Page 1
BC Group International Inc
3081 Elm Point Industrial Dr.
St. Charles, MO 63301-4333 USA
ESU-2000 Series Product Overview
A Paradigm Shift In Electrosurgery Testing
Technology and Capability Is Here
Your current ESU Analyzer just became
obsolete – no matter how new it is!
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
1 Revision 1 – June 13, 2007
Page 2

INDEX
The Next Generation in ESU Testing is Finally Here....................................................................................................................3
Electrosurgery 101 – A Basic Review of Electrosurgery ..............................................................................................................3
ESU Testing 101 – Some Testing History....................................................................................................................................7
ESU-2050: Truly Unique in the Market.........................................................................................................................................9
ESU-2050: A Replacement for the Discontinued Fluke 8920A Instrument.................................................................................11
ESU-2050: Unprecedented 1% Accuracy in ESU Testing..........................................................................................................11
ESU-2300: A More Conventional Approach...............................................................................................................................11
ESU-2400: More High-End Technology to Come.......................................................................................................................11
Common Element: Patent Pending DFA Technology.................................................................................................................11
Up To 32,768 Data Points!.........................................................................................................................................................12
ESU-2050: Precision Load Resistors Are Where Accuracy Starts.............................................................................................13
Product Development In Cooperation with ESU Manufacturers.................................................................................................14
Industry Standard Current Sensing Technology.........................................................................................................................13
Working With The Best In Current Sensing: Pearson Electronics..............................................................................................13
Current Sensing vs. Voltage Measurement................................................................................................................................13
Ensuring Quality By Taking Care Of The Details .......................................................................................................................14
Those Crazy & Exotic Pulsed Waveforms..................................................................................................................................15
ESU-2300: External Load Capabilities – Built In Non-Obsolescence.........................................................................................16
ESU-2000 Series PC Utility Software.........................................................................................................................................16
One Picture is Worth a Thousand Words – Or Up To 32,768 Data Points.................................................................................16
See The Data You Want – The Way You Want .........................................................................................................................17
Easy Setup and Operation.........................................................................................................................................................18
ESU-2050: Graphical Mode .......................................................................................................................................................18
Product Comparison Overview...................................................................................................................................................18
One-Stop-Source: We Make It Easy for You..............................................................................................................................19
Conclusion .................................................................................................................................................................................20
ESU-2050 Product Specifications ..............................................................................................................................................21
ESU-2300 Product Specifications ..............................................................................................................................................22
Technical References.................................................................................................................................................................23
About the Author ........................................................................................................................................................................24
APPENDIX A Fluke Biomedical 454A Instrument Specifications ...............................................................................................26
APPENDIX B Fluke Biomedical RF-303RS Instrument Specifications ........................................................................................27
APPENDIX C Dale Technology DALE3000 Instrument Specifications.......................................................................................31
APPENDIX D Metron QA-ES Instrument Specifications ............................................................................................................33
APPENDIX E Valleylab ForceFX Generator Output waveforms ................................................................................................34
APPENDIX F Valleylab Force 2 Generator Output Waveforms .................................................................................................36
APPENDIX G Conmed System 5000 Output Waveforms..........................................................................................................38
APPENDIX H (Pearson Electronics Model 411 Data Sheet)......................................................................................................41
APPENDIX I (Pearson Electronics Model 4100 Data Sheet) .....................................................................................................42
APPENDIX J (Vishay Dale NH-250 Data Sheet)........................................................................................................................43
APPENDIX K (Sample Microsoft Excel® Data Export Workbook) ..............................................................................................45
APPENDIX L (Tyco Healthcare / Valleylab Recommended Test Procedures)...........................................................................46
APPENDIX M (ESU-2000 Series PC Utility Software Screen Shots).........................................................................................49
APPENDIX N (History of BC Group International, Inc.)..............................................................................................................52
2 Revision 1 – June 13, 2007
APPENDICES
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 3
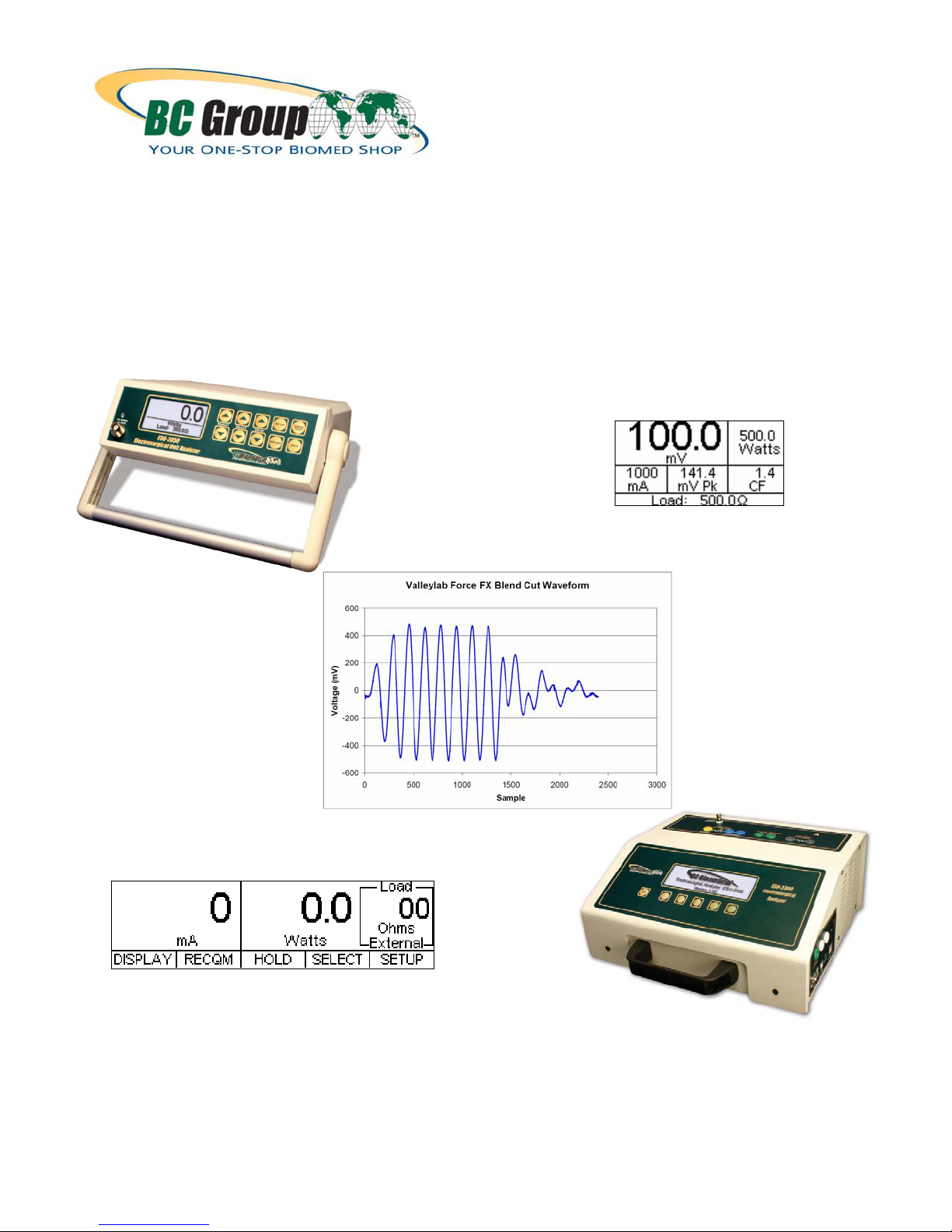
The Next Generation in ESU Testing is Finally Here
With the introduction of the new BC Biomedical ESU-2000 Series of Electrosurgery (ESU) Analyzers (the ESU2050 and ESU-2300 instruments)
technologically significant advances in electrosurgical testing to come about in well over a decade! The new BC
Biomedical ESU-2050 represents an 18-month duration major product design effort in full cooperation with some
of the leading electrosurgery generator manufacturers in the worldwide medical device market. The ESU-2050 is
the very first instrument of its kind to be introduced, specifically designed for electrosurgery generator testing, with
