Page 1

User Manual
DA-2003P
Defibrillator Transutaneous
Pacemaker Analyzer
Page 2

CONTENTS
I. MANUAL REVISION RECORD ........................................... 2
II. INTRODUCTION.................................................................. 3
2.1 Features
2.2 General Information
III. SPECIFICATIONS ............................................................... 4
3.1 Defibrillator Analyzer
3.2 Transcutaneous Pacemaker Analyzer
IV. INSTALLATION ................................................................... 8
4.1 Receipt, Inspection and Return
4.2 Setup
4.3 Power
4.4 Internal Paddles
4.5 Special Contacts
V. OPERATING ...................................................................... 10
5.1 Control Switches and Connections
5.2 Menu and Function Keys
5.3 Menu and Messages: Defibrillator Mode
5.4 Menu and Messages: Transcutaneous Pacemaker Mode
5.5 Test Result Printouts
VI. DEFIBRILLATOR MODE TESTING .................................. 16
6.1 Introduction
6.2 Test Preparation
6.3 Energy Test
6.4 Cardioversion Test
6.5 Maximum Energy Charging Time Test
6.6 Shock Advisory Algorithm Test
VII. TRANSCUTANEOUS PACEMAKER MODE TESTING .... 21
7.1 Introduction
7.2 Test Preparation
7.3 Demand Sensitivity Test
7.4 Refractory Period Test
VIII. WARRANTY ...................................................................... 25
IX. TECHNICAL SUPPORT .................................................... 25
Page 3
I. MANUAL REVISION RECORD
This record page is for recording revisions to your DA-2003P User Manual that have been published by
BC Biomedical or its authorized representatives. We recommend that only the management or facility
representative authorized to process changes and revisions to publications:
• Make the pen changes or insert the revised pages;
• Ensure that obsolete pages are withdrawn and either disposed of immediately, or marked as
superseded and placed in a superseded document file, and;
• Enter the information below reflecting that the revisions have been entered.
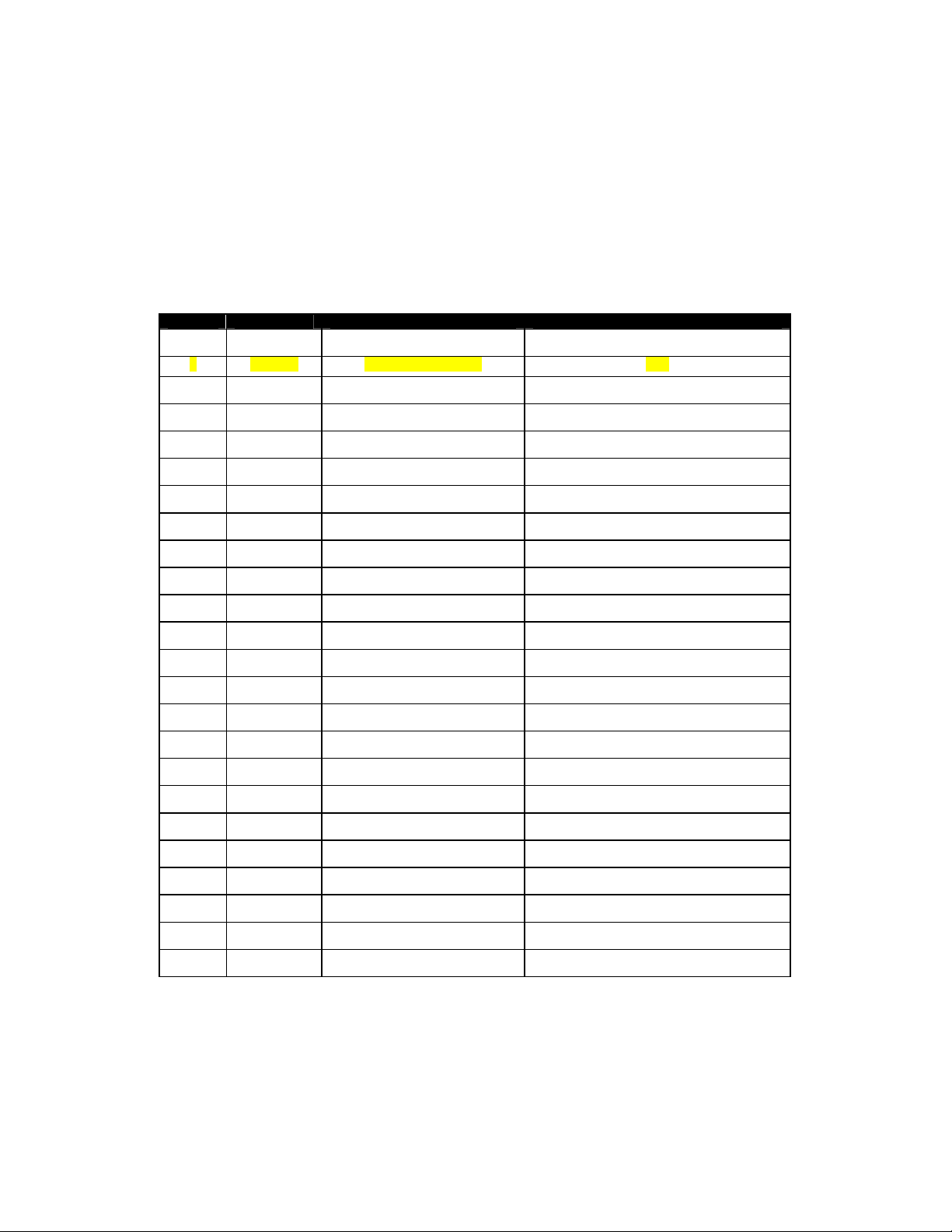
Rev No Date Entered Reason Signature of Person Entering Change
0 - Initial Release
1 11/12/04 Part Number Change KLB
2
BC Biomedical DA-2003P User Manual 11/04
Page 4

