Chattanooga Intelect Advanced Series User Manual

Moving
Rehabilitation
Forward™
!
Vacuum Electrode Module
User Manual
Operation Instructions for:
2774 - Vacuum Electrode Module with
Therapy System Cart
2785 - Vacuum Electrode Module
DJO is an ISO 13485 Certified Company

TABLE OF CONTENTS
Foreword. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Electrotherapy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-5
Precautionary Instructions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4
Electrotherapy Indications, Contraindications and Adverse Effects . . . . . . . . . . . . . . .5
Nomenclature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-7
Intelect Advanced Vacuum Electrode Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6
Symbol Definitions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7
Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8
System Set Up . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9
Electrode and Patient Preparation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-12
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Replacement Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15-16
Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Intelect® Advanced Vacuum Electrode Module

FOREWORD
This manual has been written for the owners of an Intelect Advanced Vacuum Electrode Module. It contains general information on the
operation, precautionary practices and maintenance information. In order to maximize use, efficiency and the life of your system, please
read this manual thoroughly and become familiar with the controls as well as the accessories before operating the system with the
Vacuum.
This manual contains general safety, operating, maintenance and care instructions for the optional Two (2) Channel Vacuum Electrode
Module for the owners and operators of the Intelect Advanced Therapy Electrotherapy and Combination systems. Instructions for
additional options such as sEMG, sEMG + Electrical Stimulation, Laser, Battery and Two (2) Channel Electrotherapy Modules are found in
their respective User Manuals which contain operation and installation instructions.
Specifications put forth in this manual were in effect at the time of publication. However, owing to DJO, LLC's policy of continual
improvement, changes to these specifications may be made at any time without obligation on the part of DJO, LLC.
Before administering any treatment to a patient, the users of this equipment should read, understand and follow the information
contained in this manual for each mode of treatment available, as well as the indications, contraindications, warnings and precautions.
Consult other resources for additional information regarding the application of electrotherapy.
Intelect® Advanced Vacuum Electrode Module
Product Description
The Intelect Advanced Vacuum Electrode Module is a two channel (Channels 1 and 2) vacuum electrotherapy option that allows the
clinician to have available vacuum-applied electrodes for use in electrotherapy treatment sessions.
Stay current with the latest clinical developments in the field of electrotherapy. Observe all applicable precautionary measures for
