Bitmed SLEEP&GO User Manual
SLEEP&GO
CARDIORESPIRATORY POLYGRAPH
USER’S MANUAL
534-7AB-MU2 • REV. 1.07 • 2014-06
MANUAL
ST
A
RT
SYSTEM
CO
NFIG.
19/06/10
75% 11:15

Sleep&GO Manual
Revision: 534-7AB-MU2 Rev. 1.07
All rights reserved.
Please refer to the device’s Service Manual for additional information.
This manual can be purchased through the After Sales Service.
SIBEL S.A.
Rosselló 500, 08026 Barcelona - Spain
National Sales: Tel. 93 436 00 08 e-mail: comercial@sibelmed.com
International Sales: Tel. +34 93 436 00 07
E-mail: export@sibelmed.com
Technical service: Tel. +34 93 433 54 50
E-mail: sat@sibelmed.com
Fax: +34 93 436 16 11, Website: www.sibelmed.com
SIBEL, S.A. belongs to SIBELGROUP
COPYRIGHT
No part of this publication may be reproduced, transmitted, transcribed, stored in a
back-up system or translated into any language or computer language in any form
or by any means, electronic, mechanical, optical, chemical or manual without the
express written consent from SIBEL S.A.
DISCLAIMER
SIBEL S.A. is responsible for the security, reliability and performance of this
equipment only if:
• The place where the systrem is installed or used meets the requirements for
electrical installations IEC and other applicable regulations.
• All repairs, revisions or modications, both in and out of the warranty period, are
made by technical staff of Meditel Ingeniería Médica S.L. o SIBEL S.A.
• The system is used by qualied staff in accordance with the recommendations
stated in this User’s Manual.
BRANDS
Bitmed is a registered trademark of Sibel, S.A.
534-7AB-MU2 • REV. 1.07

PRODUCT IN COMPLIANCE WITH MEDICAL DEVICE DIRECTIVE
93/42/EEC (CLASS IIa).
Thank you for choosing this product. SLEEP&GO system is designed and
manufactured with the best guarantees of quality.
Applications SLEEP&GO and its related software will open a world of
possibilities in the sleep study.
If you have any possible improvement for this product, we welcome your
suggestions may be directed to Customer Service Department.
Revised Approved
Date: 2014-06 Date: 2014-06
Technical Director Sales Director
534-7AB-MU2 • REV. 1.07
0197

Index
Sleep&Go
User’s Manual
4
534-7AB-MU2 • REV. 1.07
TABLE OF CONTENTS
SAFETY ..............................................................................7
INTENDED USE ..................................................................7
INDICATIONS FOR USE .....................................................8
LIMITATIONS IN USE. CONTRAINDICATION .....................9
WARNING AND PRECAUTIONS ..........................................9
DISPOSAL OF ELECTRICAL AND ELECTRONIC DEVICES BY
DOMESTIC USERS IN THE EUROPEAN UNION .......13
1 INSTRUCTIONS OF INSTALLATION AND USE ...............15
1.1 MODELS ....................................................................15
1.2 PACKING LIST ...........................................................16
1.3 LAYOUT OF CONTROLS, INDICATORS AND
CONNECTORS ..................................................................20
1.3.1 FRONT/TOP/RIGHT PANNEL ..................................20
1.3.2 LEFT PANNEL .......................................................... 20
1.3.3 REAR PANNEL .........................................................21
1.4 INSTALLATION AND START-UP ..................................21
1.4.1 BATTERY PLACEMENT ............................................21
1.4.2 POWER SAVING MODE ............................................22
1.4.3 BLUETOOTH MODULE INSTALLATION .....................22
1.4.4 PLACEMENT OF SENSORS AND ELECTRODES ...........22
1.4.4.1 PLACEMENT OF THE SLEEP&GO POLYGRAPH .......22
1.4.4.2 NASAL CANNULA .................................................23
1.4.4.3 THERMOCOUPLE AIRFLOW SENSOR ..................... 24
1.4.4.4 THORACIC AND ABDOMINAL EFFORT BANDS .......25
1.4.4.5 PULSE OXIMETER ................................................26
1.4.4.6 BODY POSITION AND ACTIVITY ..........................29
1.4.4.7 SNORING SENSOR ...............................................29
1.4.4.8 LIMB MOVEMENT SENSOR ...................................30
1.4.4.9 EKG ELECTRODES ................................................30
1.4.4.10 EMG. EOG AND EEG ELECTRODES .......................31
1.4.4.11 EVENT MARKER ..................................................33
2. OPERATION ................................................................. 34
2.1 WORKING MODES ...................................................... 34

Sleep&Go
User’s Manual
Index
5
534-7AB-MU2 • REV. 1.07
2.2 SYSTEM CONFIGURATION .........................................35
2.3 INITIAL DEVICE SETUP .............................................36
2.3.1 SETTING THE DATE AND TIME ................................ 36
2.3.2 SETTING THE LANGUAGE ........................................ 36
2.3.3 SETTING THE TYPE OF BATTERIES ..........................37
2.3.4 SETTING THE UNITS OF THE CPAP CHANNEL ..........37
2.4 SWITCHING ON/OFF THE BLUETOOTH MODULE ........37
2.5 MAKING POLYGRAPHY TESTS IN ONLINE MODE ........39
2.6 MAKING POLYGRAPHY TESTS IN HOLTER MODE ........39
2.6.1 STARTING AND ENDING A TEST MANUALLY............39
2.6.2 SCHEDULED TESTS .................................................40
2.6.2.1 SCHEDULING A TEST IN THE SLEEP&GO .............. 40
2.6.2.2 DISPLAYING THE SCHEDULED TESTS ...................42
2.6.2.3 CHANNEL CONFIGURATIONS ...............................43
2.7 TRANSFER AND REVIEW OF THE TESTS .....................43
2.8 FIRMWARE UPDATE ...................................................44
2.9 DEVICE OPTIONS UPDATE ......................................... 44
3. TECHNICAL SPECIFICATIONS ...................................... 46
3.1 GENERAL DATA ..........................................................46
3.2 PULSE OXIMETER TECHNICAL SPECIFICATIONS ........50
3.3 CONDITIONS OF OPERATION AND STORAGE OF
ACCESSORIES ..................................................................51
3.4 APPLICABLE STANDARDS .......................................... 51
4. SYMBOLOGY ................................................................ 55
4.1 SYMBOLOGY OF THE SLEEP&GO ................................. 55
4.2 SYMBOLOGY OF THE ACCESSORIES ........................... 56
4.3 VALIDATED ACCESSORIES ......................................... 58
5. CLEANING AND MAINTENANCE ....................................60
5.1 CLEANING .................................................................60
5.2 PREVENTIVE MAINTENANCE ...................................... 60
5.2.1 ACTIONS TO BE TAKEN BY THE USER ......................60
5.2.2 ACTIONS TO BE TAKEN BY QUALIFIED PERSONNEL 61
5.3 CORRECTIVE MAINTENANCE ......................................62
ANNEX 1. ELECTROMAGNETIC COMPATIBILITY ...............63
ANNEX 2. TROUBLESHOOTING GUIDE ............................. 68

Index
Sleep&Go
User’s Manual
6
534-7AB-MU2 • REV. 1.07

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Declaration of conformity
7
SAFETY
The Sleep&Go cardiorespiratory polygraph has been developed
by the R+D+i Department of SIBEL S.A. with the collaboration
of reference Health Centers and doctors specialized in the area of
Sleep Disorders.
