BLADDERSCAN BVI 3000 OPERATIONS Manual

BLADDERSCAN
BVI 3000
OPERATIONS & MAINTENANCE MANUAL

0900‑0383‑14‑60

BLADDERSCAN
BVI 3000
OPERATIONS & MAINTENANCE MANUAL
Effective: March 6, 2015
Caution: Federal (United States) law restricts this
device to sale by or on the order of a physician.
CONTACT INFORMATION
To obtain additional information regarding your BladderScan system,
please contact Verathon® Customer Care or visit verathon.com/contact‑us.
Corporate Headquarters:
20001 North Creek Parkway
Bothell, WA 98011 U.S.A.
800.331.2313 (US and Canada only)
425.867.1348
Fax: 425.883.2896
verathon.com
Verathon® Medical (Europe) B.V.
Linnaeusweg 11
3401 MS IJsselstein
The Netherlands
+31.30.68.70.570
Fax: +31.30.68.70.512
verathon.eu
0123
Copyright© 2014, 2015 by Verathon Inc. All rights reserved. No part of this manual may be copied or transmitted by any method
without the express written consent of Verathon Inc.
Verathon, the Verathon torch symbol, BladderScan, and the BladderScan symbol are either trademarks or registered trademarks of
Verathon Inc. All other brand and product names are trademarks or registered trademarks of their respective owners.
Information in this manual may change at any time without notice. For the most up‑to‑date information, contact Verathon Customer
Care or your local representative.

TABLE OF CONTENTS
IMPORTANT INFORMATION ..............................................................................................................................................1
OVERVIEW ...................................................................................................................................................................... 1
Product Description .......................................................................................................................................................1
Statement of Intended Use ........................................................................................................................................... 1
Essential Performance ..................................................................................................................................................1
Statement of Prescription .............................................................................................................................................1
Notice to All Users ......................................................................................................................................................... 1
SAFETY INFORMATION ................................................................................................................................................. 2
Biological Safety ............................................................................................................................................................2
Contraindications ..........................................................................................................................................................2
Precautions & Warnings ...............................................................................................................................................2
INTRODUCTION .................................................................................................................................................................. 6
COMPONENTS & FEATURES ....................................................................................................................................... 6
Control Unit ...................................................................................................................................................................7
Scanhead ...................................................................................................................................................................... 8
Batteries & Battery Charger .......................................................................................................................................... 8
SYSTEM COMPONENTS & ACCESSORIES ................................................................................................................ 9
BUTTONS, ICONS, & CONNECTIONS ..........................................................................................................................9
Battery Icon ................................................................................................................................................................... 9
Buttons ..........................................................................................................................................................................9
SETTING UP ....................................................................................................................................................................... 10
ASSEMBLING THE INSTRUMENT .............................................................................................................................. 10
Procedure 1. Charge the Batteries .......................................................................................................................10
Procedure 2. Insert or Remove a Battery .............................................................................................................12
Procedure 3. Connect the Scanhead to the Control Unit ..................................................................................... 13
Procedure 4. Load The Thermal Paper Roll ......................................................................................................... 14
Operations & Maintenance Manual: Table of Contents
i

CONFIGURING USER SETTINGS ...............................................................................................................................15
Procedure 1. Program the Name .......................................................................................................................... 15
Procedure 2. Set the Date ..................................................................................................................................... 17
Procedure 3. Set the Time ....................................................................................................................................19
Procedure 4. Adjust User Preferences .................................................................................................................21
USING THE INSTRUMENT ...............................................................................................................................................24
SCANNING A PATIENT .................................................................................................................................................25
Procedure 1. Measure Bladder Volume ................................................................................................................ 25
Procedure 2. Verify Aim & Accuracy .....................................................................................................................27
Procedure 3. Conrm the Scan Results ............................................................................................................... 29
MANAGING SCAN RESULTS ....................................................................................................................................... 31
Procedure 1. Add a Patient ID Number (Optional) ............................................................................................... 31
Procedure 2. Add Notes (Optional) ....................................................................................................................... 32
