Page 1

DEFIBRILLATOR
ANALYZERS
DA-2006
DA-2006P
ANALYZER
W/ PACER
SERVICE MANUAL
Page 2
BC BIOMEDICAL
SERVICE MANUAL
DA-2006 SERIES
TABLE OF CONTENTS
WARNINGS, CAUTIONS, NOTICES ...............................................................................ii
MANUAL REVISIONS.....................................................................................................iv
WARRANTY....................................................................................................................iv
MANUALS
DA-2006 Series User Manual (Rev 06)............................................. SECTION A
DA-CS-06 (Rev 02) ........................................................................... SECTION A
OPERATIONAL THEORY.............................................................................. SECTION B
VALIDATION / CALIBRATION .......................................................................SECTION C
PARTS ...........................................................................................................SECTION D
This Service Manual covers the following units:
• DA-2006
• DA-2006P
i
Page 3
The DA-2006 Series Analyzers are intended to be
serviced only by authorized service personnel.
Troubleshooting and service procedures
WARNING - MODIFICATIONS
The DA-2006 Series Analyzers are intended for
use within the published specifications. Any
application beyond these specifications or any
unauthorized user modifications may result in
CAUTION - SERVICE
should only be performed by
qualified technical personnel.
hazards or improper operation.
ii
Page 4
USER ASSUMES FULL RESPONSIBILITY FOR UNAUTHORIZED
EQUIPMENT MODIFICATIONS OR APPLICATION OF EQUIPMENT
OUTSIDE OF THE PUBLISHED INTENDED USE AND
SPECIFICATIONS. SUCH MODIFICATIONS OR APPLICATIONS
MAY RESULT IN EQUIPMENT DAMAGE OR PERSONAL INJURY.
BC GROUP INTERNATIONAL, INC. RESERVES THE RIGHT TO
MAKE CHANGES TO ITS PRODUCTS OR SPECIFICATIONS AT
ANY TIME, WITHOUT NOTICE, IN ORDER TO IMPROVE THE
DESIGN OR PERFORMANCE AND TO SUPPLY THE BEST
POSSIBLE PRODUCT. THE INFORMATION IN THIS MANUAL HAS
BEEN CAREFULLY CHECKED AND IS BELIEVED TO BE
ACCURATE. HOWEVER, NO RESPONSIBILITY
IS ASSUMED FOR INACCURACIES.
NOTICE – CONTACT INFORMATION
NOTICE – DISCLAIMER
NOTICE – DISCLAIMER
BC BIOMEDICAL
BC GROUP INTERNATIONAL, INC.
PO BOX 25125
9415 GENTRY AVE
ST. LOUIS, MO 63125
USA
1-800-242-8428
314-638-3800
www.bcgroupintl.com
sales@bcgroupintl.com
Service Manual DA-2006 Series Copyright © 2007
www.bcgroupintl.com Made in the USA
5/07 Rev 02
iii
Page 5

A
A
MANUAL REVISIONS
Revision # Engineering # Revisions Made
Rev 01 7395 Origination
Rev 02 395 Drawing scans inserted
LIMITED WARRANTY
WARRANTY
FROM DEFECTS IN MATERIALS AND WORKMANSHIP UNDER THE SERVICE FOR WHICH THEY
RE INTENDED. THIS WARRANTY IS EFFECTIVE FOR TWELVE MONTHS FROM THE DATE OF
SHIPMENT.
EXCLUSIONS: THIS WARRANTY IS IN LIEU OF ANY OTHER WARRANTY EXPRESSED OR
IMPLIED, INCLUDING, BUT NOT LIMITED TO ANY IMPLIED WARRANTY OF MERCHANTABILITY
OR FITNESS FOR A PARTICULAR PURPOSE.
BC GROUP INTERNATIONAL, INC. IS NOT LIABLE FOR ANY INCIDENTAL OR CONSEQUENTIAL
DAMAGES.
NO PERSON OTHER THAN AN OFFICER IS AUTHORIZED TO GIVE ANY OTHER WARRANTY OR
SSUME ANY LIABILITY.
REMEDIES: THE PURCHASER'S SOLE AND EXCLUSIVE REMEDY SHALL BE: (1) THE REPAIR OR
REPLACEMENT OF DEFECTIVE PARTS OR PRODUCTS, WITHOUT CHARGE. (2) AT THE OPTION
OF BC GROUP INTERNATIONAL, INC., THE REFUND OF THE PURCHASE PRICE.
: BC GROUP INTERNATIONAL, INC. WARRANTS ITS NEW PRODUCTS TO BE FREE
P:\Manuals\BCGroup\…\DA-2006_Series_SM_Rev02.doc
iv
Page 6
SECTION A
USER MANUAL
DA-2006 SERIES
REV 06
Page 7

DEFIBRILLATOR
ANALYZERS
DA-2006
DA-2006P
ANALYZER
W/ PACER
USER MANUAL
Page 8
BC BIOMEDICAL
DA-2006 SERIES
TABLE OF CONTENTS
WARNINGS, CAUTIONS, NOTICES ........................................................................... 2
DESCRIPTION............................................................................................................. 7
OVERVIEW.................................................................................................................. 15
DEFIBRILLATOR ANALYZER ..................................................................................... 23
MAIN SCREEN.................................................................................................. 23
ECG WAVEFORMS SCREEN .......................................................................... 31
PLAYBACK LAST PULSE SCREEN ................................................................. 35
START CHARGE TIMER SCREEN .................................................................. 37
PRINT HEADER................................................................................................ 39
SELF TEST WAVEFORM ................................................................................. 41
RUNNING A DEFIBRILLATION TEST .............................................................. 43
INTRODUCTION .................................................................................... 43
DEFIBRILLATION TEST......................................................................... 45
CARDIOVERSION TEST........................................................................ 49
CHARGE TIME TEST............................................................................. 53
SHOCK ADVISORY ALGORITHM TEST ............................................... 55
TRANSCUTANEOUS PACEMAKER ANALYZER ....................................................... 57
PACE MAIN SCREEN ...................................................................................... 57
PACER MODE SETUP SCREEN...................................................................... 63
SENSITIVITY TEST .......................................................................................... 65
REFRACTORY PERIOD TEST ......................................................................... 67
PRINT MENU SCREEN .................................................................................... 69
PLAYBACK LAST PULSE SCREEN ................................................................. 71
1
Page 9

DA-2006 Series
MESSAGES ................................................................................................................. 73
SYSTEM SETUP.......................................................................................................... 75
POWER UP SETTINGS............................................................................................... 77
AUTO SEQUENCE FUNCTION................................................................................... 79
VIEW MODE ..................................................................................................... 81
RUN MODE ...................................................................................................... 85
PROGRAMMING AUTO SEQUENCES ....................................................................... 95
MANUAL REVISIONS................................................................................................ 107
WARRANTY............................................................................................................... 107
SPECIFICATIONS ..................................................................................................... 109
NOTES....................................................................................................................... 115
2
Page 10
DA-2006 Series
WARNING - USERS
The DA-2006 Series Analyzers are for use by
skilled technical personnel only.
WARNING - USE
The DA-2006 Series Analyzers are intended for
testing only and they should never be used in
diagnostics, treatment or any other capacity
where they would come in contact with a patient.
WARNING - MODIFICATIONS
The DA-2006 Series Analyzers are intended for
use within the published specifications. Any
application beyond these specifications or any
unauthorized user modifications may result in
hazards or improper operation.
WARNING - CONNECTIONS
All connections to patients must be removed
before connecting the Device Under Test (DUT)
to the Analyzer. A serious hazard may occur
if the patient is connected when
testing with the Analyzer.
Do not connect any leads from the patient
directly to the Analyzer or DUT.
WARNING - POWER ADAPTOR
Unplug the Power Adaptor before
cleaning the surface of the Analyzer.
WARNING - LIQUIDS
Do not submerge or spill liquids on the Analyzer.
Do not operate the Analyzer if internal
components not intended for use with fluids may
have been exposed to fluid, as the internal
leakage may have caused corrosion and be a
potential hazard.
3
Page 11
DA-2006 Series
CAUTION - SERVICE
The DA-2006 Series Analyzers are intended to be
serviced only by authorized service personnel.
Troubleshooting and service procedures
should only be performed by
qualified technical personnel.
CAUTION - ENVIRONMENT
The DA-2006 Series Analyzers are intended to
function between 15 and 40 °C.
Exposure to temperatures outside this range can
adversely affect the performance of the Analyzer.
CAUTION - CLEANING
Do not immerse. The Analyzer should be
cleaned by wiping gently with a damp, lint-free
cloth.
CAUTION - INSPECTION
The DA-2006 Series Analyzers should be
inspected before each use for wear and
the Analyzer should be serviced
if any parts are in question.
NOTICE – SYMBOLS
Symbol Description
Caution
(Consult Manual for Further Information)
Center Positive
Direct Current
4
Page 12
DA-2006 Series
NOTICE – ABBREVIATIONS
A, Amps Amperes
BPM Beats Per Minute
-2
c centi- (10
C Celsius
° degree
dt Delta Time, Change in Time
DUT Device Under Test
E Energy
ECG Electrocardiogram
Euro European
Hz hertz
J Joules
k kilo- (10
kg kilograms
lbs pounds
µ micro- (10
µA microampere
µH microhertz
µV microvolt
µsec microsecond
m milli- (10
mA milliampere
mm millimeter
ms, mS,
msec
millisecond
mV millivolts
Ω ohm
P Power
ppm pulse per minute
R Resistance, ohms
Sec, S seconds
US United States
V volt
VDC Direct Current Voltage
)
3
)
-6
)
-3
)-
5
Page 13
DA-2006 Series
USER ASSUMES FULL RESPONSIBILITY FOR UNAUTHORIZED
EQUIPMENT MODIFICATIONS OR APPLICATION OF EQUIPMENT
OUTSIDE OF THE PUBLISHED INTENDED USE AND
SPECIFICATIONS. SUCH MODIFICATIONS OR APPLICATIONS
MAY RESULT IN EQUIPMENT DAMAGE OR PERSONAL INJURY.
BC GROUP INTERNATIONAL, INC. RESERVES THE RIGHT TO
MAKE CHANGES TO ITS PRODUCTS OR SPECIFICATIONS AT
ANY TIME, WITHOUT NOTICE, IN ORDER TO IMPROVE THE
DESIGN OR PERFORMANCE AND TO SUPPLY THE BEST
POSSIBLE PRODUCT. THE INFORMATION IN THIS MANUAL HAS
BEEN CAREFULLY CHECKED AND IS BELIEVED TO BE
ACCURATE. HOWEVER, NO RESPONSIBILITY
IS ASSUMED FOR INACCURACIES.
NOTICE – CONTACT INFORMATION
NOTICE – DISCLAIMER
NOTICE – DISCLAIMER
BC BIOMEDICAL
BC GROUP INTERNATIONAL, INC.
PO BOX 25125
9415 GENTRY AVE
ST. LOUIS, MO 63125
USA
1-800-242-8428
314-638-3800
www.bcgroupintl.com
sales@bcgroupintl.com
Manual DA-2006 Series Copyright © 2007
www.bcgroupintl.com Made in the USA
5/07 Rev 06
6
Page 14

