Page 1

Operator’s Manual
Absorbance Microplate Reader
ELx800
™
Page 2

.
Page 3

7331000 Rev P1
This Manual Update contains changes to the ELx800™ Absorbance Microplate Reader
Operator’s Manual. These changes will be incorporated in the next full revision of the
manual, revision Q.
Replacing and Aligning the Bulb
Chapter 5, Maintenance and Decontamination contains instructions for replacing and
aligning the bulb. These instructions do not contain enough information to help you
properly align the bulb in all cases.
When it becomes necessary to replace the bulb*, replacement kits are available from
BioTek. PN 7330513 is required for standard models, and PN 7330516 is required for
ELx800UV and ELx800NB models. The kit contains complete instructions for replacing
and aligning the bulb. Please ignore the replacement/alignment instructions currently
supplied in this manual in Chapter 5.
If you have any questions or require further assistance, please contact BioTek’s Technical
Assistance Center (TAC). Contact information is provided on page x.
* The lamp is rated for an average life of 600 hours. The intensity of the lamp will slowly
drop over time until the run-time self check detects a low signal level. The reader will
display an error code at this time, indicating that it is time to replace the bulb.
© 2006, BioTek Instruments, Inc. Page 1 of 1
Page 4

.
Page 5

ELx800™
Absorbance Microplate Reader
Operator’s Manual
October 2006
© 2006
Part Number 7331000
Revision P
BioTek® Instruments, Inc.
Page 6

ii | Preface
Notices
BioTek® Instruments, Inc.
Highland Park, P.O. Box 998
Winooski, Vermont 05404-0998 USA
All Rights Reserved
Trademarks
© 2006, BioTek
®
Instruments, Incorporated. No part of this publication may be
reproduced, transcribed, or transmitted in any form, or by any means electronic
or mechanical, including photocopying and recording, for any purpose other
than the purchaser’s use without written permission of BioTek Instruments,
Inc.
BioTek
and EL800™ are trademarks of BioTek Instruments, Inc. Microsoft
®
is a registered trademark, and ELx800™, Gen5™, KC4™, KCjunior™,
®
, Windows®,
and the Windows logo are registered trademarks of Microsoft Corporation in
the United States and other countries. All other trademarks are the property of
their respective holders.
Restrictions and Liabilities
Information in this document is subject to change and does not represent a
commitment by BioTek Instruments, Inc. Changes made to the information in
this document will be incorporated in new editions of the publication. No
responsibility is assumed by BioTek for the use or reliability of software or
equipment that is not supplied by BioTek or its affiliated dealers.
BioTek Instruments, Inc.
Page 7

Contents | iii
Contents
Notices ............................................................................................. ii
All Rights Reserved ................................................................................. ii
Trademarks ........................................................................................... ii
Restrictions and Liabilities ........................................................................ ii
Contact Information .................................................................................. x
Customer Service and Sales ..................................................................... x
Service/TAC........................................................................................... x
European Coordination Center .................................................................. x
Document Conventions ............................................................................. xi
Revision History.......................................................................................xii
Intended Use Statement .......................................................................... xv
Quality Control ....................................................................................... xv
Repackaging and Shipping........................................................................ xv
Warnings ...............................................................................................xvi
Hazards and Precautions ..........................................................................xvi
Hazards...............................................................................................xvi
Precautions......................................................................................... xvii
Directive 89/336/EEC:Electromagnetic Compatibility..................................xix
Directive 73/23/EEC Low Voltage ............................................................xix
Directive 2002/96/EC: Waste Electrical and Electronic Equipment ................xix
Directive 98/79/EC: In Vitro Diagnostics ..................................................xix
Electromagnetic Interference and Susceptibility........................................... xx
User Safety ............................................................................................ xx
Safety Symbols.......................................................................................xxi
Chapter 1: Introduction .................................................................... 1
Introducing the ELx800 Absorbance Microplate Reader ................................... 2
Hardware Features .................................................................................... 3
Software Features ..................................................................................... 3
Package Contents ..................................................................................... 4
Optional Accessories.................................................................................. 5
Specifications ...........................................................................................6
ELx800 Operator's Manual
Page 8

iv | Preface
Standard Model ...................................................................................... 6
Ultraviolet/UV Model ...............................................................................7
Narrow Beam/NB Model........................................................................... 8
Product Support & Service........................................................................ 10
Contacting the Technical Assistance Center .............................................. 10
Returning Instruments for Service/Repair................................................. 10
Chapter 2: Installation ................................................................... 11
Unpacking and Inspecting the ELx800™ ..................................................... 12
Unpacking the Instrument and Its Accessories .......................................... 13
Setting Up the ELx800 ............................................................................. 17
Operating Environment.......................................................................... 17
Electrical Connections............................................................................ 17
Power-Up and System Test .................................................................... 18
ELx800 Main Menu................................................................................ 18
Configuring Global Default Options .......................................................... 19
Connecting a Printer to the ELx800 ......................................................... 20
Setting Up the Serial Port for Communications With Other Devices................. 22
Attaching the Cable............................................................................... 22
Installing Software on the Host PC .......................................................... 22
Setting Communication Parameters......................................................... 22
Installing Additional Filters ....................................................................... 24
Checking the Reader’s Filter Table Setting ................................................ 26
Repackaging and Shipping the ELx800 ....................................................... 28
Before Repackaging the Instrument......................................................... 28
Repackaging the ELx800 and Its Accessories ............................................ 29
Preparing the Shipping Container ............................................................ 35
Chapter 3: Operation ...................................................................... 37
ELx800 Front Panel ................................................................................. 38
Overview ............................................................................................... 40
Recommendations for Achieving Optimum Performance................................ 40
System Startup ...................................................................................... 41
Main Menu ............................................................................................. 42
Define ................................................................................................... 44
BioTek Instruments, Inc.
Page 9

Contents | v
Programming Note................................................................................ 45
Selecting an Assay to Define .................................................................. 46
Editing the Assay Name ......................................................................... 46
Define (Method, Map, Formula and Curve) .................................................. 47
Defining METHOD ................................................................................. 47
Defining MAP ....................................................................................... 51
Defining FORMULA ................................................................................ 67
Defining CURVE .................................................................................... 79
Reading a Microplate ............................................................................... 86
Selecting an Assay to Run...................................................................... 87
Printing Reports ...................................................................................... 91
Editing Standard Outliers ....................................................................... 92
Printing Results .................................................................................... 94
Using the Utility Options........................................................................... 95
Setting the Date and Time ..................................................................... 95
Viewing/Editing the Filter Table............................................................... 96
Specifying Data Output and Reporting Options.......................................... 97
Selecting Read Options.......................................................................... 99
Chapter 4: Instrument Qualification ............................................. 101
Recommendations for Achieving Optimum Performance.............................. 102
Recommended Qualification Schedule ...................................................... 103
Qualification Procedures......................................................................... 104
System Test....................................................................................... 105
Checksum Test................................................................................... 107
Absorbance Plate Test ......................................................................... 108
Description ..................................................................................... 108
Requirements.................................................................................. 110
Defining the Absorbance Test Plate Parameters.................................... 111
Running the Absorbance Plate Test..................................................... 112
Test Failures ................................................................................... 113
Liquid Testing ....................................................................................... 114
Stock Solution Formulation .................................................................. 115
Liquid Test 1 ...................................................................................... 117
ELx800 Operator's Manual
Page 10

vi | Preface
Liquid Test 2 ...................................................................................... 119
Liquid Test 3 (for “UV” Models Only)...................................................... 123
Chapter 5: Maintenance and Decontamination ............................. 127
Maintenance......................................................................................... 128
Routine Cleaning Procedure .................................................................... 128
Purpose............................................................................................. 128
Tools and Supplies .............................................................................. 129
Procedure .......................................................................................... 129
Replacing the Bulb ................................................................................ 130
Decontamination Procedure .................................................................... 133
Tools and Supplies .............................................................................. 134
Procedure .......................................................................................... 134
Chapter 6: Troubleshooting and Error Codes ................................ 137
Overview ............................................................................................. 138
System Test Description......................................................................... 138
Glossary of Terms............................................................................... 138
Error Codes.......................................................................................... 139
General Errors.................................................................................... 140
Fatal Errors........................................................................................ 146
Appendix A: Computer Control ..................................................... 147
Overview ............................................................................................. 148
Controlling the Reader With Gen5™ ......................................................... 149
Setting Up Gen5 ................................................................................. 149
Problems ........................................................................................... 150
Getting Started With Gen5 ................................................................... 150
Controlling the Reader With KC4™........................................................... 152
Setting Up KC4................................................................................... 152
Problems ........................................................................................... 153
Getting Started With KC4™ .................................................................. 153
Controlling the Reader With KCjunior™ .................................................... 154
Setting Up KCjunior ............................................................................ 154
Problems ........................................................................................... 155
BioTek Instruments, Inc.
Page 11

Contents | vii
Getting Started With KCjunior™............................................................ 155
Appendix B: Using 384-Well Geometry ......................................... 157
Perform a 384-Well Plate Read in Gen5™ ................................................. 158
Perform a 384-Well Plate Read in KC4™ ................................................... 162
Perform a 384-Well Plate Read in KCjunior™............................................. 165
Appendix C: Report Format........................................................... 169
Appendix D: Comparison of the ELx800 and the EL800................. 177
Appendix E: Instructions for Programming a New Assay.............. 179
Sample ANA Screen Enzyme Immunoassay Kit.......................................... 180
Intended Use ..................................................................................... 180
Background ....................................................................................... 180
Principle of the Assay .......................................................................... 180
Materials ........................................................................................... 181
Quality Control and Results .................................................................. 182
Expected Values ................................................................................. 182
Programming the ANA Screen Enzyme Immunoassay Kit ............................ 183
Sample Human Anticardiolipin IgG Enzyme Immunoassay Kit...................... 185
Intended Use ..................................................................................... 185
Background ....................................................................................... 185
Principle of the Assay .......................................................................... 185
Materials ........................................................................................... 186
Quality Control and Results .................................................................. 187
Expected Values ................................................................................. 189
Programming the Human Anticardiolipin IgG Enzyme Immunoassay Kit ........ 190
ELx800 Operator's Manual
Page 12

viii | Preface
List of Figures
Figure 1: Removing the Power Supply and Shelf..................................... 13
Figure 2: Removing the Foam End Caps................................................ 14
Figure 3: Removing the Top Cover Mounting Screws............................... 15
Figure 4: Removing the Shipping Block ................................................. 16
Figure 5: Global Configuration Options Available Under UTIL.................... 19
Figure 6: Connectors for Printer (Parallel), Computer (Serial), and Power
Supply ........................................................................................ 21
Figure 7: Filter Wheel Assembly........................................................... 24
Figure 8: Securing the Carrier ............................................................. 29
Figure 9: Hooking the Shipping Straps Over the Post .............................. 30
Figure 10: Installing the Shipping Block ................................................ 31
Figure 11: Reinstalling the Top Cover ................................................... 32
Figure 12: Reattaching the End Caps .................................................... 33
Figure 13: Placing the Unit in the Box ................................................... 34
Figure 14: Keyboard .......................................................................... 38
Figure 15: Options Available from the Main Menu ................................... 42
Figure 16: Sample Output for the System Test (Sheet 1 of 2) ................ 106
Figure 17: Sample Output for the System Test – Autocal Analysis
(Sheet 2 of 2) ............................................................................ 107
Figure 18: Sample Standards Certificate for the Absorbance Test Plate.... 111
Figure 19: Sample Test Plate Analysis Report ...................................... 111
Figure 20: Lamp Assembly ................................................................ 133
Figure 21: Reader Configuration Dialog............................................... 160
Figure 22: Reader Settings Dialog ...................................................... 160
Figure 23: New Experiment Dialog ..................................................... 161
Figure 24: Experiment Workspace...................................................... 161
Figure 25: Procedure Dialog .............................................................. 162
Figure 26: Read Step Dialog.............................................................. 162
Figure 27: Plate Reading Dialog ......................................................... 163
Figure 28: KC4 Main Menu, System|Readers........................................ 164
Figure 29: Reader Selection Dialog..................................................... 164
Figure 30: New Data File Dialog ......................................................... 165
BioTek Instruments, Inc.
Page 13