The Sleep&Go cardiorespiratory polygraph is designed and
manufactured in accordance with the Quality Manual of SIBEL S.A.
which is in consistency with the quality standards EN 13485 and
ISO 9001 and European Directive 93/42/EEC concerning medical
devices and 2007/47/EC. According to this directive the equipment
is Class IIa. The Sleep&Go also complies with the EN 60601.1
Electrical Safety and Electromagnetic Compatibility EN 60601.1.2
standards, as specied in the ELECTROMAGNETIC COMPATIBILITY
annex.
The Sleep&Go can transmit data via Bluetooth and therefore follows
the Directive 1999/5/EC on radio equipment and telecommunications
terminal equipment. For other countries complies with FCC rules
(parts 15c) and Canada Industry (IC: 5123A-BGTWT11A).
The Sleep&Go also complies with the following directives and
regulations: Packaging and packaging waste directive 94/62/
EC; Waste Electrical and Electronic Equipment Directive (WEEE)
2002/96/EC; Regulation EC 1272/2008 on classication, labelling
and packaging of substances and mixtures (REACH).
INTENDED USE
Acquisition, storage and display of biomedical signals for the
simplied diagnosis and control of sleep-related breathing disorders
(mainly Sleep Apnea and Hypopnea Syndrome) being anyone or
any combination of the following signals: respiratory airow, snore,
CPAP pressure measurement, thoracic effort, abdominal effort,
SpO2, beats per minute, perfusion wave, body position, body
activity, extremity limb movement, and EEG-EMG-EKG signals.
Next conditions must be taken into account:
• Use in a health center, patient’s home or similar indoor use (not
for outdoor use).
• Not intended for use in moving transport vehicles.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Safety
8
• Not intended for monitoring vital signals.
• EKG signal can not be used for heart diagnosis purpose. It’s
only intended for bradycardia and tachycardia detection during
sleep analysis.
INDICATIONS FOR USE
The Sleep&Go has been designed for maximum safety. All of the
operating instructions should been read before proceeding to
operate with the system. Failure to do so may result in injuries to
the user or the patient and damage to the device and/or accessories.
The Sleep&Go has been designed for being used by a doctor or
a technician trained in the acquisition of cardiorespiratory signals
and the transmission of these signals to a PC during polygraphic
tests. The user is allowed to congure the device under these
conditions. However, it is not recommended that the conguration
of the device is changed without understanding the principles of
signal digitalizing.
Minimum age of patients is 5 years, weighing over 15 kg and
a minimum height of 70 cm. The medical staff will instruct the
patient for a correct test execution, to avoid interferences in the
measurement and to replace the sensors in case of movement.
It is therefore important that the patient can understand the
instructions given by medical staff.
The intended environments of use are hospitals, sleep centres
and sleep clinics. Tests may also be carried out at the patient’s
home, with the exception of ExG signals (EEG, EMG, EOG, EKG).
In this case the patient is only authorized to start and stop the
test, and should be adequately instructed by the doctor on this
respect. The Sleep&Go is not designed to be used outdoors, nor in
other conditions or with energy sources not covered in this manual.
Using the Sleep&Go systems does not involve any monitoring or
diagnosis of the patient.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Safety
9
LIMITATIONS IN USE. CONTRAINDICATION
The interpretation of the tests and any ensuing treatment must be
carried out by a doctor. The acceptability of a test is the responsibility
of the health personnel.
The symptoms presented by the patient before starting any test
should be considered by the health personnel.
The Sleep&Go should not be used when it is likely that the validity
of the results may be compromised due to external factors (Electro
Magnetic interference – see section EMC).
WARNINGS AND PRECAUTIONS
The Sleep&Go IS NOT CERTIFIED FOR USE IN CONTINUOUS
MONITORING, where a failure in operation may cause injuries or
the death of the patient. This product does not maintain nor does
it help to maintain the life of the patient. The term CONTINUOUS
MONITORING is specied in regulation EN60601-1. The Sleep&Go
is classied as Class IIa in accordance with Directive 93/42/EEC on
medical devices.
The pulse oximeter is not provided with physiological-type alarms.
The pulse oximeter is calibrated to display functional oxygen
saturation and requires no calibration.
The pulse oximeter waveform is not standarized.
The Sleep&Go is not intended for monitoring vital signals.
There are no applicable parts to the patient which produce
stimulation.
The system has no user-serviceable parts. Use only authorized
service and spare parts supplied by the manufacturer.
Contact of liquids with the internal parts of the device and the
connectors must always be avoided.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Safety
10
The system is only resistant to moderate splashing and dripping
(Protection level IP22: protection against access to hazardous parts
with a nger; protected against solid objects with a diameter of
12.5 mm and above; protected against water drops falling vertically
with a maximum inclination of 15 degrees of the envelope).
Do not submerge the parts of the device in any liquid. MAY CAUSE
ELECTRIC DISCHARGE.
No parts are allowed for temporary immersion.
The cleaning instructions in this manual and also in the instructions
of use of any sensor supplied but not manufactured by SIBEL S.A.
must be carefully followed.
Keep your device protected from shock and vibration. During
transportation, place all the items in the carrying case. The material
provides enough protection against small accidental impact.
Do not use the system in and MRI environment.
The system is not designed to work in an explosive environment
or in the presence of ammable anesthetics or gases of any kind.
MAY CAUSE EXPLOSION.
This product is intended for indoor use (e.g. at the patient’s home
or hospital) and is not suitable for use during patient transportation.
The polygraph is not intended to be used outdoors or with other
conditions or energy sources that are not covered in this manual.
The Sleep&Go is not protected against debrillation shocks.
Therefore, never use a debrillator on a patient connected to a
Sleep&Go system.
Do not use an electric scalpel or a high frequency surgical device
while the patient is connected to any sensors or electrodes of the
Sleep&Go.
The use of mobile phones, transmitters and similar equipment
generating radio frequency emissions and placed next to the
system is not allowed during the tests. Therefore, do not use the
system in the presence of radio equipment (mobile phones, walkietalkie ...). Follow the recommendations regarding the separation

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Safety
11
distance specied in the manufacturer’s declaration for EMC in this
manual.
Remember that when multiple devices are connected to a patient
there is a risk of accumulation of leakage current. Minimize the
number of devices.
Do not remove the device cover. The service and repair of the
device should be carried out only by trained personnel.
The Sleep&Go system is prepared to work at room temperature.
Avoid exposing any part of the system to heat sources. Also
avoid direct exposure to sunlight. Temperature changes cause
condensation and moisture. Before using the system, allow the
device to acclimate to ambient temperature. For reference, if the
temperature difference between the system and the environment is
above 10º C a 20 minutes wait time in an intermediate temperature
is recommended.
The polygraph should not be placed adjacent to or stacked with
other equipment.
The system shall be stored and used within the temperature ranges,
pressure and humidity specied in section number 3.
Artefacts in the signal may be produced as a result of ESD. A
trained operator should be able to recognize these artefacts easily.
The operator must be trained to be able to recognize the differences
between a biological signal and signal artifacts caused by patient
movements, RF interference or poor placement of the electrodes or
sensors. The presence of ESD or RF devices will not lead to wrong
conclusions. Unusable data is not considered a risk to the patient
safety.
Cables or sensors should not surround the patient’s neck, especially
when the patient is a child.
The Sleep&Go system does not increase the safety risk for patients
with pacemakers in accordance with the EN50061 standard (Medical
electrical equipment – Safety of implantable cardiac pacemakers).
