Cardinal Health MedSystem III Service bulletin

Cardinal Health
San Diego, CA
Authorized EU Representative
Cardinal Health
Basingstoke, UK
Service Bulletin 404D P/N 10999653
Service Bulletins are supplements to Cardinal Health Technical Service/Maintenance Manuals.
A list of Service Bulletins can be found at cardinalhealth.com/alaris.
Model Affected: MedSystem III® Infusion Pump Multi-Channel, Model 286X
Date: June 2008
Subject: Level of Testing Guidelines/Functional Test Updates
This supersedes Service Bulletin 404C, to provide information related to the use of Model 1561C
and
1565C AC Adapters.
PURPOSE
The purpose of this bulletin is to provide Biomedical Technicians the following:
• A Level of Testing Guidelines table, to replace all existing references to testing required after repair
(reference “Repair” chapter of Service Manual).
• A new Audio Test, and updated procedures for Electrical Safety and Run-In tests (reference “System
Functional Tests” chapter, and “Procedures Following Repair” section in “Repair” chapter of Service
Manual).
• Procedures to help troubleshoot:
NiCad battery problems (reference “NiCad Battery Problems Table” in “Troubleshooting” chapter
of Service Manual).
Instrument memory loss problems.
• Information related to release of Model 1561C and 1565C AC Adapters.
This is reference information only
Technical Inquiries: United States: 888.876.4287
Canada: 800.387.8309
United Kingdom: 0800.389.6972
International: cardinalhealth.com/alaris (for contact information)
and is not intended to suggest a need for component changes.
ALARIS® and MedSystem III® are registered trademarks of Cardinal Health, Inc. or one of its subsidiaries.
All other trademarks belong to their respective owners.
©1996, 20004, 2008 Cardinal Health, Inc. or one of its subsidiaries. All rights reserved.
Page 1 of 12
Service Bulletin 404D P/N 10999653
EXPLANATION
• The tests currently specified in the service manual as being required after repair/reassembly might
not reflect current testing requirements or recommendations. To help provide the most current
minimum test requirements and recommended tests, a Level of Testing Guidelines table has been
provided.
• The Electrical Safety and Run-In tests currently specified in the service manual have been replaced
to provide the current test procedures (Electrical Safety Test, Run-In On AC Power). An Audio Test
procedure has been provided to check the audio volume.
• Procedures have been provided to verify:
NiCad battery is being charged within specification (NiCad Battery Charge Current Test).
Current drawn from NiCad battery, when instrument is operating on battery power, is within
specification (Standby Current Test).
NiCad battery can provide sufficient instrument operating time after being discharged and
recharged (Battery Functional Test).
Current drawn from backup battery, when instrument is off, is within specification (RAM Standby
Current Test).
Models 1561/1565 (see illustrations in “Electrical Safety Test” section)
power, is double insulated as a medical grade power adapter and does not require a connection to earth
ground. Models 1561/1565 are compatible with all previously released MedSystem III
has only two connections to AC
®
Infusion Pumps.
Certification to the applicable standards for ElectroMagnetic Compatibility (EMC) has been validated by
TÜV America.
NOTE: Model 1561 is ordered as 143032 and or Model 1565 is ordered as 2861089
REFERENCES
Model 2860/2863 Technical Service Manual (identified as P/N 139981; ordered as P/N 2863012)
Applicable Field Maintenance Software Operating Instructions (FMS DFU)
Applicable Instrument's Directions For Use (DFU).
PARTS AND TOOLS REQUIRED*
• Equipment referenced, as necessary, under each test procedure in this Service Bulletin.
• Appropriate field Maintenance Software (FMS) Kit.
* These are in addition to parts, tools, and equipment referenced in the service manual.
Page 2 of 12
Service Bulletin 404D P/N 10999653
RECOMMENDED ACTION
CAUTION
Turn the instrument off and disconnect it from AC power before disassembly. Static
charges will damage instrument circuitry. Observe proper grounding techniques (use
grounding strap) to prevent possible harm to static-sensitive components.
Add this Service Bulletin to the Technical Service Manual, as a supplement to the “System Functional
Tests”, “Troubleshooting”, and “Repair” Chapters.
NOTE: Restoration of Configured Parameters, Battery History Log Memory contents,
serial number, and special message can be corrupted during repair. Field Maintenance
Software (FMS) may have been used to customize configuration settings or program drug
lists for Dose Rate Calculator. All settings should always be verified for accuracy after
repair. For reprogramming instructions, refer to the appropriate FMS DFU.