List of Figures (Continued) | ix
List of Figures (Continued)
Figure 31: Reading Dialog.................................................................... 165
Figure 32: Plate Reading Dialog ............................................................ 166
Figure 33: Setup|Reader Dialog ............................................................ 167
Figure 34: Reader Setup Dialog ............................................................ 167
Figure 35: Read Plate Dialog ................................................................ 168
Figure 36: Protocol Definition Dialog...................................................... 168
Figure 37: Example Read Method Parameters ......................................... 169
Figure 38: Samples With Calls on Matrix Report ...................................... 172
Figure 39: Curve Fit Report .................................................................. 173
Figure 40: Samples With Calls on Column Report .................................... 174
Figure 41: Column Report Without Samples............................................ 175
Figure 42: Panel Report ....................................................................... 176
Figure 43: Assay Detail Report (Sheet 1 of 2) ......................................... 177
Figure 44: Assay Detail Report (Sheet 2 of 2) ......................................... 178
List of Tables
Table 1: Recommended Qualification Schedule........................................ 103
Table 2: Typical Enzyme-Substrate Combinations and Stopping Solutions ... 115
Table 3: Test Tube Dilutions ................................................................. 120
Table 4: PBS 10X Concentrate Solution.................................................. 125
ELx800 Operator's Manual
Page 14

x | Preface
Contact Information
BioTek® Instruments, Inc.
Highland Park, P.O. Box 998
Winooski, Vermont 05404-0998 USA
Customer Service and Sales
Internet: www.biotek.com
Phone: 888-451-5171 (toll free in the U.S.)
802-655-4740 (outside the U.S.)
Fax: 802-655-7941
E-Mail: customercare@biotek.com
Service/TAC
Phone: 800-242-4685 (toll free in the U.S.)
802-655-4740 (outside the U.S.)
Fax: 802-655-3399
E-Mail: tac@biotek.com
European Coordination Center
BioTek® Instruments GmbH
Kocherwaldstrasse 34
D-74177 Bad Friedrichshall
Germany
Internet: www.biotek.de
Phone: +49 (0) 7136 9680
Fax: +49 (0) 7136 968 111
E-Mail: info@biotek.de
BioTek Instruments, Inc.
Page 15
Document Conventions
This manual uses the following typographic conventions:
Example Description
Document Conventions | xi
Warning!
Caution
DEFINE
Note:
This icon calls attention to important
A Warning indicates the potential for bodily harm and tells you
how to avoid the problem.
Caution indicates potential damage to the instrument and tells
A
you how to avoid the problem.
Text in COURIER font represents menu options as they appear on
the instrument’s display.
Bold text is primarily used for emphasis.
This icon calls attention to important information.
safety notes.
ELx800 Operator's Manual
Page 16

xii | Preface
Revision History
Revision Date Changes
A 3/95 First Release
B 7/95 Generic
C 9/95 Add Errata sheet
D 10/95 Incorporate Errata: cal plate PN cover text to UV
E 11/96 Added Panel Assay info. Added TVar parameter. Added
F 2/97 Added Notes including info on 384-well format. Updated Reuse
G 03/98 Added Liquid Test 1 and 2 to Chapter 4 –Performance
H 09/98 Modified Appendix B - Computer Control. Updated Elx800 and
I 2/99 Incorporated Manual Updates.
J 9/99 Changed printer-compatible description. Changed the number
K 1/00 Added Liquid Test 3 to test 340 nm filters. Updated screens in
L 5/03 Preface:
Appendix D to include all reports.
of Standard Curves and Panel sections. Added Appendix E –
KcJr for 384-well format.
Verification. Added Appendix F – Comparison, to show the
differences between the ELx800 and the EL800.
Added printer information.
Changed plate type information to include
384-well and 72-/96-well Terasaki plate formats. Added
reference to Quick Read on display.
EL800 model comparison table in Appendix F.
Changed European addresses.
of samples necessary if there aren't controls, standards or blanks
defined. Corrected the number of stored plates to 8. Corrected
the positions for re-use of standard curves. Removed the "M"
command from Appendix B- Computer Control.
Chapter 3. Updated chart in Appendix F.
- Updated contact information in Notices
(page iii).
- Added Document Conventions (page v).
- Corrected errors in Safety Symbol text
(page ix).
Removed "screwdriver" from Package Contents, and added
"BioTek QC Check Solutions for 405 nm" to Optional Accessories
list (page 1-7).
Removed About This Manual section (page 1-5).
Updated contact information in Technical Support (Chapter 1).
Modified appearance of display screens throughout.
Revised lamp alignment section in Chapter 2.
BioTek Instruments, Inc.
Page 17

Revision History (Continued)
L 5/03
(Cont.)
M 9/03 Preface:
N 10/04 Restructured manual according to new template.
Chapter 4:
- Changed title to "Performance Verification and
IQ/PQ/OQ Tests."
- Added IQ/PQ/OQ test procedure information.
- Clarified procedures for liquid tests.
Replaced outdated Appendix E with instructions for using KCjunior
to read 384-well microplates.
Added new Appendix G with two examples of assay kit instructions
and directions for programming an assay.
Made editorial and formatting changes throughout.
- Updated Warnings section (pages vii and viii).
- Updated Electromagnetic Compatibility section (page x).
- Added “Consult instructions for use” and “In vitro diagnostic
medical device” safety symbols (page xii).
- Expanded Intended Use Statement (page xiii).
Added Absorbance Test Plate to Optional Accessories list (Chapter 1,
page 1-8).
Changed callout in Figure 2-2 from “Place unit in bag” to “Unit in
plastic bag” (Chapter 2, page 2-2).
Added KC4 startup information to Appendix E.
Removed references to ELx800UV and EL800UV from Appendix F.
Standardized the presentation of significant digits throughout.
Changed “Abs” to “OD” throughout.
Removed references to “General Formula” throughout.
Updated Chapter 4, Performance Verification and IQ-OQ-PQ
Procedures.
Added new Chapter 5, Maintenance and Decontamination.
Added new Chapter 6, Troubleshooting and Error Codes.
Removed Computer Control portion of Appendix A; renamed
Appendix to “Controlling the Reader With KCjunior™ or KC4™.”
Added new Appendix B, Using 384-Well Geometry.
Updated sample reports in Appendix C, Report Format (formerly
Appendix D).
Removed previous Appendix A, Decontamination.
Removed previous Appendix C, Error Codes.
Added new Appendix E, Instructions for Programming a New Assay.
Revision History (Continued) | xiii
ELx800 Operator's Manual
Page 18

xiv | Preface
Revision History (Continued)
O 4/06
General:
- Changed “Bio-Tek” to “BioTek,” and “ELx800 Automated
Microplate Reader” to “ELx800 Absorbance Microplate Reader.”
- Added Gen5 references and instructions wherever KC4 and
KCjunior references and instructions were present.
Cover: Updated BioTek logo to new graphic.
Preface: Revised Hazards and Precautions, and removed Warranty
and Registration Card.
Chapter 1: Updated Package Contents, Optional Accessories, and
Specifications. Replaced 3-page Technical Support with 1-page
Product Support and Service section.
Chapter 2: Updated unpacking/packing instructions. Added new
section, “Installing Software on the Host PC,” for Gen5, KC4, and
KCjunior users.
Chapter 3: In System Startup, incorporated information from Manual
Update Rev. N2 that not all software features discussed in the manual
are available on ELx800 readers with custom programmed software.
Chapter 4:
- Changed title from “Performance Verification and IQ/OQ/PQ
Procedures” to “Instrument Qualification.”
- Add Gen5 instructions for System Test and Absorbance Plate Test.
- In Recommended Qualification Schedule, changed PQ frequency
from monthly and semiannually to monthly and quarterly, and
clarified criteria for running Liquid Tests 1, 2, or 3.
- In Absorbance Plate Test section, added a sample test plate report
(and explanatory note) illustrating that values higher than the
specified 0.000 to 2.000 OD range may not have “PASS”/”FAIL”
indications.
- For Liquid Tests 1, 2, and 3, incorporated recommendation from
Manual Update N2 to shake the plate or wait between pipetting
and read steps.
Appendix A: Changed title of appendix from “Controlling the Reader
With KCjunior or KC4” to “Computer Control.” Added new section,
“Controlling the Reader with Gen5.”
Appendix B: Added Gen5 instructions for preparing and running a
384-well plate protocol with the ELx800NB. Updated KC4 and
KCjunior sections by specifying that the ELx800NB model must be
used for running 384-well plates, and added screen shots that
illustrate selection of the ELx800NB.
BioTek Instruments, Inc.
Page 19
Revision History (Continued)
P 10/06 Ch 3 Operation, clarified instructions for running Panel assays via the
keypad.
Ch 4 Instrument Qualification, replaced incorrect sample System Test
report with a report run using an ELx800.
Ch 5 Maintenance and Decontamination, added tips for storing and
handling filters.
Appx B, Using 384-Well Geometry, inserted step 13 to select a Read
Type when using Gen5.
Intended Use Statement
The intended use of this instrument is dependent on the instrument’s rear panel label.
If there is an IVD label, then the instrument may be used for clinical, research and
development, or other nonclinical purposes. If there is no such label, then the instrument
may only be used for research and development, or for other nonclinical purposes.
Revision History (Continued) | xv
Quality Control
It is considered good laboratory practice to run laboratory samples according to
instructions and specific recommendations included in the assay package insert for the
test to be conducted. Failure to conduct Quality Control checks could result in erroneous
test data.
Repackaging and Shipping
If you need to ship the instrument to BioTek for service or
repair, contact BioTek for a Return Materials
Authorization (RMA) number, and be sure to use the
original packaging. Other forms of commercially available
packaging are not recommended and can void the
warranty. If the original packaging materials have been
damaged or lost, contact BioTek for replacement
packaging.
ELx800 Operator's Manual
Page 20

xvi | Preface
Warnings
Operate the instrument on a flat surface away from excessive
humidity.
Bright sunlight or strong incandescent light can reduce the linear
performance range of the instrument.
Measurement values may be affected by extraneous particles (such as
dust) in the microplate wells. A clean work area is necessary to
ensure accurate readings.
When operated in a safe environment according to the instructions in
this document, there are no known hazards associated with the
instrument. However, the operator should be aware of certain
situations that could result in serious injury; these may vary
depending on the instrument model.
Hazards and Precautions
Hazards
Warning! Power Rating.
cord must be connected to a power receptacle that provides voltage
and current within the specified rating for the system. Use of an
incompatible power receptacle may produce electrical shock and fire
hazards.
Warning! Electrical Grounding.
adapter to connect primary power to the external power supply. Use
of a two-prong adapter disconnects the utility ground, creating a
severe shock hazard. Always connect the power cord directly to a
three-prong receptacle with a functional ground.
Warning! Internal Voltage.
and unplug the power cord before cleaning the outer surface of the
instrument.
Warning! Liquids.
seepage into internal components creates a potential shock hazard.
Wipe up all spills immediately. Do not operate the instrument if
internal components have been exposed to fluid.
Avoid spilling liquids on the instrument; fluid
The instrument’s power supply or power
Never use a two-prong plug
Always turn off the power switch
BioTek Instruments, Inc.
Page 21

(Hazards, continued)
Hazards and Precautions | xvii
Warning! Potential Biohazards.
Some assays or specimens may
pose a biohazard. Adequate safety precautions should be taken as
outlined in the assay’s package insert. Always wear safety glasses
and appropriate protective equipment, such as chemically resistant
rubber gloves and apron.
Warning! Hot Surface.
The lamp is hot when the instrument is
turned on. Turn off the reader and allow the lamp to cool down
before attempting to replace it.
Warning! Unspecified Use.
Failure to operate this equipment
according to the guidelines and safeguards specified in this manual
could result in a hazardous condition.
Warning! Software Quality Control.
The operator must follow
the manufacturer’s assay package insert when modifying software
parameters and establishing reading methods, using the instrument’s
onboard software.
could result in erroneous test data
Warning! Reader Data Reduction Protocol.
Failure to conduct quality control checks
.
For readers with
onboard assay software, the software will flag properly defined
controls when they are out of range. The software will present the
data with the appropriate error flags for the operator to determine
control and assay validity. For readers operated via computer control,
no limits are applied to the raw absorbance data. All information
exported via computer control must be thoroughly analyzed by the
operator.
Precautions
The following precautions are provided to help avoid damage to the instrument:
Caution: Service.
service personnel. Only qualified technical personnel should perform
troubleshooting and service procedures on internal components.
Caution: Environmental Conditions.
instrument to temperature extremes. For proper operation, ambient
temperatures should remain between 18°-40°C. Performance may be
adversely affected if temperatures fluctuate above or below this
range. Storage temperature limits are broader.
ELx800 Operator's Manual
The ELx800™ should be serviced by authorized
Do not expose the
Page 22

xviii | Preface
Caution: Sodium Hypochlorite.
Do not expose any part of the
instrument to the recommended diluted sodium hypochlorite
solution (bleach) for more than 20 minutes. Prolonged contact may
damage the instrument surfaces. Be certain to rinse and thoroughly
wipe all surfaces.
Caution: Power Supply.
Only use the power supply shipped with
the instrument. Operate this power supply within the range of line
voltages listed on it.
Caution: Shipping Hardware.
Shipping straps and a shipping
block must be removed before operating the reader. They must be
reinstalled before repackaging the reader for shipment. See
, Installation.
2
Caution: Disposal.
This instrument contains printed circuit boards
Chapter
and wiring with lead solder. Dispose of the instrument according to
Directive 2002/96/EC, “on waste electrical and electronic equipment
(WEEE).”
Caution: Warranty.
protocols may
and Decontamination
void the warranty. See Chapter 5, Maintenance
Failure to follow preventive maintenance
.
BioTek Instruments, Inc.
Page 23
Hazards and Precautions | xix
Based on the testing described below and information contained
herein, this instrument bears the CE mark.
Directive 89/336/EEC:Electromagnetic Compatibility
Emissions - CLASS A
EN 61326-1:2002
EN 55022:2000 Class A, CISPR 16-1:1993, CISPR 16-2:1999
Immunity
EN 61326-1:2002
EN 61000-4-2: Electrostatic Discharge
EN 61000-4-3: Radiated EM Fields
EN 61000-4-4: Electrical Fast Transient/Burst
EN 61000-4-5: Surge Immunity
EN 61000-4-6: Conducted Disturbances
EN 61000-4-11: Voltage Dips, Short Interruptions and Variations
Directive 73/23/EEC Low Voltage
IEC 1010: “Safety requirement for electrical equipment for measurement, control
and laboratory use. Part 1, General requirements”.
Directive 2002/96/EC: Waste Electrical and Electronic Equipment
Disposal Notice
This instrument contains printed circuit boards and wiring with lead solder. Dispose
of the instrument according to Directive 2002/96/EC, “on waste electrical and
electronic equipment (WEEE).”
Directive 98/79/EC: In Vitro Diagnostics
• Product registration with competent authorities
• Traceability to the U.S. National Institute of Standards and Technology
(NIST):
Optical density measurements, and if equipped, incubator
Specific data for a particular serial number is available on request from BioTek
Instruments. See page x for contact information.
ELx800 Operator's Manual
temperature readings, are traceable to NIST.
Page 24

