Cardioline touchECG User Manual

1936
Rev. 12 – 16.06.2020
touchECG
User manual

touchECG
All rights reserved © Cardioline SpA.
CARDIOLINE® is a registered trademark of Cardioline SpA.
Android™ is a Google Inc. trademark.
This publication may not be reproduced, in whole or in part, in any form or manner, without prior written authorisation
by:
Cardioline Spa
Via Linz, 151
38121 Trento
Italy

touchECG
Contents
1. GENERAL INFORMATION ................................................................................................................................ 1
1.1. Minimum Requirements for the computer/tablet/smartphone......................................................... 1
1.2. Licensing terms ....................................................................................................................................... 1
1.3. Other important information ................................................................................................................. 2
2. SAFETY INFORMATION .................................................................................................................................... 3
2.1. Warnings on Bluetooth........................................................................................................................... 6
2.1.1. Potential sources of interference of Bluetooth ........................................................................... 6
2.1.2. Obstacles for the wireless signal ................................................................................................... 7
2.1.3. Reducing the effects of the interference of other wireless devices........................................... 7
3. SYMBOLS AND LABEL ...................................................................................................................................... 8
3.1. Explanation of the symbols .................................................................................................................... 8
3.2. Device label ............................................................................................................................................. 8
4. INTRODUCTION ............................................................................................................................................... 9
4.1. Purpose of the manual ........................................................................................................................... 9
4.2. Recipients ................................................................................................................................................ 9
4.3. Intended use............................................................................................................................................ 9
4.4. Description of the device ....................................................................................................................... 10
4.5. General overview .................................................................................................................................... 11
4.5.1. Main buttons and icons ................................................................................................................. 13
5. PREPARATION FOR USE................................................................................................................................... 18
5.1. Installing the software ............................................................................................................................ 18
5.1.1. Installation on Windows................................................................................................................. 18
5.1.2. Installation on Android ................................................................................................................... 20
5.2. Launching touchECG ............................................................................................................................... 21
5.2.1. Start-up with manual entry of Ward Id ......................................................................................... 24
5.3. Connecting and configuring the HD+ acquisition unit ......................................................................... 25
5.3.1. Connecting the HD+ acquisition unit to the Windows tablet/computer ................................... 25
5.3.2. Connecting the HD+ acquisition unit to the Android tablet/computer ..................................... 26
5.3.3. Configuring the HD+ acquisition device........................................................................................ 28
5.4. Virtual keyboard configuration (Windows version only) ..................................................................... 30
5.5. Printer configuration (Android version only) ........................................................................................ 30
5.6. Start from command line (Windows version only) ............................................................................... 33

touchECG
5.7. System installation .................................................................................................................................. 35
6. EXECUTION OF THE EXAMINATION ............................................................................................................... 36
6.1. General procedure .................................................................................................................................. 36
6.2. Before acquisition ................................................................................................................................... 36
6.2.1. Preparing the patient ..................................................................................................................... 36
6.2.2. Connecting the patient .................................................................................................................. 37
6.3. Viewing the ECG ...................................................................................................................................... 39
6.3.1. Changing the trace display mode .................................................................................................. 42
6.3.2. Leads disconnected ........................................................................................................................ 43
6.3.3. Bookmark ........................................................................................................................................ 44
6.4. Entering patient data .............................................................................................................................. 45
6.4.1. Patient window ............................................................................................................................... 45
6.4.2. Manually entering the patient's data............................................................................................ 47
6.4.3. Entering patient details from the Examinations Archive ............................................................. 48
6.4.4. Entering patient details from a work list ...................................................................................... 49
6.5. Acquisition of an ECG examination. ...................................................................................................... 51
6.5.1. Automatically acquiring an ECG examination .............................................................................. 51
6.5.2. Review mode acquisition of an ECG examination (Windows version only) ............................... 52
6.5.3. Manual mode acquisition of an ECG examination ....................................................................... 57
6.6. Previewing an ECG examination ............................................................................................................ 58
6.6.1. Changing the display and printout mode ..................................................................................... 61
6.6.2. Modifying patient details ............................................................................................................... 62
6.6.3. Modifying the automatic interpretation....................................................................................... 62
6.6.4. Identifying an urgent ECG examination ........................................................................................ 63
6.6.5. Printing out and saving an ECG examination ............................................................................... 64
6.6.6. Transmitting an ECG examination ................................................................................................. 65
6.6.7. Sending an ECG examination by email ......................................................................................... 66
6.7. Test Archive ............................................................................................................................................. 66
7. CONNECTIVITY, RECEIVING WORK LISTS AND TRANSMITTING ECG EXAMINATIONS ................................ 69
7.1. General information ............................................................................................................................... 69
7.2. Receiving a work list ............................................................................................................................... 70
7.3. Uploading an exam ................................................................................................................................. 71
7.4. Sending an examination by email .......................................................................................................... 72
7.5. Saving an examination in SCP and PDF format ..................................................................................... 73
7.6. Connectivity formats and protocols ...................................................................................................... 73

touchECG
7.6.1. GDT (in the Windows version only) ............................................................................................... 73
7.6.2. Cardioline Standard ........................................................................................................................ 74
7.6.3. Cardioline DICOM ........................................................................................................................... 75
7.6.4. Text file (in the Windows version only)......................................................................................... 75
7.6.5. XML (in the Windows version only)............................................................................................... 77
8. DEVICE SETTINGS ............................................................................................................................................. 78
8.1. General information ............................................................................................................................... 78
8.2. Summary of settings ............................................................................................................................... 80
8.2.1. PACS ................................................................................................................................................. 80
8.2.2. ECG .................................................................................................................................................. 81
8.2.3. Manual (Windows version only) .................................................................................................... 81
8.2.4. Auto ................................................................................................................................................. 82
8.2.5. Connectivity .................................................................................................................................... 82
8.2.6. Other ............................................................................................................................................... 84
8.2.7. License ............................................................................................................................................. 85
8.2.8. Safety ............................................................................................................................................... 85
8.3. Protection of the Settings ...................................................................................................................... 85
8.4. Virtual Keyboard Management (Windows version only) ..................................................................... 87
9. SET THE DEVICE IN ACCORDANCE WITH GDPR (General Data Protection Regulation) – Windows
only 88
9.1. General information ............................................................................................................................... 88
9.2. Encrypt the folder containing the database ......................................................................................... 88
9.3. Enable the audit trail .............................................................................................................................. 89
9.4. Enable the “Delete test after sending” setting. .................................................................................... 89
10. UPDATING THE SOFTWARE AND OPTIONS ................................................................................................... 90
10.1. Updating the software ............................................................................................................................ 90
10.1.1. Updating on Windows .................................................................................................................... 90
10.1.2. Updating on Android ...................................................................................................................... 90
10.2. Updating enabled options ...................................................................................................................... 90
10.2.1. General information ....................................................................................................................... 90
10.2.2. Entering the activation code.......................................................................................................... 91
11. MAINTENANCE AND TROUBLESHOOTING .................................................................................................... 92
11.1. General information ............................................................................................................................... 92
11.2. Operation check ...................................................................................................................................... 92
11.3. Bluetooth ................................................................................................................................................. 92