1% of reading accuracy and a testing methodology that is exactly the same as the one that many medical device
manufacturers currently use
2
features and functionality above and beyond competitive analyzers in this “mid-range” class. Both analyzers can
be easily upgraded in the field via the BC Biomedical Flash Update PC Utility Software in the event of a needed
firmware update. Together, these new ESU analyzers from BC Group represent an unprecedented paradigm
shift in electrosurgery testing technology, and set a new baseline for the electrosurgery test device industry. The
long-awaited next generation in ESU testing has finally arrived!
Electrosurgery 101 – A Basic Review of Electrosurgery
The following basic review on electrosurgery is derived from technical information obtained from various sources
in the public sector, including the Tyco Healthcare/ Valleylab document, Electrosurgery Self Study Guide
Copyright September 1999, Tyco Healthcare / Valleylab. This information is intended for basic review purposes
of some of the terminology and basic principles of electrosurgery technology.
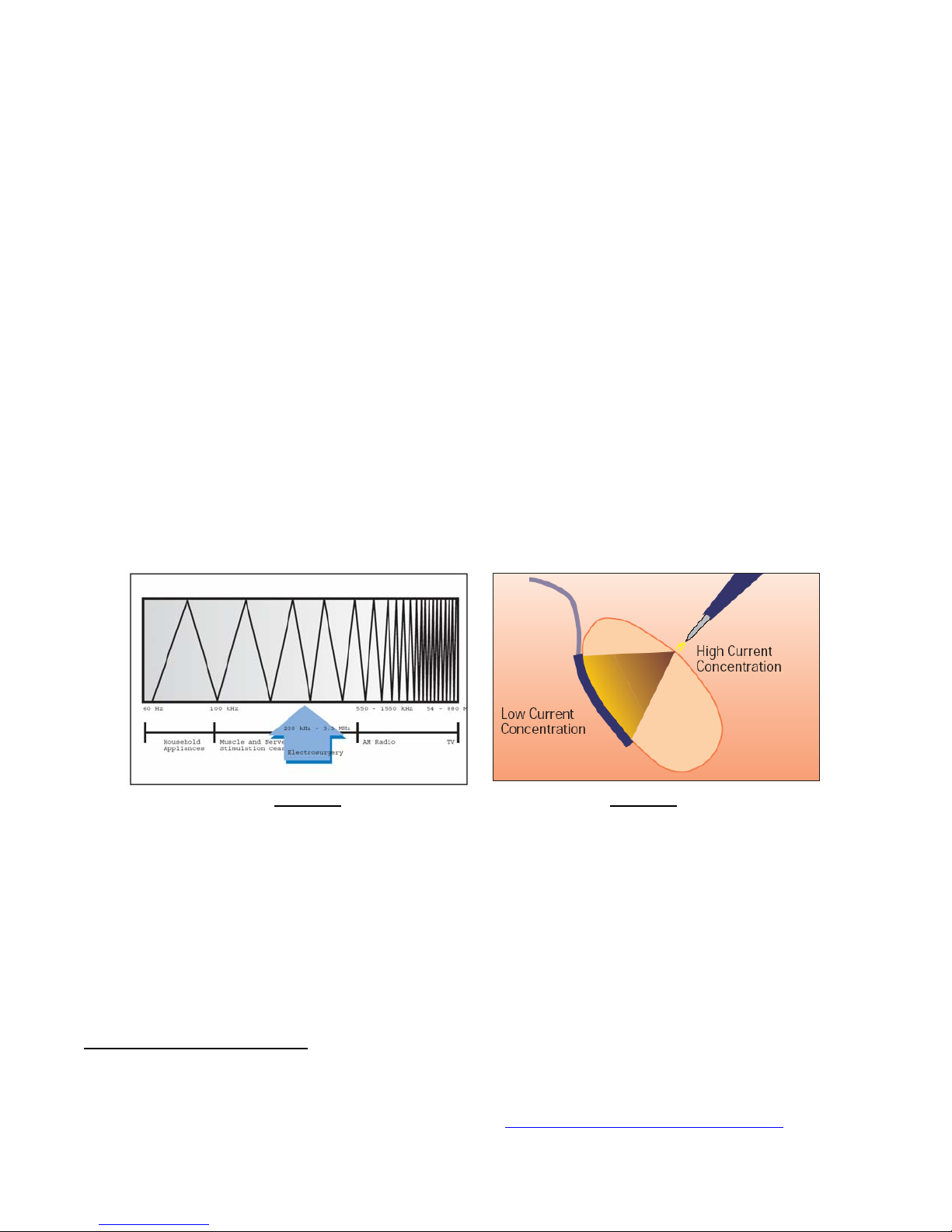
Electrosurgery generally deals with electrical signal frequencies in the range of approximately 200 kHz to 3.3 MHz
(see Figure 1). This is well above the human body’s inherent frequency range of susceptibility to the hazards of
microshock.
1
, BC Group International, Inc. brings to market, the most exciting and
. The new ESU-2300 is a more conventional “mid-range” ESU analyzer, offering
3
4
,
Figure 1 Figure 2
Frequency Spectrum Showing Range Current Density Differences
of Frequencies for Electrosurgery at Surgical Site vs. Return Path
Electrosurgery works based upon heat generated by the density (see Figure 2) of the high frequency current
being passed through human tissue. At the surgical site, the density is typically very high, resulting in high heat
and a cutting or coagulating effect. The “return path” for the high frequency current is much larger and
consequently much less current density exists at this area, which allows the high frequency energy to safely leave
the body without any adverse effects.
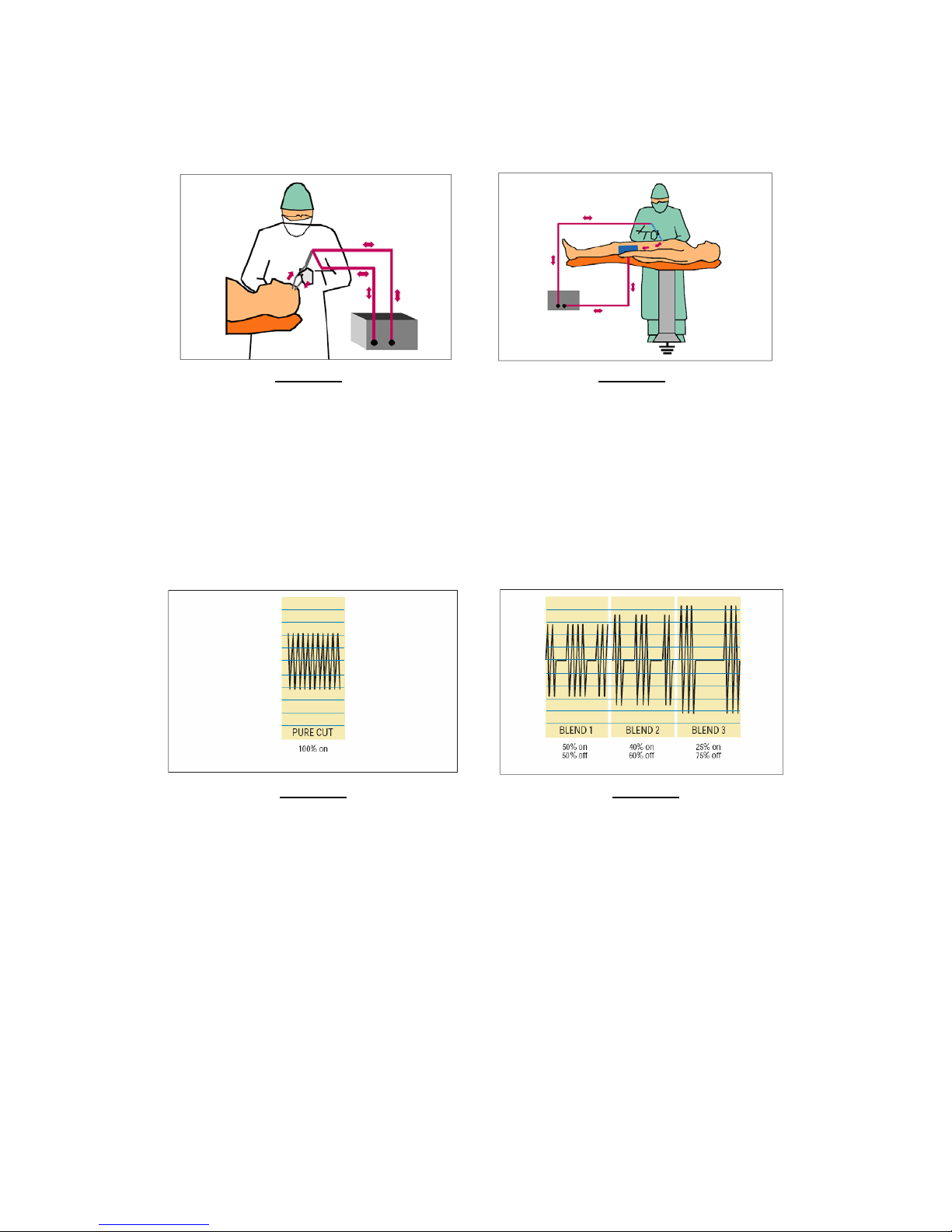
There are two basic modes of electrosurgery: bipolar and monopolar. Bipolar surgery (see Figure 3) is
accomplished by using two parallel poles in close proximity, where the flow of high frequency current is restricted
to the two poles, one being the “source” and the other being the “return path”. A patient return electrode is
typically not needed in bipolar electrosurgery applications, and because these two poles are close together, the
1
Commercial availability scheduled for July/August 2007.
2
See Appendix L for specific information regarding Tyco Healthcare / Valleylab recommended test setup procedures and recommended test equipment.
3
Sincere appreciation to Tyco Healthcare / Valleylab for the use of the illustrations in this section. Images and information are based upon the Valleylab
publication Electrosurgery Self-Study Guide, Copyright September, 1999, Authored by Br enda C. Ulmer, RN, MN, CNOR.
4
This Tyco Healthcare / Valleylab publication can be downloaded in PDF format at http://www.valleylabeducation.org/pages/list-book.html
3 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 4
voltage level and resulting applied power are lower than in monopolar electrosurgical applications. This results in
less localized tissue heating and reduced “charring” of tissue. Bipolar electrosurgery is typically used in
neurosurgical and gynecological procedures, and in other procedures where there is concern due to implanted
pacemakers and automatic defibrillators. In general, bipolar electrosurgery is safer that monopolar
electrosurgery, and the subsequent risks of high frequency burns at the return electrode site are avoided.
Figure 3 Figure 4
Electrosurgery – Bipolar Mode Electrosurgery – Monopolar Mode
Monopolar electrosurgery (see Figure 4) is a more generalized and more frequently used mode. Monopolar
electrosurgery utilizes higher voltage levels than bipolar, resulting in higher power delivered at the surgical site.
The need for a well prepared and maintained patient electrode site is of paramount concern in monopolar
electrosurgical applications, in order to prevent high frequency burns at the patient return electrode site.
The high frequency waveform produced by the electrosurgical generator determines the physiological effect of the
application of this energy to the tissue in the body. The Cut mode of an electrosurgical generator creates a
continuous waveform, as shown in Figure 5. Different degrees of hemostasis (coagulation) can be achieved by
utilizing varying degrees of “Blended” waveforms as shown in Figure 6.
Figure 5 Figure 6
Pure Cut - Pure Sinusoidal Waveform Blended Waveforms
The Coag mode (see Figure 7) of an electrosurgical generator creates a waveform with large amplitude but short
duration “spikes” to achieve hemostasis (coagulation). The surrounding tissue is heated when the waveform
spikes and then cools down (between spikes), producing coagulation of the cells. Fulguration is achieved in the
Coag mode of the electrosurgical generator, with the tip of the surgical “active electrode” held above (but not in
contact with) the tissue. Electrosurgical Desiccation is achieved in either the Cut or Coag modes of the
generator. The difference between Desiccation and Fulguration is the tip of the “active electrode” must contact
the tissue as in Figure 8 in order to achieve Desiccation. The more desired mode to achieve tissue Desiccation
through direct tissue contact is the Cut mode.
Older electrosurgical generators (those produced prior to around 1968) are generally ground-referenced devices
and must be used with extreme care to avoid unwanted “current division” and possible resulting high frequency
burns at this site (or at multiple sites). This is illustrated in Figure 9 below. Current division can occur at any point
of contact with an earth grounded point, such as the frame of the surgical table or the outer chassis of another
medical device. For the most part, these types of devices are no longer used in surgical procedures, mainly due
to advances in electrosurgical generator technology and concerns over safety.
4 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 5
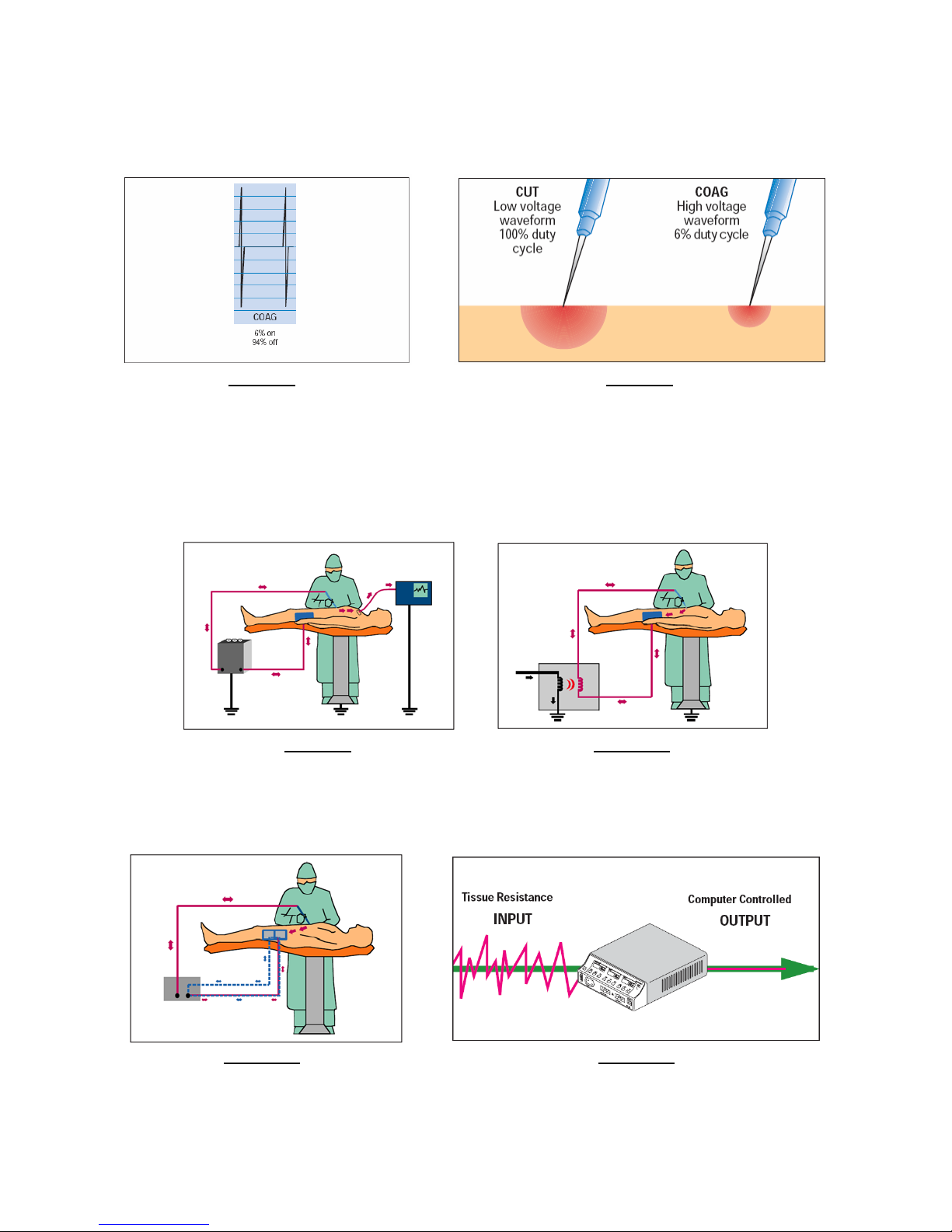
Advances in electrosurgery generator technology brought about the “solid state” generator around 1968. Along
with this more reliable and more condensed electronics technology came the introduction of the isolated-output
electrosurgical generator (see Figure 10 below), thus eliminating the concern over unwanted current division and
vastly improving patient safety. The outputs of these generators were no longer earth ground-referenced, so
even the best electrical ground-referenced contact made to the patient would not present the risk of high
frequency burns at alternate sites.
Figure 7 Figure 8
The shift in concern now focused on the quality of the patient return electrode and electrode site, and over the
succeeding years, many manufacturers introduced new monitoring techniques designed to constantly measure
the integrity of the patient electrode site in order to minimize the possibility of high frequency burns at the patient
electrode. The varying technologies introduced by the various electrosurgical generator manufacturers over the
years have generically become know in today’s market as the Contact Quality Monitor (CQM) function (see
Figure 11) of the electrosurgical generator.
Coagulation Waveform Tissue Penetration: Cut vs. Coag
Figure 9 Figure 10
Ground-Referenced Electrosurgical Generator Isolated Output Electrosurgical Generator
In more recent years, there has been a steady stream of advances in electrosurgery generator technology, one of
the most significant of which was the introduction by Tyco Healthcare / Valleylab in their Force FX Generator of
“Tissue Response Technology” in the late 90’s. This technology utilizes a constant feedback loop to the
Figure 11 Figure 12
Contact Quality Monitor (CQM) Function Tyco Healthcare / Valleylab Tissue Response Technology
Copyright June, 2007 by BC Group International, Inc.
5 Revision 1 – June 13, 2007
Author: Michael R. Erwine
Page 6

