or nitrous oxide.
Wear eye protection
NOTE: Throughout this manual, “NOTE”
indicators provide helpful information
regarding the particular area of function being
described.
2

Intelect® Neo Clinical Therapy System
COMPONENTS
Throughout these instructions the terms “left” and “right”
referring to the machine sides are from the perspective of
a user standing in front of the unit.
The Intelect® Neo Clinical Therapy System allows
installation of optional modality modules (except Vacuum
module) by the user. Speci cally designed for use with
the Intelect® Neo Clinical Therapy System, these modules
con gure the system to meet virtually every therapeutic
need that a clinician may have. The components of the
Intelect® Neo Clinical Therapy System are shown below.
NOTE: The Intelect® Neo Clinical Therapy System, when
ordered as a Tabletop System, without cart, is assembled
with Base, as shown below. The only user assembly
required is the installation of the desired Modules
described on page 7. Installation of the Vacuum module
is required by DJO-Authorized Service Persons.
Cart
SETUP
Cart with Vacuum Module
installed
Head
Base
Modules
• Stimulation Channel 1/2
• Stimulation Channel 1/2 + sEMG
• Stimulation Channel 3/4
• Laser
• Ultrasound
• Vacuum (Shown in Cart above)
3

SETUP
COMPONENTS (CONTINUED)
Intelect® Neo Clinical Therapy System
Leadwires
The available leadwires are shown below. If the user
orders Stimulation Channel 1/2 module, the box will
include the blue and green leadwires. Stimulation
Channel 3/4 is the cranberry and orange leadwires. If both
modules are ordered, the box contains all four colored
leadwires. Stimulation modules channel 1/2 with sEMG
includes blue and green sEMG leadwires.
Powercord
Leadwire Holders
4

Intelect® Neo Clinical Therapy System
HEAD TO CART ASSEMBLY
SETUP
The optional Therapy System Cart, PN 70001, is designed
for use with the Intelect® Neo Clinical Therapy System
only and allows the user to easily transport the System
from patient to patient within the clinic as well as store all
necessary accessories, supplies, and applicators used for
the various modalities of the System.
Tools required (not included): #2 Phillips screwdriver and
standard slotted screwdriver.
Remove the Intelect® Neo Clinical Therapy System from
the shipping carton. Visually inspect for damage. Report
any damage to the carrier immediately.
To assemble the Intelect® Neo Head to the Cart, follow
these steps:
1. Remove the top drawer from the Cart. Pull the drawer
open. Press the plastic tabs on both drawer slides
simultaneously in opposite directions, as shown. Pull
the drawer completely out.
3. Place the Neo Head on the cart facing toward the
drawers.
4. Fasten the Neo Head to the cart using four screws to
connect the base to the Neo Head.
2. Remove the base from the head unit first prior to
placing it on the cart. Do this by removing the four
screws from the underside of the base where they
secure to the Neo Head. Retain for use when attaching
the Neo Head to the Cart.
Screws
5. If desired, replace the closed Handles with the open
Handles. Each Handle is attached with four screws as
shown.
6. Reinstall the drawer
5

SETUP
NEO LEG TO CART ASSEMBLY/ADJUSTMENT
Intelect® Neo Clinical Therapy System
The Neo Cart is shipped without the legs attached. To
install or adjust the leg assemblies onto the Neo Cart,
follow these steps:
Neo Leg to Cart assembly/Adjustment
Tools Required:
• 3/16” Hex Key Wrench (provided)
• Flat Washer ¼” Internal diameter, quantity 6,
(provided)
• Socket Head Cap Screw ¼-20 x 1-1/4”, quantity 6
(provided)
1. Remove the bottom drawer from the Cart. Pull the
drawer open. Press the plastic tabs on both drawer
slides simultaneously in opposite directions, as shown.
Completely pull the drawer out.
2. There are two Cart height adjustments. Standard
shown on the left and lowered, shown on the right.
For initial installation, determine the desired height.
Locate three Allen-style bolts for each leg, left and
right and insert, by hand, in their respective slots. Use
the Allen wrench to secure the legs.
NOTE: To Adjust Height at a later time, simply remove
the Allen-style bolts, re-position the legs and re-insert
the bolts.
3. Reinstall the Bottom Drawer.
6

Intelect® Neo Clinical Therapy System
MODULE INSTALLATION
SETUP
All modules (except vacuum module) are installed on
from the left side (when facing the screen) of the Neo
head unit and are each installed in the same manner.
Each has color-coded lead wires that correspond to the
appropriate colored labeling on the modules. Modulespeci c Installation instructions are shown after the
generic instructions. To install the modules in the Intelect®
Neo Clinical Therapy System, follow the steps shown.
NOTE: Tools required (not included): #2 Phillips
screwdriver and standard slotted screwdriver.
The System is programmed to automatically recognize
the new Module(s), therefore, no software installation is
required.
UltrasoundLaser
3. Insert a standard slotted screwdriver (not provided)
into the top slot, pressing down with slight pressure.
Pull the faceplate off (in this example showing
electrotherapy channel 3/4).
4. The module is inserted on the left side of the Neo
Head in the slot as shown in this example (with the
Ultrasound module).
Stimulation 3/4 Stimulation 1/2
1. Ensure that the power cord is removed from the
device.
2. Remove the blank faceplate over the slot from the
left and right sides of the Neo head. (The example
displays the Ultrasound module.
5. Carefully insert the module into the slot, with 32 pins
(2x16) in first. Secure the module in place with gentle
pressure until you feel the module is seated.
7

SETUP
5. Secure the module with a screw provided at the
bottom as shown (using stimulation channel 3/4 as
example).
Intelect® Neo Clinical Therapy System
MODULE-SPECIFIC INFORMATIONMODULE INSTALLATION (CONTINUED)
Ultrasound Cable Insertion
Shown below is the Ultrasound Cable Insertion location.
6. In this example showing the laser faceplate, insert the
faceplate at the bottom and snap into place at the top,
as shown on the left and right sides (Neo allows Laser
access on the left and right sides).
7. Plug in the unit and press the power button, allow
the unit to initialize and then verify that the newly
installed module is shown as available on the Home
screen.
NOTE: Installation of the Intelect® Neo Vacuum Module
into the Cart must be performed by the Selling Dealer’s
Trained Technician.
8