BC GROUP
DA-2006 SERIES
DEFIBRILLATOR ANALYZER
The Model DA-2006 Series is a microprocessor-based instrument family that is used in the
testing of defibrillators. They measure the energy output and provide information about the
defibrillation pulse. They are used on manual, semi-automatic and automatic defibrillators
with monophasic or biphasic outputs.
The DA-2006P model additionally provides a Transcutaneous Pacemaker analysis function.
It measures and displays pacer pulse information as well as performing Refractory Period,
Sensitivity and Immunity testing.
All models have a built in 50 ohm human body simulation load as well as 12 lead ECG with
arrhythmias and performance waveforms. Additionally, they have a Centronics printer port,
a serial port, oscilloscope output, high-level ECG output, as well as provision for a battery
eliminator.
The DA-2006 Series makes viewing and selecting the desired waveforms and test data
quick and intuitive, with all operational information being available on the 240 by 64 pixel
graphic display, allowing for easy maneuvering through parameters and scrolling through
available options.
NOTE: The instrument is intended for use by trained service technicians.
7
Page 15

DA-2006
The following are highlights of some of the main features:
GENERAL
• SIMPLE TO OPERATE
• GRAPHICS DISPLAY WITH SIMULTANEOUS DETAILED STATUS OF
PARAMETERS AND SCROLLING CONTROL OF OPTIONS
• ON SCREEN VIEWING OF DEFIBRILLATOR AND PACEMAKER WAVEFORMS
• DROP DOWN CHOICE SCREENS LIST ALL OPTIONS FOR PARAMETERS
• MONOPHASIC AND BIPHASIC COMPATIBLE
• 5000 V, 1000 JOULE CAPACITY
• HIGH AND LOW RANGES
• CARDIOVERSION DELAY MEASUREMENT
• CHARGE TIME MEASUREMENT
• WAVEFORM STORAGE AND PLAYBACK
• 10 UNIVERSAL PATIENT LEAD CONNECTORS
• 25 PIN CONNECTOR FOR CENTRONICS PRINTER
• 9 VOLT BATTERY POWER
• LOW BATTERY INDICATOR
• AVAILABLE BATTERY ELIMINATOR
• DISPLAY BACKLIGHT
• FULL REMOTE OPERATION VIA RS-232
• FLASH PROGRAMMABLE FOR UPGRADES
• AUTO SEQUENCE TESTING CAPABLE OF STORING 50 CUSTOM TEST
SEQUENCES
PACEMAKER OPTION
• 26 SELECTABLE INTERNAL LOADS
• FULL PULSE ANALYSIS
• DEMAND SENSITIVITY TEST
• REFRACTORY PERIOD TESTS
• 50/60 Hz INTERFERENCE TEST SIGNALS
• INPUT TERMINALS AND CIRCUITRY PROTECTED AGAINST ACCIDENTAL
DEFIBRILLATOR DISCHARGE INTO PACEMAKER TEST TERMINALS
8
Page 16
Description
ENERGY OUTPUT MEASUREMENT GENERAL
The unit measures the energy in the output pulse of both monophasic and biphasic
defibrillators.
• PULSE TYPE: Monophasic or Biphasic
• LOAD RESISTANCE: 50 ohm +/- 1%, non-inductive (<1 µH)
• DISPLAY RESOLUTION: 0.1 Joules
• MEASUREMENT TIME WINDOW: 100 ms
• ABSOLUTE MAX PEAK VOLTAGE: 6000 Volts
• CARDIOVERSION DELAY: 0 to 6000 ms
• CARDIOVERSION RESOLUTION: 0.1 ms
ENERGY OUTPUT MEASUREMENT HIGH RANGE
The high range allows for a large pulse with high voltage and current.
• VOLTAGE: <5000 Volts
• MAX CURRENT: 120 Amps
• MAX ENERGY: 1000 Joules
• TRIGGER LEVEL: 100 Volts
• PLAYBACK AMPLITUDE: 1 mV / 1000 V Lead I
• TEST PULSE: 125 Joules +/- 20%
ENERGY OUTPUT MEASUREMENT LOW RANGE
The low range allows greater resolution on smaller pulses.
• VOLTAGE: <1000 Volts
• MAX CURRENT: 24 Amps
• MAX ENERGY: 50 Joules
• TRIGGER LEVEL: 20 Volts
• PLAYBACK AMPLITUDE: 1 mV / 1000 V Lead I
• TEST PULSE: 5 Joules +/- 20%
9
Page 17
DA-2006
ENERGY OUTPUT MEASUREMENT OTHER
OSCILLOSCOPE OUTPUT
• HIGH MEASUREMENT RANGE: 1000:1 amplitude-attenuated
• LOW MEASUREMENT RANGE: 200:1 amplitude-attenuated
WAVEFORM PLAYBACK
• OUTPUT – LEAD 1 & PLATES
• GRAPHICS SCREEN
• 200:1 Time Base Expansion
SYNC TIME MEASUREMENTS
• TIMING WINDOW: Starts at peak of each R-wave
• TEST WAVEFORMS: All waveform simulations available
CHARGE TIME MEASUREMENT
• From 0 .1 to 99.9 sec
ECG FUNCTIONS
The unit can produce a wide variety of ECG simulations. The user simply selects the
parameters that match the desired output.
• RATE: 30,40,45,60,80,90,100,120,140,160,180,200,220,240,260,280,300 BPM
• AMPLITUDE: 0.50,1.0,1.5,2.0 mV (Lead II)
ECG-PERFORMANCE FUNCTIONS
The unit can generate Sine, Square, Triangular, and Pulse waveforms with adjustable
amplitudes for performance testing.
• SINE: 0.1,0.2,0.5,5,10,40,50,60,100 Hz
• SQUARE: 0.125,2 Hz
• TRIANGLE: 2,2.5 Hz
• PULSE: 30,60,120 BPM; 60 ms WIDTH
• AMPLITUDE: 0.5,1.0,1.5,2.0 mV (Lead II)
10
Page 18

Description
ARRHYTHMIA FUNCTIONS
The unit can simulate 12 different arrhythmias.
• VENTRICULAR FIBRILLATION
• ATRIAL FIBRILLATION
• SECOND DEGREE A-V BLOCK
• RIGHT BUNDLE BRANCH BLOCK
• PREMATURE ATRIAL CONTRACTION
• EARLY PVC
• STANDARD PVC
• R ON T PVC
• MULTIFOCAL PVC
• BIGEMINY
• RUN OF 5 PVC
• VENTRICULAR TACHYCARDIA
SHOCK ADVISORY TESTS
The unit can simulate 8 different waveforms to test the shock algorithm of advanced
defibrillators:
• ASYSTOLE
• COARSE VENTRICULAR FIBRILLATION
• FINE VENTRICULAR FIBRILLATION
• MULTIFOCAL VENTRICULAR TACHYCARDIA @ 140 BPM
• MULTIFOCAL VENTRICULAR TACHYCARDIA @ 160 BPM
• POLYFOCAL VENTRICULAR TACHYCARDIA @ 140 BPM
• POLYFOCAL VENTRICULAR TACHYCARDIA @ 160 BPM
• SUPRAVENTRICULAR TACHYCARDIA @ 90 BPM
11
Page 19

DA-2006
TRANSCUTANEOUS PACER ANALYZER
The unit can test external transcutaneous pacemakers. It has a wide variety of loads and
can measure the Pacer Pulse, Demand Sensitivity and Refractory Periods (Pacing and
Sensing):
• LOAD:
• RANGE: 50,100,150,200,300,400,500,600,700,800,900,1000,1100,
1200,1300,1400,1500,1600,1700,1800,1900,2000,2100,
2200,2300 ohm
• PULSE:
• PULSE CURRENT: 4 TO 300 mA (100 ohm load)
• RATE: 30 TO 800 ppm
• WIDTH: 0.6 to 80 ms
• DEMAND SENSITIVITY:
• WAVEFORMS:
• SELECTIONS: SQUARE, TRIANGLE, HAVERSINE
• WIDTH: 10,25,40,100,200 ms
• ECG:
• AMPLITUDE – OUT: 0 to 4 mV
• PACER INPUT (50 TO 400 OHM):
• AMPLITUDE – OUT: 0 to 10 mV / 50 ohms
• RATE – IN: 30 to 120 ppm
• PACER INPUT (500 TO 2300 OHM & OPEN):
• AMPLITUDE – OUT: 0 to 100 mV
• RATE – IN: 30 to 120 ppm
• DEFIBRILLATOR PLATES:
• AMPLITUDE – OUT: 0 to 10 mV
• RATE – IN: 30 to 120 ppm
• REFRACTORY PERIOD:
• PACING: 20 to 500 ms
• SENSING: 20 to 500 ms
• 50/60 HZ INTERFERENCE TEST SIGNAL:
• ECG OUTPUT: 0,0.4,0.8,1.2,1.6,2.0,2.4,2.8,3.2,3.6,4.0 mV
• PACER INPUT 50 OHM: 0,1,2,3,4,5,6,7,8,9,10 mV
• PACER INPUT 100 OHM: 0,2,4,6,8,10,12,14,16,18,20 mV
• PACER INPUT 150 OHM: 0,3,6,9,12,15,18,21,24,27,30 mV
• PACER INPUT 200 OHM: 0,4,8,12,16,20,24,28,32,26,40 mV
• PACER INPUT 300 OHM: 0,6,12,18,24,30,36,42,48,54,60 mV
• PACER INPUT 400 OHM: 0,8,16,24,32,40,48,56,64,72,80 mV
• PACER INPUT > 500 OHM: 0,10,20,30,40,50,60,70,80,90,100 mV
• DEFIBRILLATOR PLATES: 0,1,2,3,4,5,6,7,8,9,10 mV
• INPUT CIRCUITRY PROTECTION
• INPUT CIRCUITRY IS PROTECTED AGAINST DAMAGE IN THE
EVENT OF AN ACCIDENTAL DEFIBRILLATOR DISCHARGE INTO
THE PACEMAKER TEST INPUT TERMINALS
12
Page 20