generator’s microprocessor and actually adjusts the power level output of the generator in order to provide
relatively constant power delivery (and thus a consistent surgical effect) at the surgical site, regardless of tissue
impedance.
Electrosurgery generator improvements continue, with new introductions by leading manufacturers like Tyco
Healthcare / Valleylab, Conmed (Electrosurgery Division), Erbe, Bovie, etc. on a regular basis The need for
routine testing and performance verification of these generators has not deceased due to these introductions of
new technologies. In fact, there are more features and safeguards to test for proper operation on today’s average
electrosurgical generator than ever.
Some Common Electrosurgery Terminology
Active Electrode: an electrosurgical instrument or accessory that concentrates the high frequency current at the
surgical site, thus enabling the heating effect at the site and producing the desired electrosurgical effect
Blend: an electrosurgical generator output waveform that combines the features of cut and coag waveforms,
cutting with various degrees of hemostasis (coagulation)
Contact Quality Monitor (CQM): a system that constantly monitors the impedance of the physical connection
between the patient’s body and the patient return electrode and interrupts power form the electrosurgical
generator is the quality of this connection is compromised electrically
Current Density: the amount of electrical current flow per unit of surface area – as current density increases so
does the heating of the tissue in the immediate location
Current Division: high frequency electrical current leaving the intended electrosurgical patient circuit and
following an alternate low impedance path of lesser resistance to earth ground, this introducing the possibility of
high frequency burns at the alternate earth ground contact point – typically a concern in ground-reference
generators and not isolated output generators.
Coagulation: the clotting of blood or destruction of tissue with no cutting effect – electrosurgical fulguration and
desiccation.
Cut Mode: electrosurgical mode that produces a low voltage continuous waveform optimized for tissue cutting
Desiccation: the effect of tissue dehydration and protein denaturation caused by direct contact between the
electrosurgical “active electrode” and the tissue
Fulguration: using electrical arcs (sparks) to coagulate tissue, whereby the sparks jump from the electrosurgical
“active electrode” across an air gap to the tissue
Ground-Referenced Output: an electrosurgical generator with an output that is electrically referenced to earth
ground
Isolated Output: an electrosurgical generator with an output that is not electrically referenced to earth ground
Leakage Current: electrical current that flows along an undesired pathway, usually to earth ground – in an
electrosurgical generator, RF leakage current is high frequency current that regains its ground reference and
seeks earth ground.
Patient Return Electrode: an electrically conductive plate or pad (also known as the dispersive electrode) that
recovers the high frequency current introduced into the patient’s body by the “active electrode” during
electrosurgery. This electrode minimizes the current density of this return current flow in order to minimize the
possibility of high frequency burns at this electrode site.
Radio Frequency (RF): frequencies above 100 kHz that transmit radio signals – the high frequency current
utilized in electrosurgery
Tissue Response Technology: the Tyco Healthcare / Valleylab electrosurgical generator technology that
continuously measures the impedance/resistance of the tissue in contact with the patient return electrode and
automatically adjusts the output of the generator accordingly to achieve a consistent tissue effect.
6 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 7

ESU Testing 101 – Some Testing History
Electrosurgery generator technology has undergone tremendous technological advances over the past decade,
but the technology base of ESU analyzers has remained relatively slow-moving over this same time period. The
recently discontinued Fluke Biomedical Model 454A dates back to around 1992 or 1993, and until now,
represents the culmination of research and development efforts on the behalf of competitive companies in the
area of electrosurgery testing. Here is a brief history of ESU testing devices over the past 15 to 20 years.
Analyzers are shown in the order of their introduction to the market.
No
Picture
Available
Bio-Tek Instruments RF-301: The very first offering in ESU analyzers by Bio-Tek Instruments. T his
“passive”
301 instruments in use in the field today. The design was basic and rugged.
5
RF thermocouple ammeter type instrument got the job done. There are still quite a few RF-
Neurodyne Dempsey Model 403A: The Neurodyne Dempsey (which later became Dynatech
Nevada Inc.) Model 403A was a very small-sized ESU tes ter with limited functionality. This was
a passive technology device with an RF thermocouple type analo g ammeter and a single fixed
500 Ω internal load. Meter range was 0.2 A to 1.0 A / 20 watts to 500 watts. It was the
company’s first dedicated ESU tester. There are very few of these units left in the market.
Bio-Tek Instruments RF-302: The predecessor to the Bio-Tek RF-303, the RF-302 was a
“passive” RF thermocouple ammeter type instrument. This gave an advantag e to the RF-302
above other competitive “active” type ESU analyzers available at time. T he RF-302 offered a
better high frequency range than some competitive “active” units. Bio-T ek Instruments sold qu it e
a few of these units in the market. This instrument is very similar to the BC Biomedical ESU2000A instrument that is still available today, for those customers who prefer a l egacy type RF
ammeter “passive” instrument approach to ESU generator testing.
Dynatech Nevada Model 443: The Dynatech Nevad a Model 443 was the company’ s very first
“active” type
technology, the Model 443 still utilized an analog meter. The Model 443 was discontinued
shortly after the introduction of the Model 453A.
6
design in ESU analyzers. Despite it’s active internal circuitry and measurement
Dynatech Nevada Model 453A: The predecessor to the 454A, the Dynatech Nevada Model
453A was probably the very first “Hi-Tech” ESU analyzer on the market. It utilized active
technology. Introduced in the mid 1980’s, the Model 453A was in production until the
introduction of the Dynatech Nevada Model 454A , starting in 1992 or 1993. The 453A had a
small LED 7-segment display and was a fairly large instr ument weighing well over 15 pounds.
There are still many 453A ESU analyzers in use in biomedical departments across the U.S.
today.
5
Passive technology in an ESU Analyzer refers to an instrument that does not require any external power source and simply meters the RF energy without
any electronic signal processing.
6
Active technology is an ESU Analyzer refers to an instrument that requires a power supply and has active electronic circuitry including components such as
A/D converters. Operational amplifiers, thermal converters, etc.
7 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 8

