Page 1
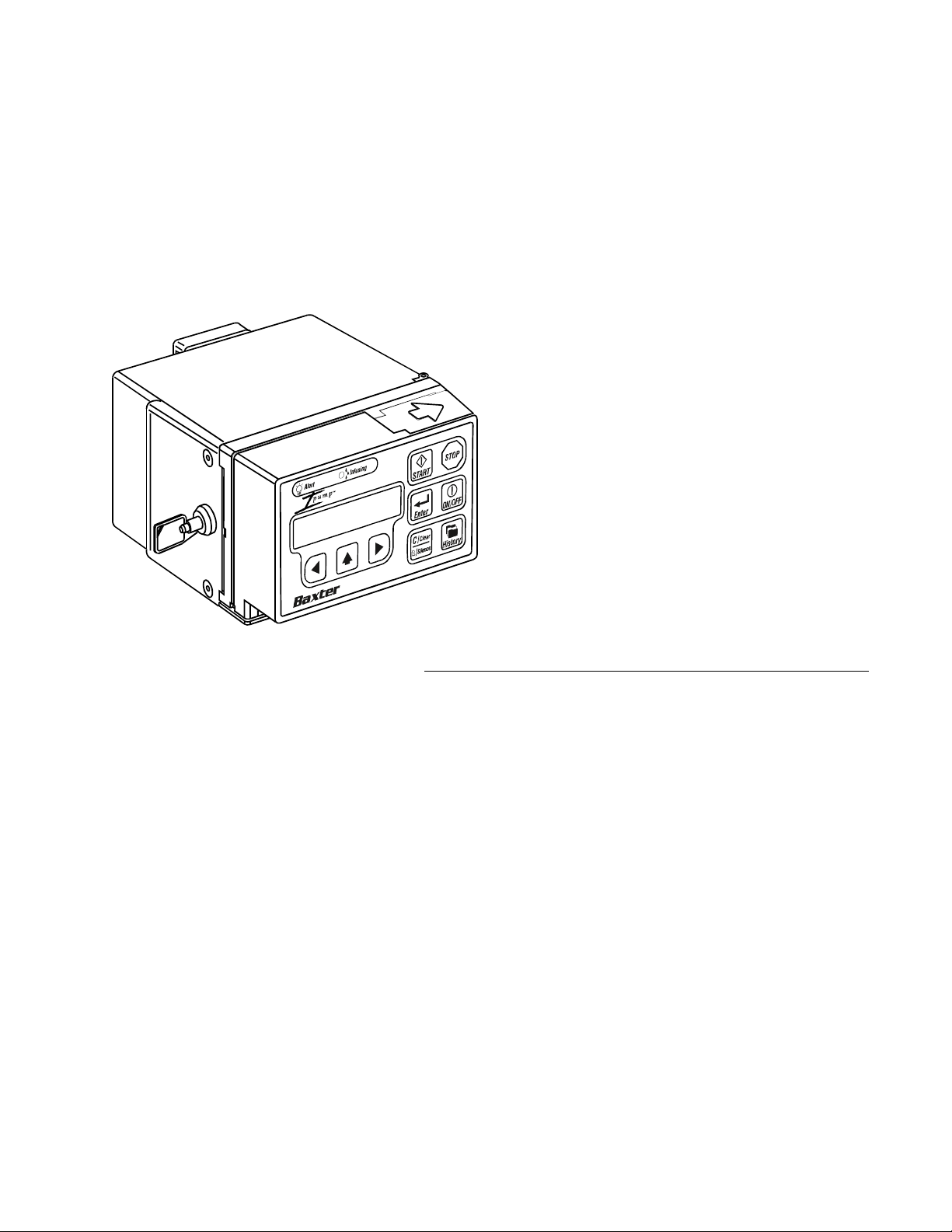
Ipump Pain Management System
SERVICE MANUAL
For use with Ipump devices with hardware revision 2 (HW Rev 2).
Page 2

Disclaimer
The information in this document has been carefully examined, and is believed to be entirely reliable. However,
no responsibility is assumed for inaccuracies. Furthermore, Baxter reserves the right to make changes to any
products herein to improve readability, function, or design. Baxter does not assume any liability arising out of
the applications or use of any product or circuit described herein; neither does it cover any license under its
patent rights nor the rights of others.
Documentation Copyrights
Duplication or distribution of this manual and any information contained within (except for the data sheets), is
strictly prohibited without the express written permission of Baxter. This manual and any information contained
within, may not be reproduced, distributed, or transmitted in any form, or by any means, for any purpose
without the express written permission of Baxter.
Computer Software Copyrights
Copyright 2006, Baxter Healthcare Corporation. All rights reserved.For use only by Baxter Healthcare
Corporation. The software contains proprietary information belonging to Baxter Healthcare Corporation. The
software must not be reproduced or disclosed to others without prior written approval. Any unauthorized use of
this information may subject the user to substantial liability.
Patent Information
This pump is protected under one or more U.S. and Foreign patents.
Trademark Information
Baxter and Ipump are trademarks of Baxter International Inc.
All other trademarks and product names appearing within this manual are the property of their respective
owners.
Copyright 1999 - 2007, Baxter Healthcare Corporation. All rights reserved.
Page 3

Chapter 1 - Introduction
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-1
Pump Accessories - - - - - - - - - - - - - - - - - - - - - - - - - - 1-2
Manual Layout - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-2
Factory Service & Assistance - - - - - - - - - - - - - - - - - - - - - 1-3
Technical Assistance, Service, & Repairs - - - - - - - - - - - - - - - 1-4
Safety Summary - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-4
Definitions - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-4
Warnings - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-5
Cautions - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-5
Notes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-6
Labeling Symbol Definitions - - - - - - - - - - - - - - - - - - - - - 1-6
Contents
Chapter 2 - Theory of Operation
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-1
System Components - - - - - - - - - - - - - - - - - - - - - - - - - 2-2
MPU Circuit - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-3
BUS Subsystem - - - - - - - - - - - - - - - - - - - - - - - - - 2-3
Keypad & Sensors - - - - - - - - - - - - - - - - - - - - - - - - 2-4
PROM - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-5
Supervisory Subsystem (SS) - - - - - - - - - - - - - - - - - - - 2-5
Power Subsystem (PS) - - - - - - - - - - - - - - - - - - - - - - 2-5
LCD Subsystem- - - - - - - - - - - - - - - - - - - - - - - - - - 2-6
Silent Shutdown Circuit - - - - - - - - - - - - - - - - - - - - - - 2-6
Motor Subsystem - - - - - - - - - - - - - - - - - - - - - - - - - 2-7
Occlusion Detection Circuit - - - - - - - - - - - - - - - - - - - - 2-7
Air Sensor Circuit - - - - - - - - - - - - - - - - - - - - - - - - - 2-7
Printer Adapter Interface Circuit - - - - - - - - - - - - - - - - - - 2-7
07-19-A8-092 Ipump Pain Management System Service Manual i
Page 4

Contents
Chapter 3 - Care & Routine Maintenance
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-1
Cleaning and Disinfecting - - - - - - - - - - - - - - - - - - - - - - - 3-1
Chapter 4 - Troubleshooting
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-1
Reviewing the Alarm Log- - - - - - - - - - - - - - - - - - - - - - - - 4-1
Troubleshooting - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-3
System Error Codes - - - - - - - - - - - - - - - - - - - - - - - - - - 4-5
Range 10 - 2V -- Peripheral/Sensor Errors - - - - - - - - - - - - - 4-5
Range 30 - 47 -- Motor Control Errors - - - - - - - - - - - - - - - - 4-6
Range 50 - 52 -- RTC Errors - - - - - - - - - - - - - - - - - - - - 4-7
Range 60 - 62 -- Power Supply Errors- - - - - - - - - - - - - - - - 4-7
Range 70 - 74 & L0 -- MPU Errors - - - - - - - - - - - - - - - - - 4-7
Range 75 - 8D -- Processing Errors - - - - - - - - - - - - - - - - - 4-8
Range 90 - 9Z & M0 - P3 -- Data Corruption Errors - - - - - - - - - 4-8
Range A0 - J1 -- Processing Errors - - - - - - - - - - - - - - - - 4-10
Chapter 5 - Functional Tests
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-1
General Information - - - - - - - - - - - - - - - - - - - - - - - - 5-2
Equipment Required - - - - - - - - - - - - - - - - - - - - - - - - - - 5-3
Optional Equipment - - - - - - - - - - - - - - - - - - - - - - - - - - 5-3
Exterior Visual Inspection - - - - - - - - - - - - - - - - - - - - - - - 5-3
Configuration Settings - - - - - - - - - - - - - - - - - - - - - - - - - 5-4
Flow Rate Accuracy Test- - - - - - - - - - - - - - - - - - - - - - - - 5-6
Test Setup - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-7
Procedure Using the Gravimetric Method - - - - - - - - - - - - - - 5-7
Procedure Using the Volumetric Method - - - - - - - - - - - - - - 5-8
Downstream Occlusion Calibration Pressure Test - - - - - - - - - - - 5-8
Operational Checks - - - - - - - - - - - - - - - - - - - - - - - - - - 5-9
Test Set Up - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-9
Power-On Self Test (POST) - - - - - - - - - - - - - - - - - - - - 5-9
Keypad Operation Test - - - - - - - - - - - - - - - - - - - - - - 5-10
Bag Cover Lock/Unlock Test - - - - - - - - - - - - - - - - - - - 5-10
ii Ipump Pain Management System Service Manual 07-19-A8-092
Page 5

Tubing Sensor Test - - - - - - - - - - - - - - - - - - - - - - - - 5-10
Occlusion Sensor Test - Downstream - - - - - - - - - - - - - - - 5-11
Occlusion Sensor Test - Upstream - - - - - - - - - - - - - - - - 5-11
Air Sensor Test - - - - - - - - - - - - - - - - - - - - - - - - - - 5-12
PCA Cable & Button Test - - - - - - - - - - - - - - - - - - - - - 5-13
AC Adapter Test (Optional) - - - - - - - - - - - - - - - - - - - - 5-13
History Retention Test (Backup Battery Check) - - - - - - - - - - 5-14
Printer Test (Optional) - - - - - - - - - - - - - - - - - - - - - - - 5-14
Unintended Shutdown Circuit Test - - - - - - - - - - - - - - - - - 5-14
Functional Test Data Sheet - - - - - - - - - - - - - - - - - - - - - - 5-15
Chapter 6 - Disassembly & Reassembly
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-2
Tools & Materials - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-2
Contents
Required Tools & Equipment - - - - - - - - - - - - - - - - - - - 6-2
Consumable Materials - - - - - - - - - - - - - - - - - - - - - - 6-3
Baxter-Created Tools & Equipment - - - - - - - - - - - - - - - - 6-3
Disassembly Procedures - - - - - - - - - - - - - - - - - - - - - - - 6-4
General Disassembly Information - - - - - - - - - - - - - - - - - 6-4
Bag Cover Assembly Removal - - - - - - - - - - - - - - - - - - 6-4
MPU PCBA Handling Guidelines - - - - - - - - - - - - - - - - - 6-5
Rear Case Assembly Removal - - - - - - - - - - - - - - - - - - 6-6
Mechanism Assembly, DDMM PCBA, & Battery Wall Removal - - - 6-7
MPU PCBA Removal - - - - - - - - - - - - - - - - - - - - - - - 6-8
ESD Flex Circuit Removal - - - - - - - - - - - - - - - - - - - - - 6-9
LCD Module Removal - - - - - - - - - - - - - - - - - - - - - - - 6-10
Optional Procedures - - - - - - - - - - - - - - - - - - - - - - - - - 6-11
3V Backup Battery Replacement - - - - - - - - - - - - - - - - - 6-11
Initializing the 3V Backup Battery - - - - - - - - - - - - - - - - - 6-12
Keypad Replacement - - - - - - - - - - - - - - - - - - - - - - - 6-12
Assembly Procedures - - - - - - - - - - - - - - - - - - - - - - - - 6-15
Torque Specifications - - - - - - - - - - - - - - - - - - - - - - - 6-15
Installing the Front Case & Keypad Assembly - - - - - - - - - - - 6-15
Installing the ESD Flex Circuit - - - - - - - - - - - - - - - - - - - 6-15
Installing the Display ESD Shield - - - - - - - - - - - - - - - - - 6-18
Installing the LCD Module - - - - - - - - - - - - - - - - - - - - - 6-18
07-19-A8-092 Ipump Pain Management System Service Manual iii
Page 6

Contents
MPU PCBA Handling Instructions - - - - - - - - - - - - - - - - - 6-19
Installing the MPU PCBA - - - - - - - - - - - - - - - - - - - - - 6-19
Installing the Battery Wall & DDMM PCBA - - - - - - - - - - - - 6-23
Installing the DDMM PCBA Hold-down Foam - - - - - - - - - - - 6-24
Installing the Mechanism Assembly - - - - - - - - - - - - - - - - 6-24
Installing the Battery Door - - - - - - - - - - - - - - - - - - - - 6-25
Pump Calibration - - - - - - - - - - - - - - - - - - - - - - - - - 6-25
Internal Inspection - - - - - - - - - - - - - - - - - - - - - - - - 6-26
Closing the Case - - - - - - - - - - - - - - - - - - - - - - - - - 6-27
Pump Functional Tests - - - - - - - - - - - - - - - - - - - - - - 6-28
Optional Assembly Procedures - - - - - - - - - - - - - - - - - - - - 6-28
Installing the Bag Cover Assembly - - - - - - - - - - - - - - - - 6-28
Chapter 7 - Internal Tests & Pump Calibration
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7-1
3V Backup Battery Test - - - - - - - - - - - - - - - - - - - - - - - - 7-2
Battery Load Test - - - - - - - - - - - - - - - - - - - - - - - - - 7-2
Battery No Load Test - - - - - - - - - - - - - - - - - - - - - - - - 7-3
Calibration Procedure - - - - - - - - - - - - - - - - - - - - - - - - - 7-4
Equipment Required - - - - - - - - - - - - - - - - - - - - - - - - 7-4
Initial Setup - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7-4
Downstream Occlusion Calibration - - - - - - - - - - - - - - - - - 7-5
Upstream Occlusion Calibration- - - - - - - - - - - - - - - - - - - 7-6
Air Sensor Calibration - - - - - - - - - - - - - - - - - - - - - - - 7-8
Downstream Occlusion Calibration Pressure Test - - - - - - - - - - 7-9
Calibration Data Sheet - - - - - - - - - - - - - - - - - - - - - - - - 7-11
Chapter 8 - Electronic Assembly Drawings
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-1
Interconnecting Wiring Diagram - - - - - - - - - - - - - - - - - - - - 8-1
Keypad Cable & Motor Connectors - - - - - - - - - - - - - - - - - - 8-2
Mechanism Assembly Flex Circuit Connector - - - - - - - - - - - - - - 8-2
MPU PCBA Assembly - - - - - - - - - - - - - - - - - - - - - - - - - 8-3
Direction & Drive Motor Module PCBA (DDMM) Daughter Board - - - - 8-4
iv Ipump Pain Management System Service Manual 07-19-A8-092
Page 7

Chapter 9 - Repair Parts
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9-1
Assembly Parts List - - - - - - - - - - - - - - - - - - - - - - - - - 9-2
Bag Cover Assembly - - - - - - - - - - - - - - - - - - - - - - - 9-2
Rear Case Assembly - - - - - - - - - - - - - - - - - - - - - - - 9-3
Pump Mechanism & Battery Compartment Assemblies- - - - - - - 9-4
MPU Board Assembly - - - - - - - - - - - - - - - - - - - - - - - 9-5
LCD Circuit Board & Front Case Assemblies - - - - - - - - - - - - 9-6
Alphabetical Parts List - - - - - - - - - - - - - - - - - - - - - - - - 9-7
Numerical Parts List - - - - - - - - - - - - - - - - - - - - - - - - - 9-9
Chapter 10 - Product Updates
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 10-1
Contents
Limited Warranty
07-19-A8-092 Ipump Pain Management System Service Manual v
Page 8
Contents
vi Ipump Pain Management System Service Manual 07-19-A8-092
Page 9

1 - Introduction
In this section Page
Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-1
Pump Accessories. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
Manual Layout . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
Factory Service & Assistance. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Technical Assistance, Service, & Repairs. . . . . . . . . . . . . . . . . . . . . . . . . . . 1-4
Safety Summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-4
Definitions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-4
Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-5
Cautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-5
Notes. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-6
Labeling Symbol Definitions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-6
Overview
The Ipump Pain Management System (hereafter referred to as the “pump”) is indicated
for the controlled delivery (continuous, intermittent, and continuous plus intermittent) of
analgesic, sedative, and anesthetic solutions through clinically acceptable routes of
administration including intravenous, subcutaneous, and epidural, and for regional (local)
analgesia applications.
This lightweight, compact pump can be battery o perated for portability or connect ed to an
AC power source for stationary use. A specially designed optional locking pole-mounting
clamp allows the pump to be attached to a standard IV pole. With the pole clamp removed,
the pump can be placed into a comfortable carrying case.
This manual contains service and maintenance information for all Ipump Pain
Management System products (product codes 2L3107, 2L3107R, and 2L3107K) with
hardware revision 2 (HW Rev 2). This information is intended for qualified biomedical
personnel and Baxter authorized service representatives.
This manual provides a basic understanding of the internal workings of the pump,
functional test procedures, troubleshooting, complete assembly/disassembly instructions,
and a replacement parts list.
For complete operational and precautionary information, pump specifications, and
cleaning instructions, refer to the Ipump Pain Management System Operator’s Manual
(p/n 07-19-x4-766). For pump installation and configuration, refer to the Ipump Pain
Management System Configuration Manual (p/n 07-19-x4-768).
Only trained, qualified personnel should perform procedures in this
CAUTION
07-19-A8-092 Ipump Pain Management System Service Manual 1-1
manual. Except for the procedures and replacement parts included in
this document, no other disassembly or repair should be attempted.
Page 10