xx | Preface
Electromagnetic Interference and Susceptibility
USA FCC CLASS A
Warning: Changes or modifications to this unit not expressly approved by
the manufacturer could void the user's authority to operate the equipment.
This equipment has been tested and found to comply with the limits for a Class
A digital device, pursuant to Part 15 of the FCC Rules.
These limits are designed to provide reasonable protection against harmful
interference when the equipment is operated in a commercial environment.
Like all similar equipment, this equipment generates, uses, and can radiate
radio frequency energy and, if not installed and used in accordance with the
instruction manual, may cause harmful interference to radio communications.
Operation of this equipment in a residential area is likely to cause interference,
in which case the user will be required to correct the interference at his/her
expense.
Canadian Department of Communications Class A
This digital apparatus does not exceed Class A limits for radio emissions from
digital apparatus set out in the Radio Interference Regulations of the Canadian
Department of Communications.
Le present appareil numerique n'met pas du bruits radioelectriques depassant
les limites applicables aux appareils numerique de la Class A prescrites dans le
Reglement sur le brouillage radioelectrique edicte par le ministere des
Communications du Canada.
User Safety
This device has been type tested by an independent laboratory and found to meet the
requirements of the following:
North America
• Canadian Standards Association CAN/CSA C22.2 No. 1010.1-92
“Safety Requirements for Electrical Equipment for Measurement, Control,
and Laboratory Use, Part 1: General Requirements”
• UL 61010A-1, 1st Edition
“Safety Requirements for Electrical Equipment for Measurement, Control,
and Laboratory Use, Part 1: General Requirements”
International
• IEC 1010
“Safety Requirements for Electrical Equipment for Measurement, Control,
and Laboratory Use, Part 1: General Requirements”
BioTek Instruments, Inc.
Page 25
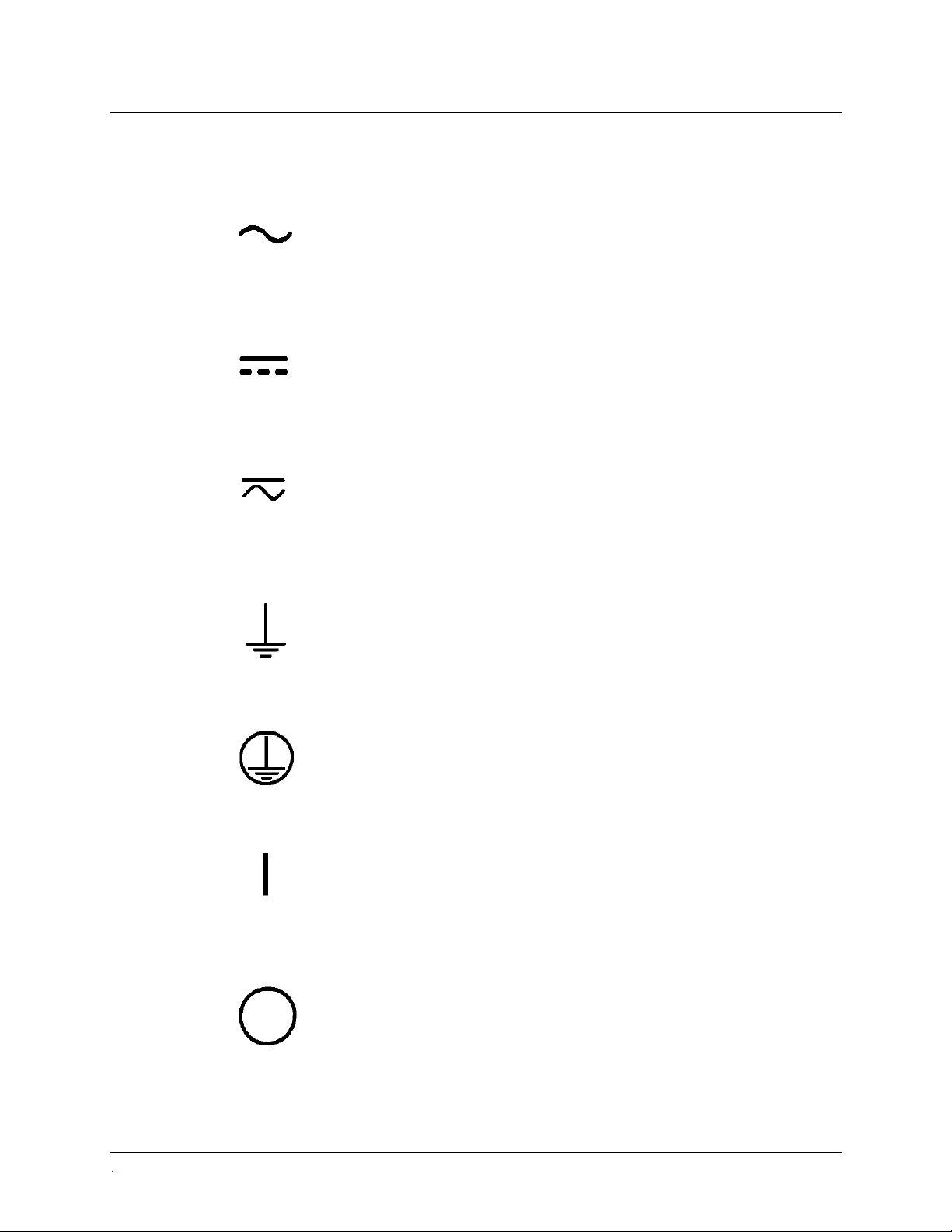
Safety Symbols
Some of the following symbols will appear on the instrument.
Alternating current
Courant alternatif
Wechselstrom
Corriente alterna
Corrente alternata
Direct current
Courant continu
Gleichstrom
Corriente continua
Corrente continua
Both direct and alternating current
Courant continu et courant alternatif
Gleich - und Wechselstrom
Corriente continua y corriente alterna
Corrente continua e corrente alternata
Safety Symbols | xxi
Earth ground terminal
Borne de terre
Erde (Betriebserde)
Borne de tierra
Terra (di funzionamento)
Protective conductor terminal
Borne de terre de protection
Schutzleiteranschluss
Borne de tierra de protección
Terra di protezione
On (Supply)
Marche (alimentation)
Ein (Verbindung mit dem Netz)
Conectado
Chiuso
Off (Supply)
Arrêt (alimentation)
Aus (Trennung vom Netz)
Desconectado
Aperto (sconnessione dalla rete di alimentazione)
ELx800 Operator's Manual
Page 26
xxii | Preface
Caution (refer to accompanying documents)
Attention (voir documents d’accompanement)
Achtung siehe Begleitpapiere
Atención (vease los documentos incluidos)
Attenzione, consultare la doc annessa
Warning, risk of electric shock
Attention, risque de choc
électrique
Gefährliche elektrische schlag
Precaución, riesgo de sacudida eléctrica
Attenzione, rischio di scossa elettrica
Warning, risk of crushing or pinching
Attention, risque d’écrasement et pincement
Warnen, Gefahr des Zerquetschens und Klemmen
Precaución, riesgo del machacamiento y sejeción
Attenzione, rischio di schiacciare ed intrappolarsi
Warning, hot surface
Attention, surface chaude
Warnen, heiße Oberfläche
Precaución, superficie caliente
Attenzione, superficie calda
Consult instructions for use
Consulter la notice d’emploi
Gebrauchsanweisung beachten
Consultar las instrucciones de uso
Consultare le istruzioni per uso
In vitro diagnostic medical device
Dispositif médical de diagnostic in vitro
Medizinisches In-Vitro-Diagnostikum
Dispositivo médico de diagnóstico in vitro
Dispositivo medico diagnostico in vitro
Separate collection for electrical and electronic
equipment
Les équipements électriques et électroniques font l’objet
d’une collecte sélective
Getrennte Sammlung von Elektro- und Elektronikgeräten
Recogida selectiva de aparatos eléctricos y electrónicos
Raccolta separata delle apparecchiature elettriche ed
elettroniche
BioTek Instruments, Inc.
Page 27
Chapter 1
Introduction
This chapter introduces the ELx800™ Absorbance Microplate Reader
and describes its hardware and software features. Also included is
contact information if technical assistance is needed.
Introducing the ELx800 Absorbance Microplate Reader ................. 2
Hardware Features.................................................................. 3
Software Features................................................................... 3
Package Contents ................................................................... 4
Optional Accessories ............................................................... 5
Specifications ......................................................................... 6
Standard Model.................................................................. 6
Ultraviolet/UV Model ........................................................... 7
Narrow Beam/NB Model ...................................................... 8
Product Support & Service ....................................................... 10
Contacting the Technical Assistance Center ........................... 10
Returning Instruments for Service/Repair .............................. 10
Page 28

2 | Chapter 1: Introduction
Introducing the ELx800 Absorbance Microplate Reader
The ELx800™ Absorbance Microplate Reader is a single-channel reader-assay system,
designed to automatically perform endpoint analysis for a variety of ELISA-based
applications. The reader can measure the optical density of solutions in 6-, 12-, 24-, 48-,
or 96-well microplates. The “NB” reader models can measure 384-well microplates and
60-, 72-, 96-well Terasaki trays as well as 6-, 12, 24-, 48-, or 96-well plates. The reader
features superior optical specifications, with an extended dynamic range of up to 3.000
absorbance units in some read modes. The wavelength range is from 400 nm to 750
nm. “UV” instruments have an extended range from 340 nm to 750 nm. Kinetic
analysis can be performed using computer control (e.g., via Gen5™, KC4™ , or
KCjunior™ running on a host PC).
Assay definitions (consisting of protocols, templates and formulas) and the data they
produce are managed by an onboard processor, via a 2-line x 24-character LCD screen
and membrane switch. Data can be stored onboard, printed, and/or uploaded to
controlling software on a host PC. The ELx800 is designed to serve as a stand-alone
system, or as part of a larger laboratory data network, sending, receiving, and
manipulating assay data as needed.
The ELx800 is backed by a superior support staff. If the ELx800 ever fails to work
perfectly, please call the Technical Assistance Center, or visit BioTek’s Web site. Refer
to the end of this chapter for contact information.
BioTek Instruments, Inc.
Page 29

Hardware Features | 3
Hardware Features
The ELx800’s hardware features include:
• Single optics channel
Wavelength range of 400-750 nm
•
ELx800UV model provides extended wavelength range of
340 to 750 nm
A 5-position filter wheel
•
A 2-line x 24-character LCD display
•
A membrane keypad with alphanumeric keys
•
X-Y carrier movement
•
Capability of reading 6-, 12-, 24-, 48- and 96-well microplates.
•
ELx800NB model extends read capability to include 384-well and
60-/72-/96-well Terasaki microplates.
External 24-volt power supply, compatible with 100-240 V~ ± 10.0%
•
@ 50 to 60 Hz
One serial COM port, 25-pin male connector
•
One parallel port, 25-pin female connector
•
Software Features
• An easy-to-use, menu-driven interface
Endpoint calculations
•
Curve Fitting, with 4-parameter, cubic, quadratic, linear, 2-P, cubic-spline, and
•
point-to-point methods
Transformation and Formula calculations for more complex mathematical
•
operations, including validations
Up to 75 assays can be programmed into memory and recalled instantly
•
Up to 10 results can be stored in memory
•
ELx800 Operator's Manual
Page 30

4 | Chapter 1: Introduction
Package Contents
The contents of the ELx800™ package include:
• ELx800 Absorbance Microplate Reader
• Power cord (part number varies according to country of use)
• Power supply (PN 61062)
• Filter wheel with 4 standard filters: 405 nm, 450 nm, 490 nm, 630 nm and one
blank filter. The UV model includes a 340 nm filter.
• Operator’s Manual (PN 7331000)
• Printer cable (PN 71072)
• Dust cover (PN 7332040)
• RS-232 serial cable (PN 75053)
• Unpacking instructions (PN 7331006); packing instructions (PN 7331034)
• Shipping document (PN 94075) that includes Warranty Statement and
Certificate of Compliance and Calibration
• Set of shipping materials (PN 7332062)
• Declaration of Conformity (PN 7331015, clinical models, or PN 7331049,
non-clinical models)
• Sample UV transparent plates and literature
BioTek Instruments, Inc.
Page 31

Optional Accessories
• Patented Bio-Cell™ Quartz vessel: to obtain 1 cm fixed pathlength absorbance
measurements (PN 7272051)
• Bio-Cell adapter plate assembly: can contain up to 8 Bio-Cells (PN 7270512)
• Lamp for ELx800NB, ELx800RNB, ELx800UV, ELx800RUV (PN 7330516)
• Lamp for ELx800 and ELx800R (PN 7330513)
• ELx800 filters (PN 7334---, plus wavelength)
340 (UV model only), 405, 415, 450, 490, 515, 540, 550, 562, 570, 590, 595,
600, 620, 630, 650, 660, 690, 750
• Terasaki plate adapter (PN 7330531) for 60-, 72-, and 96-well Terasaki plates for
ELx800NB and ELx800RNB
• Absorbance Test Plate (PN 9000547, 6-filter; or PN 7260522, 7-filter)
Optional Accessories | 5
• Gen5’s Reader Diagnostics Utility (PN 5320201) for the Absorbance Test Plate
• Absorbance Liquid Test Solutions:
BioTek Wetting Agent Solution (PN 7773002)
BioTek QC Check Solution No. 1 (PN 7120779, 25 ml; or PN 7120782,
125 ml)
• Epson LX-300 printer or equivalent (110V only) (PN 97145)
• Sheet feeder for Epson LX-300+ (PN 97144)
• HP DeskJet printer (PN 97152)
• Gen5™, KC4™, or KCjunior™ software (PNs and versions listed on biotek.com
or contact your local dealer)
• Delta-Soft Macintosh Software (PN 3070145)
• ELx800 Qualification Package (PN 7330538)
• ELx800 Service Manual (PN 7331005)
ELx800 Operator's Manual
Page 32

6 | Chapter 1: Introduction
Specifications
Standard Model
Wavelength Range:
Filters:
• The following specifications apply to 96-well, flat- or round-bottom plates,
single-wavelength measurements with a 50-second read (normal read
mode).
Absorbance Measurement
Range:
Accuracy: ± 1.0% ± 0.010 OD from 0.000 to 2.000 OD
Linearity: ± 1.0% from 0.000 to 2.000 OD at 405 nm
Repeatability (STD): ± 0.5% ± 0.005 OD from 0.000 to 2.000 OD @ 405 nm
400 to 750 nm
10 nm half-bandwidth interference filters.
User-accessible filter wheel. Up to 5 filters may be
installed on the instrument at one time.
Filters supplied: 405 nm, 450 nm, 490 nm and 630 nm.
0.000 to 3.000 OD
@ 405 nm
± 3.0% from 2.000 OD to 3.000 OD @ 450 nm
• The following specifications apply to 96-well, flat- or round-bottom plates,
single-wavelength measurements with a 30-second read (rapid read mode).
Absorbance Measurement
Range:
Accuracy: ± 2.0% ± 0.020 OD from 0.000 to 2.000 OD @ 405 nm
Linearity: ± 2.0% from 0.000 to 2.000 OD @ 405 nm
Repeatability (STD): ± 1.0% ± 0.010 OD from 0.000 to 2.000 OD @ 405 nm
• Light Source: Tungsten gas-filled bulb
• Dimensions: 42 cm x 38 cm x 18 cm (16.5" deep x 15" wide x 7" tall)
• Weight: 8 kg (18.5 lb. Maximum)
• Environment: Operating temperature 18° to 40°C
• Humidity: 10% to 85% noncondensing
• Power Supply: Input 100 to 240 V~ ± 10.0% @ 50 to 60 Hz
0.000 to 3.000 OD
Output +24 VDC, 2.1 A
BioTek Instruments, Inc.
Page 33