Intelect® Neo Clinical Therapy System
MODULE KIT CONTENTS
Electrotherapy Module Channels 1/2 – PN 70000
• Stimulation module
• Lead wires
• Dura-Stick® 5 cm (2 in) Round Disposable Electrodes
(1 pack of 4)
• Faceplates (to cover module after inserted into main
unit)
Ultrasound Module - PN 70002
• Ultrasound module
• Faceplates (to cover module after inserted into main
unit)
Electrotherapy Module Channels 3/4 – PN 70003
• Stimulation module
• Lead wires
• Dura-Stick® 5 cm (2 in) Round Disposable Electrodes
(1 pack of 4)
• Faceplates (to cover module after inserted into main
unit)
SETUP
Electrotherapy Module Channels 1/2 + sEMG – PN
70004
• Stimulation module (2 channel Stimulation with
sEMG)
• sEMG Leadwires
• Dura-Stick® plus 5 cm (2 in) (1 pack of 4) and
Dura-Stick® plus 3 cm (1.25 in) Round Disposable
Electrodes
• Faceplates (to cover module after inserted into main
unit)
Laser Module – PN 70005
• Laser module
• Protective Eyewear, 2 Pair
• Interlock
• Patient Remote, Laser Interrupt Switch
• Faceplates (to cover module after inserted into main
unit)
Vacuum Module – PN 70006
• Vacuum module
• Vacuum hoses 1&2
• Vacuum hoses 3&4
• 60mm electrodes
• Sponges
9

SETUP
THERAPY SYSTEM START-UP
Intelect® Neo Clinical Therapy System
Complete the following steps for initial setup of the
Intelect® Neo Clinical Therapy System:
1. Plug the Power cord into the back of device. Plug the
other end of the cord into an electrical outlet.
NOTE: The Power Cord may be unplugged from the
back of the cart in an emergency situation.
3. Select desired function on the Home Screen (shown
below).
2. Press the Power button located on the top left portion
of the LCD casing, as shown below:
10
Intelect® Neo Clinical Therapy System
OPERATOR INTERFACE
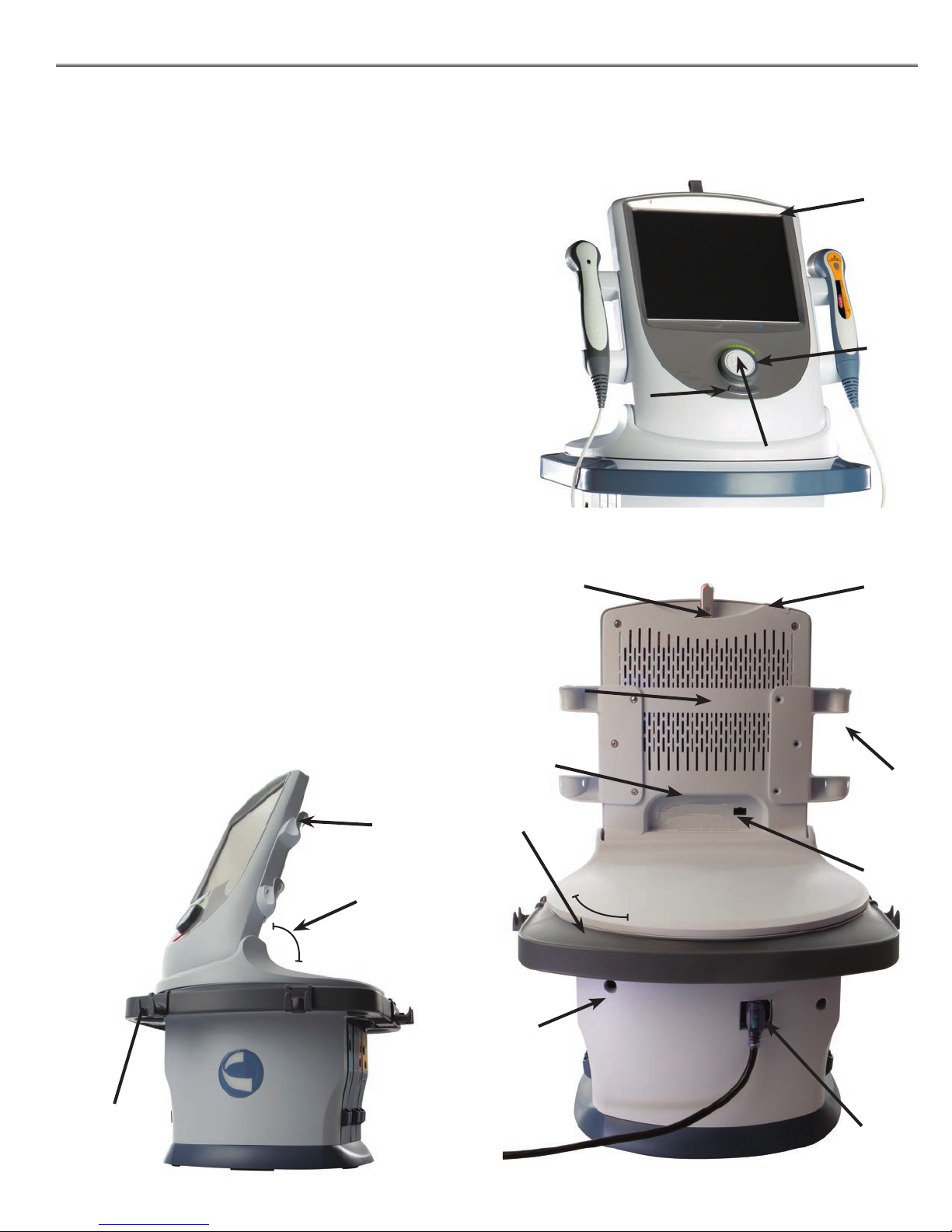
NOMENCLATURE
The Intelect® Neo Clinical Therapy System Operator
Interface contains all the functions and controls necessary
for operator access to all operator utilities, modalities, and
parameters for modi cation and system set up.
1. Color Display
2. Intensity Dial (Gray outer ring)
3. Start/Pause button
4. Stop button
5. ON/OFF switch
6. Ultrasound Applicator holder, left and right sides
7. Laser Applicator holder, left and right sides
8. Patient Remote/ Laser Interrupt Switch Port
9. Mains Power Cord
10. Rear Access Panel
11. Serial Label
12. USB Flash Drive Port (Flash Drive not included)
13. Tilt Screen
14. Swivel function
Front Controls
Rear Access Panel
12
1
2
4
3
5
15. Laser Interlock Port and Icon
16. Leadwire holders
Side Holders
45
11
10
6, 7
6, 7
14
8
º
13
º
90
15
16
9
11