Dynatech Nevada Model 454A: Until it was recently discontinued by Fluke Biomedical (the
Model 454A is no longer listed on the Fluke Biom edical web site and customers report having
been informed that the 454A is no longer availabl e from Fluke Biomedical) in favor of the more
recent Metron QA-ES (re-branded as the Fluke Biomedical QA-ES effective March 18, 2007), the
454A was probably the most popular and successful ESU anal yzer on the market. Originally
designed by Dynatech Nevada Inc., the 454A utilized industry standard current sensing
technology and offered accuracies of 5% of reading on RMS current an d 10% of range on RMS
power. For the the past decade, the 454A was considered to be an electrosurgery industry icon,
but despite this status in the market, it never really attained any level of actual customer
recommendation for any of the leading electrosurgery ge nerator manufacturers. See Appendix
A for full specifications on the discontinued Model 454A.
Fluke Biomedical RF-303RS: Originally marketed as the Bio-Tek Instruments RF-303RS, this is
the current “mid-range” ESU analyzer offering from Fluke Biomedical. The RF-303
does not
RS
utilize industry standard current sensing technology, but uses simple voltage measurement
instead. This product was designed during the period of time that Lionheart Technologies owned
and operated Bio-Tek Instruments, DNI Nevada, and Dale Technology. A concurrent compa nio n
product to the RF-303
was originally introduced in 1998 under the DNI Nevada (formerly
RS
Dynatech Nevada) brand as the Model 402A. The 402A was later re-branded as the Dale
Technology DALE3000 following the acquisition of the biomedical holdings of Lionheart
Technologies by Fluke Electronics (Fluke Biomedical) in 1 993. Instrument specifications for the
Fluke Biomedical (Bio-Tek Instruments) RF-303
Technology DALE3000 are (were) essentia lly identical. Current Fluke Biomed ical advertised specifications for the RF-303
are
+ 5% of reading or + 3 watts (whichever is greater) on RMS power and + 2.5% of readi ng or + 15m a (whichever is greater)
on RMS current. See Appendix B for full specifications on the RF-303
RS
.
, the DNI Nevada 402A, and the Dale
RS
RS
DNI Nevada Model 402A: The DNI Nevada Model 402A was the “sister product” to the Bio-Tek
Model RF-303, introduced concurrently with the RF-303 (see information above under the RF-
). Actual design, development, and manufacturing of t he 402A and the RF-3 03 took place
303
RS
at the DNI Nevada Inc. facility in Carson City, NV, under the ownership and management of
Lionheart Technologies, Inc. In order to make the two products look sufficiently different, and in
order to somehow truly differentiate the two, the 402A was given an RS232 communications port
and the RF-303 was given a battery for portable op eration. Slightly different enclosures were
also chosen, and the 402A was given an LED 7-segment display while the RF-303 was given an
LCD 7-segment display. The instrument firmware that operated the 402A and RF-303 was
common between the products, with firmware subroutines that recognized which instrument was being operated by the
microprocessor. The RS232 communications port was added to the RF-303 much later i n time, following the discontinuance
of the 402A. When Fluke Electronics (Fluke Biomed ical) acquired the biomedical holdings of Lionhea rt Technologies in 1993,
the DNI Nevada Model 402A was soon after discontinued and re-branded under the Dale Technology brand as the
DALE3000.
Dale Technology DALE3000: The DALE3000 existed in the market for less than three-years
before it was discontinued. The re-branding of the DNI Nevada Model 402A to the Dale
Technology DALE3000 was concurrent with the relocation of the Dale T echnology business from
its original location in Thornwood, NY to Carson City, NV, in the then-e xisting Fluke Biomedical
manufacturing facilities (the original Dynatech Nevada manufacturing facility and offices) in
Carson City, NV. The discontinuance of the DALE3000 was actually fairly close in time to the
Fluke acquisition of Metron AS of Trondheim, Norway, which brought the Metron QA-ES “highend” ESU analyzer to the Fluke Biomedical family of products.
BC Biomedical ESU-2000A: The BC Biomedical ESU-2000A was originally introduce d in the
year 2000, based upon strong customer dem and for a “simple but effective” legac y tester similar
to the original Bio-Tek Instruments RF-302. With accuracy of
+ 2% of full scale on current and
power, the ESU-2000A remains popular with customers today. It is still available from BC Group
International. Full instrument specifications for the ESU-2000A ESU analyzer c an be found on
the BC Group International web site at:
http://testequipmentandtools.com/acatalog/BCBiomedicalESU2000ADatasheet.pdf.
8 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 9

Fluke Biomedical / Metron QA-ES: The re-branded Metron (Trondheim, Norway) QA-ES is
the current “high-end” ESU analyzer offering from Fluke Biomedical, effective March 18,
2007. Originally introduced by Metron AS in Trondheim, Norway, the QA-ES offers accuracy
of 2% of reading for RMS current measurements. RMS power reading accuracy is not
specified by the manufacturer. The QA-ES does not utilize industr y standard c urrent sen sing
technology. See Appendix D for specifications on the Metron QA-ES v ersion of this analyzer.
Fluke Biomedical instrument specifications can be found on the manufacturer’s web site.
BC Biomedical ESU-2050: With commercial availability scheduled for July/August 2007, the
new BC Biomedical ESU-2050 represents a paradigm shift in ESU analyzer technology,
allowing customers to test their electrosurgical generators in exactly the same way as the
electrosurgery manufacturers do! With unprecedented 1% of reading accuracy, the ESU2050 is the most accurate ESU analyzer on the market, offering a dvanced level features and
functionality such as exporting waveform data sets with up to 32,768 data points to Microsoft
®
Excel
for graphing and analysis. Patent pending DFA® Technology.
BC Biomedical ESU-2300: With commercial availability sched uled for July/August 2007, the
new BC Biomedical ESU-2300 is a conventiona l design “mid-range” ESU analyzer utilizing
internal precision load resistors. Unlike other mid-range analyzers on the market, the ESU2300 utilizes industry standard current sensing technology for improved accuracy and
reliability. Like the ESU-2050, the ESU-2300 utilizes paten t pending DFA
ESU-2300 offers the ability to connect an external load resistor, thus ensu ring the availabilit y
of the required test load no matter what the value is. External load resistors can be used in
additive mode (add the external load resistor value to any of the internal load values) or
external only mode (use only the value of the external loa d resistor).
®
Technology. The
The release of the new BC Biomedical ESU-2000 Series, including the ESU-2050 and ESU-2300 analyzers
represents a paradigm shift in the level of technology offered for electrosurgery generator testing.
ESU-2050: Truly Unique in the Market
From its original product design proposal, the new BC Biomedical ESU2050 analyzer has been a totally different instrument as compared to the
traditional approach to ESU testing. Until now, traditional ESU analyzers
have had the following common elements:
• Internal load resistors (typically the most fragile element of the
conventional ESU analyzer)
• Accuracy on RMS power typically in the 5% to 10% range
• Accuracy on RMS current typically in the 2% to 5% range
• Crest factor (the ratio of V
peak
to V
16 or less
• Measurement technique: typically (less costly) voltage
measurement with the exception of the 454A which utilized
electrosurgical manufacturer industry standard current sensing
• “Active” type instruments typically utilize a thermal converter
The new ESU-2050 is a direct result of extensive collaboration with leading medical device industry
electrosurgery generator manufacturers. The ESU-2050 analyzer was designed to be 100% compatible with the
following mandates of some of the leading manufacturers in this area:
• Accuracy of 1% (give us a calibration quality instrument that can replace the legacy Fluke Electronics
Model 8920A
7
Digital Wide-Band True RMS Voltmeters currently in widespread use)
• Utilize external high-precision (1%) power resistors widely used in the OEM segment
) limitation typically around
rms
7
The Fluke 8920A was discontinued at the end of 1999 due to “product maturity” and electronic component shortages. For the detailed statement on
product discontinuance from Fluke Electronics, simply visit the following url:
9 Revision 1 – June 13, 2007
http://us.fluke.com/usen/support/safetynote/DiscontinuedProductNotice.htm.
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 10
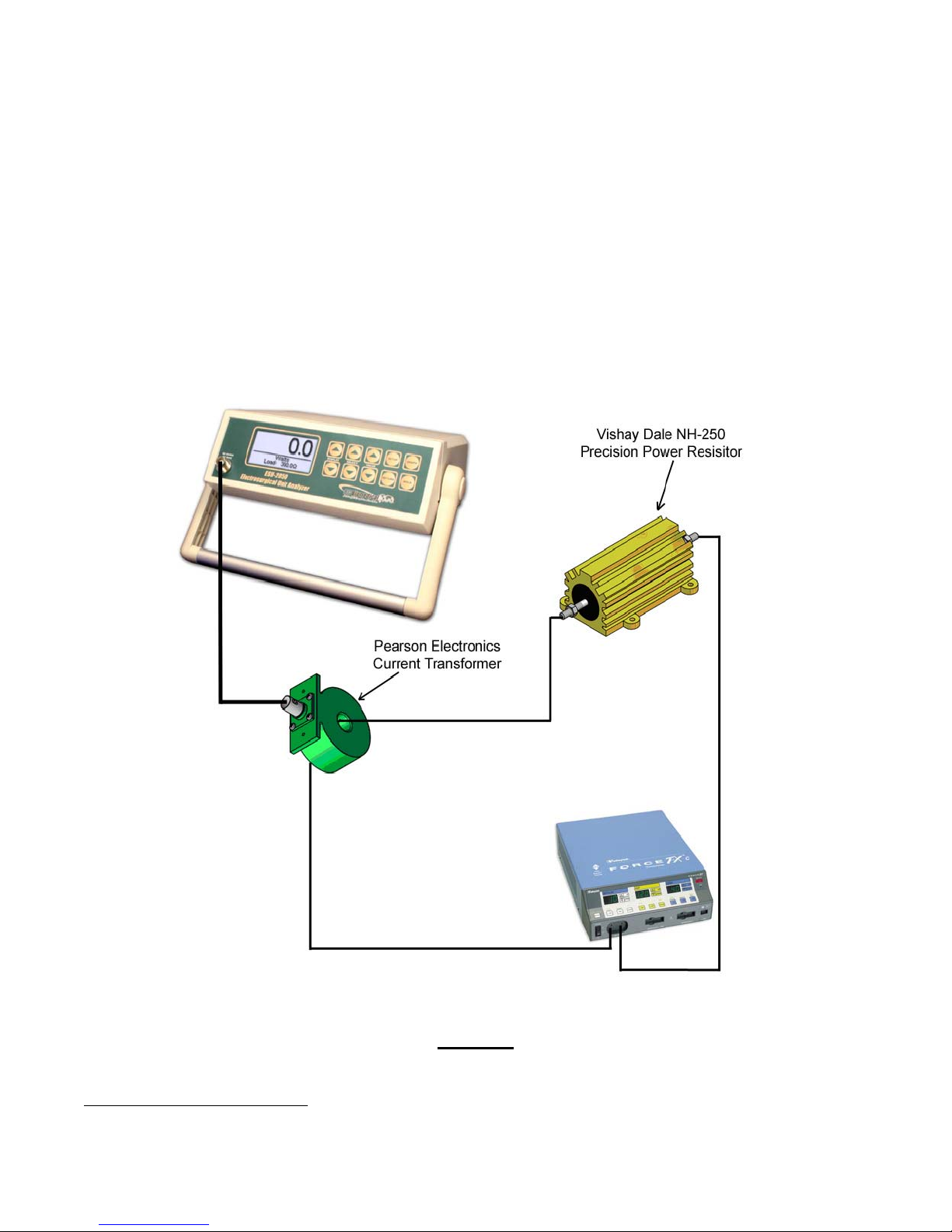
• Utilize external high-accuracy current sensing transformers of 0.1:1 and 1:1 ratio (typically Pearson
Electronics Model 411 and 4100 transformers), thus eliminating the virtual inaccuracies of commercially
available analyzers that utilize voltage measurement techniques
• Eliminate internal test load switching relays that add capacitive leakage at RF frequencies and decrease
the overall level of instrument accuracy
• Add the ability to capture and store in high resolution, the ESU output waveform
• Supply Crest Factor (CF) capability well in excess of the current industry (competitive instrument)
limitation of 16
• Make the new instrument much smaller and lighter, and more resistant to breakage during shipment than
the current industry available ESU analyzers
• In summary, give us an instrument that we can use to test the way we have tested our electrosurgery
generators over the past 10+ years!
The result of these ongoing collaborative efforts over the past 18 months is the new BC Biomedical ESU-2050
analyzer, with never before seen levels of accuracy and functionality in a commercially available ESU analyzer
8
Testing with the new ESU-2050 analyzer is remarkable easy, and requires minimal setup, as can be seen in
Figure 13 below.
.
Typical Test Setup Using the ESU-2050 ESU Analyzer – Test the Way ESU OEMs Test Their Products
8
See complete product specifications for the ESU-2050 on Page 21.
10 Revision 1 – June 13, 2007
Figure 13
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 11