Ultraviolet/UV Model
The following specifications apply to 96-well, flat- or round-bottom plates.
Specifications | 7
Wavelength Range:
Filters:
340 to 750 nm
10 nm half-bandwidth interference filters.
User-accessible filter wheel. Up to 5 filters may
be installed on the instrument at one time.
Filters supplied: 340 nm, 405 nm, 450 nm,
490 nm and 630 nm.
• Optical specifications for the 400 to 750 nm range (50-second read in normal read
mode):
Absorbance Measurement
Range:
0.000 to 3.000 OD
Accuracy: ± 1.0% ± 0.010 OD from 0.000 to 2.000 OD @ 405 nm
Linearity: ± 1.0% from 0.000 to 2.000 OD @ 405 nm
± 3.0% from 2.000 to 3.000 OD @ 450 nm
Repeatability (STD): ± 0.5% ± 0.005 OD from 0.000 to 2.000 OD @ 405 nm
• Optical specifications for the 340 to 400 nm range (50-second read in normal read
mode):
Absorbance Measurement
Range:
0.000 to 2.000 OD
Accuracy: ± 2.0% ± 0.010 OD from 0.000 to 2.000 OD
@ 340 nm
Linearity: ± 2.5% from 0.000 to 2.000 OD @ 340 nm
Repeatability (STD): ± 1.5% ± 0.005 OD from 0.000 to 2.000 OD
@ 340 nm
• Optical specifications for the 400 to 750 nm range (30-second read in rapid read
mode):
Absorbance Measurement
Range:
0.000 to 3.000 OD
Accuracy: ± 2.0% ± 0.020 OD from 0.000 to 2.000 OD
@ 405 nm
Linearity: ± 2.0% from 0.000 to 2.000 OD @ 405 nm
Repeatability (STD): ± 1.0% ± 0.010 OD from 0.000 to 2.000 OD
@ 405 nm
ELx800 Operator's Manual
Page 34

8 | Chapter 1: Introduction
• Optical specifications for the 340 to 400 nm range (30-second read):
Absorbance Measurement
Range:
Accuracy: ± 2.5% ± 0.020 OD from 0.000 to 2.000 OD
Linearity: ± 2.5% from 0.000 to 2.000 OD @ 340 nm
Repeatability (STD): ± 2.0% ± 0.010 OD from 0.000 to 2.000 OD
Narrow Beam/NB Model
Filters:
The following specifications apply to 96-well, flat- or round-bottom plates with a
50-second read (normal read mode).
0.000 to 2.000 OD
@ 340 nm
@ 340 nm
10 nm half-bandwidth interference filters.
User-accessible filter wheel. Up to 5 filters may
be installed on the instrument at one time.
Filters supplied: 405 nm, 450 nm, 490 nm and
630 nm.
Absorbance Measurement
Range:
0.000 to 3.000 OD
Accuracy: ± 1.0% ± 0.010 OD from 0.000 to 2.000 OD
@ 405 nm
Linearity: ± 1.0% from 0.000 to 2.000 OD @ 405 nm
± 3.0% from 2.000 to 3.000 OD @ 450 nm
Repeatability (STD): ± 0.5% ± 0.005 OD from 0.000 to 2.000 OD
@ 405 nm
The following specifications apply to 96-well, flat- or round-bottom plates with a
30-second read (rapid read mode).
Absorbance Measurement
Range:
0.000 to 3.000 OD
Accuracy: ± 2.0% ± 0.020 OD from 0.000 to 2.000 OD
@ 405 nm
Linearity: ± 2.0% from 0.000 to 2.000 OD @ 405 nm
Repeatability (STD): ± 1.0% ± 0.010 OD from 0.000 to 2.000 OD
@ 405 nm
BioTek Instruments, Inc.
Page 35

Specifications | 9
The following specifications apply to a 384-well plate. The minimum volume of
fluid in a well to obtain the specified performance is 80 microliters. The
specifications apply to the dual wavelength mode of read only.
Absorbance Measurement
Range:
Normal Read Mode:
0.000 to 3.000 OD
Dual wavelength (4 minutes, 45 seconds)
Accuracy: ± 2.0% ± 0.020 OD from 0.000 to 2.000 OD
@ 405 nm
Linearity: ± 2.5% from 0.000 to 2.000 OD @ 405 nm
Repeatability (STD): ± 1.5% ± 0.010 OD from 0.000 to 2.000 OD
@ 405 nm
Rapid Read Mode:
Dual wavelength (3 minutes, 25 seconds)
Accuracy: ± 2.5% ± 0.020 OD from 0.000 to 2.000 OD
@ 405 nm
Linearity: ± 2.5% from 0.000 to 2.000 OD @ 405 nm
Repeatability (STD): ± 2.0% ± 0.010 OD from 0.000 to 2.000 OD
@ 405 nm
ELx800 Operator's Manual
Page 36

10 | Chapter 1: Introduction
Product Support & Service
A superior support staff backs all of BioTek’s products. If your instrument(s) or software
ever fails to function perfectly, if you have questions about how to use or maintain it, or if
you need to send an instrument to BioTek for service or repair, please contact our
Technical Assistance Center (TAC).
Contacting the Technical Assistance Center
Our Technical Assistance Center is open from 8:30 AM to 5:30 PM (EST), Monday
through Friday, excluding standard U.S. holidays. You can send a fax or an e-mail
any time.
Phone: 800-242-4685 (in the U.S.) or 802-655-4740 (outside the U.S.)
Fax: 802-655-3399
E-Mail: tac@biotek.com
Please be prepared to provide the following information:
• Your name and company information
• A daytime phone or fax number, and/or an e-mail address
• The product name, model, and serial number
• The software part number and basecode version (available via the
keyboard by selecting
UTIL TESTS CHKSUM)
• For troubleshooting assistance or instruments needing repair, the specific
steps that produce your problem and any error codes displayed (see also
Chapter 6, Troubleshooting and Error Codes)
Returning Instruments for Service/Repair
If you need to return an instrument to BioTek for service or repair, please
contact the TAC for a Return Materials Authorization (RMA) number before
shipping the instrument. Repackage the instrument properly (see
Installation), write the RMA number on the shipping box, and ship to this
address:
BioTek Instruments, Inc.
Technical Assistance Center
100 Tigan Street
Highland Park
Winooski, Vermont 05404 USA
Chapter 2,
BioTek Instruments, Inc.
Page 37

Chapter 2
Installation
This chapter includes instructions for unpacking and setting up the
ELx800™ and instructions for connecting printers and/or serial
devices.
Unpacking and Inspecting the ELx800™ ..................................... 12
Unpacking the Instrument and Its Accessories........................ 13
Setting Up the ELx800............................................................. 17
Operating Environment ....................................................... 17
Electrical Connections ......................................................... 17
Power-Up and System Test .................................................. 18
ELx800 Main Menu ............................................................. 18
Configuring Global Default Options........................................ 19
Connecting a Printer to the ELx800 ....................................... 20
Setting Up the Serial Port for Communications With Other Devices 22
Attaching the Cable ............................................................ 22
Installing Software on the Host PC ........................................ 22
Setting Communication Parameters ...................................... 22
Installing Additional Filters ....................................................... 24
Checking the Reader’s Filter Table Setting.............................. 26
Repackaging and Shipping the ELx800 ....................................... 28
Before Repackaging the Instrument ...................................... 28
Repackaging the ELx800 and Its Accessories .......................... 29
Preparing the Shipping Container.......................................... 35
Page 38

12 | Chapter 2: Installation
Unpacking and Inspecting the ELx800™
Important! Save all packaging materials. If the ELx800™ is
The ELx800 and its accessories are securely packaged inside custom-designed shipping
materials. This packaging should protect the instrument from damage during shipping.
Inspect the shipping box, packaging, instrument, and accessories for signs of damage.
shipped to BioTek for repair or replacement, it must be carefully
repackaged, according to the instructions on pages 28 through 35
using the original packing materials (PN 7332062). The individual
packaging materials contained in PN 7332062 are listed in the
table below. Using other forms of commercially available packing
materials, or failure to follow the repackaging instructions may
void your warranty. If the original packing materials have been
damaged, replacements are available from BioTek.
If the reader is damaged, notify the carrier and your manufacturer's representative. Keep
the shipping cartons and packing material for the carrier's inspection. The manufacturer
will arrange for repair or replacement of your instrument immediately.
Refer to the unpacking instructions and
when removing the instrument and its accessories from the shipping container.
See
Repackaging and Shipping the ELx800 at the end of this chapter for complete
shipping instructions.
Packing Materials PN
Shipping container 7332036
Shelf for power supply 7332063
Left foam end cap 7332060
Right foam end cap 7332061
2-Mil poly bag (ELx800) 98085
8 ½” x 11” bubble bag (power supply) 91083
Required Tools PN
Figures 1 through 4 on the following pages
Slotted and Phillips-head screwdrivers 98145
BioTek Instruments, Inc.
Page 39

Unpacking and Inspecting the ELx800™ | 13
Unpacking the Instrument and Its Accessories
1. Carefully open the top of the box, and remove the power supply shelf
(
Figure 1).
2. Remove the manual, Declaration of Conformity, and other accessories.
Power supply
Shelf
ELx800 Operator’s Manual
Figure 1: Removing the power supply and shelf
Page 40

14 | Chapter 2: Installation
3. Lift the reader out of the box and remove the left and right foam end caps
(
Figure 2). Place the reader on a level surface and remove the instrument from
the 2-Mil plastic bag.
Left end cap
Unpacking
instructions
Unit inside 26 x 32
2-Mil poly bag
Right
end cap
Figure 2: Removing the foam end caps
BioTek Instruments, Inc.
Page 41

Unpacking and Inspecting the ELx800™ | 15
4. Carefully turn the ELx800 upside down on a level surface.
5. Use the slotted screwdriver to remove the four mounting screws from the top
cover (
Figure 3) and unhook the shipping straps from the post on the bottom
of the instrument.
6. Set aside the four screws (you will use them again in step 9).
Post
Figure 3: Removing the top cover mounting screws
Shipping straps
Top cover
ELx800 Operator’s Manual
Page 42

16 | Chapter 2: Installation
7. Turn the instrument right side up.
8. Use a Phillips screwdriver to remove the two mounting screws in the shipping
block (
Figure 4). Store the screws and shipping block with the packaging
materials.
Shipping block
Shipping
straps
Figure 4: Removing the shipping block
9. Replace the top cover: turn the instrument upside down and use the slotted
screwdriver to replace the cover’s four mounting screws.
10. Remove the shipping straps from the top of the carrier and store them with the
packaging materials.
11. Turn the instrument right side up.
BioTek Instruments, Inc.
Page 43

Setting Up the ELx800
Operating Environment
The ELx800™ is designed to operate optimally when installed on a level surface in an
area where ambient temperatures remain between 18°C (64°F) and 40°C (104°F). The
reader is sensitive to extreme environmental conditions, and these conditions should
be avoided:
•
Excessive humidity: Condensation directly on the sensitive electronic
circuitry can cause the instrument to fail internal self-checks.
•
Excessive ambient light: Bright sunlight or strong incandescent light can
reduce the linear performance range of the instrument.
•
Dust: Optical density readings may be affected by extraneous particles (such
as dust) in the microplate wells. A clean work area is necessary to ensure
accurate readings.
Setting Up the ELx800 | 17
Electrical Connections
Caution! Power Supply. Only use the specified power supply to
ensure proper operation of the unit. The ELx800 has a universal
24-VDC, 2.1 A power supply that functions from 100 to 240 V~
(± 10.0%) @ 50 to 60 Hz without external switching.
Never use a two-prong plug adapter to connect primary power to
the ELx800 power supply. Use of a two-prong adapter disconnects
the utility ground, creating a severe shock hazard. Always connect
the power supply cord directly to a three-prong receptacle with a
functional ground.
1. Connect the power cord to the external power supply.
2. Plug the rounded end of the power supply line cord into the power supply
jack on the rear of the instrument.
3. Tighten the knurled nut on the power supply outlet to ensure that the plug
does not pull out.
4. Plug the 3-prong end of the power cord into an appropriate power
receptacle.
Warning! Power Rating. The ELx800 power supply must be
connected to a power receptacle that provides voltage and current
ELx800 Operator’s Manual
within the specified rating for the system. Use of an incompatible
power receptacle may produce electrical shock and fire hazards.
Page 44

18 | Chapter 2: Installation
Power-Up and System Test
After you have installed the ELx800™ and connected the power supply, turn on
the instrument to run a system test. The on/off switch is located on the lower right
side of the base.
The
System Test begins with a check of the stepper motors and the analog power
supplies, to ensure that they have a proper input voltage level. The data flash
checksum, motor axis, and analog offset are then verified. The photodetector’s
dark current, noise, and gain are checked to ensure they fall within specific
pass/fail criteria.
If an error is detected, the reader will “chirp” and display an error code. See
Chapter 6 for a list of error codes. If no errors are detected, the reader will briefly
display SYSTEM TEST PASS.
The power-up system test does not produce a printed results report. To run the
test manually and obtain a printout of the system test values, start at the Main
Menu and press UTIL TESTS SYSTEM. See
in Chapter 4, or Connecting a Printer to the ELx800 on page 20 for more
Test
information.
System Test and Checksum
ELx800 Main Menu
Following successful power-up of the ELx800, the Main Menu appears:
R E A D Y 01:22PM 01/23 / 0 4
R E A D D EFINE REPORT U T I L
• The Main Menu permits access to all onboard functions. See Main Menu in
Chapter 3 for more information, including a diagram showing the flow of
functionality (
• The ELx800 front panel contains four circular buttons, referred to in this
manual as
each selectable option in the display. To select a menu option, simply press
its corresponding
additional instructions.
Figure 15).
“SOFT KEYS.” One SOFT KEY is positioned directly below
SOFT KEY. See ELx800 Front Panel in Chapter 3 for
BioTek Instruments, Inc.
Page 45