NOMENCLATURE
Intelect® Neo Clinical Therapy System
MODULE SLOTS ULTRASOUND APPLICATOR
1. Laser
2. Stimulation (1 & 2) / Stimulation (1&2) + sEMG
3. Ultrasound
4. Stimulation (3 & 4) – opposite side
Side Module Slots – Left Side
1
2
3
1. Applicator Head
The component of the applicator that makes
contact with the patient during Ultrasound or
Combination therapy.
2. Applicator
The assembly that connects to the system and
incorporates the Applicator.
3. LED
The component of the applicator that indicates if the
Applicator is coupled or uncoupled on the treatment area.
1
3
2
Side Module Slots – Right Side
Add 2nd shot of right side
looking from front
for US, CH3/4, Laser
4
To remove module, take right side o face plate and push
module from right side
12

Intelect® Neo Clinical Therapy System
LASER APPLICATOR
Aperture
NOMENCLATURE
NOTE: When inserting the plugs for the Ultrasound and
Laser modules, be sure to align the at side of the plug
with the at side of the slot and push in gently. This is to
avoid bending the pins in the plug.
Laser Diode
Flat side of slotFlat side of plug
Laser Head
Pause/Resume
Button
LED’s/SLD’s
Laser Head
LED Indicator
(Output Power)
LED Indicator (Output Power) This orange
light illuminates when Laser energy is being
distributed by the applicator.
13
NOMENCLATURE
Intelect® Neo Clinical Therapy System
PATIENT REMOTE/LASER INTERRUPT SWITCH PATIENT REMOTE/LASER INTERRUPT SWITCH
INSTALLATION
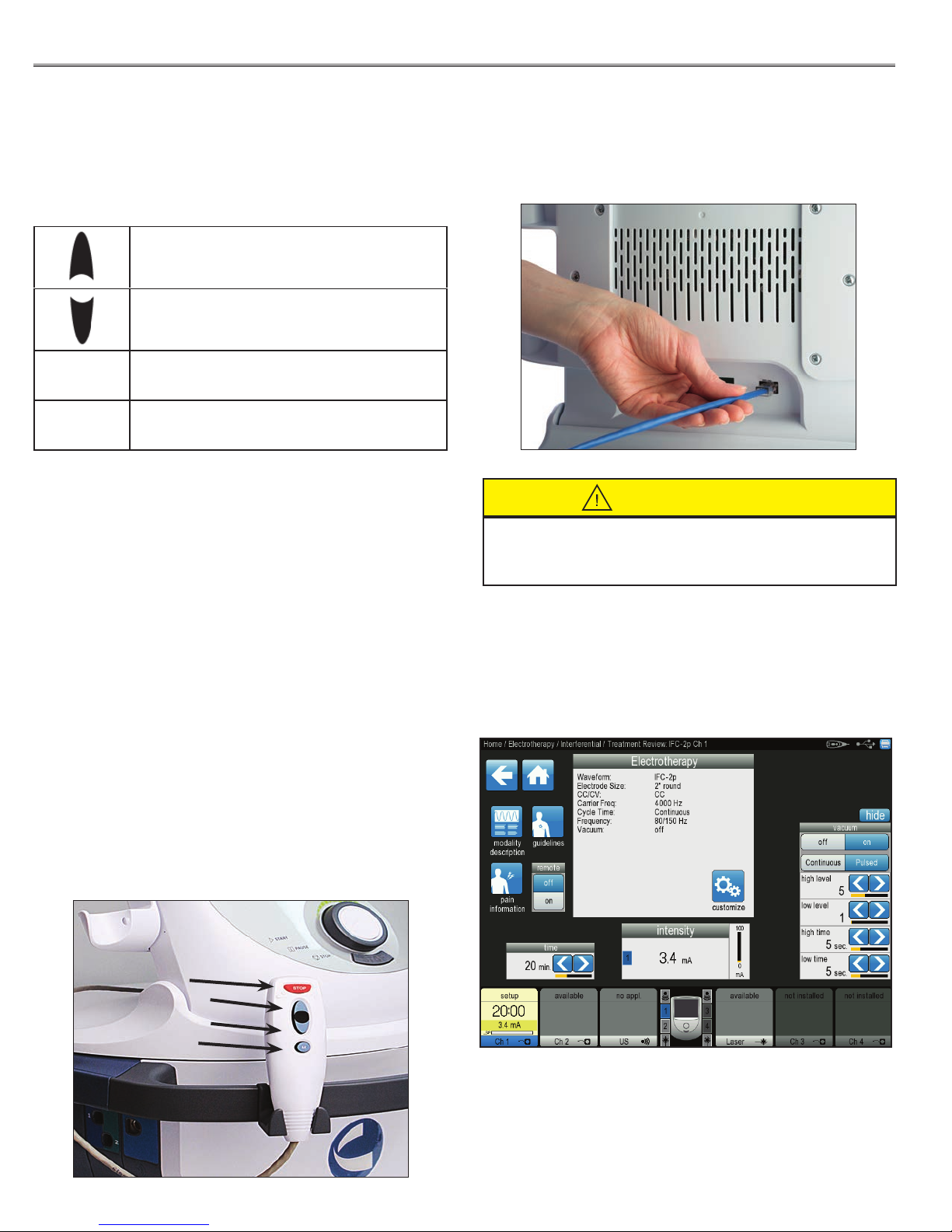
The Intelect® Neo Patient Remote/Laser Interrupt Switch
buttons are described below. By default, the remote is not
assigned to any treatment. When assigned, the buttons
function as follows:
Increase Intensity (1)
Decrease Intensity (2)
STOP STOP/Pause Treatment (3)
M Manual Stimulation (4)
Intensity Up - Operates the same as turning the Intensity
Dial clockwise for the assigned treatment.
To operate the Patient Remote/Laser Interrupt Switch,
plug the remote into the device on the Rear Access Panel
receptacle, as shown below:
!"#$%&'
Intensity Down - Operates the same as turning the
Intensity Knob counter-clockwise for the assigned
treatment.
STOP - Operates as a toggle switch between stopping and
resuming the assigned treatment.
• Operate as pause switch when assign to laser
treatment.
• Operates as pause switch when not assigned to any
treatment, it pauses all treatment.
• Operates as a toggle switch between pause and
resume if assigned to stim treatment.
M (Manual Stimulation) (Electrical Stimulation
treatments only)
When pressed for the rst time, will pause the treatment.
After the rst press, will ramp up and stimulate at desired
output if held down or will pause treatment if released.
Stop (3)
Increase (1)
Decrease (2)
Manual (4)
• Patient Remote/Interrupt Switch is to be used under supervision of
a physician or licensed practitioner only.
Complete the following steps to assign the remote to a
treatment:
1. When the remote is plugged into the unit, a Remote
icon is displayed on the screen. Shown below:
14
Intelect® Neo Clinical Therapy System
2. Press the Remote ON/OFF toggle icon to assign or
unassign the remote to the selected treatment.
The remote can be assigned to only one treatment
at a time however the remote can be reassigned as
needed.
When not in use, the Patient Remote/Laser Interrupt
Switch can be stored by hooking it onto the leadwire
holder clips in the same manner as leadwires and