1 - Introduction
Baxter Healthcare Corporation provides a one-year limited warranty for new Ipump
devices. If a pump requires warranty service, call Baxter Healthcare Corporation for
repair. Unauthorized repair of a pump before the warranty has elapsed voids the warranty.
Pump Accessories
100 mL Bag Cover 2L3218
250 mL Bag Cover 2L3220
250 mL Extended Bag Cover 2L3217
250 mL Extended Bag Cover, Amber 2L3261
500 mL Bag Cover 2L3221
Printer Adapter 2L3400
Printer Adapter Cable 2L3402
Patient Controlled Analgesia (PCA) Button B069140003RP
Locking Pole Mount Clamp 2L3211
Non-locking Pole Mount Clamp 2L3212
Pump Carrying Case (250 mL) 2L3219
AC Adapter (220-230V) 2L3205K
AC Adapter (100-120V) 2L3210
AC Adapter Holder 2L3214
Configuration Transfer Cable 2L3112
Yellow Face Plate Label 072742210
Description Catalog Number
Manual Layout
This manual is divided into the following sections:
Chapter 1 (Introduction) provides an overview of the contents of this Service Manual
and includes Warnings and Cautions concerning the use and care of this product.
Warnings and Cautions are also located where needed throughout this manual.
Chapter 2 (Theory of Operation) details the functional features of the pump. A general
overview of the pump’s operation and a functional block diagram are provided.
Chapter 3 (Care & Routine Maintenance) includes the routine maintenance and
cleaning procedures with recommended cleaning agents. Battery replacement procedures
are also included.
Chapter 4 (Troubleshooting) contains troubleshooting tables and procedures for
localizing mechanical or electronic faults. A table of System Error Codes is also included.
1-2 Ipump Pain Management System Service Manual 07-19-A8-092
Page 11

1 - Introduction
Chapter 5 (Functional Tests) provides the tests that are to be used to ensure that the
pump operates properly. Baxter recommends that these tests be performed on an annual
basis as a preventive maintenance procedure. In addition, these tests must be performed
whenever the Mechanism Assembly and/or the MPU PCBA is removed or replaced.
Chapter 6 (Disassembly & Reassembly) provides disassembly, replacement, and
reassembly instructions. Required tools and test equipment are specified. Adjustment
procedures are provided along with the required torques and tolerances. Replacement
procedures for the 3V Backup Battery and the Keypad are also included.
Chapter 7 (Internal Tests & Pump Calibration) contains the procedures required to
test the 3V Backup Battery and to calibrate the pump. The calibration procedures must be
performed after replacement of either the Mechanism Assembly or the MPU PCBA.
Chapter 8 (Electronic Assembly Drawings) contains the assembly drawings for the
interconnecting cables and flex circuits used in the pump.
Chapter 9 (Repair Parts) provides exploded view drawings and parts lists of fieldreplaceable parts and assemblies.
Chapter 10 (Product Updates) contains major updates and additional information for
the pump. This information will be listed by hardware and software revision numbers
and/or product serial number. Product Service Bulletins should also be placed in this
chapter of the manual.
Factory Service & Assistance
Baxter Healthcare Corporation provides a one-year limited warranty for each pump. (See
the inside back cover of this manual for warranty details.) If a pump requires warranty
service, call Baxter Healthcare Corporation for repair. While under Baxter Warranty,
Service Agreement (optional), or Lease Agreement, the pump must not be opened by
unauthorized personnel. Unauthorized repair of a pump before the warranty has elapsed
voids the warranty.
If factory service is desired, pumps may be returned to Baxter Healthcare Corporation for
repair. Always call for a return material authorization number before shipping any pump
to Baxter Healthcare Corporation.
When calling for service, please be prepared to provide the product code and serial
number of the pump. A brief written description of the problem should be attached to the
pump when it is returned for service.
Shipping costs for all pumps returned to Baxter shall be paid for by the customer. The
pump must be packed in its original container or in another container that will provide
adequate protection during shipment. T o ensure prompt return, a Baxter authorized service
representative must be notified before shipping any pump for repair.
Baxter Healthcare Corporation will not be responsible for unauthorized returns or for
pumps damaged in shipment due to improper packing.
07-19-A8-092 Ipump Pain Management System Service Manual 1-3
Page 12
1 - Introduction
Technical Assistance, Service, & Repairs
For technical assistance, parts ordering, and service return authorization, contact the
Baxter Healthcare Service Center:
Inside the U.S.: Call 1-800-THE-PUMP (843-7867)
Outside the U.S.: Contact your local Baxter representative.
Safety Summary
General precautions to observe while using the pump are shown below. Standards under
which this product is designed, built, and marketed are also included.
• Before operating the pump, carefully read the operator’s manual to fully understand the
pump’s functionality and to ensure safe and proper operation. An operator’s manual is
shipped with each pump.
• Although the pump has been designed and manufactured to exacting specifications, it
is not intended to replace trained personnel in the supervision of infusion therapy.
• Read and understand this manual before attempting to perform service or maintenance
on the pump.
• To ensure that the pump continues to perform within specifications, perform the
Routine Maintenance procedures described in Chapter 3 of this manual when
recommended.
• This manual has been developed with consideration to the requirements in the
International Standard, IEC 60601-2-24 (1998-02) Medical Electrical Equipment —
Part 2-24: Particular Requirements for Safety of Infusion Pumps and Controllers.
• This product is classified by Underwriters Laboratories Inc. with respect to electric
shock, fire and mechanical hazards only in accordance with UL 60601-1 Medical
Electrical Equipment - Part 1: General Requirements for Safety.
Definitions
Certain items in this manual are highlighted by special messages. The definitions of the
various types of message are provided below.
! WARNING !
CAUTION
NOTE: Provides supplemental information to the accompanying text.
1-4 Ipump Pain Management System Service Manual 07-19-A8-092
Indicates a possible hazard which, if ignored, could result in
severe personal injury or death.
Indicates a problem or unsafe practice which, if not avoided, could
result in minor or moderate personal injury, or product or property
damage.
Page 13
Warnings
1 - Introduction
! WARNING !
! WARNING !
! WARNING !
! WARNING !
! WARNING !
This pump should be repaired only by trained, qualified
personnel using Baxter-recommended parts. There are risks
associated with using anything other than Baxter-recommended
parts and procedures. Baxter will assume no responsibility for
incidents which may occur if the product was not repaired by
qualified Baxter employees.
If the pump has been dropped or appears to be damaged, it
should be taken out of service and inspected by qualified
service personnel.
To ensure safe and proper operation, read the Ipump Pain
Management System Operator’s Manual and any instructions
accompanying disposables or accessories before operating the
pump.
When attaching the pump to an IV pole, ensure it has been
securely clamped and locked.
As with all medical electronic equipment, care must be exercised
to avoid exposing this pump to powerful sources of
electromagnetic interference. Using the pump near operating
equipment which radiates high energy radio frequencies (such
as electrosurgical/cauterizing equipment, two-way radios, or
cellular telephones) may cause false alarm conditions. If this
happens, reposition the pump away from the source of
interference.
Cautions
CAUTION
CAUTION
CAUTION
CAUTION
CAUTION
CAUTION
CAUTION
CAUTION
In the U.S., use of this pump is restricted by federal law (USA) to sale
or use by, on the order of, or under the supervision of a physician or
other licensed health care professional.
DO NOT operate this infusion pump in the presence of flammable
anesthetics, ether, oxygen-enriched, or explosive atmospheres.
DO NOT expose the pump to X-rays, gamma rays, or other ionizing
radiation, or to strong electric or magnetic fields.
Wipe off spills immediately. DO NOT allow fluid or residue to remain
on the pump.
Ensure proper maintenance of the pump by following the cleaning
schedule and methods described in this manual.
Do not clean, disinfect, or sterilize any part of the pump by
autoclaving, or with ethylene oxide gas. Doing so may damage the
pump and void the warranty. Only external parts of the pump should
be disinfected.
Refer all service, repair, and calibration to trained, qualified
personnel.
To reduce the risk of electrical shock, only trained, qualified personnel
should disassemble this product.
CAUTION
07-19-A8-092 Ipump Pain Management System Service Manual 1-5
For best performance, routine maintenance procedures must be
performed. (Refer to Chapter 3 of this manual.)
Page 14

1 - Introduction
Notes
CAUTION
CAUTION
CAUTION
CAUTION
CAUTION
Wear a grounding wrist strap when disassembling and reassembling
the pump.
DO NOT lay the pump face down on any surface which could scratch
or damage the keypad or the display.
When troubleshooting the pump, do not inject or apply signals of any
kind. Damage to the pump or its sub-assemblies could result.
Motor and sensor magnets may attract metal debris to motors or
circuit boards. To prevent debris from entering the pump mechanism,
always maintain a clean work area when performing procedures
involving the pump mechanism.
To avoid personal injury, ensure that the IV pole is stable and secure.
Ensure that the pole is able to support the pump, along with any other
devices, without tipping or falling. The pole diameter should be
between 0.5" and 1.25" (1.3 cm and 3.2 cm).
Baxter requests that parties acquiring this pump:
• Promptly report the receipt of this pump to the manufacturer.
• Report the pump’s purchase, receipt in trade, return after sale, loss, destruction, or
retirement.
• If this is an initial purchase from the manufacturer, please return a signed copy of the
packing list to the manufacturer.
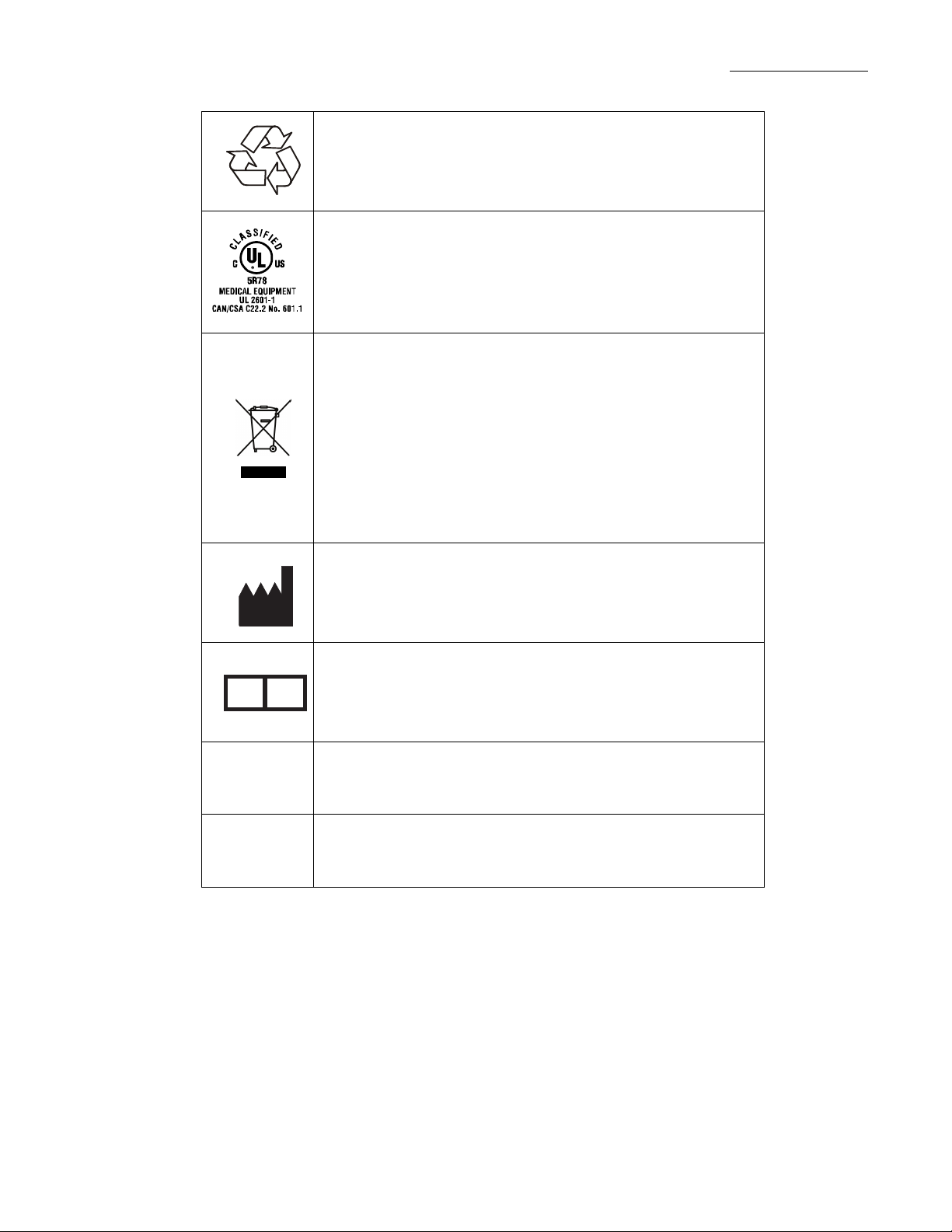
Labeling Symbol Definitions
IPX1
Drip-proof equipment: enclosed equipment protected against
dripping fluids.
Connection port for the AC to DC converter/adapter.
CAUTION, Consult Accompanying Documents
Type CF applied part. (The
indicates the level of electric shock protection for the patientcontacting parts such as the PCA button and the IV set.
UL/IEC/EN 60601-1 defines Type CF as providing greater
protection than Type B or Type BF.)
Electrostatic Sensitive Devices
(The pins of the PRINTER/COMM connector are subject to
Electrostatic Discharge and should not be touched. Refer to
page 2-7 for additional information.)
“Type CF Applied Part” symbol
1-6 Ipump Pain Management System Service Manual 07-19-A8-092
Page 15
1 - Introduction
Recyclable, dispose of properly.
This product is classified by Underwriters Laboratories Inc.
with respect to electric shock, fire, and mechanical hazards
only in accordance with UL 2601-1 (UL 60601-1),
CAN/CSA C22.2 No. 601.1, and IEC 60601-2-24.
Symbol (WEEE 2002/96/EC) Crossed-out wheeled bin
For product disposal, ensure the following:
- Do not dispose of this product as unsorted municipal waste.
- Collect this product separately.
- Use collection and return systems available to you.
Bar below bin
- Product distributed after August 13, 2005.
For more information on return, recovery, or recycling of
this product, please contact your local Baxter representative.
EC REP
REF
SN
Manufacturer
Authorized Representative in the European Community
Catalog Number
Serial Number
07-19-A8-092 Ipump Pain Management System Service Manual 1-7
Page 16
1 - Introduction
1-8 Ipump Pain Management System Service Manual 07-19-A8-092
Page 17

2 - Theory of Operation
In this section Page
Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-1
System Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-2
Ipump System Functional Block Diagram . . . . . . . . . . . . . . . . . . . . . . 2-2
MPU Circuit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
BUS Subsystem . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Keypad & Sensors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-4
PROM. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-5
Supervisory Subsystem (SS). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-5
Power Subsystem (PS) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-5
LCD Subsystem . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-6
Silent Shutdown System. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-6
Motor Subsystem . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-7
Occlusion Detection Circuit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-7
Air Sensor Circuit. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-7
Printer Adapter Interface Circuit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-7
Overview
The Ipump Pain Management System is a small, lightweight, linear peristaltic pump that
may be operated from battery or AC power. An optional pole-mounting clamp allows the
pump to be unlocked and easily removed for pump placement into a convenient carrying
case. See the list of pump accessories on page 1-2.
The user can program the pump with prescribed values for the therapy desired. A number
of security options are available in order to enter prescription parameters from the keypad.
Once programmed with prescription parameters, the pump operates with these settings
until the operator turns the pump off or re-enters the programming screens and changes the
prescription. A record of the previous prescription and therapy history are retained while
the pump is in operation or turned off. The user can choose to use the previous
prescription, review the history by pressing the
pressing the
The pump can be configured to require a key to unlock and open the pump bag cover to
change a prescription. The pump can also be configured to require either a security code,
or both a key and a security code, to gain applicable access.
The pump can be programmed for specific modes, units, and/or prescription limi ts. This is
accomplished by accessing the configuration screens during initial start-up. To access the
configuration mode refer to the Ipump Pain Management System Configuration Manual
and the Ipump Pain Management System Operator's Manual. Once programmed, the
pump will remain in that configuration until purposely changed.
HISTORY key, or clear the history by
CLEAR key.
07-19-A8-092 Ipump Pain Management System Service Manual 2-1
Page 18
2 - Theory of Operation
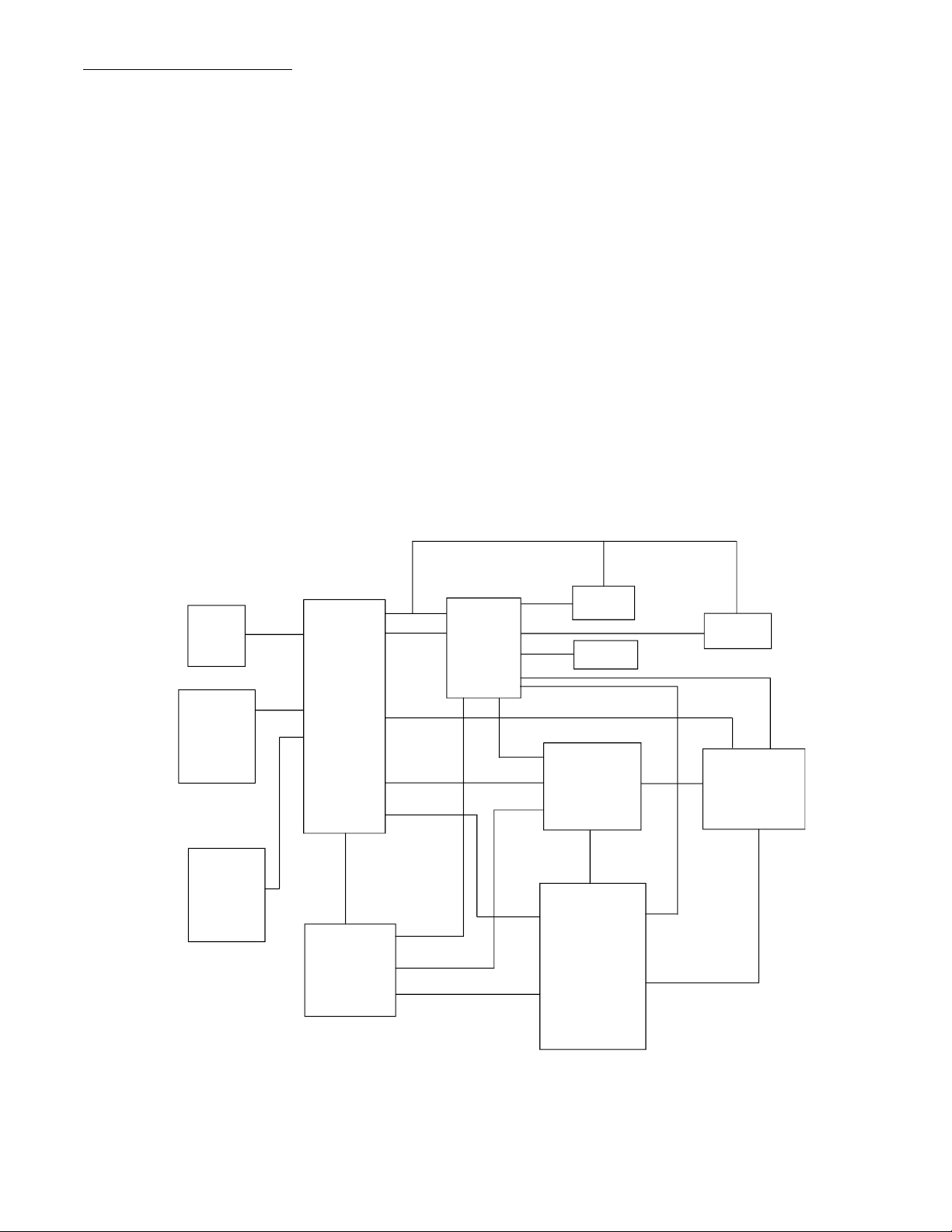
S E N S O R C I R C U I T P R I N T E R A D A P T E R I N T E R F A C E C I R C U I T
C I R C U I T M P U C I R C U I T B U S S U B S Y S T E M M O T O R S U B S Y S T E M P R O M K E Y P A D S U P E R V I S O R Y S U B S Y S T E M L C D S U B S Y S T E M P O W E R S U B S Y S T E M S I L E N T S H U T D O W N
The pump configuration can be transferred between two Ipump devices for the
configuration of multiple pumps. An optional configuration transfer cable, available from
Baxter, is required. See the list of pump accessories on page 1-2.
The remainder of this chapter provides a basic explanation of the pump's internal
operation.
System Components
The pump is divided into modules and subsystems. Figure 2-1 is a functional block
diagram of the system and not intended to illustrate component location. The m odules and
subsystems listed below are discussed later in this chapter.
• MPU Circuit • LCD Subsystem
• Bus Subsystem • Silent Shutdown Circuit
• Keypad • Motor Subsystem
• PROM • Occlusion Detection Circuit
• Supervisory Subsystem • Air Sensor Circuit
• Power Subsystem • Printer Adapter Interface Circuit
A I R
C I R C U I T
O C C L U S I O N
D E T E C T I O N
Figure 2-1. Ipump System Functional Block Diagram
2-2 Ipump Pain Management System Service Manual 07-19-A8-092
Page 19

