Page 1

Therapy System
User Manual
Operation & Installation
Instructions for:
2771- sEMG and sEMG + Stim Module
DJO is an ISO 13485 Certified Company
Page 2

TABLE OF CONTENTS
Intelect® Advanced sEMG and sEMG + Stim Module
FOREWORD . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
PRODUCT DESCRIPTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
SAFETY PRECAUTIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-7
PRECAUTIONARY DEFINITIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2
CAUTIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
WARNINGS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4
DANGERS. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
SEMG INDICATIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6
sEMG + STIM INDICATIONS, CONTRAINDICATIONS
AND ADVERSE EFFECTS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7
Indications- sEMG + Stim using VMS™, Symmetrical Biphasic (TENS), . . . .
Asymmetrical Biphasic (TENS) or Russian waveforms . . . . . . . . . . . . . . . . . . . . 7
Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Additional Precautions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Adverse Eff ects . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
NOMENCLATURE. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-10
sEMG and sEMG + STIM STANDARD ACCESSORIES . . . . . . . . . . . . . . . . . . . . . . . . . 8
SYMBOL DEFINITIONS. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9
System Hardware Symbols. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
SystemSoftware Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Operator Remote . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Battery Module. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Channel 3/4ElectrothrapyModule . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
TERMINOLOGY DEFINITIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
SPECIFICATIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-13
SEMG AND sEMG + STIM MODULE SPECIFICATIONS . . . . . . . . . . . . . . . . . . . . 11
WAVEFORM SPECIFICATIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
TENS- Asymmetrical Biphasic . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
TENS- Symmetrical Biphasic . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
VMSTM . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
High Voltage Pulsed Current (HVPC). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
INSTALLATION & REMOVAL. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14-19
GENERAL INFORMATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
sEMG Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Therapy System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
THERAPY SYSTEM PREPARATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Disconnect System Mains . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
Disconnect Leads and Accessories. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Remove Therapy System from Cart . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Remove Module. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Remove Breakout Tabs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
INSTALLING SEMG MODULE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Position sEMG Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Secure sEMG Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Install and Reinstall Additional Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Install Rear Access Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Install Cables and Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
Apply Mains Power. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
REMOVING SEMG MODULE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Prepare System. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Remove sEMG Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
sEMG Plug Kit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
PATIENT PREPARATION. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20-24
SEMG AND SEMG+STIM PATIENT PREPARATION. . . . . . . . . . . . . . . . . . . . . . . . . 20
Electrode Placement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Dura-Stick™ II Electrodes. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Install sEMG Lead Wires to System. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Install Dura-Stick™ II Electrodes. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Select Modality. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Select Body Area . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
View Electrode Placement Graphic . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
View Electrode Placement Text . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Prepare Treatment Area. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Electrode Placement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
Intra-Vaginal Probe . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
OPERATION. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .25-43
SEMG THERAPY SET UP. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Optional Patient Data Management System (PDMS). . . . . . . . . . . . . . . . . . . . 25
sEMG Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
i
Page 3

TABLE OF CONTENTS
Intelect® Advanced sEMG and sEMG + Stim Module
Prepare System and Patient. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Select sEMG Modality . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
View sEMG Description Text . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Select Edit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
Select Channel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
Set Alarm. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
Set Audio Type . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Select Target Option . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Setting Max Target. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Setting Avg Target . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
Setting Manual Target . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Set Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Start sEMG Therapy Session . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32
Stopping sEMG Therapy Session. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32
SEMG+STIM THERAPY SET UP. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
Prepare System and Patient. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
Select sEMG + Stim Modality . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
Select Edit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
Select Channel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
Set Alarm. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
Set Audio Type . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
Select Stim Waveform. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
Edit Stim. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
Select Target Option . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
Setting Max Target. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
Setting Avg Target . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
Setting Manual Target . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
Set Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
Set Auto Feature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
Start sEMG Therapy Session . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
Stopping Stim. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40
Stopping Therapy Session . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40
SET UP OF NEW SEMG DATA CARD. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41
Insert New sEMG Data Card . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Prepare System and Patient. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Set Up sEMG Therapy Session . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Enter Patient ID . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Begin Save . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .43
End Save. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43
TROUBLESHOOTING . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .44-49
ERROR CODES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 44-49
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 44
REPLACEMENT ACCESSORIES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 50
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 50
Color Series Standard Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 50
Color Series Optional Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .50
Mains Power Cords. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 50
MAINTENANCE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .51
CARING FOR THE THERAPY SYSTEM . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
Cleaning the Therapy System. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
Cleaning the Lens . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
FACTORY SERVICE. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
WARRANTY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52
ii
Page 4

FOREWORD
This manual has been written for the owners of an Intelect Advanced Therapy System, sEMG and sEMG + Stim Module. It contains general
information on the operation, precautionary practices, installation and maintenance for the Intelect Advanced sEMG and sEMG + Stim
Module. In order to maximize use, efficiency, the life of the Therapy System, and the Intelect Advanced sEMG and sEMG + Stim Module,
please read this manual thoroughly and become familiar with the Intelect Advanced sEMG and sEMG + Stim Module before operating the
system.
This manual contains general safety, operating, maintenance and care instructions as well as installation instruction for the Intelect
Advanced sEMG and sEMG + Stim Module for the users of the Intelect Advanced Therapy Two Channel and Four Channel Electrotherapy
and Combination Therapy Systems. Instructions for the Intelect Advanced Therapy System and its additional modules such as Battery,
Laser, and Vacuum Electrode modules are found in their respective User Manuals that also contain installation and operation instructions.
Specifications put forth in this manual were in effect at the time of publication. However, owing to DJO, LLC's policy of continual
improvement, changes to these specifications may be made at any time without obligation on the part of DJO, LLC.
Before administering any treatment to a patient, the users of this equipment should read, understand and follow the information
contained in this and the Therapy System User Manual for each mode of treatment available, as well as the indications, contraindications,
warnings and precautions. Consult other resources for additional information regarding the application of electrotherapy, ultrasound,
sEMG (Surface Electromyography), sEMG + Stim (Surface Electromyography with Triggered Stimulation), and Laser therapy.
PRODUCT DESCRIPTION
The Intelect Advanced sEMG and sEMG + Stim Module is designed for use with the Intelect Advanced Therapy Two and Four Channel
Electrotherapy and Combination Therapy Systems only. Additional options are available for separate purchase and can be installed by the
end user.
Stay current with the latest clinical developments in the field of Electrotherapy, Ultrasound, sEMG (Surface Electromyography) , sEMG
+ Stim (Surface Electromyography with Triggered Stimulation), and Laser Therapy. Observe all applicable precautionary measures for
treatment.
Keep informed of appropriate indications and contraindications for the use of Electrotherapy, Ultrasound, sEMG (Surface
Electromyography), sEMG + Stim (Surface Electromyography with Triggered Stimulation), and Laser Therapy.
This equipment is to be used only under the prescription and supervision of a physician or licensed practitioner.
Intelect® Advanced sEMG and sEMG + Stim Module
© 2011 DJO, LLC. All rights reserved. Any use of editorial, pictorial, or layout composition of this publication without express written consent from DJO, LLC is strictly prohibited. This
publication was written, illustrated, and prepared for distribution by DJO, LLC.
1
Page 5
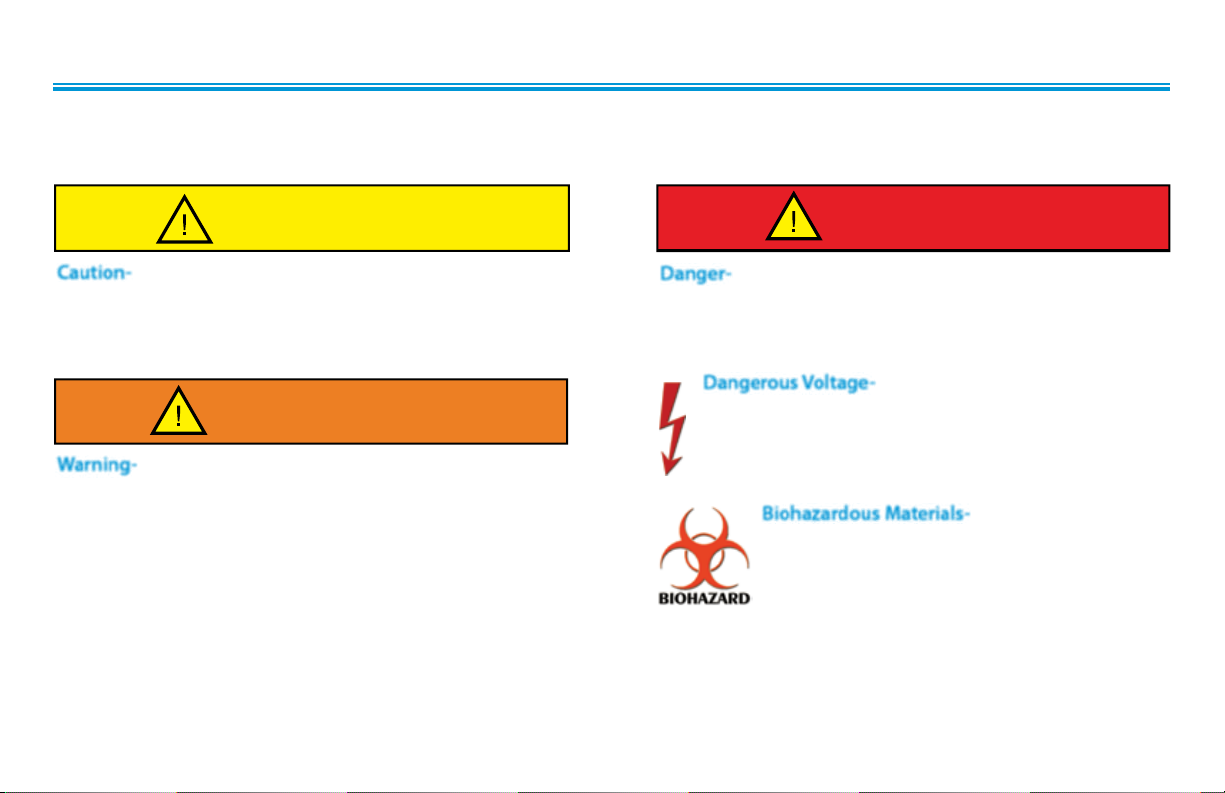
SAFETY PRECAUTIONS
DANGER
WARNING
PRECAUTIONARY DEFINITIONS
The precautionary instructions found in this section and throughout this manual are indicated by specific symbols. Understand these
symbols and their definitions before operating this equipment. The definition of these symbols are as follows;
Intelect® Advanced sEMG and sEMG + Stim Module
CAUTION
Caution-
Text with a “CAUTION” indicator will explain possible safety
infractions that could have the potential to cause minor to
moderate injury or damage to equipment.
Warning-
Text with a “WARNING” indicator will explain possible safety
infractions that will potentially cause serious injury and equipment
damage
Danger-
Text with a “DANGER” indicator will explain possible safety
infractions that are imminently hazardous situations that would
result in death or serious injury.
Dangerous Voltage-
Text with a “Dangerous Voltage” indicator serves to inform
the user of possible hazards resulting in the electrical charge
delivered to the patient in certain treatment configurations
of TENS waveforms.
Biohazardous Materials-
Text with a “BIOHAZARD” indicator serves to inform
the user of possible hazards resulting in improper
handling of components and accessories that have
come in contact with bodily fluids.
NOTE:
Throughout this manual, “NOTE” may be found. These Notes
are helpful information to aid in the particular area or function
being described.
2
Page 6
SAFETY PRECAUTIONS
CAUTIONS
Intelect® Advanced sEMG and sEMG + Stim Module
CAUTIONCAUTION
Read, understand and practice the precautionary and operating
instructions. Know the limitations and hazards associated with using
any electrical stimulation. Observe the precautionary and operational
decals placed on the unit.
• DO NOT operate the Intelect Advanced Therapy System sEMG
Module when connected to any unit other than Chattanooga
devices.
• DO NOT operate this unit in an environment where other devices
are being used that intentionally radiate electromagnetic energy in
an unshielded manner.
• DO NOT use sharp objects such as a pencil point or ballpoint pen to
operate the buttons on the control panel as damage may result.
• This unit should be operated, transported and stored in
temperatures between 15° C and 40° C (59° F and 104° F), with
Relative Humidity ranging from 30%-60%.
• Inspect Lead wires and associated connectors for signs of damage
before each use. Replace damaged lead wires immediately with
new before any treatment is applied.
• The Intelect Advanced Therapy System is not designed to prevent
the ingress of water or liquids. Ingress of water or liquids could
cause malfunction of internal components of the system and
therefore create a risk of injury to the patient.
• This equipment generates, uses and can radiate radio frequency
energy and, if not installed and used in accordance with the
instructions, may cause harmful interference to other devices in
the vicinity. However, there is no guarantee that interference will
not occur in a particular installation. Harmful interference to other
devices can be determined by turning this equipment on and off.
Try to correct the interference using one or more of the following:
Reorient or relocate the receiving device, increase the separation
between the equipment, connect the equipment to an outlet on a
different circuit from that to which the other device(s) are connected
and/or consult the factory field service technician for help.
• The Nylatex® Wraps shipped with this unit contain dry natural
rubber and may cause allergic reactions in patients with allergies
to latex.
3
Page 7
WARNING
WARNING
SAFETY PRECAUTIONS
WARNINGS
Intelect® Advanced sEMG and sEMG + Stim Module
These devices are restricted to sale by, or on the order of, a physician
or licensed practitioner. This device should be used only under the
continued supervision of a physician or licensed practitioner.
• Care must be taken when operating this equipment around other
equipment. Potential electromagnetic or other interference could
occur to this or to the other equipment. Try to minimize this
interference by not using other equipment in conjunction with it.
• Before administering any treatment to a patient you should become
acquainted with the operating procedures for each mode of
treatment available, as well as the indications, contraindications,
warnings and precautions. Consult other resources for additional
information regarding the application of sEMG + Electrical
Stimulation.
• To prevent electrical shock, disconnect the unit from the power
source before attempting any maintenance procedures.
• Keep stim electrodes separated during treatment. Electrodes in
contact with each other could result in improper stimulation or
skin burns.
• Long term effects of chronic electrical stimulation are unknown.
• Stimulation should not be applied over the anterior neck or mouth.
Severe spasm of the laryngeal and pharyngeal muscles may occur
and the contractions may be strong enough to close the airway or
cause difficulty in breathing.
• Stimulation should not be applied transthoracically in that the
introduction of electrical current into the heart may cause cardiac
arrhythmia.
• Stimulation should not be applied over swollen, infected, inflamed
areas or skin eruptions, e.g., phlebitis, thrombophlebitis, varicose
veins, etc.
• Stimulation should not be applied over, or in proximity to, cancerous
lesions.
• The safety of TENS devices for use during pregnancy or birth has
not been established.
• TENS is not effective for pain of central origin. (This includes
headache.)
• TENS devices should be used only under the continued supervision
of a physician or licensed practitioner.
• TENS devices have no curative value.
• TENS is a symptomatic treatment and as such suppresses the
sensation of pain which would otherwise serve as a protective
mechanism.
• The user must keep the device out of the reach of children.
• Electronic monitoring equipment (such as ECG monitors and ECG
alarms) may not operate properly when TENS stimulation is in use.
• Before each therapy session begins, make certain the patient has
the appropriate patient switch for the channels being used.
4
Page 8
SAFETY PRECAUTIONS
DANGER
DANGERS
• Stimulus delivered by the TENS waveforms of this
device, in certain configurations, will deliver a charge of
25 microcoulombs (µC) or greater per pulse and may be
sufficient to cause electrocution. Electrical current of this
magnitude must not flow through the thorax because it
may cause a cardiac arrhythmia.
• Patients with an implanted neurostimulation device
must not be treated with or be in close proximity to any
shortwave diathermy, microwave diathermy, therapeutic
ultrasound diathermy or laser diathermy anywhere on
their body. Energy from diathermy (shortwave, microwave,
ultrasound and laser) can be transferred through the
implanted neurostimulation system, can cause tissue
damage and can result in severe injury or death. Injury,
damage or death can occur during diathermy therapy even
if the implanted neurostimulation system is turned “off.”
• Handle, clean, and dispose of components and accessories
that have come in contact with bodily fluids according
to National, Local and Facility rules, regulations and
procedures.
Intelect® Advanced sEMG and sEMG + Stim Module
5
Page 9