ESU-2050: A Replacement for the Discontinued Fluke 8920A Instrument
The Fluke Electronics Model 8920A Digital Wide-Band True RMS Voltmeter with BNC
Input was widely adopted by electrosurgical generator manufacturers around the world.
Fluke Electronics discontinued this instrument and ceased all shipments by the end of
1999, leaving a void in the industry. Since the formal discontinuance of the 8920A,
several electrosurgical generator manufacturers have searched for a suitable
replacement instrument capable of delivering the same functionality and at the same level of accuracy as the
8920A. But none had been found up to and including the beginning of 2007. The development of the new BC
Biomedical ESU-2050 ESU analyzer was by design, intended to provide a suitable replacement for the Fluke
Electronics 8920A instruments currently in use by these manufacturers.
ESU-2050: Unprecedented 1% Accuracy In ESU Testing
The accuracy specifications (see ESU-2050 spe cifications on Page 21) of the new BC Biomedical ESU-2050 ESU
analyzer are well beyond any competitive ESU analyzer of the market today, meeting the requirements of even
the most demanding electrosurgical manufacturers. The ESU-2050 ESU analyzer allows the customer to test
according to the exact same methodology as the electrosurgery generator manufacturers test their own products.
This is an industry first! Now these manufacturers will be using the exact same instrumentation for test and
measurement that typical electrosurgery generator customers do!
ESU-2300: A More Conventional Approach
For those customers that do not wish to make the quantum leap from
conventional ESU testing methodologies to the new ESU-2050
platform, the new BC Biomedical ESU-2300 analyzer offers a more
conventional approach to ESU testing. The ESU-2300 utilizes some of
the best attributes of the ESU-2050 design:
• Patent pending DFA® Technology
• Industry standard current sensing technology via a custom
design current transformer designed specifically for the BC
Biomedical ESU-2300 by Pearson Electronics
• The latest in microprocessor design, which allows for high
speed digital acquisition and “interrogation” of the
electrosurgery generator waveform
The ESU-2300 is a mid-range analyzer (similar in price point to the Fluke Biomedical RF-303RS), but offers
superior features and accuracy (as result of the implementation of current sensing technology).
ESU-2400: More High-End Technology to Come
Is anything missing form the new BC Biomedical ESU-2000 Series lineup? What about a “high-end” analyzer?
The new BC Biomedical ESU-2400 “high-end” ESU analyzer is currently under active development. Stay tuned to
BC Group International, Inc. for more to come on the new BC Biomedical ESU-2400.
Common Element: Patent Pending DFA® Technology
The common element of all instruments in our new ESU-2000 Series is our patent pending DFA® Technology.
This technology platform allows the instrument to aggressively digitize the RF signal, analyze its components, and
provide highly accurate results. No other ESU analyzer on the market today uses this type of technology
platform. Not even the “high-end” competitive instruments offer this advanced level capability!
11 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 12
In fact, most competitive ESU analyzers on the market today utilize thermal technology, where the ESU generator
signal is fed into a thermal converter of some kind. This component measures the waveform energy through a
temperature change and provides a reading. Most commercially available “active” ESU analyzers have used this
technique for many years. The new BC Biomedical ESU-2000 Series of analyzers breaks this “old technology”
trend and introduces an exciting new level of ESU measurement technology moving forward!
Up To 32,768 Data Points!
For advanced level users, the number of A/D converter samples used in displaying the electrosurgery generator
measurement parameters can be adjusted to any of the following values: 1024, 2048, 4096, 8192, 16,384,
32,768. This setting adjusts the number of A/D converter readings used in each RMS mV computation. A higher
setting requires more computation and is slower, but results in a more stable reading. This setting also
determines the “resolution” of the stored and exported data sets for the captured electrosurgical generator
waveforms. The waveform data sets shown throughout this document and in Appendices E, F, and G all have
32,768 discrete data points.
ESU-2050 & ESU-2300: Precision Load Resistors Are
Where Accuracy Starts
If there is another industry standard among manufacturers of electrosurgery generators, it is the precision load
resistors that are commonly used in their manufacturing testing, service, and calibration functions. That is why we
chose the exact same external load resistors for use with our new BC Biomedical ESU-2050 analyzer: the proven
Vishay Dale NH-250 series. See Appendix J for additional information on these precision power re sistors.
Vishay Dale NH-250 Precision Power Resistors Commonly Used With the ESU-2050
The BC Biomedical ESU-2300 ESU ana lyzer also uses 1% precision power resistors manu factured by Riedon
Inc. (
www.riedon.com). These 225-watt rated precision resistors and are of a design that is more suitable for use
as an internal component, and have a superior accuracy specification compared to some of the resistors used in
competitive ESU analyzers.
Product Development In Cooperation with ESU Manufacturers
The product development campaign on the new BC Biomedical ESU-2050 analyzer, and the subsequent design
of the more conventional ESU-2300 analyzer has brought us into contact with some of the leading electrosurgical
manufacturers in the world market.
12 Revision 1 – June 13, 2007
Figure 14
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 13
As we move forward with ongoing product support and possible enhancements to our ESU-2000 Series of ESU
Analyzers, we will remain in contact with these manufacturers.
Industry Standard Current Sensing Technology
Virtually all of the world’s leading electrosurgery generator manufacturers use RF current sensing as their
standard means of measurement when they test, service, and calibrate their electrosurgical devices. This is why
we chose to implement the more costly but more effective current sensing technology in our new ESU-2000
Series products. The simple fact is that current sensing is more accurate and more reliable than voltage
measurement when it comes to ESU analyzers. But don’t just take our word for it. Ask your favorite
electrosurgery manufacturer which technology they approve and use.
Working With The Best In Current Sensing: Pearson Electronics
When it comes to sensing high frequency current flow, Pearson Electronics (www.pearsonelectronics.com) is one
of the very best companies in the market today. We chose to utilize their current sensing transformers with both
the ESU-2050 and ESU-2300 analyzers.
External Current Sensing Toroid Transformer by Pearson Electronics
The BC Biomedical ESU-2050 utilizes an external transformer. The Pearson Model 411 (0.1:1 ratio) and Model
4100 (1:1 ratio) are the specified transformers for use with the ESU-2050. These are the exact same
transformers currently used by many major electrosurgery generator manufacturers. Data sheets for these
transformers can be seen in Appendices H & I. For customer convenience, these external transformers are
available directly from BC Group International, Inc.
The BC Biomedical ESU-2300 utilizes an internal custom designed current transformer manufactured specifically
for BC Group International, Inc. by Pearson Electronics.
Current Sensing vs. Voltage Measurement
The simple voltage measurement technique utilized by many competitive ESU analyzers introduces several
distinct product attributes as compared to the industry standard current sensing technique:
13 Revision 1 – June 13, 2007
Figure 15
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 14

• reduced manufacturing cost for the test equipment manufacturer
• shorter product development time
• reduced accuracy for the end-user
This is why we chose to utilize the industry standard current sensing methodology in our new BC Biomedical
ESU-2050 and ESU-2300 instruments. The manufacturers of these voltage measurement based ESU analyzers
realize the shortcomings of this technology, and simply try to explain around them. The following statement is
from a User’s Manual Update
9
for a competitive product that utilizes the voltage measurement technique:
The information above is significant in two main areas. First, the explanation clearly indicates that when using the
device in question, the operator should expect measurement errors up to 35% (as compared to the industry
standard current sensing technique)! Secondly, it acknowledges that the industry accepted normal practice
by electrosurgery generator manufacturers is the superior current sensing technique.
With the discontinuance of the Fluke Biomedical Model 454A, the BC Biomedical ESU-2050 and ESU-2300 ESU
analyzers are now the only commercially available analyzers on the market today utilizing industry standard
current sensing technology!
Ensuring Quality By Taking Care Of The Details
Sometimes it comes down to the little things that ensure accuracy and a long-lasting life to your ESU analyzer.
Things like selecting the right load resistors and relays that switch in and out individual load resistors in the
internal load bank can make a big difference. The ESU-2300 utilizes switching relays rated at 10,000 volts
(isolation), 3 amps, 7500 volts (switching). The leading competitive “high-end” analyzer on the market utilizes
relays that are rated significantly lower than this. The ESU-2300 utilizes internal precision load resistors that are
rated at 1% tolerance (DC) with a power dissipation rating of 225 watts. The leading competitive “high-end” ESU
9
The complete User Manual Update can be downloaded in PDF format form the manufacturer’s web site at the following url:
http://global.flukebiomedical.com/busen/support/manuals/default.htm
14 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 15
analyzer on the market utilizes resistors that are rated at 5% tolerance (DC) with a power dissipation rating of only
175 watts. These are just a few of the subtle differences between the new BC Biomedical ESU-2000 Series of
ESU analyzers and competitive analyzers on the market today. Don’t let the perceived “promise” of a particular
brand fool you into making an inferior choice when it comes to selecting your new ESU analyzer, Do your
homework before you purchase!
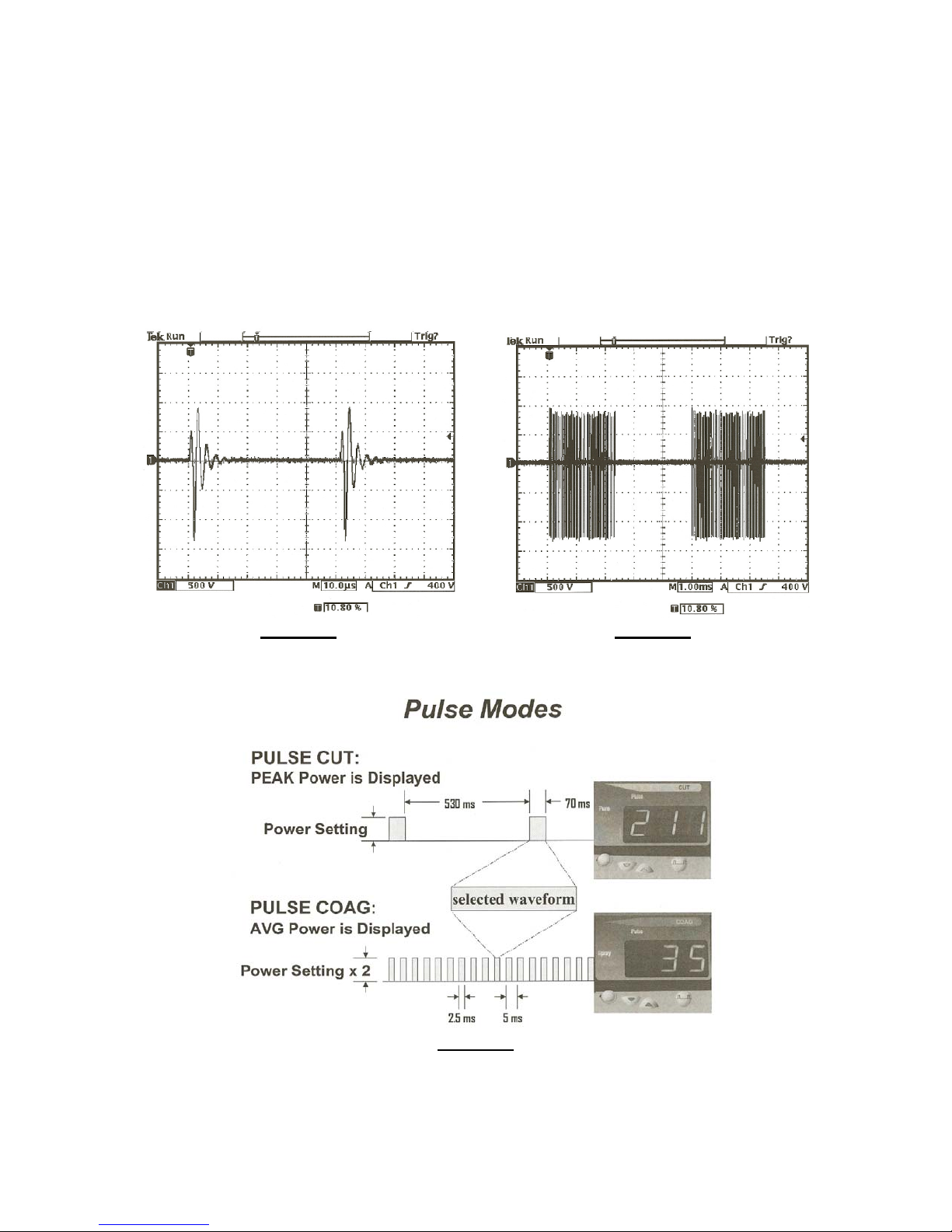
Those Crazy & Exotic Pulsed Waveforms
Conmed and Erbe are two manufacturers that offer electrosurgical generators with pulsed waveforms. Typically,
these pulsed waveforms have long single cycle time periods within which a signal is pulsed for a brief period of
time. This results in a very low duty cycle waveform that is extremely difficult to measure, let alone measure
accurately.
Figure 16 Figure 17
Conmed Spray RF Waveform Conmed Spray Pulsed Waveform
Figure 18
Conmed Pulse Waveform Modes
The BC Biomedical ESU-2050 Analyzer handles these pulsed waveforms easily, and yields accurate results time
after time, leaving all competitive units behind, wondering what hit them.
Copyright June, 2007 by BC Group International, Inc.
15 Revision 1 – June 13, 2007
Author: Michael R. Erwine
Page 16
ESU-2300: External Load Capabilities – Built In Non-Obsolescence
The most common shortcoming of any commercially available ESU analyzer is not having the correct load resistor
value available for the specific electrosurgery generator to be tested. No matter how many internal loads are
designed into a conventional ESU analyzer, you can be sure that one of the electrosurgery generator
manufacturers will eventually come along and specify a load that is not in the mix. That’s why we designed
external load resistor capability into our new ESU-2300 analyzer. Not only does the ESU-2300 allow you to
connect an external load, but you have the option of adding this external load value to the internal load selected
(additive mode) or simply using the external load for its actual value (external only mode).
Our ESU-2050 relies on external load resistors, so obsolescence due to unavailability of a specific test load is not
at all possible.
ESU-2000 Series PC Utility Software
Our BC Biomedical ESU-2000 Series Utility Software enhances the use of your BC Biomedical ESU-2050 and
ESU-2300 analyzers. When used with the ESU-2050, the software allows for export of the saved digitized
waveforms from the ESU-2050 to an Excel
®
workbook for further analysis. It also supports remote operation of
the ESU-2050 and ESU-2300. See Appendix M for sample screen shots form the utility software.
One Picture is Worth a Thousand Words – Or Up To 32,768 Data Points
We’ve all heard the old adage that “one picture is worth a thousand words”. The new BC Biomedical ESU-2050
analyzer puts some technological reality to this statement for the first time in ESU testing history. Through
utilization of the ESU-2000 Series PC Utility Software, now you can export data sets to Microsoft Excel
to 32,768 discrete data points on your electrosurgery generator’s output energy waveform. This export function
automatically creates an Excel
required. The created Excel
®
file that you can name anything you want. No knowledge of Microsoft Excel® is
®
file will automatically include all of the measurement data as well as a graphical
representation such as the one shown in Figure 19 below. See Appendix K for a sample look at the Excel
structure.
®
, with up
®
file
Microsoft Excel® (Automatically Created) Graphical Plot of Exported Electrosurgery Generator Waveform
You can then use the power of Excel
to Excel
®
, you can manipulate this data set to accomplish specific tasks, such as zooming in on a single cycle of a
specific waveform. See an example of this capability in the Figure 20 illustration below. This is a user-created
“zoomed” waveform based on the exported data.
16 Revision 1 – June 13, 2007
Figure 19
®
to analyze the data in any way you choose. Once you export the data set
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 17