Procedure 3. Print the Scan Result (Optional) ......................................................................................................34
Procedure 4. Print a Histogram of Cost Savings (Optional) ................................................................................. 36
CLEANING & DISINFECTING ...........................................................................................................................................38
Procedure 1. Clean the Instrument ....................................................................................................................... 38
Procedure 2. Disinfect the Instrument ...................................................................................................................39
MAINTENANCE & TROUBLESHOOTING ....................................................................................................................... 40
ANNUAL CERTIFICATION OF CALIBRATION ............................................................................................................40
PERIODIC INSPECTIONS ............................................................................................................................................ 40
Weekly Inspections ..................................................................................................................................................... 40
Monthly Accuracy Check ............................................................................................................................................ 40
DEVICE REPAIR ............................................................................................................................................................ 40
ii

TROUBLESHOOTING ...................................................................................................................................................41
Procedure 1. Run a Self Test ................................................................................................................................ 41
Procedure 2. Troubleshoot Power Issues ............................................................................................................. 42
Procedure 3. Troubleshoot an Error Message ...................................................................................................... 42
Procedure 4. Clear a Paper Jam ..........................................................................................................................44
Procedure 5. Test the Printer ................................................................................................................................ 45
Procedure 6. Verify Instrument Accuracy.............................................................................................................. 47
DEVICE DISPOSAL ....................................................................................................................................................... 47
WARRANTY ....................................................................................................................................................................... 48
Disclaimer of Additional Warranties ............................................................................................................................ 48
PRODUCT SPECIFICATIONS ........................................................................................................................................... 49
SAFETY & PERFORMANCE SUMMARY ....................................................................................................................49
ACCURACY RANGE ..................................................................................................................................................... 49
COMPONENT SPECIFICATIONS ................................................................................................................................ 50
Control Unit & Scanhead Specications ....................................................................................................................50
Battery Charger Specications ................................................................................................................................... 52
Battery Specications .................................................................................................................................................53
ELECTROMAGNETIC COMPATIBILITY ......................................................................................................................54
Electromagnetic Emissions.........................................................................................................................................54
Electromagnetic Immunity ..........................................................................................................................................55
Recommended Separation Distances ........................................................................................................................57
Accessory Conformance to Standards .......................................................................................................................57
SYMBOL DIRECTORY ...................................................................................................................................................... 58
GLOSSARY ........................................................................................................................................................................60
Operations & Maintenance Manual: Table of Contents
iii


IMPORTANT INFORMATION
OVERVIEW
PRODUCT DESCRIPTION
The BladderScan BVI 3000 is a B‑mode ultrasonic instrument, portable and battery‑operated, intended for
the noninvasive measurement of urinary bladder volume. A mechanical sector‑scanning transducer provides
cross‑sectional images of the bladder from twelve scan planes. Based on these images, the BladderScan
instrument automatically calculates the estimated bladder volume in milliliters (ml) and displays it on a screen.
STATEMENT OF INTENDED USE
The BladderScan BVI 3000 projects ultrasound energy through the lower abdomen of the nonpregnant patient
to obtain an image of the bladder, which is used to determine bladder volume noninvasively.
ESSENTIAL PERFORMANCE
Essential performance is the system performance necessary to achieve freedom from unacceptable risk. The
essential performance of the BladderScan BVI 3000 system is to produce ultrasonic output energy and display
numerical values for bladder volume. The system has a passively temperature‑controlled transducer assembly.
STATEMENT OF PRESCRIPTION
Caution: Federal (United States) law restricts this device to sale by or on the order of a physician.
NOTICE TO ALL USERS
The BladderScan BVI 3000 should be used only by individuals who have been trained and authorized by
a physician or the institution providing patient care. All operators should read this manual prior to using the
instrument. Failure to comply with these instructions may compromise the performance of the instrument.
Operations & Maintenance Manual: Important Information
1
SAFETY INFORMATION
BIOLOGICAL SAFETY
To date, exposure to pulsed diagnostic ultrasound has not been shown to produce adverse effects. However,
ultrasound should be used only by medical professionals when clinically indicated, using the lowest exposure
times possible commensurate with clinical utility.
The ultrasonic output power of the BladderScan BVI 3000 is not user‑adjustable and is limited to the minimum
level necessary for effective performance. Data on acoustic output levels can be found in the chapter Product
Specications on page 49.
CONTRAINDICATIONS
The BladderScan BVI 3000 instrument is not intended for fetal use or for use on pregnant patients.