SAFETY PRECAUTIONS
SEMG INDICATIONS
Indications- Surface EMG
To determine the activation timing of muscles for:
• Retraining of muscle activation
• Coordination of muscle activation
• An indication of the force produced by muscle for control and
maintenance of muscle contractions.
• Relaxation muscle training
• Muscle re-education
Intelect® Advanced sEMG and sEMG + Stim Module
6
Page 10

SAFETY PRECAUTIONS
sEMG + STIM INDICATIONS, CONTRAINDICATIONS AND ADVERSE EFFECTS
Indications- sEMG + Stim using VMS™, Symmetrical Biphasic
(TENS), Asymmetrical Biphasic (TENS) or Russian waveforms
• Stroke rehab by muscle re-education
• Relaxation of muscle spasms
• Prevention or retardation of disuse atrophy
• Increase local blood circulation
• Muscle re-education
• Maintaining or increasing range of motion
Contraindications
• This device should not be used for symptomatic local pain relief unless
etiology is established or unless a pain syndrome has been diagnosed.
• This device should not be used when cancerous lesions are present in the
treatment area.
• Stimulation should not be applied over swollen, infected, inflamed areas,
or skin erruptions, e.g. phlebitis, thrombophlebitis, varicose veins, etc.
• Other contraindications are patients suspected of carrying serious
infectious disease and or disease where it is advisable, for general medical
purposes, to suppress heat or fevers.
• Electrode placements must be avoided that apply current to the carotid
sinus region (anterior neck) or transcereberally (through the head).
• Safety has not been established for the use of therapeutic electrical
stimulation during pregnancy.
• Powered muscle stimulators should not be used on patients with cardiac
demand pacemakers.
• There should not be any use of TENS waveforms on patients with cardiac
demand pacemakers.
Additional Precautions
• Caution should be used for patients with suspected or diagnosed heart
problems.
• Caution should be used for patients with suspected or diagnosed epilepsy.
• Caution should be used in the presence of the following:
• When there is a tendency to hemorrhage following acute trauma or
fracture.
• Following recent surgical procedures when muscle contraction may disrupt
the healing process.
• Over a menstruating or pregnant uterus; over areas of the skin which lack
normal sensation.
• Some patients may experience skin irritation or hypersensitivity due to
the electrical stimulation or electrical conductive medium. The irritation
can usually be reduced by using an alternative conductive medium or an
alternative electrode placement.
• Electrode placement and stimulation settings should be based on the
guidance of the prescribing practitioner.
• Powered muscle stimulators should be used only with the lead wires and
electrodes recommended for use by the manufacturer.
• With TENS waveforms, isolated cases of skin irritation may occur at the site of
electrode placement following long term application.
• The effectiveness of TENS waveforms is highly dependent upon patient
selection by a person qualified in the management of pain patients.
Adverse Effects
• Skin irritation and burns beneath the electrodes have been reported with the
use of powered muscle stimulators.
• Potential adverse effects with TENS are skin irritation and electrode burns.
Intelect® Advanced sEMG and sEMG + Stim Module
7
Page 11
DANGER
NOMENCLATURE
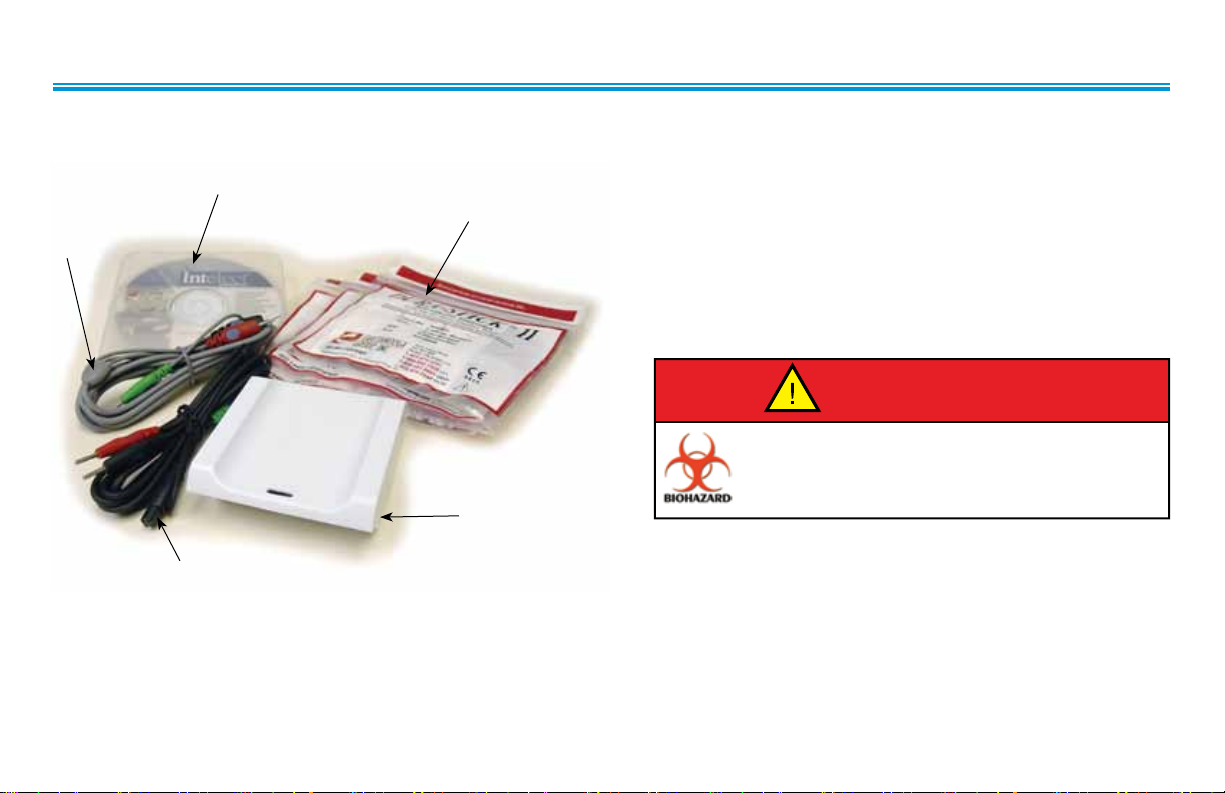
sEMG & sEMG + STIM STANDARD ACCESSORIES
5
3
2
Intelect® Advanced sEMG and sEMG + Stim Module
1. Surface EMG (sEMG) Module
2. sEMG + Electrical Stimulation Lead Wires for Channel 1
4
1
3. sEMG + Electrical Stimulation Lead Wires for Channel 2
4. Dura-Stick II 3.2 cm (1.25”) Self Adhesive disposable
Electrodes (3 packs of 4)
5. User Manual (CD-ROM)
6. Intra-Vaginal Probe (not illustrated)
Handle, clean, and dispose of components and accessories
that have come in contact with bodily fluids according
to National, Local and Facility rules, regulations and
procedures..
8
Page 12
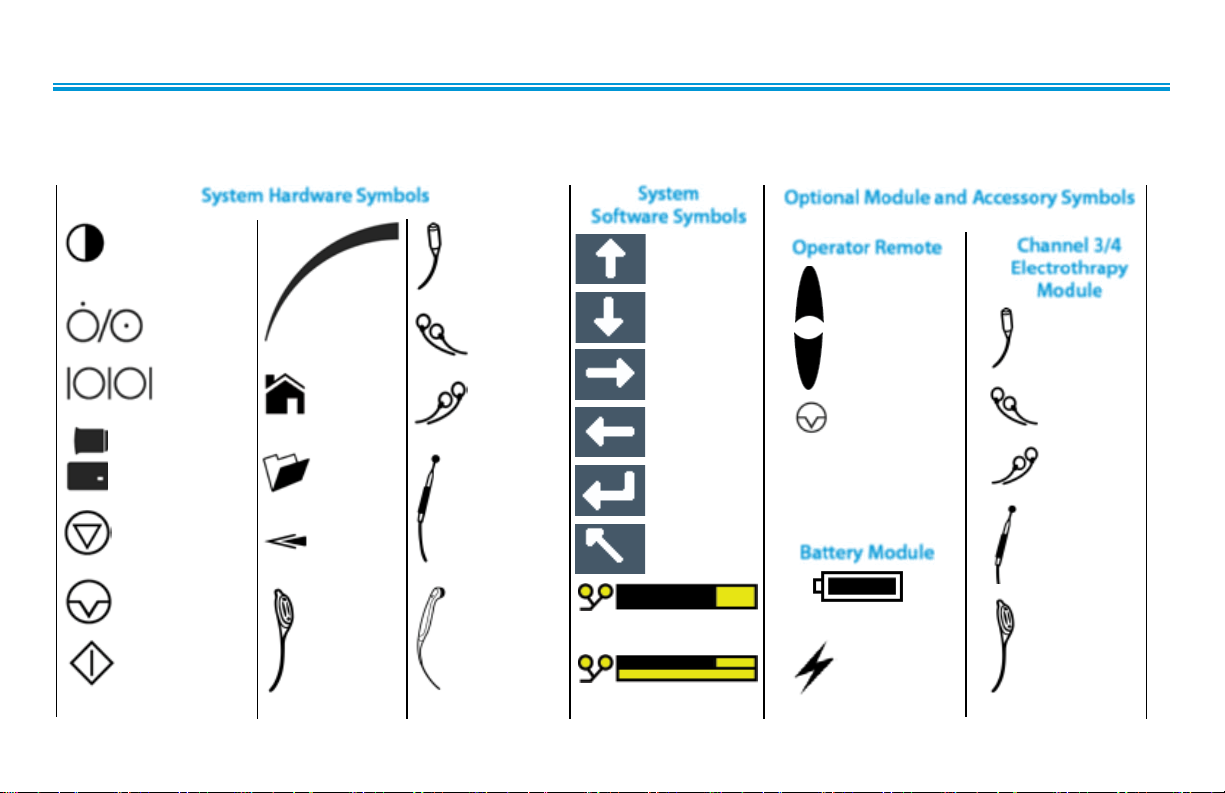
NOMENCLATURE
Intelect® Advanced sEMG and sEMG + Stim Module
SYMBOL DEFINITIONS
Below are the definitions for all of the symbols used in the Intelect Advanced hardware and software. Study and learn these symbols
before any operation of the system.
System Hardware Symbols
CONTRAST CONTROL
(NOT FUNCTIONAL ON
COLOR SYSTEMS)
ON/OFF
SWITCH
DATA
PORT
MULTI-MEDIA
AND
PATIENT CARD
STOP
TREATMENT
PAUSE
TREATMENT
START
TREATMENT
THERAPY
INTENSITY
CONTROL
HOME
CLINICAL
RESOURCES
LIBRARY
BACK
CHANNEL 1/2
OPERATOR
REMOTE
CONTROL
(OPTIONAL)
PATIENT
INTERRUPT
SWITCH
CHANNEL 1
LEAD WIRES
CHANNEL 2
LEAD WIRES
MICROCURRENT
PROBE
ULTRASOUND
APPLICATOR
System
Software Symbols
MOVE UP
MOVE DOWN
MOVE RIGHT
MOVE LEFT
ACCEPT AND
RETURN
DO NOT ACCEPT
AND RETURN
PAD CONTACT QUALITY
(SINGLE CHANNEL GRAPH)
PAD CONTACT QUALITY
(DUAL CHANNEL GRAPH)
Optional Module and Accessory Symbols
Operator Remote
Channel 3/4
Electrothrapy
INCREASE
INTENSITY
DECREASE
INTENSITY
PAUSE
TREATMENT
MANUAL
M
STIMULATION
Battery Module
CHARGE LEVEL
BATTERY
CHARGING
Module
PATIENT
INTERRUPT
SWITCH
CHANNEL 3
LEAD WIRES
CHANNEL 4
LEAD WIRES
MICROCURRENT
PROBE
CHANNEL 3/4
OPERATOR
REMOTE
CONTROL
(OPTIONAL)
9
Page 13