touchECG
11.4. Troubleshooting table ............................................................................................................................ 93
11.5. Messages and solutions table ................................................................................................................ 93
12. TECHNICAL SPECIFICATIONS ........................................................................................................................... 96
12.1. Filter features .......................................................................................................................................... 98
12.2. Harmonised standards applied .............................................................................................................. 99
12.3. Accessories .............................................................................................................................................. 99
13. WARRANTY ....................................................................................................................................................... 100

Operating System ............................
Windows: Windows 7, Windows 8.1, Windows 10
Android: Android 4.4 KitKat (API 19) or higher
Processor ........................................
Quad core 1.6 GHz or higher
RAM ................................................
Windows: 2GB or more
Android: 1 GB or more
Free space on Hard Disk ..................
8 GB or more
Monitor ...........................................
Windows: 640 x 480 pixels or more
Android: Tablet: 7” or more
Smartphone: Samsung 4.7” or more
Bluetooth ........................................
Bluetooth 2.1 +EDR
Printer .............................................
Laser (colour/BW)
Additional applications ....................
Windows: Email application which supports the EML format (only
required for the email File Upload feature)
Android: Acrobat “PDF” file format viewer
touchECG
1. GENERAL INFORMATION
1. GENERAL INFORMATION
This manual is an integral part of the device and should always be available as support material to the clinical
practitioner or the operator. Strict compliance with the information contained in this manual is an essential
prerequisite for the proper and reliable use of the device.
Have the operator read the manual thoroughly, as a great deal of the information is only described once.
1.1. Minimum Requirements for the computer/tablet/smartphone
touchECG can be installed on any computer (PC, tablet, notebook, etc.) with the following minimum
requisites:
1.2. Licensing terms
By installing the software, the terms and conditions described as follows are accepted.
Object of this agreement is the consent of a use licence for the software and the operating manual.
Cardioline SpA guarantees a personal licence, non-exclusive and non-transferable, for use of the software
and the attached documents. The software and accompanying documents are protected by copyright. The
user must comply with copyright law dispositions.
All rights relative to the software are the property of Cardioline SpA. The transferral of software onto
another computer by means of the network or data channel is not permitted.
The programs and the attached documents cannot be changed, copied, merged with other programs or
made available to third parties.
The user is deemed responsible for any damage stemming from non-compliance with the copyright, or from
violation of the conditions reported in this agreement.
1

Language
Code
English
36519163_EN
touchECG
1. GENERAL INFORMATION
1.3. Other important information
This manual was written with the utmost care. Should you find any details which do not correspond to those
contained in this manual, please inform Cardioline SpA, who will proceed to correct such inconsistencies as
soon as possible.
The information contained in this manual is subject to change without notice.
All changes will be in compliance with the regulations governing the manufacturing of medical equipment.
All trademarks mentioned in this document are property of their respective owners. Their protection is
guaranteed.
No part of this manual may be reprinted, translated or reproduced without the manufacturer's written
authorisation.
The codes relating to this manual are listed below.
2

touchECG
2. SAFETY INFORMATION
2. SAFETY INFORMATION
Cardioline SpA will be held responsible for the safety, reliability and functionality of the devices only if:
1. Assembly, modification or repair operations are carried out by Cardioline SpA or by one of its
Authorised Assistance Centres;
2. The device must be used in compliance with the instructions contained in the user manual.
Always contact Cardioline SpA should you wish to connect any devices not mentioned in this manual.
Warnings
This manual provides important information on the proper use and safety of the device. Failure to
comply with the described operating procedure, improper use of the device, ignoring the
specifications and recommendations supplied, may cause severe physical injuries to the operators,
patients and bystanders, or may damage the device.
The device cannot be modified in any way.
Do not use the device if you suspect it is malfunctioning.
The device captures and presents the data that reflects the physiological condition of the patient;
this information can be examined by specialist medical staff and will be useful in providing an
accurate diagnosis. In any event, the data cannot be used as the only means to make an accurate
diagnosis of the patient.
The operators for whom this device is intended must have the required competence regarding
medical procedures and the treatment of patients. They must also be sufficiently trained in using the
device. Have the operator carefully read and understand the contents of the operator manual and
the other annexed documents before using the device for clinical applications. Inadequate
knowledge or training could be at a greater risk for the physical safety of operators, patients and
bystanders, or could damage the device.
US Federal Legislation restricts the sale of this device to prescription by a doctor.
The device is designed for use only with the HD+ acquisition unit. Refer to the HD+ user manual for
the risks and warnings associated with it and for its user instructions.
This device may be installed on a tablet, desktop computer or laptop (hereinafter the support
device), which satisfies the minimum requirements indicated in para. 1.1. To ensure the electrical
safety of the operator and patient during its operation, the following precautions must be observed:
If the support device is battery powered, do not connect it to an external power supply (for
recharging) or other electrical equipment (e.g. to another computer via USB or to a LAN
network) when using it in the patient area.
3