Before using the system in patients with pacemakers, the operator
should check the documents accompanying the pacemaker with
respect to its certication and requirements of use and, if necessary,

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Safety
12
contact the manufacturer.
Patients must be insistently warned that they must not open the
Sleep&Go or attempt to adjust it.
Sensors or accessories in bad condition should not be used.
Use only the Sleep&Go with accessories, sensors and electrodes
provided by the manufacturer or dealer, or those that meet the
specications of this manual. The use of other sensors with the
Sleep&Go can cause damage to the device or the quality of the
signals.
Sensors should be handled by their strongest parts, which are
the connectors. Also, they should not get wet or exposed to very
abrupt changes of temperature. To clean the sensors, do not use
abrasive chemicals. Do not apply excessive stress to the sensors.
In particular, avoid bending any part of the sensors. This means
that the material should not bend more than necessary in normal
use.
The polygraph is designed to be used exclusively by medical staff,
who should be supervised and instructed by a physician.
Medical personnel should inform the patient about precautions to
be found in the WARNINGS AND PRECAUTIONS section and to be
taken when using the equipment.
Use the provided carrying case when transporting the device and
its accessories.
Do not reuse single-use accessories as there is risk of infection to
the patient.
In case of doubt or unexpected event contact the manufacturer. You
can nd contact details on page 2 of this manual.
The use of nasal cannulas is not recommended in pregnant women
or children, because they contain phtalates.
Do not leave the batteries inside the device if it won’t be used for
a long period of time.
Ensure to perform the adquisition of signals in an acustic and light
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
13
environment that allows the patient to sleeping normally.
The system cannot be used for DIRECT CARDIAC APPLICATION.
The system is not an electrocardiograph, and therefore can not be
used to generate a separable ELECTROCARDIOGRAM for diagnostic
purposes, electrocardiographic monitoring or for ambulatory
electrocardiography. The EKG signal is only intended for bradycardia
and tachycardia detection during sleep analysis.
As for the electrodes, a proper contact of the skin with the
electrodes must be achieved by using conductive paste. Otherwise,
there is a risk of inaccurate measurements. Avoid contact with
the eyes or mucus membranes of gels, collodion, alcohol, acetone
or any substance used in the placement or removal of electrodes.
Be especially careful in the usage of collodion. Always follow the
recommendations for use provided by your collodion manufacturer.
The conductive part of the electrodes and connectors, including the
ground electrode must not touch other conductive parts, including
the ground.
