Page 1

Sub-Cell®Model 96 and
Model 192
Agarose Gel
Electrophor esis Systems
Instruction Manual
Catalog Numbers
170-4500 through 170-4511
For Technical Service Call Your Local Bio-Rad Office or in the U.S. Call 1-800-4BIORAD (1-800-424-6723)
Page 2

Warranty
Bio-Rad Laboratories warrants the Sub-Cell Model 96 and Model 192 electrophoresis systems against
defects in materials and workmanship for 1 year. If any defects occur in the instrument during this
warranty period, Bio-Rad Laboratories will repair or replace the defective parts free. The following
defects, however, are specifically excluded:
1. Defects caused by improper operation.
2. Repair or modification done by anyone other than Bio-Rad Laboratories or an authorized
agent.
3. Use of fittings or other spare parts supplied by anyone other than Bio-Rad Laboratories.
4. Damage caused by accident or misuse.
5. Damage caused by disaster.
6. Corrosion due to use of improper solvent or sample.
This warranty does not apply to parts listed below:
1. Platinum Electrode Wires
For any inquiry or request for repair service, contact Bio-Rad Laboratories after confirming the
model and serial number of your instrument.
To insure the best performance from the Sub-Cell electrophoresis systems, become fully acquainted
with these operating instructions before use. Bio-Rad recommends that you first read these instructions
carefully. Assemble and disassemble the unit completely without casting a gel. After these preliminary steps, you should be ready to cast and run a gel.
Bio-Rad also recommends that all Sub-Cell system components and accessories be inspected for
damage, cleaned as recommended in this manual and rinsed thoroughly with distilled water before
use. Record the following for your records:
Model
Catalog No.
Date of Delivery
Warranty Period
Serial No.
Invoice No.
Purchase Order No.
Page 3

Table of Contents
Page
Warranty Information ..........................................................................Inside Front Cover
Section 1 General Information....................................................................................1
1.1 Introduction ................................................................................................................1
1.2 Safety..........................................................................................................................1
1.3 List of System Parts....................................................................................................3
1.4 Specifications .............................................................................................................5
Section 2 Operating Instructions................................................................................6
2.1 DNA Gel Preparation.................................................................................................6
2.2 Comb Set-up...............................................................................................................7
2.3 Casting Agarose Gels.................................................................................................8
2.4 Recirculation Ports...................................................................................................11
2.5 Electrophoresis.........................................................................................................12
2.6 Nucleic Acid Staining and Visualization.................................................................13
2.7 Note on Blotting.......................................................................................................14
Section 3 Gel and Electrophoresis Reagents Preparation......................................14
3.1 Electrophoresis Buffer Preparation..........................................................................14
3.2 DNA and RNA Gel Preparation ..............................................................................15
3.3 RNA Sample Preparation.........................................................................................15
3.4 DNA and RNA Sample Loading Dye .....................................................................16
3.5 Gel Staining Solution...............................................................................................16
Section 4 Care and Maintenance..............................................................................16
4.1 Cleaning Sub-Cell System Components..................................................................16
4.2 Compatible Cleaning Agents...................................................................................16
4.3 Maintenance Schedule .............................................................................................17
4.4 Electrode Replacement.............................................................................................17
4.5 RNase Decontamination ..........................................................................................18
Section 5 Troubleshooting..........................................................................................19
Section 6 Ordering Information ...............................................................................20
6.1 Sub-Cell Model 96 and 192 Systems.......................................................................20
6.2 Sub-Cell Model 96 and 192 System accessories.....................................................20
6.3 Related Bio-Rad Products........................................................................................21
Section 7 References...................................................................................................25
i
Page 4
Section 1
General Information
1.1 Introduction
The Sub-Cell instruments comprise a comprehensive and versatile gel electrophoresis
system that effectively separates nucleic acids using submerged agarose gels. Submarine
agarose gels are easy to cast and readily dissipate heat. These gels allow sample underlaying
and also prevent electrical field discontinuities caused by wicks or sample well dehydration.
Agarose gels are ideal for the separation of DNA restriction digestions, Polymerase Chain
Reaction (PCR)* amplified fragments, and genomic DNA and RNA prior to Southern or
Northern blotting. If operated correctly, agarose gels can effectively separate nucleic acids
from 20 base pairs to 20 kilobase pairs in length.
The Sub-Cell Model 96 and 192 electrophoresis cells have been created specifically for
multiple sample analysis. The width of each cell and the analytical combs were designed
based on the fixed spacing of multichannel pipets used to transfer samples from standard
microplates. Forty eight nucleic acid samples (plus three DNA size standards) can be visualized
in one row using the 51-well comb. If two combs are used, samples from all 96 wells of a
microplate can be analyzed on the Model 96. Four or more combs can be used on the
Model 192 for even higher throughput. The Model 96 can run gels 10 or 15 cm in length,
whereas the Model 192 can run gels 10, 15, 20 or 25 cm in length for the analysis of more samples or applications such as genomic DNA separations for Southern blotting.
The Sub-Cell systems give years of rigorous use. These rugged systems incorporate many
features that make casting and running agarose gels simple and efficient. The gel caster provides tape-free gel casting in trays and gels can be cast in the Sub-Cell bases using the gel
casting gates. Replaceable electrode cassettes provide a simple way to replace electrode wires.
A comprehensive assortment of tray sizes and multichannel pipet-compatible combs make
these systems ideal for most high throughput agarose gel applications. Recirculation ports
are provided to prevent heat and pH effects during high voltage or extended run electrophoresis.
Note: This manual contains instructions for the Sub-Cell Model 96 and Model 192
electrophoresis systems only. Bio-Rad supplies similar but smaller agarose gel
electrophoresis cells: the original Sub-Cell, Wide Mini-Sub Cell, and Mini-Sub Cell systems
and the Sub-Cell GT, Wide Mini-Sub Cell GT and Mini-Sub Cell GT systems. This
manual does not provide information concerning these smaller versions. Contact your local
Bio-Rad representative for information concerning the original Sub-Cell and
Sub-Cell GT systems.
* The Polymerase Chain Reaction (PCR) process is covered by patents owned by Hoffmann-LaRoche. Use of the PCR process
requires a license.
1.2 Safety
The Sub-Cell electrophoresis systems are designed for maximum user safety. The buffer
chambers are made of 1/4-inch (.635 cm) thick cast acrylic to create a leak-free
electrophoresis environment. The safety lids surround the entire buffer chamber to protect the
user from exposure to electrical currents. Sub-Cell systems were designed for indoor use only.
1
Page 5
Before every use, inspect the base for cracks or chips. Cracks or chips may cause the
buffer to leak from the base and cause a potential electrical hazard. Additionally, inspect all
electrical cables, banana jacks, recirculation port fittings, tubing, and plugs for loose
connections, cracks, breaks or corrosion. Do not use any part that is cracked, charred or
corroded. These parts may also cause a potential electrical shock. Contact your local Bio-Rad
representative before using a part that may be considered hazardous.
During electrophoresis inspect the base and workbench for any signs of buffer leakage.
If leaking buffer is detected disconnect the power to the cell immediately and contact your
Bio-Rad representative.
Power to Sub-Cell units is to be supplied by an external DC-voltage power supply. This
power supply must be ground isolated in such a way that the DC voltage output floats with
respect to ground. All Bio-Rad power supplies meet this important safety requirement. The
recommended power supply for this apparatus is the PowerPac 300. The PowerPac 300
contains several safety features such as no load, overload, rapid resistance change, and ground
leak detection capabilities. The maximum specified operating parameters* for the
Sub-Cell Model 96 and Model 192 systems are:
200 VDC Maximum voltage limit
70 Watts Maximum power limit
50 °C Maximum buffer temperature
4 °C – 40 °C Ambient temperature limits
* IEC 1010-1 certification applies to equipment designed to be safe at the operating parameters listed above. Additionally, both
Sub-Cell Model 96 and Model 192 have a maximum operating relative humidity of 80% for temperatures up to 31 °C decreasing
linearly to 50% relative humidity at 40 °C. Certification is valid when systems are operated at altitudes up to 2000 meters.
Current to the cell, provided from the external power supply, enters the unit through the
lid assembly, providing a safety interlock to the user. Current to the cell is broken when the
lid is removed. Do not attempt to circumvent this safety interlock, and always turn the power
supply off before removing the lid or when working with the cell in any way.
Important: These Bio-Rad instruments are designed and certified to meet IEC 1010-1*
safety standards. IEC-certified products are safe to use when operated in accordance with
this instruction manual. This instrument should not be modified in any way. Alteration of
this instrument will:
• Void the manufacturer’s warranty
• Void the IEC 1010-1 safety certification
• Create a potential safety hazard
Bio-Rad is not responsible for any injury or damage caused either by the use of this instrument for purposes other than for which it is intended or by modifications of the instrument not
performed by Bio-Rad or any authorized agent. No user-serviceable parts are contained in
this apparatus. To ensure electrical safety, do not attempt to service this apparatus.
* IEC 1010-1 is an internationally accepted electrical safety standard for laboratory instruments.
Definition of Symbols
Caution, risk of electrical shock Caution (refer to accompanying documents)
2
Page 6