touchECG
2. SAFETY INFORMATION
If the support device is powered off the mains, it may not be used in the patient area. When
used in the patient area, it must be powered with an isolation transformer and shielded
cables must be used to connect it to the LAN. The power cable shielding (when present)
must be connected to an earthing system appropriate for the area where the device is used.
This will avoid electric shocks caused by different earth potentials which could exist between
the various points of an electricity distribution system, or else by failures of the external
equipment connected to the mains.
The HD+ acquisition unit for use with the device is protected against defibrillation. Check the HD+
unit's cables for cracks and breakage before using it.
Refer to the HD+ unit's user manual for the risks and warnings associated with it.
This device is designed to be used only with the electrodes specified in this manual. Strictly follow
the correct clinical procedures to prepare the skin before the application of the electrodes and
monitor the patient in order to avoid any irritation, inflammation or other skin reactions. The
electrodes are designed for short-term applications and must be promptly removed once the
examination is complete.
The ECG electrodes may cause skin irritation; check the skin for any irritations or inflammations.
To prevent any infections, use the disposable components (e.g. the electrodes) only once. To ensure
safety and use efficiency, do not use electrodes after their expiration date.
The quality of the signal produced by the device may be adversely affected by the use of other
medical equipment such as defibrillators and ultrasound machines.
The risks associated with the use of the device together with other equipment, such as pacemakers
and other stimulators, depend on the support device on which it is installed. The signal may be
subject to disturbance in any case.
The use of the device in combination with other high frequency (HF) surgical equipment depends on
the support device in question.
The operation may be adversely affected by the presence of strong magnetic fields such as those
produced by electro surgery equipment.
The use of the device in the presence of diagnostic imaging equipment, such as Magnetic Resonance
(MR) or Computer Axial Tomography (CAT), in the same room, depends on the support device in
question.
The software reports the battery level of the HD+ unit. The low battery warning is designed to work
solely with the HD+ unit using the batteries indicated in its user manual. If the battery level is low,
replace the HD+ unit's batteries and observe its user instructions.
Using a support device equipped with a GPRS or WLAN unit may interfere with other nearby
equipment. Check with local authorities or with providers of the spectrum of your structure to find
out whether any restrictions apply to use of the device in your area.
Do not leave the patient cable unattended in the presence of children as they could be accidentally
strangled.
Do not leave the electrodes unattended in the presence of children as they could cause suffocation
if accidentally swallowed.
4

touchECG
2. SAFETY INFORMATION
If you are printing PDF files, you need to set up a corresponding program so that the document is in
no way adapted or scaled. If you are using Acrobat Reader, you need to select “Actual Size” in “Page
management and size.” Otherwise you may get nondiagnostic quality prints.
In the case of Android, we recommend using printers approved by Cardioline, for which the
diagnostic quality of prints is ensured.
Attention
The device does not require any calibration or special instrumentation for correct use and
maintenance.
The Glasgow algorithm is intended for automatic interpretation of ECGs at rest. The automatic
interpretation provided by touchECG can only be considered if HD+ is used to acquire ECGs at rest. If
HD+ is used to acquire signals while the patient is moving (for example during a stress test) the
automatic interpretation may not be valid.
The Glasgow 12-lead resting ECG Analysis Algorithm has specific criteria for patients of different
ages, gender and race. If the option is enabled, the algorithm can provide the doctor with an
automatic interpretation, which generates diagnostic messages on the ECG report. Validation is
required by a cardiologist or a doctor.
Notes
The movements of the patient may generate excessive noise and affect the quality of the ECG
tracing or the correct analysis of the device.
An appropriate preparation of the patient is important in order to guarantee a proper application of
the ECG electrodes and the correct operation of the device.
The incorrect positioning of the electrodes for the detection of the algorithm depends on the normal
physiology and on the order of the ECG leads and tries to identify the most likely exchange.
However, it is recommended to check the positioning of the electrodes of the same group (limbs or
chest).
If the electrodes are not properly connected to the patient, or one or more patient leads are
damaged, the display will indicate that the leads in question are disconnected. When the ECG is
printed, these leads will come out on paper as a square wave.
The accuracy of the measurements taken by this device complies with the specific requirement of
standard IEC 60601-2-25.
The device is a Class IIa in compliance with Directive 93/42/EEC.
The device is a “prescription device” under the terms of FDA regulations.
The HD+ unit must be connected to the support device running the software before use.
5

touchECG
2. SAFETY INFORMATION
2.1. Warnings on Bluetooth
2.1.1. Potential sources of interference of Bluetooth
It is important to learn how to minimise wireless interference that may cause slow performance and
disconnections from Bluetooth devices.
Specifically, there might be interferences and it is required to make an operation check if the following are
found:
Inability to couple a Bluetooth device to the computer/tablet
Intermittent HD+ disconnections
Loss of the ECG signal.
Possible causes of interference of the Bluetooth signal may be:
Microwave ovens: using microwave ovens close to the computer/tablet may cause interference.
Satellite TV: the coaxial cable and connectors used with some satellite dishes may cause
interference. Check whether the cables are damaged thus causing radio interferences (RF leakage).
Replace the cables if interference is suspected.
Power supply: some external electricity sources such as power lines, electrical railroad tracks and
power stations, may cause interference. Avoid placing the computer/tablet near wall power lines or
close to junction boxes.
2.4 GHz or 5 GHz telephones: cordless telephones operating in the 2.4 GHz or 5 GHz range may cause
interference with other wireless devices when used to place calls.
Wireless RF video: wireless video transmitters that operate in the 2.4 GHz or 5 GHz range may cause
interference with other wireless devices.
Wireless speakers: wireless speakers that operate in the 2.4 GHz or 5 GHz range may cause
interference with other wireless devices.
Certain external VDUs and LCD displays: certain displays emit harmonic interferences, especially in
the 2.4 GHz band between channels 11 and 14. These interferences may be stronger if using a
notebook computer with a closed display and a connected external monitor.
Inadequately shielded cables: external hard disks or other devices with poorly shielded cables can
interfere with wireless devices. If disconnecting or switching off the device reduces the
interferences, try replacing the cable that connects the device to the computer/tablet.
Other wireless devices: other wireless devices that operate in the 2.4 GHz or 5 GHz band (nearby
microwave transmitters, wireless cameras, baby monitors, WiFi devices) may cause interferences
with Bluetooth connections.
Enabling GPS operation: if the GPS function is enabled on the device in use, it may create
interference with the Bluetooth signal. If losses of Bluetooth signal packets are noted, it is
recommended to disable the GPS function.
6

Material
Potential interference
Wood
Low
Synthetic material
Low
Glass
Low
Water
Medium
Bricks
Medium
Marble
Medium
Plaster
High
Concrete
High
Bulletproof glass
High
Metal
Very high
touchECG
2. SAFETY INFORMATION
2.1.2. Obstacles for the wireless signal
The position of the device and the construction materials may affect Bluetooth performance. If possible,
avoid obstacles or move the Bluetooth device for the signal to travel through fewer obstacles. For example,
avoid placing the computer/tablet under a metal desk and the HD+ device on top of a desk.
Below is a chart showing the radio signal-blocking ability of the most common devices:
2.1.3. Reducing the effects of the interference of other wireless devices
If a lot of wireless devices are connected to the computer/tablet or nearby, it may be required to encode the
channels used by the WiFi devices.
To reduce the interference between WiFi devices and Bluetooth to the minimum try these solutions:
Change channels of the wireless network;
Connect to a 5 GHz wireless network (if possible);
Place the HD+ close to the computer/tablet.
Reduce to the minimum the number of Bluetooth devices connected to the computer/tablet or
nearby.
7
3. SYMBOLS AND LABEL
Symbol
Description
Comply with the instructions in the use manual
CE marking – compliance with the European Union directives
Refer to the user instructions
Manufacturer
3.1. Explanation of the symbols
touchECG
3. SYMBOLS AND LABEL
3.2. Device label
Windows
Android
8