2 - Theory of Operation
MPU Circuit
The pump uses a 16-bit micro-controller and an external PROM. The 16-bit microcontroller contains hardware timers, analog to digital converter, RAM and PROM, and
the microprocessor. This application of the micro-controller uses the internal RAM and
PROM whenever possible to save power, which is a major feature of this pump. The
memory expansion mode is only used when accessing functions on the external bus. The
microprocessor has eight input/output (I/O) ports which are used to control or monitor the
following functions:
• PROM • LCD Subsystem
• Motor Subsystem • Watchdog
• Keypad • Switches
• Silent Shutdown Circuit • Real Time Clock Circuit
• Occlusion Detection Circuit • Air Sensor Circuit
• Various voltages
A number of power-up tests are performed to ensure that the pump is running properly.
The power-up tests include testing of the LEDs, memory, display, beeper, backup battery,
and input voltages. If an error is detected, the processor will initiate a 2-character error
code which will produce an alert message and an audible alarm.
Included in the processor subsystem is the real time clock (RTC) circuit. The RTC
provides time of day information to the microprocessor. The RTC circuitry keeps track of
time while the pump is off, through the use of a backup battery mounted to the
microprocessor circuit board. The backup battery is also used to preserve the contents of
the microprocessor RAM when operating power drops below a minimum voltage. The
RTC also contains a small amount of RAM that is used by the system software to
determine whether there has been a loss of backup battery power.
BUS Subsystem
The BUS has the capacity to provide for a 24 bit address and 8 bit data path. The
microprocessor uses the BUS subsystem to transfer data or instructions to seven different
functions. These functions are:
• PROM • LCD Command and Data Register
• Motor Drive • Watchdog
• Keypad • Switches
• Real Time Clock (RTC)
07-19-A8-092 Ipump Pain Management System Service Manual 2-3
Page 20
2 - Theory of Operation
Keypad & Sensors
The Keypad is comprised of nine keys which enable the user to turn the pump Off and On,
enter the prescription data, and START and STOP an infusion. In addition to monitoring
each of these keys, the microprocessor also checks the status of ancillary inputs consisting
of internal switches, sensors, and connectors. Refer to Table 2-1 and Table 2-2 for a
description of the Keypad keys and ancillary inputs.
Key Description
The START key begins the operation of the pump and can also be configured to act as
a PCA button. If all of the required programming values have been entered, the START
key initiates the infusion from any programming screen.
Following the resolution of most alerts or alarms, pressing the START key resumes the
infusion if the condition no longer exists.
The STOP key must be pressed twice within 1 second to stop the operation of the
pump. After the pump is stopped, you can press the ON/OFF key to turn the pump off.
The ENTER key sets the value displayed on the Liquid Crystal Display (LCD) screen.
The ON/OFF key powers up and powers down the pump. If the pump is on, you can
press the key:
• once to deactivate the programmed settings, which can be retrieved.
• twice to power off the pump.
The CLEAR/SILENCE key either clears the data shown on the LCD screen or silences
an alert or alarm signal generated by the pump.
The HISTORY key displays the infusion history on the LCD screen. Pressing this key
again allows you to scroll through the history screens.
The left and right arrow keys move the cursor on the display screen to
the left and right.
The scroll (up arrow) key displays the next available option on the pump’s screen.
Table 2-1. Keypad Keys
2-4 Ipump Pain Management System Service Manual 07-19-A8-092
Page 21
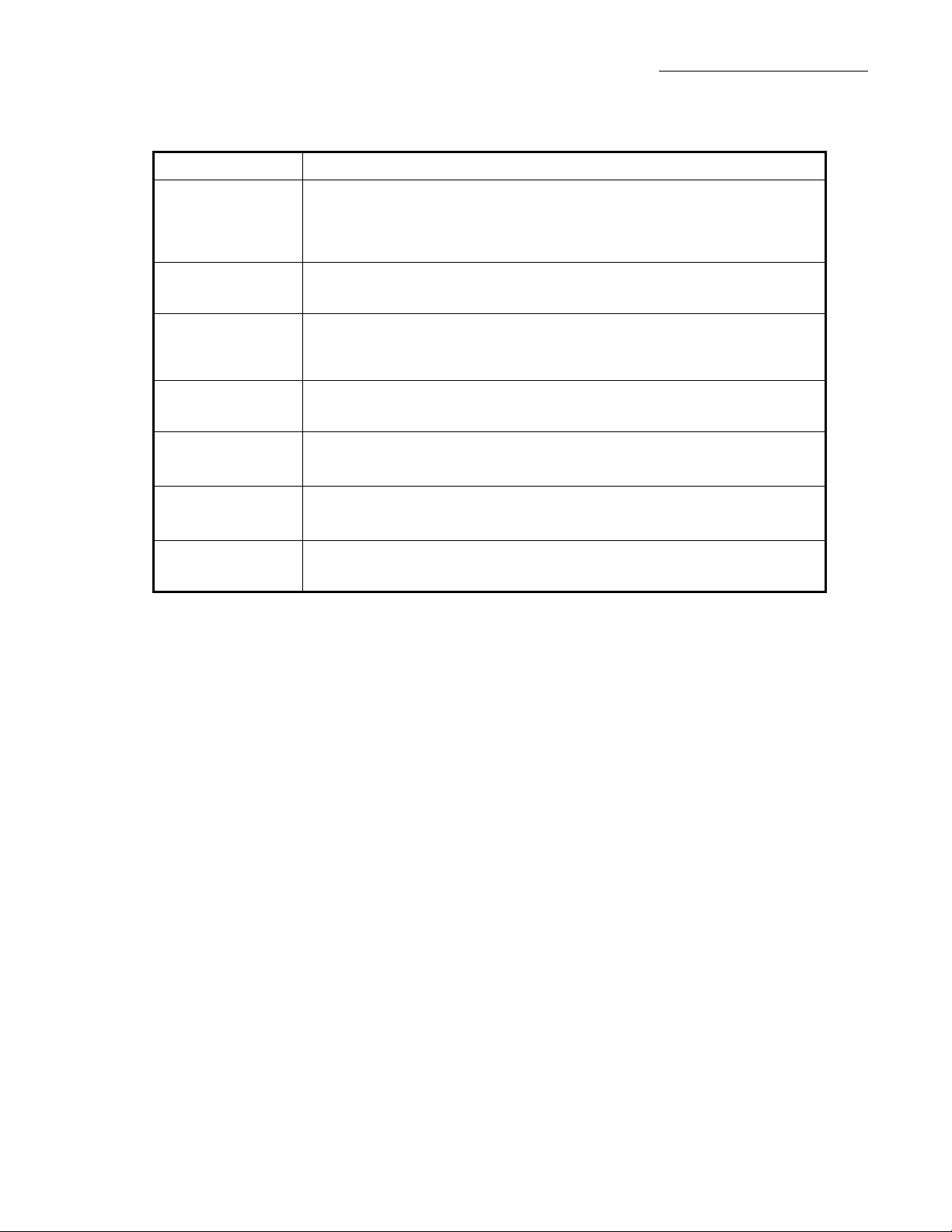
Feature Primary Function
Patient Controlled Analgesia (PCA) connector. The PCA cable connects
PCA Jack
to the pump via a phono jack and plug style connection which is
monitored by the microprocessor to determine the status of the PCA
button.
2 - Theory of Operation
Printer Jack
Bag Cover Lock
Tubing Sensor
Upstream
Occlusion Sensor
Downstream
Occlusion Sensor
Air Sensor
The printer jack allows the connection of the Baxter Printer Adapter
(p/n 2L3400), and a printer (typically a Seiko DPU-414).
An internal sensor detects when the bag cover is locked or unlocked
when the pump is configured with either security method “key + code” or
“key only.”
An internal sensor that detects when the pump tubing cover is open or
closed with the administration set properly installed.
An internal sensor that detects when an upstream occlusion occurs.
An internal sensor that detects when a downstream occlusion occurs.
An internal sensor that detects when there is air in the tubing segment
inside the pump.
Table 2-2. Ancillary Inputs
PROM
The PROM subsystem supplies data to the bus when addressed by the microprocessor to
identify the operation requested. An EEPROM is also provided in the microprocessor and
PROM subsystem to retain configuration information.
Supervisory Subsystem (SS)
The supervisory subsystem performs a major role in the start-up and shutdown of the
pump. It also monitors and responds to error situations reported by the hardware and
software. A “wellness check” is performed by the SS on some of the error detection
hardware circuitry.
The SS also provides the power for the microprocessor and the Real Time Clock (RTC).
As long as the regulated +5V remains above the backup battery voltage, the SS will
produce a +5V source for the microprocessor and the RTC. If the regulated +5V falls
below the backup battery voltage, the SS connects the backup battery to the
microprocessor and the RTC to preserve the contents of the microprocessor RAM and
provide power for RTC operation.
Power Subsystem (PS)
The power subsystem provides the required DC power for the pump from either a 9-volt
battery or an optional AC Adapter. The AC Adapter is an external device, which will
provide 10 volts DC when plugged into an AC wall outlet. The AC Adapter is connected
to the pump at its AC Adapter input jack. When power is available from both the battery
07-19-A8-092 Ipump Pain Management System Service Manual 2-5
Page 22

2 - Theory of Operation
and an AC Adapter, the PS selects the AC Adapter by default to conserve battery life. The
PS automatically switches the LCD backlight on when the AC Adapter power is present.
The PS provides regulated, partially regulated, and unregulated power. In the event that
both the battery and AC Adapter are not present, the PS, in conjunction with the SS, will
switch the microprocessor and RTC power to the backup battery. This maintains the
contents of the microprocessor RAM and keeps the RTC operational.
The unregulated voltage is used primarily to power the motor that drives the peristaltic
pump. The partially regulated voltage is used to power the buzzer and the LCD's backlight
circuit. The partially regulated supply is monitored for low voltage to shut down the
pump. The unregulated voltage is also used as a monitored voltage for the overvoltage
fault detector. The regulated voltage is supplied to all of the IC chips.
LCD Subsystem
The liquid crystal display (LCD) subsystem serves as a module for the microprocessor to
communicate infusion programming information and pump status to the user and facilitate
the entry of data from the keypad. The LCD module displays two rows of 16 characters,
with each character defined by a selection of dots from a 5 x 7 array with a cursor
underneath the array.
The LCD module can be written to by the microprocessor which supplies it with either
data or commands. Information in the LCD's memory is read by the microprocessor. For
its functional operation, the LCD module has two memories; the character generator (CG)
RAM and the display data (DD) RAM. The pump hardware has no need to distinguish
between the two types of RAM. This is accomplished by the operating software in the
microprocessor through the commands sent to the LCD module.
The backlight circuit provides power to the light emitting diodes (LEDs) inside the LCD
module to generate the necessary light for reading the display. These LEDs consume a
significant amount of power. Therefoere, when the pump is powered only by the 9-volt
battery, the display is only lit when needed. The LEDs are driven at less than the nominal
rated current. This provides a dim illumination of the display to reduce the drain on the
battery. When programming the pump on battery power, the backlight will be on. Fifteen
seconds after programming is complete, the backlight will turn off. The backlight will turn
on again when any key is pressed.
When the pump is being powered by the AC Adapter, the LEDs are on all the time. The
LEDs are supplied with nominal full rated current giving a bright backlight. As long as the
AC Adapter is providing power, the display will remain lit.
Silent Shutdown Circuit
When both the AC Adapter and 9-volt battery have been accidentally or intentionally
disconnected, the pump will notify the operator by issuing an intermittent “chirping”
sound. The power supply provides a residual voltage which maintains power to the
speaker circuit. Once this voltage has been depleted, the chirping will fade away (no less
than 20 seconds).
2-6 Ipump Pain Management System Service Manual 07-19-A8-092
Page 23

2 - Theory of Operation
Motor Subsystem
The motor subsystem has the ability to drive the DC motor in a forward or reverse
direction. The pump drive is controlled by the Direction and Drive Motor Module
(DDMM) which receives inputs from two independent shaft position encoders. The
encoder information enables precise control over the motor and therefore the fluid
delivery rate.
T o drive the motor , the microprocessor provides motor drive information using one of two
carrier frequencies. The DDMM uses a frequency discriminator to interrogate the carrier
signal and set the polarity of the voltage to the motor. Once direction is established, the
motor is controlled by the motor drive information directly from the microprocessor.
Occlusion Detection Circuit
A check is made for the possibility of blockage (occlusion) during the delivery
downstream and upstream of the pump. (NOTE: Unless the upstream occlusion alarm is
disabled in the pump’ s configuration.) During downstream occlusion, the elastic section of
the tubing set (in the area where the fingers of the peristaltic pump operate) will expand
slightly if a blockage exists. The expansion causes the deflection of a very sensitive sensor
thereby enabling the pump to sense an occlusion. During upstream occlusion, the elastic
section of the tubing set will contract slightly if a blockage exists upstream thereby
changing the deflection of a second sensor, and allowing the pump to sense the occlusion.
If the motor is operating in the reverse direction, as during the startup upstream occlusion
test, an upstream blockage will cause a slight expansion of the tubing which allows the
pump to sense the occlusion.
Air Sensor Circuit
An ultrasonic sensor is embedded in the plastic housing of the tubing door where the
tubing set is placed. When an air bubble passes through the tubing, the pump will sense
the different properties of fluid versus air and will issue an alarm when a certain amount of
air passes through that section of tubing.
Printer Adapter Interface Circuit
The interface to the printer adapter en ables the microprocessor to produce a printout of the
history data. The pump interfaces with the Baxter Printer Adapter, P/N 2L3400, and a
printer (typically a Seiko DPU-414). The printer interface is a serial port that operates on
TTL levels and provides data at a 600 baud rate.
A label is normally used to cover the PRINTER/COMM connector at the front of the
pump. This label should only be removed when the printer adapter is connected to the
pump. A second label positioned nearby indicates that this connector is sensitive to
Electrostatic Discharge (ESD).
07-19-A8-092 Ipump Pain Management System Service Manual 2-7
Page 24
2 - Theory of Operation
2-8 Ipump Pain Management System Service Manual 07-19-A8-092
Page 25