2.1 Features
II. INTRODUCTION
The DA-2003P Analyzer is a precision instrument for testing defibrillators and transcutaneous
pacemakers, and is designed to be used by trained service technicians.
The defibrillator function of the DA-2003P measures the energy output, and ensures that the defibrillator
complies with specified requirements. DA-2003P has a built-in load resistance of 50 ohm, which roughly
corresponds to the impedance of the human body. The defibrillator pads are placed on the DA-2003P
contact plates. Thus, the defibrillator is connected through the load resistance. When the defibrillator is
discharged, DA-2003P calculates and displays the energy delivered.
In the pacer function the DA-2003P tests all types of transthoracic pacemakers. The testing is menu
driven, and simple to operate. DA-2003P measures and displays a pulse’s amplitude, rate, energy and
width. It also conducts demand sensitivity tests, measuring and displaying refractory periods, and
immunity tests, which determine the pacemaker’s susceptibility to 50/60 Hz interference.
2.2 General Information
Temperature Requirements +15°C to +35°C when operating
0°C to +50°C in storage
Display
Type LCD graphic display
Alphanumeric format 6 lines, 40 characters
Data Input/ Output (2)
Parallel printer port (1); Bi-directional RS -232C
(1) for Computer control
Power
2 x 9 volt alkaline Battery Duracell
equivalent) for 20 -25 operational hours, or 240
VAC (Battery Eliminator), 115 VAC for US.
Mechanical Specifications
Housing High impact plastic case
Height 9.8 cm 3.9 in.
Width 24.8 cm 9.8 in.
Depth 28.0 cm 11.0 in.
Weight 2.06 kg (with battery) 4.5 lbs
Recommended Printer
Standard Accessories
HP DeskJet 500C / 550C and Canon BJ -10SX.
110 V or 220 V AC Adapter (P/N BC20-00429)
Internal paddle-contact adapter (P/N BC20-00430)
Snap-to-banana adapters (10 pk) (P/N BC20-00427)
DA-2003P User Manual
Protective Cover (P/N BC20-30108)
Additional Accessories
Defib. paddle adapter
(specify defibrillator type)
Pacemaker External Load Cable
(specify pacemaker type)
Soft Carrying Case (P/N BC20-30108)
Storage
Store in the carrying case in dry surroundings within the temperature range
specified, without battery. There are no other storage requirements.
Periodic Inspection
The unit should be calibrated every 12 months.
BC Biomedical DA-2003P User Manual 11/04
3
MN1604 (or
Page 5

3.1 Defibrillator Analyzer
1. Energy Output Measurement
High Range
Voltage <5000 volts
Maximum current 120 amperes
Maximum energy 1000 Joules
Accuracy
Trigger level 100 volts
Playback amplitude 1 mV/1000 V Lead I
Test pulse 100 + 4 Joules
Low Range
Voltage <1000 volts
Maximum current 24 amperes
Maximum energy 50 Joules
Accuracy
Trigger level 20 volts
Playback amplitude 1 mV/200 V Lead I
Test pulse Approx. 4 Joules
Load Resistance
Display Resolution 0.1 Joules
Measurement Time
Window
Absolute Max. Peak
Voltage
Pulse Width 100 ms
Cardioversion
Oscilloscope Output
High measure range 1000:1 amplitude-attenuated
Low measure range 200:1 amplitude-attenuated
III. SPECIFICATIONS
± 2 % of reading for >100 Joules
± 2 Joule of reading for <100 Joules
± 2% of reading for >20 Joules
± 2 Joule of reading for <20 Joules
50 ohms ± 1%, non-inductive (<1 µH)
100 ms
6000 volts
Measured time delay ± 2 ms
2. ECG Wave
Waveform Storage And Playback
Discharge can be viewed via ECG outputs and paddles.
Output: 200:1 Time Base expansion.
Sync Time Measurements
Timing window Starts - 40 ms at each R-wave peak.
Test waveforms All waveform simulations available.
Delay time accuracy
± 1 ms
Charge Time Measurement
From 0.1 seconds to 99.9 seconds.
ECG General
Lead configuration 12-lead simulation. RL, RA, LA, LL, V1-6
Output impedance Limb leads 1000 ohms to RL
V Leads 1000 ohms to RL
4
BC Biomedical DA-2003P User Manual 11/04
Page 6