PRECAUTIONS & WARNINGS
Warnings indicate that injury, death, or other serious adverse reactions may result from use or misuse of the
device. Cautions indicate that use or misuse of the device may cause a problem, such as a malfunction, failure,
or damage to the product. Throughout the manual, pay attention to sections labeled Important, as these contain
reminders or summaries of the following cautions as they apply to a specic component or use situation.
To ensure safe and reliable operation for the user and patient, please heed the following warnings and cautions.
PRECAUTIONS
CAUTION
The BladderScan BVI 3000 and related devices may contain mineral oils, batteries, and other
environmentally hazardous materials. When the instrument and/or accessories have reached the
end of their useful service life, see the section Device Disposal on page 47.
CAUTION
To avoid paper jams, never fold the end of the paper roll or cut it diagonally or to a point.
CAUTION
Failure to adhere to the following when cleaning the control unit or the scanhead may result in
permanent equipment damage and void the instrument warranty:
• Ensure that you do not immerse either component in water or a cleaning or disinfecting agent.
This may permanently damage the instrument.
®
• Do not use Cidex
Plus, as it is not recommended for use with Lexan polycarbonate.
• Do not subject any part of the BVI 3000 system to steam sterilization or ethylene oxide
sterilization.
2
CAUTION
Medical electrical equipment requires special precautions regarding electromagnetic compatibility
(EMC) and must be installed and operated according to the instructions in this manual. For more
information, see the Electromagnetic Compatibility section on page 54.
To maintain electromagnetic interference (EMI) within certied limits, the BladderScan
BVI 3000 system must be used with the cables, components, and accessories specied or
supplied by Verathon
®
. For additional information, see the System Components & Accessories
and Component Specications sections. The use of accessories and/or cables other than those
specied or supplied may result in increased emissions and/or decreased immunity of the system.
The BladderScan BVI 3000 system should not be used adjacent to or stacked with other
equipment. If adjacent or stacked use is necessary, the system should be observed to verify normal
operation in the conguration in which it will be used.
This device can radiate radio frequency energy and is very unlikely to cause harmful interference
with other devices in the vicinity. There is no guarantee that interference will not occur in a particular
installation. Evidence of interference may include degradation of performance in this device or other
devices when operated simultaneously. If this occurs, try to correct the interference by using the
following measures:
• Turn devices on and off in the vicinity to determine the source of interference
• Reorient or relocate this device or other devices
• Increase the separation between devices
• Connect the device to an outlet on a circuit different than the other device(s)
• Eliminate or reduce EMI with technical solutions (such as shielding)
• Purchase medical devices that comply with IEC 60601‑1‑2 EMC Standards
Be aware that portable and mobile radio frequency communications equipment (cellular phones,
etc.) may affect medical electrical equipment; take appropriate precautions during operation.
WARNINGS
WARNING
Do not use the BladderScan BVI 3000 instrument on:
• A patient who has open skin or wounds in the suprapubic area.
• A patient with ascites.
• A pregnant patient.
WARNING
Risk of explosion. If you use the BladderScan BVI 3000 instrument in the presence of ammable
anesthetics, the hazard of potential explosion exists.
Operations & Maintenance Manual: Important Information
3
WARNING
When using the instrument, be aware of the following conditions that can affect ultrasound
transmission and decrease the accuracy of exam results:
• Abdominal Surgery— Scar tissue, surgical incisions, sutures, and staples can affect ultrasound
transmission and accuracy. Use care when scanning patients who have had abdominal surgery
• Catheterization—A catheter in the patient’s bladder may affect the accuracy of the bladder
volume measurement; however, the volume measurement may still be clinically useful if it is
large (detecting a blocked catheter, for example).
• Obesity—Obesity may affect bladder volume measurements. Lift as much abdominal adipose
tissue out of the way of the instrument as possible. Apply more pressure to the scanhead to
reduce the amount of adipose tissue through which the ultrasound must pass.
Accuracy is compromised if you do not obtain an optimal, repeatable image.
WARNING
Electric shock hazard. Do not attempt to open the system components. This may cause serious
injury to the operator or damage to the instrument and will void the warranty. Contact Verathon
Customer Care for all servicing needs.
WARNING
Risk of electric shock or burns. Do not use the BladderScan instrument in conjunction with
high‑frequency surgical equipment.
WARNING
This product may only be cleaned and disinfected by using the approved processes provided in
this manual. Cleaning and disinfection methods listed are recommended by Verathon
®
based on
compatibility with component materials.