cables, as demonstrated. Shown below.
NOMENCLATURE
(")'%'*
• Disconnect the system from the power source before attempting
any maintenance, installation, removal or replacement procedures
to prevent electrical shock and possible damage to system.
• The laser interlock must be installed by a professional or qualified
electrician. Serious eye injury can result if the device is not
properly installed. Also, when installing the device for multiple
doors, the resistance total may not exceed 4800 ohm.
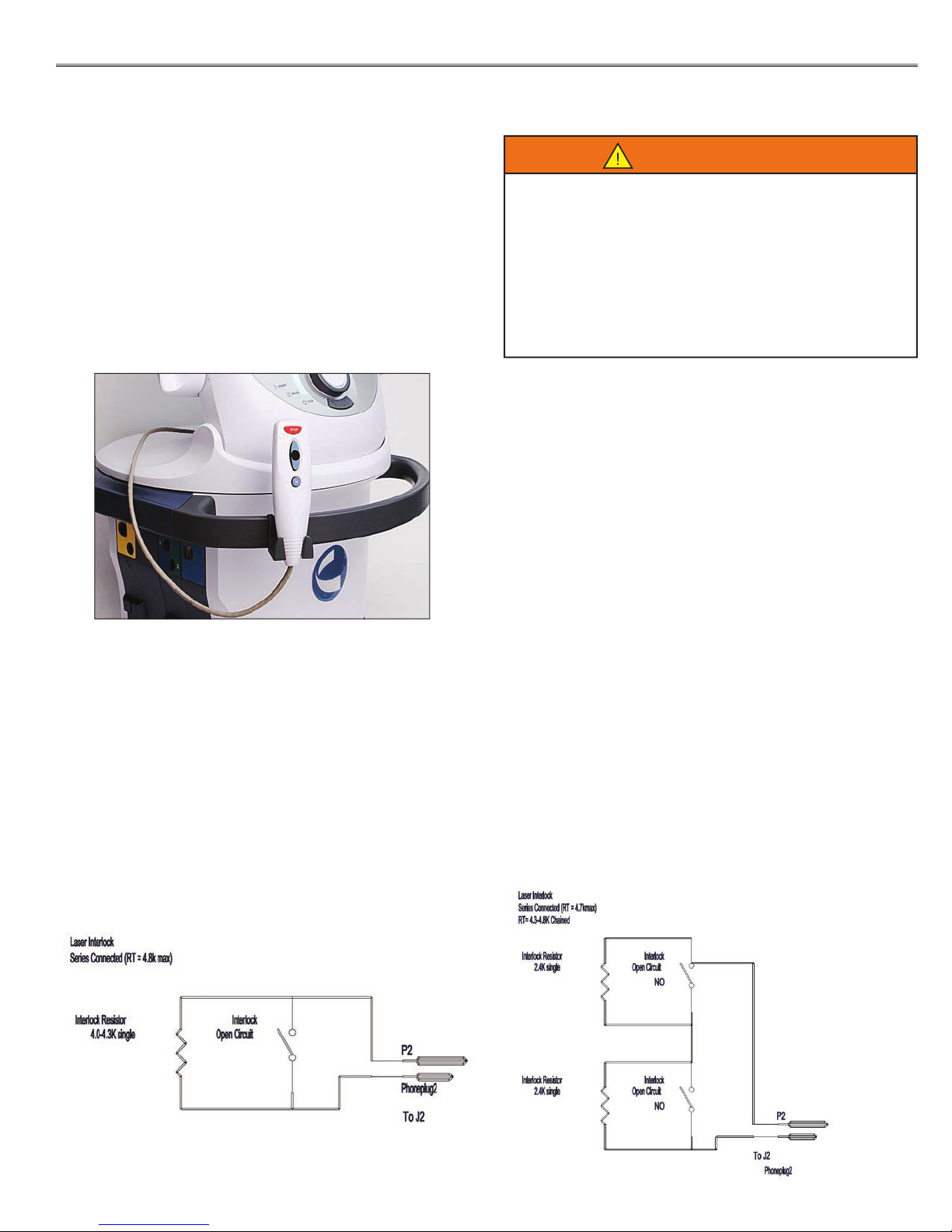
INSTALLING THE LASER INTERLOCK (DOOR
INTERRUPT SWITCH)
The Laser Interlock is an optional safety device designed
to interrupt Laser therapy if the door to the therapy room
is opened. The laser interlock kit consists of a switch
resistor and a jack. Customers must supply the necessary
cable that complies with local and international codes.
Use only quali ed electricians to install the Laser Interlock
Kit.
Diagram for Therapy Room with One Door:
Diagram for Therapy Room with Multiple Doors:
15
NOMENCLATURE
GENERAL TERMINOLOGY
The following are de nitions for the terminology used
throughout this manual. Study these terms to become
familiar with them for ease of system operation, and
control functionality of the Intelect® Neo Clinical Therapy
System.
SYSTEM SOFTWARE SYMBOLS
Intelect® Neo Clinical Therapy System
Back Arrow
Home
Increase/Decrease Parameter
Stim
Ultrasound
Combo
sEMG
Scroll Up or Down in a text box
Laser
Select
Page up
Custom Protocols
CPS
Page down
Customize
Save Data
When pressed will print the screen
contents or Patient Treatment Results
Report to the USB Flash Drive
Indicates a USB Flash Drive is
Inserted
Patient Remote/Laser Shut O Icon
Indicates the Remote is plugged in
Patient Data
Anatomical Library
16
Intelect® Neo Clinical Therapy System
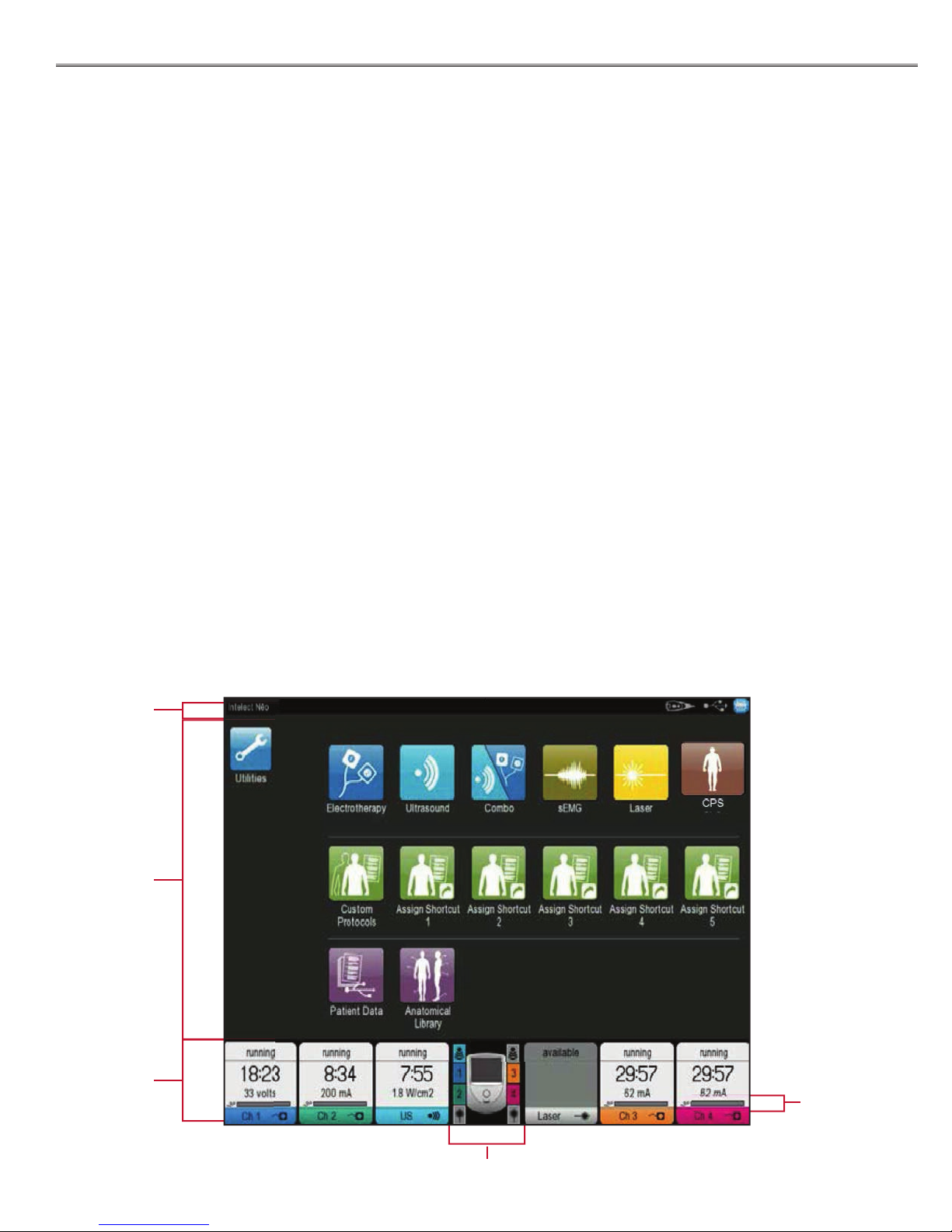
SCREEN DESCRIPTION
NOMENCLATURE
Each screen contains the following areas:
Title bar
Located at the top of each screen and lists the current
screen and previous screens back to the Home screen.
It also contains a Print Screen icon at the top right,
Patient Remote/Laser Shut O , when installed, and a USB
connectivity icon when USB Flash Drive is inserted.
Main area
• Located under the Title bar, this area displays icons
unique to the current screen.
• All screens (except the Home screen) will contain the
Back Arrow Icon to scroll to the previous screen and
the Home icon to return to the Home screen.
Channel area
Located at the bottom of each screen, this screen displays
the following status information about each channel:
Not Installed: Indicates the associated module is not
installed in the unit
Available: Indicates the channel is available for use
Setup: Indicates a treatment for the channel is
currently being setup but treatment has not yet
begun
Running: Indicates a treatment for the channel is
currently running
Paused: Indicates a treatment for the channel is
currently paused
Completed: Indicates a treatment for the channel has
completed
No applicator: Indicates there is not a valid applicator
plugged into the channel’s module (only valid for
Ultrasound and Laser)
Electrode Contact Quality Indicator
(Electrotherapy Channels Only)
Active Channels Indicator
The image below shows the Home screen with modality
and resource icons.
Title Bar
Main Area
Channels
Electrode
Contact
Quality
Indicator
Active Channels Indicator
17