All other signals are in relative proportion to Lead amplitude as follows:
The amplitudes are shown for a Lead I amplitude by 1 mV:
Lead I 1.0 mV (LA - RA)
Lead II 1.5 mV (LL - RA)
Lead III 0.5 mV (LL - LA)
V Lead 1.5 mV (V - 1/3 (LL+LA+RA))
High Level Output (ECG Jack)
1/4" standard phone-jack with an amplitude of 1V/mV of low level Lead II signal
Defibrillator Contact Plates
Same amplitude as Lead I low level ECG.
1 mV between contact surfaces.
Playback
200 to 1 time-base expansion of defibrillator pulse by playback to ECG Leads
Manual ECG Performance Test
DC Pulse 4 seconds 1.0 mV
Square wave 2 Hz 1.0 mV p-p biphasic
Triangular wave 2 Hz 1.0 mV
Sine 0.1, 0.2, 0.5, 10, 40, 50, 60, and 100 Hz
Amplitude 0.5, 1.0, 1.5, 2.0 mV (Lead II)
Accuracy
± 5 % (Lead II 1.0 mV)
ECG Performance Test
Gain/Damping 2 Hz square wave
Frequency Response
Low Frequency 4 second DC pulse
Band Pass 10 Hz sine
Monitor -3dB point: 40 Hz sine
Power Line Notch
50 Hz sine
Filter
Linearity 2 Hz triangle wave
Normal Sinus
Rates 30, 60, 80, 120, 180, 240 and 300 BPM.
Accuracy
±1% of selection
Amplitudes 0.5, 1.0, 1.5 and 2.0 mV (Lead II)
Accuracy
±5 % (Lead II 1.0 mV)
Automatic ECG Rate Test
Arrhythmia Selections
vfib Ventricular Fibrillation
afib Atrial Fibrillation
blk II Second degree A-V block
RBBB Right Bundle Branch Block
PAC Premature Atrial Contraction
PVC_E Early PVC
PVC_STD PVC
PVCRonT R on T PVC
mfPVC Multifocal PVC
bigeminy Bigeminy
run5PVC Bigeminy Run of 5 PVCs
vtach Ventricular Tachycardia
BC Biomedical DA-2003P User Manual 11/04
5
Page 7

Shock Advisory Test Algorithms
ASYS Asystole
SVTa_90 Supraventricular Tachycardia
PVT_140
PVT_ 160
MVT_140
MVT_160
CVF Course Ventricular Fibrillation
FVF Fine Ventricular Fibrillation
6
BC Biomedical DA-2003P User Manual 11/04
Page 8

3.2 Transcutaneous Pacemaker Analyzer
1. TEST LOAD RANGE
50 to 2300 ohms in step of:
50 ohm up to 200 ohms
100 ohm from 200 up to 2300 ohms
Accuracy
Oscilloscope Output
50 - 150 ohm 10.24:1 amplitude attenuation
200 - 500 ohm 41:1 amplitude attenuation
600 - 2300 ohm 164:1 amplitude attenuation
2. PULSE MEASUREMENTS
Amplitude 4 to 300 mA (100 ohm load)
Accuracy
Max. Amplitude 300 mA all loads
Rate 30 to 800 Pm
Accuracy
Pulse width 0.6 to 80 ms
Accuracy
50 - 1300 ohm ±1%
1400 - 2300 ohm ±1.5 %
±5 % or ±0.5 mA
±1% or 2 Pm
±1% or ±0.3 ms
3. DEMAND SENSITIVITY TEST
Waveforms Square(SQR), Triangle(TRI), and Havemine (SSQ)
ECG output Amplitude 0 - 4 mV
Pacer input (Load depended)
Amplitude (50 ohm) 0 10 mV
Resolution (50 ohm)
Amplitude: (≥500 ohm) 0 - 100 mV
Resolution: (≥500 ohm) 1 mV
Defib. Pads Amplitude 0 10 mV
Resolution 0.1 mV
Waveform width 10, 25, 40, 100 and 200 ms
Pacer rate 30 to 120 Pm
Immunity Test
50/60 Hz Interference Signal
ECG output 0 - 4 mV peak in steps of 0.4 mV
Pacer input (Load dependent)
0 - 10 mV peak in steps of 1 mV (50 ohm)
Defibrillator pads 0 - 10 mV peak in steps of 1 mV
Resolution 40 µV
40 µV
0 - 100 mV peak in steps of 10 mV (≥500 ohm)
4. Refractory Period Measurement
20 to 500 ms (both Pacing and Sensing) Accuracy: ±2 ms
BC Biomedical DA-2003P User Manual 11/04
7
Page 9