NOMENCLATURE
TERMINOLOGY DEFINITIONS
Below are the definitions for all of the unique terminology used throughout this manual. Study these and become familiar with these
terms for ease of system operation, component and control functionality of the Intelect Adavnced Therapy System. Some of these terms
and definitions refer to a specific button, control, or module on the system. Refer to page 9 for symbol definitions.
GENERAL TERMINOLOGY
Back Button
The dedicated button on the System, below the display, that
each time pressed takes the user back one screen at a time.
Previous Page Button
The button used in some modalities and functions that will take
the user back one page when reading multiple pages of text.
UP and DOWN Arrows
Controls used in various modality parameter screens to navigate
or change a value up or down within the parameter.
Electrotherapy
Refers to the electrical muscle or nerve stimulation modalities of
the system.
System
The primary system with all controls and functions.
sEMG Module
The Module installed into the bottom cavity of the System and
utilized by the System to cause the sEMG and sEMG + Stim
modalities to function.
sEMG
Abbreviation for the Surface Electromyography modality.
sEMG + Stim
Abbreviation for Surface Electromyography with Triggered
Electrical Stimulation modality.
Intelect® Advanced sEMG and sEMG + Stim Module
10
Page 14

SPECIFICATIONS
sEMG AND sEMG + STIM MODULE SPECIFICATIONS
DEPTH
WIDTH
HEIGHT
Intelect® Advanced sEMG and sEMG + Stim Module
DIMENSIONS
Width . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8.3 cm (3.25”)
Depth . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9.2 cm (3.625”)
Height . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3 cm (1.187”)
Input Impedance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .> 1,000,000 ohm
Input Sensitivity. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . < 1.0 µV RMS
Frequency Range . 15 Hz-300 Hz with CMMR of > 120 dB CMMR
at 50/60 Hz > 180 dB
Weight . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 105 g (3.8 oz)
Product Type
Internally Powered . . . . . . . . . .Intelect Advanced Therapy System
Electrical Class . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Class I
Electrical Type. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Type BF
For Continuous Operation
Regulatory Compliance
UL/IEC/EN 60601-1
IEC/EN 60601-1-2
IEC 60601-2-10
11
Page 15
SPECIFICATIONS
DANGER
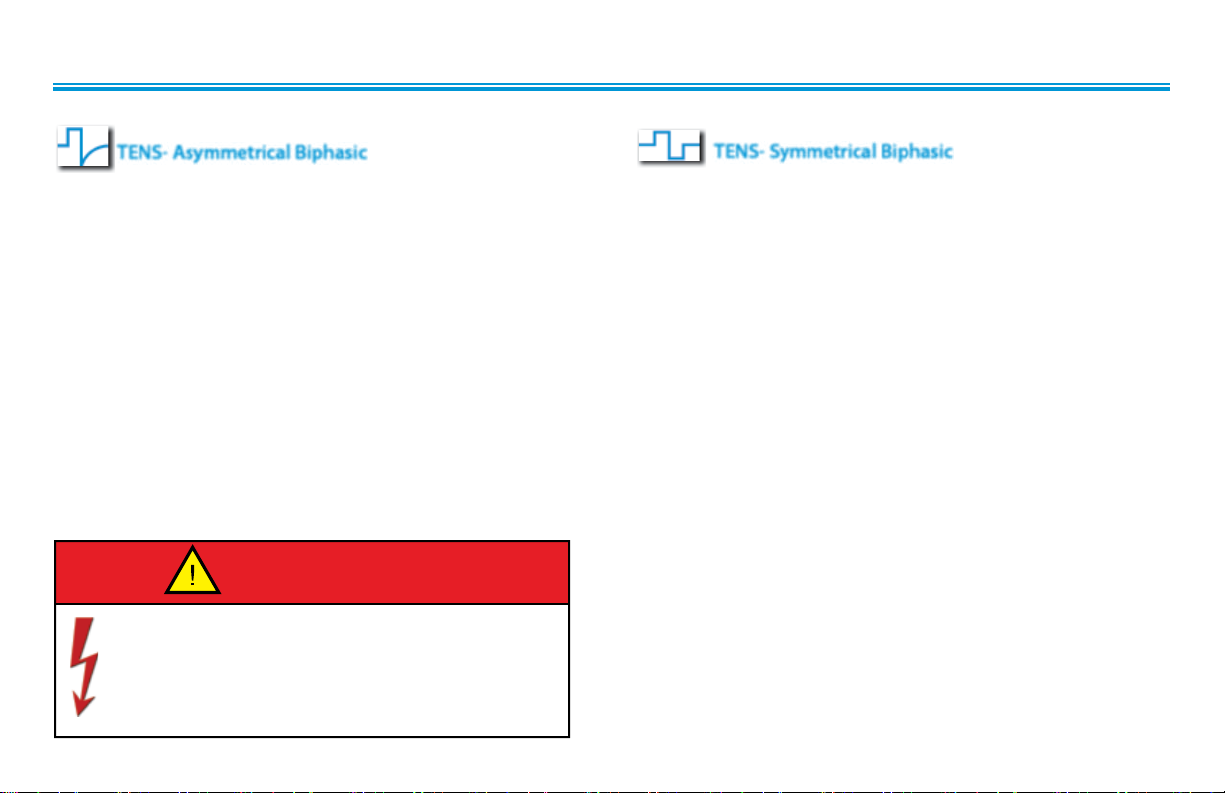
WAVEFORM SPECIFICATIONS
Intelect® Advanced sEMG and sEMG + Stim Module
TENS- Asymmetrical Biphasic
The Asymmetrical Biphasic waveform has a short pulse duration.
It is capable of strong stimulation of the nerve fibers in the skin
as well as of muscle tissue. This waveform is often used in TENS
devices. Because of its short pulse, the patient typically tolerates
the current well, even at relatively high intensities.
Output Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Electrodes
Output Intensity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0-110 mA
Phase Duration . . . . . . . . . . . . . . . . . . . . . . . . . Adjustable 20-1,000 µsec
Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-250 Hz
Mode Selection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . CC or CV*
Burst Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0-10 bps
Frequency Modulation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0-250 Hz
Amplitude Modulation . . . . . . . . . . . . Off, 40%, 60%, 80% and 100%
Treatment Time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-60 minutes
Stimulus delivered by the TENS waveforms of this device,
in certain configurations, will deliver a charge of 25
microcoulombs (µC) or greater per pulse and may be sufficient
to cause electrocution. Electrical current of this magnitude
must not flow through the thorax because it may cause a
cardiac arrhythmia.
TENS- Symmetrical Biphasic
The Symmetrical Biphasic waveform has a short pulse duration
and is capable of strong stimulation of nerve fibers in the skin
and in muscle. This waveform is often used in portable muscle
stimulation units, and some TENS devices. Because of its short
pulse duration, the patient typically tolerates the current well,
even at relatively high intensities.
Output Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Electrodes
Output Intensity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .0-80 mA
Phase Duration . . . . . . . . . . . . . . . . . . . . . . . . . Adjustable 20-1,000 µsec
Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-250 Hz
Mode Selection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . CC or CV*
Burst Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .0-4 bps
Frequency Modulation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0-250 Hz
Amplitude Modulation . . . . . . . . . . . . Off, 40%, 60%, 80% and 100%
Treatment Time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-60 minutes
*CC= Constant Current
CV= Constant Voltage
12
Page 16
SPECIFICATIONS
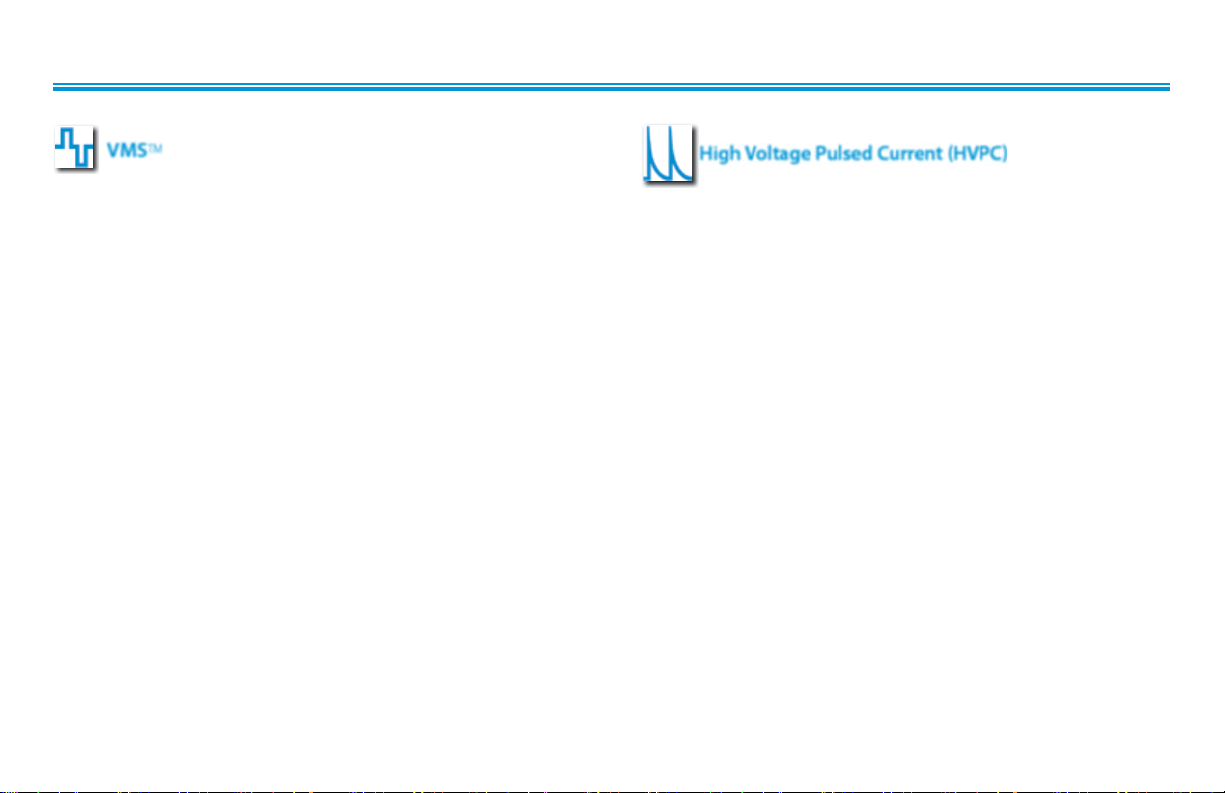
WAVEFORM SPECIFICATIONS (continued)
TM
VMS
Intelect® Advanced sEMG and sEMG + Stim Module
High Voltage Pulsed Current (HVPC)
VMS is a symmetrical biphasic waveform with a 100 µsec interphase
interval. Because the pulse is relatively short, the waveform has a low
skin load, making it suitable for applications requiring high intensities,
such as in muscle strengthening protocols.
Output Mode: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Electrodes
Output Intensity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0-255 mA
Channel Mode . . . . . . . . . . . . . . . . . . . . . . . . . Single, Reciprocal, Co-Contract
Phase Duration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20-1000µsec
Mode Selection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . CC or CV*
Anti-Fatigue . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Off or On
Set Intensity . . . . . . . . . . . . . . . . . . . . Individual Channel Intensity Setting in
Reciprocal and Co-Contract modes
Cycle Time . . . . . . . . . . .Continuous, 5/5, 4/12, 10/10, 10/20, 10/30, 10/50
Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-200 pps
Ramp . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .0.5 sec, 1 sec, 2 sec, 5 sec
Treatment Time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-60 minutes
Available on Channels . . . . . . . . . . . . . . . . . . . . . . . . 1 & 2, 3 & 4 Option
The High Voltage Pulsed Current (HVPC) has a very brief pulse
duration characterized by 2 distinct peaks delivered at high
voltage. The waveform is monophasic (current flows in one