DISPOSAL OF ELECTRICAL AND ELECTRONIC DEVICES BY
DOMESTIC USERS IN THE EUROPEAN UNION
Never dispose of the Sleep&Go, its accessories and its
batteries in the household trash. It must be disposed
of properly and may need to be recycled in accordance
with the statutory requirements in your country.
• Materials according to the RoHS Directive 2011/65/UE.
Conform from July 22th, 2014.
The device uses a lithium battery and could use an optional
NiMh battery.
For devices commercialised before July 22th, 2014: The
SleepSense sensor for limb movement contain mercury.
All Sleepsense sensors (except the cannulas) contain Pb in the
solderings.
All Sleepsense sensors (except the cannulas) may also contain
PBB and PBDE.
The cables for electrodes (Ref. 08093) contain Pb in the
soldering.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
14
The interconnection bridge (Ref: 02741) and the cable adapter
(Ref. 01644) contain Cadmium.
• Materials according to the Medical Device Directive: two
of the sensors that may be used in combination with the
device, contains phtalates (Sleepsense cannulas and Pro-Tech
cannulas). The device and all the accesories are latex free.
• Materials according to the REACH regulation: neither the device
nor its accessories use any hazardous substance according to
REACH regulation.
• In the event that the device or its accessories are infected at
the time of recycling, it must be disinfected or disposed by
following the national regulations regarding the disposal of
infected products.
Information on proper disposal is available from your dealer or
from Technical Support at SIBEL S.A.
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
15
1. INSTRUCTIONS OF INSTALLATION AND USE
1.1 MODELS
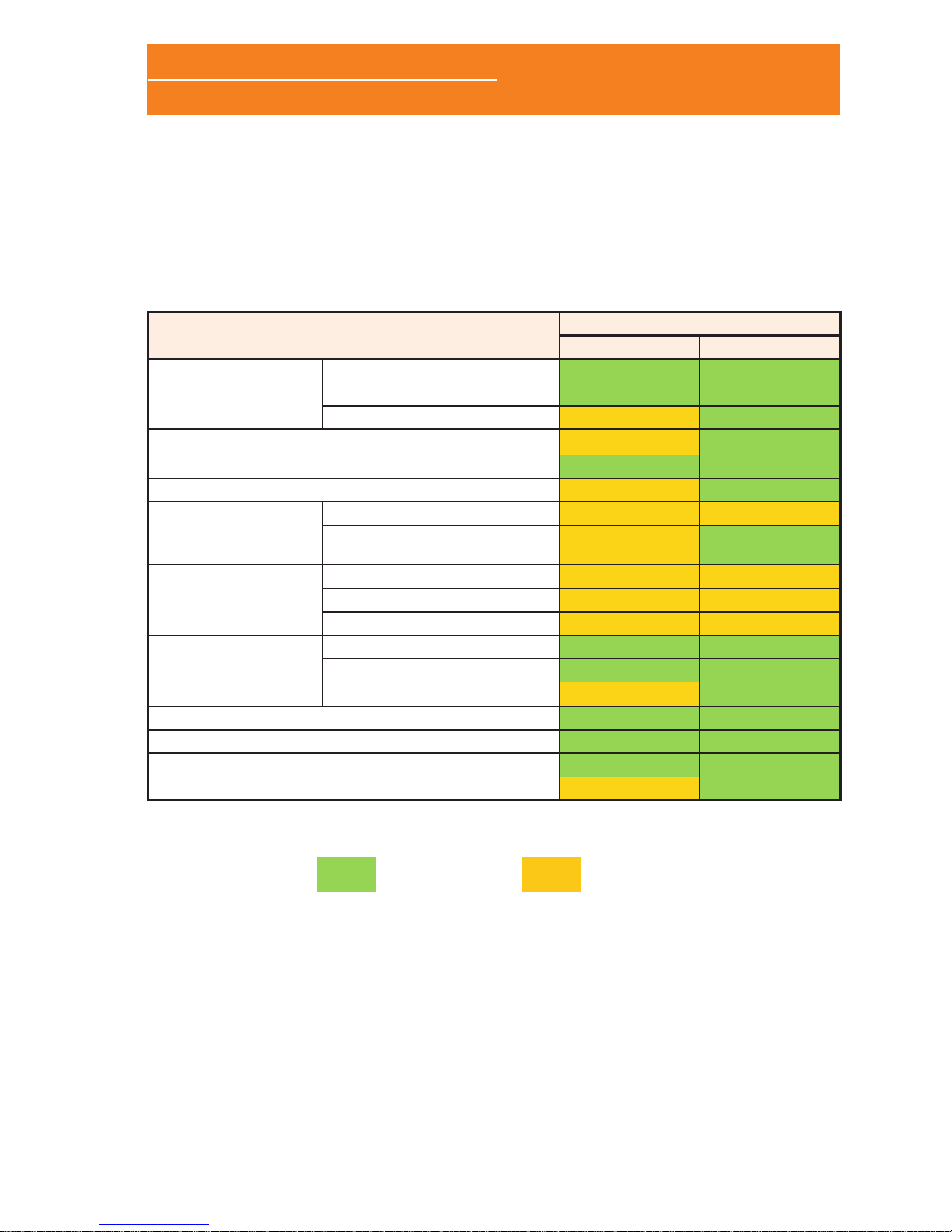
The Sleep&Go cardiorespiratory polygraph is available in two
different models (A and B) with the following features:
Sleep&Go
Channels
A model B model
Nasal cannula
Airow
Snore
CPAP
Thermocouple
Inductive plethysmography band (thoracic)
Inductive plethysmography band (abdominal)
Auxiliary channel
External snore
Limb movements
External EXG
module
EXG 1
EXG 2
EXG 3
External Xpod
SpO
2
BPM
Pulse wave
Position
Activity
Marks
Bluetooth (real time tests)
Default Opon
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
16
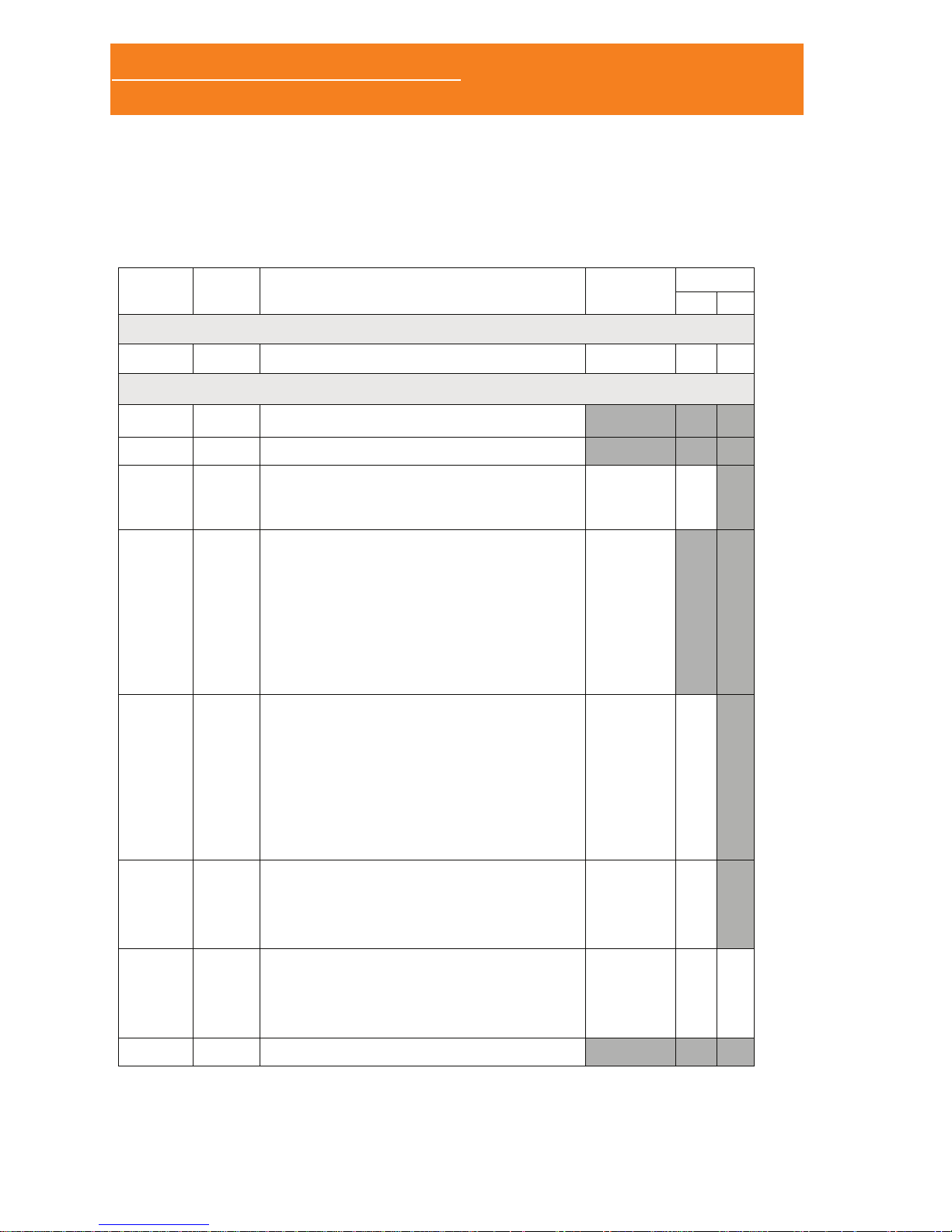
1.2 PACKING LIST
RELACIÓN DE CONTENIDO / PACKING LIST 534-708-120 REV. 4
SCREEN&GO/SLEEP&GO 2014-06
(*)
CUANDO SE ENTREGA LA REFERENCIA 08089 JUNTO CON UN SCREEN/SLEEP&GO NO SE INCLUYE LA REFERENCIA 01420 (PAQUETE DE 2 BANDAS
INDUCTIVAS TAMAÑO XL), PUESTO QUE YA ESTÁ INCLUIDA EN LA REFERENCIA 08088. LA REFERENCIA 01420 SE INCLUIRÁ SÓLO SI EL MÓDULO 08089 SE
SUMINISTRA POR SEPARADO.
WHEN THE REFERENCE 08089 IS SUPPLIED WITH A SCREEN / SLEEP & GO DOES NOT INCLUDE REFERENCE 01420 (PACK OF 2-BAND INDUCTIVE SIZE XL),
SINCE IT IS ALREADY INCLUDED IN THE REFERENCE 08088. THE REFERENCE 01420 IS INCLUDED ONLY IF THE MODULE 08089 IS DELIVERED SEPARATELY.
1/4
MODELOS/ MODELS
CÓDIGO
CODE
CANT.
QTY.