Figure 20
Microsoft Excel Graphical Plot of Exported Electrosurgery Generator Waveform
With Zoom Manipulation of the Waveform Cycle
A sample Microsoft Excel® exported data set can be viewed in Appendix K. This example illustrates the
fundamental structure of the resulting Excel
on a single page. The original Excel
®
by a graph of the displayed data. This Excel
Biomedical ESU-2000 Series PC Utility Software. No knowledge of Excel
®
workbook, but has been reduced significantly in size to allow display
workbook contains 32,768 rows of actual measurement data, accompanied
®
workbook is created automatically by the export function of the BC
®
is required.
See The Data You Want – The Way You Want
Both the BC Biomedical ESU-2050 and ESU-2300 offer the capability of multiple parameter screens for viewing
test measurement parameters. The ESU-2050 allows you to select screens with 1 to 5 measurement parameters
for display. You can even select which parameters you want displayed in each window of the screen. For user
convenience, the value of load resistance is always displayed on the screen. See Figure 21 below.
ESU-2050: Display the Number of Measurement Parameters That You Want
The ESU-2300 offers multiple display screens for viewing instrument parameters and setup information. See
Figure 22 below for some examples of the wide screen layout of the ESU-2300’s LCD display with backlighting.
17 Revision 1 – June 13, 2007
Figure 21
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 18

Figure 22
ESU-2300: Multiple Options Are Available for Display of Measurement Parameteters
Easy Setup and Operation
The user interface of both the BC Biomedical ESU-2050 and ESU-2300 is extremely intuitive and easy to learn.
On-screen selection of test loads and other setup parameters is extremely easy.
Figure 23
The “learning curve” for new users of the ESU-2050 and ESU-2300 ESU analyzers is short and both instruments
have an extremely intuitive user interface.
The ESU-2050 and ESU-2300 User Interface is Easy to Learn
ESU-2050: Graphical Mode
The BC Biomedical ESU-2050 offers a graphical mode in which you can easily view the captured electrosurgical
generator waveform. You can even zoom in on the waveform for a closer look, and save it to any one of three
storage locations. Stored waveforms can have up to 32,768 discrete data points.
The ESU-2050 Graphical Mode Offers Advanced Capabilities
Up to three captured waveforms can be saved in the ESU-2050 for export to the ESU-2000 Series PC Utility
Software at any time.
The ESU-2000 Series PC Utility Software also works with the ESU-2300. See Appendix M for some screen shots
of the ESU-2000 Series PC Utility Software in action.
Figure 24
18 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 19

Product Comparison Overview
A brief overview of the BC Biomedical ESU-2000 Series as compared to leading competitive products on today’s
market is as follows:
Feature / Product BC Biomedical
Industry standard current sensing
technology
High-speed digital waveform processing
and analysis
Store digitized high resolution ESU
waveforms
Export ESU waveform data to Microsoft
®
Excel
Internal load resistors No Yes Yes Yes Yes
External load resistor capability Yes Yes Yes
RF Leakage Current Testing Yes
CQM Testing (Internal) No
Displayed parameters Power (watts)
RS232 Communications Port Yes Yes Yes Yes Yes
USB Communications Port Yes Yes No No No
Battery Operation No Yes No Yes No
Pulse waveform compatibility Yes No No No No
Accuracy specification for RMS current
Accuracy specification for RMS power
Easy Flash ROM field upgrades via PC Yes Yes No No No
First introduced to market (year) 2007 2007 1993 1998 2002
Manufactured In (Country) USA USA USA USA Norway
U.S. List Price $ 4,495 $ 3,495 $ 4,442 $ 3,439 $ 4,720
10
11
ESU-2050
Yes Yes Yes No
Yes
Patent Pending
Externally using
Externally per
Specifications
Peak Voltage
RMS Voltage
1% of Reading 2.5% of Reading 5% of Reading 2.5% of Reading 2% of Reading
1% of Reading 5% of Reading 10% of Reading 5% of Reading Not Specified
®
DFA
Technology
Yes No No No No
Yes Yes No No No
Perform Test
1:1 Current
Transformer
Perform
OEM
RMS Current
Crest Factor
Test Load Ω
For a more thorough product comparison, please visit
product comparison sheet on the ESU-2000 Series in Adobe Acrobat PDF format.
BC Biomedical
ESU-2300
Yes
Patent Pending
®
DFA
Technology
Yes Yes Yes Yes
Yes Yes
Power (watts)
RMS Current
Test Load Ω
Fluke
Biomedical
454A
(Discontinued)
No No No
Proprietary Load
Modules Only
Limited
Power (watts)
RMS Current
Peak to Peak
Voltage
Crest Factor
Test Load Ω
Fluke
Biomedical
RF303RS
Voltage
Measurement
No No
Yes
Limited
Power (watts)
RMS Current
Test Load Ω
Metron QA-ES
&
Fluke
Biomedical
QA-ES
No
Voltage
Measurement
Yes
Limited
Power (watts)
RMS Current
Peak to Peak
Voltage
Crest Factor
www.bcgroupintl.com/compare.htm and download the
10
This is an abbreviated specification. See the full specification in the product specifications at the end of this document or in the Appendices.
11
This is an abbreviated specification. See the full specification in the product specifications at the end of this document or in the Appendices.
19 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 20

One-Stop-Source: We Make It Easy for You
BC Group International, Inc. does whatever it takes to make it easy on our customers. Our customers who
purchase the ESU-2050 won’t have to go shopping elsewhere for their Pearson Electronics current sensing
transformers or Vishay Dale NH-250 precision load resistors. BC Group offers these product accessories directly
to the customer, without the standard long lead times from the original manufacturers.
Conclusion
At BC Group International, Inc., we would rather spend our time and effort designing and bringing new and
innovative products such as the new ESU-2050 and ESU-2300 ESU Analyzers to market under the BC
Biomedical brand, than waste it re-branding and re-launching 5 to 10 year old technology base products.
Innovative new products are what we are all about; not new photography and product overlays. We’ll let those
types of “development” efforts up to our competitors. We hold the technology needs of our customers in the
highest regard, and we are confident that our ongoing development efforts, in cooperation with some of the
medical device industry’s most significant players, will be recognized by our customers for what they are – a
sincere desire to lead the biomedical test and measurement industry through a continuous launch of exciting new
products. Our new BC Biomedical ESU-2050 and ESU-2300 ESU Analyzers are simply the most recent evidence
of our real mission. Stay tuned for much more to come!
Typical Test Setup Using the ESU-2050 ESU Analyzer – Test the Way ESU OEMs Test Their Products
20 Revision 1 – June 13, 2007
Figure 25
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 21