1.3 List of System Parts
Each Sub-Cell system comes with the components listed in Table 1.1. Check your
instrument to insure all items are present. Note any damage to the unit which may have
occurred during shipping. Notify Bio-Rad Laboratories if any items are missing or damaged
(see Figure 1.1 for part descriptions, on the following page).
Table 1.1 Sub Cell System Components
Item Quantity
Base (buffer chamber) 1
Gel Casting Gates 2
Safety Lid and Cables 1
UVTP Gel Tray 1
Comb (51 well, 1.5 mm thick) 1
Comb Holder 1
Leveling Bubble 1
Gel Caster (optional) 1
Instruction Manual 1
3
Page 7
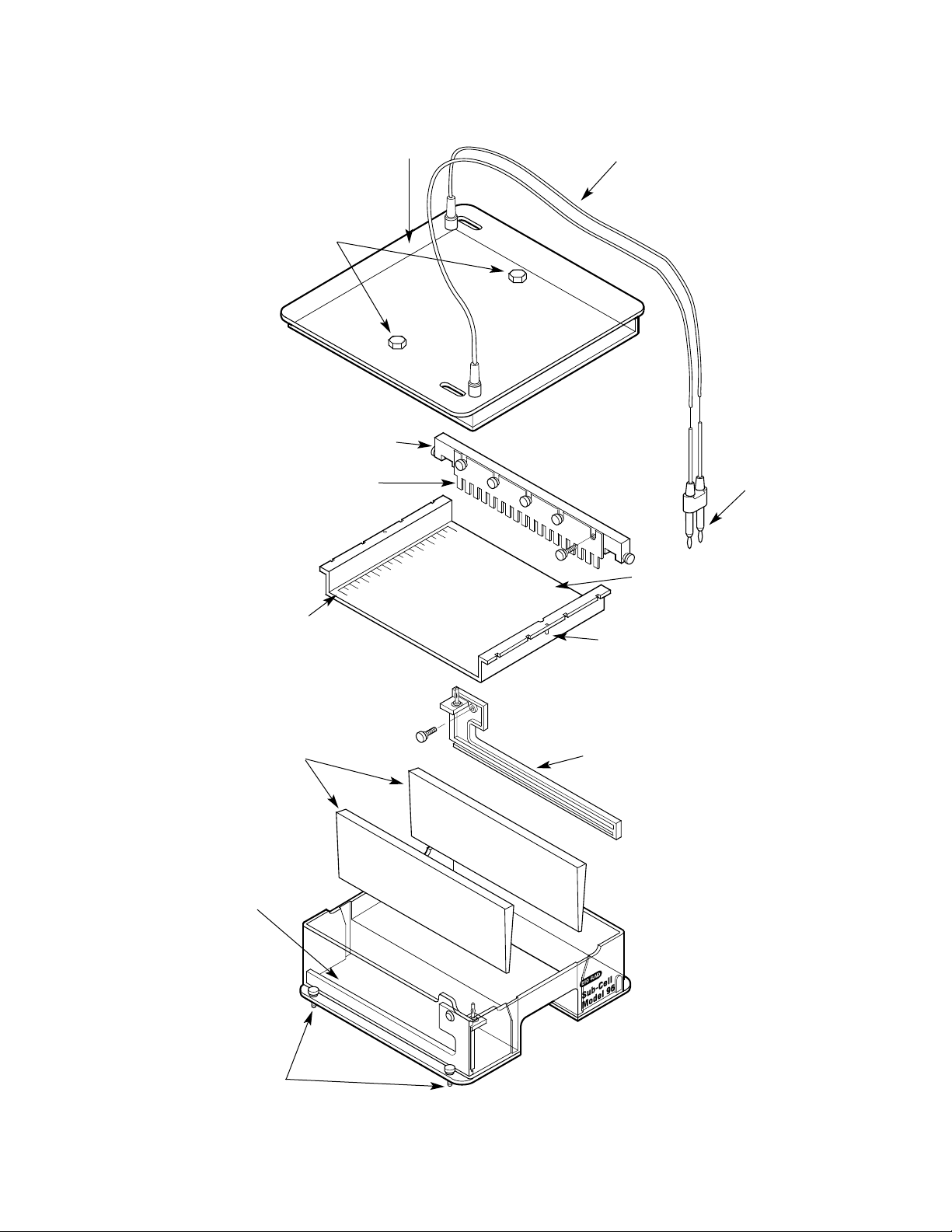
Figure 1.1 Sub-Cell Model 96 and Model 192 Parts
4
Safety Lid
Electrical
Cables
Recirculation
Port Plugs
Comb Holder
Comb
Electrical
Leads
UVTP Gel Tray
Comb Holder Slot
Fluorescent
Ruler
Banana Plug/Electrode
Wire Assembly
Gel Casting
Gates
Base
(Buffer Chamber)
Leveling
Feet
Page 8