direction only). The high voltage causes a decreased skin
resistance making the current comfortable and easy to tolerate.
Output Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Electrodes or Probe
Output Intensity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0-500 V
Polarity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Positive or Negative
Ramp . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .0.5 sec, 1 sec, 2 sec, 5 sec
Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Peak Current or Volts
Sweep . . . . . . . . . . . . . .Continuous, 80/120 pps, 1/120 pps, 1/10 pps
Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-120 pps
Cycle Time . . . . . .5/5, 4/12, 10/10, 10/20, 10/30, 10/50, Continuous
Treatment Time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-60 Minutes
Available on Channels . . . . . . . . . . . . . . . . . . . . . . . . 1 & 2, 3 & 4 Option
*CC= Constant Current
CV= Constant Voltage
13
Page 17
INSTALLATION & REMOVAL
WARNING
GENERAL INFORMATION
sEMG Module
The Intelect Advanced (Color Series) Electrotherapy and
Combination Therapy Systems have, as standard equipment, an
sEMG Module factory installed.
A second sEMG Module may be installed onto the Optional
Channel 3/4 Electrotherapy Module only. The sEMG Module will
not install to any other optional modules such as the Battery or
Laser Modules.
The sEMG Module is available as an option for the Intelect
Advanced (Monochromatic) Therapy System.
These instructions assume that there is no sEMG Module installed
on the Therapy System in order to provide the proper installation
instructions for future use should it become necessary.
Disconnect the system from the power source (outlet or remove
battery module if installed) before attempting any maintenance,
installation, removal, or replacement procedures to prevent electrical
shock and possible damage to system.
Intelect® Advanced sEMG and sEMG + Stim Module
Therapy System
If installing the sEMG Module on a Therapy System equipped
with a Battery, Laser, or the Channel 3/4 Electrotherapy Module
and it is desired to install the sEMG Module onto the Therapy
System, it will be necessary to remove the Battery, Laser, or
Channel 3/4 Electrotherapy Module. Refer to the respective
Module User Manual for Instructions.
Installing the sEMG Module on the System will make the sEMG
and sEMG + Stim modalities available for Channels 1/2. If installed
on the Optional Channel 3/4 Electrotherapy Module, sEMG and
sEMG + Stim modalities will be available on Channels 3/4.
If two sEMG Modules are installed, one on the Therapy System
and one on the Channel 3/4 Electrotherapy Module, sEMG and
sEMG + Stim modalities will be available to all four Channels.
However, only two channels at a time may be used; either
Channels 1/2 or Channels 3/4.
This section provides general instructions to prepare the Therapy
System and the optional Channel 3/4 Electrotherapy Module for
installation of the sEMG and sEMG + Stim Module(s).
14
Page 18

INSTALLATION & REMOVAL
WARNING
THERAPY SYSTEM PREPARATION
Disconnect System Mains
Disconnect the system from the power source (outlet or remove
battery module if installed) before attempting any maintenance,
installation, removal, or replacement procedures to prevent electrical
shock and possible damage to system.
Intelect® Advanced sEMG and sEMG + Stim Module
Remove Module
Place the System upside down on a
smooth surface. Place a soft, clean fabric or
cushion under the System Display Lens to
prevent damage.
Disconnect the Power Cord from the
power supply.
Remove the Rear Panel and disconnect the
Power Cord from the System.
REAR
ACCESS
PANEL
DISCONNECT
POWER CORD
Disconnect Leads and Accessories
Remove the Front Access Panel and disconnect all accessories.
DISCONNECT ALL ACCESSORIES
Remove Therapy System from Cart
To remove Therapy System from Cart, refer
to the Therapy System Cart User Manual.
15
Remove Channel 3/4 Electrotherapy,
Battery, or Laser Module if equipped. Refer
to the respective User Manual for the
Module being removed.
Page 19

INSTALLATION & REMOVAL
CAUTION
THERAPY SYSTEM PREPARATION (continued)
Remove Breakout Tabs
Using a flat blade screwdriver, carefully
remove the four sEMG Breakout Tabs on
the system bottom.
Be careful not to cause any damage to the PC Board Contacts that
will be exposed once the Breakout Tabs are removed.
BREAKOUT
TABS
NOTE:
Twist the screwdriver to break tabs free.
If installing on the Channel 3/4
Electrotherapy Module, remove tabs from
the Module in the same manner.
Intelect® Advanced sEMG and sEMG + Stim Module
16
Page 20

INSTALLATION & REMOVAL
INSTALLING
Position sEMG Module
Position the sEMG Module so that the two
mounting tabs are inserted into the System
or Channel 3/4 Electrotherapy Module
mounting slots.
MOUNTING
SEMG MODULE
Secure sEMG Module
Push the upper portion of the sEMG
Module until it snaps and is locked into
position.
TABS
Intelect® Advanced sEMG and sEMG + Stim Module
Install and Reinstall Additional Module
If desired, install a Channel 3/4, Battery
or Laser Module to the System, refer to
the respective module User Manual for
installation instructions prior the following
procedures.
Install Rear Access Panel
Insert the Power Cord into the System
Mains connector. Install the Rear Access
Panel.
MOUNTING
SLOTS
17
CONNECT
POWER CORD
REAR
ACCESS
PANEL
NOTE:
If installing the System to a Therapy System
Cart, refer to the Therapy System Cart User
Manual for proper instructions.
Page 21

INSTALLATION & REMOVAL
WARNING
INSTALLING
Install Cables and Accessories
Install all cables and accessories to the Front
Access Panel. Refer to page 9 for Symbol
Definitions.
SEMG MODULE (continued)
CONNECT ALL CABLES
AND ACCESSORIES
Apply Mains Power
Plug Power Cord into an
approved outlet.
Turn the System On
using the On/Off
Switch. The System will
automatically recognize
the added Module and
display a configuration
change message.
Read and carefully
follow the instructions
on the Screen.
Intelect® Advanced sEMG and sEMG + Stim Module
Verify that the Module installed is the Module displayed in the message
BEFORE pressing the START Button. If it is not, DO NOT press the START
Button. Turn the System OFF and back ON. If the problem continues, call
the selling dealer or DJO Technical Support immediately.
DO NOT USE THE SYSTEM until all necessary repairs are made by a
Technician certified by DJO. If use is attempted before repairs are made,
the System may operate unpredictably and has the potential of causing
injury to the patient or damage to the System's internal components.
18
Page 22

