Cardioline ar2100adv User Manual

ar2100adv
user manual
english

This User Manual has been prepared with the objective of giving the user all the
information necessary to make the best use of the CARDIOLINE® ar2100adv.
General information
et medical devices SpA, continuously in search of technological improvement and
customer satisfaction, reserves the right to modify this publication without prior
notice at any time.
All rights reserved © et medical devices SpA ITALY.
CARDIOLINE® is a registered trademark of et medical devices SpA
CARDIOLINE® product support services
For any questions about a CARDIOLINE product:
consult the documentation and other printed material included in the package;
consult any guidelines available.
If you find no solution, you can obtain further information by contacting your
CARDIOLINE supplier.
Before calling, check you have the available documentation to hand and the product
nearby. It may also be necessary to supply the following information:
serial number and product reference number, if available;
type of hardware available, including any network hardware fitted;
operating system used, for software products;
exact contents of any error messages displayed;
description of the operation being executed when the problem occurred;
description of any action taken to solve the problem.
um_ar2100adv_cardioline_02_eng1 Rev. 02/sr/GZ 20/03/2007 Ref: 36519098
2

Contents
1 Introduction 5
1.1 How to read the manual 6
1.2 Information and recommendations relating to safe use 7
1.3 The electrocardiograph 9
Front view 9
Side view 9
Parts, symbols and controls 10
2 Installation and initial preparation 13
2.1 Selecting the installation site 13
2.2 Loading the thermal paper 13
2.3 Power supply; control and management of the rechargeable battery 14
Recharging the battery 14
2.4 How to switch on the electrocardiograph 15
2.5 How to switch off the electrocardiograph 15
Auto power off 16
3 Preparation for use: the menu 17
3.1 How to access the menu 17
3.2 Structure of the menu 17
3.3 Menu-activated operation and personalization of the electrocardiograph 20
"Personalise mode" 20
The “ECG archive” 21
“Settings” 21
“Tools” 24
4 Preparing for an ECG recording 25
4.1 Connecting the patient cable 25
4.2 Preparing the patient and applying the electrodes 25
4.3 Select recording characteristics operating mode, print format, speed,
sensitivity, filters 26
Operating mode 26
Print format 27
Speed of recording on paper 28
Sensitivity of recording on paper 28
Recording filters 28
5 Recording of a rest ECG 30
3
5.1 Patient data entry 30
5.2 Recording in manual mode 31
5.3 Recording in automatic mode 31
Automatic calculation of ECG parameters 32
Automatic ECG interpretation 32
Copy of an automatic ECG recording 33
ECG memory: saving a recording 34
ECG memory: archive management 34
Saving to Personal Computer archive 34
5.4 Recording in ECG Autotimer mode 35
5.5 Recording in "PC ECG" mode 36
5.6 “Paper Saving” mode” 36
5.7 Recording in “HRV Analysis" mode 37
5.8 Recording in “Arrhythmia mode” 37
5.9 Defibrillation! 38
6 Management and control of electrocardiograph functionalities 39
6.1 Disconnected electrodes, potential defibrillation 39
6.2 Batteries low or in need of recharging 39
6.3 Print system control. Out of paper 40
6.4 Status messages and error indication: description and related event 40
6.5 Troubleshooting 41
7 Maintenance 42
7.1 Self-test 42
7.2 Replacing the thermal paper 43
7.3 How to clean the device and the electrodes 43
7.4 Periodic checks 43
8 Technical specifications 44
Basic accessories supplied 45
4

1 Introduction
ar2100adv combines optimised performance in multichannel ECG recording
with all the features of reliability, modularity, versatility and upgradeability
that characterise the latest generation of CARDIOLINE®
electrocardiographs.
ar2100adv is an electrocardiograph with dual power supply (mains and
rechargeable internal batteries), which in the basic configuration will:
record an ECG exam in automatic, manual and timed mode;
reproduces the ECG signal on 210 mm paper in various formats thanks
to high resolution thermal printer: 3, 6x1, 6x2, “Full Page 1” (3x4+R for
1 page), “Full Page 3” (3x4+3R for 1 page) and 12 channels;
store the most recent recording in automatic mode and print additional
copies.