WARNING
Availability of cleaning and disinfection products varies by country, and Verathon is unable
to test products in every market. For more information, please contact Verathon Customer
Care at 1.800.331.2313 or your local representative. For additional contact information, visit
verathon.com/contact‑us.
WARNING
Ensure that you follow the manufacturer’s instructions for handling and disposing of the cleaning
and disinfection solutions provided in this manual.
4
WARNING
Cleaning is critical to ensuring the component is ready for disinfection. Failure to properly clean the
device could result in a contaminated instrument after completing the disinfection procedure.
WARNING
In order to maintain electrical safety, use only the provided medical‑approved power supply, battery
charger, and batteries.
WARNING
To reduce the risk of electrical shock, use only the accessories and peripherals recommended by
Verathon
®
.
WARNING
No modication of this equipment is allowed.
WARNING
The battery charger, power supply, and power cables are not intended for patient contact. Ensure
six feet (two meters) is maintained between the patient and these components.
WARNING
Risk of explosion, re, or serious injury. The BladderScan BVI 3000 system is battery‑powered.
Failure to note the following when handling the battery may result in serious injury or damage:
• Never short‑circuit the battery by bringing the battery terminals into contact with any other
conductive object.
• Never expose the battery to abnormal shock, vibration, or pressure.
• Do not disassemble, heat above 60°C (140°F), or incinerate the battery.
• Keep battery out of reach of children and in original package until ready to use.
• If the battery is leaking or its case is cracked, put on protective gloves to handle it, and discard
it immediately. Always dispose of used batteries in compliance with all applicable laws and
regulations.
• Put insulating tape, such as cellophane tape, on the electrodes during transportation in order to
avoid a possible short circuit, re, or electrical shock.
Operations & Maintenance Manual: Important Information
5

INTRODUCTION
COMPONENTS & FEATURES
The BladderScan BVI 3000 consists of four main components: the scanhead, the control unit, rechargeable
batteries, and the battery charger. In addition, you may purchase optional accessories and additional supplies
such as ultrasound gel, thermal paper for the printer, a mobile cart, or a carrying case. For more information
about available accessories, see System Components & Accessories on page 9 or contact Verathon®.
This section describes the main components and their parts and features.
Figure 1. BladderScan BVI 3000 Control Unit and Scanhead
6

CONTROL UNIT
The control unit provides all operating controls for the scanning procedure by means of six buttons. The
measured bladder volume and a target‑shaped aiming icon are clearly displayed on the LCD screen, helping the
operator to achieve accurate measurement results.
Figure 2. Control Unit Components
Battery‑eject button
Battery
Light sensor
Function buttons (5)
Printhead release lever and
manual paper‑feed wheel
Power button
Paper well
Scanhead cable port
Operations & Maintenance Manual: Introduction
7

SCANHEAD
The scanhead transmits and receives ultrasound, automatically moving its internal transducer 360º to scan
twelve planes and produce a three‑dimensional image of the urinary bladder. The scanhead is connected to the
control unit by means of a cable.
Figure 3. Scanhead Components
Cable connector
Cable
Scan button
Patient orientation icon
Dome
BATTERIES & BATTERY CHARGER
Two NiMH rechargeable batteries are included with the BladderScan BVI 3000. One battery can be recharged in
the battery charger while the other is being used to power the BVI 3000. This ensures that there is no instrument
downtime due to battery charge.
Figure 4. Battery Charger
Battery charger
Battery contacts
Power adapter
Figure 5. Rechargeable Batteries (2)
Battery contacts
8
SYSTEM COMPONENTS & ACCESSORIES
WARNING
To reduce the risk of electrical shock, use only the accessories and peripherals recommended by
Verathon
Table 1. Components & Accessories
BladderScan BVI 3000 control unit
BladderScan BVI 3000 scanhead
BladderScan BVI 3000 battery charger
Battery, 7.2 volt (V), 2 provided
Printer paper
BladderScan BVI 3000 In‑Service CD, containing the operations & maintenance manual and a video tutorial
Acoustic coupling gel
Carrying case (Optional)
Mobile cart (Optional)
®
.