Figure 1.2 Sub-Cell Model 96 and Model 192 Gel Caster Parts
1.4 Specifications
Sub-Cell Model 96
Base Footprint (L x W x H) 29 .5 cm x 29.0 cm x 9.0 cm
Base Buffer Volume* 2.0 L
Base Gel Size 25 x 10 cm
Gel Tray Sizes 25 x 10 cm
25 x 15 cm
Sub-Cell Model 192
Base Footprint (L x W x H) 39.5 cm x 29.0 cm x 9.0 cm
Base Buffer Volume* 3.0 L
Base Gel Size 25 x 15 cm
Gel Tray Sizes 25 x 10 cm
25 x 15 cm
25 x 20 cm
25 x 25 cm
5
Comb and
Comb Holder
UVTP Gel Tray
Gel Caster Leveling
Bubble
Leveling Feet
Movable Wall
Fixed Wall
Page 9
Contruction
Base Cast Acrylic
Gel Casting Gates Anodized Aluminum
Safety Cover Cast Acrylic
Banana Plug/Electrode Cassette Polycarbonate
Banana Plugs Gold-Plated Brass, 4.4 cm Length
Electrodes Platinum, 0.25 mm Diameter
Electrical Cables Dual, 20 AWG, Tinned Copper Wire Cable
Flame-Retardant Polyurethane Insulation jacket
Electrical Leads Nickel Silver
Gel Tray UV-Transparent Acrylic Plastic (UVTP)
Combs Machined Acrylic
Comb Holder Polycarbonate
Gel Casting Device Polycarbonate
0.64 cm Silicon Foam
* Base buffer volumes will vary depending on the size and thickness of gel used.
Section 2
Operating Instructions
Note: Refer to Section 3 for information on preparation of RNA gels. See References
1 and 2 for more information on DNA and RNA electrophoresis.
2.1 DNA Gel Preparation
DNA agarose gels can be used to separate and visualize DNA of various sizes. Before
casting an agarose gel, consult Table 2.1 to determine the appropriate percent agarose gel to
use based on the size of DNA to be separated.
Procedure
1. Determine the amount of agarose (grams) and volume needed. Use Tables 2.1 and 2.2
as a guide for agarose concentration and gel volume requirements.
Example: For a 1% agarose gel, add 1 gram of agarose to 100 ml of electrophoresis buffer.
Table 2.1 Gel Concentration Required for DNA Separation
1-2
Gel Concentration % DNA Size (Kbp)
0.50 1 – 30
0.75 0.8 – 12
1.00 0.5 – 10
1.25 0.4 – 7
1.50 0.2 – 3
2-5* 0.01 – 0.5
* Sieving agarose such as Bio-Rad AmpliSize®agarose.
6
Page 10

Table 2.2 Gel Volume Requirements
Gel Size 0.5 cm thick 0.75 cm thick 1.0 cm thick
Bases
25 x 10 cm (Model 96) 125 ml 185 ml 250 ml
25 x 15 cm (Model 192) 185 ml 280 ml 375 ml
Trays
25 x 10 cm 125 ml 185 ml 250 ml
25 x 15 cm 185 ml 280 ml 375 ml
25 x 20 cm 250 ml 375 ml 500 ml
25 x 25 cm 310 ml 465 ml 625 ml
2. Add the agarose to a suitable container (e.g., 500-ml Erlenmeyer flask, Wheaton bottle, etc.).
Add the appropriate amount of electrophoresis buffer (see Section 3) and swirl to sus-
pend the agarose powder in the buffer. If using an Erlenmeyer flask, invert a 50 ml
Erlenmeyer flask into the open end of the 500-ml Erlenmeyer flask containing the agarose.
The small flask acts as a reflux chamber, thus allowing long or vigorous boiling without
much evaporation.
Note: Place a mark on the flask at the liquid level. If evaporation occurs, water can be
added to bring the volume back to the original liquid level.
3. The agarose can be melted by boiling on a magnetic hot plate or in a microwave oven.
Caution: Always wear protective gloves, goggles, and a lab coat while preparing and
casting agarose gels. Boiling molten agarose or the vessels containing hot agarose can
cause severe burns if allowed to contact skin. Molten agarose can become super-heated
and boil over vessels when swirled which can also cause severe burns.
Magnetic Hot Plate Method
4a. Add a stir bar to the undissolved agarose solution. Heat the solution to boiling while
stirring on a magnetic hot plate. Use the appropriate size container to allow bubbles or
foam to disrupt before rising to the neck of the container.
5a. Boil the solution until all of the small translucent agarose particles are dissolved. Set aside
to cool to 50-60 °C before pouring.
Microwave Oven Method
4b. Place the gel solution into the microwave. Using a low to medium setting, set the timer for
a minimum of 5 minutes, stopping the microwave oven every 30 seconds and swirling the
container gently to suspend the undissolved agarose. This technique is the fastest and
safest way to dissolve agarose.
5b. Boil and swirl the solution until all of the small translucent agarose particles are dissolved.
Set aside to cool to 50-60 °C before pouring.
2.2 Comb Set-up
Comb and Comb Holder Set-up
The comb holder used for the Model 96 and 192 was designed to incorporate all the
necessary features required for any agarose gel application. The comb holder allows for
adjustable comb height and can be adjusted so that the comb can be placed anywhere on the
7
Page 11

base stage or UVTP tray. The following instructions describe how to manipulate the comb and
comb holder for obtaining comb height and comb holder position on a UVTP tray or base stage.
Adjusting and Setting Comb Height
1. Loosen the five thumbscrews from the front plate of the comb holder.
2. Align the slots of the well-forming comb with the thumbscrews on the comb holder. Insert
the slots over the shaft (threaded portion) of the thumbscrews and tighten until the flat head
(shoulder) of the screws come in contact with the comb.
3. Place the comb holder assembly on the cell base or UVTP tray and adjust the height of
the comb to the desired distance from the surface of the base stage or tray (typically 1-2 mm).
Tighten all five screws once the full-length of the comb is at a uniform distance from the
base stage or tray.
Adjusting and Setting Comb Position on UVTP Tray or Base Stage
1a. Turn the two thumbscrews clockwise on the sides of the comb holder until resistance is
felt. With the screws in this position, the comb holder can be placed into the comb slots
of the base and UVTP tray for gel casting.
OR
1b. Turn the two thumbscrews counterclockwise on the sides of the comb holder until the
shaft (threaded portion) of the thumbscrews can no longer be seen in the comb holder
notches. With the screws in this position, this will allow the comb holder assembly to be
placed anywhere on the base or UVTP tray. The comb can be secured to the tray or base
by turning the thumbscrews clockwise until resistance is felt.
2.3 Procedures for Casting Agarose Gel Slabs
There are several ways to cast agarose submarine gels for the Model 96 and Model 192.
Gels may be cast with or without UV-transparent plastic (UVTP) trays directly on the stage
of the Sub-Cell bases using the gel casting gates. Gels may also be cast on UVTP trays with
the aid of the gel caster or with standard laboratory tape.
Casting Gels on the Base Stages
1. Level the Sub-Cell base using the leveling bubble provided.
2. Slide the gel casting gates into the slots at opposite ends of the gel stage.
3. Place the comb(s) into the appropriate slot(s) of the base so that the sample wells are near
the cathode (black) (refer to Section 2.2 for comb adjustments). DNA samples will migrate
towards the anode (red) during electrophoresis.
4. When the solution of agarose has cooled to 60 °C (Section 2.1), pour the molten agarose
between the gates.
Warning: Hot agarose (>60 °C) may cause the cell to warp or craze and will decrease the
lifetime of the cell. Warping may also result in sample wells of uneven depth.
5. Allow 30 – 60 minutes for the gel to solidify at room temperature.
6. Carefully remove the comb and then remove the gel casting gates from the gate slots of
the base.
7. Submerge the gel beneath 4 to 6 mm of electrophoresis buffer (Section 3.1).
8
Page 12