4. INTRODUCTION
4.1. Purpose of the manual
This manual covers the touchECG product.
The manual represents a guide for the execution of the following operations:
Reasonable use of the device, of the function keys and of the sequence of menus.
Preparation of the device for use (Section 5)
Acquisition, printing and storage of ECG traces (Section 6)
Connectivity and transmission of ECG tracings. (Section 7)
touchECG
4. INTRODUCTION
System configuration (Section 8)
Updating the device (Section 10)
Troubleshooting (Section 11)
4.2. Recipients
This manual is intended for professional healthcare operators. They are therefore presumed to have specific
knowledge of medical procedures and terminology, as required by clinical practice.
4.3. Intended use
touchECG is designed to monitor and diagnose cardiac function. However, a Cardiologist must validate the
results of the analysis run by the ECG.
touchECG is intended for use in hospitals, clinics and outpatient departments of any size. It is also suitable
for use by medical staff at the patient's home and in an emergency (ambulance).
touchECG is designed for use with the Cardioline HD+ device. The Cardioline HD+ device acquires the ECG
signal and sends it via Bluetooth to the PC on which the touchECG software is installed. touchECG software is
designed to only work with the Cardioline HD+ device.
The device acquires, analyses, displays and prints out electrocardiograms.
The device interprets the data for review by a doctor.
The device is intended for use by a doctor or by healthcare personnel on behalf of an authorised
doctor in clinical facilities. It is not intended as the only means for determining the diagnosis.
The device's interpretation of the ECG analysis is only significant if used together with an additional
analysis by a physician and with an assessment of all the patient's other significant data.
9

touchECG
4. INTRODUCTION
The device can be used on adult and paediatric patients.
The device must not be used as a physiological monitoring of vital signs.
4.4. Description of the device
touchECG is a 12-lead diagnostic electrocardiograph which displays, acquires, prints and stores ECG traces
for adults and children. It also calculates the principal global ECG parameters.
The device can be supplied with the optional 12-lead Glasgow resting ECG interpretation algorithm, with
specific criteria for patients of different age, sex and race. If this option is enabled, the algorithm can provide
the referring physician with an automatic report, generating diagnostic messages in the ECG report.
For further information on the resting ECG interpretation algorithm, see the Instruction Manual for doctors
for its use with adults and children (see list of accessory equipment).
The device can be configured with the DICOM® function.
The device installs on any PC, tablet or notebook with the minimum requisites listed in para. 1.1.
It prints out in the following formats: standard or Cabrera 3, 3+1, 3+3, 6 or 12 channel in automatic mode,
and 3, 6 or 12 printout channels of the rhythm strip.
The touchECG device includes:
1. Dongle with touchECG software
2. User manual
The device can be sold as a system that may include, depending on the sale configurations:
1. HD+ unit
2. HandyVAQ suction-operated patient cable
3. Computer (tablet or all-in-one PC)
4. Thermal battery-powered printer (Windows version only)
5. Trolley (tablet model or digital model)
Each device in the system comes with its own user manual and accessories.
Note: the Glasgow Algorithm Interpretation is always loaded on the device but can be enabled or disabled
according to the acquired options. The Glasgow interpretation program can only be enabled or disabled by
using the activation code provided by Cardioline (see Para. 10.2).
The Glasgow interpretation program runs the automatic interpretation as well as the readings on the ECG
traces. In any case, if the Glasgow interpretation program is disabled, only the readings are printed, whereas
the automatic interpretation is not printed.
10