Setting Up the ELx800 | 19
R
P
P
E
Configuring Global Default Options
The ELx800™ contains several global configurable options, such as date and time, report
output, and plate reading preferences. These options are accessed via the Select Utility
Option menu (
SETUP: Set the current date and time, as well as the date and time formats.
•
•
OUTPUT: Specify where plate data should be sent, to a printer, a computer, or to
both. Additional options include report format (Column and/or Matrix), and
whether or not to print standard curves.
•
READ: Enable or disable read-time prompting for Plate ID, Sample ID, and Sample
Count. Specify whether or not to read in Rapid mode.
Figure 5 below), and include:
READY 09:14AM 09/08/04
READ DEFINE REPORT UTIL
SELECT UTILITY OPTION:
TESTS SETUP OUTPUT READ
ROMPT FOR PLATE ID? YES
YES NO
EPORT OUTPUT: PRINT
RINT COMPUTER BOTH
DIT SETUP INFORMATION:
DATE TIME FILTER *MORE
Figure 5: Global configuration options available under UTIL
Note: Refer to Using the Utility Options in Chapter 3 for more
specific information on setting up the ELx800.
ELx800 Operator’s Manual
Page 46

20 | Chapter 2: Installation
Connecting a Printer to the ELx800
Connect the printer to the ELx800™ only if you are running the
The ELx800 has a parallel port (LPT1) to allow connection to Epson-compatible
printers or HP Deskjet
Options
on the next page. The parallel port requires a 25-pin D-sub connector. A parallel
cable (PN 71072) designed to connect the reader to a printer is provided with the
ELx800.
instrument in standalone mode. If you are using BioTek’s Gen5™,
KCjunior™, or KC4™ software, skip this step, and go to page 22,
Setting Up the Serial Port for Communications With Other
Devices.
™ printers. (See Specifying Data Output and Reporting
in Chapter 3 for more information.) The port is illustrated in Figure 6,
To attach a printer to the ELx800:
1. Turn the reader off.
2. Place the printer in a location adjacent to the ELx800.
3. Attach one end of the cable to the parallel port on the printer.
4. Attach the other end of the cable to the parallel port on the ELx800.
5. Tighten the securing screws on both ends of the cable.
6. Turn on the reader, and then turn on the printer.
To avoid system instability, be sure to connect the printer to the
Note: For the latest list of compatible printers, consult the BioTek
Web site (www.biotek.com), or call BioTek Instruments' Technical
Assistance Center (refer to Chapter 1 for contact information).
reader before powering up the reader.
BioTek Instruments, Inc.
Page 47

Setting Up the ELx800 | 21
Serial port
Parallel port
(printer port)
Power supply jack
Figure 6: Connectors for printer (parallel), computer (serial), and power supply
ELx800 Operator’s Manual
Page 48

22 | Chapter 2: Installation
Setting Up the Serial Port for Communications With Other Devices
Before serial communication can be initiated between the ELx800™ and another device
(such as a host PC running BioTek’s Gen5™, KCjunior™ or KC4™ software), the
communication parameters must match between the devices.
The ELx800 has a 25-pin serial (RS-232) port located on the rear panel of the
instrument. The serial port allows the reader to communicate with a computer, using
standard communications software and/or RS-232 protocols.
information on required protocols for computer control of the reader.
The serial port also allows field upgrades of the ELx800 software.
Attaching the Cable
1. Power down the computer and the ELx800.
Appendix A contains
2. Connect the appropriate serial cable to both machines. The serial port on
the reader is a DTE configuration with a 25-pin (pin-male) D-sub connector.
3. Power up the reader and the computer.
4. Ensure that the ELx800 and the computer are operating with the same
communications settings.
Installing Software on the Host PC
Refer to the Gen5 Getting Started Guide or Help system, or to the KC4 or KCjunior
User’s Guides for complete instructions for installation of the software. Refer also
to
Appendix A for basic software installation and configuration instructions.
Setting Communication Parameters
The reader’s default communication parameters are:
• 9600 Baud Rate
• 8 Data Bits
• 2 Stop Bits
• No Parity
The baud rate can be changed to 1200 or 2400 bps, if necessary.
The Data Bits, Stop Bits, and Parity settings cannot be changed.
BioTek Instruments, Inc.
Page 49

Setting Up the Serial Port for Communications With Other Devices | 23
To change the baud rate from the instrument keypad:
1. At the Main Menu, press UTIL.
R E A D Y 12:45PM 01/23 / 0 4
R E A D D EFINE REPORT U T I L
2. At the SELECT UTILITY OPTION screen, press SETUP.
S E L E C T UTILITY OPTION :
T E S T S S ETUP OUTPUT RE A D
3. At the EDIT SETUP INFORMATION screen, press *MORE, and then RS232
to continue.
E D I T S E TUP INFORMATIO N
D A T E TIME FILTER *M O R E
E D I T S E TUP INFORMATIO N ?
R S 2 3 2 CALPLATE *M O R E
4. The SELECT BAUD RATE screen will appear, showing the currently
defined Baud Rate:
S E L E C T BAUD RATE: 960 0
1 2 0 0 2400 9600 VI E W
• Select the desired baud rate.
• Select VIEW, if you wish, to see the reader’s other communication
settings.
R S 2 3 2 S ETTING: NO PAR I T Y
2 S T O P - BITS 8 DATA-B I T S
To change the baud rate (or other communications settings) in KC4™ or
KCjunior™ software, refer to the KC4 or KCjunior User Guides, or to
A, Computer Control
9600 and cannot be changed.
ELx800 Operator’s Manual
Appendix
. In Gen5™ software, the baud rate for the ELx800 is set at
Page 50

24 | Chapter 2: Installation
Installing Additional Filters
Installed in the internal, five-position filter wheel are the filters that come standard
with the ELx800™ (standard models have 405, 450, 490, 630 nm filters; the UV model’s
filter set is 405, 450, 490, 630 and 340 nm).
Important! Keep track of all filter locations. The physical
location of the filters must match the filter locations mapped in
the reader’s software filter table. The filter wheel must have no
empty locations; all locations must be filled with either a filter or
a blank plug. Install all filters with the arrow denoting the light
direction pointing downward.
Filter wheel cover
Thumbscrew
Filter
Optics arm
Filter wheel
Figure 7: Filter wheel assembly
BioTek Instruments, Inc.
Page 51

Installing Additional Filters | 25
If you wish to install additional filters, or change the filter locations:
1. Turn off the unit and disconnect the power cord.
2. Tape down the carrier in the home (full out) position to prevent movement
while the instrument is being handled.
3. Carefully turn the instrument upside down on a level surface.
4. Using a slotted screwdriver, remove the four screws from the bottom of the
instrument (see
5. Hold on to both the instrument base and the top case to prevent the
detachment of the case from the body of the instrument.
6. Carefully turn the instrument right side up and remove the top cover.
With the cover off, the instrument’s mechanics and optics are exposed. The
filter wheel is housed within the small black box (filter wheel cover) directly
under the end of the optics arm (
7. Remove the four thumbscrews around the perimeter of the cover. The filter
wheel sits on a pin and can be lifted off.
Figure 3 on page 15).
Figure 7 on the previous page).
8. Remove the filters by turning the wheel upside down over a cloth. The filters,
which are labeled with the wavelength and light direction arrow, should easily
slide out.
9. When replacing the filters in the wheel, handle them from the edges. Do not
touch the glass portion of the filter. Clean any filters that appear dirty with lens
paper and isopropyl alcohol.
10. Once the filters are installed in the filter wheel, place the wheel back on the pin
in the base of the instrument, making sure the filter wheel is sitting flat and
that it meshes with the filter wheel drive gear.
11. Install the filter wheel cover with the four thumbscrews.
Note: The cover can only be installed one way.
12. Place the top cover on the base, and carefully turn the instrument upside
down.
13. Reinstall the 4 screws with washers to hold the top cover in place.
14. Connect the power supply and cable to the rear of the instrument.
Important! Store unused filters in a cool, dry place away
from direct sunlight. The filters can be wrapped in a piece
of lens paper to protect them from scratches and dust
accumulation.
ELx800 Operator’s Manual
Page 52

26 | Chapter 2: Installation
Checking the Reader’s Filter Table Setting
After installing new filters, ensure that the ELx800’s filter table (the reader’s
software reference for filter locations) matches the physical location of the filters.
To check or change the software filter table:
1 Power up the reader. At the Main Menu Screen, press UTIL to display
the SELECT UTILITY OPTION menu.
R E A D Y 9:45AM 01/31 / 0 3
R E A D D EFINE REPORT U T I L
S E L E C T UTILITY OPTION :
T E S T S S ETUP OUTPUT RE A D
2 From the SELECT UTILITY OPTION menu, press SETUP. The Edit
Setup Information screen appears on the display.
E D I T S E TUP INFORMATIO N :
D A T E TIME FILTER *M O R E
3 From this menu, press FILTER.
4 The wavelength for Filter #1 will be displayed. To advance to Filter 2,
press ENTER.
5 To change the filter wavelength number, use the NUMERIC keypad to
enter a number at the cursor location. The cursor will automatically
advance to the next editable field. Press ENTER to save the entry and
move to the next filter on the filter table.
BioTek Instruments, Inc.
Page 53

Installing Additional Filters | 27
E N T E R
F I L T E R # 1 WAVELENGTH:4 0 5
When the last filter has been entered, the software exits the filter
routine, and displays the following screen:
E D I T S E TUP INFORMATIO N :
D A T E TIME F ILTER *M O R E
6 Press the Main Menu key to return to the main menu.
ELx800 Operator’s Manual
Page 54

28 | Chapter 2: Installation
Repackaging and Shipping the ELx800
IMPORTANT! Failure to properly repackage the reader increases
If you need to ship the ELx800™ Reader to BioTek for service or repair, be sure to use
the original packing. Other forms of commercially available packing are not
recommended and can
If the original packing materials have been damaged or lost, contact BioTek for
replacement packing (see
information).
the likelihood of damage to the instrument during shipping. The
shipping system was designed to stabilize the reader’s mechanical
mechanisms, which would otherwise be free to move around
during shipping.
void the warranty.
Product Support & Service in Chapter 1 for contact
Warning! If the reader has been exposed to potentially
hazardous material, decontaminate it to minimize the risk to
all who come in contact with the reader during shipping,
handling, and servicing.
Decontamination prior to shipping is required by U.S.
Department of Transportation regulations.
Before Repackaging the Instrument
1. Decontaminate the reader before repackaging it. (See Chapter 5,
Maintenance and Decontamination
procedure.)
2. Once the reader is clean, follow the instructions on the next few pages to
repackage the instrument.
, for the Decontamination
BioTek Instruments, Inc.
Page 55

Repackaging and Shipping the ELx800 | 29
r
Repackaging the ELx800 and Its Accessories
Refer to Figures 8 through 13 when repackaging the ELx800™.
1. Move the carrier to the home position.
2. Turn off the instrument and unplug the power supply.
3. Wrap the shipping straps around the carrier (Figure 8).
Carrie
Shipping
straps
Figure 8: Securing the carrier
ELx800 Operator’s Manual
Page 56

30 | Chapter 2: Installation
4. While holding the carrier, carefully turn the instrument over and hook the
shipping straps over the post (
5. Using a slotted screwdriver, remove the four top cover mounting screws.
6. Lift the instrument off the cover and turn it over.
Mounting screws
Figure 9).
Shipping
straps
Figure 9: Hooking the shipping straps over the post
Post
Top cover
BioTek Instruments, Inc.
Page 57

Repackaging and Shipping the ELx800 | 31
7. Install the shipping block: place the groove in the shipping block over the
shaft and use a Phillips screwdriver to attach the two mounting screws
(
Figure 10).
Shipping
block
Shaft
Figure 10: Installing the shipping block
ELx800 Operator’s Manual
Page 58

32 | Chapter 2: Installation
8. Turn the instrument over and install the top cover that was removed in
step 6 (
Figure 11).
Mounting screws
Top cover
Figure 11: Reinstalling the top cover
BioTek Instruments, Inc.
Page 59

Repackaging and Shipping the ELx800 | 33
9. Turn the instrument right side up, place it in the 2-Mil plastic bag, then
attach the left and right end caps (
Figure 12).
Left end cap
26 x 32 2-Mil
poly bag
Right
end cap
10. Place the instrument with the end caps into the box (Figure 13).
ELx800 Operator’s Manual
Figure 12: Reattaching the end caps
Page 60

34 | Chapter 2: Installation
11. Place the shelf into the notched-out area in the end cap, and the power
supply into an 8” x 11 ½” bubble bag. Place the power supply on the shelf.
(
Figure 13).
Shelf
Power supply
Figure 13: Placing the unit in the box
BioTek Instruments, Inc.
Page 61

Preparing the Shipping Container
1. Obtain a Return Materials Authorization (RMA) number from BioTek’s
Technical Assistance Center through BioTek’s Web site, fax, or e-mail
address listed in
2. When obtaining the RMA, explain whether the reader requires calibration,
cleaning, periodic maintenance, warranty work, and/or repair. Make a note
of any error messages displayed and their frequency.
3. Provide BioTek with the name and contact information of a person who
may be contacted if questions arise.
4. Close the box and tape it shut.
5. Write “RMA” and the RMA number in large, clear letters on the outside of
the shipping container, and ship the instrument to the BioTek address
provided in the
Chapter 1.
Product Support & Service section of Chapter 1.
Repackaging and Shipping the ELx800 | 35
ELx800 Operator’s Manual
Page 62

36 | Chapter 2: Installation
BioTek Instruments, Inc.
Page 63

Chapter 3
Operation
This chapter includes instructions for operating the ELx800™ and its
software.
ELx800 Front Panel ................................................................. 38
Overview ............................................................................... 40
Recommendations for Achieving Optimum Performance................ 40
System Startup ...................................................................... 41
Main Menu ............................................................................. 42
Define ................................................................................... 44
Define (Method, Map, Formula and Curve).................................. 47
Defining METHOD............................................................... 47
Defining MAP ..................................................................... 51
Defining FORMULA.............................................................. 67
Defining CURVE ................................................................. 79
Reading a Microplate ............................................................... 86
Selecting an Assay to Run ................................................... 87
Printing Reports...................................................................... 91
Editing Standard Outliers..................................................... 92
Printing Results.................................................................. 94
Using the Utility Options .......................................................... 95
Setting the Date and Time................................................... 95
Viewing/Editing the Filter Table ............................................ 96
Specifying Data Output and Reporting Options........................ 97
Selecting Read Options ....................................................... 99
Page 64