Removable Tray (UVTP) Gel Casting
Casting gels on the base stage with UVTP tray
1. Level the cell using the leveling bubble provided.
2. Place the UVTP tray on the cell base stage.
Note: The Sub-Cell Model 96 system requires the 25 x 10 cm UVTP tray for casting in
the base. Sub-Cell Model 192 system requires the 25 x 15 cm UVTP tray for casting in
the base.
3. Slide the gel casting gates into the slots at opposite ends of the base stage. Ensure the
gates are evenly seated in the slots and the gates uniformly contact all edges of the UVTP
tray. The weight of the gates provide a tight seal to avoid any leakage problems during gel
casting.
4. Place the comb(s) into the appropriate slot(s) of the trays so that the sample wells are
near the cathode (black). DNA samples will migrate towards the anode (red) during elec-
trophoresis.
5. Prepare the desired concentration and amount of agarose in 1x electrophoresis buffer
(see section 2.1). When the agarose solution has cooled to 50-60˚ C pour the molten
agarose between the gates.
Warning: Hot agarose (>60 ˚C) may cause the tray to warp or craze and will decrease the
lifetime of the tray. Warping may also result in sample wells of uneven depth.
6. Allow 30 – 60 minutes for the gel to solidify at room temperature.
7. Carefully remove the comb from the solidified gel. Remove the gel casting gates.
8. Submerge the gel beneath 2 to 6 mm of 1x electrophoresis buffer (see Section 3, Gel and
Electrophoresis Reagent Preparation). Use greater depth overlay (more buffer) with
increasing voltages to avoid pH and heat effects.
Gel Caster Method
1. Level the gel caster on the lab bench using the leveling bubble provided.
2. Disengage and slide the movable wall to the open end of the gel caster by turning and
lifting the cam peg upward.
3. Place the UVTP tray against the fixed wall of the gel caster.
4. Slide the movable wall against the edge of the UVTP tray (Figure 2.1).
5. To seal the open tray ends, engage the cam peg by turning and pressing downward simul-
taneously.
6. Once the cam peg has dropped down into the appropriate slot, turn the peg in either
direction until resistance is felt. This action seals the ends of the tray for casting.
9
Page 13

Figure 2.1 Sealing the UVTP tray for gel casting.
7. Place the comb(s) into the appropriate slot(s) of the tray (refer to Section 2.2 for comb
adjustments).
8. When the solution of agarose has cooled to 60 °C (Section 2.1), pour the molten agarose
onto the tray.
Warning: Hot agarose (>60 °C) may cause the tray to warp or craze and will decrease the
lifetime of the tray. Warping may also result in sample wells of uneven depth.
9. Allow 30 – 60 minutes for the gel to solidify at room temperature.
10. Carefully remove the comb from the solidified gel.
11. Disengage the cam peg by turning and lifting upward. Slide the movable wall away from
the tray. Remove the tray from the gel caster.
Note: While the gel is solidifying, a light seal is formed between the gasket and the gel
(especially for low percentage agarose gels (<0.8%). Carefully lift the tray on one side to
release the seal.
12. Place the tray onto the leveled Sub-Cell base so that the sample wells are near the cathode
(black). DNA samples will migrate towards the anode (red) during electrophoresis.
13. Submerge the gel beneath 4 to 6 mm of electrophoresis buffer (Section 3.1).
10
Movable Well
Lift cam lever up
UVTP Gel Tray
Fixed wall
Leveling Feet
Gel Caster
Leveling Bubble
Engage and seal
(press down and rotate)
Slide Forward
Page 14

Tape Method
1. Seal the ends of the UVTP gel tray securely with strips of standard laboratory tape. Press
the tape firmly to the edges of the gel tray to form a fluid-tight seal.
2. Level the gel tray on a leveling table or workbench using the leveling bubble provided with
the instrument.
3. Place the comb(s) into the appropriate slot(s) of the tray (refer to Section 2.2 for comb
adjustments).
4. When the solution of agarose has cooled to 60 °C (Section 2.1), pour the molten agarose
onto the tray.
Warning: Hot agarose (>60 °C) may cause the tray to warp or craze and will decrease the
lifetime of the tray. Warping may also result in sample wells of uneven depth.
5. Allow the gel to solidify at room temperature for 30 – 60 minutes.
6. Carefully remove the comb from the solidified gel.
7. Remove the tape from the edges of the gel tray. Be careful when removing tape so the gel
does not slide off the tray.
8. Place the tray onto the leveled Sub-Cell base so that the sample wells are near the cathode
(black). DNA samples will migrate towards the anode (red) during electrophoresis.
9. Submerge the gel under 4 to 6 mm of electrophoresis buffer.
2.4 Recirculation Ports
Buffer recirculation is not required for most run conditions on the Sub-Cell systems. We
recommend buffer recirculation for extended run times (over 2 hours) or for high voltage
run conditions (150-200 volts). This will prevent lane distortion that can arise from uneven
heating or buffer pH gradients. If recirculation is desired, the buffer recirculation kit
(Bio-Rad catalog number 170-4537) contains the adapters required to connect the pump
tubing to the Sub-Cell lid.
1. Carefully remove the port plugs from the safety lid.
2. Turn clockwise and tighten the elbow-shaped recirculation port fitting into the threaded
port holes (Figure 2.2).
Note: There should be at least three threads extending below the bottom surface of the
safety lid.
Figure 2.2. Connecting the recirculation ports and tubing.
11
Elbow-shaped
Fitting
Safety Lid
Recirculation Port
Straight Fitting
Tubing
Page 15

3. Attach and tighten (10 lb.-in. torque) the straight fitting to the elbow-shaped fitting.
4. Connect tubing to the elbow-shaped fittings on the safety lid. Connect the other end of the
tubing to a suitable buffer recirculation pump (Section 6.3). Attach the tubing clips at all
tubing/fitting connections to insure that tubing does not disengage during electrophoresis.
5. Recirculate the buffer at a rate of 300-500 ml/min. Pumping at a higher rate will cause the
gel to float or slide off the tray causing variable sample migration rates during
electrophoresis. Check for any leaking in the fitting, tubing, and pump connections before
turning on the power supply and starting electrophoresis.
Note: If recirculation port fittings are to be removed, always cover the port holes by
replacing the port plugs (use 5 lb.-in. torque to tighten).
2.5 Electrophoresis
Once the agarose gel has solidified, sample loading and electrophoresis can begin. Agarose
gels can be run in many different types of electrophoresis buffers. Nucleic acid agarose gel
electrophoresis is usually conducted with either Tris-Acetate-EDTA (TAE) buffer or TrisBorate-EDTA (TBE) buffer. While TAE buffer provides faster electrophoretic migration of
linear DNA and better resolution of supercoiled DNA, TBE buffers have a stronger buffering
capacity and are less conductive than TAE buffers and therefore are used for longer or higher
voltage electrophoresis runs.
Note: Because of the higher voltages and resulting higher currents often used with the
Model 96 and Model 192, it is strongly recommended that only TBE buffers be used for
electrophoresis. TBE buffers have a stronger buffering capacity and are less conductive.
Thus, pH or temperature gradient formation during extended electrophoresis will be
reduced. If pH or temperature gradients cause uneven sample migration reduce the voltage,
add more buffer or recirculate the buffer during electrophoresis to eliminate these effects
(Section 2.4). Bio-Rad offers premixed 10x TBE buffers as well as individual buffer
reagents for use with the Sub-Cell systems (Section 6.3).
1. When placing the gel tray into the base, make sure that the sample wells are at the cathode
(black). DNA samples will migrate towards the anode (red) during electrophoresis.
2. Prepare the desired concentration of electrophoresis buffer (the electrophoresis buffer
used should be identical to the type used for gel preparation).
3. Submerge the gel under 4 to 6 mm of electrophoresis buffer. Do not fill buffer above the
max. buffer mark on the Sub-Cell base.
4. Prepare samples for gel loading. The maximum sample loading volume for Bio-Rad
combs is listed in Section 6.2. Loading volume is dependent upon the type of comb used
(i.e., well thickness and length) and thickness of the gel.
5. Once loading volume is determined, samples are made dense for underlaying into sample
wells by using standard nucleic acid sample loading dyes (refer to Section 3.4 for sample
loading dye preparation). Add loading dye to a final 1x concentration.
6. Load the samples into the wells using standard pipets or multichannel pipets.
Note: Sample wells are often difficult to see. Well visualization can be enhanced by placing
black paper or tape under the base or tray where comb placement or well formation is
common.
7. Place the lid on the DNA cell carefully. Do not disturb the samples. The Sub-Cell systems
lid attaches to the base in one orientation only. To attach the lid correctly, match the red
and black banana jacks on the lid with the red and black banana plugs of the base.
12
Page 16