treatment.
Keep informed on appropriate indications and contraindications for the use of electrotherapy.
This equipment is to be used only under the prescription and supervision of a licensed practitioner.
The Intelect Vacuum Electrode Module is not for sale in the United States of America.
© 2011 DJO, LLC. All rights reserved. Any use of editorial, pictorial, or layout composition of this publication without express written consent from DJO, LLC is strictly prohibited. This publication
was written, illustrated, and prepared for distribution by DJO, LLC.
1
ELECTROTHERAPY
PRECAUTIONARY INSTRUCTIONS
The precautionary instructions found in this section and throughout this
manual are indicated by specific symbols. Understand these symbols and
their definitions before operating this equipment. The definition of these
symbols are as follows:
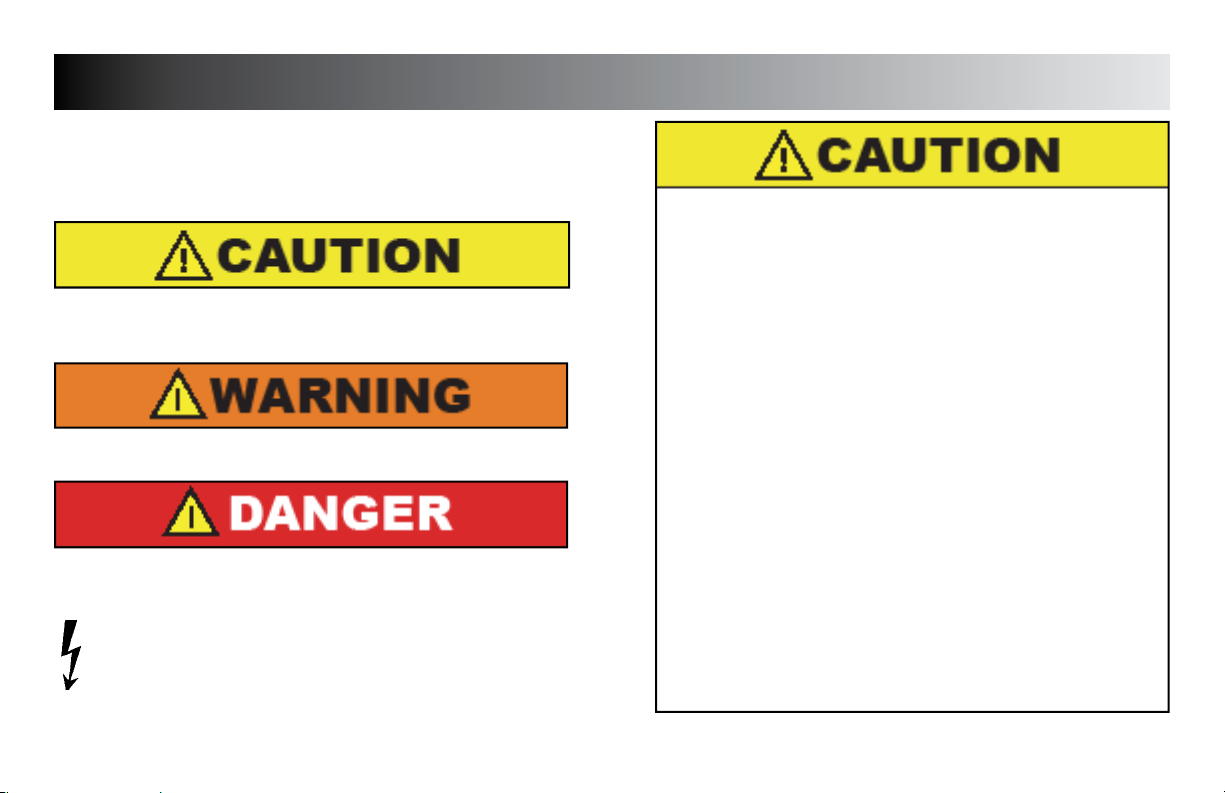
Text with a “CAUTION” indicator will explain possible Safety infractions
that could have the potential to cause minor to moderate injury or damage
to equipment.
Text with a “WARNING” indicator will explain possible Safety infractions
that will potentially cause serious injury and equipment damage.
Text with a “DANGER” indicator will explain possible Safety infractions
that are imminently hazardous situations that would result in death or
serious injury.
DANGEROUS VOLTAGE
Text with a “DANGEROUS VOLTAGE” indicator serves to inform the
user of possible hazards resulting in the electrical charge delivered to
the patient in certain treatment configurations of TENS waveforms.
NOTE: Throughout this manual “NOTE” may be found. These notes are
helpful information to aid in the particular area or function being described.
Intelect® Advanced Vacuum Electrode Module
• Read, understand and practice the precautionary and operating
instructions. Know the limitations and hazards associated with using
any electrical stimulation device. Observe the precautionary and
operational decals placed on the unit.
• The Intelect Advanced Vacuum Electrode Module is designed to
operate only when properly installed in the Intelect Advanced
Therapy System Cart and properly connected to an Intelect
Advanced Therapy System.
• DO NOT operate the system in an environment of shortwave
diathermy use.
• DO NOT use sharp objects such as a pencil point or ballpoint pen to
operate the buttons on the control panel as damage may result.
• This unit should be operated, transported and stored in
temperatures between 15° C and 40° C (59° F and 104° F), with
Relative Humidity ranging from 30%-60%.
• Before each use, inspect Vacuum Electrode Cups and Lead Hoses
for cracks and damage which may not allow the vacuum to properly
secure the electrodes.
• The Intelect Advanced Vacuum Electrode Module is not designed to
prevent the ingress of water or liquids. Ingress of water or liquids
could cause malfunction of internal components of the system and
therefore create a risk of injury to the patient.
• Drain the Vacuum Electrode Module water reservoir regularly to
prevent excessive accumulation from electrode sponge water.