DESCRIPCIÓN
DESCRIPTION
SCREEN&GO
SLEEP&GO
A B
POLÍGRAFO / POLYGRAPH
_______ 1 SCREEN&GO/SLEEP&GO SN: 347 - _______
Accesorios Estándar / Standard Accessories
06312 1
BANDA DE SUJECIÓN TAMAÑO GRANDE (L) /
FASTENING BELT LARGE SIZE (L)
08049 1
CÁNULA DESECHABLE NASAL /
DISPOSABLE NASSAL CANNULA
08087
06309
1
1
MÓDULO TERMOPAR (INCLUYE ACTIVACIÓN DEL
CANAL) / THERMOCOUPLE MODULE (INCLUDES
CHANNEL ACTIVATION)
• SENSOR TERMOPAR / THERMOCOUPLE SENSOR
08088
06314
01420
07678
1
1
1
1
MÓDULO DE ESFUERZO TORÁCICO INDUCTIVO
(INCLUYE ACTIVACIÓN DEL CANAL) /
INDUCTIVE THORACIC EFFORT MODULE (INCLUDES
CHANNEL ACTIVATION)
• INTERFAZ DE AMPLIFICACIÓN BANDA INDUCTIVA
ESFUERZO TORÁCICO / THORAX EFFORT
INDUCTIVE BAND AMPLIFICATION INTERFACE
• BANDA INDUCTIVA ELÁSTICA TAMAÑO EXTRA
GRANDE (XL) / ELASTIC INDUCTIVE BAND XTRA
LARGE SIZE (XL)
• ANILLA SUJECIÓN / FASTENING RING
08089
06308
01420
(*)
07678
1
1
1
(*)
1
MÓDULO DE ESFUERZO ABDOMINAL INDUCTIVO PARA
SLEEP&GO (INCLUYE ACTIVACIÓN DEL CANAL) /
INDUCTIVE ABDOMINAL EFFORT MODULE FOR
SLEEP&GO (INCLUDES CHANNEL ACTIVATION)
• INTERFAZ DE AMPLIFICACIÓN BANDA INDUCTIVA
ESFUERZO ABDOMINAL / ABDOMINAL EFFORT
INDUCTIVE BAND AMPLIFICATION INTERFACE
• BANDA INDUCTIVA ELÁSTICA TAMAÑO EXTRA
GRANDE (XL) / ELASTIC INDUCTIVE BAND XTRA
LARGE SIZE (XL)
• ANILLA DE SUJECIÓN / FASTENING RING
---
08090
06310
1
1
CANAL AUXILIAR (MOVIMIENTO EXTREMIDADES)
PARA SLEEP&GO (INCLUYE ACTIVACIÓN DEL CANAL) /
AUXILIARY CHANNEL (LIMB MOVEMENT) FOR
SLEEP&GO (INCLUDES CHANNEL ACTIVATION)
• KIT SENSOR MOVIMIENTO EXTREMIDADES / LIMB
MOVEMENT SENSOR KIT
---
08091
06346
1
1
CANAL AUXILIAR (RONQUIDO) PARA SLEEP&GO
(INCLUYE ACTIVACIÓN DEL CANAL) /
AUXILIARY CHANNEL (SNORING) FOR SLEEP&GO
(INCLUDES CHANNEL ACTIVATION)
• SENSOR RONQUIDO PIEZOELÉCTRICO /
PIEZOELECTRIC SNORE SENSOR
---
08060 1
TARJETA MEMORIA MICRO SD CON ADAPTADOR SD /
MICROSD MEMORY CARD WITH SD ADAPTER
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
17
RELACIÓN DE CONTENIDO / PACKING LIST 534-708-120 REV. 4
SCREEN&GO/SLEEP&GO 2014-06
2/4
03673 2
PILAS ALCALINAS AA 1.5V /
AA 1.5V ALKALINE BATTERY
08011 1
MALETÍN TRANSPORTE SCREEN&GO
CARRYING CASE SCREEN&GO
--- ---
08010 1
MALETÍN TRANSPORTE SLEEP&GO /
CARRYING CASE SLEEP&GO
---
08070
______
08072
08071
1
1
1
1
CD SOFTWARE BITMEDLAB (CON LICENCIA) /
CD BITMEDLAB SOFTWARE (WITH LICENSE)
• MANUAL DE USO / USER’S MANUAL
(Doc.534-740-MU_)
• MÓDULO BASE DE DATOS / DATABASE MODULE
• MÓDULO ANALISIS AUTOMÁTICO EVENTOS /
AUTOMATIC EVENTS DETECTION MODULE
______ 1
SCREEN&GO MANUAL DE USO /
SCREEN&GO USER’S MANUAL (Doc. 534-70S-MU_)
--- ---
______ 1
SLEEP&GO MANUAL DE USO /
SLEEP&GO USER’S MANUAL (Doc. 534-7AB-MU_)
---
______ 1
GUÍA RÁPIDA SCREEN - SLEEP&GO /
SCREEN-SLEEP&GO QUICK GUIDE (Doc. 534-7AB-GR_)
Accesorios Opcionales / Optional Accessories
08094 1
LECTOR TARJETAS MEMORIA USB PARA PC /
USB MEMORY CARD READER FOR PC
08073 1
MÓDULO BITMED VISION PARA BITMEDLAB /
BITMED VISION MODULE FOR BITMEDLAB
---
06976
08098
08012
07677
1
1
1
1
KIT DE PULSIOXIMETRÍA ADULTOS PARA SCREEN&GOSLEEP&GO (Canales: SPO
2
, BPM) / ADULT PULSE
OXIMETRY KIT FOR SCREEN&GO-SLEEP&GO (Channels:
SpO
2
, BPM)
• MODULO PULSIOXIMETRIA XPOD /
XPOD PULSE OXIMETRY MODULE
• SENSOR PULSIOXIMETRIA SOFT (ADULTO) /
SOFT PULSE OXIMETRY SPO2 SENSOR (ADULT)
• MUÑEQUERA DE SUJECIÓN / WRISTBAND
08069
08098
08013
07677
1
1
1
1
KIT DE PULSIOXIMETRÍA PEDIÁTRICO PARA
SCREEN&GO/SLEEP&GO (Canales: SPO
2
, BPM) /
PEDIATRIC PULSE OXIMETRY KIT FOR
SCREEN&GO/SLEEP&GO(Channels: SpO
2
, BPM)
• MODULO PULSIOXIMETRIA XPOD /
XPOD PULSE OXIMETRY MODULE
• SENSOR PULSIOXIMETRIA SOFT (PEDIÁTRICO)/
SOFT PULSE OXIMETRY SPO2 SENSOR (PEDIATRIC)
•
MUÑEQUERA DE SUJECIÓN / WRISTBAND
08078 1
OPCIÓN FIRMWARE CANAL DE RONQUIDO POR PRESIÓN
/ PRESSURE SNORING CHANNEL FIRMWARE OPTION
08079 1
OPCIÓN FIRMWARE CANAL DE POSICIÓN CORPORAL /
BODY POSITION CHANNEL FIRMWARE OPTION
08080 1
OPCIÓN FIRMWARE CANAL DE ACTIGRAFÍA /
ACTIGRAPHY CHANNEL FIRMWARE OPTION
08081 1
OPCIÓN FIRMWARE CANAL DE MARCAS DEL PACIENTE /
PATIENT’S MARKS CHANNEL FIRMWARE OPTION
08082 1
OPCIÓN FIRMWARE CANAL DE PRESIÓN CPAP /
CPAP PRESSURE CHANNEL FIRMWARE OPTION
---
08083 1
OPCIÓN FIRMWARE CANAL DE ONDA DE PULSO /
PULSE WAVE CHANNEL FIRMWARE OPTION
---
08084 1
OPCIÓN FIRMWARE MÓDULO BLUETOOTH /
BLUETOOTH MODULE FIRMWARE OPTION
---
08022
08251
1
1
MÓDULO DE SENSORES EXG /
EXG SENSOR MODULE
• PIEZA ANTI APERTURA TAPA BATERÍAS /
BATTERY COVER BLOCKING PIECE
---
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
18
RELACIÓN DE CONTENIDO / PACKING LIST 534-708-120 REV. 4
SCREEN&GO/SLEEP&GO 2014-06
3/4
08085
08701
01644
02741
1
1
3
3
KIT DE ELECTRODOS EEG PARA SLEEP&GO / EEG
SENSOR KIT FOR SLEEP&GO
• ELECTRODO CUCHARA ORO EXG (PAQUETE 10U) /
EXG GOLD CUP ELECTRODES (PACKAGE 10PC)
• CABLE ADAPTADOR 1MM A 1.5MM /
CABLE ADAPTER 1MM TO 1.5MM
• PUENTE DE INTERCONEXIÓN /
INTERCONNECTION BRIDGE
---
08086
08093
01027
01644
1
1
1
3
KIT DE ELECTRODOS ECG PARA SLEEP&GO / EKG
SENSOR KIT FOR SLEEP&GO
• CABLES PARA ELECTRODOS DE CORCHETE (PAQUETE
10U) / CABLES FOR BUTTON STUD TYPE ELECTRODES
(PACKAGE 10 PC)
• ELECTRODO DE ECG (PAQUETE 50 uni.