38 | Chapter 3: Operation
ELx800 Front Panel
READY 9:45AM 09/12/04
READ DEFINE REPORT UTIL
7
4
1
0
8
G
D
A
H
56
E
23
B
.
CLEAR
Main
Main
Menu
Menu
STOP
9
F
C
Shift
Figure 14: Keyboard
Options
Options
ENTER
Previous
Previous
Screen
Screen
READ
BioTek Instruments, Inc.
Page 65

ELx800 Front Panel | 39
The keypad has four SOFT KEYS, one below each selectable menu
option. Press a
Main Menu, press the leftmost
SOFT KEY to make a selection. For example, from the
SOFT KEY to select READ, the
rightmost to select UTIL.
Exit the current screen and return to the
Main
Menu
Menu while defining or modifying an assay automatically saves the
Main Menu. Pressing Main
current settings.
Cycle through available options within a screen. For example, press
Options
Options within the Select Assay Number screen to cycle through
the names of the onboard assays.
ENTER
Pressing
ENTER generally saves the current screen settings and
advances to the next screen in a series.
Previous
Screen
Pressing
Previous Screen generally saves the current screen settings
and returns control to the screen most previously viewed.
Press
CLEAR
CLEAR to reset a numeric value to 0, or to clear all characters
when editing an assay name.
Generation
screen to “clear” a previously defined manual map.
Tip: Press Shift + Clear at the Map
Move the cursor to the left in data-entry screens.
Move the cursor to the right in data-entry screens.
READ
Initiate a plate read.
STOP
7G
Shift + 7G
Halt the read currently in progress.
Press a number to enter it in a data-entry screen.
Press Shift + a number (for example, Shift + 7G) to enter the
letter, then press
ELx800 Operator’s Manual
Options to advance through the alphabet.
Page 66

40 | Chapter 3: Operation
Overview
IMPORTANT! Do not turn on the instrument until the
The ELx800™ features a 25-pad keypad and a 2-line x 24-character LCD display, allowing
you to access the reader’s program menus and print test results. The reader’s bidirectional
serial port allows computer control of the instrument, and provides the means for
downloading additional assay definition files to the instrument. This chapter describes the
operation of the
open (configurable) assays onboard the ELx800.
carrier shipping straps and shipping block have been
removed.
Recommendations for Achieving Optimum Performance
Microplates should be perfectly clean and free of dust or bottom scratches. Use new
microplates from sealed packages. Do not allow dust to settle on the surface of the
solution; use microplate covers when not reading the plate. Filter solutions to remove
particulates that could cause erroneous readings.
Although the ELx800 supports standard flat, U-bottom, and V-bottom microplates,
optimum performance is achieved with optically clear, flat-bottomed wells.
Non-uniformity in the optical density of the well bottoms can cause loss of accuracy,
especially with U- and V-bottom polyvinyl microplates. Check for this by reading an
empty microplate. Dual-wavelength readings can eliminate this problem, or bring the
variation in density readings to within acceptable limits for most measurements.
Inaccuracy in pipetting has a large effect on measurements, especially if smaller volumes
of liquid are used. For best results, use at least 100 μl per well in a 96-well plate and 25 μl
in a 384-well plate.
Dispensing solution into 384-well plates often traps air bubbles in the wells, which may
result in inaccurate readings. A dual-wavelength reading method usually eliminates these
inaccuracies; however, for best results, remove the air bubbles by degassing the plate in a
vacuum chamber before reading.
The inclination of the meniscus can reduce reading accuracy in some solutions, especially
with small volumes. Agitate the microplate before reading to help bring this problem
within acceptable limits. Use Tween
normalize the meniscus. Some solutions develop menisci over a period of several minutes.
This effect varies with the brand of microplate and the solution composition. As the center
of the meniscus drops and shortens the light path, the density readings change. The
meniscus shape will stabilize over time.
®
20, if possible (or some other wetting agent) to
BioTek Instruments, Inc.
Page 67

System Startup | 41
s
System Startup
To turn on the ELx800™, press the on/off switch on the right side of the reader’s base. The
ELx800 will perform a System Test, displaying the screens shown below until initialization
is complete. During this period, all keys are inactive.
If the instrument fails the System Test, a chirp will sound, and an error code will display.
• Refer to
information.
• Refer to
and
Technical Assistance Center (TAC).
System Self-Test and Checksum Test in Chapter 4 for more
Chapter 6, Troubleshooting and Error Codes to interpret error codes,
Chapter 1, Introduction for information on contacting BioTek Instruments’
P o we r u p Sequence Vx.x x
I n it i a l izing...
B i o - T e k I n
S y st e m Self-Test
IMPORTANT! Some ELx800 readers have custom
programmed software installed. Not all features of the
software discussed in this Operator’s Manual are available
on custom instruments.
truments
Please contact BioTek’s Technical Assistance Center at 800-242-4685 if you have any
questions about the software on your reader.
ELx800 Operator’s Manual
Page 68

42 | Chapter 3: Operation
S
T
P
R
Main Menu
Following successful power-up of the ELx800™, the Main Menu appears:
R E A D Y 9:45AM 01/31 / 0 3
R E A D D EFINE REPORT U T I L
The Main Menu permits access to all reader functions (
•
READ option (or, press the key labeled READ on the keyboard) for plate-
reading prompts. You will be asked to select from a list of preprogrammed
assays.
•
DEFINE option: Allows the creation of a reading and data reduction protocol.
You will be prompted to select an assay from an assay definition list.
Figure 15):
•
REPORT option: Allows printing of results and protocol descriptions. You will
be prompted for the name of a previously run assay with valid data.
•
UTIL option: You will be prompted to select options from the list of onboard
utilities.
READY 09:14AM 09/08/04
READ DEFINE REPORT UTIL
ELECT UTILITY OPTION:
ESTS SETUP OUTPUT READ
RINT REPORT:
ESULT MAP ASSAY LIST
SELECT ASSAY NUMBER: 53
NAME: 53_Bordetella M
SELECT ASSAY NUMBER: 53
NAME: 53_Bordetella M
Figure 15: Options available from the Main Menu
BioTek Instruments, Inc.
Page 69

Main Menu | 43
The Quick Read assay default DEFINE settings are shown below, and cannot be edited,
except where noted.
Method
• Single Wavelength 405 nm (editable)
Map
• 96-well plate geometry
Important: On some Absorbance Microplate Readers,
Assay 01 has been designed to allow for quick and
simple programming. It appears as “_Quick Read” on
the display. Most of the options available in Assays
2-55, and described in this section, are unavailable for
programming within Quick Read. You can quickly
access the Quick Read assay by selecting READ from
the main menu.
• Blank on Air
• Automap
• Map starting location A1
• Samples only (no blanks, standards or controls)
• Sample count prompted at runtime (can be turned off in UTIL|READ options)
ELx800 Operator’s Manual
Page 70

44 | Chapter 3: Operation
Define
The Main Menu option DEFINE allows you to define the data acquisition and reduction
parameters for a new assay, or modify previously defined assays stored in memory.
1. Start at the Main Menu and select DEFINE to display the SELECT ASSAY
NUMBER screen.
S E L E C T ASSAY NUMBER:0 1
N A M E : H B S -A G 1
2. Select an assay to define or modify, and then press ENTER. See Selecting an
Assay to Define
screen will appear.
If you are modifying/selecting a PANEL assay (#99), see page 83 for
instructions.
on page 46 for detailed instructions. The EDIT ASSAY NAME
N A M E : H B S -A G 1
- / : SP A C E
3. (Optional) Edit the assay name, and then press ENTER. See Editing the Assay
Name
on page 46 for detailed instructions. The DEFINE menu will appear:
D E F I N E :
M E T H O D MAP FORMULA CU R V E
The following options are available within the DEFINE menu:
METHOD: Define the wavelength type (single or dual), wavelength(s), and
•
plate geometry (page 47).
•
MAP: Specify the plate layout, using blanks, controls, standards, and/or
samples. Choose to map the plate manually, or let the software map it
automatically (page 51).
•
FORMULA: Define cutoff, transformation, and validation formulas. Create
variables to be used within formulas (page 67).
•
CURVE: Specify a curve fit type and x/y axis types (lin/log). Specify whether
or not standard outliers can be edited, and then the method by which they will
be edited. Enable or disable the extrapolation feature (page 79).
BioTek Instruments, Inc.
Page 71

Programming Note
Assays with certain criteria may “lock up” during the “Calculating Results…”
phase of data reduction (see
corrected by modifying assay criteria as follows:
Define | 45
Beginning the Plate Read on page 90). This can be
• Specify
at least one (01) sample when mapping the plate, and/or
• Change PROMPT SAMPLE COUNT? to YES.
• Set CURVE FIT to NONE if you do not have a standard curve as part of the
assay.
1 From the Main Menu, press DEFINE MAP, and continue to press
ENTER until ENTER NUMBER OF SAMPLES is displayed. Enter at least
one sample.
The software will lock up if zero (00) samples are selected.
E N T E R N UMBER OF
S A M P L E S : 0 1
2 From the Main Menu, press UTIL READ, and continue to press
ENTER until PROMPT SAMPLE COUNT? is displayed. Select YES.
P R O M P T SAMPLE COUNT? Y E S
Y E S NO
3 From the Main Menu, press DEFINE SELECT ASSAY NUMBER:
ENTER. At the DEFINE: menu, press CURVE to display CURVE-FIT
TYPE: and select NONE.
C U R V E - F IT TYPE: NONE
N O N E L INEAR QUAD *M O R E
ELx800 Operator’s Manual
Page 72

46 | Chapter 3: Operation
Selecting an Assay to Define
To select an assay to define or modify, start at the Main Menu and select DEFINE
to display the SELECT ASSAY NUMBER screen.
S E L E C T ASSAY NUMBER:0 1
N A M E : H B S -A G 1
1. Use the numeric keys to enter the number of any predefined Assay
Definition Files stored in the reader’s memory, or the
advance one assay at a time. The cursor is positioned at the first editable
field, and advances automatically. The numeric range depends on the
number of assays stored in the reader’s memory.
The ELx800 has 55 “open” assays available; the EL800 has only 10.
2. Press ENTER to advance to the EDIT ASSAY NAME screen. You may
change the default assay name to a more descriptive one (see
Assay Name
below):
Options key to
Editing the
CLEAR: Clears the reader’s display.
MAIN MENU: Returns the display to the Main Menu screen.
PREVIOUS SCREEN: Returns the display to the previous screen.
ENTER: Saves the current settings and advances to the next screen.
Editing the Assay Name
Use the EDIT NAME screen to edit the name currently assigned to the assay. The
assay name can contain up to 16 alphanumeric characters.
N A M E : HBS-AG1
- / : SP A C E
• The cursor is positioned at the first editable field (e.g., under “H”). Use the
alpha and numeric keys to change the assay name.
• Use the
above the cursor. The characters will cycle through the alphabet (A-Z), with
a space following Z.
• Use the
next editable field. The cursor will wrap around the edit field.
Options key to sequentially advance the character positioned
left and right arrow keys to move the cursor to the previous or
• Use the
• Use
CLEAR key to remove the assay name from the display.
SOFT KEYS 1, 2, 3, and 4 to select a dash, forward slash, colon, or
space for inclusion in the assay name.
BioTek Instruments, Inc.
Page 73

Define (Method, Map, Formula and Curve) | 47
Define (Method, Map, Formula and Curve)
The DEFINE screen allows you to edit the Method, Map, Formula, or Curve Fit
parameters for the currently selected assay.
D E F I N E :
M E T H O D MAP FORMULA CU R V E
Press the
• METHOD: Specify the wavelength type, wavelength, filter(s), and plate
• MAP: Specify mapping information.
• FORMULA: Access the formula entry screens.
• CURVE FIT: Specify curve-fit options.
SOFT KEY beneath the displayed option to access the following functions:
geometry.
Defining METHOD
Defining a method includes selecting:
• Single or dual wavelength
• Filter(s)
• Plate geometry
The options appear on the display in the order that they
were programmed in the assay. If the assay contains a closed
variable (i.e., an element of the assay definition that you
cannot access or modify), the entry screen is skipped.
ELx800 Operator’s Manual
Page 74

48 | Chapter 3: Operation
Single or Dual Wavelength
W A V E L E N GTH: DUAL
S I N G L E DUAL
1. Select SINGLE or DUAL wavelength.
2. Press ENTER to continue.
The WAVELENGTH selection screen allows you to select SINGLE or DUAL
wavelength for the assay.
If SINGLE wavelength is chosen, the reader measures the optical density of each
well with a single filter.
If DUAL wavelength is chosen, each well is read twice, each time with a different
filter. The microplate is not removed from the reading chamber between the two
measurements. The final reported optical density is the difference between the two
readings (the delta OD). Dual-wavelength readings can significantly reduce optical
interference caused by scratched or fingerprinted microplates, since the scratches
or fingerprints reduce the amount of light on both wavelengths.
The currently selected wavelength appears on the top line of the display, and the
available options appear on the bottom.
MEAS Selection
The MEAS selection screen allows you to select the filter(s) for the assay.
M E A S : 4 5 0 REF:630
4 0 5 4 5 0 4 9 0 6 3 0
1. Select the wavelength.
2. Use the right arrow key to move the cursor to REF and then select the
Reference Filter.
3. Press ENTER to move to the next screen.
BioTek Instruments, Inc.
Page 75

Define (Method, Map, Formula and Curve) | 49
Plate Type
For 6- to 384-well standard plates, the plate types and sizes included in the
software onboard the ELx800™ are based on the brands listed below. For best
measurement results, use these brands when operating the ELx800 via its onboard
software.
Plate Type Brand
6-well Costar
12-well Corning
24-well Corning
48-well Costar
96-well Costar
384-well
96 T
72 T
60 T
*Used only on ELx800NB version
Nunc square
Terasaki
Terasaki
Terasaki
*
*
*
*
The PLATE TYPE selection screen allows you to select the geometry of the plate
that will be used for the assay. Press *MORE to cycle through the available options.
Note: The NB version of the reader includes choices for 384-well, or
72- and 96-well Terasaki plate formats. An adapter (PN 7330531) is
available from BioTek if Terasaki plates are to be used with the NB
reader.
P L A T E T YPE: 96 WELLS
2 4 4 8 9 6 3 8 4
P L A T E T YPE: 96 WE L L S
6 1 2 2 4 * M O R E
ELx800 Operator’s Manual
Page 76