PRECAUTIONARY INSTRUCTIONS
!"#$%&' !"#$%&'
Intelect® Neo Clinical Therapy System
• Read, understand, and practice the precautionary and operating
instructions. Know the limitations and hazards associated with using
any electrical stimulation, laser device or ultrasound device. Observe
the precautionary and operational decals placed on the unit.
• All modalities should be routinely checked before each use to
determine that all controls function normally, especially that the
intensity control does properly adjust the intensity of the ultrasonic
power output in a stable manner. Also, determine that the treatment
time control does actually terminate ultrasonic power output when
the timer reaches zero.
• DO NOT use sharp objects such as a pencil point or ballpoint pen to
operate the buttons and touchscreen on the control panel.
• This unit should be operated at 10°C to 45°C and 0% to 90% Relative
Humidity. The unit should be transported and stored at 0°C to 60°C
and 0% to 95% Relative Humidity.
• Handle Ultrasound Applicator and Laser Applicator with care.
Inappropriate handling may adversely affect its characteristics.
• Before each use, inspect Ultrasound Applicator for cracks, which may
allow the ingress of conductive fluid.
• Failure to use and maintain the Intelect® Neo System, its modules, and
its accessories in accordance with the instructions outlined in this
manual will invalidate the warranty.
• DO NOT permit foreign materials, liquids or cleaning agents to enter
the unit, including, but not limited to, inflammables, water, and
metallic objects from entering the unit, to prevent unit damage,
malfunction, electrical shock, fire, or personal injury.
• If you have difficulty operating the unit after carefully reviewing this
user manual, contact your DJO dealer for assistance.
• DO NOT remove the front and back covers. Doing so may cause unit
damage, malfunction, electrical shock, fire, or personal injury. There
are no user-serviceable parts inside the unit. If a malfunction occurs,
discontinue use immediately and consult dealer for repair service.
• Use of parts or materials other than DJO’s can degrade minimum
safety.
• The Intelect® Neo Clinical Therapy System is not designed to prevent
the ingress of water or liquids. Ingress of water or liquids could cause
malfunction of internal components of the system and therefore
create a risk of injury to the patient.
• Inspect Applicator cables and associated connectors before each use.
• Device is designed to comply with electromagnetic safety standards.
This equipment generates, uses, and can radiate radio frequency
energy and, if not installed and used in accordance with instructions,
may cause harmful interference to other devices in the vicinity.
However, there is no guarantee that interference will not occur in a
particular installation. Harmful interference to other devices can be
determined by turning this equipment on and off. Try to correct the
interference using one or more of the following:
• Reorient or relocate the receiving device
• Increase the separation between the equipment
• Connect the equipment to an outlet on a different circuit from that
to which the other device(s) are connected and consult the factory
field service technician for help.
• Consult your authorized DJO dealer for help.
• Do not operate this unit when connected to any unit other than DJO
devices or accessories specifically described in user or service manuals.
• Use of controls, adjustments or performance of procedures other than
those specified herein may result in hazardous exposure to Laser
energy.
• The Intelect® Neo Vacuum Electrode Module is designed to operate
only when properly installed in the Intelect® Neo Clinical Therapy
System Cart.
• DO NOT operate the system in an environment of shortwave diathermy
use.
• Before each use, inspect Vacuum Electrode Cups and Lead Hoses for
cracks and damage which may not allow the vacuum to properly
secure the electrodes.
• Drain the Vacuum Electrode Module water reservoir regularly to
prevent excessive accumulation from electrode sponge water.
• Periodic flushing of the Vacuum system and the Vacuum reservoir are
required to maintain factory functionality of the Vacuum Electrode
Module. Refer to the Maintenance Section of this manual for proper
instructions.
• DO NOT disassemble, modify, or remodel the unit or accessories. This
may cause unit damage, malfunction, electrical shock, fire, or personal
injury.
18