Thanks to the flexibility of the software used and to the infrared interface,
the ar2100adv can be adapted at any given moment to suit your individual
requirements. The range of “options” offered is particularly generous and
there are no restrictions or constraints, as the selection can be made either
at the moment of purchase or later on at your clinic or surgery without
having to interrupt day-to-day activity.
In just a few minutes, your ar2100adv can be equipped with:
- “memory option”: storage of up to 40 full ECG exams, with no need to
print out immediately on paper (“paper saving” mode);
- "ECG parameters option": automatic ECG parameter measurement
program;
- "ECG signal interpretive option": a useful and dependable diagnostic
support provided by the program;
- "arrhythmia option": a program enabling detection of arrhythmia events
during continuous recording;
- “HRV analysis option”: a program enabling detection of variations in
heart rate;
• “PC archive option": for saving the exam to archive stored in a personal
computer running CARDIOLINE software. The data upload to the PC is
made by use of the wireless “IR” interface; no direct connection to the
PC is required.
• “PC-ECG option": for real time display of the twelve leads on your
computer screen to allow management of patient medical records and
archiving of exams in digital format using CARDIOLINE software. The
software has an optional module for automatic interpretation of the ECG
signal.
For more information on available options, contact your selected dealer.
5

CONGRATULATIONS ON YOUR PURCHASE. Your new computerised
electrocardiograph CARDIOLINE® has been designed and built in
compliance with the applicable regulations in force at the time when et
medical devices SpA, Cavareno (Trento) - ITALY drew up this manual. et
medical devices operates in accordance with the requirements for quality
management systems defined by EN ISO 9001: 2000 and EN ISO 13485:
2003 standards. The system is covered by a Nemko Certification AS (Cert.
N. 800278). Your new electrocardiograph has also been built in compliance
with the Medical Device Directive 93/42/EEC and is therefore marked by the
relevant CE0470 mark.
1.1 How to read the manual
In order to ensure the CARDIOLINE® ar2100adv is operated in a safe and
correct manner, and to appreciate its ease of use and high reliability, the
user instructions must be read carefully.
This documentation describes the functions of your electrocardiograph
including those provided by all the possible "options" available. It is
therefore possible that some of the functions described may not be present
in the model you have purchased. For details of the options, consult the
"firmware configuration" chart that accompanies each individual appliance.
This symbol allows you to identify the functions not provided on all
models, which must be requested specifically at the time of purchase.
This symbol allows you to identify the functional, behavioural and
operational aspects that may be conditioned by the type of configuration
selected during the step of “Preparation for use: the menu”.
When a given key is depicted in the body of a sentence or a paragraph,
press the corresponding key on the device to perform the action.
The structure of this manual allows you to approach the use of the
electrocardiograph according to your level of knowledge. If you have already
had experience with CARDIOLINE® equipment, the initial fast-track part of
each paragraph will allow you to begin working immediately. In the
continuation of the paragraph, on the other hand, the single aspects of
operation are discussed in more depth.
The manual gives detailed information on the use of the model ar2100adv
in traditional ECG procedures, and an introduction to the use of particular
functionalities involving interaction with software and a Personal Computer.
For instructions on the use of the software applications for Personal
Computer, consult the special online guides.
The quick guide to the electrocardiograph (at power-up the display shows
1
the message “ ? Press 1 ”:
operations linked to the single commands presented in the manual.
6
Q
to obtain the printout) sums up the

7
Further information and clarifications can be requested directly from:
CARDIOLINE® - Supporto Prodotto
Strada Rivoltana Nuova, 53, I - 20060 Vignate (MI) ITALIA
e-mail: et.service@etmed.biz
tel. +39 02 95 05 181 fax: +39 02 95 66 013
1.2 Information and recommendations relating to
safe use
- The electrical system used by the device must be in accordance with the
standard in force.
- Always use the equipment according to the instructions in this manual.
- The device is equipped with a set of standard accessories. For reasons
of safety, reliability and conformity with the Medical Devices Directive
93/42/EEC, use only original accessories or accessories approved by the
manufacturer.
- The device is equipped with a special long-life thermal head writing
system, which allows maximum writing precision. To avoid frequent and
costly replacements and repairs, always use the original paper or paper
approved by the manufacturer. The manufacturer will not accept liability
for any damage to the device or any other adverse effect caused by the
use of unsuitable paper.