) /
ECG ELECTRODE (PACKAGE 50 units)
• CABLE ADAPTADOR 1MM A 1.5MM /
CABLE ADAPTER 1MM TO 1.5MM
---
06346 1
SENSOR RONQUIDO PIEZOELÉCTRICO / PIEZOELECTRIC
SNORE SENSOR
---
07679 1
CÁNULA NASAL DESECHABLE PROTECH (PAQUETE DE
60u) / DISPOSABLE PROTECH NASAL CANNULA (PACK
OF 60u)
07680 1
CÁNULA ORO-NASAL DESECHABLE PROTECH (PAQUETE
DE 30u) / DISPOSABLE PROTECH ORO-NASAL CANNULA
40cm (PACK OF 30u)
07681 1
CÁNULA ORO-NASAL DESECHABLE SLEEPSENSE
(PAQUETE DE 5u) / DISPOSABLE SLEEPSENSE ORO-
NASAL CANNULA 60cm (PACK OF 5u)
08049 1
CÁNULA NASAL DESECHABLE SLEEPSENSE /
DISPOSABLE SLEEPSENSE NASAL CANNULA
06311 1
BANDA DE SUJECIÓN TAMAÑO PEQUEÑO (S) /
FASTENING BELT SMALL SIZE (S)
06313 1
BANDA DE SUJECIÓN TAMAÑO EXTRA GRANDE (XL) /
FASTENING BELT XTRA LARGE SIZE (XL)
01425 1
BANDA INDUCTIVA ELÁSTICA TAMAÑO PEQUEÑA (S) /
ELASTIC INDUCTIVE BAND SMALL SIZE (S)
01424 1
BANDA INDUCTIVA ELÁSTICA TAMAÑO MEDIANA (M) /
ELASTIC INDUCTIVE BAND MEDIUM SIZE (M)
01421 1
BANDA INDUCTIVA ELÁSTICA TAMAÑO GRANDE (L) /
ELASTIC INDUCTIVE BAND LARGE SIZE (L)
01420 1
BANDA INDUCTIVA ELÁSTICA TAMAÑO EXTRA GRANDE
(XL) / ELASTIC INDUCTIVE BAND XTRA LARGE SIZE (XL)
01417 1
BANDA INDUCTIVA ELÁSTICA TAMAÑO EXTRA EXTRA
GRANDE (XXL) / ELASTIC INDUCTIVE BAND XTRA XTRA
LARGE SIZE (XXL)
08701 1
ELECTRODO CUCHARA ORO EXG (PAQUETE 10u) / EXG
GOLD CUP ELECTRODES (PACK OF 10u)
---
08093 1
CABLES PARA ELECTRODOS DE CORCHETE (PAQUETE DE
10u) / CABLES FOR BUTTON STUD TYPE ELECTRODES
(PACK OF 10u)
---
01027 1
ELECTRODO DE ECG (PAQUETE DE 50u) /
ECG ELECTRODE (PACK OF 50u)
---
ESTÁNDAR
STANDARD
OPCIONAL
OPTIONAL
---
NO DISPONIBLE
NOT AVAILABLE

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
19
RELACIÓN DE CONTENIDO / PACKING LIST 534-708-120 REV. 4
SCREEN&GO/SLEEP&GO 2014-06
4/4
ADVERTENCIA:
• LOS ARTÍCULOS Y CANTIDADES RELACIONADAS ANTERIORMENTE HAN SIDO CUIDADOSAMENTE COMPROBADAS.
EN CASO DE FALTAS O DESPERFECTOS PROCEDAN A COMUNICÁRNOSLO LO MAS PRONTO POSIBLE.
•
SI DETECTA CUALQUIER DAÑO EN EL EMBALAJE, CONTACTE CON SU DISTRIBUIDOR ANTES DE PROCEDER A LA
INSTALACIÓN DEL EQUIPO.
• NO SE DEBE DESPRENDER DE LOS EMBALAJES, BOLSAS, ETC. HASTA QUE VERIFIQUE TOTALMENTE EL CORRECTO
FUNCIONAMIENTO DEL EQUIPO.
• EN CASO DE DEVOLUCIÓN DE MATERIAL O EQUIPO EN DEPOSITO, ROGAMOS NOS LO ENVÍEN EN PERFECTO
ESTADO, COMPLETO DE ACCESORIOS Y DEBIDAMENTE EMBALADO. CUALQUIER DESPERFECTO OCASIONADO
PROVOCARÍA UN CARGO CORRESPONDIENTE EN LA REPARACIÓN O EN LA REPOSICIÓN.
WARNING:
• THE ITEMS AND QUANTITIES RELATED BEFORE HAVE BEEN CAREFULLY CHECKED. IN CASE OF ANY PART IS
MISSING OR IS DAMAGED, NOTIFY US AS QUICKLY AS YOU CAN.
• IF YOU DETECT ANY DAMAGE IN THE PACKAGING, CONTACT WITH YOUR DISTRIBUTOR BEFORE PROCEEDING TO
INSTALL IT.
• DO NOT THROW AWAY THE PACKAGING, BAGS, ETC. UNTIL THE CORRECT FUNCTIONING OF THE DEVICE IS
VERIFIED
• IN THE CASE OF RETURNING THE GOODS, IT WILL BE APPRECIATED THAT YOU SEND THE DEVICE IN PERFECT
ORDER, WITH ALL THE ACCESSORIES AND PROPERLY PACKAGED. ANY DAMAGE SUFFERED WILL MAKE A CHARGE
CORRESPONDING TO REPAIR OR NEW PARTS.
PREPARADO/PREPARED BY: ................................. FECHA/DATE: .............