Intelect® Neo Clinical Therapy System
PRECAUTIONARY INSTRUCTIONS
(")'%'*
• This device should be used only under the continued supervision of a
physician or licensed practitioner.
• Be sure to read all instructions for operation before treating patient.
• Make certain the unit is electrically grounded by connecting only to a
grounded electrical service receptacle conforming to the applicable
national and local electrical codes.
• Care must be taken when operating this equipment around other
equipment. Potential electromagnetic or other interference could
occur to this or to the other equipment. Try to minimize this
interference by not using other equipment in conjunction with it.
• The safety of TENS waveforms for use during pregnancy or birth has
not been established.
• TENS is not effective for pain of central origin. (This includes
headache.)
• TENS waveforms have no curative value.
• Electronic monitoring equipment (such as ECG monitors and ECG
alarms) may not operate properly when electrical stimulation is in use.
(")'%'*
• Clean applicators after each use, otherwise it can lead to cross
contamination and infection.
• Clean vacuum electrodes, sponges and hoses before each use. Lack of
proper cleaning and maintenance may lead to cross-contamination
and infection.
• When the laser module is not in use, it should be protected against
unqualified use.
• Do not treat through clothing.
• Stop treatment immediately if patient experiences discomfort or pain.
• Do not apply laser on an area of skin that has lotion or ointments
applied as burns may occur.
• Do not use laser on or over a tattoo.
• The laser head must be cleaned with a disinfectant cleaner
(i.e. Virex® II 256) or germicidal cloth (i.e. PDI Sani-Cloth® Plus/Hb)
between each therapy session. Ensure no liquids enter into the laser
head while cleaning. Do not use any chlorine-based cleaners on the
laser head.
• TENS is a symptomatic treatment, and as such, suppresses the
sensation of pain which would otherwise serve as a protective
mechanism.
• Inspect the plastic lens of the laser head for blemishes, deformation,
pitting, scratches, discoloration, and cleanliness before each use.
• Do not drop the applicator or unit on hard surfaces or submerge in
water. These actions will damage the applicator and unit. Damage
resulting from these conditions is not covered under the warranty.
• Use of controls or adjustments or performance of procedures other
than those specified herein may result in hazardous exposure to Laser
energy.
• This device should be kept out of the reach of children.
• Use of accessories other than those specified in this User Manual may
increase electrical emissions and decrease electrical immunity of the
device.
• DO NOT operate this unit in an environment where other devices are
being used that intentionally radiate electromagnetic energy in an
unshielded manner.
• Contaminated sponges, electrodes, leadwires, and gel can lead to
infection.
• Use of contaminated sponge or electrodes with corrosion with vacuum
system can result in possible infection or skin irritation.
• Use of electrode with degraded hydro-gel can result in infection to the
skin burn.
• The color of skin, age of lesion, depth of lesion, sensitivity of the
patient, tissue type and medications that increase sensitivity to light
may affect therapy.
• Powered muscle stimulators should be used only with the leads and
electrodes recommended for use by the manufacturer.
• In the event of all 300-Level or a 200-Level error message that cannot
be resolved, immediately stop all use of the system, and contact the
dealer or DJO for service. Errors and Warnings in these categories
indicate an internal problem with the system that must be tested by
DJO or a Trained Technician before any further operation or use of the
system.
• Use of a system that indicates an Error or Warning in these
categories may pose a risk of injury to the patient, user, or
extensive internal damage to the system.
• Use of controls or adjustments or performance of procedures other
than those specified herein may result in hazardous exposure to
ultrasonic energy.
• Before administering any treatment to a patient you should become
acquainted with the operating procedures for each mode of treatment
available, as well as the indications, contraindications, warnings and
precautions. Consult other resources for additional information
regarding the application of each mode of treatment.
• Disconnect the system from the power source before attempting any
maintenance, installation, removal or replacement procedures to
prevent electrical shock and possible damage to system.
• Use of electrode on multiple patients can lead to infection.
19