IV. INSTALLATION
4.1 Receipt, Inspection and Return
1. Inspect the outer box for damage.
2. Carefully unpack all items from the box and check to see that you have the following items:
• DA-2003P Defibrillator/Transcutaneous Pacemaker Analyzer
• 110 V or 220 V AC Adapter
• Internal paddle-contact adapter
• Ground contact adapter
• 10 pack, Snap-to-banana adapter
• DA-2003P User Manual
3. If you note physical damage, or if the unit fails to function according to specification, inform the
supplier immediately. When BC Biomedical or the company’s representative, is informed, measures
will be taken to either repair the unit or dispatch a replacement. The customer will not have to wait for
a claim to be investigated by the supplier. The customer should place a new purchase order to ensure
delivery.
4. When returning an instrument to BC Biomedical, or the company representative, fill out the address
label, describe what is wrong with the instrument, and provide the model and serial numbers. If
possible, use the original packaging material for return shipping. Otherwise, repack the unit using:
• A reinforced cardboard box, strong enough to carry the weight of the unit.
• At least 5 cm of shock-absorbing material around the unit.
• Nonabrasive dust-free material for the other parts.
Repack the unit in a manner to ensure that it cannot shift in the box during shipment.
BC Biomedical’s product warranty is on page 26 of this manual. The warranty does not cover freight
charges. C.O.D. will not be accepted without authorization from BC Biomedical or its representative.
4.2 Set-up
1. Equipment connection is as shown in the typical setup below.
8
BC Biomedical DA-2003P User Manual 11/04
Page 10

4.3 Power
1. Main On/Off Switch. DA-2003P should remain off for at least 5 seconds
before switching on again, in order to allow the test circuits to discharge fully.
2. Low Battery Power. If battery power falls below 6.9 volts (± 0.3 volts), the
display will show 'Change battery, and reset system'. This means that the battery
should either be replaced or the instrument should be connected to a battery
eliminator. The main switch has to be switched off and then on again in order to
use the instrument.
Do not use mercury, air or
NOTE
carbon-zinc batteries.
3. Changing Batteries. Open the compartments in the base of the
instrument, replace the old batteries with new ones, and close the compartment
covers. Use 9-volt alkaline batteries (Duracell
MN1604 or similar).
4. Battery Eliminator. BC Biomedical’s AC Adapter plug-in power supply
Remove the batteries and
NOTE
disconnect the AC Adapter if
you do not intend to use the
DA-2003P for an extended
period of time.
transformer allows you to use the DA-2003P anywhere a standard electrical
outlet is available. To attach the AC Adapter insert the adapter’s small connector
into the micro jack labeled “Batt. Elim. 9V DC” on the right rear of the unit. Plug
the large connector into the nearest standard electrical outlet.
4.4 Internal Paddles
To be able to test defibrillators with internal paddles, an internal paddle adapter has to be used. These
contacts have a banana plug that is attached to the standard paddle contact, and which is protected by
a plastic insulation washer.
4.5 Special Contacts
Certain defibrillators (automatic models and those with pacer options) have special contacts that are
fastened to the electrodes attached to the patient. BC Biomedical has special adapters to suit the
majority of these defibrillators. These are available as accessories. They are more or less the same as
the internal pad adapter except that they have a special adapter on the top, which matches the contact
on the defibrillator.
Defibrillator paddle adapter (specify defibrillator type)
Pacemaker external load cable (specify type pacemaker type)
9
BC Biomedical DA-2003P User Manual 11/04
Page 11

5.1 Control Switches and Connections
Front Panel
V. OPERATING
DA-2003P
1. Power Switch
2. Mode Switch
3. LCD Display
4. Function Keys
5. Contact Surfaces
6. Low Level ECG Connectors
7. Pacer Input Connectors
Turns the power on and off.
Switches between PACE and Low / High
ranges of defibrillator energy.
Shows messages, test results and function
menus.
Fl - F5 are used to select the functions shown
on the bottom line of the LCD display, i.e., for
selecting the function that is directly above the
key.
The defibrillator’s paddles are placed on these
so that the discharged energy passes through
the instrument in defib. mode and that the pacer
signal passes through the instrument with a
fixed 50 ohm load in the PACE mode.
10 color-coded 4 mm safety terminals with
snap-to-banana adapters.
The pacer output cables are connected to
these so that the pacer signal passes
through the instrument with a variable load
selectable from 50 to 2300 ohms
.
10
BC Biomedical DA-2003P User Manual 11/04
Page 12

Rear Panel
8. High Level ECG Jack
1/4” standard phone-jack for amplitude of 1 V/mV
of low level Lead 1 signal.
9. Oscilloscope Output
10. RS-232 Serial Port
11. Printer Outlet Port
12. Location of Batteries
BNC-contact for attenuated signal in real time.
9-pin D-sub
14-25 pin D-sub
2 compartments in the base of the instrument can
be opened to replace the batteries.
13. Battery Eliminator
Socket
5.2 Menu and Function Keys
Battery contact for connecting 9V 30 mA battery
eliminator.
The DA-2003P uses display and programmable function keys to provide flexibility and control over the
operations. The upper part of the screen displays messages, status and results. The menu bar is at the
bottom of the display. The function keys are numbered from Fl to F5.
A function is selected by pressing the key located directly under the Menu Item displayed in the menu
bar. A menu unit is written in capital letters. The menu has three pages. The next pages of the menu
are selected by pressing more-2, more-3, or more-1.
5.3 Menu and Messages: Defibrillator Mode
1. Startup Screen. The following screen will be displayed for 2 seconds after the DA-2003P has
been switched on.
DA-2003P
4
11
BC Biomedical DA-2003P User Manual 11/04
Page 13