INSTALLATION & REMOVAL
WARNING
REMOVING
The sEMG Module should only be removed for service related problems.
SEMG MODULE
Intelect® Advanced sEMG and sEMG + Stim Module
Prepare System
Prepare the Therapy System for removal of
the sEMG Module. Refer to Page 15.
Disconnect the system from the power source (outlet or remove
battery module if installed) before attempting any maintenance,
installation, removal, or replacement procedures to prevent electrical
shock and possible damage to system.
Remove sEMG Module
Insert a Flat Blade
Screwdriver into the
Release Slot and pry
against the Locking Tab
of the sEMG Module
until the sEMG Module
is free from the
Therapy System.
LOCKING
TAB
RELEASE
SLOT
sEMG Plug Kit
The sEMG Plug Kit, part number 28027,
is designed to protect the exposed PC
Board contacts on the bottom of the
Therapy System and the Channel 3/4
Electrotherapy Module when the sEMG
Module has been removed.
The sEMG Plug Kit must be installed when
an sEMG Module has been removed for
service related problems and the Therapy
System will be put back into service
without the sEMG module.
The sEMG Plug Kit is installed and
removed in the same fashion as an sEMG
Module. Refer to page 17 for installation
instructions.
19
Page 23

PATIENT PREPARATION
WARNING
SEMG AND SEMG+STIM PATIENT PREPARATION
Electrode Placement
• Examine the skin for any wounds and clean the skin.
• Apply the electrodes on the treatment area.
• Ensure the electrodes are applied securely to the skin.
• Ensure good contact between each electrode and the skin.
• Check the electrode contact regularly during the treatment.
• Examine the skin again after the treatment.
• Choose electrodes that fit the anatomy.
• View the Electrode Placement recommendations in the
Treatment Review screen for the particular modality being used
for treatment as a reference point only prior to administering
treatment.
• Follow electrode manufacturer instructions.
• Keep electrodes separated during treatment. Electrodes in contact
with each other could result in improper stimulation or skin burns.
• Output current density is related to electrode size. Improper
application may result in patient injury. If any question arises as to
the proper electrode size, consult a licensed practitioner prior to
therapy session.
• Powered muscle stimulators should be used only with the leads
and electrodes recommended for use by the manufacturer.
Intelect® Advanced sEMG and sEMG + Stim Module
Dura-Stick™ II Electrodes
Chattanooga Dura-Stick II
Electrodes are a self adhesive,
single patient, one time use
disposable product designed
specifically for use with
Chattanooga Electrotherapy
systems.
It is recommended that
Dura-Stick II Electrodes be used
whenever possible to ensure
the highest level of contact with
the treatment area and most
uniform delivery of the prescribed
treatment.
Properly dispose of Dura-Stick II
Electrodes upon completion of the
therapy session.
20
Page 24

PATIENT PREPARATION
SEMG AND SEMG+STIM PATIENT PREPARATION (continued)
Install sEMG Lead Wires to System
Remove the Front Access Cover from the
Therapy System and remove the existing
Lead Wires. Connect the sEMG Lead wires
to the channel(s) desired for use with the
sEMG or sEMG + Stim modality.
CONNECT SEMG LEAD WIRES
Replace the Front Access Cover.
NOTE:
Two Channels may be used for sEMG
Therapy at the same time.
If four electrotherapy channels are
available, a maximum of only two may be
used at a time and must be channels 1/2
or 3/4.
Install Dura-Stick™ II Electrodes Select Modality
Connect a Dura-Stick II 3 cm (1.25”)
disposable electrode to each lead. These
electrodes are designed for use with
Chattanooga equipment and will provide
an accurate reading of sEMG activity.
Leave electrodes on the protective
backing until treatment area has been
prepared.
ACTIVE (BLACK)
LEAD
REFERENCE
ACTIVE (RED) LEAD
Intelect® Advanced sEMG and sEMG + Stim Module
Press the sEMG or sEMG + Stim button as
required for the therapy prescribed.
PRESS SEMG OR SEMG+STIM BUTTON
(GREEN) LEAD
21
Page 25

PATIENT PREPARATION
SEMG AND SEMG+STIM PATIENT PREPARATION (continued)
Select Body Area
Press the Electrode Placement button to
view the Select Body Area screen.
ELECTRODE PLACEMENT BUTTON
Press the Up and Down Arrow buttons
until the area desired is highlighted.
NOTE:
The Page Up and Page Down buttons
allow navigation either at the top or
bottom of the available selections.
UP ARROW BUTTON
DOWN ARROW
BUTTON
Intelect® Advanced sEMG and sEMG + Stim Module
View Electrode Placement Graphic
Press the Accept and Return Arrow button
to view the specific electrode placement
graphic.
ACCEPT AND RETURN
ARROW BUTTON
NOTE:
As illustrated, the two Black electrodes
represent Active electrodes and the one
White electrode represents the Reference
electrode.
22
Page 26

PATIENT PREPARATION
SEMG AND SEMG+STIM PATIENT PREPARATION (continued)
View Electrode Placement Text
Press the Next Page button to view
text relating to the specific electrode
placement selected and typical conditions
of the area.
NEXT PAGE BUTTON
Press the Next Page button to view
additional text.
Press the Prev Page button to review the
previous page.
Press the Back button to return to the
Select Body Area screen.
Press the Back button a second time to
return to the Treatment Review screen.
Intelect® Advanced sEMG and sEMG + Stim Module
Prepare Treatment Area
Examine the skin for any wounds.
Thoroughly clean the treatment area by
scrubbing the skin with medical grade
alcohol.
PREV PAGE
BUTTON
23
NEXT PAGE
BUTTON
BACK
BUTTON
SCRUB AREA WITH
MEDICAL GRADE
ALCOHOL
NOTE:
Thorough and proper cleaning of the
treatment area to remove any topical
medication and cream film as well as
loose skin particles from the treatment
area is critical to the skin contact and
reception of the Electrodes during sEMG
and sEMG + Stim therapy.
Page 27

PATIENT PREPARATION
WARNING
SEMG AND SEMG+STIM PATIENT PREPARATION (continued)
Electrode Placement
Using Dura-Stick™ II 3 cm (1.250")
electrodes, place the Active (red and
black lead) electrodes in the center of the
muscle belly and parallel with the muscle
fibers.
Position the Reference electrode (green
lead) in close proximity of the treatment
area. Review the specific Electrode
Placement graphic for positioning of the
Reference (green lead) electrode.
REFERENCE
(GREEN) LEAD
ACTIVE
(BLACK)
LEAD
ACTIVE
(RED)
LEAD
NOTE:
The electrodes may be placed for specific,
general and quasi-specific biofeedback
muscle or muscle group activity.
Using small electrodes and placing them
closer together will render a more specific
reading of muscle activity during sEMG
and sEMG + Stim therapy.
The Active electrodes may
be placed farther apart to
obtain a general reading
of a muscle or muscle group
activity during the session.
DJO recommends
using only Dura-Stick™ II
electrodes to obtain the
most accurate sEMG
feedback.
Follow the electrode
manufacturer instructions.
Trimming or cutting electrodes may
interfere with the reception of sEMG data
and may affect the delivery of electrical
stimulation in the sEMG + Stim Modality.
Intelect® Advanced sEMG and sEMG + Stim Module
Intra-Vaginal Probe
If using the Intra-Vaginal Probe for
Incontinence Therapy, plug the active
ends of the sEMG lead wire (red and black)
into the Intra-Vaginal Probe.
The Intra-Vaginal Probe is for single
patient use only. Refer to the instructions
packaged with the probe for proper use,
care and disposal.
• Keep stim electrodes separated during sEMG + Stim treatment.
Electrodes in contact with each other could result in improper
stimulation or skin burns.
• Long term effects of chronic electrical stimulation are unknown.
• Stimulation should not be applied over the anterior neck or
mouth. Severe spasm of the laryngeal and pharyngeal muscles
may occur and the contractions may be strong enough to close
the airway or cause difficulty in breathing.
24
Page 28

OPERATION
SEMG THERAPY SET UP
General Information
The Intelect Advanced sEMG modality reads and records the sEMG biofeedback activity of a muscle or muscle group by sensing the
electrical impulses generated during a voluntary muscle contraction and relax cycle. These signals are accurately relayed to the Intelect
Advanced Therapy System through 3 cm (1.250") Dura-Stick™ II Disposable Electrodes.
sEMG can be beneficial in the area of muscle retraining therapy by setting target values and charting the patient progress in reaching
those goals in a specific muscle or muscle group.
Within this section, general setup procedures of the various parameters of sEMG are explained. Also, setup and use of the optional sEMG
Data Card for recording sEMG Data is demonstrated.
Optional Patient Data Management System (PDMS)
The sEMG data can be recorded onto the optional
sEMG Data Card and viewed in graph form via the
optional Patient Data Management System connected
to a Windows
session activity, as well as see progress the patient may
be making through therapy as well as, saving the data
and printing patient graphs and reports.
Illustrated is an example of the sEMG Graph through
the optional Patient Data Management System (PDMS)
®
PC. This allows the clinician to record
Intelect® Advanced sEMG and sEMG + Stim Module
25
Page 29

OPERATION
SEMG THERAPY SET UP (continued)
Intelect® Advanced sEMG and sEMG + Stim Module
sEMG Screen
The Intelect Advanced sEMG screen affords access to all of the sEMG parameters and functions. The area surrounding the screen has 10
parameter modification buttons. However, only six are used for the sEMG modality. Below is a general description of each of the sEMG
parameter buttons. Each sEMG parameter button is explained in greater detail in the following pages of this section.
1. Channel
Select Channel 1, Channel 2, or Channel 1+2. Channel 1+2 is the Default Unit
Setting.
1
3
5
2. Target
2
Select Average, Max, or Manual Target. Max Target is the Default Unit Setting.
3. Alarm
4
Set Alarm to sound Above, Below, or at Target. Above Target is the Default Unit
Setting.
6
4. Capture Target
Captures target value while patient is performing a series of muscle contractions.
5. Audio
Allows selection of Constant, Pulsed, or Dynamic audio types. Constant is the
Default Unit Setting.
6. Volume
Set volume level to Low, Medium, High, or Off. Medium is the Default Unit Setting.
26
Page 30

OPERATION
SEMG THERAPY SET UP (continued)
Prepare System and Patient
Prepare Therapy System and patient. Refer
to pages 20 through 24.
Select sEMG Modality
Press the sEMG button on the Home
screen.
SEMG
BUTTON
View sEMG Description Text
Press the sEMG Description Text button to
view text explaining the rationale of sEMG
Therapy.
SEMG DESCRIPTION TEXT
BUTTON
Intelect® Advanced sEMG and sEMG + Stim Module
Press the Next Page button to view
additional text.
Press the Prev Page button to review
previous page.
Press the Back button to return to the
Treatment Review screen.
PREV PAGE
BUTTON
NEXT PAGE
BUTTON
BUTTON
BACK
NOTE:
Refer to page 24 for Electrode Placement.
27
Page 31