In this section Page
Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-1
Cleaning and Disinfecting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-1
Overview
The Ipump device is designed to provide reliable service with only minor routine
maintenance. A periodic functional inspection of the pump should be performed at least
every six months to assure proper operation. The procedures in Chapter 5, “Functional
T ests”, must be used to ensure that the pump operates properly . A review of the Alarm Log
should also be performed to identify system errors encountered by the pump. Refer to
Chapter 4, “Troubleshooting”, for details on reviewing the Alarm Log.
Baxter recommends performing preventive maintenance on an annual basis and cleaning
after every use. For convenience, the pump can be configured to give the operator an alert
whenever maintenance is due. Refer to the Ipump System Configuration Manual for
details.
3 - Care & Routine Maintenance
Cleaning and Disinfecting
The exterior surfaces of the pump may be cleaned with a cloth, sparingly dampened with
any of the cleaners listed in the table below. Follow the manufacturer's instructions for
diluting concentrated cleaners. After use, pumps should be cleaned/disinfected with an
agent from the list below before being used on another patient. Spills and dirt should be
cleaned off the pump as quickly as possible.
Recommended Cleaner Manufacturer Cleaner Disinfectant
Soapy water N/A XXX
A solution of 10% bleach and water N/A XXX XXX
LpH STERIS Corporation XXX XXX
Septisol STERIS Corporation XXX XXX
Super-Edisonite Colgate-Palmolive Co. XXX
TOR or Hi-TOR
Plus
CAUTION
Table 3-1. Approved Cleaners and Disinfectants
The Ipump device and the AC Adapter are not waterproof and should
not be immersed. Avoid getting liquids inside the pump or permanent
damage may result. Do not use alcohol for cleaning. Sterilization via
ETO, steam, etc. should not be attempted.
Huntington Professional Products XXX XXX
07-19-A8-092 Ipump Pain Management System Service Manual 3-1
Page 26
3 - Care & Routine Maintenance
3-2 Ipump Pain Management System Service Manual 07-19-A8-092
Page 27
4 - Troubleshooting
In this section Page
Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
Reviewing the Alarm Log. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
System Error Codes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Range 10 - 2V -- Peripheral/Sensor Errors. . . . . . . . . . . . . . . . . . . . . . . 4-5
Range 30 - 47 -- Motor Control Errors. . . . . . . . . . . . . . . . . . . . . . . . . . 4-6
Range 50 - 52 -- RTC Errors. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-7
Range 60 - 62 -- Power Supply Errors . . . . . . . . . . . . . . . . . . . . . . . . . . 4-7
Range 70 - 74 & L0 -- MPU Errors . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-7
Range 75 - 8D -- Processing Errors . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-8
Range 90 - 9Z & M0 - P3 -- Data Corruption Errors . . . . . . . . . . . . . . . 4-8
Range A0 - J1 -- Processing Errors . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-10
Overview
CAUTION
CAUTION
Pumps under warranty must be returned to the factory for repair. Unauthorized
disassembly/repair will void your warranty. When a pump malfunctions, perform the
following to see if pump operation can be restored. Ensure that the:
• batteries are installed and not depleted.
• batteries are installed correctly (proper polarity).
If this does not restore the pump operation, refer to the Troubleshooting Chart, Table 4-1.
! WARNING !
Only trained, qualified personnel and Baxter authorized service
representatives should perform procedures in this manual.
Contact Baxter Healthcare Corporation to arrange any needed
service support or if you have any questions while servicing the
pump.
The pump must only be serviced by a trained biomedical
engineering technician or Baxter Healthcare personnel.
Reviewing the Alarm Log
Troubleshooting an Ipump device should begin with a review of the alarm log. This
section describes the steps involved for performing this review. For system errors
identified in the alarm log review, refer to the System Error Codes Tables in this chapter to
help determine the component or assembly that may be contributing to the failure.
07-19-A8-092 Ipump Pain Management System Service Manual 4-1
Page 28

4 - Troubleshooting
1. Unlock the Bag Cover and press the <ON/OFF> key to turn the pump on. The Bag
Cover must be open.
2. If the language option is “None,” the display will read “PRESS ENTER FOR
ENGLISH.” The display will cycle through the choices. Press the <ENTER> key
while the ENGLISH choice is being displayed.
3. If a language has been previously configured, the display will be blank and will automatically continue to the next step.
4. When the display reads “SOFTWARE VERSION XX.XX.XX,” hold down the left
arrow key until “TESTING MEMORY” is displayed. “TESTING MEMORY” will
only be displayed momentarily followed by “CONFIGURATION XXXXX.”
5. The display will then read “000 ENTER CONFIG CODE.” Using the left, right and up
arrow keys, input the code 2-1-5 and press the <ENTER> key.
6. Upon entry to the Configuration Set mode, the display will read “CONFIGURATION
PRESS ENTER.” DO NOT PRESS THE ENTER KEY AT THIS TIME.
7. Press the <HISTORY> key .
8. The display will read “ALARM LOG.”
9. Press the right cursor key to display the first system alarm entry. Each system alarm
log entry will be displayed as follows:
SYSTEM ERROR XX
MM/DD/YY HH:MMXM
10. Record all alarms and their associated dates and times.
11. Continue to press the right cursor key to display the next system alarm log entry. The
display will eventually read “END OF ALARM LOG.”
NOTE: If the Alarm Log is empty, the display will read “END OF ALARM LOG.”
12. At the “END OF ALARM LOG” display, press the <ENTER> key. The pump will
then display “CONFIGURATION PRESS ENTER.” To clear the System Alarm Log,
proceed to the next step. T o reta in the System Alarm Log, press the <ON/OFF> key to
turn the pump off.
13. Press the <CLEAR/SILENCE> key. The display will read “RESET CONFIG?”
14. Choose NO and press the <ENTER> key. The display will read “CLEAR HISTORY?” (if there is a history).
15. Choose NO and press the <ENTER> key. The display will read “CLEAR ALARM
LOG?”
16. Choose YES and press the <ENTER> key. The pump will clear the System Alarm
Log and the display will momentarily read “ALARM LOG CLEARED.”
17. When the display reads “CONFIGURATION PRESS ENTER,” press the <ON/OFF>
key to turn the pump off.
4-2 Ipump Pain Management System Service Manual 07-19-A8-092
Page 29
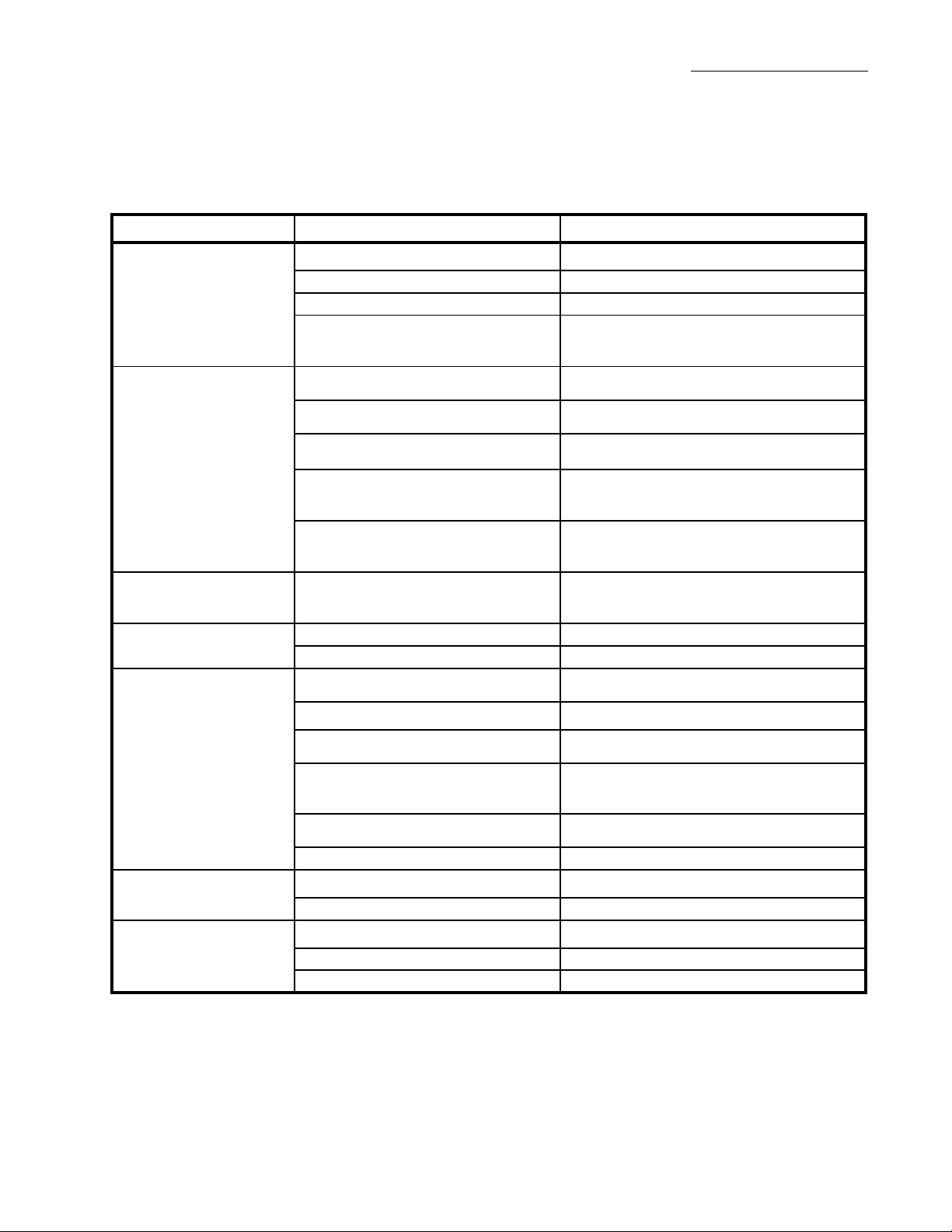
Troubleshooting
The information in this chapter is written for repair to the board or module level. Except
for those items listed, circuit board components are not available from Baxter Healthcare.
Refer to Chapter 6 for disassembly procedures and Chapter 9 for repair parts information.
Symptom Possible Cause Solution
No power (9V)
No power (AC)
(No AC Plug icon)
Constant audible alarm –
no display when battery
or AC Adapter plugged in
No audio alarm Defective Buzzer. Replace the Buzzer.
Constant occlusion alarm
No air alarm or constant
Constant alarm, no LCD
display when ON/OFF
key pressed
Dead/Contaminated 9-volt Battery. Check/replace the 9-volt Battery.
Broken battery leads. Replace the Battery Contact Assembly.
Battery installed with wrong polarity. Remove and re-install the 9-volt Battery.
Defective MPU Board.
Poor AC Adapter connection to pump.
Defective AC Adapter.
Defective AC Connector on the Front
Case.
Defective or disconnected AC Power
Connector inside pump.
Defective MPU Board.
Defective MPU Board. Replace the MPU Board.
Defective MPU Board. Replace the MPU Board.
Damaged/disconnected wiring to Flex
Cable.
Defective occlusion sensors. Replace the Mechanism Assembly.
Defective/damaged Flex Cable or
connector on Mechanism Assembly.
Dirty or jammed Occlusion Sensors.
Defective/damaged J14 connector on
MPU Board.
Defective MPU Board. Replace the MPU Board.
Defective Air Sensor. Replace the Mechanism Assembly.
Air Sensor Disabled. Check the pump configuration.
Bent or broken pin on LCD Module. Replace the LCD Module.
Defective LCD Module. Replace the LCD Module.
Defective MPU Board. Replace the MPU Board.
Check for 9-volt line at J10 connector. If it is
present, replace the MPU Board. If not,
replace the battery contact assembly.
Ensure that the red dots are aligned and
the connector is plugged in fully.
Check the output of the AC Adapter for
10 VDC. Replace the AC Adapter.
Replace the Front Case Assembly.
Check for proper installation of the AC
power connector onto the MPU Board at
J4. Replace the front case assembly.
Check for 10 volts at connector J4. If
present, replace the MPU Board. If not,
replace the Front Case Assembly.
Check wiring to the Flex Cable and resolder
as necessary.
Replace the Mechanism Assembly.
Clean the Occlusion Sensors on the
Mechanism Assembly or replace the
assembly.
Replace the MPU Board.
Table 4-1. Troubleshooting Chart
4 - Troubleshooting
07-19-A8-092 Ipump Pain Management System Service Manual 4-3
Page 30

4 - Troubleshooting
Symptom Possible Cause Solution
LCD not working or
segments missing
No backlighting
“Check Tubing
Placement” screen will
not clear
“Cover Is Unlocked”
alarm will not clear
No input from front panel
keypad
Will not retain memory Low or dead 3V Backup Battery.
Will not accept attempts/
injections from PCA
switch
Will not print
System Error 32 Loose Motor Connector. Tighten the Motor Connector J2 on DDMM.
System Error 33
Defective LCD Module. Replace the LCD Module.
Defective MPU Board. Replace the MPU Board.
Poor connection between J23 and the
LCD Module.
Defective LCD Module. Replace the LCD Module.
Defective MPU Board. Replace the MPU Board.
Tubing segment improperly installed
or not installed.
Dirty or disconnected Flex Cable
Connector to J14 on MPU.
Defective Microswitch.
Defective/damaged Flex Cable. Replace the Mechanism Assembly.
Defective MPU Board. Replace the MPU Board.
Defective Reed Switch on Rear Case. Replace the Rear Case Assembly.
Disconnected Reed Switch.
Missing Magnet (Bag Cover Latch). Repair/replace the Bag Cover.
Defective Lock Assembly. Repair/replace the Bag Cover.
Defective MPU Board. Replace the MPU Board.
Defective Keypad.
Disconnected Keypad Connector. Connect the Keypad Connector.
Bad contact between Keypad Flex
and MPU Board.
Defective MPU Board. Replace the MPU Board.
Defective PCA Cable. Replace the PCA Cable.
Defective PCA Connector on MPU
Board.
Defective MPU Board. Replace the MPU Board.
Defective Printer Connector on MPU
Board.
Defective Motor. Replace the Mechanism Assembly.
Defective/damaged Mechanism
Assembly.
Check to ensure the backlight connector is
properly installed (pins 15 & 16 of J23).
Ensure the tubing set is installed properly.
Refer to the operator’s manual for proper
installation.
Clean and tighten the Flex Cable
Connector.
With a tubing segment properly installed,
check continuity between pins 13 and 14
of the mechanism flex circuit connector
(refer to connector pin-out in Figure 8-3).
If the circuit remains “open,” replace the
Mechanism Assembly.
Connect the Reed Switch Connector to the
MPU Board.
With power removed, check the Keypad for
continuity while pressing the suspected key
(refer to the Keypad pin-out in Figure 8-2).
Check the connector pins and clean/repair
as needed.
Replace the 3V Backup Battery. (Refer to
the 3V Backup Battery Test in Chapter 6.)
Replace the MPU Board.
Check for bent Printer Connector pins.
Replace the Printer Connector if necessary.
Replace the Mechanism Assembly.
Table 4-1. Troubleshooting Chart (Continued)
4-4 Ipump Pain Management System Service Manual 07-19-A8-092
Page 31

System Error Codes
NOTE: If an error code appears on the display, remove all power, then restart the pump. A
problem is indicated if the error persists. Due to the fact that error codes shutdown
the pump, it is difficult to troubleshoot without swapping out suspected assemblies.
As all error codes are software generated, the MPU PCBA is always suspected.
The following tables contain a listing of all error codes that the pump can generate. This
list is provided for reference purposes only.
Range 10 - 2V -- Peripheral/Sensor Errors
Failures specific to components external to the MPU such as switches or the EEPROM.
Code Cause
10 key held, even after warning
11 display RAM failure, pattern 1
12 display RAM failure, pattern 2
13 character generator RAM failure
4 - Troubleshooting
15 display is not responding expeditiously
16 red LED is not functioning
18 green LED is not functioning
19 EEPROM didn't ack write address command preceding read
1A EEPROM didn't ack high address preceding read
1B EEPROM didn't ack low address preceding read
1C EEPROM didn't ack read command
1D EEPROM didn't ack write address command preceding write
1E EEPROM didn't ack high address preceding write
1F EEPROM didn't ack low address preceding write
1G EEPROM didn't ack send byte
1H EEPROM didn't ack write address command within timeout
20 trying to read beyond end of EEPROM
21 trying to write beyond end of EEPROM
22 event log trying to write beyond end of EEPROM
23 system log trying to write beyond end of EEPROM
24 EEPROM read after write failure
25 (Not Used)
26 Rx data int, but Rx buffer is empty
27 error while printing history data
28 Tx could not send config data
29 Tx could not send config data after NAK
07-19-A8-092 Ipump Pain Management System Service Manual 4-5
Page 32

4 - Troubleshooting
2A Tx could not send memory dump data
2B pump turned on by other than the on/off key
2C CRC failure on internal ROM (8000-ffff)
2D CRC failure on external ROM (20000-3ffff)
2E external watchdog circuitry failure during reset test
2F external watchdog circuitry failure during shutdown
2G cat_fmt_str, string too long
2H noise on sensor condition in start_air_sensor
2J upstream occlusion sensor failure
2K downstream occlusion sensor failure
2L air percentage is greater than 100
2M air to_percent macro, attempt to divide by 0
2N Stuck downstream occlusion sensor
2V Stuck upstream occlusion sensor
Range 30 - 47 -- Motor Control Errors
Failures specific to the control of the motor.
Code Cause
30 no forward motion after several control intervals
31 motor runaway
32 can't reach desired speed
33
34
35
36
37 motor moving when it should be stopped
38 motor moving when it should be stopped
39 motor drive transistor failure
40 motor should be stopped, but it is backing up
main encoder counts 25% over nominal value
for one motor rev
main encoder counts 25% under nominal value
for one motor rev
main encoder counts 3% over nominal value
for 8 motor revs
main encoder counts 3% under nominal value
for 8 motor revs
41 motor should be going forward, but it is moving backwards
42 speed nearing mechanical limits
43 not stopping quickly enough
44 Invalid motor control state
4-6 Ipump Pain Management System Service Manual 07-19-A8-092
Page 33

45 Motor should be moving backwards, but it is moving forwards
Attempt to move motor backwards other than during Startup
46
up occlusion test
47 Motor moving while attempting to change direction
Range 50 - 52 -- RTC Errors
Failures specific to real-time clock functionality.
Code Cause
50 RTC vs. system clock comparison error
51 Time read back = time written
52 RTC RAM failure
4 - Troubleshooting
Range 60 - 62 -- Power Supply Errors
Failures specific to power supply voltage checks.
Code Cause
60 power supply voltage is out of range
61 no detectable power source
62 no detectable power source
Range 70 - 74 & L0 -- MPU Errors
Failures specific to the operation of the microprocessor such as the contents of a mode
register being incorrect.
Code Cause
70 a-d converter timeout in voltage check
71 a-d converter timeout in occlusion check
72 illegal interrupt
73 internal watchdog timeout
74 ROM check stack overflow error
L0 CPU test failed
07-19-A8-092 Ipump Pain Management System Service Manual 4-7
Page 34