2. Main Menu
a. Main Menu Bar (Page 1) - Mode switch in Low or High position.
b. Second Menu Bar (Page 2)
c. Third Menu Bar (Page 3)
3. ECG WAVES (F1).
Choose desired wave by pressing UP (F2) or DOWN (F3). Save this under ‘Wave” in the
STATUS field by pressing SELECT (F4). Press CANCEL (F5) to cancel selection.
4. ADV. ALG. (Advisory Algorithms) (F2).
These ECG algorithms are meant to test the analysis and prompting feature of automatic and
semi-automatic defibrillators. Choose desired selection by pressing UP (F2) or DOWN (F3).
Save this under ‘Wave” in the STATUS field by pressing SELECT (F4). Press CANCEL (F5)
to cancel selection.
BC Biomedical DA-2003P User Manual 11/04
12
Page 14

5. CHARGE TIME (F3). Used to test the battery and charging capacitor in the defibrillator. It
changes the text ‘Delay’ to ‘Chrg ‘ in the RESULT field in the main menu.
6. PRINT HEADER (F4). Automatically writes a heading for the new test protocol.
7. WAVE AMPL. (Wave Amplitude) (F1).
Choose desired amplitude by pressing UP (F2) or DOWN (F3). Save this under ‘Ampl” in
the STATUS field by pressing SELECT (F4). Press CANCEL (F5) to cancel selection.
8. PLAY PULSE (F2) enables playback of the last discharge.
9. PERF. WAVE (Performance ECG) (F3).
Choose desired wave by pressing UP (F2) or DOWN (F3). Save this under ‘Wave” in the
STATUS field by pressing SELECT (F4). Press CANCEL (F5) to cancel selection.
10. SYSTEM TEST (F1) .
Note
DA-2003P has an internally
generated test pulse. The control
pulse is set at 1.2 Joules in the
Low range and 28.5 Joules in the
High range. The test pulse is not
a calibration pulse, and should not
be used as an indication of the
general accuracy of the
instrument. The test pulse is a
good control for testing functions.
Choose a test variant by pressing UP (F2) or DOWN (F3) or TEST PULSE (F1). Press
CANCEL (F5) to cancel selection. For ‘ECG0’, ‘ECG+’ and ‘ECG-’ see Chapter 6,
Control and Calibration. For ‘A/D-read’, see paragraph 7.3.7, page 7-5. Memory’ is for
factory testing. Also, see paragraph 4.3.5, page 4-3.
11. REMOTE CONTR. (Remote Control) (F4) enables communication with a PC with test
automation software.
13
BC Biomedical DA-2003P User Manual 11/04
Page 15

5.4 Menu and Messages: Defibrillator Mode
1. Startup Screen. The following screen will be displayed for 2 seconds after the DA2003P has been switched on.
DA-2003P
2. Main Menu
a. Main Menu Bar (Page 1) - Mode switch in PACE position.
b. Second Menu Bar (Page 2)
3. SELECT LOAD (F1)
Choose desired PACER load by pressing UP (F2) or DOWN (F3) and then SELECT
(F4). Press CANCEL (F5) to cancel selection.
4. SELECT NOISE (F2)
14
BC Biomedical DA-2003P User Manual 11/04
Page 16

Choose desired noise for the immunity test by UP (F2) or DOWN (F3) and then
SELECT (F4). Press CANCEL (F5) to cancel selection.
5. PRINT HEADER (F3). Automatically writes a heading for the new test protocol.
6. PRINT RESULT (F3). Prints the results of measurements.
7. SELECT WAVE (F2)
Choose desired waveform for the sensitivity test by pressing UP (F2) or DOWN (F3)
and then SELECT (F4). Press CANCEL (F5) to cancel selection.
8. SENS. TEST (Sensitivity Test) (F2). Sensitivity is the QRS minimum amplitude (mV)
required to cause the pacemaker to operate in the demand mode. This waveform is
delayed from the pacer pulse so that it is outside the pacing refractory period. See
‘Sensitivity Measurements’ in Chapter 5.
9. REF. PER TEST (F3). Used to test time interval (ms) if the pacemaker is insensitive to
any external inputs, the maximum time interval after the generation of a pacer pulse
and maximum time interval after a QRS wave. See ‘Pacing Refractory Period’ and
‘Sensing Refractory Period’ in Chapter 5.
5.5 Test Result Printouts
1. Defibrillator Mode. DA-2003P automatically prints out the test results, via the printer
output, after each discharge generated. Select PRINT HEADER (F4) if you want to print
out a page with a new header.
2. Pace Mode. DA-2003P prints out the test results, after the measurements, when you
press PRINT RESULT (F4) in the Main menu.
15
BC Biomedical DA-2003P User Manual 11/04
Page 17