- Do not subject the device to impact or excessive vibrations.
- Do not allow liquids to penetrate inside the device. If this should
accidentally occur, have the device tested by an Authorised Assistance
Centre to verify its functional efficiency, before using it again.
- Make sure that the value of the supply voltage corresponds to that
indicated on the data plate of the device.
- If you are using the device in connection with others, ensure that: all
connections are made by skilled persons; all connections comply with
safety regulations; all other devices connected respond likewise to
regulations. Non-compliance with regulations can cause physical harm to
the patient connected and to the person operating the device. Should it
be difficult to obtain the necessary information for assessing the risk of
the individual connections, apply directly to the manufacturers
concerned or avoid making the connections.
- In the event of other equipment being connected directly or indirectly to
the patient, check for the possible risks caused by the sum of the
leakage currents on the body of the patient.
- The device is protected against defibrillation discharges in accordance
with IEC standard 601-1-25; to ensure that the signal is restored, use
only original electrodes or electrodes responding to IEC and AAMI
standards.
- If an electrosurgical scalpel is in use, the patient cable should be
disconnected from the device.
- At all events, when defibrillators or high-frequency surgical devices are
being used at the same time, it is essential to take the greatest care. If

there is any doubt when such devices are in use, disconnect the patient
from the electrocardiograph temporarily.
- The device recognises the impulses generated by a pacemaker and does
not interfere with its operation, as prescribed by standards in use at the
time of drafting this manual.
- Avoid exposing the equipment to extreme temperatures, excessive dust
or dirt, and very salty or damp environments; observe the ambient
conditions described in detail under the "Technical specifications”
heading.
- Periodically check the efficiency of all accessories and of the device
itself. Contact the Authorised Assistance Centre whenever the device
seems to be operating irregularly. To prolong the life of your device,
have it checked periodically by an Authorised Assistance Centre.
- Warning: The electrocardiograph can be used for intracardial
applications.
- Warning: It is therefore necessary before activating the equipment, to
make sure of the connection to ground (normally secured by the power
supply cable). If grounding of the main electrical service is not certain,
do not connect the device and use it powered only by the rechargeable
internal battery.
- Warning: do not use the device in the presence of anaesthetics or
volatile gases!
- Warning: devices for medical applications must be used only by
persons who by virtue of training or practical experience are able to
ensure maximum safety and effectiveness in operation. Operators must
in any event read this manual carefully and familiarise themselves with
the instrument before using it on a patient.
- Warning: the indications obtained using automatic interpreting
programs or other diagnostic aids must be reviewed and countersigned
by a qualified medical person!
- Warning: the device is provided with an IR interface for the transfer of
data to other devices. The IR interface must not be masked, even
accidentally, as this will adversely affect its capability and its operation,
interrupting and preventing the correct flow of data.
- The manufacturer will acknowledge liability for the safety, reliability and
functional efficiency of the device only if:
o modifications and repairs are performed by the
manufacturer or by an Authorised Assistance Centre;
o the a.c. mains power supply of the building responds to
current regulations;
o the device is operated according to user instructions;
o any accessories in use are those approved by the
manufacturer.