4 - Troubleshooting
Range 75 - 8D -- Processing Errors
Failures specific to abnormal processing conditions encountered during operation, such as
a stack overflow, or a watchdog timeout.
Code Cause
75 bus_count exceeds maximum limit
76 unknown event received by task
77 tried to remove total not in list
78 software timer out of range
79 B0_isr held off for more than 60ms.
80 stack overflow in BB check_stack()
81 BYTE-BOS stack overflow
82 operations task stack overflow
83 user interface stack overflow
84 serial monitor stack overflow
85 UIT message buffer overflow
86 stop_rx routine called when it should not have been
87 delivery attempt before delay elapsed
88
89 volume given not within +/- 0.5% during bolus
8A
8B volume given not within +/- 0.5% during basal or continuous
8C attempt to infuse at 0 rate
8D BYTE-BOS failure, returned to main
attempt to stop injection when no injection
in progress
volume given not within +/- 0.5% during
PCA injection
Range 90 - 9Z & M0 - P3 -- Data Corruption Errors
Failures specific to Data Corruption Errors -- Error detected during testing of data validity.
Code Cause
90 UIT invalid state during prime
91 UIT invalid state during bolus
92 UIT invalid state during display of SOT
93 UIT invalid state updating history attempts/injections
94
95 UIT invalid state displaying event history
4-8 Ipump Pain Management System Service Manual 07-19-A8-092
UIT invalid state displaying history total given
Page 35

96 UIT invalid state displaying held key info
97 UIT invalid state displaying system errors list
98 UIT invalid state while setting modes
99 UIT invalid state while setting units
9A UIT invalid state while attempting to send replication data
9B UIT invalid state at end of replication
9C UIT invalid state found in auxil table
9D invalid field requested in get_hist()
9E invalid field requested in get_hist_time()
9F history checksum failure, data corrupted
9G invalid field requested in write_history()
9H error in getting rx data units for printing
9J 0 concentration in rx data for printing
9K error in getting rx data units for printing
4 - Troubleshooting
9L configuration checksum failure, data corrupt at startup
9M configuration checksum failure, data corrupt in get_cf()
9N configuration checksum failure, data corrupt in get_cf_string()
9P rx checksum failure, data corrupted at get_rx_units()
9Q rx checksum failure, data corrupted at get_rx_mode()
9R rx checksum failure, data corrupted at get_rx()
9S invalid field requested in argument to get_rx()
9T invalid field requested in argument to set_rx()
9U rx checksum failure, data corrupted at get_rx_all()
9V cur_state is corrupt
9W branch_state is corrupt
9X sw_status is corrupt at soft_status()
9Y sw_status is corrupt at set_soft_status()
9Z pump_status is corrupt at check_status()
M0 pump_status is corrupt at set_status()
M1 pump_status is corrupt at clear_status()
M2 tot_entry list too long in add_entry()
M3 tot_entry list too long in rem_entry()
M4 tot_entry list too long in upd_totals()
M5 invalid address passed to calc_checksum()
M6 LCD line 1 string length too long
M7 LCD line 2 string length too long
M8 EEPROM read after write error in reset_event_log()
07-19-A8-092 Ipump Pain Management System Service Manual 4-9
Page 36

4 - Troubleshooting
M9 EEPROM read after write error in reset_event_log()
N0 EEPROM read after write error in log_event()
N1 EEPROM read after write error in log_event()
N2 EEPROM read after write error in reset_syserr_log()
N3 EEPROM read after write error in reset_syserr_log()
N4 EEPROM read after write error in log_syserr()
N5 EEPROM read after write error in log_syserr()
N6 checksum error on get_next_event()
N8 checksum error on log_syserr()
N9 checksum error on get_next_syserr()
P0 checksum error on get_prev_syserr()
P2 checksum error on event log only in start_logs()
P2 checksum error on syserr log only in start_logs()
P3 checksum error on both logs in start_logs()
Range A0 - J1 -- Processing Errors
Failures specific to abnormal processing conditions encountered during operation variable out of range.
Code Cause
A0 invalid type passed to checkpump()
A1 invalid state in f329_init()
A2 invalid date format in f135()
A3 invalid cursor position in date set, proc12()
A4 invalid cursor position in date set, proc12()
A5 invalid cursor position in date set, proc12()
A6 invalid cursor position in time set, proc24()
A7 invalid cursor position in set_dt_display()
A8 invalid cursor position in set_dt_display()
A9 invalid cursor position in set_dt_display()
B0 invalid state in f234_enter()
B1 invalid state in f303_enter()
B2 invalid state in f304_enter()
B3 invalid state in f310_enter()
B4 invalid state in f319_init()
B5 invalid state in f320_init()
4-10 Ipump Pain Management System Service Manual 07-19-A8-092
Page 37

B6 invalid state in f325_init()
B7 invalid state in f324_init()
B8 invalid state in f330_init()
B9 invalid state in f334_init()
C0 invalid state in f334_init()
C1 invalid state in f335_init()
C2 invalid state in f335_enter()
C3 invalid state in f336_init()
C4 invalid state in f336_enter()
C5 invalid state in f337_enter()
C6 invalid state in f340_init()
C7 invalid state in f345_init()
C8 invalid state in f405()
C9 invalid state in f405_io()
4 - Troubleshooting
D0 invalid reason to stop bolus
D1 illegal event f425()
D2 illegal dose limit type f615_init()
D3 illegal menu item in f730_enter()
D4 illegal menu item in f734_enter()
D5 illegal menu item in f740_enter()
D6 illegal menu item in f750_enter()
D7 illegal menu item in Build766Display()
D8 illegal menu item in f766()
D9 illegal menu item in Build770Display()
E0 illegal menu item in f770()
E1 illegal menu item in f810_enter()
E2 illegal event in proc_i()
E3 illegal event in io_proc_o()
E4 illegal event in io_proc_cur()
E5 illegal str_buf len in io_bcd_to_string()
E6 illegal str_buf len in io_bcd_to_string()
E7 illegal event in proc_menu()
E8 illegal event in proc_menu1()
E9 illegal event in proc_menu2()
F0 illegal event in proc_text()
F1 illegal unit type in get_unit_text()
F2 invalid LED request
07-19-A8-092 Ipump Pain Management System Service Manual 4-11
Page 38

4 - Troubleshooting
F3 invalid audio request
F4 negative number passed to lrtoa()
F5 invalid unit for this print request
F6 decimal point precision out of range
F7 number too big in round_value()
F8 string too long to display
F9 precision too large in sprintg()
G0 unrecognized format in sprintg()
G1 string produced by cpystr() too long for display
G2 unknown timer in process_msg()
G3 unknown key in process_msg()
G4 invalid silence until time requested
G5 invalid config item requested at get_cf()
G6 invalid config item requested at get_cf_string()
G7 invalid config item requested at cf_cond()
G8 invalid config item requested at set_cf()
G9 invalid config item requested at set_cf_string()
H0 invalid timer_id in set_timer
H1 invalid timer_id in kill_timer
H2 invalid clock register requested
H3 variable out of range in hextobcd()
H4 invalid clock register requested in rtc_rd_time()
H5 variable out of range in bcdtohex()
H6 invalid month in days_in_month()
H7 invalid alert time in check_bag_volume()
H8
H9 invalid unit for this print request
J0 invalid field width in 1toaw
J1 invalid field width in 1rtoaw
illegal menu item in restart
pm cycle
4-12 Ipump Pain Management System Service Manual 07-19-A8-092
Page 39

5 - Functional Tests
In this section Page
Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-2
Equipment Required . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-3
Optional Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-3
Exterior Visual Inspection. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-3
Configuration Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-4
Flow Rate Accuracy Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-6
Test Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-7
Procedure Using the Gravimetric Method . . . . . . . . . . . . . . . . . . . . . . . 5-7
Procedure Using the Volumetric Method . . . . . . . . . . . . . . . . . . . . . . . . 5-8
Downstream Occlusion Calibration Pressure Test . . . . . . . . . . . . . . . . . . . . 5-8
Operational Checks. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-9
Test Set Up . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-9
Power-On Self Test (POST) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-9
Keypad Operation Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-10
Bag Cover Lock/Unlock Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-10
Tubing Sensor Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-10
Occlusion Sensor Test - Downstream. . . . . . . . . . . . . . . . . . . . . . . . . . . 5-11
Occlusion Sensor Test - Upstream . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-11
Air Sensor Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-12
PCA Cable & Button Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-13
AC Adapter Test (Optional) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-13
History Retention Test (Backup Battery Check) . . . . . . . . . . . . . . . . . . 5-14
Printer Test (Optional) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-14
Unintended Shutdown Circuit Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-14
Functional Test Data Sheet . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-15
Overview
The pump design includes extensive self-tests which continually monitor the pump’s
operation. These checks occur during normal operation of the pump. When an alarm or
fault condition is detected, the pump generates an error message, flashing LED indicators
and/or an audible alarm. These indicators warn the user of the detected fault. The pump
will stop operating until the fault condition has been corrected.
The test procedures in this chapter ensure that the pump operates properly. It is
recommended that anyone performing these functional tests become familiar with the
07-19-A8-092 Ipump Pain Management System Service Manual 5-1
Page 40

5 - Functional Tests
pump operating procedures contained in the Ipump Pain Management System Operator’s
Manual. To properly perform these tests, the pump must be reset to the specific
configuration settings provided in the procedure on page 5-4.
Pumps that fail any of the tests in this chapter are to be repaired using the information
provided in Chapter 4, "Troubleshooting" and Chapter 6, "Disassembly & Reassembly",
or returned to a Baxter Service Center for repair. Before returning the pump for repair,
record the failure mode and the pump’s setup prior to and during the failu re. Call a Baxter
representative for a Service Authorization Number and the procedure for returning a pump
for repair.
It is recommended that the results of the functional tests in this chapter be recorded on a
copy of the Functional Test Data Sheet provided at the end of this chapter. A copy of the
Functional Test Data Sheet should be kept as a preventive maintenance record for each
pump.
General Information
The following notes provide nice-to-know information about conditions which could
occur during the procedures provided in this chapter.
NOTE: In the procedures that follow, keystroke requirements are shown enclosed within
brackets. Example: <ENTER>. (This does not apply to prescription entries.)
NOTE: If the pump alarm activates, press <CLEAR/SILENCE> to silence the alarm.
NOTE: The pump will alarm if left in programming mode for longer than 3 minutes.
NOTE: Press <ENTER> to return to the “ENTER CODE” screen and resume a procedure
from the previous code entry step.
NOTE: The pump display goes blank when the pump is on battery power and the keypad
is inactive for more than approximately 15 seconds. Press any key to restore the
previous screen.
NOTE: The 9-volt battery icon is present in the upper right-hand corner of the display
when a 9-volt battery is installed in the pump.
NOTE: Press <ON/OFF> once to power on the pump.
NOTE: Press <ON/OFF> twice to power off the pump. (The current prescription data will
be erased from the pump programming options, but will be retained in History.)
NOTE: If <ON/OFF> is pressed too rapidly, the pump may not detect the second press.
NOTE: To stop priming or an infusion press <STOP> twice within one second.
NOTE: If <STOP> is pressed too rapidly, the pump may not detect the second press.
NOTE: During the test procedures, record all applicable information on the Functional
Test Data Sheet.
5-2 Ipump Pain Management System Service Manual 07-19-A8-092
Page 41

Equipment Required
• Pump with a fresh 9-volt battery installed
• PCA Cable (refer to the table on page 1-2)
• Empty tubing set (see Operator’s Manual)
• Syringe, 60 cc (or equivalent) with Luer lock connection (see Operator’s Manual)
• Magnet (small enough to fit into the Rear Case Latch)
• Distilled water
• (2) 250 mL bags - one filled with fluid (see Operator’s Manual)
• Fluid-filled tubing set - with fluid-filled bags
• Slide clamp or equivalent
• Stopwatch or timer (minutes and seconds)
• Fluid pressure gauge (minimum range 0 to 30 psi)
• Scale with minimum of two decimal place gram readout (for use with gravimetric test)
• 20 mL minimum burette (Recommended: ASTM class A burette with 0.2 mL
graduation resolution or better.)
5 - Functional Tests
Optional Equipment
• AC Adapter (2L3210)
• Printer Adapter (2L3400), Printer Adapter Cable (2L3402), and printer
Exterior Visual Inspection
The pump should be inspected for the parameters listed below. Upon completion of this
inspection, check off PASS or FAIL on the Functional Test Data Sheet, and record any
pertinent comments. If the pump fails any of these inspections, ensure that the applicable
service is performed on the pump before it is made available for patient use.
1. Pump Case - Verify that the Pump Case is free of visible damage and free of any indi-
cation of fluid ingress.
2. Bag Cover - Verify that the Bag Cover is properly positioned and secured to the pump.
Verify that it opens and closes freely and without binding.
3. Bag Cover Lock - Verify that the Bag Cover Lock turns freely when locking and
unlocking the cover. (Align the Bag Cover with the pump and hold closed. Push the
key into the lock before turning it.)
4. Battery Door - Verify that the Battery Door operates freely and closes securely when
a battery is in place.
5. Keypad - Verify that the entire Keypad is secured to the case and is not lifting up at
the edges. Ensure that the Keypad is free of damage.
6. Tubing Door - Verify that the Tubing Door opens freely and that the latching mecha-
nism operates properly when the door is closed.
7. Pumping Fingers - Verify that the pumping fingers are not loose or missing.
8. Tube Pathway - Ensure that the pathway is clean and clear of any obstructions.
07-19-A8-092 Ipump Pain Management System Service Manual 5-3
Page 42

5 - Functional Tests
9. Labels - Verify the presence of the following labels:
• PRINTER/COMM Connector Cover label (under the Front Case)
• Electrostatic Discharge (ESD) Warning label (under the Front Case)
• Rear label (on the Rear Case)
• Serial Number label (on the Rear Case)
• Battery Polarity label (inside the Battery Compartment)
Configuration Settings
Prior to performing the test procedures in this chapter, the pump must be reconfigured to
the specific settings provided in the following procedure. Perform the steps that follow:
1. Make sure the 9-volt battery is inserted properly or connect the optional AC adapter.
2. Unlock the Bag Cover.
3. Press the <ON/OFF> key. The display reads:
PERFORMING POWER
ON SELF TESTS
then moves to:
PRESS ENTER FOR
↑ ENGLISH
and the display scrolls through the operational languages available in the pump.
(If the display goes blank, press any key to restore the display.)
4. When the desired language is displayed, press the <ENTER> key. The display reads:
SOFTWARE VERSION
X.XX.XX
5. Press and hold down the
display automatically proceeds through the following screens:
W key. A continuous beeping sound will be heard, and the
TESTING
MEMORY
and:
CONFIGURATION
XXXXX
NOTE: If these screens fail to appear, the pump has not entered the Configuration Mode.
Press <ON/OFF> twice to turn the pump off, then start again at Step 3 above.
5-4 Ipump Pain Management System Service Manual 07-19-A8-092
Page 43

5 - Functional Tests
6. Release the W key. The beeping sound will stop. The display reads:
000 ENTER
↑ CONFIG CODE
7. Use the W or X key to move from column to column and the S key to enter 215, then
press the <ENTER> key to enter the Configuration Set Mode. The display reads:
CONFIGURATION
PRESS ENTER
8. Press the <ENTER> key. The display reads:
RESET CONFIG?
↑ NO
9. Use the S key to move between NO and YES. When YES is shown on the display,
press the <ENTER> key. The display reads:
SETTING DEFAULT
CONFIGURATION
then moves to:
CLEAR ALARM LOG?
↑ NO
10. Use the S key to move between NO and YES. When YES is shown on the display,
press the <ENTER> key. The display reads:
CONFIGURATION
PRESS ENTER
11. Press the <ENTER> key, then press the
S key one time so the display reads:
SELECT GROUP:
↑ LIMITS
12. Press the <ENTER> key five times, with a pause between each press for the display
information to change, until the display reads:
XX.X mL/hr MAX
↑ BASAL RATE
07-19-A8-092 Ipump Pain Management System Service Manual 5-5
Page 44

5 - Functional Tests
13. Use the W or X key to move from column to column and the S key to enter
15.0 mL/hr as the Maximum Basal Rate, then press the <ENTER> key three times.
The display reads:
then returns to:
14. Press the S key one time so the display reads:
15. Press the <ENTER> key seven times until the display reads:
SAVING
LIMITS
SELECT GROUP:
↑ LIMITS
SELECT GROUP:
↑ CONTROLS
AIR DETECTION
↑ OFF
16. Press the S key once so the display changes from “OFF” to “LOW”, then press the
<ENTER> key. The display reads:
SAVING
CONTROLS
then returns to:
SELECT GROUP:
↑ CONTROLS
17. Press the <ENTER> key twice to turn the pump off. The pum p is now reconfigured to
the Factory Default settings except for the Maximum Basal Rate which is set to 15.0
mL/hr, and the Air Detection feature which is set to LOW.
NOTE: To reconfigure the pump for the operational features selected by your institution,
follow the steps in the Ipump Pain Management System Configuration Manual.
Flow Rate Accuracy Test
The following procedure should be used to verify the flow rate accuracy of the pump. The
performance of commercially available automated rate testing equipment has not been
evaluated by Baxter for use on the Ipump device.
NOTE: Both a Gravimetric Method and a Volumetric Method of measurement have been
provided. The performance of either method is acceptable. Record all appropriate
information on the Functional Test Data Sheet.
5-6 Ipump Pain Management System Service Manual 07-19-A8-092
Page 45

