
Instruction Manual
3-D Flow Chamber Device
TABLE OF CONTENTS
Section 1: General Information
1.1 Flow rate chart. . . . . . . . . . . . . . . . . . . . . . . . . . . 3
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
1.2
1.3 System Overview
System Contents . . . . . . . . . . . . . . . . . . . . . . . . . .4-5
1.4
Section 2: Cell Culture on Upper and Lower Inserts
2.1 Coating the Membrane disk
2.2 Growing the Cells
Section 3: Flow Chamber Set-Up & Use
3.1 Preparation . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
3.2 Setting up the Tubing . . . . . . . . . . . . . . . . . . . . . . . .8-9
3.3 Priming the Tubing . . . . . . . . . . . . . . . . . . . . . . . . . 10
3.4 Filling the Reservoirs and Assembly of 3D Chamber . . . . . . . . . . . 11-13
3.5 Introducing Circulating Cells to Flow Chamber. . . . . . . . . . . . . . . 13
3.6 Recovery of Cells from Chamber . . . . . . . . . . . . . . . . . . .14-16
3.7 Cleaning the Chmaber after Use . . . . . . . . . . . . . . . . . . . . 17
Section 4: Tips and Troubleshooting
4.1 Media Considerations . . . . . . . . . . . . . . . . . . . . . . . . 18
4.2 Pump Controller
4.2 Flow Rate Chart . . . . . . . . . . . . . . . . . . . . . . . . . . 23
. . . . . . . . . . . . . . . . . . . . . . . . . . 4
. . . . . . . . . . . . . . . . . . . . . . 6
. . . . . . . . . . . . . . . . . . . . . . . . .6-7
. . . . . . . . . . . . . . . . . . . . . . . . .19-22
2
SECTION 1 GENERAL INFORMATION
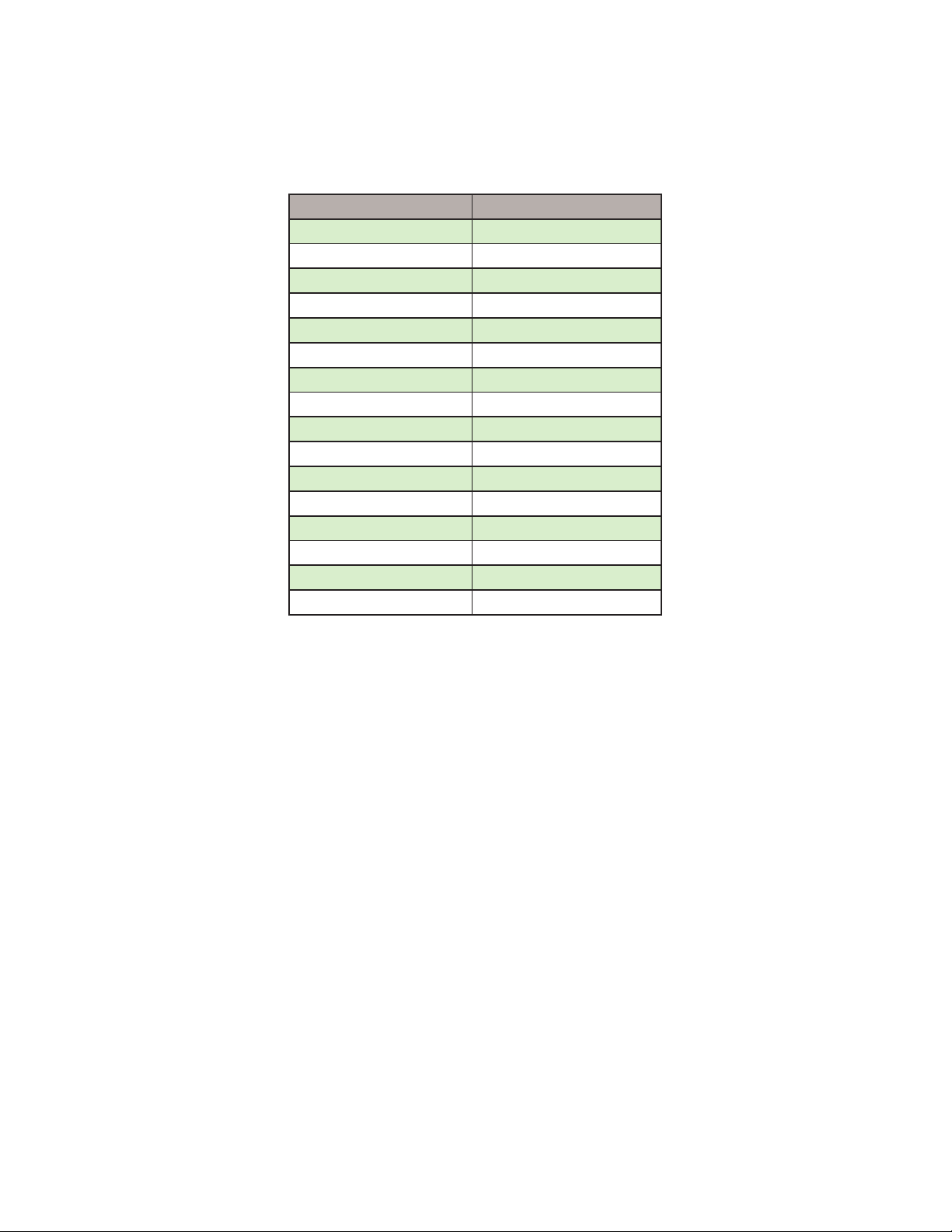
1.1 Flow rate chart for Pump Controller for Quick Reference
ml/min dyn/cm2
0.2 0.7
0.3 1
0.4 1.3
0.5 1.6
0.6 1.9
0.7 2.3
0.8 2.6
0.9 2.9
1 3.3
2 6.6
3 9.8
5 16.3
10 35.7
20 71.6
30 107
44 157.4
1.2 References
Please go to this link: http://www.jove.com/video/50959/a-novel-three-dimensional-ow-chamber-device-to-study-chemokine
44ml/min is the maximum ow rate for the Pump Controller.
3-D Flow Chamber Device Instructions April 2014
3
www.cbsscientific.com