8
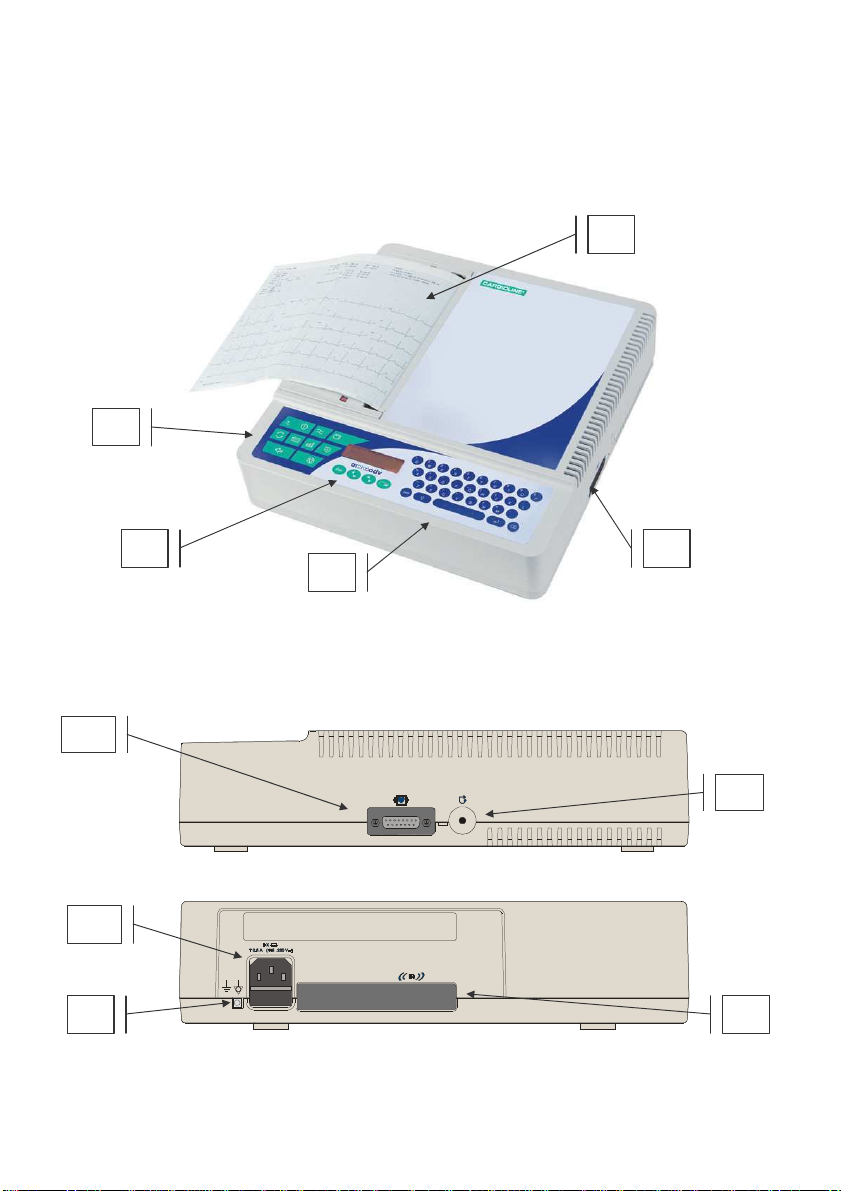
1.3 The electrocardiograph
4
6
9 8
7
1
3
4 2
5
In order to simplify the installation and the use of your electrocardiograph,
it is recommended that you become familiar with the component parts and
with the logic of its operation.
Front view
Side view
9
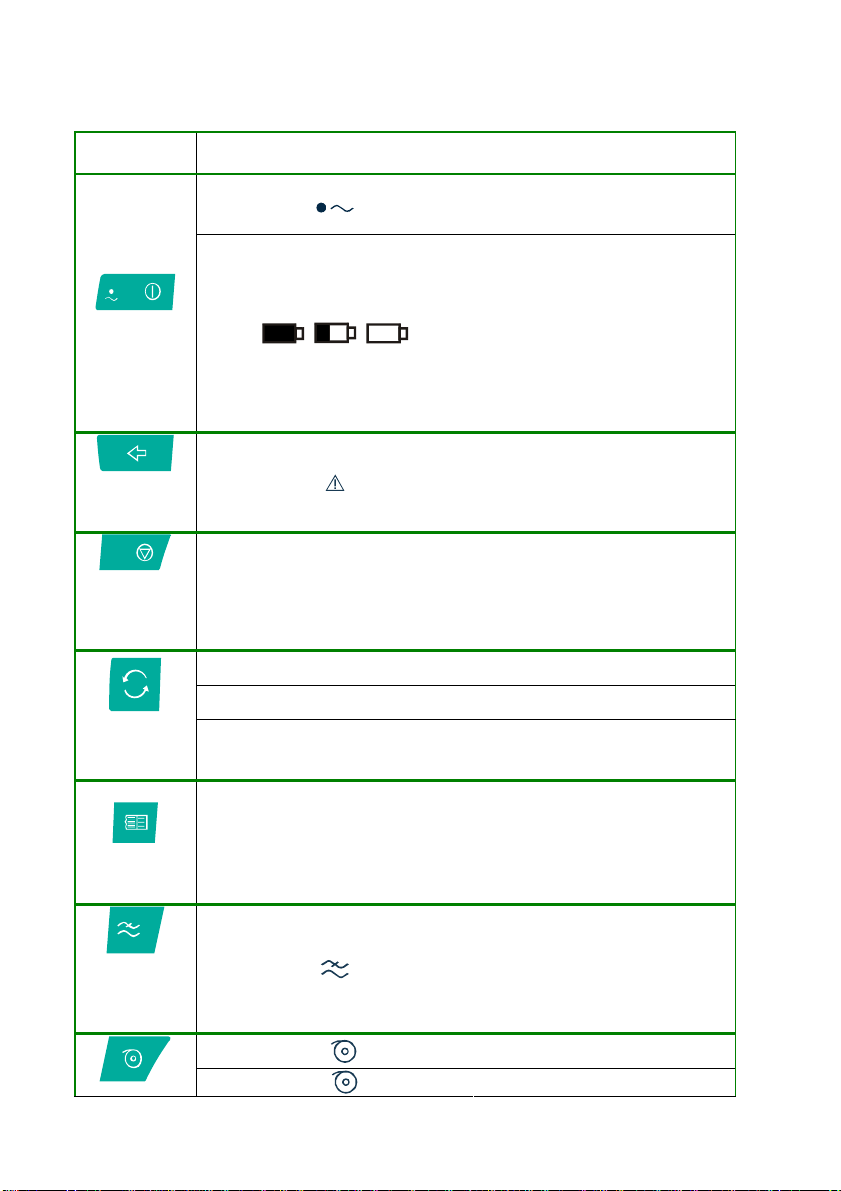
Parts, symbols and controls
25
1. Keyboard:
Function
key
on/ off
Messages & Symbols displayed /
Associated LED
- LED on: device connected
to mains power; internal
battery charging
-
“full” symbol: battery
charged
-
“part empty” symbol
battery power less than
30%
- “empty” symbol: internal
battery flat; the device
must be connected to the
mains power for
recharging
select start
operating
mode
interrupt
current
operation;
stop
select
operating
mode
select print
format :
a.c. mains
and muscle
interference
filter
-
Automatic mode:
3x1, 3x2, 6x1, 6x2, 12, Full
Page 1, Full Page 3.
- Manual mode:
3, 6, 12.
Auto
Man
Personalised
50
- indicated electrodes not
connected or insufficient
contact; saturation
- Automatic recording
- Manual recording
- Recording mode selected
in configuration phase
(“Personalised mode”)
- filter on
- paper speed 25 mm/s
- paper speed 50 mm/s
10
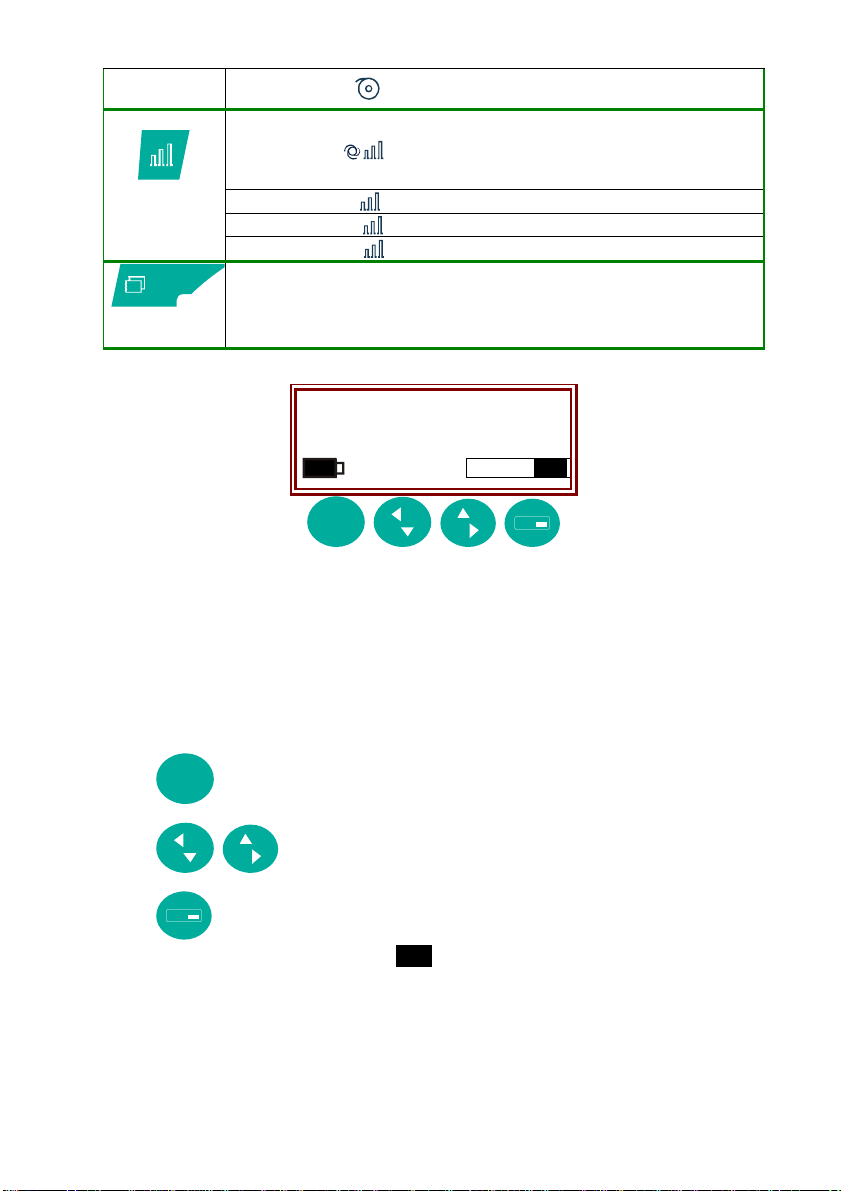
select paper
5
speed
select
recording
sensitivity
copy last
recording
5
10
20
- paper speed 5 mm/s
- automatic sensitivity: the
device optimises the ratio
between n° channels and