ESU-2050 Product Specifications
12
INPUT RANGE
Voltage (RMS): 2.0 – 700.0 mV RMS
Input Resolution: 0.1 mV RMS
Voltage (Peak): 1000.0 mV
Resolution: 0.1 mV
Frequency: 10 kHz – 10 MHz
Accuracy: 0.5 mV,
< 50 mV
1% of reading, > 50 mV, up to 1 MHz
3% of reading, > 50 mV, 1 to 10 MHz
CALCULATED RANGES
Current (with 0.1:1 CT): 7000 mA RMS
Resolution: 1 mA
Current (with 1:1 CT): 700.0 mA RMS
Resolution: 0.1 mA
Wattage: 999.9 Watts
Resolution: 0.1 Watt
Crest Factor: 1.4 to 500
Resolution: 0.1
INPUT IMPEDANCE
50 Ohms
INPUT COMPATIBILITY
RF Current Transformer (50 ohm): Pearson Electronics 411 or 4100 (Typical)
RF Current Transformer Attenuation: 0.1:1 or 1:1 User Selectable
OTHER
Display: LCD Graphical 128 x 64 Pixels with Backlight
Setup Memory: EEPROM, All Parameters
Memory Retention: 10 Years without Power
Operating Range: 15 to 30 Degrees C
Storage Range: -40 to 60 Degrees C
Construction: Enclosure – ABS Plastic
Face – Lexan, Back Printed
Size: 3.4” H x 9.1” W x 8.0” D
Weight:
Connections: Input: BNC
Output: Serial DB-9 or USB
Power Supply Adapter: 6 VDC, Cen ter Positive, 300 mA
Power Consumption: ON: less than 150 mA
OFF: less than 40 ua
Data Storage (Internal): 3 Sets of 32,768 Data Points
< 3 lbs
12
Specifications are accurate as of the date of release of this document. Specifications are subject to change without prior notice.
21 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 22

ESU-2300 Product Specifications
13
Measurement
Method
Power
Range
Resolution
Accuracy
Industry Standard Current
Sensing Using RF Current
Transformer (Pearson
®
Coil)
Weight
1.0 to 400.0 Watts RMS
0.1 Watts
+ 5% Reading or + 3 Watts
Physical
Enclosure
Electrical
Power Supply
(whichever is greater)
Voltage
Current
Range
Resolution
Accuracy
Frequency
30 to 2500 mA RMS
1 mA
+ 2.5% Reading or + 15 mA
(whichever is greater)
Battery
General
Display
Limits
Bandwidth
Crest Factor
Voltage
Ventilation
10 kHz to 10 MHz
1.4 to 500
10,000 Peak
Oscilloscope Output
Setup Memory
Memory Retention
Operating Range 15 to 35 Degrees C
Loads
Main Test Load
Range
Resolution
Accuracy
Duty Cycle
Auxiliary Test Load
Fixed
Accuracy
Rating
CQM Test Load
Range
Resolution
Accuracy
Storage Range -40 to 60 Degrees C
Humidity Limit
50 to 750 Ω
50 Ω
+ 1% (DC)
50% (1 minute period)
Connections
200 Ω
+ 1% (DC)
225 Watts
(Contact Quality Monitor test
load is an independent variable
load)
1 to 500 Ω
1 Ω
+ 2%
6.0” x 13.5” x 12.0”
12.5 lbs
External Universal Power
Supply: 12 VDC Output
83 to 264 VAC
47 to 63 Hz
Sealed Lead Acid
6 VDC, 7.2 AH
LCD Graphical 256 x 64
Pixels with Backlight
Internal Fan, variable speed,
over-temperature protected,
Fan rotor sensor
Isolated (uncalibrated), BNC
Connector
EEPROM, All Parameters
10 Years without Power
90% Non-Condensing
Oscilloscope: BNC
Communications: USB &
RS232 DB-9
External Loads: 4mm Safety
Sockets
13
Specifications are accurate as of the date of release of this document. Specifications are subject to change without prior notice.
22 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 23

Technical References
• Tyco Healthcare / Valleylab Electrosurgery Self-Study Guide, Copyright Tyco Healthcare Valleylab,
September, 1999 (Author: Brenda C. Ulmer, RN, MN, CNOR)
• Fluke Biomedical / DNI Nevada Model 454A Electrosurgical Analyzer Operating Manual, Copyright Fluke
Electronics / Fluke Biomedical
• Fluke Biomedical / Bio-Tek Instruments RF-303 Electrosurgical Analyzer Operator’s Manu al, Copyright
September, 1998 Fluke Electronics / Fluke Biomedical
• Metron QA-ES Operator’s Manual, Copyright Fluke Electronics / Fluke Biomedical
• DNI Nevada Model 402A Operating Manual, Copyright Fluke Electronics / Fluke Biomedical
• Dale Technology DALE3000 Instruction Manual, Copyright Fluke Electronics / Fluke Biomedical
• Tyco Healthcare / Valleylab Force 2 Electrosurgical Generator Service Manual, Copyright 2004
• Tyco Healthcare / Valleylab Force FX Electrosurgical Generator Service Manual, Copyright 2006
• Tyco Healthcare / Valleylab LigaSure Vessel Sealing System Service Manual, Copyright 2004
• Electrosurgical Generators: Guide to Performance and Safety Testing (ISBN 09661128-1-4), Copyright
Tektran Incorporated (Available for downloading as a PDF at
• Principles of Electrosurgery (ISBN 0-9661128-0-6), Copyright Tektran Incorporated (Available for
downloading as a PDF at
www.tektran.com)
• Understanding Electrosurgery, Bovie / Aaron Medical (Publication # MC-55-049-001 Rev 1), Copyright
July, 2003
• Application Note: Electrosurgical Analyzer Primer, Copyright Fluke Electronics / Fluke Biomedical
(Available for downloading as a PDF from the Fluke Electronics / Fluke Biomedical website at
http://global.flukebiomedical.com/busen/support/appnotes/default.htm#)
• ANSI/AAMI HF18-2001 Standard, Electrosurgical Devices
www.tektran.com)
23 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 24

About the Author
Michael R. Erwine has been involved in the medical device industry since 1975, with his roots being in biomedical
and clinical engineering. Since January, 2006 Mr. Erwine has been employed by BC Group International, Inc.
and is currently fulfilling multiple roles including Strategic Accounts Manager, Worldwide Dealer Network
Manager, Product Manager, as well as other functions. He also manages the BC Group International, Inc.
Western Regional Office in Carson City, NV. Since 1989, Mr. Erwine has worked for or with (under strategic
alliances, marketing partnerships, corporate family structure, etc.), the following major biomedical test and
measurement manufacturers and service providers:
• Spectrum Technologies Inc. (Elysburg, PA)
• Dynatech Nevada Inc. (Carson City, NV)
• Metron AS (Trondheim, Norway)
• Bender GmbH (Grunberg, Germany)
• Datrend Systems (Burnaby, Canada)
• Lionheart Technologies Inc. (Carson City, NV)
• DNI Nevada Inc. (Carson City, NV)
• Bio-Tek Instruments Corp. (Burlington, VT)
• Ultramedic Ltd (Liverpool, England)
• Labatoire Gamida (Eaubonne, France)
• Dale Technology Inc. (Thornwood, NY)
• Fluke Biomedical Corp. (Carson City, NV)
• Fluke Electronics Corp. (Everett, WA)
• Metron USA (Grand Rapids, MI)
During his career track, Mr. Erwine has served in the following capacities, just to name a few:
• Product Manager
• Project Manager
• Product Validation Engineer
• Commercial Manager
• National Sales Manager
• International Sales Manager
• Strategic Accounts Manager
• VP of Sales & Marketing
• Executive Vice President
• General Manager
In the electrosurgery testing specialty area, Mr. Erwine has interfaced and worked with major medical device
manufacturers such as Tyco Healthcare (Valleylab), Conmed (Electrosurgery Division), Erbe USA, Bovie, etc.
While at Dynatech Nevada Inc., Mr. Erwine successfully launched the industry icon Model 454A ESU Analyzer.
Following the acquisition of Dynatech Nevada, Inc. by Lionheart Technologies in 1997, Mr. Erwine launched the
DNI Nevada Model 402A ESU Analyzer and assisted the Bio-Tek Instruments sales and marketing staff in the
launch of their very first “active” ESU testing device in the way of the concurrent Bio-Tek Model RF-303 (currently
offered as the Fluke Biomedical RF-303
Mr. Erwine has worked heavily with Tyco Healthcare / Valleylab and Conmed Electrosurgery Division since
January, 2006 on the development and functionality of the new BC Biomedical ESU-2050 analyzer, and has also
interfaced with Erbe USA, Bovie, Megadyne, and others during the BC Biomedical ESU-2000 Series development
project. Mr. Erwine continues to work with all of these medical device manufacturers in his various roles at BC
Group International, Inc. The design and functionality of the new BC Biomedical ESU-2000 Series of ESU
Analyzers is strongly rooted in these collaborative efforts with some of the same medical device manufacturers
with whom Mr. Erwine has historically worked.
If you have questions concerning anything in this document or on the ESU-2000 Series of Electrosurgery
Analyzers in general, please feel free to e-mail the author at
RS
).
merwine@bcgroupintl.com.
24 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 25

APPENDICES
All documents and information contained in the following Appendices have been
obtained from various sources within the public sector, and do not represent confidential
information or trade secrets of any kind. Recognition of ownership and copyright is
hereby given to the companies and manufacturers whose product and other types of
information are represented here in this document for informational purposes. Original
ownership and copyright of this documentation remains with these companies.
25 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 26

APPENDIX A
Fluke Biomedical 454A Instrument Specifications
(Source: Fluke Biomedical Model 454A Operating Manual)
26 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 27

APPENDIX B
Fluke Biomedical RF-303RS Instrument Specifications
(Source: Fluke Biomedical RF-303 Operator’s Manual)
27 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 28

APPENDIX B (Continued)
Fluke Biomedical RF-303RS Instrument Specifications
(Source: Fluke Biomedical RF-303 Operator’s Manual)
28 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 29

APPENDIX B (Continued)
Fluke Biomedical RF-303RS Instrument Specifications
(Source: Fluke Biomedical RF-303 Operator’s Manual)
29 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 30

APPENDIX B (Continued)
Fluke Biomedical RF-303RS Instrument Specifications
(Source: Fluke Biomedical RF-303 Operator’s Manual)
30 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 31

APPENDIX C
Dale Technology DALE3000 Instrument Specifications
(Source: Dale Technology Product Catalog)
31 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 32

APPENDIX C (Continued)
Dale Technology DALE3000 Instrument Specifications
(Source: Dale Technology Product Catalog)
32 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 33

APPENDIX D
Metron QA-ES Instrument Specifications
(Source: Metron QA-ES Product Data Sheet)
33 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 34