4.5. General overview
The program windows are divided into three main areas:
Top bar (1):
at the top, it displays the window's main information, e.g. patient first and last name, messages and
so on.
Side bar (2):
on the right, it contains the window's function buttons.
Two different displays are available, that may be selected from the Settings page (see para. 8.2.6):
˗ “Complete user interface”, showing all available controls,
˗ “Quick user interface", showing some controls only.
Some buttons, such as trace speed, width, filter and so on, allow you to select parameter values and
change when clicked on to reflect the current value. The trace speed button, for instance, allows you
to select a different value with each click, and cycles through the possible rates (5, 10, 25, 50 mm/s).
It changes to display the currently selected value (5, 10, 25 or 50).
Other buttons activate/deactivate their value and change to reflect this.
touchECG
4. INTRODUCTION
Central area (3):
the central area displays the contents of the window, for example the traces in the main window.
Lower bar (4):
at the bottom, depending on the window, it is used to show the continuous rhythm track or to show
informational messages.
11
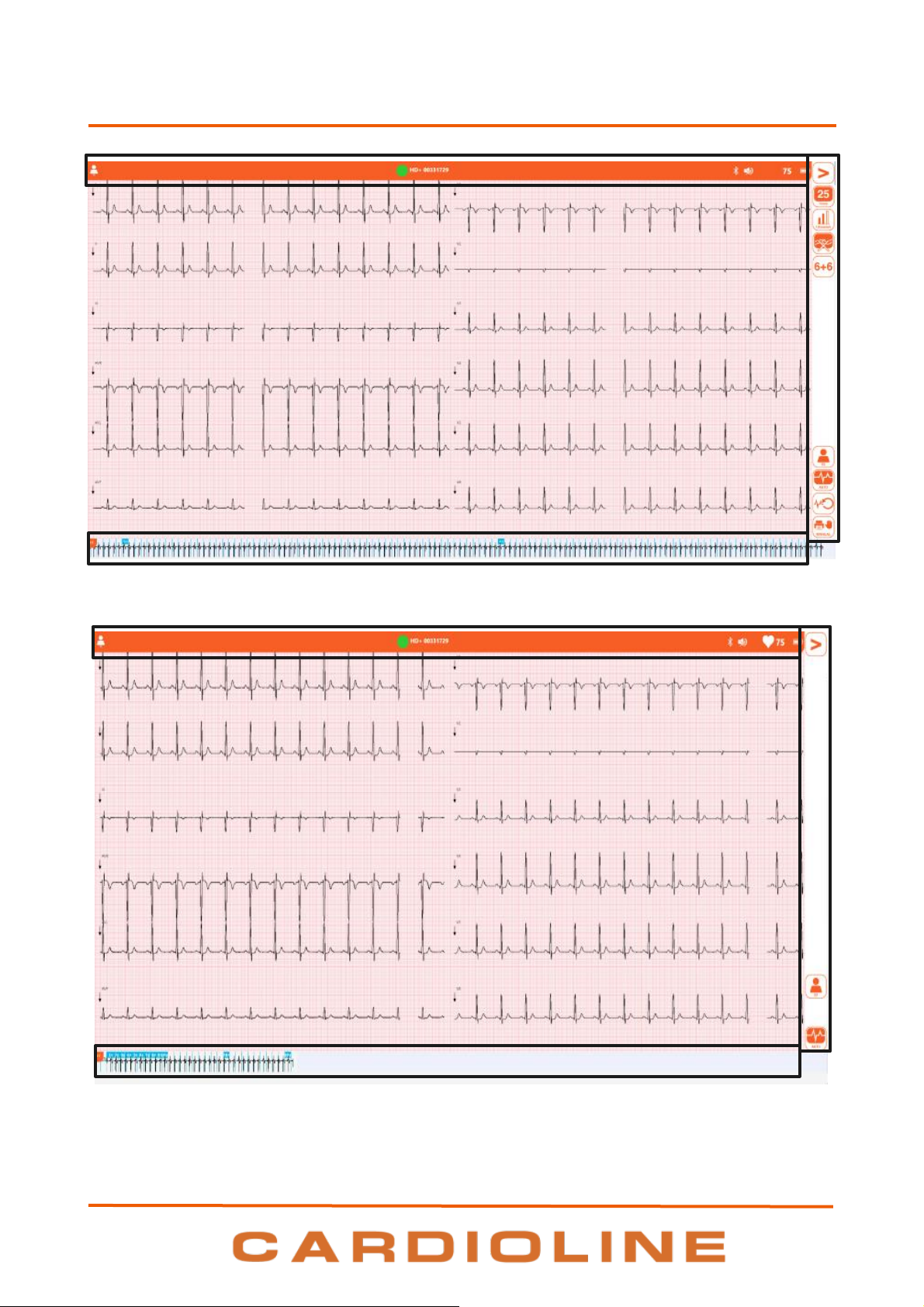
1
2
3 4 1
2
3
4
touchECG
4. INTRODUCTION
Main window with full User Interface
12
Main window with quick User Interface
Icon
Description
Patient first and last name (if entered)
HD+ connected / not connected
QRS sound on / off
Attention!
Cardiac frequency (with value in bpm)
HD+ battery level
Number of tests to be sent
Number of pages to print (only for Windows version)
Button
Version
Name
Description
Main window
Menu 1
Open menu
Opens the secondary menu.
C
Speed
Selects the trace speed
5, 10, 25, 50 mm/s
C
Amplitude
Selects the trace amplitude
5, 10, 20 mm/mV
touchECG
4. INTRODUCTION
4.5.1. Main buttons and icons
We list all commands available in touchECG.
For a detailed description of the commands and their functions, refer to the chapters describing the
respective application windows.
Top bar
Side bar
C indicates the controls only featured in the full user interface
W indicates the controls only featured in the Windows version
13
C
Muscle filter
Selects the muscle filter
25 Hz, 40 Hz, 150 Hz, off
Note: The 150 Hz filter only works on 1000 Hz
acquisitions and is only available in the
Windows version.
Note: The 25 Hz filter is stronger than the 40
Hz filter.
Setting the filter to “off” disables muscle
filtering of the traces.
C
Format
Selects the trace format
6+6, 3x1 (I-II-II), 3x1 (aVL, aVR, aVF), 3x1 (v1,
v2, v3), 3x1 (v4, v5, v6), 12x1
Id
Opens the patient database for entering the
patient details.
Auto
Starts automatic 10s ECG acquisition
C W
Review
Starts acquisition in review mode
Available in the Windows version only.
C W
Manual
Starts/stops manual mode acquisition
Available in the Windows version only.
Menu 2
Close menu
Closes the secondary menu.
Examination archive
Opens the examination archive
Settings
Opens the settings window
W
Launch App
Launches an external application (if so
configured in the settings)
Close
Closes touchECG
Patient window
New patient
Opens a new patient
Search
Searches for a patient in the examinations
archive
Work list
Opens the work list window
touchECG
4. INTRODUCTION
14
OK
Closes the patient window and saves the
entered data.
Back
Closes the patient window without saving the
entered data.
Examinations archive window
Display
Displays the selected examination
Transmit
Transmits the selected examination
Transmit all
Transmits all untransmitted examinations
Printing
Prints out the selected examination
Send email
Sends the selected examination by email (as
a PDF report)
Remove
Deletes the selected examination
Ok
Closes the examinations archive and saves
the database data for the selected
examination.
Back
Closes the examinations archive without
saving the database data for the selected
examination.
Work list window
Update
Updates the work list by downloading it
again.
Delete
Deletes the order selected from the list
(locally, not on the server from which the list
has been downloaded).
OK
Closes the window, saves the selection and