available space
- sensitivity 5 mm/mV
- sensitivity 10 mm/mV
- sensitivity 20 mm/mV
2. Display: to manage operating functions and patient data
Auto FP1 ►
Recording …
♥ 68
Menu
Esc
In normal operating mode:
line 1: information on recording parameters selected;
line 2: operating information and error messages;
line 3: battery charge status, heart rate, menu;
▲▼◄► indicate the presence of additional menu options and
information.
Esc
cancel operation, delete text, return to previous menu.
scroll menus and information.
Menu access and selection. Execute action highlighted on
lower right of display (e.g.
Select
).
3. QWERTY alphanumeric keyboard for patient data management.
11

SMB
displays symbol map and special characters. to
select. to copy symbol into text.
delete text.
confirm.
4. CF type patient cable connector protected against defibrillation as
indicated by the symbol.
5. Paper compartment door.
6. Reset button : used to re-establish normal operating conditions in
the event of an error that cannot be managed using the keyboard.
7. “Mains line” connector.
8. Equipotential earth connection / functional.
9. IR infrared interface.
12

2 Installation and initial preparation
This section describes the operations to be performed before using your new
CARDIOLINE® ar2100adv electrocardiograph. Suggestions are given for
"selecting the installation site" and "recommendations for safe use in
conformity with current statutory regulations" are indicated. Also introduced
are the operations involved in preparing the electrocardiograph for use,
such as "loading the thermal paper", "power supply”; “control and
management of the rechargeable internal battery", "switching on and off",
"the menu", "set-up".
2.1 Selecting the installation site
Your electrocardiograph complies with European directives on
electromagnetic compatibility. The absence of emissions damaging to radio
and telecommunications transmissions is therefore assured, as also is
protection from interference emitted by other systems and equipment
Nevertheless, in order to protect your device from other equipments not in
conformity with the aforementioned directives:
- avoid the use of mobile phones near the electrocardiograph;
- place the electrocardiograph as far as possible from electrical power
lines and sources of static electricity. The ECG signal can be disturbed if
the electrocardiograph is placed near sources of high voltage or
electricity lines;
- avoid placing the electrocardiograph close to other diagnostic or
therapeutic equipment (e.g. X-ray machines, ultrasound machines,
electrically operated beds, etc.) that could be a source of excessive
interference and ECG signal distortion;
- if it is impossible to position the electrocardiograph at a distance from
other electrical equipment, switch the other equipment off while
recording an ECG.