COMPONENTS
ACCESSORIES
BUTTONS, ICONS, & CONNECTIONS
BATTERY ICON
The battery icon, located in the upper‑right corner of the LCD, indicates the power status of the battery currently
installed in your instrument.
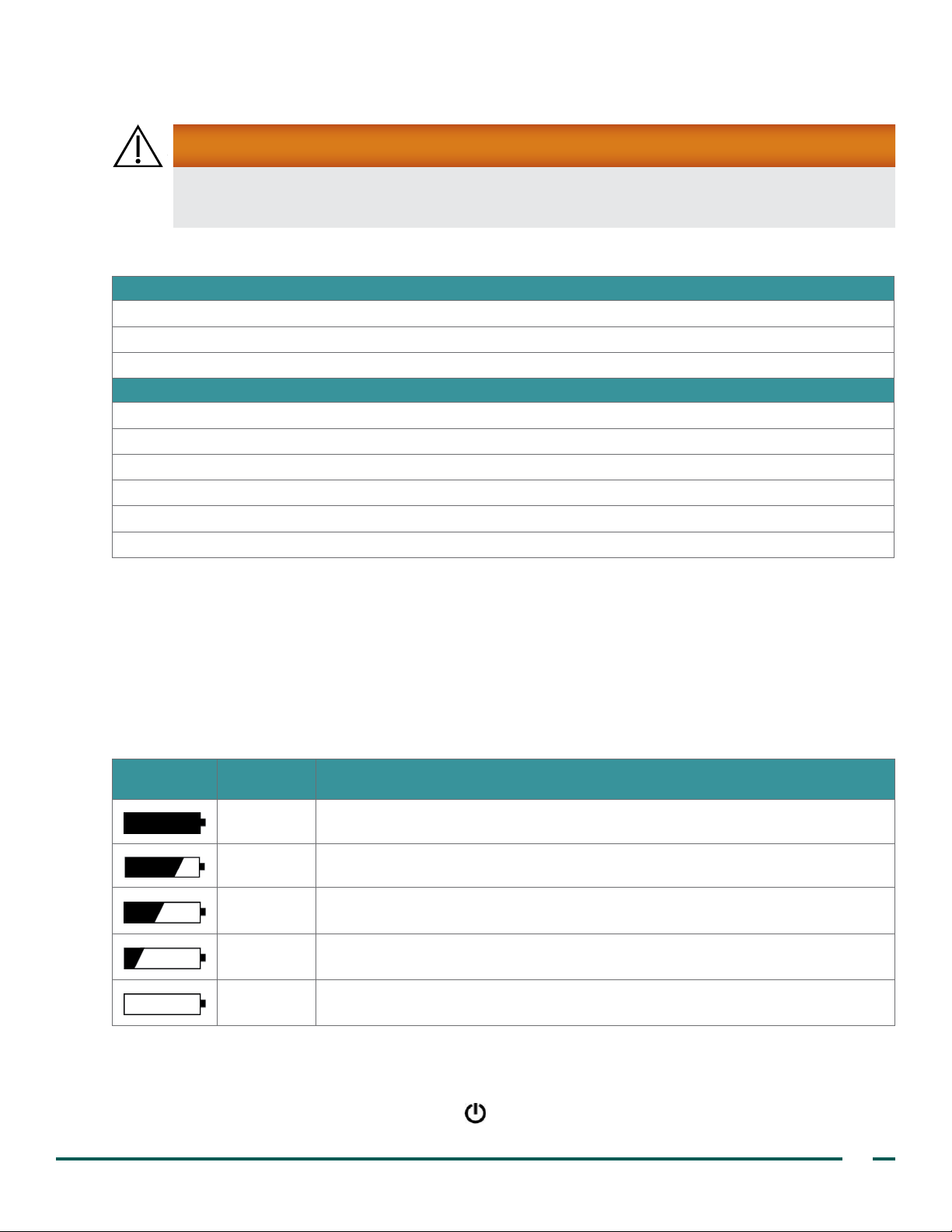
Table 2. Battery Icons
ICON STATUS
BUTTONS
The ve buttons below the LCD screen change functions based on the content currently displayed on the
screen. The button marked with the power symbol turns the BVI 3000 control unit on or off.
PERCENT
REMAINING
100% A fully darkened battery icon indicates that the battery is fully charged and ready for use.
75% A battery icon that is almost full indicates a partially discharged battery.