8. Power requirements vary depending on gel thickness, length and concentration, and type
of electrophoresis buffer used. Refer to Table 2.3 for relative sample migration rate for the
Sub-Cell Model 96 and Model 192 systems. Also, review Table 2.4 for DNA size migration
with sample loading dyes.
Note: Buffer recirculation is not required for most standard DNA and RNA agarose gel
electrophoresis. For most electrophoresis, TBE buffer is recommended. If buffer
recirculation is required, use the recirculating ports (Section 2.4).
Table 2.3 Relative Sample Migration Rates*
Bromophenol Blue
Cell Type Voltage migration rate
Sub-Cell Model 96 200 V 5.15 cm/hr
Sub Cell Model 192 200 V 6.20 cm/hr
* Note: These sample migration rates were determined based on a 0.5 cm thick 1.0% agarose gel using Bio-Rad Molecular Biology
Certified Agarose in 1x TBE buffer diluted from Bio-Rad Premixed 10x TBE Buffer). Migration rates will vary depending on the
voltage, current, and type of agarose or buffer used.
Table 2.4 DNA Size Migration with Sample Loading Dyes
Agarose
Concentration (%) Xylene Cyanol Bromophenol Blue
0.5 – 1.5 4-5 Kbp 400-500 bp
2.0 – 3.0 * 750 bp 100 bp
4.0 – 5.0* 125 bp 25 bp
* Sieving agarose such as Bio-Rad AmpliSize agarose.
9. With the desired power requirements, begin electrophoresis. If using buffer recirculation,
electrophorese for 15 minutes before turning the pump ON.
Note: Buffer recirculation is optional for gels that require short run times. Gels run at
higher voltages (200 volts) may require recirculation to prevent heat or pH effects.
Recirculate the buffer at a rate of 300-500 ml/min. Do not pump at a higher rate, it will
cause the gel to float or slide off the tray causing variable sample migration rates during
electrophoresis.
10. After electrophoresis is complete, turn off the power. If using buffer recirculation, do not
turn the pump OFF and do not disconnect the tubing from the safety lid. Lift the safety lid
with the pump still ON and empty the buffer contained in the tubing and pump into the
base buffer chamber. When the tubing is empty, turn the pump OFF and disconnect the
tubing if desired.
2.6 Nucleic Acid Staining and Visualization
Gels can be removed from the base or gel tray for nucleic acid staining. The gel can also
remain on the UVTP gel tray for staining.
Ethidium Bromide Staining Procedure
1. Place the gel into the appropriate volume of 0.5 µg/ml ethidium bromide (EtBr) and stain
for 15–30 minutes. Use enough staining solution to cover the entire gel.
Caution: Ethidium bromide is a suspected carcinogen and should be handled with extreme
care. Always wear gloves, eye glasses and a laboratory coat. Dispose of used EtBr solutions
13
Page 17

and gels appropriately (Review EtBr Material Safety Data Sheet [MSDS] for proper
disposal methods).
2. Destain the gel for 10-30 minutes in dH2O using the same volume used for staining.
Note: Ethidium Bromide can be removed from the DNA with extended destaining. This
will cause lower sensitivity of detection. However, insufficient destaining will create
higher background fluorescence.
3. Rinse the gel briefly with dH2O once to remove any residual staining solution.
4. Place the gel on a UV transilluminator for nucleic acid visualization and analysis. DNA-
Ethidium Bromide complexes may be illuminated with UV light of 254, 302, or 366 nm.
Sensitivity decreases with illumination at higher wavelength. However, nicking of DNA
will increase below 302 nm. Table 2.5 indicates the percentage of transmittance of UV
light through 1/4″ (.635 cm) UV-transparent plastic.
Note: Nucleic acids in the gel can be visualized through the UVTP trays. If a UVTP tray
is not used, place household plastic wrap between the UV transilluminator and the gel to
avoid contaminating the transilluminator with nucleic acids or EtBr.
Table 2.5 Percent UV Transmittance through 1/4” (.635 cm)
UV Transparent Plastic
Approximate %
Wavelength (nm) Transmittance
254 0
302 80
360 90
5. Photograph the gel using standard cameras and film (e.g., Bio-Rad Standard Polaroid Gel
Documentation System) or with CCD-based digitized image analysis systems (e.g., Bio-Rad
Gel Doc™ 1000). Gels are generally photographed with a yellow, orange, or red inter-
ference filter. Red filters generally give the cleanest background. Bio-Rad offers a full-line
of standard photography and CCD-based imaging systems for nucleic acid gel analysis.
2.7 Note on Blotting
Nucleic acids within the gel can be transferred to membranes using the techniques of
Southern and Northern blotting. It is beyond the scope of this instruction manual to include
blotting procedures. Consult References 1 and 2 for blotting techniques. Bio-Rad offers a
full-line of nitrocellulose and positively-charged nylon membranes, as well as vacuum and
electrophoretic blotting apparatus for Southern and Northern blotting (Section 6.3).
Section 3
Gel and Electrophoresis Reagents Preparation
3.1 Electrophoresis Buffer Preparation
DNA agarose gel electrophoresis is usually conducted with either Tris-Acetate-EDTA
(TAE) or Tris-Boric Acid-EDTA (TBE). While TAE provides faster electrophoretic migration
of linear DNA and better resolution of supercoiled DNA, TBE buffers have a stronger buffering
capacity for longer- or higher-voltage electrophoresis runs. Bio-Rad offers premixed
50x TAE and 10x TBE buffers for use with the Sub-Cell systems. RNA formaldehyde gels
require a MOPS [3-(N-morpholino)-propanesulfonic acid] electrophoresis buffer.
14
Page 18

1x Tris-Acetate-EDTA (TAE) — 40 mM Tris (pH 7.6), 20 mM Acetic Acid, and 1 mM EDTA.
50x Stock (1 liter): dissolve in 600 ml distilled water:
Tris Base (FW = 121) 242.0 g
Glacial acetic acid 57.1 ml
0.5 M EDTA (pH 8.0) 100.0 ml
Fill to a final volume of one liter with distilled water.
1x Tris-Boric Acid-EDTA (TBE) — 89 mM Tris (pH 7.6), 89 mM Boric Acid, 2 mM EDTA
10x Stock (1 liter): dissolve in 600 ml distilled water:
Tris Base (FW = 121) 108 g
Boric Acid (FW = 61.8) 55 g
0.5 M EDTA (pH 8.0) 40 ml
Fill to a final volume of one liter with distilled water.
1x MOPS Buffer (RNA Gels) — 0.02 M MOPS [3-(N-morpholino)-propanesulfonic acid]
(pH 7.0), 8 mM Sodium Acetate, 1 mM EDTA (pH 8.0)
5x Stock (1 liter): dissolve in 600 ml DEPC-treated distilled water:
MOPS 20.6 g
3 M Sodium Acetate (DEPC treated) pH 7.4 13.3 ml
0.5 M EDTA (DEPC-treated) pH 8.0 10.0 ml
Fill to a final volume of one liter with DEPC-treated distilled water.
Caution: DEPC is a suspected carcinogen. Always wear gloves, eye glasses and a
laboratory coat. Use caution when handling DEPC containing solutions. Consult the
DEPC MSDS (Material Safety Data Sheet) for more information.
3.2 DNA and RNA Gel Preparation
DNA Agarose Gels
(See Section 2.1)
RNA Agarose Formaldehyde Gels
1-2
For 100 ml of a 1% agarose formaldehyde gel prepare as follows:
1.6% melted agarose 62 ml
5x MOPS Electrophoresis Buffer (1x final concentration) 20 ml
12.3 M (37.5%) Formaldehyde (2.2 M final concentration) 18 ml
Caution: Formaldehyde solutions and formaldehyde vapors are toxic. When handling
solutions or gels that contain formaldehyde use a chemical hood. Always wear gloves, eye
glasses and a laboratory coat while using formaldehyde. See the formaldehyde MSDS
for more safety information.
3.3 RNA Sample Preparation
1-2
Prior to loading RNA onto an agarose formaldehyde gel prepare each RNA sample as
follows:
3.0 µl RNA in DEPC-treated water
5.0 µl 5x MOPS Buffer (final concentration 1.67x)
4.5 µl 12.3 M Formaldehyde (final concentration 3.7 M)
12.5 µl Formamide (final concentration 50% v/v)
Caution: Formamide is a teratogen. Always wear gloves, eye glasses and a laboratory
coat. Use caution when handling formamide. Consult the formamide MSDS for more
information.
15
Page 19