Description
ACCESSORIES
BC20 - 40032 INTERNAL PADDLE ADAPTERS (2 adapters)
BC20 - 21103 BATTERY ELIMINATOR (120 VAC) (US Version)
BC20 - 21101 BATTERY ELIMINATOR (220 VAC) (Euro Version)
BC20 - 00427 PLASTIC ELECTRODE PLATES (2 plates)
OPTIONAL ACCESSORIES
BC20 - 30108 BC BIOMEDICAL MEDIUM SOFT SIDED CARRYING CASE
BC20 - 41341 COMMUNICATION CABLE (DB 9 M to DB 9 F)
BC20 - 00420 PHYSIO-CONTROL DEFIB / PACE TEST CABLE
BC20 - 00421 MARQUETTE DEFIB / PACE TEST CABLE
BC20 - 00423 ZOLL DEFIB/PACE TEST CABLE
BC20 - 00424 PHYSIO-CONTROL PACE ONLY TEST CABLE
BC20 - 00425 ZOLL PACE ONLY TEST CABLE
BC20 - 00426 HP / AGILENT / LAERDAL / AAMI
DEFIB / PACE TEST CABLE
13
Page 21

DA-2006
This page intentionally left blank.
14
Page 22

OVERVIEW
This section looks at the layout of a DA-2006 and gives descriptions of the elements that
are present.
LCD Graphical Display:
10 Universal
Patient Lead
Connectors:
RA R
LA L
RL(-) N
LL F
V1 C1
V2 C2
V3 C3
V4 C4
V5 C5
V6 C6
Back Light Key
for turning on
and off the
backlight
Shows Parameters for Test
Data and Waveforms
5 Light Touch Keys
for Dynamic
functions:
These keys are
labeled in the
bottom portion of
the screen and
change function
based on operating
mode.
Range Key
for selecting
Defib input
range high or
low
Large Defib Plates:
Fixed 50 ohm input
load for Defibrillator
testing
9V Battery
Compartment
(Rear)
15
Page 23
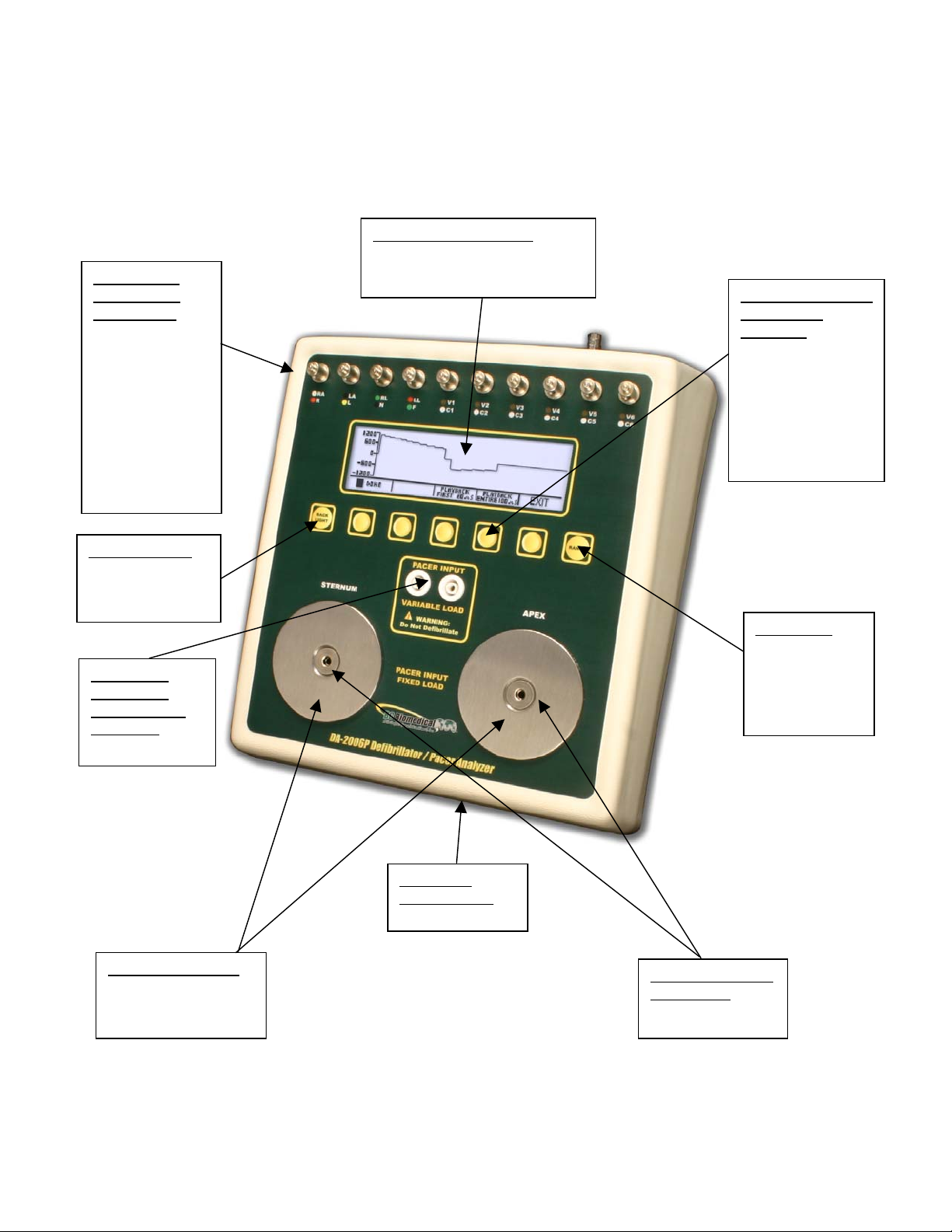
DA-2006 Series
This section looks at the layout of a DA-2006P and gives descriptions of the elements that
are present.
10 Universal
Patient Lead
Connectors:
RA R
LA L
RL(-) N
LL F
V1 C1
V2 C2
V3 C3
V4 C4
V5 C5
V6 C6
LCD Graphical Display:
Shows Parameters for Test
Data and Waveforms
5 Light Touch Keys
for Dynamic
functions:
These keys are
labeled in the
bottom portion of
the screen and
change function
based on operating
mode.
Back Light Key
for turning on
and off the
backlight
Pacemaker
Input Jacks
Variable Load
(optional):
50-2300 ohms
Large Defib Plates:
Fixed 50 ohm input
load for Defibrillator
testing
Range Key
for selecting
Defib input
range high or
low and
pace mode
9V Battery
Compartment
(Rear)
Pacer Input Jacks
Fixed Load:
50 ohm
16
Page 24

Overview
A
r
This section looks at the layout of the back and gives descriptions of the elements that are
present.
Oscilloscope
output
BNC connector
for easy
viewing of input
waveforms
Power Switch
Parallel Port
C Adaptor/
Battery
Eliminator
2.1 mm Micro
High Level
ECG output
RCA Jack
Serial Port
Female 9-Pin
D-Sub
Connecto
Female 25-Pin
D-Sub
Connector
NOTE
The DA-2006 and the DA-2006P offer the same
features, with the DA-2006P having the addition
of a Transcutaneous Pacemaker Analyzer
function (See Pacemaker Analyzer section
for more details).
17
Page 25
DA-2006 Series
General Operation
The unit is controlled by 7 light touch keys. They allow the user to move around within the
displayed parameters, select the desired options, choose a specific category and control
the setup for the unit. When a key is depressed there is an audio click when it is accepted,
or a razz tone if the key is invalid.
A large LCD graphics display with backlight provides the user with information about the
current status of the device configuration options, test results and more. The display
identifies the function of each key on a dynamic basis. As the operation mode changes,
the key functions change to suit the operating mode.
Range Key
The key scrolls through the ranges of the DA-2006 Series analyzers. Depressing
the key will allow the user to select between High Defibrillator Range (1000J max), Low
Defibrillator Range (50J max) and, with the DA-2006P, Pacemaker Range. The default
mode on power up is High Defibrillator Range.
Backlight Key
The Graphic LCD display may be viewed with or without the backlight. Depressing any key
will activate the backlight. However, since the backlight will drain the battery if left on, it will
automatically shut off after a user programmable delay when running on battery power.
The key is provided to toggle the backlight on or off at any time.
18
Page 26
Overview
Function Keys
There are five keys that are used to provide general operational control. The
functions of the keys vary depending on the current screen. The section of the screen
just above the key indicates its current meaning.
NOTE: Only functions that are available to the user will be visible at any given time.
Sample Function Key Labeling
ECG Waveforms
The microprocessor has stored in its memory all of the digitalized waveforms. It sends the
waveforms to a D/A converter, which generates an accurate analog representation. The
waveform is then sent through resistor networks, developing the appropriate signals on the
output terminals.
19
Page 27
DA-2006 Series
Universal Patient Lead Connectors
The 10 Universal Patient Lead Connectors allow for 12 lead ECG simulations. AHA and
IEC color-coded labels are located on the face of the unit to aid in connecting the
corresponding U.S. and International Patient Leads.
AHA Label IEC Label Description
RA R Right Arm
LA L Left Arm
RL N
LL F Left Leg
V1
V2
V3
V4
V5
V6
C1
C2
C3
C4
C5
C6
Right Leg
(reference or ground)
V Leads (V1-V6)
(U.S. and Canada)
also referred to as pericardial,
precordial or unipolar chest leads
Chest Leads (C1-C6)
(International)
20
Page 28