50%
25%
0%
When the battery icon is half‑darkened, the battery is partially discharged. This is
the most common display, shown during the majority of the battery’s charge life.
An almost empty battery icon indicates that the battery is nearly discharged. While a
few more scans can still be performed, the battery should be replaced at this point.
When the battery is fully discharged, the battery icon is completely clear and the
BVI 3000 does not work. The discharged battery must be replaced with a charged one.
DESCRIPTION
Operations & Maintenance Manual: Introduction
9
SETTING UP
ASSEMBLING THE INSTRUMENT
PROCEDURE 1. CHARGE THE BATTERIES
WARNING
The battery charger, power supply, and power cables are not intended for patient contact. Ensure
six feet (two meters) is maintained between the patient and these components.
WARNING
Risk of explosion, re, or serious injury. The BladderScan BVI 3000 system is battery‑powered.
Failure to note the following when handling the battery may result in serious injury or damage:
• Never short‑circuit the battery by bringing the battery terminals into contact with any other
conductive object.
• Never expose the battery to abnormal shock, vibration, or pressure.
• Do not disassemble, heat above 60°C (140°F), or incinerate the battery.
• Keep battery out of reach of children and in original package until ready to use.
• If the battery is leaking or its case is cracked, put on protective gloves to handle it, and discard
it immediately. Always dispose of used batteries in compliance with all applicable laws and
regulations.
• Put insulating tape, such as cellophane tape, on the electrodes during transportation in order to
avoid a possible short circuit, re, or electrical shock.
WARNING
In order to maintain electrical safety, use only the provided battery charger and batteries.
The BladderScan BVI 3000 draws very little power when it is turned off; however, if you do not plan to use your
instrument for several weeks, you should remove the battery to prevent it from discharging.
The battery that is not in use should be stored in the charger so that it remains fully charged. There is no danger
of overcharging the battery.
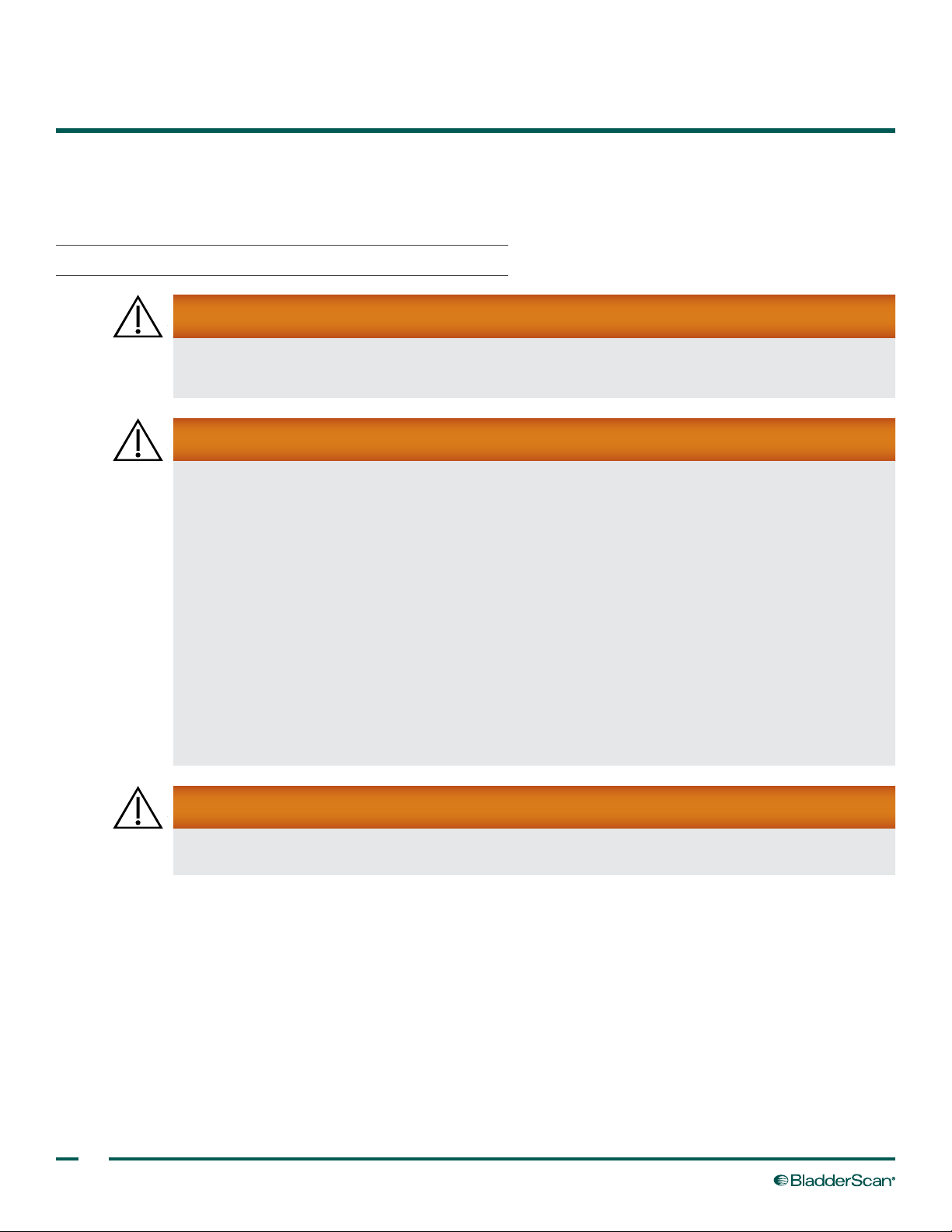
1. To maintain accessibility, plug in the charger only where it can be easily unplugged.
10

2. Align the battery as shown in the following gure, and then slide the battery into the recess on the top of
the charger.
3. Monitor the color indicator lights on top of the battery charger in order to determine the battery’s
charging status:
• Solid Green—When the battery is low on charge, charging begins in the fast‑charge mode. During
fast‑charge mode, the green light is solid. For a fully discharged battery, fast‑charge mode lasts about
two to three hours.
• Quickly Flashing Green—When the battery reaches 80% of its charge level, the charger starts to