loads the selected patient data.
Back
Closes the window without saving the
selection or loading the selected patient
data.
Settings
Update license
Updates the program's active options.
Save
Closes and saves the settings.
touchECG
4. INTRODUCTION
15
Back
Closes without saving the settings
Examination preview window
Menu 1
Open menu
Opens the secondary menu.
Speed
Selects the trace speed
25, 50 mm/s
Amplitude
Selects the trace amplitude
5, 10, 20 mm/mV
Muscle filter
Selects the muscle filter
25 Hz, 40 Hz, 150 Hz, off
Note: The 150 Hz filter only works on 1000 Hz
acquisitions and is only available in the
Windows version.
Note: The 25 Hz filter is stronger than the 40
Hz filter. Setting the filter to “off” disables
muscle filtering of the traces.
Format
Selects the trace format
6x2, 3x4, 3x4+1, 3x4+3, 12x1
Urgent
Turns the "urgent" status of the examination
on/off.
Transmit
Transmits the acquired examination
Printing
Prints out the acquired examination
Save / Update
Saves / updates the acquired examination
Back
Closes the window and returns the main
window in real time
Menu 2
Close menu
Closes the secondary menu.
W
Calipers
Enables / disables the calipers tool.
Available in the Windows version only.
Send email
Sends the acquired examination by email (as
a PDF report)
touchECG
4. INTRODUCTION
16
W
Launch App
Launches an external application (configured
in the settings)
Available in the Windows version only.
Review window
Speed
Selects the trace speed
25, 50 mm/s
Amplitude
Selects the trace amplitude
5, 10, 20 mm/mV
Muscle filter
Selects the muscle filter
25 Hz, 40 Hz, 150Hz, off
Note: The 150 Hz filter works only on
acquisitions at 1000 Hz. The 25 Hz filter is
stronger than the 40 Hz filter.
Setting the filter to “off” disables muscle
filtering of the traces.
Format
Selects the trace format
6+6, 12x1
Id
Opens the Patient database for entering the
patient details.
Auto
Acquires a 10s ECG corresponding to the
selected portion of the trace.
Back
Closes the window and returns the main
window in real time
touchECG
4. INTRODUCTION
17
5. PREPARATION FOR USE
5. PREPARATION FOR USE
5.1. Installing the software
5.1.1. Installation on Windows
Installation on Windows operating system may take place in two ways:
Insert the supplied CD into your computer (possibly provided as part of the system) and:
a. double click on the setup.exe file, if an internet connection is available
Note: this mode uses the ClickOnce technology described below.
b. Click on the touchECG.application file, if an internet connection is not available.
touchECG
Note: this mode uses the ClickOnce technology described below.
c. double-click on the touchECG_Setup.msi file
Note: this mode uses the ClickOnce technology described below.
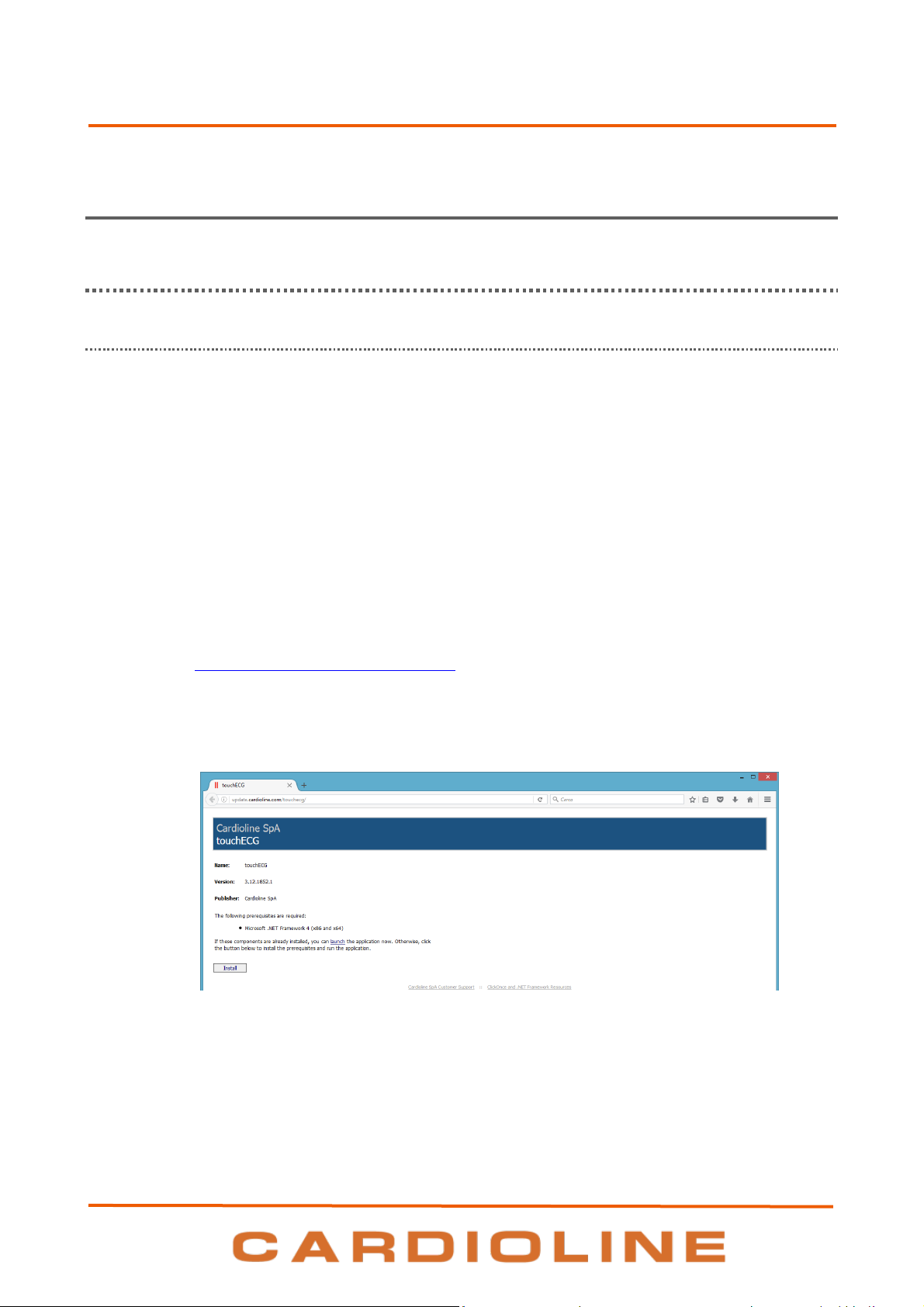
Download the installation software from the internet, if an internet connection is available, by
typing: ttp://update.cardioline.com/touchecg in the address bar of the browser and clicking Install
Note: this mode uses the ClickOnce technology described below.
Note: if there is no Internet connection to install the application, the touchECG.application file in the
installation archive must be launched.
The software installation is launched automatically after having launched the setup program and is
completed when the touchECG program begins and a desktop icon is created.
18
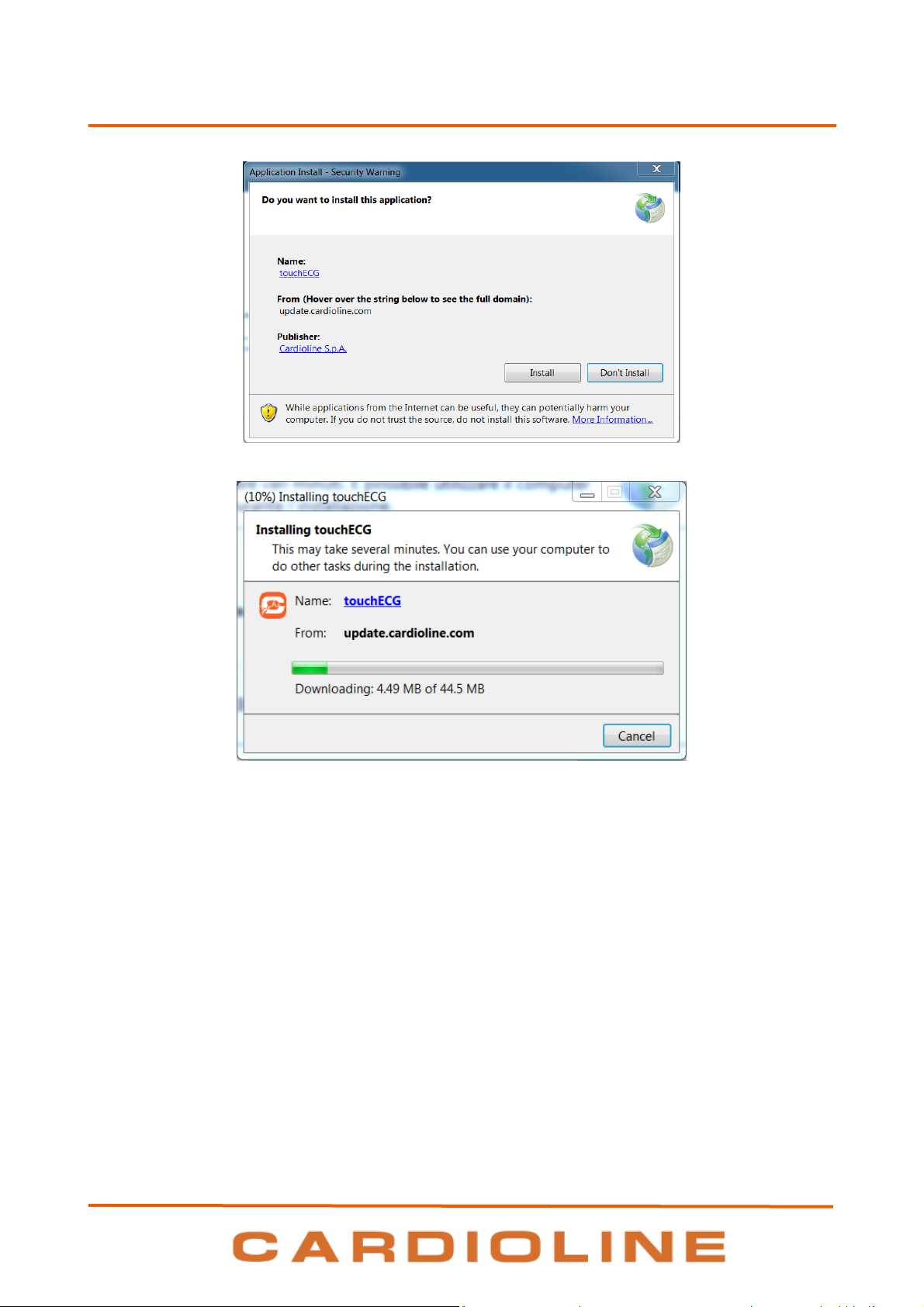
touchECG
5. PREPARATION FOR USE
Confirmation window
Installation progress window
The installation modes that use the Microsoft ClickOnce technology make it possible to:
Install the program automatically;
Manage the automatic updates of the program, by downloading them directly from internet when
available and when an internet connection is available.
19
5. PREPARATION FOR USE
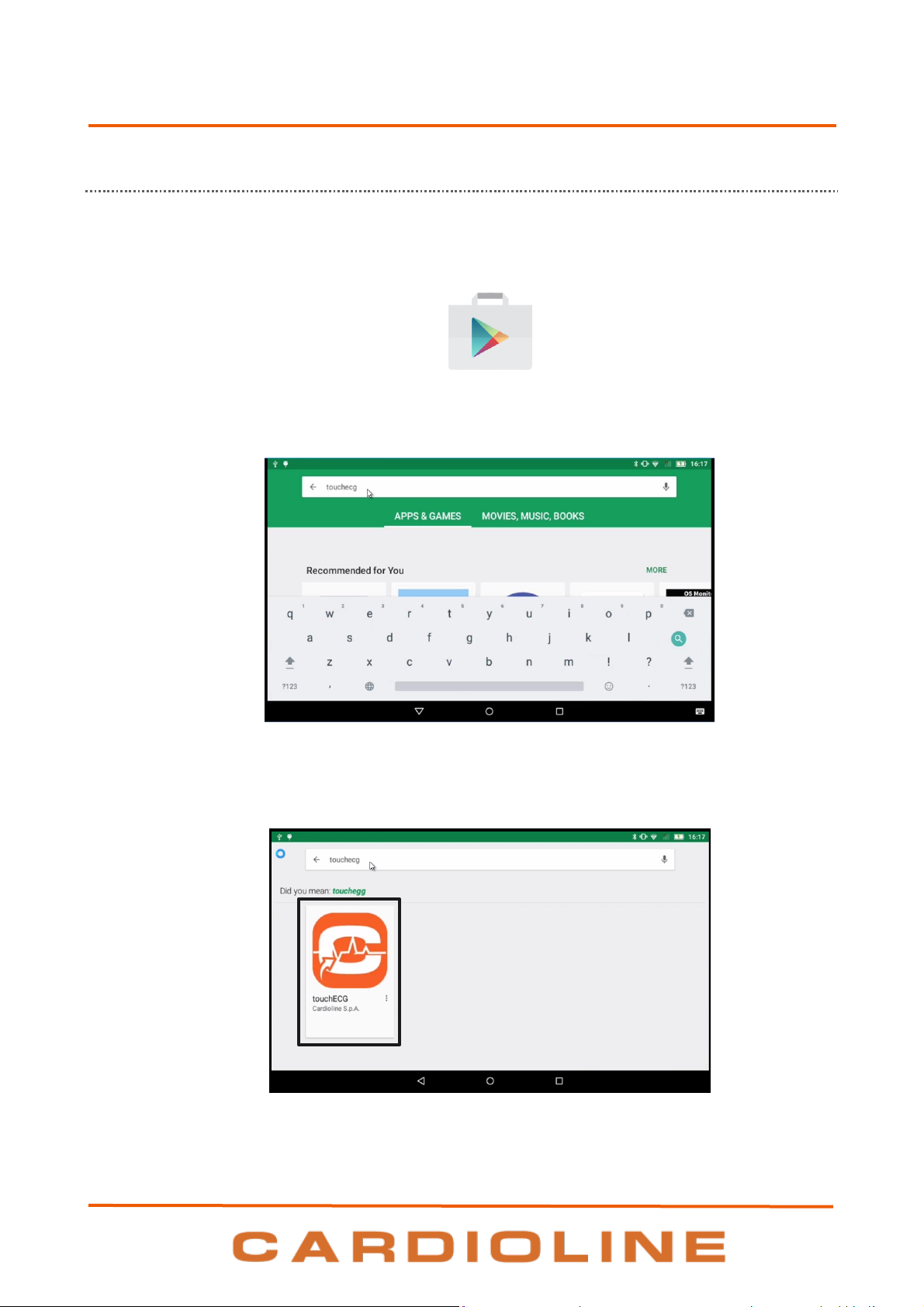
5.1.2. Installation on Android
It is possible to install TouchECG on Android operating system simply from the Android Play Store:
1. Launch the Play Store by clicking on its icon (in the App list)
touchECG
2. Search for “touchECG” in the search bar.
3. Select TouchECG and click on INSTALL.
Play Store Icon
Search for “touchECG”
20