Overview
High Level Output (+)
A high level ECG output signal (200 X Amplitude Setting) is available on the RCA jack
located on the rear of the unit.
Serial Port
A female 9-pin D-Sub connector is provided for the connection of the unit to a PC or laptop
serial port (e.g. Com 1). This link is then used for either remote control or flash
downloading of software upgrades.
Parallel Port
A female 25-pin D-Sub connector is provided for the connection of a printer via a
Centronics parallel interface.
Oscilloscope Output
A BNC connector is provided to connect an oscilloscope to the unit. This output is a 200:1
attenuated version of the input to the Defibrillator Plates.
21
Page 29

DA-2006 Series
Power Switch
A rocker switch is provided on the rear of the unit to turn the power on and off.
Power Supply
The unit utilizes two 9 Volt Alkaline Batteries in the bottom battery compartments. When
the unit detects a LOW BATTERY condition (10% Battery Life), a warning window will
appear once per minute to alert the user.
Battery Eliminator
The unit has a 2.1 mm micro jack for connecting a 10-Volt AC battery eliminator. The
adapter will power the unit, but will not charge the battery.
22
Page 30

DEFIBRILLATOR ANALYZER
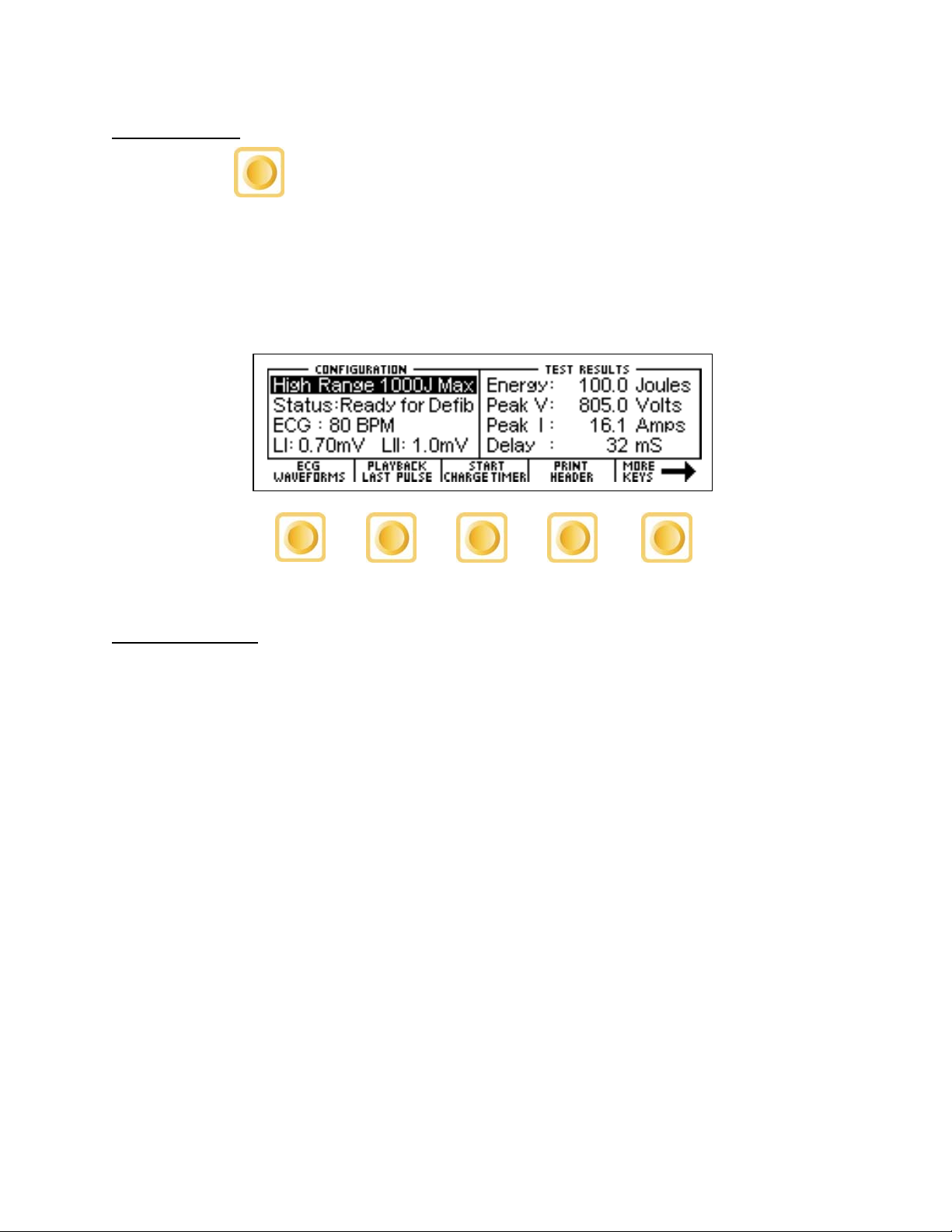
When the DA-2006 is first powered up, the Defibrillator Analyzer MAIN SCREEN will be
displayed. This screen shows the current CONFIGURATION, the TEST RESULTS and the
available FUNCTION KEYS. All defibrillator tests are run from the MAIN SCREEN. When
the unit detects an input of greater than 100 Volts on the Defibrillator Plates (20 Volts in
Low Range), it will automatically begin a test.
The default configuration is the High Range Defibrillator mode. This mode allows for a
MAIN SCREEN
waveform of up to 1000 Joules to be analyzed.
The following is a sample screen for this mode:
23
Page 31

Defibrillator Analyzer
The key may be used to toggle the unit to the Low Range Defibrillator mode. This
mode allows for waveforms up to 50 Joules to be analyzed. The Defibrillator Analyzer
works the same in both ranges. The lower range simply provides for a higher resolution for
pulses with smaller amplitudes.
The following is a sample screen for this mode:
NOTE: The key will also put the DA-2006P into the Transcutaneous Pacemaker
Analyzer mode (See Pacemaker Analyzer section for more information).
24
Page 32

Main Screen
CONFIGURATION
The CONFIGURATION section of the MAIN SCREEN displays the current setup of the unit.
RANGE
The first line displays the range value for the pulse. It may be either 1000 Joules or 50
Joules max. This setting may be changed using the key.
NOTE: This line also allows for the selection of the Pacer Analyzer that is an option
available in the DA-2006P model. The key will toggle to Pacer to put the unit into
the Pacemaker Analyzer mode (See Pacemaker Analyzer section for more information).
STATUS
This line provides information about the current status of the analyzer.
ECG
This line displays the selection that is active on the ECG terminals. This setting may be
changed in the ECG WAVEFORMS screen.
AMP
This line displays the amplitude that has been selected for the ECG terminals. This setting
may be changed in the ECG WAVEFORMS screen.
25
Page 33

Defibrillator Analyzer
TEST RESULTS
The TEST RESULTS section of the MAIN SCREEN displays the results of the last pulse. It
will continue to be displayed until the power is turned off, another test is run or the range is
changed.
NOTE: The unit automatically starts a test when it sees a voltage greater than 100 Volts on
the Defibrillator Plates (20 Volts in Low Range).
NOTE: Test results are immediately sent to the printer port as soon as the data is
available.
ENERGY
This line displays the total energy of the last pulse.
PEAK V
This line displays the peak voltage of the last pulse.
PEAK I
This line displays the peak current of the last pulse.
26
Page 34

Main Screen
DELAY
This line normally displays the delay from the peak of the R wave until the start of the Defib
Energy pulse. The line is replaced by the CHARGE TIME if this test has been run (see
START CHARGE TIMER SCREEN for more information).
CHG TIME
This line displays if the Charge Timer has been run. It shows the time required to charge
the Device Under Test (DUT). This test is started with the key.
27
Page 35

Defibrillator Analyzer
eys
Secondary Functio
eys
FUNCTION KEYS
The FUNCTION KEYS section of the MAIN SCREEN displays the current functions of the
keys found below the display. These keys allow for navigation to supporting screens and
initiation of specific features.
Primary Function K
n K
ECG WAVEFORMS
This key enters the ECG WAVEFORMS screen where all ECG parameters are set.
PLAYBACK LAST PULSE
This key enters the PLAYBACK LAST PULSE screen where a graphical representation of
the last pulse may be viewed and sent out.
START CHARGE TIMER
This key brings up the CHARGE TIMER screen and starts the pre-warn timer. It is used to
test the charge time for the defibrillator.
PRINT HEADER
This key sends the Report Header to the printer.
MORE KEYS
These keys toggle between the Primary and Secondary Function Keys.
28
Page 36

Main Screen
AUTO SEQUENCES
This key brings up the AUTO SEQUENCE MENU, which is used to view or run the Auto
Sequences stored in the unit.
SELF TEST WAVEFORM
This key sends an internal test pulse to the unit, allowing for the display of the results to
give an indication that the system is working properly.
DA-2006 SETUP
This key brings up the SYSTEM CONFIGURATION SCREEN, which allows for adjusting
the various system configuration parameters.
29
Page 37