OPERATION
SEMG THERAPY SET UP (continued)
Select Edit
Press the Edit button on the Treatment
Review screen to edit sEMG parameters as
prescribed.
EDIT
BUTTON
Select Channel
Press the Channel Button until the
prescribed channel is displayed in the icon.
Therapy System
Available selections are 1, 2 or 1+2.
Channel 3/4 Electrotherapy Module
Available selections are 3, 4, or 3+4.
CHANNEL
BUTTON
NOTE:
A maximum of two channels may be
used to administer sEMG Therapy. Either
channels 1+2 or 3+4. sEMG will not
function as a four channel modality.
Intelect® Advanced sEMG and sEMG + Stim Module
Set Alarm
Set the alarm trigger in relation to the
target, by pressing the Alarm button until
the desired selection is displayed in the
Alarm icon.
The available selections are:
Above- Alarm sounds when a muscle
contraction surpasses the target.
Target- Alarm sounds when a muscle
contraction reaches the target.
Below- Alarm sounds when a muscle
contraction is below the target.
ALARM
BUTTON
NOTE:
If Volume is Off, no alarm will be heard.
28
Page 32

OPERATION
SEMG THERAPY SET UP (continued)
Set Audio Type
Audio sets the type of sound heard when
the muscle contraction reaches the Alarm
setting.
Press the Audio button until the desired
audio type is displayed in the Audio icon.
The available selections are;
Constant- Continuous sound. ________
Pulsed- Fast, short beeps. . . . . . . . . . . . . . .
Dynamic- Slow, long beeps. _ _ _ _ _ _
ALARM
BUTTON
Select Target Option
Three options are available for setting the
sEMG modality target:
Max- Maximum of muscle contractions in
10 seconds.
Avg- Average of maximums achieved in
15 seconds of muscle contractions.
Manual- Set Target manually.
Press the Target button until the prescribed
target option is displayed in the Target icon.
TARGET
BUTTON
Intelect® Advanced sEMG and sEMG + Stim Module
Setting Max Target
Make certain Target Max is displayed in the
Target icon.
Press the Capture Target button. Have the
patient begin contracting the muscle and
press the Begin Capture button. Patient
should contract the muscle as hard as
possible during the preset 10 second
contraction period.
CAPTURE TARGET
BUTTON
BEGIN CAPTURE
BUTTON
NOTE:
If volume is Off, no Audio type will be
heard.
NOTE:
The capture may be stopped by pressing
the End Capture Button. The System will
then select the maximum contraction level
achieved during the preset 10 second
contraction period.
29
Page 33

OPERATION
SEMG THERAPY SET UP (continued)
During the preset 10 second contraction
period, the Therapy System captures
and retains the maximum level of the
contraction the patient performed, then
automatically displays the Adjust sEMG
Target screen.
Use the Up and Down Arrow buttons to
adjust the Target percentage displayed at
the bottom of each channel column.
Press the Accept and Return Arrow button
to set the Target.
Setting Avg Target
Make certain Target Avg is displayed in the
Target icon.
Press the Capture Target button. Have the
patient begin contracting and releasing the
muscle. Press the Begin Capture button.
The patient should contract and release the
muscle as many times as possible during
the preset 15 second contraction period.
CAPTURE TARGET
BUTTON
Intelect® Advanced sEMG and sEMG + Stim Module
During the preset 15 second contraction
period, the Therapy System captures and
retains the average maximum level of the
contractions the patient performed, then
automatically displays the Adjust sEMG
Target screen.
Use the Up and Down Arrow buttons to
adjust the Target percentage displayed at
the bottom of each channel column.
Press the Accept and Return Arrow button
to set the Target.
UP ARROW
BUTTON
ACCEPT AND
RETURN ARROW
BUTTON
DOWN ARROW
BUTTON
BEGIN CAPTURE
BUTTON
NOTE:
During the preset 15 second contraction
period, the Therapy System captures and
retains the average maximum level of all
contractions that the patient performed.
30
UP ARROW
BUTTON
ACCEPT AND
RETURN ARROW
BUTTON
DOWN ARROW
BUTTON
Page 34

OPERATION
SEMG THERAPY SET UP (continued)
Setting Manual Target
Make certain Target Manual is displayed in
the Target icon.
Press the Adjust Target button.
ADJUST TARGET
BUTTON
Press the Up and Down Arrow buttons to
adjust Target to prescribed level.
Press the Accept and Return Arrow button.
UP ARROW
BUTTON
ACCEPT AND
RETURN
ARROW BUTTON
DOWN ARROW
BUTTON
Intelect® Advanced sEMG and sEMG + Stim Module
Set Volume
Press Volume button until the desired
volume level is displayed in the Volume
icon.
The available volume levels are Low,
Medium, High, and Off.
VOLUME
BUTTON
31
NOTE:
If Volume is Off, no alarm will be heard
when contraction reaches the target
setting.
Page 35

OPERATION
SEMG THERAPY SET UP (continued)
Start sEMG Therapy Session
Have patient perform contractions as
prescribed.
As the patient contracts the muscle, the
Vertical Scale, for the channel being used,
will begin to fill from bottom to top. When
the scale reaches the Target the alarm will
sound.
CHANNEL
TARGET
DISPLAYED
Stopping sEMG Therapy Session
Press the Stop Button. The Completed
Treatment Review Screen will display.
Intelect® Advanced sEMG and sEMG + Stim Module
NOTE:
To record the sEMG Data to an sEMG Data
Card, refer to the sEMG Data Card Section of
this Manual for Set Up and use of the sEMG
Data Card.
To save the session parameters to a Patient
Data Card, refer to the Intelect Advanced
User Manual for set up and use of the
Patient Data Card.
The sEMG Data Card cannot be used to
save session parameters.
VERTICAL
SCALE
SCALE
TARGET POINT
EACH
CONTRACTION
MAXIMUM
32
Page 36

OPERATION
SEMG+STIM THERAPY SET UP
General Information
The Intelect Advanced sEMG + Stim modality utilizes sEMG biofeedback activity coupled with triggered electrical muscle stimulation using
selected electrotherapy waveforms for the maximum benefit in muscle retraining. The sEMG + Stim will not function as a multi-channel
modality. It is designed for single channel use only. However, it is available to all electrotherapy channels.
The Electrical Muscle Stimulation is triggered when the muscle contraction (sEMG portion of the therapy) reaches the target. sEMG stops
and the muscle is then electrically stimulated for a period. After stimulation, the patient is given a short rest period and then repeats the
muscle contraction, attempting to reach the target to again trigger the electrical stimulation. This is repeated throughout the therapy
session.
Session parameters can be stored on a Patient Data Card. The Patient Data Card can be used with the optional Patient Data Management
System for adding session notes and printing reports. Several sessions can be stored on a Patient Data Card. Any of the sessions stored on
the Patient Data Card can be recalled in the Therapy System for future use.
It is recommended that each patient be assigned a Patient Data Card. Refer to the Intelect Advanced User Manual for Patient Data Card
setup and use instructions.
The sEMG portion of sEMG + Stim modality is used to force the patient to contract the muscle to a prescribed target. The data cannot be
recorded or stored on the Patient Data Card or the sEMG Data Card.
Intelect® Advanced sEMG and sEMG + Stim Module
33
Page 37

OPERATION
SEMG+STIM THERAPY SET UP (continued)
Prepare System and Patient
Prepare Therapy System and patient. Refer
to pages 20 through 24.
Select sEMG + Stim Modality
Press the sEMG + Stim button on the Home
screen.
SEMG+STIM
BUTTON
Select Edit
Press the Edit button on the Treatment
Review screen to edit sEMG + Stim
parameters as prescribed.
EDIT
BUTTON
Intelect® Advanced sEMG and sEMG + Stim Module
Select Channel
Press the Channel button until the
prescribed channel is displayed in the icon.
Therapy System
Available selections are 1 or 2.
Channel 3/4 Electrotherapy Module
Available selections are 3 or 4.
CHANNEL
BUTTON
NOTE:
The sEMG + Stim will not function as a
multi-channel modality. It is designed
for single channel use only. However, it is
available to all electrotherapy channels.
34
Page 38

OPERATION
SEMG + STIM THERAPY SET UP (continued)
Set Alarm
Set the alarm trigger in relation to the
target, by pressing the Alarm button until
the desired selection is displayed in the
Alarm icon.
The available selections are:
Above- Alarm sounds when a muscle
contraction surpasses the target.
Target- Alarm sounds when a muscle
contraction reaches the target.
Below- Alarm sounds when a muscle
contraction is below the target.
ALARM
BUTTON
NOTE:
If Volume is Off, no alarm will be heard.
Set Audio Type
Audio sets the type of sound heard when
the muscle contraction reaches the Alarm
setting.
Press the Audio button until the desired
audio type is displayed in the Audio icon.
The available selections are;
Constant- Continuous sound. ________
Pulsed- Fast, short beeps. . . . . . . . . . . . . . .
Dynamic- Slow, long beeps. _ _ _ _ _ _
AUDIO
BUTTON
NOTE:
If volume is Off, no Audio type will be
heard.
Intelect® Advanced sEMG and sEMG + Stim Module
Select Stim Waveform
Press the Stim button.
STIM
BUTTON
ACCEPT
UP ARROW
BUTTON
AND
RETURN
ARROW
BUTTON
DOWN ARROW
BUTTON
Press the Up and Down Arrow buttons until
the prescribed waveform is highlighted.
Press the Accept and Return Arrow button.
35
Page 39

OPERATION
SEMG + STIM THERAPY SET UP (continued)
Edit Stim
Press the Stim button.
EDIT STIM BUTTON
BACK
BUTTON
Refer to the Therapy System User Manual
for Stim Editing procedures.
After editing is complete, press the Back
button.
NOTE:
Stim treatment time is fixed and cannot be
changed.
Intelect® Advanced sEMG and sEMG + Stim Module
Select Target Option Setting Max Target
Three options are available for setting the
sEMG modality target:
Max- Maximum of muscle contractions in
10 seconds.
Avg- Average of maximums achieved in
15 seconds of muscle contractions.
Manual- Set Target manually.
Press the Target button until the prescribed
target option is displayed in the Target icon.
TARGET
BUTTON
Make certain Target Max is displayed in the
Target icon.
Press the Capture Target button. Have the
patient begin contracting the muscle and
press the Begin Capture button. Patient
should contract the muscle as hard as
possible during the preset 10 second
contraction period.
CAPTURE TARGET
NOTE:
The capture may be stopped by pressing
the End Capture Button. The System will
then select the maximum contraction level
achieved during the preset 10 second
contraction period.
BUTTON
BEGIN CAPTURE
BUTTON
36
Page 40

OPERATION
to set the Target.
SEMG + STIM THERAPY SET UP (continued)
During the preset 10 second contraction
period, the Therapy System captures
and retains the maximum level of the
contraction the patient performed, then
automatically displays the Adjust sEMG
Target screen.
Use the Up and Down Arrow buttons to
adjust the Target percentage displayed at
the bottom of each channel column.
Press the Accept and Return Arrow button
to set the Target.
UP ARROW
BUTTON
ACCEPT AND
RETURN ARROW
BUTTON
Setting Avg Target
Make certain Target Avg is displayed in the
Target icon.
Press the Capture Target button. Have the
patient begin contracting and releasing the
muscle. Press the Begin Capture button.
The patient should contract and release the
muscle as many times as possible during
the preset 15 second contraction period.
CAPTURE TARGET
BUTTON
BEGIN CAPTURE
BUTTON
Intelect® Advanced sEMG and sEMG + Stim Module
During the preset 15 second contraction
period, the Therapy System captures and
retains the average maximum level of the
contractions the patient performed, then
automatically displays the Adjust sEMG
Target screen.
Use the Up and Down Arrow buttons to
adjust the Target percentage displayed at
the bottom of each channel column.
Press the Accept and Return Arrow button
to set the Target.
UP ARROW
BUTTON
ACCEPT AND
RETURN ARROW
BUTTON
DOWN ARROW
BUTTON
NOTE:
During the preset 15 second contraction
period, the Therapy System captures and
retains the average maximum level of all
contractions that the patient performed.
37
DOWN ARROW
BUTTON
Page 41