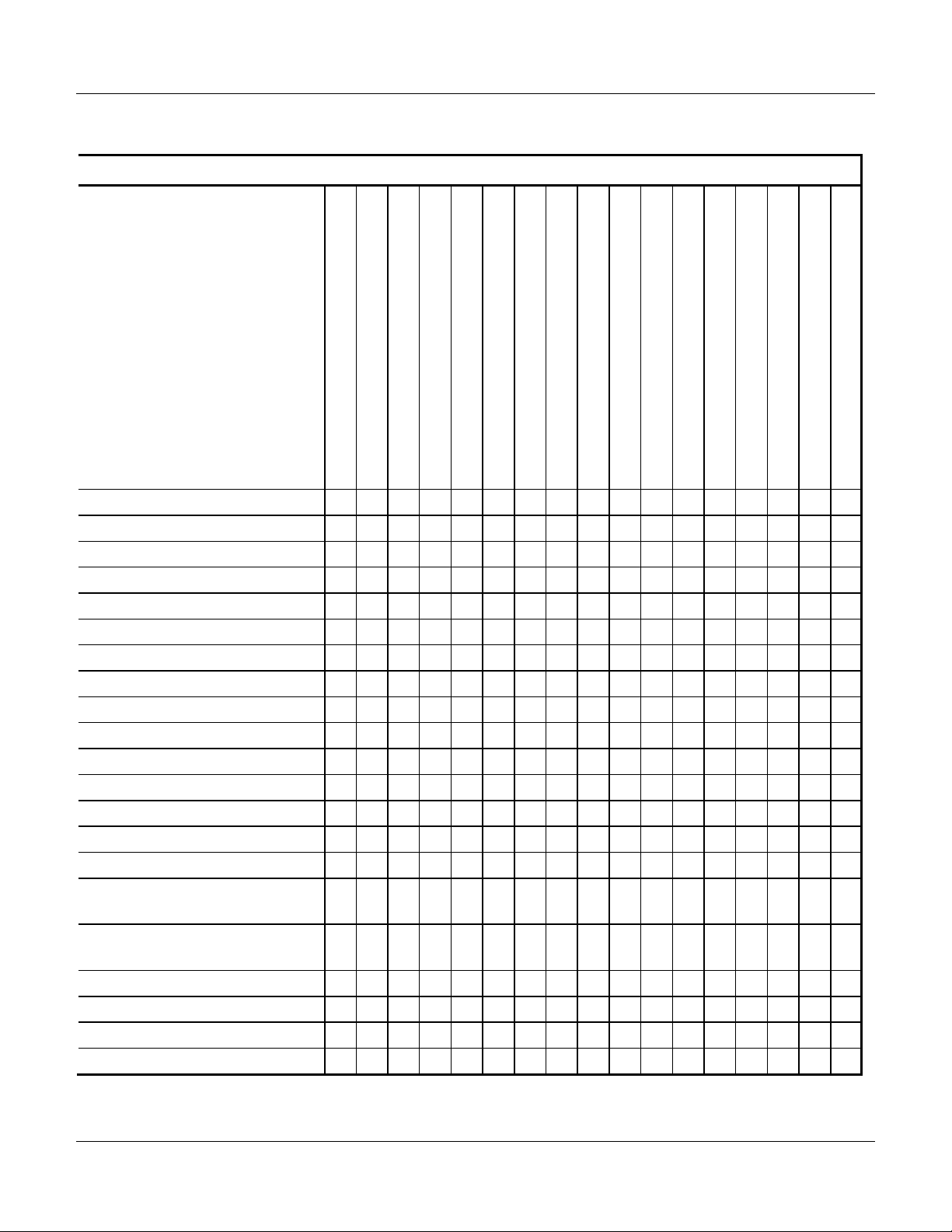
1. Level of Testing Guidelines
Any instrument repair or disassembly requires testing, determined by the level of the repair/
disassembly. Use the Level of Testing Guidelines, Table 1, in place of the test requirements
specified in the “Repair” chapter of the Service Manual.
Repair or replacement of the following parts does not require testing unless part of a higher level
repair, as listed in Table 1.
• Chassis Mounts
• Dust Cap
• Lower Housing Assembly
• Universal Pole Clamp
Page 3 of 12
Service Bulletin 404D P/N 10999653
RECOMMENDED ACTION (Continued)
Table 1 Level of Testing Guidelines
Tests to Perform ·
O = Required
= Optional
Repair/Replacement of Ð
No Fault Found
Adapter
Air-in-Line Sensor
Audio PCBA (Side Bd. Assy.)
Backup Battery
Battery Pack
Display Module
Drive Module Assembly
EPROM Module
Main Housing Assembly
MEA (Logic Board Assy.)
Optomodule
Power Supply Board Assembly
Power Supply Fuse
Pressure Transducer
Pump Latch Assembly and Shaft
Seal (case was removed)
Battery Charge Current Test
Standby Current Test
RAM Standby Current Test
Pump Latch Height Adjustment (7.3.2)
Valve Actuator Height Verification (7.3.6)
Full Calibration using FMS (5.0)
O O
O O
O
O O O O O O O O
O O
O
O O O O
O O O
O O O O O O O O O O
O O O O
O O O
O O O O O
O O O O O O
Electrical Safety Test (3.1)
Power Tests (3.2)
Cassette and Sensor Test (3.3)
O O
O O
O O
O O
O O
O O
O O
O O
PSOD Test (3.4)
FSOD Test (3.5)
AIL Test (3.6)
Volume Accuracy Test (3.7)
Watchdog Audio Test (3.9)
Audio Test
Battery Functional Test
Run-In AC Power
O
O
O
O
Pump Latch Assy. and Shaft Seal
(case was not removed)
Pump Latch Movable Jaw
Pump Shaft
Slide Link
Valve Actuator Assembly
O
O O O O O O O O
O
O O O O O O O O
Page 4 of 12
O O
O O
O O
O
O
O
O
 Loading...
Loading...