5 - Functional Tests
0
T
0
9.9
Test Setup
1. Use a syringe to fill a fluid bag with a minimum of 100 mL of distilled water.
2. Remove all the air from the fluid bag, then cap it.
3. Install the tubing set into the pump.
4. Uncap the fluid bag and attach it to the tubing set.
5. Install the bag into the pump’s Bag Cover, close and lock the Bag Cover.
6. Turn on the pump and program it as follows:
Mode = Continuous
Units = mL
Bag volume = 100 mL
7. Prime the pump until all the air is removed from the tubing set and fluid bag.
NOTE: Use either the Gravimetric Method or the Volumetric Method to determine the flow
rate accuracy of the pump.
Procedure Using the Gravimetric Method
1. Set the scale to read in grams.
2. Attach the distal end of the tubing set to an empty 250 mL bag (output bag).
3. Deliver two additional priming volumes to ensure flow to the output bag.
4. Disconnect the output bag, cap it, weigh it, and record this as the “start weight” on the
Functional Test Data Sheet. After recording the start weight, reconnect the output bag
to the tubing set.
5. Complete the pump program as follows:
Rate = 9.9 mL/h
Bolus = 00.0 mL
6. Press the <START> key and observe the message “TESTING UP OCCLUSION”.
Start the stopwatch as soon as the display reads “CONTINUOUS 9.9 mL/hr”.
7. Run the pump for at least 1 hour (but no more than 2 hours), then simultaneously
unlock the bag cover and stop the stopwatch. Shut the pump off.
8. Disconnect the outp ut b ag, cap it, and weigh it. Record this weight as the “end weight”.
9. Record the stopwatch reading in seconds.
10. Use the following formulas to calculate the rate error:
Rate
ErrorRate
⎛
⎜
hr
⎝
()
% ×
est
07-19-A8-092 Ipump Pain Management System Service Manual 5-7
mL
()( )
⎞
=
⎟
⎠
=
()()
9.9
−
−
TimeElapsed
RateTest
10
WeightStartWeightEnd
sec
×
360
Page 46

5 - Functional Tests
0
T
0
9.9
11. Record the test rate and the rate error on the Functional Test Data Sheet.
12. If the rate error is equal to or less than 8%, the pump passes this test. Otherwise, the
pump fails. Record the results on the Functional Test Data Sheet.
Procedure Using the Volumetric Method
1. Attach the distal end of the tubing set to a 25 mL burette.
2. Deliver two additional priming volumes to ensure flow to the burette.
3. Record the volume reading on the burette as the “start volume” on the Data Sheet.
4. Complete the pump program as follows:
Rate = 9.9 mL/h
Bolus = 00.0 mL
5. Press the <START> key and start the stopwatch as soon as the display reads
“CONTINUOUS 9.9 mL/hr”.
6. Run the pump for at least 1 hour (but no more than 2 hours), then simultaneously
unlock the bag cover and stop the stopwatch. Shut the pump off.
7. Record the volume reading on the burette as the “end volume”.
8. Record the stopwatch reading in seconds.
9. Use the following formulas to calculate the rate error:
est
10. Record the test rate and the rate error on the Functional Test Data Sheet.
11. If the rate error is equal to or less than 8%, the pump passes this test. Otherwise, the
pump fails. Record the results on the Functional Test Data Sheet.
⎜
hr
⎝
()
ErrorRate
% ×
mL
⎛
Rate
()( )
⎞
=
⎟
⎠
=
()()
9.9
−
−
TimeElapsed
RateTest
10
VolumeStartVolumeEnd
sec
×
360
Downstream Occlusion Calibration Pressure Test
1. Close-up the pump and install a fluid-filled tubing set into the pump.
2. Connect the set to a pressure gauge with a minimum range of 0 to 30 psi.
3. Program the Pump as follows:
• Mode = PCA
• Units = mL
• Set fluid volume = 0100 mL
5-8 Ipump Pain Management System Service Manual 07-19-A8-092
Page 47

4. Program the Pump as follows:
•PCA DOSE = 1.0 mL
• DELAY = 3 minutes
• 1 HR LIMIT = 10.0 mL
• BOLUS = 05.0 mL
5. Start the bolus infusion and let the pump run until it goes into a downstream occlusion
alarm.
6. Observe the Occlusion Pressure value and record the reading on the Functional Test
Data Sheet.
• If the reading is 22 ±10 psi, the pump passes this test.
Operational Checks
This series of tests checks the follow ing pump features: Power On Self Test (POST),
Keypad, Bag Cover Lock, Tubing Sensor, Occlusion Sensors, Air Sensor, PCA Cable &
Button, AC Adapter (optional), history retention, printer port (optional), and unintended
shutdown circuit.
Record the results of each test on a copy of the Functional T est Data Sheet located at the
end of this chapter.
These tests are designed to be performed in one continuous sequence.
5 - Functional Tests
Test Set Up
1. Ensure a 9-volt battery is installed in the pump.
2. Remove any tubing set from the pump.
3. Plug the PCA cable into the pump.
4. Plug the AC Adapter into the pump (optional).
Power-On Self Test (POST)
NOTE: Read the entire POST process before proceeding.
1. Press the <ON/OFF> key to turn the pump on.
2. Verify that the following events occur:
• If the AC Adapter is plugged into the pump, the backlight will illuminate.
• The pump beeps once initially. The red Alert and green Infusing LEDs turn on.
• All LCD segments momentarily light up as 2 rows of rectangular test pattern segments.
The Alert and Infusing LEDs flash at a rapid rate.
“PERFORMING POWER ON SELF TESTS” is displayed. The Alert and Infusing
•
LEDs continue to flash at a rapid rate.
• If the language setting option is NONE, the pump will display
ENGLISH”
press the
then press the
and will scroll through the choices. When “ENGLISH” is displayed,
<ENTER> key. (You may also press the © key to scroll to “ENGLISH”,
<ENTER> key.)
“PRESS ENTER FOR
07-19-A8-092 Ipump Pain Management System Service Manual 5-9
Page 48

5 - Functional Tests
• “SOFTWARE VERSION XX.XX.XX” is displayed (where XX.XX.XX is the
current version) as the pump sounds a stutter beep. The Alert LED is flashing at an
approximately one-second interval. The Infusing LED is off.
• The ID label will be displayed if previously configured.
• The Date and Time will appear on the display followed by
“ENTER OR CLEAR.”
a. If the date and time are correct, press the
b. If not, press the
<ENTER> key.
“UNLOCK THE COVER” is displayed.
•
3. Record the results on the Functional Test Data Sheet.
<CLEAR> key and correct the Date and Time, then press the
<ENTER> key.
Keypad Operation Test
1. Press each key on the keypad. Verify that the pump beeps three times after each key press.
NOTE: The beeps following the ON/OFF and STOP keys will be delayed one second. DO
NOT PRESS the ON/OFF key twice as this will turn off the pump.
2. Record the results on the Functional Test Data Sheet.
Bag Cover Lock/Unlock Test
1. Unlock the Bag Cover.
2. The pump will go into alarm and display
3. Lock the Bag Cover.
4. The display will read
“000 ENTER CODE”. Unlock the Bag Cover one more time.
“LOCK THE COVER”.
5. The pump will go into alarm and display
6. Lock the Bag Cover.
7. Record the results
on the Functional Test Data Sheet.
“COVER IS UNLOCKED”.
Tubing Sensor Test
1. Press the <ENTER> key and continue through the settings to program the pump as
follows:
Mode = BASAL + PCA
Units = mL
Bag volume = 100 mL
2. At the
3. The screen will display “CHECK TUBING PLACEMENT”, the red LED will flash,
and the audible alarm will sound. If so, the pump passes this test. (The
SILENCE key may be pressed to silence the alarm.) Record the results on the Func-
tional Test Data Sheet.
“START TO PRIME, ENTER TO PROCEED”
screen, press the <
CLEAR/
START>
key.
5-10 Ipump Pain Management System Service Manual 07-19-A8-092
Page 49

Occlusion Sensor Test - Downstream
1. Unlock the Bag Cover.
5 - Functional Tests
2. The screen will display
audible alarm will sound. (The
“COVER IS UNLOCKED”, the red LED will flash and the
CLEAR/SILENCE key may be pressed to silence the
alarm.)
3. Open the Bag Cover, open the T ubing Door, install a primed tubing set into the pump,
then close the Tubing Door.
4. Close and lock the Bag Cover. The security code screen will appear. Enter the security
code (123) and press the
<ENTER> key.
5. Program the pump as follows:
Mode = BASAL + PCA
Units = mL
Bag volume = 100 mL
6. At the
<ENTER> key.
“START TO PRIME, ENTER TO PROCEED” screen, press the
7. Program the pump as follows:
PCA dose = 1.0 mL
Delay = 3 minutes
Basal rate = 5.0 mL/h
1 Hr. limit = 20.0 mL
Bolus = 1.0 mL
8. At the “
key. The pump will display,
ST AR T BEGINS RX, ENTER REVIEWS RX” screen, press the <START>
“TESTING UP OCCLUSION” while the pump per-
forms the startup upstream occlusion test.
9. When the display reads,
“BOLUS INFUSING XX.X”, clamp the distal (downstr eam)
end of the tubing set within approximately 3 inches of the pump. After a short period,
the display will read,
“DOWNSTREAM OCCLUSION”, the red LED will flash and
the audible alarm will sound. If so, the pump passes the test. If not, check that the tubing set is properly clamped and, if necessary, repeat this test.
10. Record the results on the Functional Test Data Sheet.
Occlusion Sensor Test - Upstream
NOTE: Since the pump is currently configured with Factory Default settings, the
upstream occlusion detection feature is ON.
1. Unlock the Bag Cover.
2. The screen will display
audible alarm will sound. (The
alarm.)
3. Open the Bag Cover, open the Tubing Door and make sure the tubing set is fully
primed. Manual priming is preferred. After the set is primed, close the Tubing Door.
4. Place a slide clamp on the solution bag outlet to occlude the line.
“COVER IS UNLOCKED”, the red LED will flash, and the
CLEAR/SILENCE key may be pressed to silence the
07-19-A8-092 Ipump Pain Management System Service Manual 5-11
Page 50

5 - Functional Tests
5. Close and lock the Bag Cover. The security code screen will appe ar. Enter the security
code (123) and press the
6. Program the pump as follows:
Mode = BASAL + PCA
Units = mL
Bag volume = 100 mL
<ENTER> key.
7. At the
“START TO PRIME, ENTER TO PROCEED” screen, press the
<ENTER> key.
8. Program the pump as follows:
PCA dose = 1.0 mL
Delay = 3 minutes
Basal rate = 5.0 mL/h
Hr. limit = 20.0 mL
Bolus = 1.0 mL
9. At the
<
an upstream
play reads
NOTE: The pump may go into therapy prior to detecting the occlusion. But the occlusion
“START BEGINS RX, ENTER REVIEWS RX” screen, press the
START> key . “TESTING UP OCCLUSION” is displayed while the pump performs
occlusion test. Verify that the pu mp goes int o an audi ble alarm an d the di s-
“UPSTREAM OCCLUSION”.
should be detected prior to 0.5 mL of fluid delivery.
10. Record the results on the Functional Test Data Sheet.
Air Sensor Test
NOTE: The pump is currently configured with Factory Default settings and with the air
detection feature set to LOW so this test can be performed.
NOTE: This procedure is written to run after the Upstream Occlusion test without power-
ing off the pump. If power is cycled or the Tubing Door is opened prior to this procedure, the user must ensure the IV set is primed and that the startup upstream
occlusion test has been successfully completed prior to continuing.
1. Unlock the Bag Cover.
2. The screen will display
audible alarm will sound. (The
“COVER IS UNLOCKED”, the red LED will flash, and the
CLEAR/SILENCE key may be pressed to silence the
alarm.)
3. Without opening the Tubing Door, open the Bag Cover, clamp the solution bag, and
disconnect it from the tubing set.
4. Close and lock the Bag Cover. The security code screen will appe ar. Enter the security
code (123) and press the
<ENTER> key.
5. Program the pump as follows:
Mode = BASAL + PCA
Units = mL
Bag volume = 100 mL
PCA dose = 1.0 mL
5-12 Ipump Pain Management System Service Manual 07-19-A8-092
Page 51

Delay = 3 minutes
Basal rate = 5.0 mL/h
1 Hr. limit = 20.0 mL
Bolus = 2.0 mL
5 - Functional Tests
6. Press the <
play. Verify that the pump goes into an audible alarm, with the display reading
IN TUBING.” Record the results on the Functional Test Data Sheet.
START> key to begin the bolus and monitor the volume infused on the dis-
“AIR
PCA Cable & Button Test
NOTE: Since the pump is currently configured with Factory Default settings, the
PCA Button REQUIRED feature is ON.
1. Unlock the Bag Cover.
2. The screen will display
audible alarm will sound. (The
alarm.)
3. Open the Bag Cover and the Tubing Door, and remove the tubing set from the pump.
Connect the tubing set to the bag, remove the clamp from the bag, and manually prime
the tubing set.
4. Reinstall the tubing set into the pump.
5. Close and lock the Bag Cover. The security code screen will appear. Enter the security
code (123) and press the
6. Program the pump as follows:
PCA dose = 1.0 mL
Delay = 3 minutes
Basal rate = 5.0 mL/H
1 Hr. limit = 20.0 mL
Bolus = 00.0 mL
“COVER IS UNLOCKED”, the red LED will flash, and the
CLEAR/SILENCE key may be pressed to silence the
<ENTER> key.
7. Press the
lowed by
<START> key. The display will read “TESTING UP OCCLUSION” fol-
“BASAL + PCA” and the green LED will flash.
8. Wait a minimum of 3 minutes then press the PCA button 4 times. The pump will beep
each time the PCA button is pressed.
9. Unplug the PCA cable from the pump. The screen will display
CONNECTED”, both LEDs will flash, and the audible alarm will sound.
10. Re-insert the PCA cable into the pump. The screen will display
“PCA BUTTON NOT
“BASAL + PCA”, the
green LED will flash, and the audible alarm will be off.
11. If all the observations in steps 8, 9 and 10 have occurred, the pump passes this test.
Record the results on the Functional Test Data Sheet.
AC Adapter Test (Optional)
1. If present, disconnect the AC Adapter from the pump. The backlight will turn off and
after approximately 2 seconds, the pump will sound a stutter beep and the icon in the
upper right hand corner of the display will change to the battery symbol.
07-19-A8-092 Ipump Pain Management System Service Manual 5-13
Page 52

5 - Functional Tests
2. Re-connect the AC Adapter into the pump. The backlight will turn on and the
displayed icon will change to the plug symbol.
3. If all the observations in steps 1 and 2 have occurred, the pump passes this test. Record
the results on the Functional Test Data Sheet.
History Retention Test (Backup Battery Check)
1. Press the <ON/OFF> key to turn the pump off. Unplug the AC Adapter (if present)
and remove the 9-volt battery. After approximately 1 minute, re-insert the 9-volt battery and the AC Adapter (if present) and turn the pump on.
2. Scroll through the history screens as described in the
tem Operator’s Manual. Verify that the prescription is correct and that the INJ/ATT
screen indicates 1 INJ 4 ATT.
3. If the history screens are accurate, the pump passes this test. Record the results on the
Functional Test Data Sheet.
Ipump Pain Management Sys-
Printer Test (Optional)
To perform this test, it will be necessary to remove the label covering the printer port
connector at the front of the pump. After this test, replace the label over the printer port
connector.
1. Connect the Printer Adapter to the printer.
2. Insert the Printer Cable to the Printer Adapter and the pump printer connector.
3. Turn on the printer.
4. Ensure that the active light is illuminated on the Printer Adapter.
5. Press the
6. Verify that the pump provides a history printout.
7. If the printout is accurate, the pump passes this test.
8. Replace the label over the printer port connector. Record the results on the
Functional Test Data Sheet.
<PRINT/STOP> key on the Printer Adapter.
Unintended Shutdown Circuit Test
1. Remove the 9-volt battery.
2. Ensure that the pump “chirps” and the red Alert LED flashes.
3. Press the
chirping is silenced.
4. Reinstall the 9-volt battery, ensuring that proper battery polarity is observed.
5. Record the results on the Functional Test Data Sheet.
Operatonal Checks are now complete. Ensure the Functional Test Data
Sheet is properly reviewed and signed.
5-14 Ipump Pain Management System Service Manual 07-19-A8-092
<CLEAR/SILENCE> key and ensure that the LED stops flashing and the
Page 53

5 - Functional Tests
Functional Test Data Sheet
Record the results of the Ipump device functional tests on this Data Sheet. This sheet may
be reproduced. Pumps that fail any of these tests must be servi ced before be ing put int o use.
Pump S/N: ___________________HARDWARE REV:________ SOFTWARE REV:_____________
VISUAL INSPECTION
PASS FAIL COMMENTS
FLOW RATE ACCURACY TEST
Gravimetric Method
Start Weight End Weight Elapsed Time Test Rate Rate Error
Volumetric Method
Start
Volu me
End Volume Elapsed Time Test Rate Rate Error
Results
PA SS FA IL
Results
PAS S FA IL
Comments:
DOWNSTREAM OCCLUSION CALIBRATION PRESSURE TEST
PASS FAIL OCCLUSION PRESSURE
OPERATIONAL CHECKS
Feature
Power-On Self Test (POST)
Keypad Operation Test
Bag Cover Lock/Unlock Test
Tubing Sensor Test
Occlusion Sensor Test - Downstream
Occlusion Sensor Test - Upstream
Air Sensor Test
PCA Cable & Button Test
AC Adapter Test (Optional)
History Retention Test
Printer Test (Optional)
Unintended Shutdown Circuit Test
Test Results
PA SS FA IL
Comments
Signature: _________________________________________ Date: ______________
Reviewed by: _______________________________________ Date: ______________
07-19-A8-092 Ipump Pain Management System Service Manual 5-15
Page 54

5 - Functional Tests
5-16 Ipump Pain Management System Service Manual 07-19-A8-092
Page 55

6 - Disassembly & Reassembly
In this section Page
Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-2
Tools & Materials . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-2
Required Tools & Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-2
Consumable Materials . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-3
Baxter-Created Tools & Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-3
Disassembly Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4
General Disassembly Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4
Bag Cover Assembly Removal. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4
MPU PCBA Handling Guidelines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-5
Rear Case Assembly Removal . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-6
Mechanism Assembly, DDMM PCBA, & Battery Wall Removal. . . . . 6-7
MPU PCBA Removal. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-8
ESD Flex Circuit Removal . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-9
LCD Module Removal . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-10
Optional Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-11
3V Backup Battery Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-11
Initializing the 3V Backup Battery. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-12
Keypad Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-12
Assembly Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-15
Torque Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-15
Installing the Front Case & Keypad Assembly . . . . . . . . . . . . . . . . . . . 6-15
Installing the ESD Flex Circuit. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-15
Installing the Display ESD Shield . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-18
Installing the LCD Module. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-18
MPU PCBA Handling Guidelines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-19
Installing the MPU PCBA . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-19
Installing the Battery Wall & DDMM PCBA. . . . . . . . . . . . . . . . . . . . . 6-23
Installing the DDMM PCBA Hold-down Foam. . . . . . . . . . . . . . . . . . . 6-24
Installing the Mechanism Assembly. . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-24
Installing the Battery Door . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-25
Pump Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-25
Internal Inspection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-26
Closing the Case . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-27
Pump Functional Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-28
Optional Assembly Procedures. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-28
Installing the Bag Cover Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-28
07-19-A8-092 Ipump Pain Management System Service Manual 6-1
Page 56

