Braemar FUSION User Manual

Braemar, Inc.
Fusion
Wireless
Recorder

Braemar Limited Warranty
Braemar products are warranted to be free from manufacturing and
material defects for a period of one (1) year from the date of shipment
from Braemar to the original purchaser.
Excluded from this warranty are expendable supply items including, but
not limited to, electrodes, lead wires, patient cables and batteries. This
warranty does not apply to any product which Braemar determines has
been modified or damaged by the customer.
Except for the express warranties stated above, Braemar disclaims
all warranties including implied warranties of merchantability and
fitness. The stated express warranties are in lieu of all obligations
of liabilities on the part of Braemar for damages, including but not
limited to, special indirect or consequential, arising out of or in
connection with the use or performance of Braemar products.
Any action for breach of warranty shall be commenced within one (1)
year of said breach or be forever barred. Any repairs made to the
product which are not covered by the warranty shall be billed to the
customer.
Document Number: 600-0645-00
Revision: 05
Date: February 2009

Fusion Wireless Recorder
1
2
Fusion Wireless Recorder
Table of Contents
Overview...................................................................................................2
Precautions...............................................................................................2
Disclaimer.................................................................................................3
Recorder Components..............................................................................4
Setup Steps ..............................................................................................5
Electrode Application and Placement .......................................................5
1/2/3 Channel Electrode Placement .........................................................6
3 Channel (5 lead) Electrode Placement (1st option)………………7
3 Channel (5 lead) Electrode Placement (2nd option)………………8
Recorder Preparation ...............................................................................9
Establishing the Home Link ....................................................................13
Troubleshooting (page 1 of 2).................................................................16
Service and Maintenance .......................................................................18
Service Items and Accessories...............................................................18
Equipment Symbols................................................................................19
Specifications .........................................................................................20
Overview
The Fusion Wireless Recorder is a battery operated, solid state recorder
designed to record symptomatic heart arrhythmias.
The Fusion Recorder provides up to 20 days of total recording time for 3
channels, 30 days of total recording time for 1 or 2 channels with the AA
Lithium battery pack.
The Fusion Recorder is enhanced with Arrhythmia Detection firmware
which will capture and automatically record asymptomatic, infrequent, or
elusive heart arrhythmia events such as Bradycardia, Tachycardia, Pause,
and Atrial Fibrillation.
Once an event is recorded, the event ECG is automatically transferred via
a digital cellular link. If a digital cellular link is not available, the event
ECG can be transferred by Bluetooth to a phone line via a Home Link
Bluetooth modem.
Precautions
A. Patient leads must be removed from electrodes before defibrillation.
B. Observe local laws for disposal of batteries.
C. Do not leave the batteries in the Recorder when it is not in use.
Damage from corrosion could result.
D. Patient should be instructed to avoid close proximity to heavy
electrical equipment or other sources of electromagnetic
interference.
E. Use only the provided battery pack. Observe polarity when inserting
F. Recorder is not for infant use.
G. No automatic analysis algorithm can replace data review by a
qualified physician. Review and confirmation of analysis results is
required.
H. Patients should seek immediate medical attention if they experience
symptoms that concern them.
Electromagnetic Emissions.....................................................................21
Electromagnetic Immunity ......................................................................21
Recommended Separation Distances ....................................................24
Fusion Wireless Recorder
3
4
Fusion Wireless Recorder
Disclaimer
Operation of the Fusion Recorder may be subject to governmental and
business restrictions, including but not limited to air travel and hospital
visitations.