Defibrillator Analyzer
This page intentionally left blank.
30
Page 38

ECG WAVEFORMS SCREEN
The DA-2006 ECG output can be connected in 3, 5 or 12 lead configurations. Pressing the
key from the MAIN SCREEN will allow the user to configure the waveform that
is used for the ECG output.
The following is a sample of the ECG waveform configuration screen:
ECG GROUP WAVEFORM
Disabled None
30,40,45,60,80,90,
NSR
AED
Arrhythmias
Performance
100,120,140,160,
180,200,220,240,
260,280,300 BPM
Asystole
Coarse Vfib
Fine Vfib
Multifocal Vtach 140
Multifocal Vtach 160
Polyfocal Vtach 140
Polyfocal Vtach 160
SupraVent Tach 90
Vfib
Afib
Second Deg Block
RBBB
PAC
PVC Early
PVC STD
PVC R on T
MF PVC
Bigeminy
Run of 5 PVC
Vtach
0.125, 2 Hz Square
2, 2.5 Hz Triangle
0.1,0.2,0.5,5,10,40,5
0,60,100 Hz Sine
30, 60, 120 BPM
Pulse
AMPLITUDE
Lead I 0.35 mV Lead II 0.5 mV
Lead I 0.70 mV Lead II 1.0 mV
Lead I 1.05 mV Lead II 1.5 mV
Lead I 1.40 mV Lead II 2.0 mV
31
Page 39

Defibrillator Analyzer
The ECG Group, Waveform and Amplitude can be selected using to highlight
the parameter and using to open a drop down menu of all the options for the
highlighted parameter.
Use to scroll to the desired option. Then is used to accept the
new setting. The key can be used to return to the ECG waveform
configuration screen without making a new selection.
The key is used to return to the MAIN SCREEN.
32
Page 40

ECG Waveforms Screen
The following is a brief description of how the DA-2006 simulates the available Arrhythmias:
Abbreviation Arrhythmia Description
Irregular waveform with no real
Vent Fib –
Fine
Atrial Fib
2nd Deg Heart
Block
Rt Bundle
Branch Block
PAC
PVC Early
PVC Std
PVC R on T
Multifocal
PVCS
Ventricular Fibrillation
Atrial Fibrillation
Second Degree Heart Block
Right Bundle Branch Block
Premature Atrial Contraction
Early Type 1
Premature Ventricular Contraction
Standard Type 1
Premature Ventricular Contraction
R on T Type 1
Premature Ventricular Contraction
Multifocal
Premature Ventricular Contraction
P-wave or clear R-R interval and a low
signal level
(Continuous)
Absence of P-wave, irregular P-R
interval rate and a high level signal
(Continuous)
80 BPM with increasing
P-R interval for four beats
(160, 220, 400, 470 ms)
followed by a P wave without a QRS
(Continuous)
80 BPM with Normal P-wave and P-R
interval but wider QRS complexes
(Continuous)
NSR of 80 BPM with Periodic
Abnormal 25% early P waves
(PAC, 7 NSR)
(Continuous)
NSR of 80 BPM with periodic left focus
premature ventricular beats with 33%
premature timing
(PVC Type 1, 9 NSR)
(Continuous)
NSR of 80 BPM with periodic left focus
premature ventricular beats with 20%
premature timing
(PVC Type 1, 9 NSR)
(Continuous)
NSR of 80 BPM with periodic left focus
premature ventricular beats with 65%
premature timing, placing R on the
previous T
(PVC Type 1, 9 NSR)
(Continuous)
NSR of 80 BPM with Type 1 and Type
2 PVCs (PVC Type 1, 2 NSR, PVC
Type 2, 2 NSR)
(Continuous)
33
Page 41

Defibrillator Analyzer
Abbreviation Arrhythmia Description
NSR of 80 BPM with every other beat
Bigeminy
Run of 5
PVCs
Vent Tach
Bigeminal Rhythm
Run of 5 Premature Ventricular
Contractions
Ventricular Tachycardia
a Type 1 PVC
(Continuous)
NSR of 80 BPM with periodic group of
5 Type 1 PVCs
(5 PVC Type 1, 36 NSR)
(Continuous)
160 BPM, No P-wave,
Beats similar to Type 1 PVC
(Continuous)
34
Page 42

PLAYBACK LAST PULSE SCREEN
The DA-2006 can display a graphical representation of the last pulse. This screen may be
accessed by pressing the key from the Defibrillator Analyzer MAIN
SCREEN. The playback allows the user to view the Defibrillator pulse in a time-expanded
form. Samples are stored internally at 0.1 ms intervals. The PLAYBACK LAST PULSE
SCREEN shows these samples expanded by a time factor of 200.
In playback mode, the samples are shown on the display and sent out the ECG leads,
Defibrillator Plates and the High Level output. The following is a sample of the waveform
that is shown in the display:
The scale shown on the screen is automatically adjusted to provide the maximum
resolution available.
The key can be used to pause the screen at any point while a pulse is being
played back. This key replaces the key when a pulse is being played back.
The key can be used to play (continue) the waveform if it has been paused.
This key replaces the key.
35
Page 43

Defibrillator Analyzer
The key starts a playback of only the first 20 ms of the waveform.
The key starts a playback of the entire 100 ms of the waveform.
At any time, the key or key can be depressed to return to the
MAIN SCREEN.
36
Page 44

START CHARGE TIMER SCREEN
A special timer has been incorporated into the DA-2006 to analyze the charging circuit of
the Device Under Test (DUT). The START CHARGE TIMER SCREEN can be accessed
by pressing the key from the MAIN SCREEN. To synchronize the charge
timer with the defibrillator charge time, a Pre-Warning Countdown period is started. When
the timer reaches zero, the defibrillator charge should be initiated. The following is an
example of the countdown timer:
When the timer reaches zero, a beep will sound and the charge timer will begin counting
up. The following is an example of the count up timer:
37
Page 45

Defibrillator Analyzer
The DUT should be discharged as soon as it becomes charged. When the DUT is
discharged, the timer will automatically stop. The display will show the results of the
Defibrillator pulse analysis as well as the time required to charge the DUT:
Charge Timer
Results
At any time, the key can be depressed to end the timer and return to the
MAIN SCREEN.
38
Page 46

PRINT HEADER
The DA-2006 provides a header for recording test data as well as the results of each pulse
that is discharged into the unit. Test results are immediately sent to the printer port as soon
as the data is available. The header is sent by pressing the key from
the MAIN SCREEN.
The status line of the configuration section will indicate that the header has been sent to the
printer.
39
Page 47

Defibrillator Analyzer
The following is the print header and sample data that are used for the Defibrillator
Analyzer mode.
NOTE: Printing the header also resets the test number printed on the data sheet.
NOTE: In the test results, the user must manually write the power setting of the DUT.
40
Page 48

SELF TEST WAVEFORM
The DA-2006 has built in test waveforms that will give an indication that the system is
working properly. The Self Test Waveform may be sent by pressing the key
from the MAIN SCREEN.
After the waveform has been sent, the results will be reflected in the test results section of
the MAIN SCREEN and the PLAYBACK LAST PULSE SCREEN. The Self Test Waveform
is not calibrated, but will provide a waveform that is approximately 125 Joules when
configured for the High Range and 5 Joules when configured for the Low Range.
The following is an example of the MAIN SCREEN with the results of the Self Test
Waveform:
The following is an example of the PLAYBACK LAST PULSE SCREEN, showing a
graphical representation of the Self Test Waveform:
41
Page 49

Defibrillator Analyzer
This page intentionally left blank.
42
Page 50

RUNNING A DEFIBRILLATOR TEST
All connections to patients must be removed
before connecting the Device Under Test (DUT)
to the Analyzer. A serious hazard may occur
WARNING - CONNECTIONS
if the patient is connected when
testing with the Analyzer.
Do not connect any leads from the patient
directly to the Analyzer or DUT.
INTRODUCTION
The DA-2006 will analyze the pulse output of a monophasic or biphasic defibrillator. The
primary measure of the output is the Energy that it contains. Other information deals with
the maximum voltage and current as well as the pulse timing with respect to the R-wave.
The human body has characteristic impedance that may vary, but 50 ohms is used for
comparative defibrillator testing. The DA-2006 has a large internal 50 ohm non-
inductive, high-power resistor to simulate a human body. It is sized to accept repeated
pulses at the maximum energy levels.
43
Page 51

Defibrillator Analyzer
The energy contained in a pulse is determined mathematically based on the fact that
the energy is defined as the integral of the power curve. The following formulas
describe the basic computation:
Energy = E = P dt
Power = P = V
2
/ R => E = V2 / R dt = V2 dt / R
This computation is implemented digitally by taking timed samples of the voltage every
100 µsec for 100 msec (1000 readings). Each value is then squared and divided by the
resistance (50 ohms). The sum of these 1000 values times 10 is then the Energy in
Joules (Watt Seconds) contained in the pulse.
44
Page 52

Running a Defibrillator Test
The setup for a Defibrillation Test is dependent on the physical hardware involved. For
the sake of this example we will assume a standard defibrillator with 5 lead ECG.
DEFIBRILLATION TEST
WARNING
(1) Connect ECG leads to the corresponding universal connector on the DA-2006.
This section is provided as a guide to familiarize
the user with the DA-2006 Series. It is not
intended to provide the necessary test sequence
for every Defibrillator. The user must consult the
manufacturer’s manual for each DUT to
determine the correct test procedure to follow.
The connectors are marked with both the AHA and International color codes.
(2) Turn on the DA-2006.
(3) The unit will come up in the “High Range Defibrillator” mode. This range is used
for normal adult testing.
NOTE: If it is desirable to run a test at 50 Joules or less with a peak
voltage of 1000 Volts or less, the unit may be changed to the “Low Range
Defibrillator” mode using the key.
(4) Select “Ventricular Fibrillation” from the ECG WAVEFORM SCREEN with an
amplitude of 1 mV. This is necessary for most automatic defibrillators.
45
Page 53