6 - Disassembly & Reassembly
Overview
This chapter of the manual contains procedures to assist in the disassembly and
reassembly of the pump and the removal and replacement of parts and assemblies. It is
highly recommended that the procedures provided in this chapter be used whenever
disassembly and repair is required.
For most assemblies and unique parts, specific reassembly procedures are provided. In
some cases, there is only the need to reverse the steps used for the disassembly procedure.
Torque values for fasteners are provided where needed.
In some procedures, special tools or equipment may be required if disassembly continues
past a certain point. Information about these special needs is included with the appropriate
procedures.
Additional information, such as exploded view drawings and their associated parts lists, is
contained in Chapter 9 and may be of assistance during these procedures.
CAUTION
CAUTION
CAUTION
Tools & Materials
Required Tools & Equipment
The following tools are recommended for use in maintaining and repairing the pump. The
repair procedures in this chapter assume these tools or their equivalent are available.
Soldering station (use small tip and keep
temperature low to prevent damage to PCB
pads)
3/16" Hex Nut driver (with an outer diameter
less than 0.26”) or equivalent
Flat Blade Screwdriver Magnifying system, 3x eye piece or equivalent
Phillips Screwdriver - #1 Potentiometer Adjustment Tool
Scale (Minimum resolution of two decimal place
gram readout)
Torque Screwdriver (22 - 28 in-oz) Stop Watch or Timer
Needle-Nose Pliers Wire Cutters
Only qualified, Baxter-trained personnel should attempt to repair the
pump.
It is strongly recommended that the pump be serviced in a static-free
environment. When performing any repairs on the pump, exercise
extreme caution to protect the circuit boards from static discharge. The
inspection or repair station, all equipment, and personnel should be
properly grounded.
After the pump is completely reassembled, perform the Functional Test
Procedures provided in Chapter 5 of this manual.
Required Tool Required Tool
Digital Multi-Meter (DMM) capable of reading
Resistance and Voltage.
Test leads (small - must include mini-clips on
both ends)
Pressure Gauge (PG-2000 - minimum range of
0 - 50 psi)
Table 6-1. Required Tools & Equipment
6-2 Ipump Pain Management System Service Manual 07-19-A8-092
Page 57

6 - Disassembly & Reassembly
Consumable Materials
The following consumable items are used where called out in the repair procedures in this
chapter. You may order these materials from Baxter.
Consumable Item Purpose
Small Applicator Swabs (foam or cotton) For applying lubricants and adhesives
Syringe (60cc) with Luer Lock Connector For pump testing and calibration
Fluid-filled Tubing Set (with fluid-filled bag) For pump testing and calibration
Empty Tubing Set (optional: pumping segment) For pump testing and calibration
250 mL Fluid Bags (Qty 2) For pump testing and calibration
Slide Clamp or Equivalent For pump testing and calibration
Permabond 792 For mounting the Keypad Spacer
Loctite 425 General Mounting adhesive
GLPT Red Insulating Varnish (p/n 109002) Electrical Insulating Material
Nyogel 760G
Solder For electrical connections
Flux Paste For electrical connections
Isopropyl Alcohol Adhesive remover
9-volt Battery For operating pump as required
3M/Scotch Filament Tape 898 To place around the Backup Battery as needed
Black Permanent Marker To mark guiding lines during parts replacement
Paper Towels To wipe up spills or excess lubricants
Flex Circuit contact preservative
(Do Not Use on Keypad Flex Cable!)
Table 6-2. Consumable Materials
Baxter-Created Tools & Equipment
The following tools and equipment are required for the proper assembly or adjusting the
pump mechanics. These are available from Baxter.
Part Number Description
AS3AL4002 Ipump Calibration Gauge
B069290000 Ipump High Point Upstream Calibration Gauge
B069290001 Ipump Low Point Upstream Calibration Gauge
2L3400 Printer Adapter
2L3402 Printer Adapter Cable
2L3510 Anti-Siphon Set
Table 6-3. Baxter-created Tools & Equipment
07-19-A8-092 Ipump Pain Management System Service Manual 6-3
Page 58

6 - Disassembly & Reassembly
Disassembly Procedures
General Disassembly Information
• The MPU Printed Circuit Board Assembly (PCBA) cons ists of 3 inter connected circ uit
boards referred to as the “Primary PCB”, “Secondary PCB”, and the “Connector PCB”.
• The Daughter PCBA is also referred to as the DDMM PCBA.
• Numbers in parentheses refer to the numbered parts in the drawing associated with the
procedure.
NOTE: Prior to disassembly, remove all accessories and batteries.
NOTE: During disassembly or movement of the MPU PCBA and flexes, follow the MPU
PCBA Handling Guidelines provided (refer to Figure 6-2).
NOTE: During disassembly, note the orientation and routing of all cables and connectors.
Failure to do so may result in improper operation and/or damage to the pump upon
reassembly.
NOTE: During disassembly, keep track of all hardware and avoid leaving loose hardware
in the pump upon reassembly. Failure to do so may result in improper operation
and/or electrical damage to the circuit boards.
Bag Cover Assembly Removal
1. Refer to Figure 6-1. Unlock and open the Bag Cover (1).
2. Remove the three pan head screws (3). Save the screws for use during reassembly.
3. Remove the Hinge Cover (4).
4. (Optional, if required) Remove the Mounting Plate (5), remove the two screws (2) that
secure it to the Bag Cover (1).
Figure 6-1. Bag Cover Removal
6-4 Ipump Pain Management System Service Manual 07-19-A8-092
Page 59

6 - Disassembly & Reassembly
MPU PCBA Handling Guidelines
When the MPU PCBA is removed ( either partially or fully) during this procedure, the
assembly must be handled as indicated in Figure 6-2.
Figure 6-2. MPU PCBA Handling Guideline
07-19-A8-092 Ipump Pain Management System Service Manual 6-5
Page 60

6 - Disassembly & Reassembly
Rear Case Assembly Removal
1. Refer to the previous disassembly instructions to remove the following items:
Bag Cover Assembly
2. Refer to Figure 6-3. Place the pump face down on a flat, clean surface.
3. Remove the six screws (2) and six flat washers (3) from the Rear Ca se Assembly (1).
The Rear Case contains the Primary PCB. This circuit board is
connected to the Secondary PCB located in the Front Case via a
semi-rigid Flex Circuit (4). The Secondary PCB is also attached to the
CAUTION
Gently lift the Rear Case (1) from the rest of the pump assembly and lay it over on its back.
4.
Connector PCB by a similar Flex Circuit. Extreme care must be taken
to prevent any damage to these Flex Circuits. To remove the circuit
boards from the Front and Rear Cases, refer to the MPU PCBA
Removal procedure later in this chapter.
Figure 6-3. Rear Case Assembly Removal
6-6 Ipump Pain Management System Service Manual 07-19-A8-092
Page 61

6 - Disassembly & Reassembly
Mechanism Assembly, DDMM PCBA, & Battery Wall Removal
NOTE: When the Mechanism Assembly is replaced, the pump must be calibrated.
1. Refer to the previous disassembly instructions to remove the following items:
Bag Cover Assembly
Rear Case Assembly
2. The Battery Door (6) is free to be removed after the Rear Case is removed.
3. Refer to Figure 6-4. Remove the screw (3) and lift off the Motor Retainer Bracket (4).
Carefully disconnect the six-pin motor connector (2) from J2 on the DDMM PCBA (10).
4. Locate the fifteen-pin Flex Cable Connector (9), hold it by the edges, and carefully lift
up from J14 on the Secondary PCB. (DO NOT pull on the Flex Cable.)
5. Lift the Mechanism Assembly (1) out of the Front Case.
6. To remove the Battery Wall (5), desolder the 30 ga jumper wire (11) from the solder
pad on the DDMM PCBA. Carefully lift the DDMM PCBA straight up from J6 (8)
and slide horizontally from under the ESD Flex Circuit Ground Tab (7). Slide the Battery Wall from under the ESD Flex Circuit Ground Tab (7) and lift out of the pump.
NOTE: For ease of parts identification, the ESD Flex Circuit is not shown in Figure 6-4.
Figure 6-4. Mechanism Assembly, DDMM PCBA, and Battery Wall Removal
07-19-A8-092 Ipump Pain Management System Service Manual 6-7
Page 62

6 - Disassembly & Reassembly
MPU PCBA Removal
NOTE: When the MPU PCBA is replaced, the pump must be calibrated.
1. Refer to the previous disassembly instructions to remove the following items:
Bag Cover Assembly
Rear Case Assembly
Mechanism Assembly, DDMM PCBA, and Battery Wall
2. Refer to Figure 6-5. Remove the jumper from J11 on the Primary PCB (7).
3. Remove the three screws (6) and three lock washers (8) that hold the Primary P CB (7 )
to the Rear Case Assembly (11).
4. Carefully lift the Primary PCB (7) and disconnect the Reed Switch Cable (10) from J1
(9) on the under side.
5. The Rear Case (11) can now be moved aside, using care not to damage the DDMM
Hold-down Foam (12).
CAUTION
Use extreme care to not damage the flex circuits when disconnecting
and handling the MPU PCBA.
Figure 6-5. MPU PCBA Removal
6-8 Ipump Pain Management System Service Manual 07-19-A8-092
Page 63

6 - Disassembly & Reassembly
NOTE: If Backup Battery replacement is required, proceed to the “Optional Procedures”
topic in this chapter.
6.
Disconnect the ten-pin Keypad Flex Cable (5) from J8/J9 (3) on the Secondary PCB (4).
7. Disconnect the two-pin Battery Harness Connector (17) from J10 (2) on the Secondary
PCB (4).
8. Use caution to not damage the ESD Flex Circuit Ground Tab under the Negative
Battery Connector (15), and carefully remove the Battery Harness.
9. Remove the three screws (1) and three lock washers (8) that attach the Secondary PCB
to the Front Case Assembly (16).
NOTE: There is a 16-pin connector, J23, on the underside of the Secondary PCB (4)
which connects to a mating connector on the LCD Module (13).
10. Use a small screwdriver at the back edges of the Secondary PCB (4) to gently lift the
PCB from the LCD Module (13) below.
11. Raise the front of the Secondary PCB to access the mounting screws for the Connector
PCB (18) below.
12. Disconnect the two pin AC connector (14) from J4 (19) on the Connector PCB (18).
13. Remove the four screws (20) and four lock washers (8) that hold the Connector PCB
(18) to the Front Case Assembly (16).
14. The complete MPU PCBA can now be carefully lifted from the Case.
ESD Flex Circuit Removal
1. Refer to the previous disassembly instructions to remove the following items:
Bag Cover Assembly
Rear Case Assembly
Mechanism Assembly, DDMM PCBA, and Battery Wall
MPU PCBA
2. Refer to Figures 6-6 and 6-7. Starting at the rear of the Front Case, carefully raise the
ESD Flex Circuit and release the two tabs from around the keypad cable.
Figure 6-6. ESD Flex Circuit and Display ESD Shield
07-19-A8-092 Ipump Pain Management System Service Manual 6-9
Page 64

6 - Disassembly & Reassembly
3. Continue in a clockwise direction and carefully remove the ESD Flex Circuit from the
Front Case.
Figure 6-7. ESD Flex Circuit & LCD Module Removal
LCD Module Removal
1. Refer to the previous disassembly instructions to remove the following items:
Bag Cover Assembly
Rear Case Assembly
Mechanism Assembly, DDMM PCBA, and Battery Wall
MPU PCBA
ESD Flex Circuit
2. Refer to Figure 6-7. Remove the three standoffs (1), one screw (2), and one lock
washer (3), that secure the LCD Module (4) to the Front Case Assembly.
NOTE: To remove the three standoffs, it will be necessary to use a 3/16" nutdriver with an
outer diameter less than 0.26".
3. Lift the LCD Module away from the Front Case Assembly.
4. Remove the clear Display ESD Shield (5) by turning over the Front Case. The Display
ESD Shield should fall out. Remove by hand if necessary.
6-10 Ipump Pain Management System Service Manual 07-19-A8-092
Page 65

Optional Procedures
3V Backup Battery Replacement
To determine if the Backup Battery needs to be replaced, perform the 3V Backup Battery
Test located in Chapter 7 of this manual. If the battery must be replaced, follow the
procedures below.
1. Refer to the previous disassembly instructions to remove the following items:
Bag Cover Assembly
Rear Case Assembly
Primary PCB
2. Refer to Figure 6-8. On the Primary PCB, ensure the 2-pin jumper (1) has been
removed from connector J11, located next to the Backup Battery (2).
6 - Disassembly & Reassembly
Figure 6-8. Backup Battery Replacement
3. Once the Primary PCB is removed from the Rear Cover, hold the Mechanism Assem-
bly in position and carefully turn over the Front Case Assembly and the Primary PCB.
4. Using care not to overheat the circuit board, carefully desolder the Backup Battery.
5. If tape is not present on the replacement 3V Backup Battery (p/n 6465634), apply a 20
mm long piece of 3M/Scotch Filament Tape 898 over the battery.
6. Ensure the replacement 3V Batte ry is positioned prop er ly, then insert the battery le ads
through the circuit board.
7. Use proper soldering technique and solder the new battery to the board. If necessary,
trim off the leads.
8. Carefully turn the entire assembly right side up.
9. Connect the Reed Switch cable to J1 on the underside of the Primary PCB. Use three
screws and 3 lock washers to reinstall the Primary PCB onto the Rear Case.
NOTE: DO NOT install the 2-pin jumper (1) on connector J11 at this time.
10. While ensuring that no flex cables or wires are caught between the Front and Rear
Cases, close the Pump Case and hold the pump together while continuing to the next
topic to initialize the 3V Backup Battery.
07-19-A8-092 Ipump Pain Management System Service Manual 6-11
Page 66

6 - Disassembly & Reassembly
Initializing the 3V Backup Battery
The Backup Battery must be initialized as follows:
1. Ensure the 2-pin jumper is removed from J11 on the Primary PCB then insert a 9-volt
Battery into the pump.
2. Press the
ON/OFF key to power up the pump. The pump will perform its Power On
Self-Test.
3. Check that all the display segments light up during this self-test routine.
NOTE: After replacing the 3V Backup Battery, the initial screen display that normally
shows “Performing Power on Self Test” may show a different language or be
unrecognizable. The pump will continue to start up normally, and subsequent message screens will display normally. Future power up sequences will display “Per-
forming Power on Self Test” as usual.
4. After the self-test is complete, select ENGLISH. The display must read “NO RX
PRESS ENTER”
. If not, remove the 9-volt battery and allow the pump to sit idle for
a period of time (~30 seconds) then re-initialize the 3V battery, starting at step 2.
5. If any of the LCD display segments were not lit during the self-test, abort this initialization, remove the 9-volt battery, and perform the appropriate steps to troubleshoot
and/or replace the LCD Module.
6. If all the LCD display segments light up during the self-test (do not turn the pump
OFF), open the Rear Case, and install the 2-pin jumper connector (1) onto J11 on the
Primary PCB.
7. Once the 2-pin jumper is installed onto J11, turn the pump off.
8. Press the ON/OFF key and ensure the pump properly performs the Power On SelfTest, then turn the pump back OFF.
Keypad Replacement
If replacing the Keypad on the Front Case, obtain either a Keypad with Spacer kit
(p/n B069610006RP).
1. Refer to the previous disassembly instructions to remove the following items:
Bag Cover Assembly
Rear Case Assembly
Mechanism Assembly
MPU PCBA
LCD Module
ESD Flex Circuit
NOTE: A keypad cannot be reused after it has been removed from the Front Case.
2. Position the Front Case with the Keypad facing up.
3. Use a small tool to lift a corner of the Keypad, then carefully peel it completely away
from the Front Case. Under the Keypad, a Keypad Spacer is held in position with
Permabond 792.
4. Carefully remove the Keypad Spacer from the Front Case, then pull the 10-pin Keypad
Cable through the slot.
6-12 Ipump Pain Management System Service Manual 07-19-A8-092
Page 67

6 - Disassembly & Reassembly
5. Clean off all residual adhesive from the Front Case (3) using Isopropyl alcohol, then
immediately wipe it dry.
6. Refer to Figure 6-9. Manually create a permanent crease in the new Keypad Cable.
This bend must be approximately 90°.
Figure 6-9. Keypad Cable
7. Remove the small piece of adhesive liner from behind the Keypad Cable.
8. Refer to Figures 6-10, 6-11, and 6-12. Insert the new Keypad Cable through the open-
ing in the Front Case.
Figure 6-10. Keypad Replacement
9. Refer to Figure 6-10. Apply a small amount of Permabond 792 onto both ledges on the
Front Case where the Spacer will sit.
10. Refer to Figure 6-11. Place the Spacer into its position on the Front Case as shown,
and hold in place for a minimum of 10 seconds. The upper edge of the Spacer must be
against the upper edge of the opening as illustrated.
07-19-A8-092 Ipump Pain Management System Service Manual 6-13
Page 68

6 - Disassembly & Reassembly
Figure 6-11. Front Case and Keypad Assembly
11. Remove the remaining portion of the paper liner from the back of the Keypad.
12. Beginning with the top edge of the Keypad, place the Keypad into position on the
Front Case.
13. Press the entire Keypad down onto the Front Case from the center of the Keypad outward. Ensure that all the edges of the Keypad sit flat inside the raised ridge around the
front of the pump.
14. Allow the assembly to cure for a minimum of 24 hours at room temperature.
6-14 Ipump Pain Management System Service Manual 07-19-A8-092
Page 69