1.3 System Overview
The 3-D Flow Chamber Device is a specialized laminar ow chamber that enables the unique ability
to quantitate cellular motility through a semi-permeable membrane in the presence of shear stress.
Relevant applications for the 3-D Flow Chamber Device include transmigration, chemotaxis, vascular
permeability, and complex in vitro modeling of 3D cell cultures such as the blood brain barrier. Fur-
ther, it can be used to hold material coupons in place of the membranes for analysis of biolm activity.
1.4 System Contents
The following components ship with the 3-D Flow Chamber Device.
Complete System Includes:
• Flow Chamber Device with 3 reservoirs
• 3 upper membrane inserts
• 3 lower membrane inserts
• Tubing and connectors
• Carrying tray
• Respirator to allow gas exchange and bubble escape
4
Respirator (Bubble Trap)Pump Controller
Carrying Tray (optional)
Flow Cell (disassembledl)
Reagent Reservoir (3x)
3-D Flow Chamber Device Instructions April 2014
Upper Inserts
5
Lower Inserts
www.cbsscientific.com

SECTION 2 CELL CULTURE ON UPPER & LOWER INSERTS
2.1. Coat the membrane disk with the relevant substrate to support cell adhesion
a. Using aseptic technique (sterile forceps) place the dry membrane disk on a dry sterile petri dish
The top membrane is positioned so that the membrane support (clear ring) is on the bottom and the
membrane is facing up.
The bottom membrane is positioned so that the membrane support is facing up – the membrane disk
will look like a little cup.
b. Carefully dispense the coating of interest (example: bronectin, poly-l-lysine) onto the membrane
surface. The membrane is hydrophobic, be sure that the liquid covers the entire surface. It will
bead up. It will take approximately 500μl to cover the top membrane and the same amount will ll
the bottom membrane cup.
c. Cover up the petri dish and incubate at the desired temperature for the desired duration.
d. Wash the coating as necessary. The membrane is now ready for use with cells.
2.2 Growing cells on the coated membrane
6

a. Remove any liquid remaining from the coating and dispense the cells onto the membrane as shown above. The
liquid will bead up as it did when coating.
b. Place the covered petri dish in the incubator and incubate for the desired amount time – most cells will attach
within 1 hour.
c. Fill the dish with medium so that the membranes are oating. The top membrane is prone to lling up
with air – if this happens you can pick the membrane up with forceps and pipet some of the media
into the underside of the membrane and then slowly lay it down onto the media so that it is wet from
below.
d. Place the dish in the incubator. The cells can be stained with a live cell dye to facilitate viewing them
on the membranes (example: calcein AM or cell tracker dyes).
e. Observe cells and plan to use immediately when they reach the desired density. Conuent HUVEC
cells stained with calcein AM are shown below.
3-D Flow Chamber Device Instructions April 2014
7
www.cbsscientific.com

SECTION 3 FLOW CHAMBER SET-UP & USE
3.1 Preparation
a. Assemble all of the needed components in the area where you will set up the chamber
(the chamber, the tubing, forceps, pump, respirator, the clamps, and connectors)
b. Prepare chemokines, buffers, etc.
c. If you are going to perform your experiments at 37˚C, pre-warm all the media to avoid
spontaneous bubble formation.
3.2 Setting Up the Tubing
The 3D Flow Chamber Device is intended to be connected in a closed loop for most
experiments - the loop will consist of the chamber, tubing, pump tubing (is a xed length
and has color coded stops attached) and the respirator.
a. Set up the pump tubing and clip tubing into the pump.
b. Complete the tubing loop
8

c. Connect tubing coming from Respirator to the chamber (above).
3-D Flow Chamber Device Instructions April 2014
9
www.cbsscientific.com

3.3 Priming the Tubing in Preparation for an Experiment.
a. Prepare a 50 ml tube with your buffer of interest place the tubing (right side, not the Respirator side) into
the tube. Run the pump so that it is pumping in the direction of the Respirator. A ow rate of ~2ml/min is a
good starting point. Make sure the pinch clamp is closed on the Respirator side.
b. Fill the Respirator, cover it and release the pinch clamp when ready to connect to 3D chamber.
10