Defibrillator Analyzer
(5) Place the Defibrillator Paddles on the DA-2006 contact plates. The APEX is on
the right and the STERNUM is on the left.
NOTE: Reversing the paddles will not cause any damage to the unit or
error in the energy reading. However, it will cause the polarity of the
oscilloscope output and the playback waveform to be inverted.
(6) Holding the paddles firmly in place, charge the Defibrillator and discharge it into
the DA-2006.
Observe all precautions noted by the Defibrillator
WARNING
Manufacturer when using the Defibrillator.
(7) The DA-2006 will automatically sense the voltage rise across the internal 50 ohm
load and begin taking readings. After the sampling is done (100 ms) the unit will
compute and display the results.
a. The power pulse is available at the oscilloscope output in real time at
200:1 signal attenuation when in low range and 1000:1 signal attenuation
when in high range.
b. After the computation, the pulse is automatically played back at a 200:1
time base expansion (200 times slower) on both the ECG leads and the
Paddle plates. The signal level is 1 mV per 1000 Volts on Lead 1.
c. At the same time, the test results are sent to the printer.
46
Page 54

Running a Defibrillator Test
(8) The Status line will change to indicate the various steps as they are being done.
(9) At the end of the process the results are continuously displayed in the Test
Results section of the MAIN SCREEN. They will remain there until another test
is performed, the range is changed or the power is turned off.
(10) The user may repeat the playback of the waveform at any time by changing to
the PLAYBACK LAST PULSE SCREEN using the key. In this
screen the pulse may be viewed in 20 msec segments and paused for review.
NOTE: The pulse is sent to the ECG and Paddle outputs at the same time
it is being displayed on the screen.
47
Page 55

Defibrillator Analyzer
This page intentionally left blank.
48
Page 56

Running a Defibrillator Test
A Cardioversion Test is simply an energy test with special attention being given to the
timing. The DA-2006 continuously monitors for the R-wave timing and displays, if
possible, the delay between the R-wave and the pulse. In Cardioversion testing, the
Defibrillator is set to deliver a pulse based on a specific delay after the R-wave.
CARDIOVERSION TEST
WARNING
This section is provided as a guide to familiarize
the user with the DA-2006 Series. It is not
intended to provide the necessary test sequence
for every Defibrillator. The user must consult the
manufacturer’s manual for each DUT to
determine the correct test procedure to follow.
(1) Connect ECG leads to the corresponding universal connector on the DA-2006.
The connectors are marked with both the AHA and International color codes.
(2) Turn on the DA-2006.
(3) The unit will come up in the “High Range Defibrillator” mode. This range is used
for normal adult testing.
NOTE: If it is desirable to run a test at 50 Joules or less with a peak
voltage of 1000 Volts or less, the unit may be changed to the “Low Range
Defibrillator” mode using the key.
49
Page 57

Defibrillator Analyzer
(4) Select the desired ECG Waveform and Amplitude to be tested from the choices
on the ECG WAVEFORM SCREEN.
NOTE: The selected waveform must contain a QRS complex.
(5) Set the Defibrillator to Synchronized Cardioversion mode.
(6) Place the Defibrillator Paddles on the DA-2006 contact plates. The APEX is on
the right and the STERNUM is on the left.
NOTE: Reversing the paddles will not cause any damage to the unit or
error in the energy reading. However, it will cause the polarity of the
oscilloscope output and the playback waveform to be inverted.
(7) Holding the paddles firmly in place, charge the Defibrillator and discharge it into
the DA-2006.
WARNING
Observe all precautions noted by the Defibrillator
Manufacturer when using the Defibrillator.
50
Page 58

Running a Defibrillator Test
(8) The DA-2006 will automatically sense the voltage rise across the internal 50 ohm
load and begin taking readings. After the sampling is done (100 ms) the unit will
compute and display the results.
a. The power pulse is available at the oscilloscope output in real time at
200:1 signal attenuation when in low range and 1000:1 signal attenuation
when in high range.
b. After the computation, the pulse is automatically played back at a 200:1
time base expansion (200 times slower) on both the ECG leads and the
Paddle plates. The signal level is 1 mV per 1000 Volts on Lead 1.
c. At the same time, the test results are sent to the printer.
(9) The Status line will change to indicate the various steps as they are being done.
(10) At the end of the process the results are continuously displayed in the Test
Results section of the MAIN SCREEN. They will remain there until another test
is performed, the range is changed or the power is turned off.
NOTE: Special note should be made of the “Delay: xxx msec” line in the
results. This will show the delay between the peak of the R-wave and the
start of the Pulse.
51
Page 59

Defibrillator Analyzer
The user may repeat the playback of the waveform at any time by changing to the
PLAYBACK LAST PULSE SCREEN using the key. In this screen the pulse
may be viewed in 20 msec segments and paused for review.
NOTE: The pulse is sent to the ECG and Paddle outputs at the same time
it is being displayed on the screen.
52
Page 60

Running a Defibrillator Test
The charging time of a Defibrillator is nothing more than a measurement of the time
required for the Defibrillator to charge. It is used to test the battery, charging circuitry
and capacitor. The DA-2006 provides a simple way to start and stop the timer. It also
records the results.
This section is provided as a guide to familiarize
CHARGE TIME TEST
WARNING
the user with the DA-2006 Series. It is not
intended to provide the necessary test sequence
for every Defibrillator. The user must consult the
manufacturer’s manual for each DUT to
determine the correct test procedure to follow.
(1) Turn on the DA-2006.
(2) The unit will come up in the “High Range Defibrillator “ mode. This range is used
for normal adult testing.
(3) Set the Defibrillator to its maximum power setting.
(4) Depress the key.
53
Page 61

Defibrillator Analyzer
(5) While the Pre-Warning Countdown is running, place the Defibrillator Paddles on
the DA-2006 contact plates. The APEX is on the right and the STERNUM is on
the left.
NOTE: Reversing the paddles will not cause any mage to the unit or error
in the energy reading. However, it will cause the polarity of the
oscilloscope output and the playback waveform to be inverted.
(6) Holding the paddles firmly in place, wait until the Pre-Warning Countdown
equals zero and then immediately start charging the Defibrillator.
(7) As soon as the DUT is fully charged, discharge it into the DA-2006.
(8) At the end of the process the results are continuously displayed in the Test
Results section of the MAIN SCREEN. They will remain there until another test
is performed, the range is changed or the power is turned off.
NOTE: The last line in the Test Results section of the screen will show
Observe all precautions noted by the Defibrillator
Manufacturer when using the Defibrillator.
WARNING
“Chg Time: xxx.x sec”
54
Page 62

Running a Defibrillator Test
SHOCK ADVISORY
ALGORITHM TEST
The Shock Advisory Algorithm Test works with the analysis and prompting functions on
automatic and semiautomatic Defibrillators. These circuits look at ECG waveforms and
prompt the user to “Shock” or “No Shock” based on national and international
guidelines. The following table outlines these guidelines:
ECG SIGNALS
Asystole
Supra Ventricular Tachycardia @ 90 BPM
Polyfocal Ventricular Tachycardia @ 140 BPM
Multifocal Ventricular Tachycardia @ 140 BPM
SHOCK ADVISORY ALGORITHM TEST
ACTION
No Shock
No Shock
No Shock
No Shock
Coarse Ventricular Fibrillation
Fine Ventricular Fibrillation
Polyfocal Ventricular Tachycardia @ 160 BPM Shock
Multifocal Ventricular Tachycardia @ 160 BPM Shock
Shock
Shock
WARNING
This section is provided as a guide to familiarize
the user with the DA-2006 Series. It is not
intended to provide the necessary test sequence
for every Defibrillator. The user must consult the
manufacturer’s manual for each DUT to
determine the correct test procedure to follow.
55
Page 63

Defibrillator Analyzer
(1) Connect ECG leads to the corresponding universal connector on the DA-2006.
The connectors are marked with both the AHA and International color codes.
(2) Turn on the DA-2006.
(3) The unit will come up in the “High Range Defibrillator” mode. This range is used
for normal adult testing.
(4) Select the desired AED Waveform and Amplitude to be tested from the choices
on the ECG WAVEFORM SCREEN.
(5) Set the Defibrillator to analyze the ECG waveform in the automatic or
semiautomatic mode.
(6) Observe and record the response of the Defibrillator to the various waveforms.
56
Page 64

TRANSCUTANEOUS PACEMAKER ANALYZER
The DA-2006P can analyze pacemaker pulses as well as determine Refractory periods and
Sensitivity levels of on-demand pacemakers. For maximum versatility, the DA-2006P has
26 internally selectable pacemaker loads ranging from 50 ohms to 2300 ohms. The
DA-2006P can also test the noise immunity of the DUT by generating a 50 or 60 Hz noise
waveform with amplitude up to 100 mV. For sensitivity testing, the DA-2006P can output
a Square, Triangle or Haversine waveform with widths ranging from 10ms to 200ms. The
input circuitry of the DA-2006P is protected against damage in the case of an accidental
defibrillator discharge into the Pacemaker Input terminals.
The key is used to change to the Pacemaker Analyzer mode.
PACE MAIN SCREEN
The Pacemaker Analyzer MAIN SCREEN shows the current CONFIGURATION, the TEST
RESULTS and the available FUNCTION KEYS.
The following is a sample of the PACE MAIN SCREEN:
NOTE: The Test Results section of the PACE MAIN SCREEN contains eight lines of data
that can be toggled to view the first 4 lines or the second 4 lines (See TEST RESULTS
section of manual for more information).
57
Page 65

Pacemaker Analyzer
CONFIGURATION
The CONFIGURATION section of the PACE MAIN SCREEN displays the current setup of
the unit.
LOAD
This line displays the selected load. This setting may be changed in the PACE MODE
SETUP screen. The load choice determines what impedance is used at the pacemaker
input as well as whether the unit uses the Pacer Input Terminals or the Defibrillator Plate
Input Terminals.
NOISE
This line displays the selected noise output. This setting may be changed in the PACE
MODE SETUP screen.
WAVE
This line displays the selected output waveform. This setting may be changed in the PACE
MODE SETUP screen. The selected waveform is the output to the pacer on the ECG
Terminals, Pacer Terminals and Defibrillator Plate Terminals.
58
Page 66