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
20
1.3 LAYOUT OF CONTROLS, INDICATORS AND
CONNECTORS
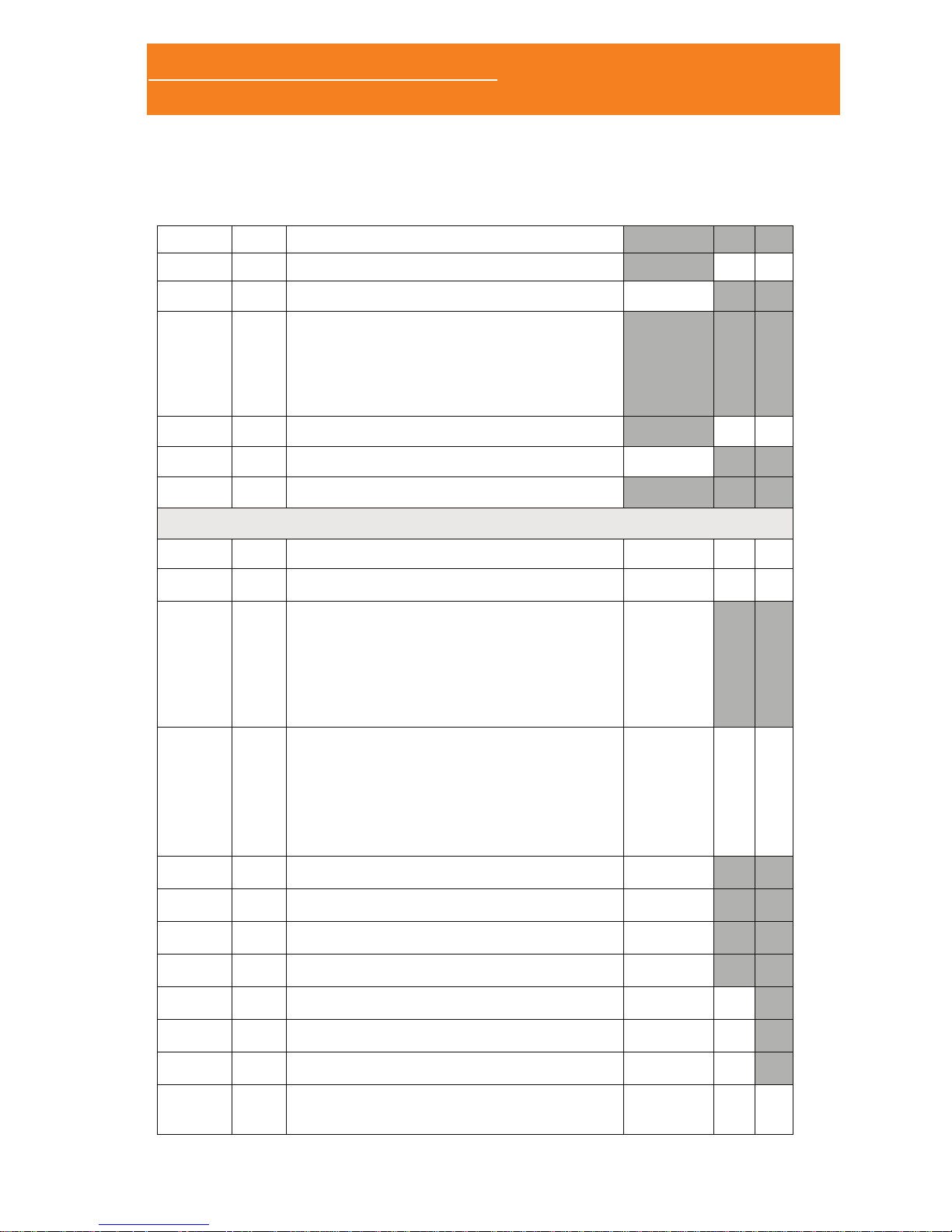
1.3.1 FRONT/TOP/RIGHT PANNEL
SpO2: XPod pulse
oximeter connector
Color LCD
Status LED
Joystick
Ther: thermocouple airow
sensor connector
Tho: thoracic effort band
connector.
Abd: abdominal effort band
connector.
Aux: auxiliary channel
connector (suitable for both
snore and limb movement
sensors)
ExG: ExG module
connector
Nasal: cannula
nasal cannula
connector
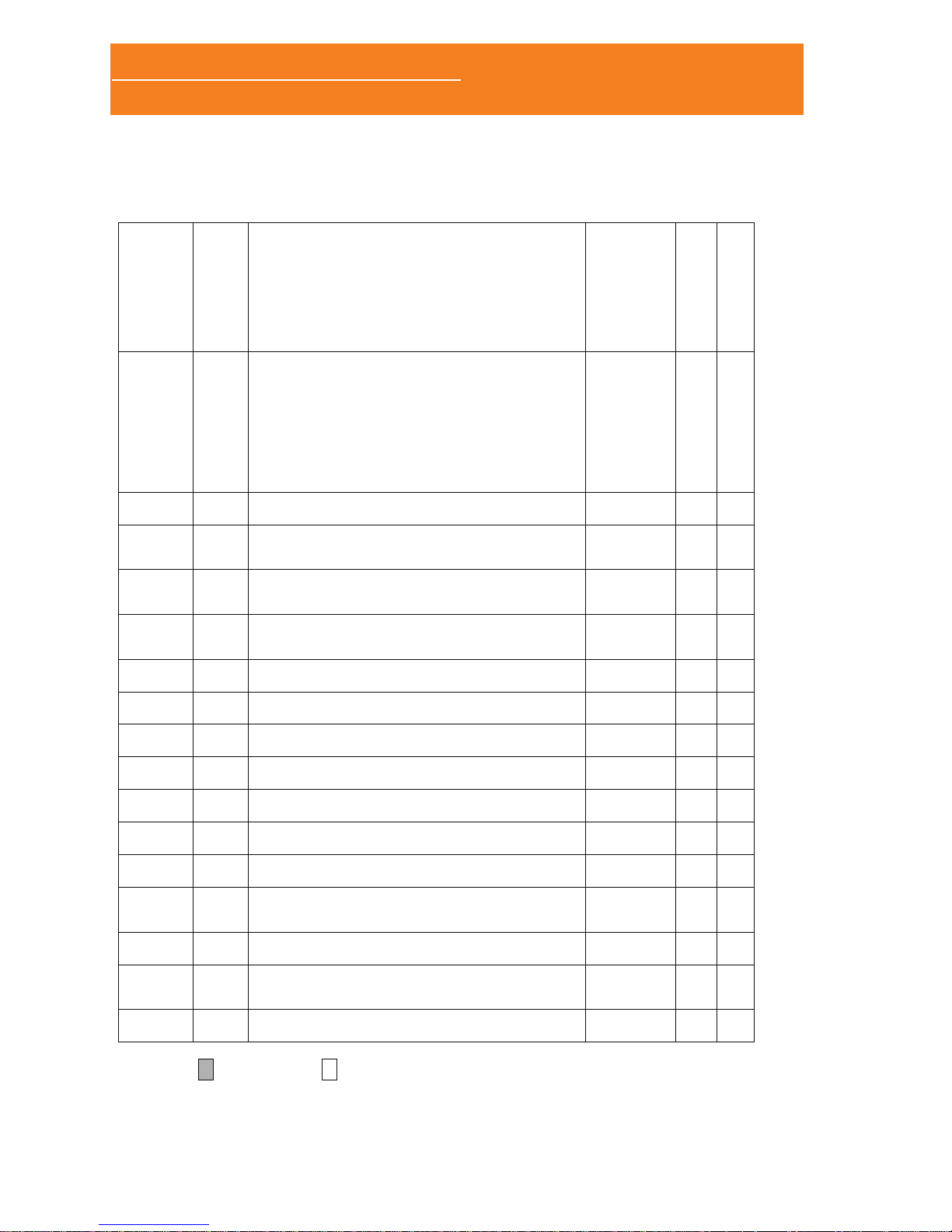
1.3.2 LEFT PANNEL
MicroSD memory
card slot
ON/OFF button
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
21
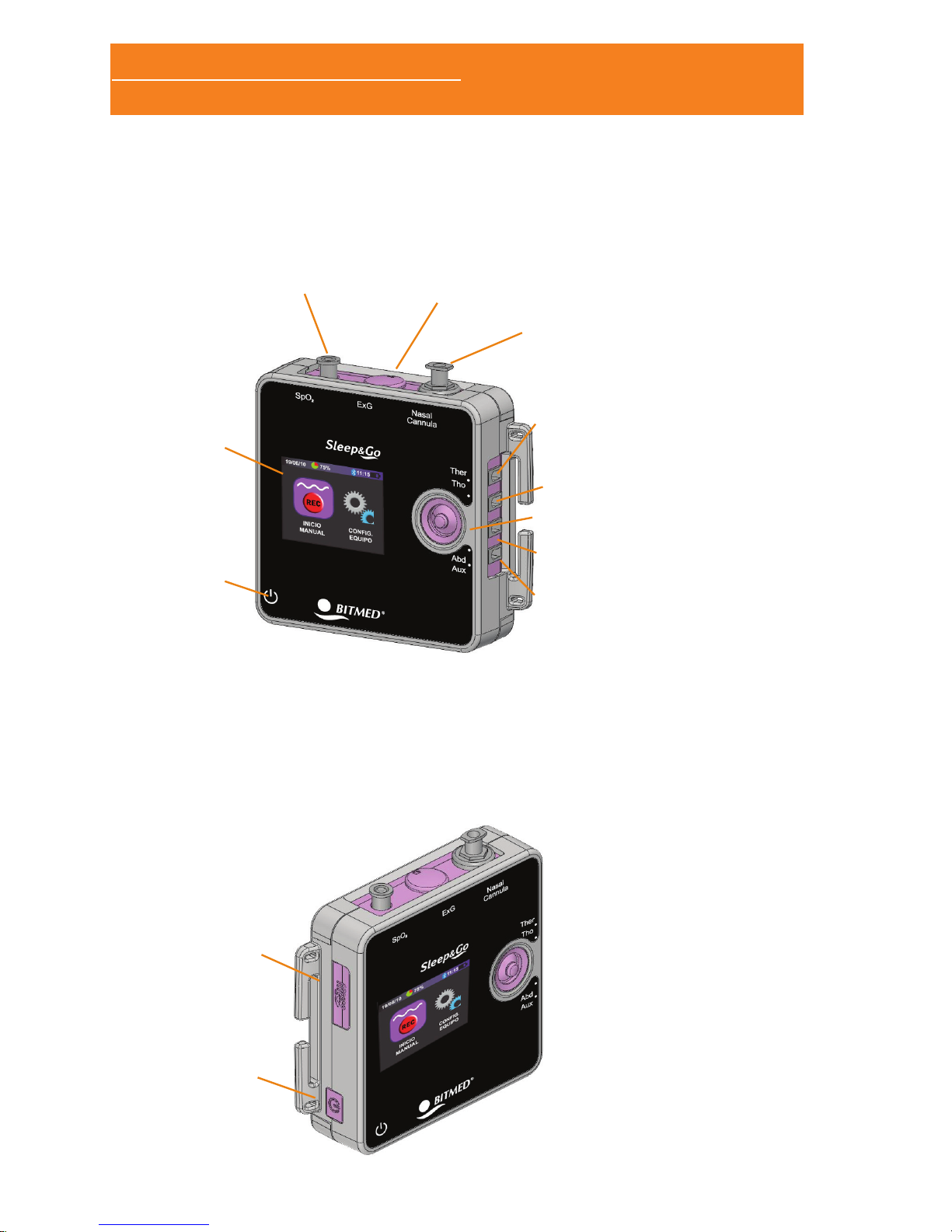
1.3.3 REAR PANNEL
08251 – Battery cover
blocking piece (only with
EXG module).
Battery compartment
(2x AA alkaline or
rechargeable batteries).
1.4 INSTALLATION AND START-UP
This system is made of solid-state professional components