touchECG
5. PREPARATION FOR USE
.
Select TouchECG and click on INSTALL
4. Wait for the installation to be completed. Completion of the installation is notified in the top bar of
the home page or in the standby screen.
Installation completed
5.2. Launching touchECG
To launch the touchECG program, simply click on its desktop icon or in the applications list.
21
touchECG icon
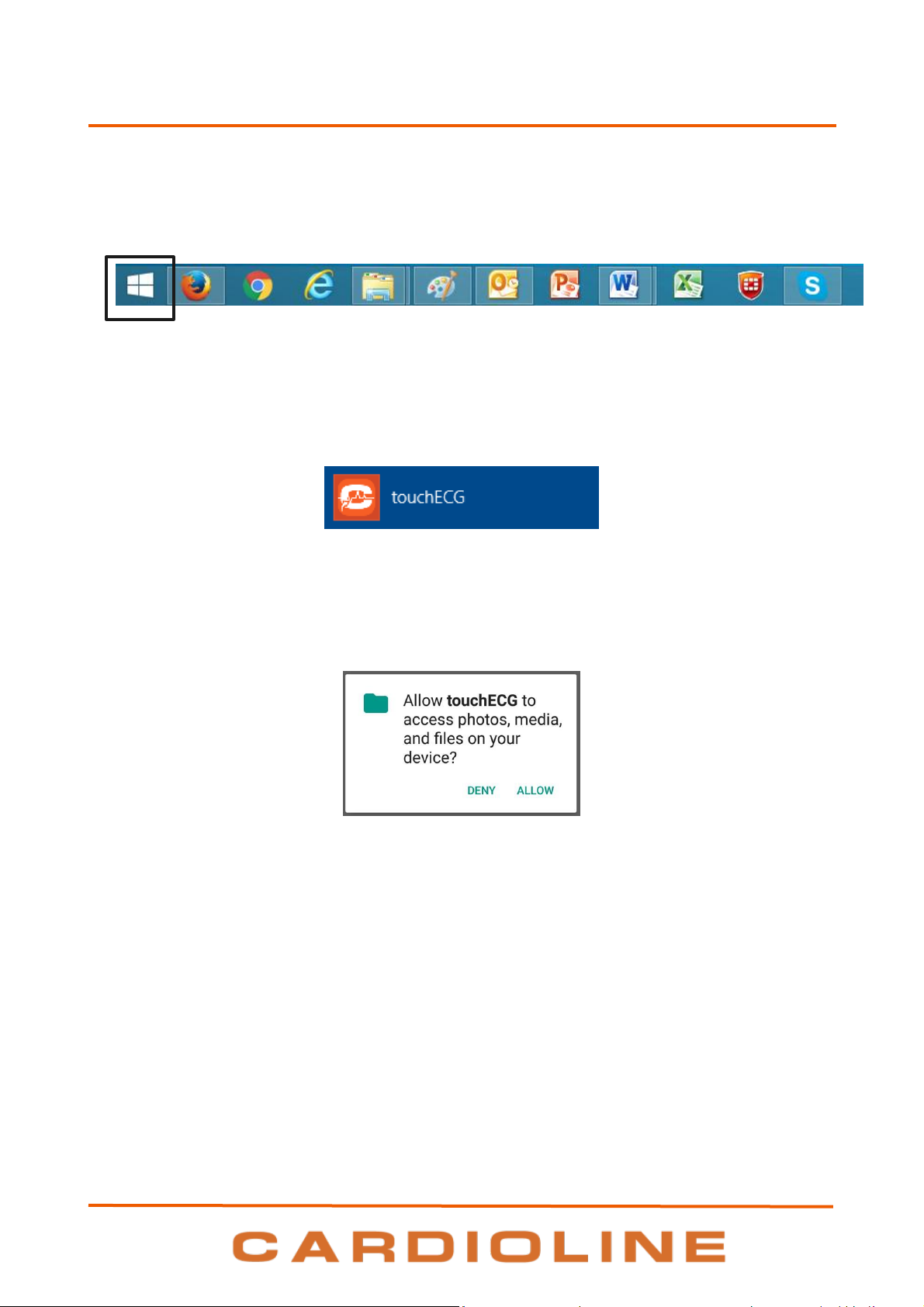
touchECG
5. PREPARATION FOR USE
Alternatively, you can select the program from the list of installed programs:
1. On Windows operating system, click on the Windows button in the application bar (see picture
below) to open the main Windows menu.
Windows button
On Android operating system, go the applications list.
2. In the list of applications (App) select touchECG.
Select touchECG
NOTE: from TouchECG version 3.42 onwards, with Android operating system, the first time the program is
launched a message is displayed, asking permission to access to the device’s memory.
Permission for access must be granted, otherwise the program ends.
Permissions may be entered manually from the menu Settings > Permissions > Memory permissions.
Note: The program starts up with “Quick user interface” default
Note: if the “Boot Screen” mode is selected (see par. 5.2.1) TouchECG is started with a home screen that lets
you enter the Ward ID.
22