PRECAUTIONARY INSTRUCTIONS
Intelect® Neo Clinical Therapy System
(")'%'*
• Keep electrodes separated during treatment. Electrodes in contact
with each other could result in improper stimulation or skin burns.
• Long term effects of chronic electrical stimulation are unknown.
• Stimulation should not be applied over the anterior neck or mouth.
Severe spasm of the laryngeal and pharyngeal muscles may occur and
the contractions may be strong enough to close the airway or cause
difficulty in breathing.
• Stimulation should not be applied transthoracically in that the
introduction of electrical current into the heart may cause cardiac
arrhythmia.
• Stimulation should not be applied over swollen, infected, and
inflamed areas or skin eruptions, e.g., phlebitis, thrombophlebitis,
varicose veins, etc.
• Stimulation should not be applied over, or in proximity
to, cancerous lesions.
• Electrotherapy output current density is related to electrode size.
Improper application may result in patient injury. If any question
arises as to the proper electrode size, consult a licensed practitioner
prior to therapy session.
• The Intelect® Neo Clinical Therapy System optional modules and
associated accessories are designed for use only with the Intelect® Neo
Clinical Therapy System.
• Remove the Ultrasound or Laser Applicator by pulling the cable
connector only. DO NOT remove by pulling the cable.
• Vacuum electrodes should not be used on patients with thin, papery
skin. Vacuum may lead to contact difficulty and bruising.
• Vacuum electrodes are not suitable for patients who are taking
steroids, due to the likelihood of bruising.
• Output current density is related to electrode size. Improper
application may result in patient injury. If any question arises as to the
proper electrode size, consult a licensed practitioner prior to therapy
session.
• Do not apply the Ultrasound Applicator to the patient during the Head
Warming period. Applicator must remain in Applicator Hook during the
Head Warming period.
• Some patients are more sensitive to laser output (i.e., patients taking
medications that increase sensitivity to light) and may experience a
reaction similar to a heat rash.
• Before each Laser use, clean the plastic lens with a clean cloth. Make
certain to apply with a clean cloth. Failure to clean the lens between
patient therapy sessions could cause beam fragmentation, which may
reduce the effectiveness of the treatment.
+"'*,)
• Stimulus delivered by the TENS waveforms of this
device, in certain configurations, will deliver a charge of
25 microcoulombs (µC) or greater per pulse and may be
sufficient to cause electrocution. Electrical current of this
magnitude must not flow through the thorax because it
may cause a cardiac arrhythmia.
• Handle, clean and dispose of components and accessories
that have come in contact with bodily fluids according to
National, Local and Facility rules, regulations and
procedures.
• This unit is considered to be a Class 3B Laser product and
thus emits visible and invisible Laser radiation (IR). Avoid
direct eye exposure to the Laser beam. The symbol to the
left is located on the back of the applicator and indicates
the active radiant surface (the area on the applicator that
emits infrared Laser energy and the direction of the beam
of light).When the unit is on, not all wavelengths are
visible to the naked eye. Therefore, when performing any
operational or functional check, always wear Chattanooga
laser protective eyewear.
• The solvents of adhesives and flammable solutions used
for cleaning and disinfecting should be allowed to
evaporate before the unit is used.
• DO NOT connect the unit to an electrical supply without
first verifying that the power supply is the correct voltage.
Incorrect voltage may cause unit damage, malfunction,
electrical shock, fire, or personal injury. Your unit was
constructed to operate only on the electrical voltage
specified on the Voltage Rating and Serial Number Plate.
Contact your DJO dealer if the unit is not properly rated.
• Laser protective eyewear should be worn during laser
treatment by the operator and patient to block infrared
light energy from the eyes during treatment.
• DO NOT point the Laser beam directly into human or
animal eyes. The lens of the eye does not detect the
invisible, coherent Laser beams, potentially resulting in
permanent retinal damage.
• Device is not designed to be used in oxygen rich
environment. Explosion hazard if the device is used in the
presence of flammable anesthetic mixture with air,
oxygen, or nitrous oxide.
20

Intelect® Neo Clinical Therapy System
ELECTROTHERAPY INDICATIONS
INDICATIONS
Indications
The Intelect® Neo Clinical Therapy System o ers VMS, VMS
Burst, VMS-FR, Russian, TENS, TENS HAN, High Voltage
Pulsed Current (HVPC), Interferential, and Premodulated
Waveforms, providing the following bene ts:
• Relaxation of muscle spasms
• Prevention or retardation of disuse atrophy
• Increase local blood circulation
• Muscle re-education
• Maintaining or increasing range of motion
• Immediate postsurgical stimulation of calf muscles to
prevent venous thrombists
Additional Indications for Microcurrent, Interferential,
Premodulated, VMS™, VMS™ Burst, VMS™ FR, TENS, TENS
HAN Waveforms:
• Symptomatic relief and management of chronic,
intractable pain
• Post-traumatic acute pain
• Post-surgical acute pain
Indications for DC (Direct Current) Mode:
• Relaxation of muscle spasm
Indications for Iontophoresis
• Calcium deposits, calcific tendinitis
• Edema
• Myofascial trigger points (chronic)
• Scar tissue, adhesions
• Scleroderma
• Gouty arthritis
• Wounds, ulcers
• Inflammation
• Pain management
• Achilles Tendinopathy
• Carpal Tunnel Syndrome
• Epicondylitis
• Gouty arthritis
• Plantar Fasciitis
Contraindications
The Intelect® Neo Clinical Therapy System should NOT be
used under the following conditions:
• Do not use for symptomatic local pain relief unless
etiology is established or unless a pain syndrome has
been diagnosed
• Do not use when cancerous lesions are present in the
treatment area
• Do not apply stimulation over swollen, infected,
inflamed areas or skin eruptions (e.g., phlebitis,
thrombophlebitis, varicose veins, etc.)
• Do not use when patient is suspected or known to
have infectious disease and/or disease where it is
advisable, for general medical purposes, to suppress
heat or fevers
• Do not place electrode placements to the carotid sinus
region (anterior neck) or transcerebrally (through the
head)
• Do not use on pregnant women. Safety has not
been established for the use of therapeutic electrical
stimulation during pregnancy
• Do not use powered muscle stimulators or TENS
waveforms on patients with cardiac demand
pacemakers
Indications for FES:
• Stimulation of the muscles of the leg and ankle of
partially paralyzed patients to provide flexion of the
foot and thus improve the patient’s gait.
21

INDICATIONS
ELECTROTHERAPY INDICATIONS (CONTINUED)
Additional Precautions
• Use caution for patients with suspected or diagnosed
heart problems
• Use caution for patients with suspected or diagnosed
epilepsy
• Use caution in the presence of the following:
- When there is a tendency to hemorrhage
following acute trauma or fracture
- Following recent surgical procedures when
muscle contraction may disrupt the healing
process
- Over a menstruating or pregnant uterus
- Over areas of the skin that lack normal sensation
• Some patients may experience skin irritation or
hypersensitivity due to the electrical stimulation
or electrical conductive medium. The irritation can
usually be reduced by using an alternative conductive
medium or an alternative electrode placement
• Electrode placement and stimulation settings
should be based on the guidance of the prescribing
practitioner
• Powered muscle stimulators should be used only with
the lead wires and electrodes recommended for use
by the manufacturer
• With TENS waveforms, isolated cases of skin irritation
may occur at the site of electrode placement following
long-term application
• The effective management of pain by TENS waveforms
is highly dependent upon patient selection by a
person qualified in pain management
Intelect® Neo Clinical Therapy System
Adverse E ects
• Skin irritation and burns beneath the electrodes
have been reported with the use of powered muscle
stimulators
• Potential adverse effects with TENS are skin irritation
and electrode burns.
22