3.4 DNA and RNA Sample Loading Dye
1-2
A convenient 10x sample buffer stock consists of 50% glycerol, 0.25% bromophenol
blue, and 0.25% xylene cyanole FF in 1x electrophoresis buffer. Prepare only 1-10 ml of the
10x loading dye.
3.5 Gel Staining Solution
Add 10 mg of ethidium bromide to 1 ml distilled water. Bio-Rad offers pre-mixed
EtBr solutions (10 mg/ml). Store reagent in the dark.
Section 4
Care and Maintenance
4.1 Cleaning Sub-Cell System Components
1. All Sub-Cell systems parts should be washed with a mild detergent solution in warm
water. If necessary, use a soft-bristled brush or sponge to remove dried buffer salts or
agarose.
Note: Be careful not to snag or break the electrode wire in the base while cleaning.
2. Rinse all parts thoroughly with warm water or distilled water and air dry, if possible.
3. To clean recirculation ports and tubing, simply pump distilled water into the tubing to
rinse. Thoroughly empty tubing of liquid before use.
4.2 Compatible Cleaning Agents
Chemically compatible cleaners must be used to ensure long life of parts. These include:
• Aqueous solutions of soaps and mild detergents:
Bio-Rad Cleaning Concentrate (catalog number 161-0722)
Dishwashing Liquid
• Organic Solvents:
Hexane
Aliphatic Hydrocarbons
Do not leave plastic parts to soak in detergents more than 30 minutes. A short detergent
rinse typically is all that is required.
Caution: Do not use the following chemicals to clean Sub-Cell parts. Exposure to these
chemicals may cause the plastic parts to crack, craze, etch or warp.
• Chlorinated Hydrocarbons
Carbon Tetrachloride
Chloroform
• Aromatic Hydrocarbons
Benzene
Phenol
Toluene
Methyl Ethyl Ketone
Acetone
• Alcohols
Methanol
Ethanol
Isopropyl Alcohol
16
Page 20

Do not use abrasive or highly alkaline cleaners on Sub-Cell parts. Do not expose Sub-Cell
parts to temperatures >60 °C. Do not sterilize Sub-Cell parts by autoclaving or dry heat.
4.3 Maintenance Schedule
Item Look For Frequency Action
All Parts Dried salts, agarose, Each Use Clean parts as
grease, and dirt described in Section 4.1
Electrical cables Breaks or fraying Each Use Replace Cables
Trays Chips or cracks Each Use Replace Tray
Electrode Wires Breaks Each Use See Section 4.4
(Electrode Cassette
Replacement)
Cable Connections Looseness Weekly Replace Banana Jacks
(Banana Jacks or Banana Plug Holders
and Plugs)
Base Crazing, cracks, Monthly Replace Base
or leaks
Recirculation Looseness or cracks Each Use Tighten or Replace
Tubing
4.4 Electrode Replacement
The Sub-Cell systems allow easy, hassle-free replacement of broken electrode wires by simply
removing the banana plug/electrode wire assembly and ordering a new assembly from Bio-Rad
(Figure 4.1). See Ordering Information (Section 6.2) for catalog numbers and part descriptions.
Instructions
1. Remove the thumb screw from the side wall of the base to release the banana plug/electrode
wire assembly. Do not discard this thumb screw (keep this screw with the base).
2. Remove the broken wire assembly from the base and discard the broken assembly.
3. Insert the new electrode assembly ensuring the electrode wire guard is properly seated into
the electrode wire guard slot in the bottom of the base.
4. Replace and tighten the thumb screw to secure the assembly in the base.
Figure 4.1 Replacement of banana plug/electrode wire assembly.
17
Page 21

4.5 RNase Decontamination
Sub-Cell parts can be cleaned with a mild detergent and treated for 10 minutes with 3%
hydrogen peroxide (H2O2) and then rinsed with 0.1% DEPC- (diethyl pyrocarbonate) treated
distilled water to eliminate RNases prior to using the Sub-Cell systems for RNA gels
1-2
. Do
not soak Sub-Cell parts in DEPC water. Consult references
1-2
for other suggestions regarding
the use of DEPC in RNase decontamination.
Caution: DEPC is a suspected carcinogen. Always wear gloves, eye glasses and a
laboratory coat. Use caution when handling DEPC-containing solutions. Consult the
DEPC MSDS for more information.
Do not attempt to RNase decontaminate Sub-Cell parts using extreme dry heat.
Note: Several commercial products are also available for eliminating RNase contamination.
RNaseZAP™(Ambion) or RNase AWAY™(Molecular Bio-Products) are safe, simple and
effective methods that if used properly do not craze or fog the Sub-Cell parts. See
manufacturer instructions for proper use.
18
Page 22

Section 5
Troubleshooting
Symptoms Probable Causes Solutions
Slanted lanes (bands)
Curved line or distortion of
lanes (bands)
Differential relative mobilities
Curved bands, smiles
Ragged bands
Band smearing and streaking
Bands sharp but too few bands
seem
High MW bands sharp/Low MW
bands smeared
Gels crack
• Gel not fully solidified.
• Comb warped or at an angle.
• Bubbles in sample wells.
• Sample spilled out of wells.
• Unit not leveled.
• Gel floated or slid off tray.
• Sample overload.
• Temperature or pH buffer
gradients
• Sample density incorrect.
• Sample well deformed.
• Excessive power or heating.
• Agarose has improper endosmosis (-mr).
• Salt concentration in sample
too high.
• Excessive power and heating.
• Sample spilled out of well.
• Incomplete digest, nuclease
contamination, bad enzyme.
• Sample wells cast through the
gel. Sample leaks along bottom of running surface.
• Sample overload.
• Too high gel percentage.
• Incomplete digest.
• Gel percentage too low.
• Too high voltage gradient
especially with low melting
temperature agarose or low gel
strength gels.
• Let gel solidify for at least
30-60 minutes.
• Check alignment of comb.
• Remove bubbles prior to
electrophoresis.
• Samples should have proper
density. Apply carefully.
• Level unit. Place on steady
work bench.
• Recirculate at a rate of
300-500 ml/min.
• Reduce the amount of sample
loaded.
• Reduce load.
• Add more buffer.
• Recirculate buffer.
• See sample application
instructions.
• Carefully remove comb, especially from soft gels. Be sure
gel has solidified. Cooling soft
gels aids in comb removal.
Add buffer to help lubricate
removal of the comb.
• Reduce voltage. See electrophoresis instructions.
• Consult Bio-Rad about
agarose.
• Reduce salt concentration to
≤ 0.1 M.
• Reduce voltage. See electrophoresis instructions
• Take care in applying sample.
Increase gel thickness for large
sample volumes.
• Heat sample. Check enzyme
activity. Digest sample further.
• Comb should be placed 1 to
2 mm above the base of the running surface. Add buffer to help
lubricate removal of the comb.
• Dilute sample.
• Lower gel percentage.
• Check enzyme activity, digest
further.
• Increase gel percentage.
• Switch to polyacrylamide.
• Reduce voltage. Run gel at
lower temperature.
19
Page 23