“top off” the charge, and the green light ashes quickly. At this point, you may use the battery in the
BVI 3000 instrument.
• Slowly Flashing Green—If the green light ashes slowly upon inserting a battery, then the battery level
is too low for fast‑charge mode. The charger slowly charges the battery until the power level is high
enough to begin fast‑charge mode. When the battery power level is high enough, fast‑charge mode
begins automatically.
• Solid Amber—The amber light indicates that the battery temperature is stabilizing before recharging can
begin. This may occur when the battery is taken from a very cold or warm environment, or if the battery is
defective. If the light remains amber for over an hour, the battery is defective and must be replaced.
Operations & Maintenance Manual: Setting Up
11

PROCEDURE 2. INSERT OR REMOVE A BATTERY
INSERT A BATTERY
1. Ensure the battery has been properly charged according to the instructions in the procedure Charge the
Batteries on page 10.
2. If a battery is already in the control unit, remove it according to the instructions in the following section
Remove a Battery.
3. Align the charged battery with the battery well as shown in the following gure.
Battery contacts facing up
4. Slide the battery into the well and press until the battery clicks into place.
REMOVE A BATTERY
1. Next to the battery well, press the battery‑eject button. The battery is released from the control unit.
Battery‑eject button
2. Slide the battery out of the control unit.
3. Recharge or store the removed battery in the charger according to the instructions in the procedure Charge
the Batteries on page 10.
12

PROCEDURE 3. CONNECT THE SCANHEAD TO THE CONTROL UNIT
1. Align the scanhead cable connector so that the arrow is facing up.
Arrow on scanhead
cable connector
2. Press the connector directly into the port until you hear a click.
3. If you want to disconnect the scanhead from the control unit, grip the black plastic ring on the cable
connector, and then pull straight out. Do not twist the cable or connector.
Operations & Maintenance Manual: Setting Up
13
PROCEDURE 4. LOAD THE THERMAL PAPER ROLL
If the instrument is experiencing a paper jam, see the procedure Clear a Paper Jam on page 44.
The BladderScan BVI 3000 has an automatic paper‑loading mechanism. When you insert the paper roll
correctly, the instrument automatically feeds the paper through the instrument and prepares for printing.
1. On the top of the control unit, open the paper well door.
2. If there is an empty paper roll in the paper well, remove it.
3. If you are loading a new paper roll, cut off the rst inch of the new paper.
4. Ensure that the end of the paper roll is cut straight. Do not fold the end of the paper roll, cut it diagonally, or
cut it to a point.