• Periodic flushing of the system and reservoir are required to
maintain Factory functionality of the Vacuum Electrode Module.
Refer to the Maintenance Section of this manual for proper instructions.
2
ELECTROTHERAPY
Precautionary Instructions (continued)
Intelect® Advanced Vacuum Electrode Module
• This equipment generates, uses and can radiate radio frequency energy
and, if not installed and used in accordance with the instructions,
may cause harmful interference to other devices in the vicinity.
However, there is no guarantee that interference will not occur in a
particular installation. Harmful interference to other devices can be
determined by turning this equipment on and off. Try to correct the
interference using one or more of the following: reorient or relocate
the receiving device, increase the separation between the equipment,
connect the equipment to an outlet on a different circuit from that to
which the other device(s) are connected and/or consult the factory
field service technician for help.
• This device should be used only under the continued supervision
of a licensed practitioner.
• Make certain the unit is electrically grounded by connecting only to a
grounded electrical service receptacle conforming to
national and local electrical codes.
• This device should be kept away from children.
• Care must be taken when operating this equipment around other
equipment. Potential electromagnetic or other interference could
occur to this or to the other equipment. Try to minimize this
interference by not using other equipment in conjunction with it.
• Powered muscle stimulators should be used only with the leads
and electrodes recommended for use by the manufacturer.
the applicable
• Before administering any treatment to a patient, become acquainted
the operating procedures for each mode of treatment available, as well
as the indications, contraindications, warnings and precautions. Consult
resources for additional information regarding the application of
other
Electrotherapy.
• To prevent electrical shock, disconnect the unit from the power source
before attempting any maintenance procedures.
• Keep electrodes separated during treatment. Electrodes in contact
with each other could result in improper stimulation or skin burns.
• Long term effects of chronic electrical stimulation are unknown.
• Stimulation should not be applied over the anterior neck or mouth.
Severe spasm of the laryngeal and pharyngeal muscles may occur and
the contractions may be strong enough to close the airway or cause
difficulty in breathing.
• Stimulation should not be applied transthoracically in that the
introduction of electrical current into the heart may cause cardiac
arrhythmia.
• Stimulation should not be applied over swollen, infected, inflamed areas
or skin eruptions, e.g., phlebitis, thrombophlebitis, varicose veins, etc.
• Stimulation should not be applied over, or in proximity to, cancerous
lesions.
• Output current density is related to electrode size. Improper application
may result in patient injury. If any question arises as to the proper
electrode size, consult a licensed practitioner prior to therapy session.
• Vacuum electrodes should not be used on patients with thin, papery
skin. Vacuum may lead to contact difficulty and bruising.
• Vacuum electrodes are not suitable for patients who are taking
steroids due to the likelihood of bruising.
3
with
ELECTROTHERAPY
Precautionary Instructions (continued)
Intelect® Advanced Vacuum Electrode Module
• The safety of TENS waveforms for use during pregnancy or birth
has not been established.
• TENS is not effective for pain of central origin. This includes
headache.
• TENS should be used only under the continued supervision of a
physician.
• TENS waveforms have no curative value.
• TENS is a symptomatic treatment and as such suppresses the
sensation of pain which would otherwise serve as a protective
mechanism.
• The user must keep the device out of the reach of children.
• Electronic monitoring equipment (such as ECG monitors and ECG
alarms) may not operate properly when TENS stimulation is in use.
• In the event that an Error message or Warning beginning with a 2 or
3 appears, immediately stop all use of the system and contact the
dealer or DJO for service. Errors and Warnings in these categories
indicate an internal problem with the system that must be tested
by DJO or a Field Service Technician certified by DJO before any further operation or use of the system. Use of a system that indicates an
Error or Warning in these categories may pose a risk of injury
to the patient, user or extensive internal damage to the system.
• Stimulus delivered by the TENS waveforms of this device,
in certain configurations, will deliver a charge of 25
microcoulombs (µC) or greater per pulse and may be
sufficient to cause electrocution. Electrical current of this
magnitude must not flow through the thorax because it
may cause a cardiac arrhythmia.
4
 Loading...
Loading...