Section 6
Ordering Information
6.1 Sub-Cell Model 96 and Model 192 Systems
Catalog
Number Product Description
Sub-Cell Model 96 Systems
170-4540 Sub-Cell Model 96/PowerPac 300 System, 100/120 V
170-4542 Sub-Cell Model 96/PowerPac 300 System, 220/240 V
170-4500 Sub-Cell Model 96, with 25 x 10 cm tray and Gel Caster
170-4501 Sub-Cell Model 96, with 25 x 15 cm tray and Gel Caster
170-4502 Sub-Cell Model 96, with 25 x 10 cm tray
170-4503 Sub-Cell Model 96, with 25 x 15 cm tray
Sub-Cell Model 192 Systems
170-4541 Sub-Cell Model 192/PowerPac 300 System, 100/120 V
170-4543 Sub-Cell Model 192/PowerPac 300 System, 220/240 V
170-4504 Sub-Cell Model 192, with 25 x 10 cm tray and Gel Caster
170-4505 Sub-Cell Model 192, with 25 x 15 cm tray and Gel Caster
170-4506 Sub-Cell Model 192, with 25 x 20 cm tray and Gel Caster
170-4507 Sub-Cell Model 192, with 25 x 25 cm tray and Gel Caster
170-4508 Sub-Cell Model 192, with 25 x 10 cm tray
170-4509 Sub-Cell Model 192, with 25 x 15 cm tray
170-4510 Sub-Cell Model 192, with 25 x 20 cm tray
170-4511 Sub-Cell Model 192, with 25 x 25 cm tray
6.2 Sub-Cell Model 96 and Model 192 Systems Accessories
Catalog
Number Product Description
Sub-Cell Model 96 Accessories
170-4512 Sub-Cell Model 96 Base
170-4513 Sub-Cell Model 96 Safety Lid, with cables
170-4514 Model 96 Gel Caster
170-4518 Electrode Assembly (Anode) – Red
170-4519 Electrode Assembly (Cathode) – Black
170-4520 Gel Casting Gates
170-4521 UV Transparent Tray, 25 x 10 cm
170-4522 UV Transparent Tray, 25 x 15 cm
170-4525 Comb Holder
170-4537 Buffer Recirculation Kit
20
Page 24

Catalog
Number Product Description
Sub-Cell Model 192 Accessories
170-4515 Sub-Cell Model 192 Base
170-4516 Sub-Cell Model 192 Safety Lid, with cables
170-4517 Model 192 Gel Caster
170-4518 Electrode Assembly (Anode) – Red
170-4519 Electrode Assembly (Cathode) – Black
170-4520 Gel Casting Gates
170-4521 UV Transparent Tray, 25 x 10 cm
170-4522 UV Transparent Tray, 25 x 15 cm
170-4523 UV Transparent Tray, 25 x 20 cm
170-4524 UV Transparent Tray, 25 x 25 cm
170-4525 Comb Holder
170-4537 Buffer Recirculation Kit
Sub-Cell Model 96 and 192 Comb Specifications
Catalog Well Thickness Well Width Well Volume
Number Number (mm) (mm) Capacity* (µl)
170-4526 26 0.75 6.0 22.50
170-4527 26 1.5 6.0 45.00
170-4528 51 0.75 3.0 11.25
170-4529 51 1.5 3.0 22.50
170-4530 2 0.75 97 364.0
170-4530 4 0.75 46 172.5
170-4531 2 1.5 97 727.5
170-4531 4 1.5 46 345.0
All Sub-cell Model 96 and Model 192 combs require a comb holder (170-4525)
* Well volume capacity determined based on 0.5 cm thick gel
6.3 Related Bio-Rad Products
Contact your local Bio-Rad representative concerning the following products for nucleic
acid electrophoresis and blotting.
Catalog
Number Product Description
Sub-Cell GT Systems
170-4400 Sub-Cell GT System
170-4401 Sub-Cell GT System, with 15 x 10 cm tray
170-4402 Sub-Cell GT System, with 15 x 15 cm tray
170-4403 Sub-Cell GT System, with 15 x 20 cm tray
21
Page 25

Catalog
Number Product Description
Sub-Cell GT Systems
(continued)
170-4404 Sub-Cell GT System, 15 x 25 cm tray
170-4405 Wide Mini-Sub Cell GT System
170-4406 Mini-Sub Cell GT System
170-4481 Sub-Cell GT System, with 15 x 10 cm tray and Gel Caster
170-4482 Sub-Cell GT System, with 15 x 15 cm tray and Gel Caster
170-4483 Sub-Cell GT System, with 15 x 20 cm tray and Gel Caster
170-4484 Sub-Cell GT System, with 15 x 25 cm tray and Gel Caster
170-4485 Wide Mini-Sub Cell GT System and Gel Caster
170-4486 Mini-Sub Cell GT System and Gel Caster
Power Supplies
165-5050 PowerPac 300 Power Supply, 100/120 V
165-5051 PowerPac 300 Power Supply, 220/240 V
Buffer Recirculation Pump Systems
170-2929 Buffer Recirculating Pump, 120/100 V
170-2930 Buffer Recirculating Pump, 220/240 V
Zeta-Probe®Positively-Charged Nylon Blotting Membranes
161-0153 Sheets, 9 x 12 cm, 15
161-0154 Sheets, 10 x 15 cm, 15
161-0155 Sheets, 15 x 15 cm, 15
161-0156 Sheets, 15 x 20 cm, 15
161-0157 Sheets, 20 x 20 cm, 15
161-0158 Sheets, 20 x 25 cm, 3
161-0159 Roll, 30 cm x 3.3 m, 1
161-0165 Roll, 20 cm x 3.3 m, 1
Zeta-Probe GT (Genomic Tested) Positively Charged Nylon
Blotting Membranes
161-0190 Sheets, 9 x 12 cm, 15
161-0191 Sheets, 10 x 15 cm, 15
161-0192 Sheets, 15 x 15 cm, 15
161-0193 Sheets, 15 x 20 cm, 15
161-0194 Sheets, 20 x 20 cm, 15
161-0195 Sheets, 20 x 25 cm, 3
161-0196 Roll, 30 cm x 3.3 m, 1
161-0197 Roll, 20 cm x 3.3 m, 1
22
Page 26

Catalog
Number Product Description
Supported Nitrocellulose Membrane (0.45 micron)
161-0090 Sheets, 7 x 8.4 cm, 10
161-0091 Sheets, 10 x 15 cm, 10
161-0092 Sheets, 15 x 15 cm, 10
161-0093 Sheets, 20 x 20 cm, 10
161-0094 Roll, 30 cm x 3 m, 1
Supported Nitrocellulose Membrane (0.20 micron)
161-0095 Sheets, 7 x 8.4 cm, 10
161-0096 Sheets, 15 x 15 cm, 10
161-0097 Roll, 30 cm x 3 m, 1
Vacuum Blotting Apparatus
165-5000 Model 785 Vacuum Blotter
165-5001 Model 785 Vacuum Blotter System, 120 VAC
165-5002 Model 785 Vacuum Blotter System, 220/240 VAC
Semi-Dry Transfer Cells
170-3940 Trans-Blot®SD Semi-Dry Electrophoresis Transfer Cell
170-3948 Trans-Blot SD System, 100/120 VAC
170-3949 Trans-Blot SD System, 220/240 VAC
UV Crosslinking Chamber
165-5031 GS Gene Linker®UV Chamber, 120 VAC
165-5032 GS Gene Linker UV Chamber, 220 VAC
165-5033 GS Gene Linker UV Chamber, 240 VAC
165-5034 GS Gene Linker UV Chamber, 100 VAC
Gel Reagents
162-0019 Low Melt Preparative Grade Agarose, 100 g
162-0133 Molecular Biology Certified Agarose, 500 g
162-0126 High Strength Analytical Grade Agarose, 500 g
162-0144 Amplisize Agarose, 50 g
170-8200 AmpliSize®DNA Size Standard, 50-2,000 bp
170-8210 DNA Size Standard, 1-4.2 Kb ladder
170-8220 DNA Size Standard, 0.7-8.4 Kb
161-0404 Bromophenol Blue, 10 g
161-0423 Xylene Cyanole FF, 25 g
161-0433 Ethidium Bromide Solution, 10 ml, 10 mg/ml
23
Page 27