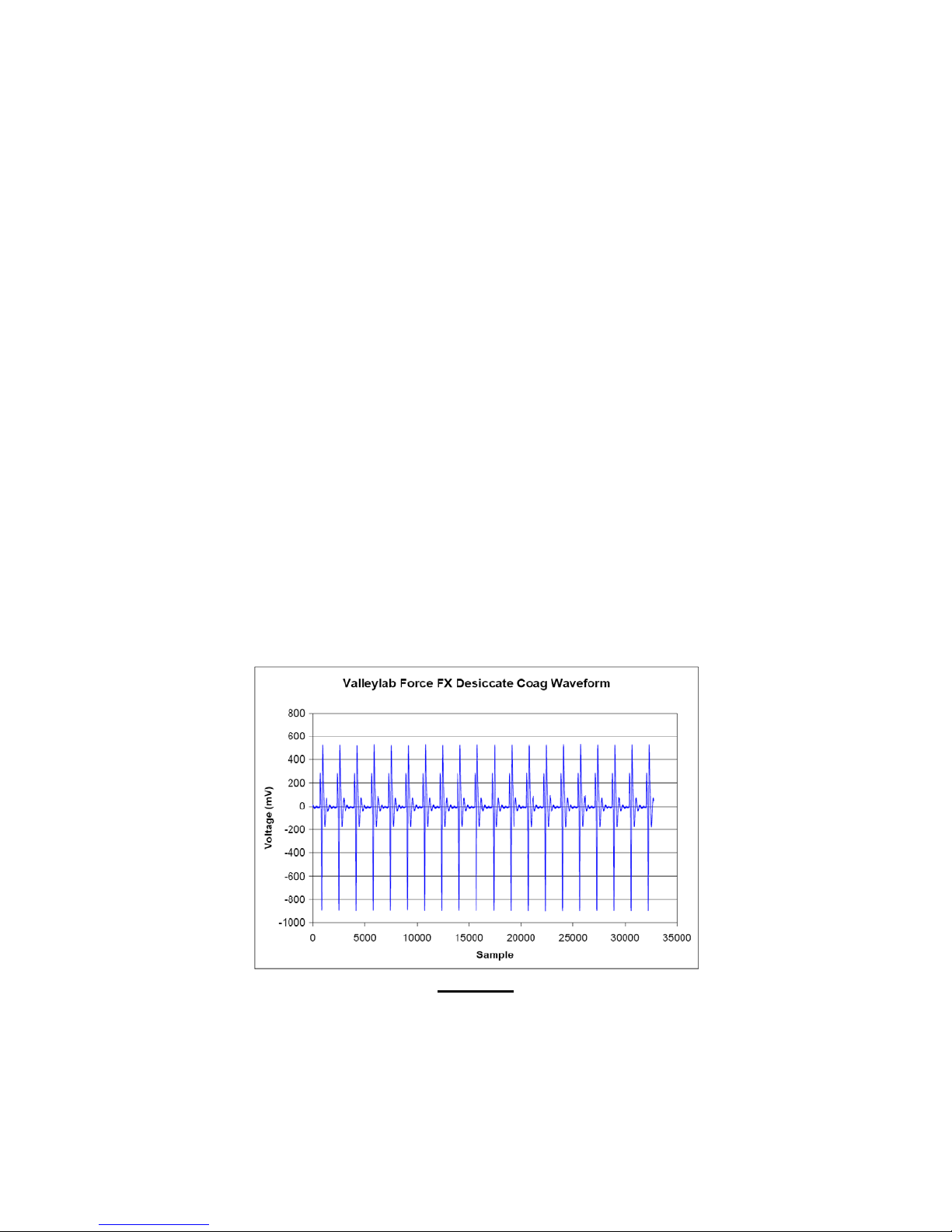
APPENDIX E
Valleylab Force FX Generator Output Waveforms
(Source: BC Biomedical ESU-2000 Series PC Utility Software Excel® File Export14)
14
Graphed data shown in the left hand set of illustrations above was automatically created by Excel® as part of the normal data export from the ESU-2000
Series PC Utility Software. No user knowledge of Excel
within Excel
34 Revision 1 – June 13, 2007
®
by the user, based upon the data exported from the ESU-2000 Series PC Utility Software.
®
for graphing purposes is required at this level. The graphs on the right were individually created
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 35

APPENDIX E (Continued)
Valleylab Force FX Generator Output Waveforms
(Source: BC Biomedical ESU-2000 Series PC Utility Software Excel® File Export15)
15
Graphed data shown in the left hand set of illustrations above was automatically created by Excel® as part of the normal data export from the ESU-2000
Series PC Utility Software. No user knowledge of Excel
within Excel
35 Revision 1 – June 13, 2007
®
by the user, based upon the data exported from the ESU-2000 Series PC Utility Software.
®
for graphing purposes is required at this level. The graphs on the right were individually created
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 36

APPENDIX F
Valleylab Force 2 Generator Output waveforms
(Source: BC Biomedical ESU-2000 Series PC Utility Software Excel® File Export16)
16
Graphed data shown in the left hand set of illustrations above was automatically created by Excel® as part of the normal data export from the ESU-2000
Series PC Utility Software. No user knowledge of Excel
within Excel
36 Revision 1 – June 13, 2007
®
by the user, based upon the data exported from the ESU-2000 Series PC Utility Software.
®
for graphing purposes is required at this level. The graphs on the right were individually created
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 37

APPENDIX F (Continued)
Valleylab Force 2 Generator Output waveforms
(Source: BC Biomedical ESU-2000 Series PC Utility Software Excel® File Export17)
17
Graphed data shown in the left hand set of illustrations above was automatically created by Excel® as part of the normal data export from the ESU-2000
Series PC Utility Software. No user knowledge of Excel
within Excel
37 Revision 1 – June 13, 2007
®
by the user, based upon the data exported from the ESU-2000 Series PC Utility Software.
®
for graphing purposes is required at this level. The graphs on the right were individually created
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 38

APPENDIX G
Conmed System 5000 Output Waveforms
(Source: BC Biomedical ESU-2000 Series PC Utility Software Excel® File Export18)
18
Graphed data shown in the left hand set of illustrations above was automatically created by Excel® as part of the normal data export from the ESU-2000
Series PC Utility Software. No user knowledge of Excel
within Excel
38 Revision 1 – June 13, 2007
®
by the user, based upon the data exported from the ESU-2000 Series PC Utility Software.
®
for graphing purposes is required at this level. The graphs on the right were individually created
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 39

APPENDIX G (Continued)
Conmed System 5000 Output Waveforms
(Source: BC Biomedical ESU-2000 Series PC Utility Software Excel® File Export19)
19
Graphed data shown in the left hand set of illustrations above was automatically created by Excel® as part of the normal data export from the ESU-2000
Series PC Utility Software. No user knowledge of Excel
within Excel
39 Revision 1 – June 13, 2007
®
by the user, based upon the data exported from the ESU-2000 Series PC Utility Software.
®
for graphing purposes is required at this level. The graphs on the right were individually created
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 40

APPENDIX G (Continued)
Conmed System 5000 Output Waveforms
(Source: BC Biomedical ESU-2000 Series PC Utility Software Excel® File Export20)
20
Graphed data shown in the left hand set of illustrations above was automatically created by Excel® as part of the normal data export from the ESU-2000
Series PC Utility Software. No user knowledge of Excel
within Excel
40 Revision 1 – June 13, 2007
®
by the user, based upon the data exported from the ESU-2000 Series PC Utility Software.
®
for graphing purposes is required at this level. The graphs on the right were individually created
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 41

APPENDIX H
Pearson Electronics Model 411 Data Sheet
(Source: www.pearsonelectronics.com)
41 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 42

APPENDIX I
Pearson Electronics Model 4100 Data Sheet
(Source: www.pearsonelectronics.com)
42 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 43

APPENDIX J
Vishay Dale NH-250 Data Sheet
(Source: www.vishay.com)
43 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 44

APPENDIX J (Continued)
Vishay Dale NH-250 Data Sheet
(Source: www.vishay.com)
44 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 45

APPENDIX K
Sample Microsoft Excel® Data Export Workbook
(Limited in format to what can be displayed on this page)
21
21
The illustration above shows the left hand portion of a graph that is automatically created during the data export process from the ESU-2000 PC Utility
Software. No knowledge of Excel
illustration purposes. The actual Excel
file size is approximately 1.5 MB.
45 Revision 1 – June 13, 2007
®
is required to get this data and the resulting graph into Excel®. The measurement data shown above is abbreviated for
®
spreadsheet created by the data export function contains a total of 32,768 rows of actual measurement data. Excel®
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 46

APPENDIX L
Valleylab Recommended Test Procedure
(Source: Valleylab Force 2 Electrosurgical Generator Service Manual)
22
22
For their Force 2 Electrosurgical Generator, Valleylab recommends a current sensing test setup utilizing a Pearson Electronics Model 411 current
transformer and Dale NH-250 precision resistors. The Fluke 8920A is no longer available and is being replaced with the BC Biomedical ESU-2050.
46 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 47

APPENDIX L (Continued)
Valleylab Recommended Test Procedure
(Source: Valleylab Ligasure Vessel Sealing Generator Service Manual)
23
23
For their Ligasure Vessel Sealing Generator, Valleylab recommends a current sensing test setup utilizing a Pearson Electronics Model 411 current
transformer and Dale NH-250 precision resistors. The Fluke 8920A is no longer available and is being replaced with the BC Biomedical ESU-2050.
47 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 48

APPENDIX L (Continued)
Valleylab Recommended Test Procedure
(Source: Valleylab Force FX-C Electrosurgical Generator Service Manual)
24
24
For their Force FX-C Electrosurgical Generator, Valleylab recommends a current sensing test setup utilizing a Pearson Electronics Model 411 current
transformer and Dale NH-250 precision resistors. Measurement procedures are outlined as above. The Fluke 8920A is no longer available and is being
replaced with the BC Biomedical ESU-2050.
48 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 49

APPENDIX M
ESU-2000 Series PC Utility Software Screen Shots
25
25
Version 1.1 shown – software subject to change with new features and functionality added through routine updates.
49 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 50

APPENDIX M (Continued)
ESU-2000 Series PC Utility Software Screen Shots
26
26
Version 1.1 shown – software subject to change with new features and functionality added through routine updates.
50 Revision 1 – June 13, 2007
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 51

APPENDIX M (Continued)
ESU-2000 Series PC Utility Software Screen Shots
27
Microsoft Excel
®
File Screen Shot - See Footnote
28
27
Version 1.1 shown – software subject to change with new features and functionality added through routine updates.
28
After the ESU waveform is exported from The ESU-2000 Series PC Utility Software to Microsoft Excel®, this file is automatically created. No user
knowledge of Microsoft Excel
51 Revision 1 – June 13, 2007
®
is required to create this file or the accompanying graph.
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
Page 52

APPENDIX N
History of BC Group International, Inc.
BC Group was founded in 1988. The company was
formed as a sales and service organization to
handle the test equipment needs of the worldwide
biomedical engineering community.
Originally, BC Group sold only test equipment
manufactured by other companies. Then, in 2000,
we began manufacturing our own product line under
the now familiar Green and Gold BC Biomedical
brand.
In January of 2005, Lloyd Industries, the company
that engineered and manufactured most of BC
Biomedical brand products, purchased BC Group.
This acquisition has allowed our products and
services to expand at an even faster pace. BC
Group, under the “BC Biomedical” brand, is now the
second largest manufacturer of biomedical test and
measurement equipment in the world.
Our philosophy with BC Biomedical products runs
counter to the current trend of “one-size-fits-all”. We
offer families of products that provide the users with
a choice of models so they can pick the features
they need and at a price they can afford.
We are working closely with leading medical device
manufacturers worldwide, in the continuing
development of new and innovative test and
measurement products for the biomedical
engineering community. For 2007, our new ESU2000 Family of Electrosurgery Analyzers and our
new ULT-2000 Series of Ultrasound Electrical
Leakage Current Testers are just the latest evidence
of this important collaboration with medical device
manufacturers worldwide. We have a lot more in
store for the future, so keep checking our website for
the latest additions to the BC Biomedical product
line.
Website:
E-mail: merwine@bcgroupintl.com
52 Revision 1 – June 13, 2007
www.bcgroupintl.com
Copyright June, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
 Loading...
Loading...