Additional equipment classification information as required in EN 60601-1
A. EQUIPMENT not suitable for use in the presence of a
FLAMMABLE ANAESTHETIC MIXTURE WITH AIR OR WITH
OXYGEN OR NITROUS OXIDE
B. IPX0 Ordinary Equipment (enclosed equipment without protection
against ingress of water)
C. Internally Powered Equipment
D. Mode of Operation - Continuous Operation
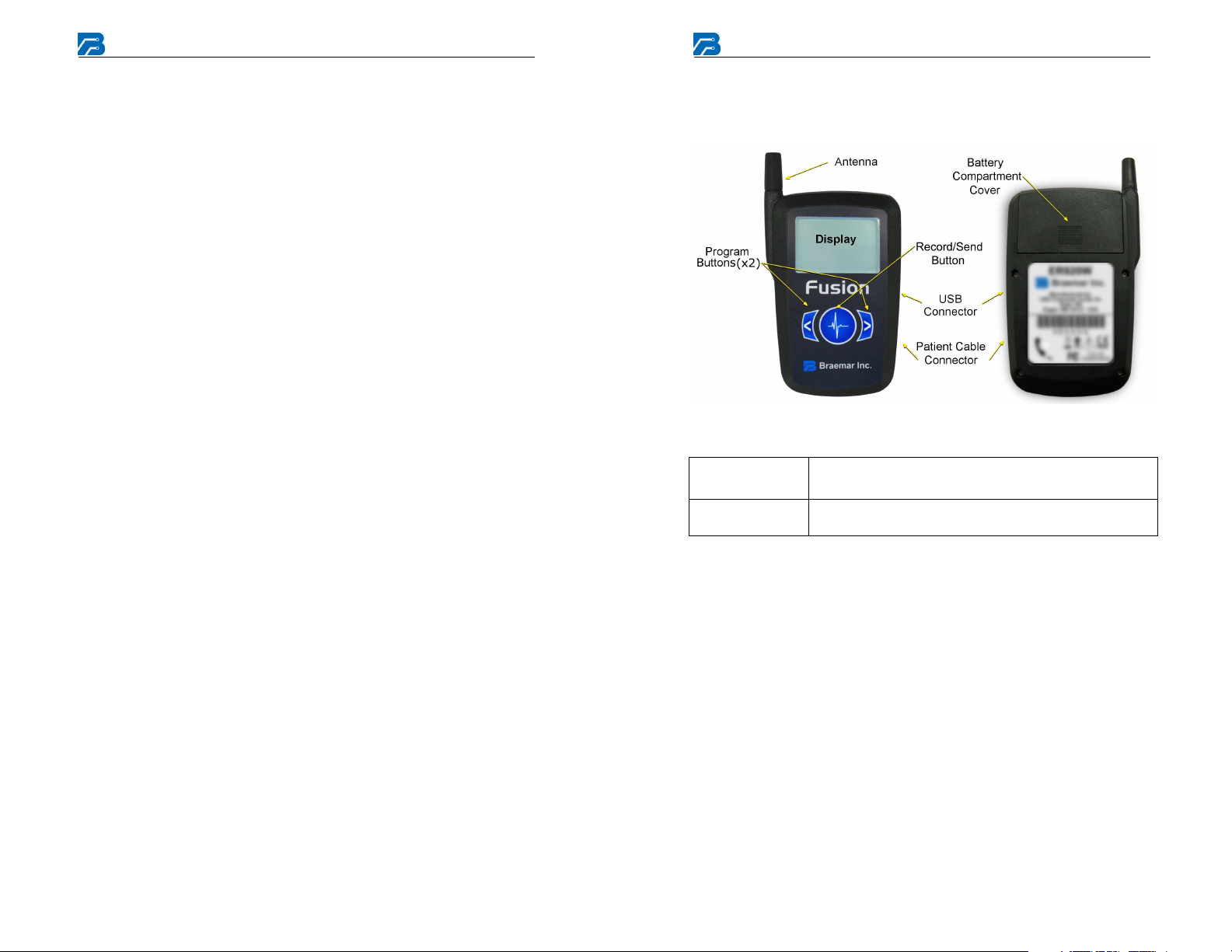
Recorder Components
Batteries
Patient Cable
Caution: U.S. Federal law restricts this device to sale by or on the
order of a physician.
3.6V AA Lithium battery pack. Insert into battery
compartment observing polarity symbols.
To adjust, move plastic slip rings up or down to keep
leads together. To lengthen, pull leads apart.
Fusion Wireless Recorder
5
6
Fusion Wireless Recorder
Setup Steps
This manual is designed to allow a technician to follow the instructions
page by page to setup the Fusion Recorder. Here is the general layout:
1. Connect leads and electrodes to patient.
2. Prepare Recorder for recording.
A. Choose/Setup program options you want to use.
B. Erase all previous data.
3. Connect Patient Cable to Recorder.
Electrode Application and Placement
For each electrode lead wire:
1. Snap the electrode onto the lead wire.
2. Remove the protective backing from the adhesive side of the
electrode.
3. Apply the electrode to the patient’s skin per Electrode Placement
diagram in this manual or as instructed by the physician.
Notes:
A. It is recommended that trained medical personnel instruct the
patient in the proper application of electrodes.
B. Use good quality long term electrodes. Braemar recommends
the use of low impedance Holter electrodes. Instruct patient to
apply fresh electrodes regularly. (Usually on a daily basis.)