OPERATION
SEMG + STIM THERAPY SET UP (continued)
Setting Manual Target
Make certain Target Manual is displayed in
the Target icon.
Press the Adjust Target button.
ADJUST TARGET
BUTTON
Press the Up and Down Arrow buttons to
adjust Target to prescribed level.
Press the Accept and Return Arrow button.
UP ARROW
BUTTON
ACCEPT AND
RETURN
ARROW BUTTON
DOWN ARROW
BUTTON
Intelect® Advanced sEMG and sEMG + Stim Module
Set Volume
Press Volume button until the desired
volume level is displayed in the Volume
icon.
The available volume levels are Low,
Medium, High, and Off.
VOLUME
BUTTON
38
NOTE:
If Volume is Off, no alarm will be heard
when contraction reaches the target
setting.
Page 42

OPERATION
SEMG + STIM THERAPY SET UP (continued)
Set Auto Feature
The sEMG + Stim Auto feature is designed
to work in conjunction with the Target
settings. When On, the Auto feature will
automatically adjust the maximum target
as the patient fatigues.
Press the Auto button until Off or On is
displayed in the Auto icon.
AUTO
BUTTON
Start sEMG Therapy Session
After all parameters for sEMG and Stim have
been completed, press the Start sEMG +
Stim button.
START SEMG+STIM
BUTTON
Intelect® Advanced sEMG and sEMG + Stim Module
Have patient perform contractions as
prescribed.
When the contraction reaches the target,
the alarm will sound, the screen will
change, and Stim will begin. The patient
should hold the contraction throughout
the short period of Stim.
39
NOTE:
The Stim Intensity may be adjusted only
during the Stim portion of the sEMG
+ Stim therapy session.
Page 43

OPERATION
SEMG + STIM THERAPY SET UP (continued)
After Stim is complete, the screen will
change and the patient should relax the
contraction.
After the Relax period is complete the
screen will change to Go.
Repeat the process throughout the
prescribed treatment time or until the
patient is fatigued.
Stopping Stim
Stim may be stopped at any time during
the session by pressing the Stop sEMG +
Stim button.
STOP SEMG+STIM
BUTTON
Stim may be restarted at any time during
the session by pressing the Start sEMG +
Stim button.
NOTE:
With Stim Off, the sEMG portion of the
session may continue.
Intelect® Advanced sEMG and sEMG + Stim Module
Stopping Therapy Session
Press the Stop button. The Completed
Treatment Review screen will display.
NOTE:
Refer to the Intelect Advanced User Manual
to save the session parameters to a Patient
Data Card.
The sEMG Data Card cannot be used to
save session parameters.
sEMG data, from an sEMG + Stim therapy
session, cannot be recorded to an sEMG
Data Card or Patient Data Card.
40
Page 44

OPERATION
SET UP OF NEW sEMG DATA CARD
General Information
The Intelect Advanced Therapy System incorporates an sEMG Data Card recording device that allows the recording of sEMG data from sEMG
therapy sessions. After recording the sEMG data, it can be imported, saved to, and viewed through a Windows® PC equipped with the optional
Patient Data Management System. The Patient Data Management System software and Card Reader are designed to allow importing and easy
access to the sEMG data, printing of reports, adding session notes, and adding other sEMG specific information to the sEMG data through a
Windows® PC.
The Therapy System recording device allows recording only of the sEMG Data. No sEMG Data may be recorded from the PC and saved to
the sEMG Data Card for use in the Therapy System. However, the sEMG session parameters may be saved to a Patient Data Card (refer to the
Therapy System User Manual) and recalled in the Therapy System.
Each time an existing sEMG Data Card is used for another sEMG therapy session, the existing sEMG data is overwritten with the new session
data.
It is recommended that each patient be assigned an sEMG Data Card. sEMG Data cannot be saved or recorded to the Patient Data Card.
Intelect® Advanced sEMG and sEMG + Stim Module
41
Page 45

OPERATION
SET UP OF NEW sEMG DATA CARD (continued)
Insert New sEMG Data Card
Insert a new sEMG Data Card into the
system access port as shown below. The
Therapy System will automatically format
the new sEMG Data Card and a verification
message will appear.
Press any button to continue.
INSERT NEW SEMG DATA CARD
PRESS ANY
BUTTON
TO CONTINUE
Prepare System and Patient
Prepare Therapy System and patient. Refer
to pages 20 through 24.
Set Up sEMG Therapy Session
Set up the patient's prescribed sEMG
therapy session. Refer to pages 25
through 32 of this manual for sEMG
modality set up.
Press the Setup sEMG Card button.
SETUP SEMG CARD
BUTTON
Intelect® Advanced sEMG and sEMG + Stim Module
Enter Patient ID
Select the row of alpha or numeric
characters by pushing the button beside
the corresponding row. Select the desired
character in the row by pressing the row
button until the desired letter is framed.
SELECT ROW AND CHARACTERS
42
Page 46

OPERATION
SET UP OF NEW sEMG DATA CARD (continued)
When the desired character is framed, press
the Accept and Return Arrow button. The
character selected will display in the top of
the screen and the cursor will advance to
the next position.
To move back a character, press the Left
Arrow button.
CHARACTER
DISPLAYED
To discard entire entry, press the back
Button.
Repeat this procedure until the desired
Patient ID is entered.
After Patient ID is entered, press the Save
button.
SAVE
BUTTON
Intelect® Advanced sEMG and sEMG + Stim Module
Begin Save
Press the Begin Save button. A message will
appear verifying that sEMG data is being
recorded. After the message has been
removed, have the patient perform the
prescribed contractions.
BEGIN SAVE BUTTON
LEFT
ARROW
BUTTON
ACCEPT AND RETURN
ARROW BUTTON
To delete a character, press the Left Arrow
button until the character to delete is
framed. Press the Delete button.
End Save
To stop recording, press the End Save
button.
The sEMG Data Card will record
approximately 7 minutes of sEMG data.
43
Page 47

TROUBLESHOOTING
Intelect® Advanced sEMG and sEMG + Stim Module
ERROR CODES
General Information
The Intelect Advanced Therapy Systems use error messages and warnings to inform the user of problems or potential problems with the
system, modality, or accessories. These are numbered so the user can possibly correct the problem without the aid of service personnel.
Use the following Troubleshooting Charts to define the error codes and locate the probable cause and possible remedies before
contacting the dealer or factory for technical service.
Code
Number
100 Warning Overcurrent A. Check Electrodes and Lead Wires. Make certain Lead Wires are not damaged and are properly
101 Warning Shorted Lead Wires A. Check Electrodes and Lead Wires. Make certain Lead Wires are not damaged and are properly
102 Warning Bad Contact Quality A. Make certain Electrodes are making proper contact with the treatment area.
103 Warning Blank Patient ID Properly enter Patient ID. Refer to Therapy System User Manual for Patient Data Card instructions.
104 Warning 1. Blank Protocol Name
106
107
Type
Message
Warning
Warning
Probable Cause Possible Remedies
2. Blank Sequence Name
1. Attempting to delete factory set Sequence
2. Attempting to delete Clinical Protocol
connected to the system. Make certain Lead Wires are properly connected to the Electrodes and
that electrodes are not damaged and are making proper contact with treatment area.
B. Replace Lead Wires and Electrodes.
connected to the system. Make certain Lead Wires are properly connected to the Electrodes and that
electrodes are not damaged and are making proper contact with treatment area.
B. Replace Lead Wires and Electrodes.
B. Make certain Lead Wires are properly connected to Electrodes.
C. Replace Electrodes and Lead Wires.
Properly enter Protocol or Sequence Name. Refer to the appropriate section of the Therapy System
User Manual.
Cannot delete factory set Clinical Protocols or factory set Sequences.
44
Page 48

TROUBLESHOOTING
ERROR CODES (continued)
Intelect® Advanced sEMG and sEMG + Stim Module
Code
Number
108 Warning Attempting to save additional User Protocols or Sequences after
109
110
111
112 Warning Ultrasound Applicator disconnected from system during treatment
113 Warning Attempting to perform Ultrasound treatment with no Applicator
114 Warning Ultrasound Applicator is not calibrated. Attempt to use a known good Applicator. If problem continues, contact dealer or DJO for Service.
115 Warning Ultrasound Applicator is too hot. Allow Ultrasound Applicator Sound Head to cool to ambient temperature.
116
117
Type Message Probable Cause Possible Remedies
Delete some User Protocols or Sequences. Refer to appropriate section of the Therapy System User
Manual for instructions.
A. User Protocols- No protocols have been saved in the system. Refer to Therapy System User Manual
to save User Protocols.
B. Sequences- No User Sequences have been saved in the system. Refer to Therapy System User
Manual to save Sequences.
A. Connect Ultrasound Applicator to system.
B. If Ultrasound Applicator is connected, reset system by turning power switch Off and On.
C. If problem persists, connect a known good Ultrasound Applicator. If problem continues, contact
dealer or DJO for service.
A. Connect the desired Ultrasound Applicator to the system.
B. If Ultrasound Applicator is connected, reset system by turning power switch Off and On.
C. If problem persists, connect a known good Ultrasound Applicator. If problem continues, contact
dealer or DJO for service.
A. Properly insert the Patient Data Card into the system port. Refer to Therapy System User Manual
for new and existing Patient Data Card instructions.
B. Use a known good Patient Data Card.
C. Make certain a Patient Data Card and not an sEMG Data Card is being used.
D. If problem continues, contact dealer or DJO for service.
Warning
Warning
Warning
Warning
Warning
system memory has reached the maximum allowed (200).
Attempting to access protocols or sequences and none are found
in the system.
session.
connected to the system.
1. No Patient Data Card is inserted into the system.
2. Attempted to use an Invalid Patient Data Card.
45
Page 49

TROUBLESHOOTING
Intelect® Advanced sEMG and sEMG + Stim Module
ERROR CODES (continued)
Code
Number
118 Warning Attempting to save additional User Protocols or Sequences after
119
120
121
122
123 Warning Patient Data Card is full. Erase Patient Data Card. Refer to Therapy System User Manual for instructions.
124 Warning Patient Treatment Data already saved. A. Cannot save same data again on Patient Data Card.
125 Warning Multimedia Card (MMC) not in system port. A. Properly insert the MMC card into the system port.
126 Warning No valid channels are available for attempted treatment. A. Complete existing treatment before attempting to start another.
127
128
129 Warning sEMG Data Card full. sEMG Data Card faulty. Insert a known good sEMG Data Card. If problem continues, contact dealer or
Type
Message
Warning
Warning
Warning
Warning
Warning
Warning
Probable Cause Possible Remedies
system memory has reached the maximum allowed (200).
1. Attempted to read a treatment from Patient Data Card that is
not a valid treatment for the system
2. Attempted to use a Non-Patient Data Card.
3. No Patient Data Card inserted into system port.
4. Unknown type of smart card inserted into system.
1. No sEMG Channels are available for treatment.
2. No sEMG Module installed or detected by system.
Delete some User Protocols or Sequences. Refer to appropriate section of the Therapy System User
Manual for instructions.
A. Use a Patient Data Card with proper treatment data for the system.
B. Properly insert a Patient Data Card.
C. Insert a known good Patient Data Card.
D. If problem persists, insert a known good Patient Data Card. If problem continues, contact
dealer or DJO for service.
B. Use a new Patient Data Card to resave data.
C. Erase Patient Data Card and resave treatment data.
B. Insert a known good MMC Card. If problem continues, contact dealer or DJO for service.
B. Reset Therapy System by turning main power switch Off and On.
A. Wait until current treatment is complete.
B. Reset Therapy System by turning main power switch Off and On.
C. Make certain sEMG Module is properly installed. Refer to sEMG Module User Manual for installation
instructions.
D. Replace sEMG Module with known good sEMG Module.
E. If problem continues, contact dealer or DJO for Service.
DJO
for Service.
46
Page 50