manufactured under stringent quality controls. However,
accidents may occur during the transportation or storage, being
convenient an initial checking of the device and accessories prior
to installation. If you detect a deterioration in the package, please
contact the transport company and your supplier immediately,
prior to installation. Do not dispose the packaging until completely
verifying the proper functioning of the system.
Use only accessories described in this manual. The use of not
recommended accessories could adversely affect both the patient
safety and the equipment.
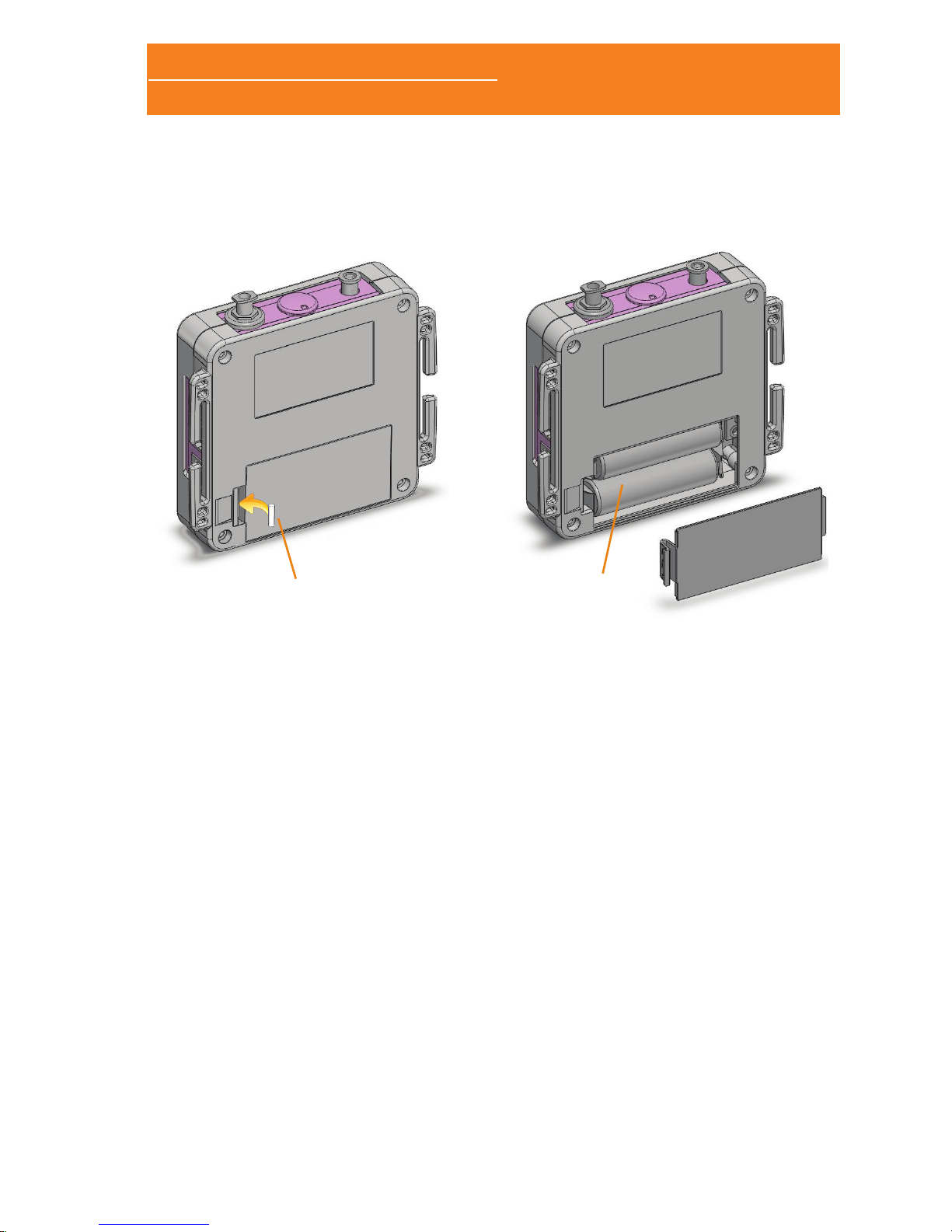
1.4.1 BATTERY PLACEMENT
The Sleep&Go system uses two 1.5V AA batteries. You can use
alkaline or rechargeable batteries with, at least, 2450mA/h. Using
smaller capacity batteries diminishes the autonomy. The autonomy
in normal use is about 12 hours operating in online mode and 24
hours in holter mode.
 Loading...
Loading...