VI. DEFIBRILLATOR MODE TESTING
6.1 Introduction
The defibrillator function of the DA-2003P measures the energy output, and ensures that the
defibrillator complies with specified requirements.
roughly corresponds to the impedance of the human body. The defibrillator pads are placed on the DA2003P contact plates. Thus, the defibrillator is connected through the load resistance. When the
defibrillator is discharged, DA-2003P will calculate and display the energy delivered.
Defibrillator energy is defined as an integral of the moment of the discharged energy from the
defibrillator. The energy is equal to the square of the voltage, divided by the load resistance.
It has a built-in load resistance of 50 ohms, which
E = Ø p dt = Ø V2 / R dt = Ø V2 dt / R
DA-2003P measures and records the voltage pulse every 100 µs, 1000 times, for a total time of 100
ms. The squares of the voltages are then summed, multiplied by 100 µs, and divided by the load
resistance, 50 ohms.
1000 1000
E = Ø (V2) • dt / R = Ø (V2) • 100 µs / 50
ohms
The unit for energy is 'joule', which is equal to Ws (Watt second).
6.2 Test Preparation
1. If checking ECG monitoring, prompting, or triggering from the ECG, connect the low level or
high level ECG connectors to the ten 4 mm AHA color-coded safety terminals or standard
phone jack, as appropriate.
2. Switch the DA-2003P on. The following will be displayed in the LCD display for about two
seconds:
DA-2003P
3. The following main menu will then appear. It will show LOCAL.
16
BC Biomedical DA-2003P User Manual 11/04
Page 18

6.3 Energy Test
1. Select a suitable energy range using the mode switch.
• Use the HIGH range for normal adult testing.
• Use the LOW range for low energy testing, where the energy does not exceed 50 Joule
and the peak voltage does not exceed 1200 volts.
2. Securely place the defibrillator paddles on the DA-2003P contact plates, and discharge the
defibrillator. The APEX (+) pad should be connected to the right-hand plate, and the STERNUM
pad to the left plate. This ensures correct signal polarity for the oscilloscope output. A reversal
of this configuration will not damage the DA-2003P, nor will it give incorrect energy readings.
However, the polarity of the oscilloscope output will simply be reversed. The discharge from the
defibrillator is transferred to the DA-2003P’s load resistance.
3. DA-2003P calculates the energy delivered over the load resistance and displays the result in
joules under RESULT.
If the maximum voltage for a
Note
selected range is exceeded, the
LCD display will show ‘WARNING!
Overload’
APEX (+) pad → right plate
STERNUM pad → left plate
DA-2003P also shows the energy measured, the maximum voltage and the maximum current in
the energy wave. Following the discharge from the defibrillator, DA-2003P shows a playback of
the wave from the ECG output. A new pulse can be generated when the LCD display shows
'LOCAL'.
4. Following a discharge from the defibrillator, the instrument shows a playback of the wave from
the ECG output. The display will thus be in playback mode. When this is shown in one line, DA2003P automatically prints out the result.
5. The discharged pulse can be repeated. To do this press more-2 (F5) to advance to page 2 of
the main menu.
Press PLAY PULSE (F2). The display will show 'Oper: Playback,' and displays the result in
joules under RESULT.
Following playback, the apparatus is ready to receive a new discharge from the defibrillator. The
display will show 'LOCAL'.
BC Biomedical DA-2003P User Manual 11/04
17
Page 19

6. When testing automatic defibrillators, it is quite common to have to select 'vfib' from the ECG
menu 'ECG WAVE' for the 'ventricular fibrillation' wave. Automatic defibrillators typically do not
fire without seeing 'v-fib'.
6.4 Cardioversion Test
1. Select ECG WAVE (F1) from the main menu.
2. The ECG Wave menu opens. DA-2003P includes the following ECG wave selection for cardioversion
tests, or for the testing of electrocardiograph monitors.
Normal Sine Rates: 30, 60, 80, 120, 180, 240 and 300 BPM.
ECG Arrhythmia types as follows:
vfib Ventricular Fibrillation
afib Atrial Fibrillation
blk II Second degree A-V block
RBBB Right Bundle Branch Block
PAC Premature Atrial Contraction
PVC_E Early PVC
PVC_STD PVC
PVCRonT R on T PVC
mfPVC Multifocal PVC
bigeminy Bigeminy
run5PVC Bigeminy Run of 5 PVCs
vtach Ventricular Tachycardia
Select a desired wave by pressing UP (F2) or DOWN (F3). Save this under ‘Wave” in the STATUS
field by pressing SELECT (F4). Press CANCEL (F5) to cancel selection.
3. DA-2003P includes the following ECG wave amplitude options:
0.5 mV, 1.0 mV, 1.5 mV and 2.0 mV. To change wave amplitude select more-2 on the main menu to
advance to page 2. Select WAVE AMPL. (F1).
18
BC Biomedical DA-2003P User Manual 11/04
Page 20