5. Turn the BVI 3000 on by pressing the power button.
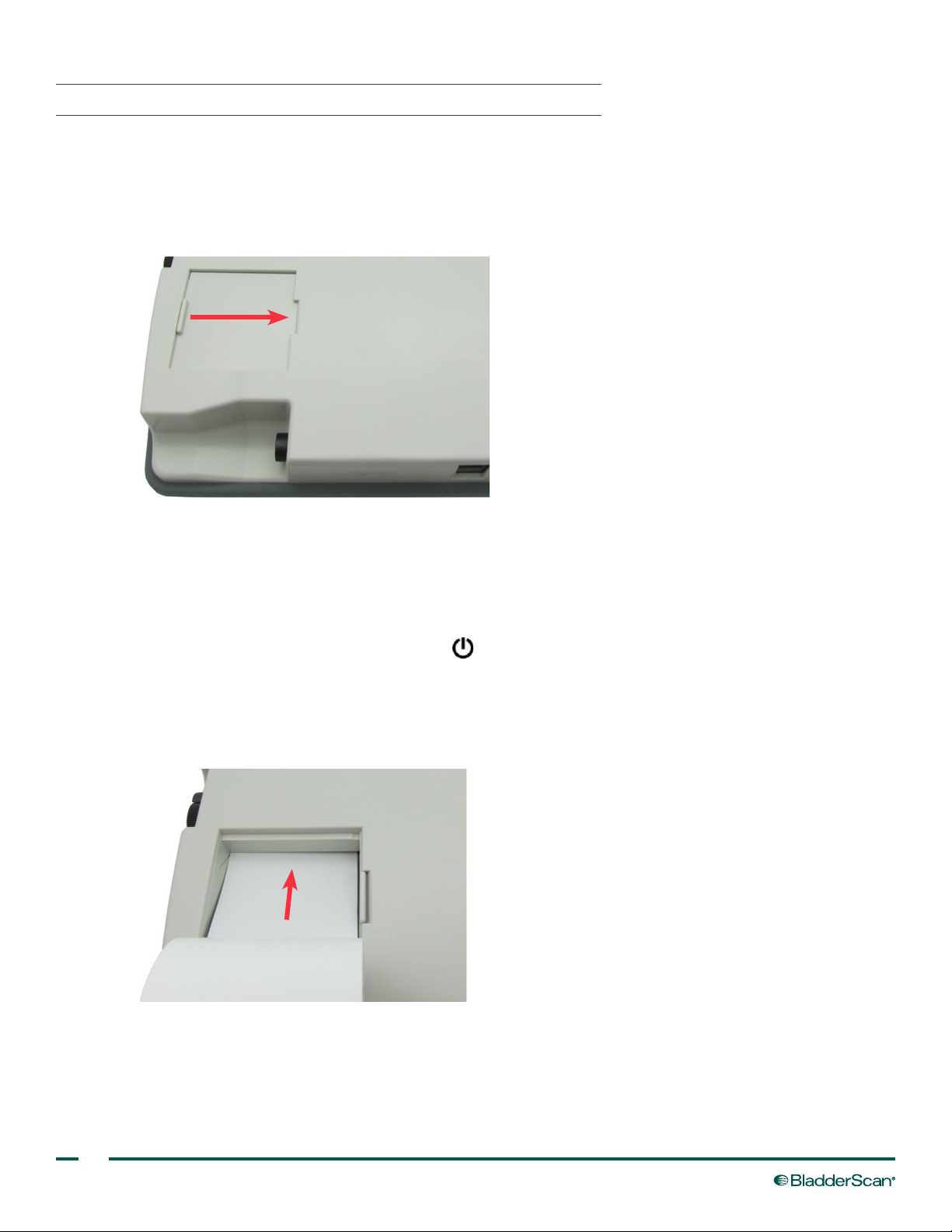
6. Insert the end of a new paper roll, with the thermal side down, into the paper input slot. The
BVI 3000 senses the presence of the paper and automatically feeds the paper through the instrument.
Note: To verify that you are loading the paper with the thermal side down, ick your nail over the paper. If a
black mark appears, this is the thermal side.
7. Place the paper roll in the paper well.
8. Close the paper well door. The instrument is ready to print.
14
 Loading...
Loading...