Intelect® Neo Clinical Therapy System
SEMG & STIM INDICATIONS
INDICATIONS
Indications
• Stroke rehab by muscle re-education
• Relaxation of muscle spasms
• Prevention or retardation of disuse atrophy
• Increase local blood circulation
• Muscle re-education
• Maintaining or increasing range of motion
Indications for EMG alone:
To determine the activation timing of muscles for:
• Retraining of muscle activation
• Coordinating of muscle activation
Any indication of the force produced by muscle for
control and maintenance of muscle contractions.
• Relaxation muscle training
• Muscle re-education
Indications for incontinence:
Provide biofeedback for the purpose of rehabilitation of
weak pelvic floor muscles for the treatment of urinary
Incontinence.
Contraindications
• The Intelect® Neo Clinical Therapy System should not
be used under the following conditions:
• Do not use for symptomatic local pain relief unless
etiology is established or unless a pain syndrome has
been diagnosed
• Do not use when cancerous lesions are present in the
treatment area
• Do not apply stimulation over swollen, infected,
inflamed areas or skin eruptions (e.g., phlebitis,
thrombophlebitis, varicose veins, etc.)
• Other contraindications are patients suspected of
carrying serious infectious disease and or disease
where it is advisable, for general medical purposes, to
suppress heat or fevers
• Do not place electrode placements to the carotid sinus
region (anterior neck) or transcerebrally (through the
head)
• Safety has not been established for the use of
therapeutic electrical stimulation during pregnancy
• Do not use powered muscle stimulators or TENS
waveforms on patients with cardiac demand
pacemakers
Additional Precautions
• Use caution for patients with suspected or diagnosed
heart problems
• Use caution for patients with suspected or diagnosed
epilepsy
• Use caution in the presence of the following:
- When there is a tendency to hemorrhage
following acute trauma or fracture
- Following recent surgical procedures when
muscle contraction may disrupt the healing
process
- Over a menstruating or pregnant uterus
- Over areas of the skin that lack normal sensation
• Some patients may experience skin irritation or
hypersensitivity due to the electrical stimulation
or electrical conductive medium. The irritation can
usually be reduced by using an alternative conductive
medium or an alternative electrode placement.
• Electrode placement and stimulation settings
should be based on the guidance of the prescribing
practitioner
• Powered muscle stimulators should be used only with
the lead wires and electrodes recommended for use
by the manufacturer
• With TENS waveforms, isolated cases of skin irritation
may occur at the site of electrode placement following
long term application
• The effective management of pain by TENS waveforms
is highly dependent upon patient selection by a
person qualified in the management of pain patients
Adverse E ects
• Skin irritation and burns beneath the electrodes
have been reported with the use of powered muscle
stimulators
• Potential adverse effects with TENS are skin irritation
and electrode burns
23

INDICATIONS
ULTRASOUND INDICATIONS LASER INDICATIONS
Intelect® Neo Clinical Therapy System
Indications
Application of therapeutic deep heat for the treatment of
selected sub-chronic and chronic medical conditions such
as:
• Relief of pain, muscle spasms and joint contractures
• Relief of pain, muscle spasms and joint contractures
that may be associated with:
- Adhesive capsulitis
- Bursitis with slight calcification
- Myositis
- Soft tissue injuries
- Shortened tendons due to past injuries
and scar tissues
• Relief of sub-chronic, chronic pain and joint
contractures resulting from:
- Capsular tightness
- Capsular scarring
Contraindications
• This device should not be used for symptomatic local
pain relief unless etiology is established or unless a
pain syndrome has been diagnosed
• This device should not be used when cancerous
lesions are present in the treatment area
• Other contraindications are patients suspected
of carrying serious infectious disease and disease
where it is advisable for general medical purposes to
suppress heat or fevers
• This device should not be used over or near bone
growth centers until bone growth is complete
• This device should not be used over the thoracic area
if the patient is using a cardiac pacemaker
• This device should not be used over a healing fracture
• This device should not be used over or applied to the
eye
• This device should not be used over a pregnant uterus
• Tissue necrosis might result if the device is used on
ischemic tissues in individuals with vascular disease,
where the blood supply would not keep up with the
metabolic demand
Additional Precautions
Additional precautions should be used when ultrasound is
used on patients with the following conditions:
• Over an area of the spinal cord following a
laminectomy, i.e., when major covering tissues have
been removed
• Over anesthetic areas
• On patients with hemorrhagic diatheses
Indications
To provide topical heating for the following:
• Increasing local blood circulation
• Relieving minor muscle and joint aches, pains, and
stiffness
• Relaxing muscles
• Relieving muscle spasms
• Relieving minor pain and stiffness associated with
arthritis
• Promoting nerve regeneration, bone growth, and
ligament repair
• Healing wounds
Contraindications
The Intelect® Neo Clinical Therapy System Laser should
NOT be used:
• Where analgesia may mask progressive pathology,
and where the practitioner would normally avoid
the use of any other analgesia in order to retain the
beneficial aspects of pain
• For direct aim into the eyes of humans or animals over
areas injected with steroids in the past 2-3 weeks
• Over areas that are suspicious or contain potentially
cancerous tissue
• Over areas of active hemorrhage
• Over a pregnant uterus
• Over the neck (thyroid or carotid sinus region) or chest
(vagus nerve or cardiac region of the thorax)
• Directly over areas with open wounds, unless covered
with a clear protective barrier
• Treatment over sympathetic ganglia
• For symptomatic local pain relief unless etiology
is established or unless a pain syndrome has been
diagnosed
• On patients suspected of carrying serious infectious
disease and/or disease where it is advisable, for
general medical purposes, to suppress heat or fevers
• Over or near bone growth centers until bone growth is
complete
• Over the thoracic area if the patient is using a cardiac
pacemaker
• Over or applied to the eye
• On ischemic tissues in individuals with vascular
disease where the blood supply would be unable to
follow the increase in metabolic demand and tissue
necrosis might result
24
 Loading...
Loading...