Catalog
Number Product Description
Electrophoresis Buffers
161-0733 10x Tris/Boric Acid/EDTA (TBE), 1 L
161-0743 50x Tris/Acetic Acid/EDTA (TAE), 1 L
161-0719 Tris, 1 kg
161-0751 Boric Acid, 1 kg
161-0729 EDTA, 500 g
DNA Gel Image Analysis and Documentation Systems
170-3742 Standard Polaroid®Documentation System, 120 VAC
170-3746 Standard Polaroid Documentation System, 100 VAC
170-3747 Standard Polaroid Documentation System, 220/240 VAC
170-7520 Gel Doc®1000 UV Gel Documentaion System-PC, 100 VAC
170-7521 Gel Doc 1000 UV Gel Documentaion System-PC, 120 VAC
170-7522 Gel Doc 1000 UV Gel Documentaion System-PC, 220/240 VAC
170-7525 Gel Doc 1000 UV Gel Documentaion System-Mac, 100 VAC
170-7522 Gel Doc 1000 UV Gel Documentaion System-Mac, 120 VAC
170-7522 Gel Doc 1000 UV Gel Documentaion System-Mac, 220/240 VAC
24
Page 28

Section 7
References
1. Sambrook, Fritsch, and Maniatis, Molecular Cloning, A Laboratory Manual, Second Edition, Cold
Spring Harbor Laboratory Press, 1989
2. Current Protocols in Molecular Biology, Greene Publishing Associates and Wiley-Interscience,
1989
Additional Reading
1. Kopchick, J.J., Cullen, B.R. and Stacey, D.W., Anal. Biochem, 115, 419 (1981).
2. Southern, E., Methods in Enzymol., Academic Press, N.Y., 68, 152 (1979).
3. The Bio-Rad Silver Stain – Bulletin 1089.
4. Bittner, M., Kupferer, P. and Morris, C.F., Anal. Biochem., 102, 459 (1980).
5. Bio-Rad Trans-Blot Cell Operation Instructions – Bulletin 1082.
6. Winberg, G. and Hammarskjold, M.L., Nucleic Acids Res., 8, 253 (1980).
7. Jytatekadze, T.V., Axelrod, V.D., Gorbulev, V.G., Belzhelarskaya, S.N. and Vartikyan, R.M.,
Anal. Biochem., 100, 129 (1979).
8. Dretzen, G., Bellard, M., Sassone-Corsi, P. and Chambon, P., Anal. Biochem., 112, 295 (1981).
25
Page 29

Molecular
M1704498 Rev A
Bio-Rad
Laboratories
Bioscience Group
2000 Alfred Nobel Drive
Hercules, California 94547
Telephone (510) 741-1000
Fax: (510) 741-5800
Australia,
Austria,
Belgium,
Canada,
China,
Denmark,
Finland,
France,
Germany,
India,
Italy,
Bio-Rad Laboratories S.r.l.,Via Cellini, 18/A, 20090 Segrate Milano • Phone 02-21609 1 • Fax 02-21609-399
Japan,
The Netherlands,
New Zealand,
Pacific,
Singapore,
Spain,
Sweden,
Switzerland,
United Kingdom,
Bio-Rad Laboratories Pty Limited, Block Y Unit 1, Regents Park Industrial Estate, 391 Park Road, Regents Park, NSW 2143 • Phone 02-805-5000 • Fax 02-805-1920
Bio-Rad Laboratories Ges.m.b.H., Auhofstrasse 78D, 1130 Wien • Phone (1) 877 89 01 • Fax (1) 876 56 29
Bio-Rad Laboratories S.A./N.V., Begoniastraat 5, 9810 Nazareth Eke • Phone 09-385 55 11 • Fax 09-385 65 54
Bio-Rad Laboratories (Canada) Ltd., 5671 McAdam Road, Mississauga, Ontario L4Z 1N9 • Phone (905) 712-2771 • Fax (905) 712-2990
Bio-Rad Laboratories, 14, Zhi Chun Road, Hai Dian District, Beijing 100088 • Phone (01) 2046622 • Fax (01) 2051876
Bio-Rad Laboratories, Symbion Science Park, Fruebjergvej 3, DK-2100 Copenhagen • Phone 39 17 9947 • Fax 39 27 1698
Bio-Rad Laboratories, Business Center Länsikeskus, Pihatörmä 1A SF-02240, Espoo, • Phone 90 804 2200 • Fax 90 804 1100
Bio-Rad S.A., 94/96 rue Victor Hugo, B.P. 220, 94 203 Ivry Sur Seine Cedex • Phone (1) 49 60 68 34 • Fax (1) 46 71 24 67
Bio-Rad Laboratories GmbH, Heidemannstraße 164, D-80939 München/Postfach 450133, D-80901 München • Phone 089 31884-0 • Fax 089 31884-100
Bio-Rad Laboratories, C-248 Defence Colony, New Delhi 110 024 • Phone 91-11-461-0103 • Fax 91-11-461-0765
Nippon Bio-Rad Laboratories, 7-18, Higashi-Nippori 5-Chome, Arakawa-ku, Tokyo 116 • Phone 03-5811-6270 • Fax 03-5811-6272
Bio-Rad Laboratories B. V., Fokkerstraat 10, 3905 KV Veenendaal • Phone 0318-540666 • Fax 0318-542216
Bio-Rad Laboratories Pty Ltd., P. O. Box 100-051, North Shore Mail Centre, Auckland 10 • Phone 09-443 3099 • Fax 09-443 3097
Bio-Rad Laboratories, Unit 1111, 11/F., New Kowloon Plaza, 38, Tai Kok Tsui Road, Tai Kok Tsui, Kowloon, Hong Kong • Phone 7893300 • Fax 7891257
Bio-Rad Laboratories (Singapore) Ltd., 221 Henderson Rd #05-19, Henderson Building, Singapore 0315 • Phone (65) 272-9877 • Fax (65) 273-4835
Bio-Rad Laboratories, S. A. Avda Valdelaparra 3, Pol. Ind. Alcobendas, E-28100 Alcobendas, Madrid • Phone (91) 661 70 85 • Fax (91) 661 96 98
Bio-Rad Laboratories AB, Gärdsvägen 7D, Box 1276, S-171 24 Solna • Phone 46-(0)8-735 83 00 • Fax 46-(0)8-735 54 60
Bio-Rad Laboratories AG, Kanalstrasse 17, Postfach, CH-8152 Glattbrugg • Phone 01-809 55 55 • Fax 01-809 55 00
Bio-Rad Laboratories Ltd., Bio-Rad House, Maylands Avenue, Hemel Hempstead, Herts HP2 7TD • Free Phone 0800 181134 • Fax 01442 259118
SIG 101295 Printed in USA
 Loading...
Loading...