Main Screen
TEST RESULTS
The TEST RESULTS section of the PACE MAIN SCREEN displays the results of the last
test. It will continue to be displayed until the power is turned off or another test is run.
The Test Results section of the PACE MAIN SCREEN contains eight lines of data that can
be toggled to view the first 4 lines or the second 4 lines by pressing the key.
RATE
This line displays the rate of the pacemaker pulse that is present at the selected load.
WIDTH
This line displays the width of the pacemaker pulse that is present at the selected load.
AMP
This line displays the current of the pacemaker pulse that is present at the selected load.
59
Page 67

Pacemaker Analyzer
ENERGY
This line displays the energy of the pacemaker pulse that is present at the selected load.
SENS PADS
This line displays the sensitivity at the pads for the selected waveform during the last
Sensitivity Test.
SENS ECG
This line displays the sensitivity at the ECG leads for the selected waveform during the last
Sensitivity Test.
PACED RP
This line displays the paced refractory period detected at the selected load during the last
Refractory Period Test.
SENSED RP
This line displays the sensed refractory period detected at the selected load during the last
Refractory Period Test.
60
Page 68

Main Screen
eys
Secondary Functio
eys
FUNCTION KEYS
The FUNCTION KEYS section of the PACE MAIN SCREEN displays the current functions
of the keys found below the display. These keys allow for navigation to supporting screens
and initiation of specific features.
PACE MODE SETUP
This key enters the PACE MODE SETUP SCREEN where all pace parameters are chosen.
SENSITIVITY TEST
Primary Function K
n K
This key activates a Sensitivity Test.
REFRACTORY PERIOD TEST
This key activates a Refractory Period Test.
TOGGLE TEST RESULTS
This key toggles the test result section to view the first 4 lines or the second 4 lines of data.
MORE KEYS
These keys toggle between the Primary and Secondary Function Keys.
PRINT MENU
This key enters the PRINT SCREEN that allows the printing of the header or the test data.
61
Page 69

Pacemaker Analyzer
PLAYBACK LAST PULSE
This key enters the PLAYBACK LAST PULSE screen where a graphical representation of
the last pulse may be viewed and sent out.
AUTO SEQUENCES
This key brings up the AUTO SEQUENCE MENU, which is used to view or run the Auto
Sequences stored in the unit.
DA-2006 SETUP
This key brings up the SYSTEM CONFIGURATION SCREEN, which allows for adjusting
the various system configuration parameters.
62
Page 70

PACER MODE SETUP SCREEN
The DA-2006P can be configured to run a large number of tests under various load
conditions. This screen is used to configure the unit for these tests. The pacemaker
configuration screen is accessed by pressing the key from the PACE MAIN
SCREEN. In this screen, the user can select the desired Load, the output Noise waveform
and the Sensitivity Test waveform.
The following is a sample of the Pacemaker configuration screen:
Defib Plates Input (50Ω)
LOAD
50 ohm
100 ohm
150 ohm
200 ohm
200 ohm
300 ohm
400 ohm
500 ohm
600 ohm
700 ohm
800 ohm
900 ohm
1000 ohm
1100 ohm
1200 ohm
1300 ohm
1400 ohm
1500 ohm
1600 ohm
1700 ohm
1800 ohm
1900 ohm
2000 ohm
2100 ohm
2200 ohm
2300 ohm
Open
NOISE
10 mV 50 Hz
9 mV 50 Hz
8 mV 50 Hz
7 mV 50 Hz
6 mV 50 Hz
5 mV 50 Hz
4 mV 50 Hz
3 mV 50 Hz
2 mV 50 Hz
1 mV 50 Hz
NONE
1 mV 60 Hz
2 mV 60 Hz
3 mV 60 Hz
4 mV 60 Hz
5 mV 60 Hz
6 mV 60 Hz
7 mV 60 Hz
8 mV 60 Hz
9 mV 60 Hz
10 mV 60 Hz
WAVEFORM
10 ms Square
25 ms Square
40 ms Square
100 ms Square
200 ms Square
10 ms Triangle
25 ms Triangle
40 ms Triangle
100 ms Triangle
200 ms Triangle
10 ms SSQ
25 ms SSQ
40 ms SSQ
100 ms SSQ
200 ms SSQ
63
Page 71

Pacemaker Analyzer
The Load, Noise and Waveform can be selected using to highlight the
parameter and using to open a drop down menu of all the options for the
highlighted parameter.
Use to scroll to the desired option. Then is used to accept the
new setting. The key can be used to return to the Pacemaker configuration
screen without making a new selection.
The key is used to return to the PACE MAIN SCREEN.
64
Page 72

The Sensitivity Test is used to determine the smallest waveform that the pacemaker can
detect. For this test, the selected waveform is generated outside of the refractory period of
the pacemaker. The DA-2006P uses a successive approximation approach to determine
the smallest output waveform that can be detected by the pacemaker. The Sensitivity Test
SENSITIVITY TEST
may be initiated by pressing the key from the PACE MAIN SCREEN.
WARNING
While this test is running, the following display will show the progress of the test:
This section is provided as a guide to familiarize
the user with the DA-2006P. It is not intended to
provide the necessary test sequence for every
Pacemaker. The user must consult the
manufacturer’s manual for each DUT to
determine the correct test procedure to follow.
At any time, the key can be depressed to stop the test and return to the
PACE MAIN SCREEN.
65
Page 73

Pacemaker Analyzer
At the end of the test, the display will show the pacemaker amplitude sensitivity at the
Pacer Terminals and the ECG Terminals.
66
Page 74

For on-demand pacemakers, the pacemaker should ignore any ECG activity after a pacer
pulse for a specific period of time. This time period is known as the Refractory Period. The
Paced Refractory Period is the time after the pacemaker pulse that ECG activity is ignored.
If an ECG pulse is present inside the refractory period, it is ignored. If an ECG pulse is
detected outside of the refractory period, the pacemaker will re-synchronize to the sensed
ECG pulse. For each sensed ECG pulse, there is a second refractory period. This is
known as the Sensed Refractory Period. It is the period of time after the sensed ECG
pulse that ECG activity is ignored. The Refractory Period Test may be initiated by pressing
the key from the PACE MAIN SCREEN.
REFRACTORY PERIOD TEST
WARNING
While the Refractory Period test is running, the display will indicate the progress of the test:
This section is provided as a guide to familiarize
the user with the DA-2006P. It is not intended to
provide the necessary test sequence for every
Pacemaker. The user must consult the
manufacturer’s manual for each DUT to
determine the correct test procedure to follow.
NOTE: It is important that the pulse rate does not change for the duration of the Refractory
Test.
67
Page 75

Pacemaker Analyzer
At any time, the key can be depressed to stop the test and return to the
PACE MAIN SCREEN.
When the test is completed, the display will update with the Paced Refractory Period and
Sensed Refractory Period in the Test Results.
68
Page 76

PRINT MENU SCREEN
The DA-2006P allows the user to print the latest Pacemaker Analysis data or a header.
The PRINT MENU SCREEN is accessed by pressing the key from the
PACE MAIN SCREEN.
The following is an example of the print menu screen:
The header is sent by pressing the key.
The test data is sent by pressing the key.
The key can be depressed to return to the PACE MAIN SCREEN.
69
Page 77

Pacemaker Analyzer
The following is the print header and sample data that are used for the Pacemaker
Analyzer mode:
NOTE: Since Pacemaker pulses are normally continuous, the test data must be printed
manually via the Print Menu.
NOTE: Printing the header also resets the test number printed on the data sheet.
70
Page 78

PLAYBACK LAST PULSE SCREEN
The DA-2006P can display a graphical representation of the last pulse. This screen may
be accessed by pressing the key from the PACE MAIN SCREEN. The
playback allows the user to view the Pacemaker pulse in a time-expanded form. Samples
are stored internally at 0.1 ms intervals. The PLAYBACK LAST PULSE SCREEN shows
these samples expanded by a time factor of 200.
In playback mode, the samples are shown on the display and sent out the ECG leads,
Defibrillator Plates, and the High Level output. The following is a sample of the waveform
that is shown in the display:
The scale shown on the screen is automatically adjusted to provide the maximum
resolution available.
The key can be used to pause the screen at any point while a pulse is being
played back. This key replaces the key when a pulse is being played back.
The key can be used to play (continue) the waveform if it has been paused.
This key replaces the key.
71
Page 79

Pacemaker Analyzer
The key starts a playback of only the first 20 ms of the waveform.
The key starts a playback of the entire 100 ms of the waveform.
At any time, the key or key can be depressed to return to the
MAIN SCREEN.
72
Page 80

MESSAGES
INPUT OVERLOAD
The “Warning Input Overload Check Range” message can display during Defibrillator
testing. The range should be checked to see if it should be changed to High Range for the
current Joule setting.
NO PULSE (DA-2006P Only)
The “Test Cancelled No Pulse for 3 seconds” message can display during Refractory or
Sensitivity Pacer testing. The settings should be checked and the test rerun.
73
Page 81

DA-2006 Series
SENSITIVITY TOO HIGH (DA-2006P Only)
The “Test Cancelled DUT Sensitivity too high” message can display during Pacer testing.
This happens when the Pacemaker does not detect the pulse generated by the DA-2006P.
It could be that it is connected improperly or set to Async mode. This can occur during
either the Sensitivity or Refractory test modes.
LOW BATTERY
This message indicates that the batteries are low and should be replaced.
EXITING AUTO SEQUENCE TESTING
The “Exit Auto Sequence Test All Data Will be Lost!” message will display in the Auto
Sequencing Mode when is pushed. If the data is needed, it should be
printed prior to exiting.
74
Page 82