Also, to avoid the effect of ambient conditions when recording ECG:
- record in a room where the temperature is between 20 and 25 degrees
Centigrade. This precaution prevents the patient from feeling cold, which
could increase shivering and contribute to muscle tremor;
- record using the battery, disconnecting the device from the mains power
supply. This avoids presence of mains power disturbance of the recorded
ECG signal.
2.2 Loading the thermal paper
CARDIOLINE® ar2100adv is able to reproduce the ECG signal both on
thermal paper in Z-fold packs. Thermal paper in rolls with can also be used.
It’s necessary to insert the page format “Letter”, refers to the following § for
the configuration menu. To correctly load the different types of paper:
13

If using paper in packs:
a. Open the paper compartment.
b. Prepare a new pack and position it in the compartment. Check that the
red mark on the paper is on the upper left of the pack.
c. Position the paper, centring it between the two paper guides. Close the
cover, positioning the paper between the rubber roller and the device
case.
If using rolls of paper:
d. Open the paper compartment and remove the “roll guide”. To avoid
losing the “guide”, place it in a safe place. If replacing an empty roll,
retrieve the core before throwing away the empty roll.
e. Insert the core in a new roll of paper and place in the paper
compartment, fitting the pins into the guides provided. Check that the
black mark on the paper is on the upper part of the paper holder.
f. Position the paper, centring it between the two paper guides. Close the
cover, positioning the paper between the rubber roller and the device
case.
Caution: use only original thermal paper or paper approved by the manufacturer.
The use of paper that does not respond to the manufacturer's specifications could
jeopardise the correct operation of the device.
2.3 Power supply; control and management of the
rechargeable battery
Your electrocardiograph uses a dual power supply system: a.c. mains and a
rechargeable lead battery.
The rechargeable battery is housed inside the device, and is protected
against short circuits.
Caution: before using the device, it is necessary to go through a complete cycle of
recharging of the battery!
Before connecting the electrocardiograph to the a.c. supply with the cable
supplied, check that the mains voltage is the right voltage for the device.
Caution: when the device is connected to the mains, the batteries are recharged
automatically, even during use.
To gain maximum benefit from the characteristics of the dual power supply
system, follow the indications given below.
Recharging the battery
The battery must be recharged when the power indicator symbol is part
14
empty : the reserve charge is lower than 30%.
Connect the electrocardiograph to the mains: Led
lit. Complete
recharging of the battery requires at least 24 hours.
For longer life, the battery should be allowed to run down and recharged
completely at least every two months.
A complete recharge allows the recording of up to 220 complete ECGs
(automatic recording mode, 6 channel print format, speed 25 mm/s,
complete with analysis, two pages).
If the battery should be completely discharged (symbol ), it is still
possible to make an ECG recording by connecting the device to the a.c.
mains supply.
The average life of the battery is more than 300 complete
discharge/recharge cycles.
Warning: do not dispose of a spent battery as ordinary refuse or litter. If the battery
appears to need replacing, consult an Authorised Assistance Centre.
Warning: the device must be connected only to mains with earth connection,
realised following the current regulations.
2.4 How to switch on the electrocardiograph
for at least two seconds.
The display lights up. for at least two seconds. The display lights up.
Warning: if the symbols and are displayed, internal power is insufficient
and the battery must therefore be recharged by connecting the device to the mains
(see heading “Power supply; ...”). The battery will recharge even if the device is in
use.
2.5 How to switch off the electrocardiograph
.
The display turns off. The settings for the last recording remain stored
in the memory. To see the effect of switching off on the last automatic
recording see "Copy an automatic ECG recording".
Warning: switching off is not enabled 1. during the transmission of an ECG to a PC;
2. during the self-test routine; 3. if "set-up" mode is active. In these cases, first stop
the device and then switch off.
15
 Loading...
Loading...