TROUBLESHOOTING
ERROR CODES (continued)
Intelect® Advanced sEMG and sEMG + Stim Module
Code
Number
130 Warning Another treatment is running while attempting to set up and
131 Warning Treatment Room Door Lockout is breached. A. Make certain Treatment Room Door is completely closed.
132 Warning Attempted to start a laser treatment but no Laser Applicator is
133 Warning Laser Applicator became unplugged while performing a laser
134 Warning Entered incorrect laser PIN. A. Enter correct Laser PIN number.
135 Warning Control Board software upgrade warning. Upgrade Control Board software to latest version. Contact dealer or DJO for latest software upgrade and
136 Warning Stim Board Main software upgrade warning. Upgrade Stim Board software to latest version. Contact dealer or DJO for latest software upgrade and
Type
Message
Probable Cause Possible Remedies
perform a Laser Therapy treatment.
plugged in.
treatment.
A. Allow existing treatment to complete before starting Laser Therapy.
B. If no other treatment is running, reset Therapy System by turning main power switch
Off and On.
B. Make certain the Lockout cable is connected to the system.
C. Replace Lockout to System cable with a known good cable.
D. Contact department responsible for installation of the Treatment Room Door Lockout mechanism for
maintenance or repair.
E. If problem continues, send Laser Module to factory for service.
A. Connect desired Laser Applicator to the system.
B. If Applicator is connected, reset Therapy System by turning main power switch Off and On.
C. Connect a known good Laser Applicator.
D. If problem continues, send Laser Module to factory for service.
A. Connect desired Laser Applicator to the system.
B. If Laser Applicator is connected, reset Therapy System by turning main power switch
Off and On.
C. Connect a known good Laser Applicator.
D. If problem continues, send Laser Module to factory for service.
B. If problem continues, send Laser Module to factory for service.
instructions.
instructions.
47
Page 51

TROUBLESHOOTING
ERROR CODES (continued)
Intelect® Advanced sEMG and sEMG + Stim Module
Code
Number
137 Warning Stim Board Main software upgrade warning. Upgrade Stim Board sSoftware to latest version. Contact dealer or DJO for latest software upgrade and
138 Warning Ultrasound Board software upgrade warning. Upgrade Ultrasound Board software to latest version. Contact dealer or DJO for latest software upgrade
139 Warning Laser Board software upgrade warning. Upgrade Laser Board software to latest version. Contact dealer or DJO for latest software upgrade and
140 Warning MMC software upgrade warning. Upgrade MMC software to latest version. Contact dealer or DJO for latest software upgrade and
141 Warning Battery Module software upgrade warning. Upgrade Battery software to latest version. Contact dealer or DJO for latest software upgrade and
142 Warning A Laser Protocol was selected but no Laser Module is installed
143 Warning A Laser Protocol was selected but no Laser Applicator connected
144 Warning Wrong Laser Applicator connected to system for the protocol
Type
Message
on system.
to system.
selected.
Probable Cause Possible Remedies
instructions.
and instructions.
instructions.
instructions.
instructions.
Install Laser Module to Therapy System. Refer to Laser User Manual for installation instructions.
A. Connect proper Laser Applicator to the system.
B. If Laser Applicator is connected, reset Therapy System by turning main power switch
Off and On.
C. Connect a known good Laser Applicator.
D. If problem continues, send Laser Module to factory for service.
A. Connect correct Laser Applicator to the system.
B. If Applicator is connected, reset Therapy System by turning main power switch Off and On.
C. Connect a known good Laser Applicator.
D. If problem continues, send Laser Module to factory for service.
48
Page 52

TROUBLESHOOTING
WARNING
ERROR CODES (continued)
Intelect® Advanced sEMG and sEMG + Stim Module
Code
Number
145 Warning Patient Data Card button on Home screen was pressed with no
Type
Message
Probable Cause Possible Remedies
Patient Data Card installed into system port and no treatment
currently being performed.
In the event that an Error message or Warning appears beginning with
a 2 or 3, immediately stop all use of the system and contact the dealer
or DJO for service. Errors and Warnings in these categories indicate an
internal problem with the system that must be tested by DJO or a Field
Service Technician certified by DJO before any further operation or use
of the system.
Use of a system that indicates an Error or Warning in these categories
may pose a risk of injury to the patient, user, or may cause extensive
internal damage to the system.
Properly insert a Patient Data Card, set up and perform the treatment, and save data to Patient Data
Card.
49
Page 53

REPLACEMENT ACCESSORIES
Intelect® Advanced sEMG and sEMG + Stim Module
General Information
When ordering replacement or optional accessories, provide the respective part number, description and quantity desired.
Color Series Standard Features
Order No. Description Qty
2765CS Two Channel Electrotherapy System (or) 1
2762CC Two Channel Combination System 1
27378 Electrotherapy Accessory Kit- Includes the following: 1
27312 Channel 1 Lead Wire 1
27313 Channel 2 Lead Wire 1
10648 Nylatex® Wrap 2
79967 6 x 8 cm Carbon Electrodes 4
79970 6 x 8 cm Electrode Sponges 4
42044 7 cm (2.75") Round Disposable Electrodes (4 per pack) 40/case
27469 Patient Interrupt Switch for Channels 1/2 1
27335 5 cm
4248 Conductor
27085 Anatomical/Pathological Library (MMC Card) 1
27465 Patient Data Card 5
2771 sEMG Module (Factory Installed) 1
27567 sEMG Accessory Kit- Includes the Following 1
27321 sEMG Channel 1 (A) Lead Wire 1
27322 sEMG Channel 2 (B) Lead Wire 1
77725 Intravaginal Probe 1
42061 3.2 cm (1.25") Round Disposable Electrode Pack (4 per pack) 3
27455 User Manual (CD-ROM) 1
2
Ultrasound Applicator (Combination Systems Only) 1
TM
Transmission Gel- 9 oz Bottle (Combination Systems Only) 24/case
Order No. Description
2770 Two Channel Electrotherapy Module
2767 NiMH Battery Module
2766 Laser Therapy Module
2771 sEMG Module
27567 sEMG Accessory Kit
2785 Vacuum Electrode Module
2774 Vacuum Electrode Module w/Cart
2775 Therapy System Cart
2768 Patient Data Management System- Includes
27779 Version 1.0 PC Software (Windows)
27176 Card Reader
27300 USB Cable
27167 sEMG Data Card
27516 sEMG Data Card Sleeve
27780 User Manual (on Software CD)
27508 Operator Remote (Ch 1/2)
27079 Operator Remote (Ch 3/4)
27333 1 cm
27334 2 cm
27336 10 cm
Color Series
Optional Accessories
the following:
2
US Applicator (Combination Only)
2
US Applicator (Combination Only)
2
US Applicator (Combination Only)
Mains Power Cords
Order No.
21284 Euro 1
78121 US 1
20971 Australian 1
20972 Swiss 1
20973 UK 1
20974 Danish 1
20975 Japanese 1
20976 Indian 1
20977 Israeli 1
NOTE:
The Power Cord shipped with
the System will accommodate
the electrical requirements for
the country of use.
Type Qty
50
Page 54

MAINTENANCE
CARING FOR THE THERAPY SYSTEM SERVICE
Cleaning the Therapy System
With the system disconnected from the power source, clean the
system with a clean, lint free cloth moistened with water and
mild antibacterial soap. If a more sterile cleaning is needed, use a
cloth moistened with an antimicrobial cleaner.
Do not submerse the system or modules in liquids. Should the
unit accidentally become submersed, contact the dealer or
DJO Service Department immediately. Do not attempt to use a
system that has been wet inside until inspected and tested by a
Service Technician Certified by DJO
Do not allow liquids to enter the ventilation holes in the optional
modules. This could permanently damage the modules.
Cleaning the Lens
Clean the Therapy System Screen Lens using NOVUS® Polish System.
Contact Novus at: www.novuspolish.com
Should the unit require service, warranty, or repair, please contact
the selling dealer or your local DJO customer service.
Ultrasound Applicators require annual calibration, from the date
placed in service, by the Factory or a Service Technician certified
by DJO.
Intelect® Advanced sEMG and sEMG + Stim Module
NOVUS is the Registered Trademark of NOVUS Inc.
51
Page 55

WARRANTY
DJO, LLC ("Company") warrants that the Intelect Advanced Electrotherapy System sEMG and sEMG + Stim Module ("Product") is free of defects in material and workmanship. This warranty shall remain in effect for two years (24 months) from the date of original consumer purchase. If this Product fails to function during the two year warranty
period due to a defect in material or workmanship, Company or the selling dealer will repair or replace this Product without charge within a period of thirty (30) days from
the date on which the Product is returned to the Company or the dealer.
All repairs to the Product must be performed by a service center authorized by the Company. Any modifications or repairs performed by unauthorized centers or groups will
void this warranty.
The warranty period for accessories is 180 days. Accessories consist of sEMG Lead Wires, Electrodes and Nylatex®.
This warranty does not cover:
• Replacement parts or labor furnished by anyone other than the Company, the selling dealer or a certified Company service technician.
• Defects or damage caused by labor furnished by someone other than Company, the selling dealer or a certified Company service technician.
• Any malfunction or failure in the Product caused by product misuse, including, but not limited to, the failure to provide reasonable and required maintenance or any use
that is inconsistent with the Product User's Manual.
• DJO, LLC is not responsible for injury or damage resulting from modifications or service performed by non-authorized DJO, LLC service personnel.
COMPANY SHALL NOT BE LIABLE IN ANY EVENT FOR INCIDENTAL OR CONSEQUENTIAL DAMAGES.
Some locations do not allow the exclusion or limitation of incidental or consequential damages, so the above limitation or exclusion may not apply to you.
To Obtain Service From Company or the selling dealer under this warranty:
1. A written claim must be made within the warranty period to the Company or the selling dealer.
2. The Product must be returned to the Company or the selling dealer by the owner.
This warranty gives you specific legal rights and you may also have other rights which vary from location to location.
The Company does not authorize any person or representative to create for it any other obligation or liability in connection with the sale of the Product.
Any representative or agreement not contained in the warranty shall be void and of no effect.
THE FOREGOING WARRANTY IS IN LIEU OF ALL OTHER WARRANTIES, EXPRESSED OR IMPLIED,
INCLUDING ANY WARRANTY OR MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE.
Intelect® Advanced sEMG and sEMG + Stim Module
52
Page 56

DJO is an ISO 13485 Certied Company
DJO France SAS
Centre Europeen de Fret
3 rue de Bethar
64990 Mouguerre, France
T: + 33 (0) 5 59 52 86 90
F: + 33 (0) 5 59 52 86 91
djoglobal.eu/fr_FR
© 2011 DJO, LLC. All rights reserved.
27455_H SEMG (English)
 Loading...
Loading...