50 | Chapter 3: Operation
P L A T E T YPE: 96 WE L L S
4 8 9 6 9 6 H * M O R E
P L A T E T YPE: 96 WE L L S
9 6 M * M O R E
Press *MORE to cycle through the available options.
6: 6-well (2 x 3) 96: 96-well (8 x 12)
12: 12-well (3 x 4) 96H: 96-well Hellma Quartz (8 x 12)
24: 24-well (4 x 6) 96M: 96-well Metric (8 x 12, 9 mm well
48: 48-well (6 x 8) spacing)
BioTek Instruments, Inc.
Page 77

Defining MAP
The MAP GENERATION screen allows you to edit or specify the following options
in the assay:
Define (Method, Map, Formula and Curve) | 51
• Automatic or manual map generation
• Mapping direction
• Replication direction
• Blank Map Selection
• Blanking constant
• Number of Blanks
• Location of Blanks
• Number of Standards
• Number of Standard Replicates
• Averaging of Standards
• Concentration and Location of Standards
• Number of Controls
• Control Type Definition
• Number of Control Replicates
• Control Location
• Number of Samples
• Number of Sample Replicates
• Sample Location
Important! The NB reader using 384-well geometry does
not have access to map, formula, or curve options. The
384-well plate type is preset for the map to have 384
samples. This does not include any blanking, controls, or
standards. (Selecting 6-, 12-, 24-, 48-, or 96-well geometries
enables data reduction capabilities.)
ELx800 Operator’s Manual
Page 78

52 | Chapter 3: Operation
MAP screens appear in the order that they were defined in the assay. If the
assay has a closed variable, the screen for this variable is omitted.
D E F I N E :
M E T H O D MAP FORMULA CU R V E
At the DEFINE options screen, select MAP to begin the plate map process.
Map Generation
“Map Generation” represents the method by which blanks, controls, standards,
and/or samples are assigned to specific locations on the plate.
M A P G E N ERATION: MANUA L
A U T O M ANUAL
• Select AUTOMATIC PLATE MAP GENERATION to instruct the software
to automatically generate a plate map after the blanks, controls,
standards, and/or samples have been defined.
• Select MANUAL PLATE MAP GENERATION to indicate that the well
assignments will be performed manually (by the user) at Define and/or
Read time.
• Press
ENTER to save the selection and continue.
Use the SHIFT-CLEAR keys to clear any previously defined
manual map.
BioTek Instruments, Inc.
Page 79

Define (Method, Map, Formula and Curve) | 53
Mapping Direction
This option allows you to specify how the blank, control, standard, or sample
groups will be mapped on the plate. The well types can be listed in column
format (down) or in row format (across). The currently selected Mapping
Direction appears on the top line of the display, and the available options
appear on the bottom.
M A P P I N G DIRECTION:DOW N
D O W N A CROSS
• Select DOWN to map down the column.
• Select ACROSS to map across the row.
• Press
ENTER to save the selection and continue.
Replication Direction
This option allows you to specify how replicates are mapped on the plate.
The currently selected Replication Direction appears on the top line of the
display, and the available options appear on the bottom.
R E P D I R ECTION:ACROSS
D O W N A CROSS
• Select DOWN to map the replicates down the column, following the
direction of the map listing.
• Select ACROSS to map the replicates across (in a paired format). As an
example, two replicates can be placed in A1 and A2 wells. The third
replicate would follow in B1. The next standard control, or sample,
would follow in B2.
• Press ENTER to save the selection and continue.
Examples of mapping directions are shown on the next page.
ELx800 Operator’s Manual
Page 80

54 | Chapter 3: Operation
Examples of Mapping Directions
Map Direction DOWN, Rep Direction DOWN:
1 2 3 4 5 6 7 8 9 10 11 12
A STD1 STD5 SMP
B STD1 STD5 SMP
C STD2 PC SMP
D STD2 PC
E STD3 NC
F STD3 NC
G STD4 SMP
H STD4 SMP
Map Direction ACROSS, Rep Direction ACROSS:
1 2 3 4 5 6 7 8 9 10 11 12
A STD1 STD1 STD2 STD2 STD3 STD3 STD4 STD4 STD5 STD5 PC PC
B NC NC SMP SMP SMP SMP SMP SMP
C
D
E
F
G
H
Map Direction DOWN, Rep Direction ACROSS:
1 2 3 4 5 6 7 8 9 10 11 12
A STD1 STD1
B STD2 STD2
C STD3 STD3
D STD4 STD4
E STD5 STD5
F PC PC
G NC NC
H SMP SMP
Map Direction ACROSS, Rep Direction DOWN:
1 2 3 4 5 6 7 8 9 10 11 12
A STD1 STD2 STD3 STD4 STD5 PC NC SMP SMP
B STD1 STD2 STD3 STD4 STD5 PC NC SMP SMP
C
D
E
F
G
H
BioTek Instruments, Inc.
Page 81

Define (Method, Map, Formula and Curve) | 55
Start Mapping at Well Location
The Start Mapping at Well Location screen is only shown if automatic mapping
is selected. This option allows you to enter the location of the well that will be
the starting point for automatic mapping.
S T A R T M APPING
A T W E L L LOCATION:A01
• Use the
cursor location. For any well location, only the
the first character and
numeric and alpha keys to enter a letter or number at the
alpha keys are active for
numeric for the second and third characters. The
valid entry range is from A01 to the last well on the plate, depending on
the plate type and the number of blanks, standards, controls, and/or
samples defined in the assay.
• Press
ENTER to save the well location and continue.
ELx800 Operator’s Manual
Page 82

56 | Chapter 3: Operation
Blank Map
This option allows you to select which blanking method to apply to the assay.
The blanking options, AIR, FULL and CONSTANT; ROW and COLUMN; and
P-ACROSS and P-DOWN are displayed on three screens.
B L A N K M A P : F U L L
A I R FULL CONST *M O R E
B L A N K M A P : F U L L
R O W C OLUMN *M O R E
B L A N K M A P : F U L L
P - A C R OSS P-DOWN * M O R E
• Select the BLANK MAP type (see the descriptions on the next page).
• Press *MORE to cycle through the available options: ROW or COLUMN, and
P-ACROSS or P-DOWN.
• Press
ENTER to save the well location and continue.
BioTek Instruments, Inc.
Page 83

Define (Method, Map, Formula and Curve) | 57
Blank Map Definitions
AIR performs an initial reading on “air” just prior to the plate read,
and uses that value as the blank value. This value is subtracted from
each well on the plate.
FULL enables a single blank well or an average of blank wells to be
subtracted from the whole plate.
CONST (Constant) allows entry of a user-specified absorbance value.
This value will be subtracted from each well on the plate.
Tip for using CONST: Use a blank value from the first plate, or a
blanking plate, to save space on subsequent assay plates.
ROW enables a single blank well or an average of blank wells to be
selected for each row. The maximum number of blanks is 48. The
blank (or average) will be subtracted from each well in the row. Use
manual mapping to position blanks, controls, standards, and
samples.
COLUMN enables a single blank well or an average of blank wells to
be placed in each column. Since the maximum number of blanks is
12, and if all 12 columns are used, each column can have only one
blank. Manual mapping is recommended in this case. Replicates
follow in the same column as the first well of each sample, even if
the ACROSS direction has been specified for replicates.
P-ACROSS enables a blank in every even-numbered column to be
subtracted from the well to the left of it in every odd column.
Manual mapping is recommended to set up the appropriate map by
placing the standards, controls, and samples in only the odd
columns.
P-DOWN enables a blank in the B, D, F and H rows to be subtracted
from the well above in the A, C, E and G rows. Manual mapping is
recommended to set up the appropriate map by placing the
standards, controls, and samples in only the A, C, E, and G rows.
ELx800 Operator’s Manual
Page 84

58 | Chapter 3: Operation
Constant Blank Value
This entry screen only appears when a CONSTANT BLANK map is selected.
Enter the value to be subtracted from each well on the plate.
E N T E R
B L A N K I N G CONSTANT: 1. 2 0 0
• Use the numeric keys to enter the value. The range is 0.000 to 3.000.
The cursor is positioned at the first editable field and advances
automatically.
• Press
• Press
CLEAR to clear the value on the display.
ENTER to continue.
Number of Blanks
The NUMBER OF BLANKS field allows you to enter the number of blank wells
in the assay. This entry screen is only displayed when Full, Column, or Row
blank maps
E N T E R N UMBER OF
B L A N K S : 0 2
• Use the
• Use the
is selected. Any previously defined value is displayed.
numeric keys to enter the number of blanks. The range is 0 to
48.
CLEAR key to clear the NUMBER OF BLANKS value from the
display.
• Press
ENTER to continue.
BioTek Instruments, Inc.
Page 85

Define (Method, Map, Formula and Curve) | 59
Blank Location
The BLANK LOCATION screen allows you to define where the blank well or
wells occur on the microplate. This screen only appears if
was selected.
E N T E R T HE LOCATION OF
B L A N K # 1 : A 1 2
manual mapping
• Use the
numeric and alpha keys to enter a Blank Location, based upon
the plate geometry.
• Use the arrow keys to move the cursor to the next or previous editable
field. The cursor is positioned beneath the first editable field.
• Press
ENTER to continue.
Number of Standards
This option allows you to enter the number of standard groups that will be
used in the assay. Any previously defined value will be displayed on the
screen.
Note: If the number of standards is altered, the number of
replicates for the standard automatically reverts to 1.
E N T E R N UMBER OF
S T A N D A R D S : 0 2
• Use the
range depends on the selected curve fit method. The maximum number
of standards is 12. The minimum is 4 for 4-P fit, cubic, cubic spline, and
logit-log; 3 for quadratic; and 2 for linear and point-to-point.
• Press
• Press
ELx800 Operator’s Manual
numeric keys to enter the NUMBER OF STANDARDS. The valid
CLEAR to clear the value on the display.
ENTER to continue.
Page 86

60 | Chapter 3: Operation
O
Number of Standard Replicates
This option allows you to enter the number of replicates per standard group in
the assay. Any predefined value appears on the display.
E N T E R N UMBER
F
S T A N D A R D REPLICATES: 0 2
• Use the numeric keys to enter the NUMBER OF STANDARD
REPLICATES. The range is 1 to 8 replicates. The software will verify
that the number of replicates, multiplied by the number of standards,
does not exceed the number of wells on the plate.
• Press
• Press
CLEAR to clear the value on the display.
ENTER to continue.
Average Standards
The AVERAGE STANDARDS option allows you to select whether or not to
average the replicates of each standard group. This average is used to calculate
the standard curve instead of using the individual replicates of each standard.
If the number of standard replicates is 1, this option is not available.
A V E R A G E STANDARDS? YE S
Y E S N O
• Select YES to average the replicates for each standard group, and then
use the group averages when calculating the standard curve.
• Select NO to use the individual standard replicates when calculating the
standard curve.
• Press
ENTER to continue.
BioTek Instruments, Inc.
Page 87

Define (Method, Map, Formula and Curve) | 61
Standard Concentrations
The Standard Concentration field allows you to enter the predicted or expected
concentration value for each standard group. If
selected, the replicate locations must also be defined.
C O N C N O F LOCATIO N
S T D 1 : 0 R E P # 1 : A 0 1
manual mapping was
• Use the
numeric and alpha keys and the decimal point key to enter
standard concentration values. The range is 0.00001 to 999999. The entry
cannot exceed six characters including the decimal point.
• If automatic mapping is selected, each replicate’s location is available
for viewing only. Pressing
entry for the next standard.
• If manual mapping is selected, the location must be defined. Pressing
ENTER from the standard concentration entry moves the cursor to the
LOCATION field. Pressing
the concentration value entry for the next standard.
Valid Well Locations
When defining the replicate locations, only the alpha keys are active for
the first character and
characters and numeric entries are based on the selected plate geometry.
The following table lists acceptable entries for well locations based on plate
geometry:
ENTER advances to the concentration value
ENTER from the LOCATION field advances to
numeric for the second and third characters. Valid
ELx800 Operator’s Manual
Plate Type Range
6-Well A01-B03
12-Well A01-C04
24-Well A01-D06
48-Well A01-F08
96-Well A01-H12
96H A01-H12
96M A01-H12
Page 88

62 | Chapter 3: Operation
Reuse of Standard Curves
The ELx800™ has the ability to reuse a standard curve that has already been
established.
Limitations of the Reuse of Standard Curves
Standard curves can only be reused in assay positions 31 through
55. Each of these positions can only store one standard curve.
Standard curves cannot be reused on panels (see page 83 for Panel
Definition).
Standard curves will be stored in memory with the Assay Name,
Standard Concentrations, Replicate Counts, and Optical Density
values for each standard replicate.
Stored standard curves can only be reused for the assay that the
curve was originally generated on (e.g., the curve for Assay 53
cannot be applied to samples on a plate to be run in Assay 51).
To reuse a standard curve, you must first program an assay (in
positions 31 through 55) and then run the assay. During the defining
process, you will be prompted to enter the number of standards, the
number of standard replicates, and the standard concentrations. The
following screen will appear after these prompts:
R E U S E S TANDARD CURVE? Y E S
Y E S N O
After the assay has been run, the results have been calculated, and the reports
have been generated, the reader will prompt if this standard curve should be
stored in memory. The following display will appear:
S A V E S T ANDARD CURVE? Y E S
Y E S N O
Select YES to store the curve for use at a later time. The next time a plate is to
be read using this assay, the instrument will prompt if there are standards on
the plate. Select NO to discard the curve.
BioTek Instruments, Inc.
Page 89