SYSTEM SETUP
The SYSTEM SETUP SCREEN allows for the configuration of the system settings. The
settings can be selected using to highlight the parameter and using
to allow the editing of the parameter. The keys are used to edit
the setting, then is used to accept the new setting. The key can
be used to return to the configuration screen without making a new selection.
The key is used to return to the MAIN SCREEN.
The following is a brief description of the parameters and the available range of settings:
Parameter Description Range
Off – Always off
1-20 sec – The elapsed time after
Backlight Timed
which the backlight will automatically
turn off.
Off, 1-20 sec,
Always On
Always On – The backlight will be
manually controlled by backlight key)
Auto Sequence
Timer
Sets the delay between Auto
Sequence tests when the test
passes.
1-20 sec
Displays current life of the batteries.
Battery Life
At 5%, a warning screen will appear.
At 10%, the unit will power down
5-100%
(Read Only)
automatically.
Power up with
Selects the values that will be used
when the unit is first turned on. It is
also used to Set the Custom
Defaults, if used. (See Power Up
Settings).
Default/Last/
Custom/
Set Custom
Defaults
Software Displays current software program. (Read Only)
75
Page 83

DA-2006 Series
This page intentionally left blank.
76
Page 84

POWER UP SETTINGS
The DA-2006 Series allows the user to tailor the settings that the unit will have on Power
Up. The “Power Up With” parameter in the System Setup Menu allows for the selection of
either Default or Custom selections.
DEFAULT
If this option is selected the following settings will be used every time the unit is turned on.
Range – Defib, High Range mode
ECG – Output Disabled
Pacemaker Load – 100 ohm
Pacemaker Noise Waveform- None
Pacemaker Output Waveform – 40 ms Square wave.
CUSTOM
If this option is selected, the user may save a unique set of default parameters and the unit
will recall them every time the power is turned on.
SET CURRENT AS CUSTOM
The user simply configures the unit to the desired default conditions, selects this option and
presses . The current configuration is then saved as the Custom Power up
values.
77
Page 85

DA-2006 Series
This page intentionally left blank.
78
Page 86

AUTO SEQUENCE FUNCTION
The DA-2006 Series allows the user run up to 50 pre-programmed sequences of tests
(Auto Sequences). The tests are configured with an easy to use PC program. Each test
can be configured to test Defibrillator, Transcutaneous Pacemaker or both. (For
programming Auto Sequences, see the Auto Sequence Programming section). Once
configured, the tests are then downloaded to the DA-2006 unit through the RS232 serial
interface.
The AUTO SEQUENCE SCREEN is accessed using the key.
In this menu, the keys are used to select the desired
test. The key can be used to enter the VIEW MODE
which will allow the user to view the programmed test options of the
selected test. The key will run the selected test and
enter the RUN MODE which will step the technician through the
AUTO SEQUENCES
LifePak 4
LifePak 5
LifePak 6
LifePak 6S
LifePak 8P
LifePak 9P
LifePak 9PM
LifePak 10
LifePak 10P
LifePak 10PM
HP 78660A
HP XLPM
Nihon Kohden 7000
Laerdal HS 2000
Marquette 1500PM
Zoll PD 2000
Zoll M-Series DSW
Zoll AED Plus
Blank Tests 20-50
programmed test as well as identify whether each step has passed
or failed based upon the pre-programmed test limits that are part of
each Auto Sequence.
79
Page 87

Auto Sequence Function
The following table shows the possible test sequence with the details and options that can
be selected using the PC program:
Test Description Fields Options
Defibrillator Test Sequence
Steps 1-20 xxx Joules
Energy Level Limits 0-99%
VFIB ECG Output yes/no
Do Test? yes/no
Energy Level Limits xxx Joules
Max Allowed Charge
Time
x sec
Steps 1-3 xxx Joules
Energy Level Limits 0-99%
Steps Up to 10
Waveform Outputs
and Amplitudes
x Waveform Group
x Waveform
Lead II = x.x mV
Steps 1-20
Pulse Rate,
Pulse Amplitude and
Load settings
Limits for
Rate and Amplitude
xxx ppm
xx mA
xxx ohms
0-99%
Do Test? yes/no
Pulse Rate and
Load
xxx ppm
xxx ohms
Steps 1-5
Pulse Rate,
Load and
Output Waveform
xxx ppm
xxx ohms
x Waveform
Do Test? yes/no
Defib Energy Tests
Maximum Energy
Test
Cardioversion Tests
ECG Performance
Test
Pulse
Rate and Amplitude
Tests
Asynchronous Test
Demand Mode Tests
Refractory Test
Measures defibrillator
discharge energy
Measures time required
for defibrillator to charge
to maximum energy
Measures Cardioversion
Delay
Tests defibrillator
ECG input
Pacemaker Test Sequence (DA-2006P Only)
Measures Pacemaker
Pulse Rate and
Amplitude
Tests Pacemaker
Asynchronous Mode
Measures Pacemaker
Sensitivity at Pacemaker
Pads and ECG leads
Measures Paced
Refractory Period and
Sensed Refractory Period
80
Page 88

VIEW MODE
The VIEW MODE allows the user to look at the test configuration. Each test setting will be
shown, as well as the test limits that identify a valid or invalid test result. The screens that
are displayed in the VIEW MODE are determined by the Auto Sequence selected on the
AUTO SEQUENCE SCREEN and its configuration as defined with the PC program.
The following screens are examples of what could be shown in the VIEW MODE if all test
options are selected:
NOTE: If any particular test option is disabled using the PC Program, it will not be shown in
the VIEW MODE.
DEFIBRILLATOR ENERGY TESTS:
Test Settings
.
.
.
Energy Limits
VFIB Option
81
Page 89

Auto Sequence Function
DEFIBRILLATOR MAXIMUM ENERGY TESTS:
Max Energy
Max Energy Test Limits
DEFIBRILLATOR CARDIOVERSION TESTS:
.
.
.
Test Settings
Test Limits
82
Page 90

View Mode
DEFIBRILLATOR ECG PERFORMANCE TEST:
NOTE: The individual selected waveforms are not displayed in the VIEW MODE.
PACEMAKER PULSE AND AMPLITUDE TESTS: (DA-2006P Only)
Waveform Selection
Test Settings
.
.
PACEMAKER ASYNCHRONOUS MODE TEST: (DA-2006P Only)
.
Test Limits
Pulse Rate Setting
83
Page 91

Auto Sequence Function
PACEMAKER DEMAND MODE TESTS:
(DA-2006P Only)
.
.
PACEMAKER REFRACTORY TEST: (DA-2006P Only)
.
Test Settings
Refractory Selection
84
Page 92

A
p
RUN MODE
The RUN MODE allows the user to run the test configuration. The screens that are
displayed in the RUN MODE are determined by the Auto Sequence selected on the AUTO
SEQUENCE SCREEN and its configuration as defined with the PC program.
Running an Auto Sequence will provide a consistent, guided procedure for testing
equipment. This is a semi-automated process that will provide immediate feedback to the
user if the DUT passes or fails individual tests. A programmable timer is provided to
automatically progress through the test when a given test passes. This timer is set in the
Auto Sequence Timer parameter in the SYSTEM SETUP SCREEN.
NOTE: If any particular test option is disabled using the PC Program, it will not be shown in
the RUN MODE.
NOTE: Some tests, like Performance Waveforms, do not have quantitative analyses and
therefore require the user to manually progress through the test.
The following sample screen shows the common elements present during the RUN MODE:
Instructions
Selected
uto Sequence
Current
Test Ste
Test Results
for Settings and
Actions
NOTE: All Data
Test Results
Passed
will be lost when
this key is used.
Failed
Exit Key
for exiting the
RUN MODE
Last Step Key
for returning to
the last step
Next Step Key
for advancing
to the next step
Print Test Results Key
for sending all results
to the printer
85
Special Action Key
Capture Readings,
Get New Readings,
Start
Page 93

Auto Sequence Function
The following screens may be shown in the RUN MODE if all test options are selected:
DEFIBRILLATOR ENERGY TESTS:
Test Setup and Action
Test Passed
Test Failed
86
Page 94

Run Mode
DEFIBRILLATOR MAXIMUM ENERGY TESTS:
Test Setup and Action
Charge Timer Warning
Charge Timer Running
Results
87
Page 95

Auto Sequence Function
DEFIBRILLATOR CARDIOVERSION TESTS:
Test Setup and Action
Test Passed
DEFIBRILLATOR ECG PERFORMANCE TEST:
Waveform
NOTE: Some tests, like Performance Waveforms, do not have quantitative analyses and
therefore require the user to manually progress through the test.
88
Page 96

Run Mode
PACEMAKER PULSE AND AMPLITUDE TESTS:
(DA-2006P)
Test Setup and Action
Results
NOTE: If the test fails or new readings are desired, the Get New Readings Key can be
used to replace the current readings. The current readings will be lost, even if they are
from a test that passed.
89
Page 97

Auto Sequence Function
PACEMAKER ASYNCHRONOUS MODE TEST:
(DA-2006P Only)
Test Setup and Action
Results
NOTE: If the test fails or new readings are desired, the Get New Readings Key can be
used to replace the current readings. The current readings will be lost, even if they are
from a test that passed.
90
Page 98

Run Mode
PACEMAKER DEMAND MODE TESTS:
(DA-2006P Only)
Test Setup and Action
Sensitivity Test Running
Results
NOTE: If the test fails or new readings are desired, the Get New Readings Key can be
used to replace the current readings. The current readings will be lost, even if they are
from a test that passed.
91
Page 99

Auto Sequence Function
PACEMAKER REFRACTORY TEST:
(DA-2006P Only)
Test Setup and Action
Refractory Test Running
Results
NOTE: If the test fails or new readings are desired, the Get New Readings Key can be
used to replace the current readings. The current readings will be lost, even if they are
from a test that passed.
92
Page 100

Run Mode
EXITING AUTO SEQUENCE TESTING MESSAGE
The “Exit Auto Sequence Test All Data Will be Lost!” message will display in the Auto
Sequencing Mode when is pushed. If the data is needed, it should be
printed prior to exiting.
93
 Loading...
Loading...