The Wave Amplitude Menu appears:
Select the desired amplitude by pressing UP (F2) or DOWN (F3). Save this under ‘Ampl” in the
STATUS field by pressing SELECT (F4). Press CANCEL (F5) to cancel selection.
4. Set the defibrillator to synchronized cardioversion mode. Discharge the defibrillator over the
instrument's load resistance.
5. DA-2003P measures the time delay in milliseconds (ms) between the top of the 'R' wave and the
discharging of the defibrillator pulse. This delay will be shown in the LCD display as: 'Delay: xxx ms'.
DA-2003P also shows the energy measured, the maximum voltage and the maximum current in the
energy wave. Following the discharge from the defibrillator, DA-2003P shows a playback of the wave
from the ECG output. A new pulse can be generated when the LCD display shows 'LOCAL'.
6.5 Maximum Energy Charging Time Test
1. The charge time function is used to test the battery and the charging capacitor in the defibrillator.
2. Set the defibrillator to maximum energy.
3. Securely place the defibrillator paddles on the DA-2003P contact plates, and discharge the
defibrillator. The APEX (+) pad should be connected to the right-hand plate, and the STERNUM pad
to the left plate. This ensures correct signal polarity for the oscilloscope output. A reversal of this
configuration will not damage the DA-2003P, nor will it give incorrect energy readings. However, the
polarity of the oscilloscope output will simply be reversed. The discharge from the defibrillator is
transferred to the DA-2003P’s load resistance.
4. Select CHARGE TIME (F3) from the main menu and the charge button on the defibrillator
simultaneously.
APEX (+) pad → right plate
STERNUM pad → left plate
When the defibrillator is charged, discharge it through the instrument.
5. Charging time will be shown in the display as ‘Chrg T: xx.x MS’ under RESULT.
19
BC Biomedical DA-2003P User Manual 11/04
Page 21

6.6 Shock Advisory Algorithm Test
1. This tests the analysis and prompting of automatic and semi-automatic defibrillators. A series of
arrhythmia is available for analysis by the defibrillator that should then prompt the user to ‘shock’ of
‘no shock,’ in accordance with national and international guidelines, as shown below:
ASYS No shock
SVTa_90 No shock
PVT_140 No shock
MVT_140 No shock
CVF Shock
FVF Shock
PVT_160 Shock
MVT_160 Shock
2. Select ADV. ALG. (F2) from the main menu.
3. The Advisory Algorithms Menu opens.
Select the desired rhythm by pressing UP (F2) or DOWN (F3). Save this under ‘Wave” in the
STATUS field by pressing Select. Press CANCEL (F5) to cancel selection. The ECG signal is output
through the low-level ECG connectors, high-level ECG connector, and paddle contact plates on the
DA-2003P.
4. Set the defibrillator to analyze the ECG rhythm and operate in the automatic and semi-automatic
mode.
5. Records the defibrillator’s response.
20
BC Biomedical DA-2003P User Manual 11/04
Page 22

VII. TRANSCUTANEOUS PACEMAKER TESTING
7.1 Introduction
DA-2003P tests all types of transthoracic pacemakers. The testing is menu driven, and simple to
operate. DA-2003P measures and displays a pacer pulse’s amplitude, rate, energy and width. It also
conducts demand sensitivity tests, measuring and displaying refractory periods, and immunity tests,
which determine the pacemaker’s susceptibility to 50/60 Hz interference.
7.2 Test Preparation
1. Connect the pacer output cables to the pacer input connectors.
2. Switch the mode switch to ‘PACE’ mode.
3. Turn the DA-2003P on. The following will be displayed in the LCD display for about two seconds:
DA-2003P
4. The following main menu will then appear.
5. Press SELECT LOAD (F1). The following load options will appear:
The load range is 50 to 2300 ohms in steps of 50 ohms up to 200 ohms, and 100 ohms from 200 up
to 2300 ohms
Select the desired noise form by pressing UP (F2) or DOWN (F3) and then Select (F4). Press
CANCEL (F5) to cancel the selection. After selection the main menu will reappear.
21
BC Biomedical DA-2003P User Manual 11/04
Page 23

6. Select the desired waveform by pressing UP (F2) or DOWN (F3) and then SELECT (F4). Press
CANCEL (F5) to cancel the selection. After selection the main menu will reappear.
7. For Immunity Testing Only. The immunity test determines the pacemaker’s susceptibility to 50/60
Hz interference signals. If you desire to test immunity simultaneously with other testing, press
SELECT NOISE (F2). The following load options will appear:
Select the desired noise form by pressing UP (F2) or DOWN (F3) and then SELECT (F4). Press
CANCEL (F5) to cancel the selection. After selection the main menu will reappear.
7.3 Demand Sensitivity Test
1. General. Sensitivity is the minimum QRS amplitude (mV) required to cause the pacemaker to
operate in the demand mode. During sensitivity measurement three different waveforms are
selectable with widths varying in steps from 10 to 200 ms. This waveform is delayed from the pacer
pulse so that it is outside the pacing refractory period. DA-2003P then checks whether this wave is
sensed or not by the pacemaker.
If it is not sensed, a message 'exceeded' is displayed which means that the pacemaker needs an
amplitude more than 100 mV for sensing at that setting. If the wave is sensed, DA-2003P then
reduces the amplitude in steps until it reaches the lowest value required for the pacemaker to sense
it. (The internal algorithm used converges to the lowest value in the least number of cycles.) This
lowest value is the sensitivity.
2. Procedure
a. From the main menu press more-2, then SELECT WAVE (F1).
22
BC Biomedical DA-2003P User Manual 11/04
Page 24