Define (Method, Map, Formula and Curve) | 63
S T A N D A R DS ON PLATE ? N O
Y E S N O
If YES is chosen, a new standard curve will be generated. The plate map is not
changed. (If “Prompt for Sample ID” is enabled in the UTIL section, you will be
prompted to enter the number of samples. See
Using the Utility Options in
this chapter for more information on the UTIL options.)
If NO is chosen, the stored standard curve will be used. If
Auto mapping had
been used to originally map the standards, blanks, controls and samples
defined for this assay, the map will be automatically regenerated without the
standards, beginning in well xxx (where xxx was chosen as the starting well in
the map, usually well A01). If
the map is
not regenerated – the reader will not produce results for the well
manual mapping was used to map the plate,
positions that originally were standards. Auto mapping is recommended, if
the standard’s curves will be routinely reused.
Number of Controls
The NUMBER OF CONTROLS screen allows you to enter the number of control
groups that will be used in the assay. Any previously defined value will
appear on the display.
E N T E R N UMBER OF
C O N T R O L S : 0 2
• Use the
the assay. For example, if the assay requires one or more positive
control wells and one or more negative control wells, enter 02.
• The valid entry range depends on the number of locations on the plate
that are undefined. The maximum number of control groups is 8.
ELx800 Operator’s Manual
numeric keys to enter the NUMBER OF CONTROLS groups in
Page 90

64 | Chapter 3: Operation
O
Control Type
This option allows you to enter the type of control used in the assay. Any
previously defined Control Type will be displayed on the screen.
C O N T R O L # 1 : P C
P C N C H P C * M O R E
C O N T R O L # 1 : P C
L P C CTL1 CTL2 *M O R E
C O N T R O L # 1 : P C
C T L 3 CTL4 *M O R E
• Choose one control identifier for each type of control in your assay. The
available options are:
Control, Low Positive Control, CTL1, CTL2, CTL3, CTL4.
• After choosing an identifier for CONTROL# 1, press
Positive Control, Negative Control, High Positive
ENTER to choose
the identifier for the next control.
Number of Control Replicates
The NUMBER OF CONTROL REPLICATES entry screen is displayed if the
number of control groups is greater than 0.
E N T E R N UMBER
R E P L I C A T E S O F P C: 0 2
• The well ID associated with CONTROL# 1 appears first. Press
advance to the next control.
• Use the
numeric keys to enter a value for Number of [Control]
Replicates.
F
ENTER to
• The valid entry range is from 1 to 12 replicates. The software
automatically performs a check to ensure the number of replicates,
multiplied by the number of controls, does not exceed the number of
undefined wells remaining on the plate.
BioTek Instruments, Inc.
Page 91

Define (Method, Map, Formula and Curve) | 65
Location of Controls
Use this option to enter the location of controls in the assay.
C O N T R O L #1 LOCATI O N
T Y P E : P C REP#1: A 0 2
The displayed location field can only be edited if manual
mapping was selected (see page 52). This screen is not
accessible in auto map.
• Use the numeric and alpha keys to enter the well location for Rep #1
of Sample Group #1. Press
ENTER to advance to the next replicate or
sample group.
Number of Samples
The number of sample groups on the plate can be defined here, and/or it can
be defined at run-time if UTIL READ PROMPT FOR SAMPLE COUNT?
is set to YES. See
Selecting Read Options on page 99 for more information.
E N T E R N UMBER OF
S A M P L E S : 2 4
• Use the numeric keys to enter the number of sample groups on the
plate.
• The range is 0 to the number of undefined well locations remaining on
the plate. For example, if there are no controls, blanks, or standards
defined on a 96-well plate, the maximum number of samples is 96, and
the minimum number of samples is 1.
ELx800 Operator’s Manual
Page 92

66 | Chapter 3: Operation
Number of Sample Replicates
After the number of sample groups is entered, the NUMBER OF SAMPLE
REPLICATES entry screen is presented.
E N T E R N UMBER OF
S A M P L E REPLICATES: 0 2
• Use the
• The valid range is from 1 to 12 replicates. The software automatically
performs a check to ensure that the number of replicates multiplied by
the number of samples does not exceed the number of undefined wells
remaining on the plate.
Sample Location
If MANUAL MAP GENERATION is selected and samples are defined, the
locations for each sample replicate must be specified.
S A M P L E #1 LOCATIO N
REP# 1: C 0 2
• Use the
of Sample Group #1. Press
sample group.
numeric keys to enter the number of sample replicates.
numeric and alpha keys to enter the well location for Rep #1
ENTER to advance to the next replicate or
BioTek Instruments, Inc.
Page 93

Defining FORMULA
The ELx800™ supports three types of formulas (Cutoff, Transformation, and
Validation), as well as the ability to program variables for use within formulas. Up
to three types of Validation formulas may be defined (Blank, Control, and Assay
Validation).
Formula definition is not available in the EL800 version.
To define formulas:
1. Start at the Main Menu and select DEFINE.
2. Select the assay then press ENTER to display the DEFINE options screen.
D E F I N E :
M E T H O D MAP FORMULA CU R V E
Define (Method, Map, Formula and Curve) | 67
3. Select FORMULA. The SELECT FORMULA TYPE screen will appear
(described on the next page).
Calculation Structure
During data reduction, formulas are processed in the order shown below. The
number of permitted formulas of each type is shown as well.
• Blank Validation 0-1
• Control Validation 0-4
• Assay Validation 0-4
• Transformations 0-1
• Cutoff Formulas 0-1
• Curve-Fit Analysis (if a curve-fit method is defined)
To capture and manipulate the raw data using 384-well
microplates with the ELx800NB, you must use Gen5™, KC4™,
or KCjunior™. Gen5, KC4, and KCjunior are PC-based software
programs you can use to set up your assay, communicate with
the ELx800 to run the assay, and then manipulate the raw data
that is automatically retrieved from the reader. Refer to
Appendix B for additional information.
ELx800 Operator’s Manual
Page 94

68 | Chapter 3: Operation
Formula Type
The ELx800™ supports three types of formulas, as well as the ability to define
variables for use within Transformation formulas.
Note: GENERAL formulas are not used.
S E L E C T FORMULA TYPE:
C U T O F F TRANS VAL *M O R E
S E L E C T FORMULA TYPE:
G E N E R A L T R A N S - V A R * M O R E
• CUTOFF formulas are used to classify results. During data reduction,
results are evaluated against the cutoff formulas, and each well is
assigned a user-specified label (POS, NEG, or EQUIV).
•
TRANSformation formulas are applied to the raw data in preparation
for further data reduction and/or curve fit calculation.
•
VALidation formulas can be used to determine whether or not blanks
and/or controls are valid. In addition, Assay Validation formulas can
be used to determine whether or not the entire assay should be
considered valid.
• The
TRANS-VAR option allows you to define a variable to be used in
transformation formulas.
BioTek Instruments, Inc.
Page 95

Define (Method, Map, Formula and Curve) | 69
Formula Entry
After the formula type is selected, the FORMULA ENTRY screen appears. Each
formula can contain a maximum of 24 characters. Spaces are not necessary.
Note: In formulas, “OD” is used to represent the optical density
value.
F O R M U L A # 1 :
M A T H O THER MAP FUN C T N
P C ; X > 1 . 2 0 0
M A T H O THER MAP FUN C T N
• After a moment, the FORMULA #1: prompt disappears, and the formula
can be entered. Use the options found under MATH, OTHER, MAP, and
FUNCTN to “build” the formula.
To cycle through the available MATH, OTHER, MAP, or FUNCTION
options, continue to press the appropriate
press the MATH
When the desired option appears, press the
select it and advance to the next editable field.
Press the
Press
When a formula is complete, press
• Select
• Select OTHER to insert an opening “ (” or closing “) ” parenthesis, or
logical operators AND or OR.
• Select MAP to insert a well ID such as BLK;x or NC;1.
• Select FUNCTN to insert a mathematical function such as LOG or SQRT.
CLEAR to delete the item above the cursor.
MATH to insert a mathematical symbol such as +, %, or <=.
The reader software checks the formulas for errors
during data reduction. A syntax error in a formula will
result in a “Token Error” on results reports.
SOFT KEY several times to see +, -, *, /, %, =, etc.
left arrow key to move the cursor to the left.
ENTER to continue.
SOFT KEY. For example,
right arrow key to
ELx800 Operator’s Manual
Page 96

70 | Chapter 3: Operation
MATH
The following mathematical symbols can be used in formulas:
+
Addition
==
Equal to
-
*
/
%
Subtraction
Multiplication
Division
Percent
>
>=
<
<=
Greater than
Greater than or equal to
Less than
Less than or equal to
OTHER
The following additional symbols can be used in formulas:
(
)
AND
OR
Left parenthesis
Right parenthesis
Logical AND
Logical OR
MAP
The available MAP options depend on the formula type and the current plate map.
MAP options resemble BLK;x (mean of the blank wells), NC;1 (the first NC well),
or OD (every well).
FUNCTION
The following functions can be used in formulas:
LOG10 Log Base 10 ALOG Anti Log
ALOG10 Anti Log Base 10 LOG Log
AB Absolute Value SQRT Square Root
PWR Power
EXAMPLES
LOG10- Log Base 10-
ALOG- Anti Log- ALOG (0.69314718) = 2
Log10 2 = 0.301029995
ALOG10- Anti Log Base 10-
LOG- Log- LOG 2 = 0.69314718
ALOG10 (0.30102995) = 2
AB- Absolute Value- AB (-1) = 1 SQRT- Square Root- SQRT 2 = 1.4142
PWR- Power- (10 PWR 2) = 100
BioTek Instruments, Inc.
Page 97

Define (Method, Map, Formula and Curve) | 71
Validation Formulas
Validation formulas can be used to determine whether or not blanks
and/or controls are valid. In addition, Assay Validation formulas can be
used to determine whether or not the entire assay should be considered
valid.
See
Formula Type on page 68 for instructions on selecting an assay and
accessing the SELECT VALIDATION TYPE screen.
S E L E C T VALIDATION TYP E :
C O N T R O L ASSAY BL A N K
Control and Blank Validation Formulas
Blank Validation is used to ensure that the OD values for the blank
replicates, or for the blank mean, meet certain criteria. Control Validation
serves the same purpose as Blank Validation, but it applies to the control
replicates or control mean. If the criteria are not met, results are considered
suspect, and the message “RESULTS INVALID! BLANK (OR CONTROL)
VALIDATION FAILED” appears on results reports.
One blank validation formula can be defined.
Up to four control validation formulas can be defined.
Define the plate map (via DEFINE MAP) before creating blank or
control validation formulas.
Blank/Control validation can be performed on individual replicates
(BLK, PC), or on the group mean (BLK;x, NC;x).
Examples
If an assay protocol states that
Each blank well on a plate should have an OD of less than 0.050, the
formula is: BLK < 0.050
Each Positive Control replicate must have an OD higher than 1.000,
but less than 2.500, this can be accomplished with one formula:
PC>1.000ANDPC<2.500
Or with two separate formulas:
PC > 1.000 and PC < 2.500
Negative Control mean must have an OD of less than 0.100, the
formula is: NC;x < 0.100
ELx800 Operator’s Manual
Page 98

72 | Chapter 3: Operation
Number of Required Controls/Blanks
If a control or blank validation formula is defined, enter the number of
valid controls or blanks that must meet the criteria established by that
formula.
P C : N U M B E R O F V A L I D
R E P L I C A TES REQUIRED: 0 2
Use the numeric keys to enter the NUMBER OF REQUIRED
CONTROLS. The range is 1 through the number of defined replicates
of a control or blank.
Press the CLEAR key to clear the displayed value.
Press ENTER to save the displayed value and advance to the next
screen, or use the
the menu structure.
Previous Screen key to move backward through
Assay Validation Formulas
Assay Validation formula(s) establish a set of criteria used to determine
whether or not an assay can be considered valid. If the criteria are not met,
results are considered suspect, and the message “RESULTS INVALID!
Assay validation failed” appears on results reports.
Up to 4 assay validation formulas can be defined.
Define the plate map (via DEFINE MAP) before creating assay
validation formulas.
Examples
If an assay protocol states that for the assay to be valid:
The mean of the negative controls must be less than 0.100. The
formula is: NC;x<0.100
The mean of the positive controls must be greater than the mean of
the negative controls. The formula is: PC;x>NC;x
BioTek Instruments, Inc.
Page 99

Define (Method, Map, Formula and Curve) | 73
Transformation Formulas
Transformation formulas can be used to transform raw or blanked absorbance
data in preparation for further data reduction, including curve-fit analysis.
See
Formula Type on page 68 for instructions on selecting an assay and
accessing the Transformation Formula definition screen.
• If a blanking method is selected in the assay, transformation formulas
are applied to the blanked absorbance values; otherwise, they are
applied to the raw data. Turn to page 67 to review the results
calculation structure.
• One transformation formula may be defined per assay.
• A transformation formula can be simple (ex. (OD/2)*100 to multiply
all wells on the plate by 100), or more complex with the inclusion of a
predefined Transformation Variable (see TVAR, below).
Simple Transformation Formulas
“Simple” transformation formulas are typically applied to all wells on the
plate. For example:
To divide the OD in each well on the plate by 2 and then multiply
by 100, the formula is: (OD/2)*100
Transformation Variable (TVAR)
For more complex transformations, a Transformation Variable (TVAR) can
be defined for use within a transformation formula. This variable defines
the scope of the transformation: whether to apply the transformation to all
of the wells on the plate (OD), or just to the sample wells (SMP).
S C O P E V ARIABLE: O D
S M P OD
ELx800 Operator’s Manual
Page 100

74 | Chapter 3: Operation
If SMP is chosen:
- The transformation formula will be applied to the
sample wells
only.
- SMP and any other well identifiers (BLK, PC, NC, STD, etc.)
defined will become available as MAP options when building the
transformation formula.
Example:
The assay plate map contains 2 NC wells and 2 PC wells. The
remainder of the map is filled with samples.
The assay data reduction requires that the mean of the NC be
subtracted from all the samples on the plate.
The transformation formula is: SMP-NC;x
If OD is chosen:
- The formula definition screen will appear so that you can define a
formula for use within the transformation formula.
- Use the formula keys (Math, Other, Map and Function) to define
the Transformation Variable (TVAR). Once the variable has been
defined, it can be used in a transformation formula. The TVAR
will be available as a MAP option when building the
transformation formula.
Example:
The assay plate map has 2 blanks, 1 control well in duplicate
(CTL1), 1 negative control well in triplicate (NC), and 5 standards
in duplicate (STD1-STD5).
The assay data reduction states:
- Subtract the mean of CTL1 from the mean of the NC. Subtract the
difference from all ODs on the plate.
- Divide the result of the above by the mean of the NC less the mean
of CTL1, and then multiply by 100.
BioTek Instruments, Inc.
 Loading...
Loading...