3.4. Filling the Reservoirs and Assembling the 3D Chamber.
a. If you are using 2 membranes as a sandwich ll the reservoir with ~800 ul of chemokine or buffer. If you are using
the single top membrane ll the chamber with ~1.5ml of chemokine or buffer.
b. Work quickly! If using the double membrane, rst pop the membranes together and then lay them into the reservoir.
If using a single membrane, lay it over the buffer using forceps. In either case, tamp the membranes down ush with
the top of the chamber and remove excess buffer if it overows from the reservoir using a pipet.
3-D Flow Chamber Device Instructions April 2014
11
www.cbsscientific.com

c. Close the chamber.
12

d. Start the pump (direction towards the respirator) - 1-2 mls/min. Tilt the chamber slightly upwards to facili-
tate bubble clearance from the chamber. You also may tap it lightly to dislodge any bubbles. Once buffer
is coming out the other side of the chamber attach the tubing to close the loop.
e. Reduce the pump speed to desired level for experiment (see appendix).
3.5. Introducing Circulating Cells to the Flow Chamber without Disrupting the Flow.
a. Prepare cells to desired density. 1-10million/ml is a common range although varying levels may need to
be tested to ensure compatibility with the application.
b. With the pump running, dispense the cells into the respirator as shown.
c. If desired, place entire apparatus including the pump into a tissue culture incubator.
3-D Flow Chamber Device Instructions April 2014
13
www.cbsscientific.com

3.6. How to Recover Cells from the Chamber
At the end of the experiment, if desired cells can be collected from the reservoirs and the sandwich
space created by the membranes. Before stopping the pump and terminating the experiment – be
sure to have tubes ready for cells from reservoirs and have clean petri dishes to take the membrane
sandwiches apart.
a. First stop the pump. Close both pinch valves.
b. Working quickly – take chamber apart on a paper towel or absorbent mat. Remove excess cham-
ber liquid.
14

3-D Flow Chamber Device Instructions April 2014
15
www.cbsscientific.com

16

3.7. Cleaning the System After Use.
• The membranes are meant to be disposed of after use according to the biosafety guidelines of
your institute.
• The durable parts of the system (chamber, tubing, connectors, Respirator, etc...) can be de-
contaminated by placing them in a solution of 10% bleach for 20 minutes. Tubing can either be
disposed of outright or ushed with a bleach solution if re-use is desired. A
void using alcohols,
uv or other harsh cleaners as they may adversely affect the surfaces. After soaking in bleach,
be sure to rinse each part very well with sterile RO water.
• For further cleaning (recommended), a solution containing lab detergent (e.g. Alconox) can be
used at the manufacturer’s recommended strength followed by thorough rinsing with distilled
water.
• The components of the system can be sterilized either by gamma irradiation or ethylene oxide
treatment. DO NOT AUTOCLAVE.
3-D Flow Chamber Device Instructions April 2014
17
www.cbsscientific.com

SECTION 4 ADDITIONAL TIPS AND TROUBLESHOOTING
4.1. Media Considerations
• All uids should be equilibrated to temperature to avoid bubble formation.
• Maintenance of pH by CO2 buffering must be achieved by placing the respirator into a Tissue
culture incubator with a loose tting Petri dish cover on the top of it allowing gas exchange
into the system.
If you would like to bypass the Respirator, an alternative is to use medium that can be buffered
without CO2 such as CO2-independent media (Invitrogen cat# 18045088) which can be supplemented with typical additives such as growth factors and FBS to grow cells. For example,
for HUVEC, CO2-independent media is supplemented with the EGM-2 bullet kit (Lonza)
4.2. Pump Controller
18

3-D Flow Chamber Device Instructions April 2014
19
www.cbsscientific.com

20

3-D Flow Chamber Device Instructions April 2014
21
www.cbsscientific.com

The tubing loop diameter controls the range of ow rates for the system. The range of ow
rates per tube diameter (mm) is shown below. The system includes tube loops with diameters
of both 1.22 mm and 1.52 mm to enable ow rates from 0.079 ml/min to 12 ml/min. The relationship of ow rate to shear is shown in the next section.
22

Flow rate chart for Pump Controller
ml/min dyn/cm2
0.2 0.7
0.3 1
0.4 1.3
0.5 1.6
0.6 1.9
0.7 2.3
0.8 2.6
0.9 2.9
1 3.3
2 6.6
3 9.8
5 16.3
10 35.7
20 71.6
30 107
44 157.4
44ml/min is the maximum ow rate for the Pump Controller.
3-D Flow Chamber Device Instructions April 2014
23
www.cbsscientific.com

CONTACT INFORMATION
Online Ordering
www.cbsscientic.com
Telephone
Local and International: 858-755-4959
Toll Free: 800-243-4959
Sales E-mail Address
sales@cbssci.com
Technical Service E-mail Address
technicalservice@cbssci.com
Mailing Address
P.O. Box 856
Del Mar, CA 92014
Shipping Address
10805 Vista Sorrento Parkway, Ste 100
San Diego, CA 92121
24
 Loading...
Loading...