b. The following menu will be displayed:
c. Select the desired waveform by pressing UP (F2) or DOWN (F3) and then Select
(F4). Press CANCEL (F5) to cancel the selection. After selection the main menu will
reappear.
d. Select SENS. TEST (F2). The following display will appear:
e. Upon completion of testing the results will be displayed under RESULT. Press
SENS. TEST. CANCEL (F5) to cancel the test.
7.4 Refractory Period Test
1. General. This test is used to test the time interval in milliseconds (ms) during which the pacemaker is
insensitive to any external inputs. DA-2003P does this by measuring the maximum time interval after
the generation of a pacer pulse, and maximum time interval after a QRS wave.
a. Refractory Period. A time interval in milliseconds, during which a pacemaker is
insensitive to any external inputs. If a QRS is detected during this period, the
pacemaker ignores it. On the other hand, if a QRS is detected outside the refractory
interval, then the pacemaker resets its internal timer and the next pacer pulse is
generated after a delay of one time period from this QRS wave.
b. Paced Refractory Period. The maximum time interval after the generation of a
pacer pulse during which time the presence of a QRS wave is ignored.
The measurement of paced refractory period takes a few cycles of the pacemaker
output. First, DA-2003P measures the pacer-to-pacer interval T. Then, it puts out a
BC Biomedical DA-2003P User Manual 11/04
23
Page 25

2. Procedure
square wave 40 milliseconds wide, delayed by delay time D, which is more than the
pacing refractory period, from the last pacer pulse. The pacemaker senses this square
wave. The delay time D is gradually decremented in subsequent cycles until the square
waveform is not sensed by the pacemaker. The maximum value of the delay time D, for
which the pace maker does not sense the square wave, is the paced refractory period.
c. Sensed Refractory Period. The maximum time interval after a QRS wave is
sensed by the pacemaker during which time the presence of a second QRS wave is
ignored.
The sensed refractory period is measured in a similar manner, except that DA-2003P
now generates two square waves instead of one. The first square wave is generated
at a fixed time delay from a pacer pulse, which is greater than the paced refractory
period. The pacemaker always senses this square wave.
The second square wave is generated at a delay D from the first square wave. The
initial value of D is selected to be greater than the sensed refractory period.
Therefore the first time the pacemaker is on it also senses the second square wave.
In subsequent cycles, the delay 'D' is gradually reduced until the pacemaker is
unable to sense the second square wave. The maximum value of D, for which the
pacemaker does not sense the second square wave, is the sensed refractory period.
a. From the main menu press more-2. Press REF. PER. TEST (F3).
b. The following display will appear while testing:
c. Upon completion of testing the results will be displayed under RESULT. Press REF.
PER. CANCEL (F5) to cancel the test.
24
BC Biomedical DA-2003P User Manual 11/04
Page 26

VIII. WARRANTY
BC Group warrants that the DA-2003P Defibrillator Analyzer will substantially conform to published
specifications and to the documentation, provided that it is used for the purpose for which it was
designed. BC Group will, for a period of twelve (12) months from date of purchase, replace or repair any
defective analyzer, if the fault is due to a manufacturing defect. In no event will BC Group or its local
representatives be liable for direct, indirect, special, incidental, or consequential damages arising out of
the use of or inability to use the DA-2003P Defibrillator Analyzer, even if advised of the possibility of such
damages. BC Group or its local representatives are not responsible for any costs, loss of profits, loss of
data, or claims by third parties due to use of, or inability to use the DA-2003P Defibrillator Analyzer.
Neither BC Group nor its local representatives will accept, nor be bound by any other form of guarantee
concerning the DA-2003P Defibrillator Analyzer other than this guarantee. Some jurisdictions do not
allow disclaimers of expressed or implied warranties in certain transactions; therefore, this statement may
not apply to you.
IX. TECHNICAL SUPPORT
BC Biomedical’s DA-2003P Defibrillator Analyzer is backed by a superior support staff. If the DA-2003P
ever fails to work perfectly, please contact the Technical Support Staff.
Written Communications
You may write a letter with your comments and send it to:
BC Biomedical
BC Group International, Inc.
PO Box 25125
9415 Gentry Ave
St. Louis, MO USA 63125
OR
E-mail: sales@bcgroupintl.com
Phone Support
You can telephone the Technical Assistance Center at 314-638-3800 or 1-800-242-8428 between
8:00 AM and 4:30 PM Central Standard Time (CST) Monday through Friday, except holidays.
Whichever method of contact you choose, please provide the following information:
• Product name and serial number
• Revision level of your software
• The specific steps which reproduce your problem
• Any error codes displayed on screen
• A daytime phone number, fax number, and/or email address (if available)
• Your name / company
25
BC Biomedical DA-2003P User Manual 11/04
 Loading...
Loading...