C. Proper preparation of the patient's skin is absolutely essential
for obtaining a quality ECG recording. The skin surface where
the electrodes will be placed should be cleaned with alcohol,
allowed to dry, and abraeded.
D. Any loose electrode needs to be replaced.
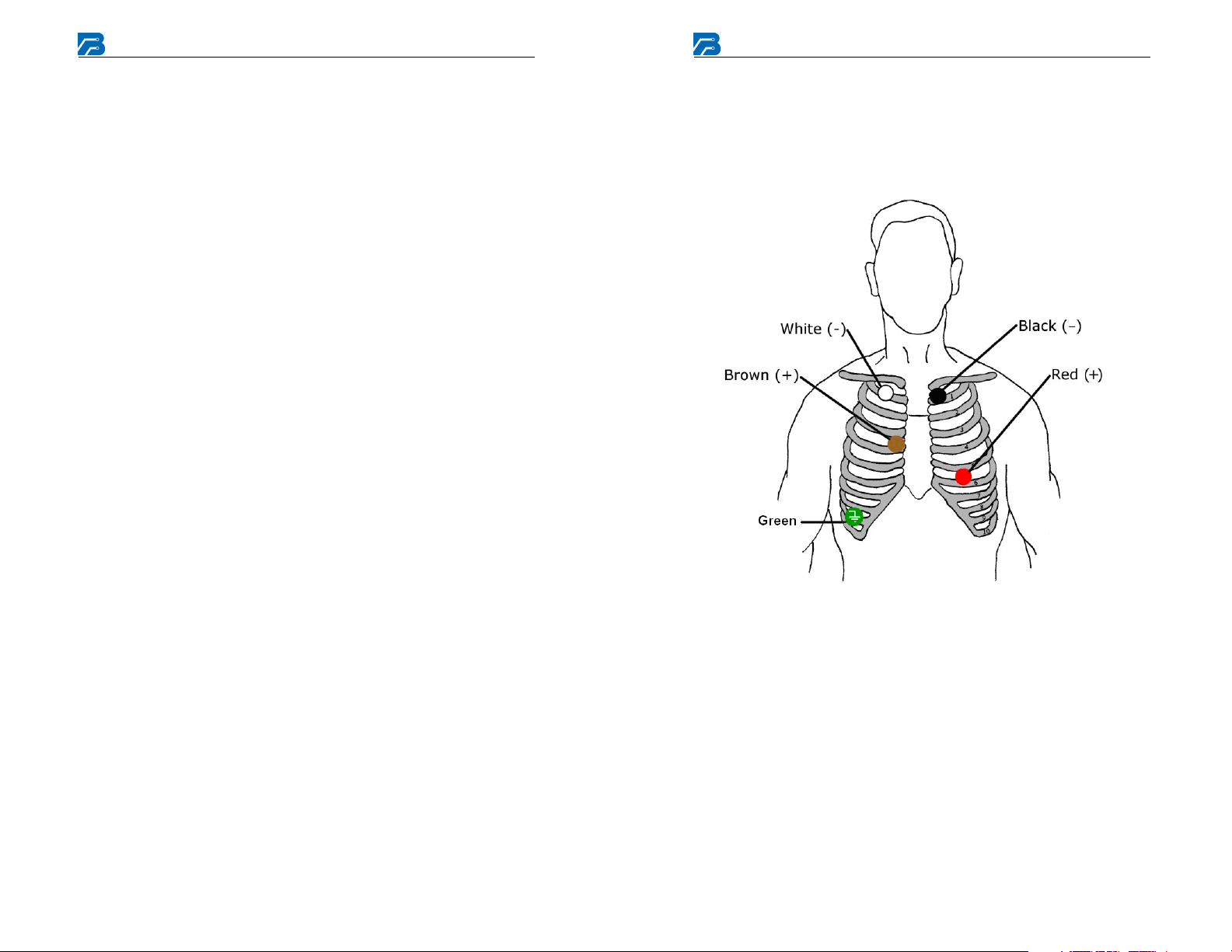
1/2/3 Channel Electrode Placement
This is a typical electrode placement. Refer to Analysis System software
and the physician for recommended positioning.
1, 2, and 3 Channel Electrode Placement
Channel 1 = Red (1+), White (1-),
Green (ground (RL))
Channel 2 = Red (1+), White (1-/2-), Black (2+),
Green (ground (RL))
Channel 3 = Brown (1+), Red (1-/2-), Black (2+,3+),
White (3-), Green (ground (RL))
 Loading...
Loading...