Once the program is launched the following window is displayed:
touchECG
5. PREPARATION FOR USE
Start page
If no HD+ has been configured yet (see para. 5.3), TouchECG will start in DEMO mode showing a screen with
sample traces. Video traces are marked with the word “DEMO,” just as saved and printed tests.
Demo mode can be also enabled by selecting HD+ “Demo” in settings (see para. 8.2).
23
DEMO Mode

touchECG
5. PREPARATION FOR USE
For the ClickOnce version for Windows, every week, when the program is closed, the software automatically
searches for any available updates and alerts to this with a dialogue window.
Dialogue window”
If any updates are available, you will be prompted to download them.
Click on OK to download and install the updates; click on Ignore to skip this step. If you ignore the updates,
the program will no longer check for them when it launches. After a week, the automatic check for updates
is resumed.
Once the updates have been downloaded, they install automatically and a dialogue window displays the
progress.
5.2.1. Start-up with manual entry of Ward Id
It is possible to set up TouchECG for it to start with a window to manually enter the Ward ID. This feature is
useful if the device is often moved between wards with different Ward ID or in case of replacement
equipment.
If the Boot Screen setting is enabled (see par. 8.2), upon start-up the window pictured below is displayed, in
which the Ward ID may be entered.
The entered Ward ID is also saved in the program settings and becomes the default one.
24
Boot Screen for entering the Ward ID.
 Loading...
Loading...