Assembly Procedures
NOTE: During assembly, screws should be tightened to torques listed in the procedures.
NOTE: After replacing either the Mechanism Assembly or any Circuit Board Assembly,
the pump must be calibrated. Refer to Chapter 7. Failure to properly calibrate the
pump may result in occlusion detection or air-in-line detection failures.
NOTE: After repair is complete, all pumps must pass the general checks in Chapter 7 and
the functional tests in Chapter 5.
Torque Specifications
Torque requirements for fasteners (typically 22 - 28 in-oz.) are provided as needed within
the specific replacement procedures in this chapter. When specific torques requirements
are not given, use care to not over tighten those fasteners during assembly.
Installing the Front Case & Keypad Assembly
Two options exist for replacement of the Front Case and Keypad Assembly. Steps 1
and 2 below will assist in determining which replacement option to use.
6 - Disassembly & Reassembly
1. If replacing the Front Case and Keypad Assembly, obtain p/n B 069180004RP, skip the
remaining steps in this procedure,
procedure.
and continue to “Installing the ESD Flex Circuit”
2. If replacing the Keypad on the Front Case, obtain a Keypad with Spacer kit
(p/n B069610006RP). For proper installation, refer to the Keypad Replacement procedure located earlier in this chapter.
Installing the ESD Flex Circuit
1.
Refer to Figure 6-12. Pri or to inst alling th e ESD Flex Circuit (p /n B069110002), ensure
the Serial Number Label is attached to the Front Case as shown. If replacing the Front
Case, remove the label from the old Front Case and apply it to the new one as shown.
Figure 6-12. Serial Number Label Location
07-19-A8-092 Ipump Pain Management System Service Manual 6-15
Page 70

6 - Disassembly & Reassembly
2. Refer to Figure 6-13. Make sharp right angle creases at locations 1, 2, 3, and 4 (respectively in order) as indicated. The bend point location is indicated by the notches on the
ESD Flex Circuit and the bend direction is shown.
Figure 6-13. ESD Flex Circuit Preparation
3. Refer to Figure 6-13. Make gentle bends at locations 5, 6, and 7, (respectively in
order) as indicated. DO NOT crease these bends. See Figure 6-14 for proper results.
Figure 6-14. ESD Flex Circuit
4. Refer to Figure 6-15. Position the ESD Flex Circuit around the perimeter of
the Front Case, then bend the ground tab around the battery compartment wall.
Figure 6-15. ESD Flex Circuit Installation
6-16 Ipump Pain Management System Service Manual 07-19-A8-092
Page 71

6 - Disassembly & Reassembly
NOTE: In the following step, use caution not to damage the ESD Flex Circuit when
installing the negative battery contact.
5. Install the negative contact of the battery harness into position over the ESD Flex
Circuit Ground Tab as shown in Figure 6-16.
NOTE: If there is corrosion or rust on the battery contacts, replace the harness.
Figure 6-16. Negative Battery Connector Installation
6. Refer to Figure 6-17. Make sure the two tabs of the ESD Flex Shield are placed in the
recess edge of the slot for the 10-pin keypad cable as shown.
Figure 6-17. ESD Tabs & Shield Positioning
7. Perform the ESD Flex Circuit Continuity test as follows:
a. Set the multimeter to
“Continuity” testing mode and verify the continuity of the
ESD Flex Circuit by probing the negative (-) battery contact point and the conduc tive coating on the outer surfaces of the ESD Flex Circuit. Pay particular attention to
the conductive tabs.
b. If continuity is not found, reposition or replace the ESD Flex Circuit as needed.
07-19-A8-092 Ipump Pain Management System Service Manual 6-17
Page 72

6 - Disassembly & Reassembly
Installing the Display ESD Shield
1. Lay the Front Case with Keypad face down.
2. If present, peel the frosted plastic sheet from the clear Display ESD Shield
(p/n B069610005).
NOTE: Ensure there is no dirt, dust, or lint on the Display ESD Shield.
3. Refer to Figure 6-17. Place the Display ESD Shield into the recess around the display
opening.
Installing the LCD Module
1. If present, remove the protective film from the LCD Module Display
(p/n B069494000).
2. Refer to Figure 6-18. Set the LCD Module in the Front Case recess over the ESD
Shield as shown.
3. Attach the LCD Module to the Front Case with three hex standoffs using a nut driver
with an outer diameter less than 0.26” (TE1193).
4. Install the mounting screw and lock washer.
5. Torque the hex standoffs and the mounting screw to 22 – 28 in-oz.
6. Route the AC connector wires as shown in Figure 6-18. The ferrite must be positioned
under the LCD Module.
Figure 6-18. LCD Module Installation
7. Inspect the Front Case to ensure:
a. The LCD Module Assembly is secured by 3 standoffs and one
screw.
6-18 Ipump Pain Management System Service Manual 07-19-A8-092
Page 73

6 - Disassembly & Reassembly
MPU PCBA Handling Instructions
When the MPU PCBA is removed ( either partially or fully) during this procedure, the
assembly must be handled as indicated in Figure 6-19.
Figure 6-19. MPU PCBA Handling Guideline
Installing the MPU PCBA
Prior to installing the MPU PCBA, use a 3X eye piece (or other equivalent magnifying
system) to perform a visual inspection of connector J14. For proper operation of the
Mechanism Assembly, sensors, and switches, ensure there is no damage, foreign material,
or corrosion in or around the connector. If any signs of these contaminants are present,
clean the connector or replace the MPU PCBA.
Also, refer to Chapter 7,
Check. If the battery does not pass the tests, replace the Backup Battery before installing
the MPU PCBA into the pump.
07-19-A8-092 Ipump Pain Management System Service Manual 6-19
“Tests & Calibration”, and perform the 3V Backup Battery
Page 74

6 - Disassembly & Reassembly
1. Refer to Figure 6-20. Ensure that the flex circuit between the Secondary PCB and the
Connector PCB has an
2. Refer to Figure 6-20. Ensure that the flex circuit between the Primary PCB and the
Secondary PCB has a
Figure 6-20. MPU PCBA Flex Circuit Bends
3. Refer to Figure 6-21. Plug the 2-pin connector from the Reed Switch on the Rear Case
Assembly into J1 on the bottom of the Primary P CB as shown.
NOTE: The orientation of the red lead is next to the outside edge of the Primary PCB.
“S-Shaped” bend with the bend radii approximately equal.
“hump-shaped” bend.
Figure 6-21. Reed Switch Connection
4. Refer to Figure 6-22. Lay the Rear Case Assembly on its back and place the Primary
PCB in place over the shorter mounting bosses.
5. If necessary, remove the J11 jumper to access the screw mounting hole on the PCB.
6. Secure the Primary PCB onto the Rear Ca se using three 2-56 X 1/4" Pan Head Screws
(p/n 5101101) and three #2 Lock Washers (p/n 5110049) as shown in Figure 6-22.
Torque screws to 22 - 28 in-oz.
6-20 Ipump Pain Management System Service Manual 07-19-A8-092
Page 75

7. Re-install the J11 jumper.
6 - Disassembly & Reassembly
Figure 6-22. Installed Primary PCB
8. Inspect the Front Case to ensure:
a. The 2-pin connector from the Reed Switch is fully inserted onto
J1 underneath the Primary PCB.
9. In the Front Case, carefully move the AC connector cable out of the way, then gently
place the Connector PCB into position as shown in Figure 6-24.
NOTE: The AC connector wires should come up around the edge of the board near the
flex cable.
10. Ensure that the Printer Connector and the PCA Connector align with their respective
port holes on the Front Case as shown in Figure 6-23.
Figure 6-23. Connector Alignment
07-19-A8-092 Ipump Pain Management System Service Manual 6-21
Page 76

6 - Disassembly & Reassembly
11. Secure the Connector PCB onto the Front Case using four 2-56 X 1/4" Pan Head
Screws and four #2 Lock Washers as shown in Figure 6-24. Torque screws to
22 - 28 in-oz.
Figure 6-24. Installed Secondary & Connector PCBs
12. Refer to Figure 6-24. Plug the 2-pin AC connector into J4 on the Connector PCB with
the Black wire toward the outside of the Pump.
13. If not already attached, install the Positive Battery Terminal onto the Front Case.
14. T o position and secure the Secondary PCB, move the battery contact wire carefully out
of the way.
15. While lining up the Secondary PCB screw holes with the stand off holes, carefully
press the 16-pin connector, J23, on the bottom of the Secondary PCB down onto the
mating connector on the LCD Module. Ensure that the two LEDs align properly with
their respective holes in the Front Case.
16. Secure the Secondary PCB to the Front Case using three 2-56 X 1/4" Pan Head Screws
and three #2 Lock Washers. Torque screws to 22 - 28 in-oz.
17. Plug the Keypad Cable into J8/J9 on the Secondary PCB.
18. Gently tuck the excess slack of the AC Connector wires between the Secondary PCB
and the LCD Module.
19. Plug the 2-pin Battery Contact Connector into J10 on the Secondary PCB with the
black wire toward the outside of the pump.
20. Position the Ferrite so that it ends up between the Secondary PCB and the Connector
PCB and gently tuck the Battery Contact wires under the Secondary PCB.
21. Inspect the Front Case to ensure:
a. The 16 pin connector, J23 on the bottom of the Secondary PCB is
properly connected to the mating connector on the LCD Module.
b. The 2-pin AC Connector is fully inserted onto J4 on the Connector
PCB with the BLACK WIRE toward the outside of the pump.
6-22 Ipump Pain Management System Service Manual 07-19-A8-092
Page 77

6 - Disassembly & Reassembly
Installing the Battery Wall & DDMM PCBA
1. Refer to Figure 6-25. Place the Battery Wall (p/n B069120008RP) on top of the
Battery Compartment, ensuring it is positioned under the ESD Flex Circuit Tab.
2. Position the DDMM PCBA (p/n B069130010) on top of the Battery W a ll. Ensure that
the PCBA is also positioned under the ESD Flex Circuit Tab. Carefully plug the 6-pin
connector of the DDMM PCBA down onto J6 of the Secondary PCB.
Figure 6-25. DDMM PCBA Installation
3. Solder the end of the wire that runs through the Ferrite Bead on the Secondary PCB to
the ground wire pad on the DDMM PCBA. See required wire routing in Figure 6-26.
Dress the wire under the DDMM PCBA if the wire length is too long.
Figure 6-26. Ground Wire Soldering
07-19-A8-092 Ipump Pain Management System Service Manual 6-23
Page 78

6 - Disassembly & Reassembly
Installing the DDMM PCBA Hold-down Foam
1. Refer to Figure 6-27. If not currently in place, attach two pieces of Hold-down Foam
(p/n B069090000), one on top of the other, to the Rear Case as shown.
Figure 6-27. Hold-down Foam Installation
Installing the Mechanism Assembly
1. Refer to Figure 6-28. Slide the Mechanism Assembly (p/n B069120016RP) down into
position on the Front Case, being careful not to pinch any of the existing wires. Ensure
both the Motor Cable and the Flex Cable are not trapped under the Mechanism Assembly. The ribs and bosses of the Front Case must be engaged into their respective slots
on the ends of the Mechanism Assembly.
Figure 6-28. Installing the Mechanism Assembly
2. To ensure proper operation of the Mechanism Assembly, sensors, and switches, perform a visual inspection using a 3X eye piece or other equivalent magnifying system
6-24 Ipump Pain Management System Service Manual 07-19-A8-092
Page 79

6 - Disassembly & Reassembly
to check the Flex Cable connector to ensure that there is no damage, dirt, and/or bend
in the connector and that there is no foreign material or corrosion on the connector.
3. Refer to Figure 6-28. Grasp the edges of the 15-pin Flex Cable connector from the
mechanism assembly and insert it into J14 on the Secondary PCB. DO NOT press on
the Flex Cable itself.
4. Refer to Figure 6-28. Apply Nyogel 760G onto the pins of J2 connector on the DDMM
PCBA. Plug the six-pin motor connector from the Mechanism Assembly into J2 on the
DDMM PCBA (note pin 1 orientation). Ensure the Motor Connector is fully seated
into J2. Wipe off excess Nyogel 760G using a cotton swab or cloth.
5. Refer to Figure 6-28. Apply Loctite 425 to the threads of a 2-56 x 3/8" Screw (p/n
5101103). Use this screw to secure the Retainer Bracket, ESD Flex Circuit, DDMM
PCBA, and Battery W all to the pump housing. The ring of the ESD Flex Circuit should
be positioned between the DDMM PCBA and the Retainer Bracket. Ensure the
DDMM PCBA connector is properly seated on J6 of the Secondary PCB, then tighten
the screw with a torque of 22-28 in-oz.
6. Inspect the Front Case to ensure:
a. J1 of the DDMM Board is seated properly onto J6 of the
Secondary PCB.
Installing the Battery Door
1. Place the Battery Door (p/n B069620008) into its position on the Front Case as shown
in Figure 6-29, with the pin of the door in the slot of the Front Case.
Figure 6-29. Battery Door Installation
Pump Calibration
At this point in the assembly process, it is a requirement that the Calibration Procedure in
Chapter 7 be performed. The Calibration Procedure must be performed whenever the
Mechanism Assembly and/or the MPU PCBA is removed or replaced. When the Pump
Calibration procedure is compl ete, return t o this point in t he m anual and continue w ith the
remaining checks and assembly procedures.
07-19-A8-092 Ipump Pain Management System Service Manual 6-25
Page 80

6 - Disassembly & Reassembly
Internal Inspection
Prior to closing the Pump Case, it is recommended that a qualified technician complete
this internal inspection. Inspect the pump to ensure the following:
INTERNAL INSPECTION
FRONT CASE ASSEMBLY
The Secondary PCB is secured by 3 screws. (Only 2 screws are visible when all PCBAs are
a.
installed.)
b. The Keypad Cable is connected to J8 and J9 on the Secondary PCB.
The 2-pin AC Connector is fully inserted onto J4 on the Connector PCB with the BLACK WIRE
c.
toward the outside of the pump.
The Serial Number Label inside the Front Case behind the ESD Flex Circuit is present and
d.
matches the Serial Number on the Rear Case.
e. There is no breakage on the Front Case internal or external surfaces.
WIRE HARNESS & FLEX CIRCUIT
a. All Wire Harnesses are routed properly and not pinched.
b. The Flex Circuit between the Secondary PCB and the Connector PCB has an “S-shape” bend.
MECHANISM ASSEMBLY
There are four pieces of filament tape - two on top of the Mechanism Assembly, one on the
a.
back of the mechanism, and one on the motor.
b. The Motor Connector Harness is not pinched.
c. The Serial Number Label of the Mechanism Assembly is present on the Motor.
The Mechanism Assembly Flex Cable connector is fully inserted into J14 of the Primary PCB.
d.
(Do not push on the Flex Cable!)
BATTERY WALL AND ESD SHIELD
a. The DDMM PCBA and Retainer Bracket are secured to the battery wall with a screw.
b. The Motor Connector is connected properly to J2 on the DDMM PCBA.
REAR CASE ASSEMBLY
a. The Primary PCB is secured by 3 screws.
b. Integrated Circuit U26 is fully inserted in its socket.
c. The jumper is properly positioned on Connector J11.
d. The 3V Backup Battery is sitting up straight.
e. The S/N Label and OVERLAY are present on the outside of the Rear Case.
IMPORTANT ACTIONS BEFORE AND AFTER CLOSING THE FRONT AND REAR
HOUSING ASSEMBLIES.
a. There is no loose hardware.
Figure 6-30. Completed Internal Pump Assembly
6-26 Ipump Pain Management System Service Manual 07-19-A8-092
Page 81

6 - Disassembly & Reassembly
Closing the Case
1. Refer to Figure 6-31. Check that the Motor Cable is completely clear of the Battery
Wall hole located just above and to the left of the Mechanism Assembly.
Figure 6-31. Mounting Hole Location
2. Refer to Figure 6-32. Carefully fold the Rear Case Assembly over the Front Case
Assembly as shown.
NOTE: For proper assembly, the 3V Backup Battery must end up between the Keypad
Cable and the Front Case.
3. Being careful not to pinch any wires or Flex Circuits, carefully press the case together.
Figure 6-32. Aligning the Front and Rear Cases
4. Move the Pump Case back and forth to check for any loose hardware and remove any
found.
5. Secure the Front and Rear Cases together using six 2-56 x 3/8” Pan Head Screws (p/n
5101103) and six #2 Flat Washers (p/n 5143011). Torque screws to 22 - 28 in-oz.
6. Inspect the closed Pump Case to ensure:
a. The Front and Rear Cases fit together evenly and there
is no loose hardware.
b. The Battery Door opens and closes properly.
07-19-A8-092 Ipump Pain Management System Service Manual 6-27
Page 82

6 - Disassembly & Reassembly
Pump Functional Tests
The Pump Functional Test procedures are located in Chapter 5. These tests must be
performed whenever the Mechanism Assembly and/or the MPU PCBA is removed or
replaced. Only after passing these functional tests, can the pump be placed back into service.
Optional Assembly Procedures
Installing the Bag Cover Assembly
1. Refer to Figure 6-33. Align the metal Hinge against the recess on the Rear Case and
place the Hinge Cover (p/n 4909620001) over the Hinge, oriented so that the notched
area is aligned so the label on the back of the pump can be seen.
2. Assemble the 250E Bag Cover (p/n 2L3217) to the pump using three 2-56 x 1/4" Pan
Head Screws (p/n 5101101) as shown in Figure 6-33. Torque screws to 22 - 28 in-oz.
Figure 6-33. Bag Cover Assembly
NOTE: Service repairs/upgrades may require the installation of different bag covers.
2L3217 250 mL Extended Bag Cover
2L3218 100 mL Bag Cover
2L3220 250 mL Bag Cover
2L3221 500 mL Bag Cover
2L3261 250 mL Extended Bag Cover, Amber
NOTE: A hinge cover is not required on a 500 ML Bag Cover.
3. Inspect the pump to ensure:
a. The Bag Cover’s internal and external surfaces are not cracked or
broken.
6-28 Ipump Pain Management System Service Manual 07-19-A8-092
Page 83

7 - Internal Tests & Pump Calibration
In this section Page
Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-1
3V Backup Battery Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-2
Calibration Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-4
Equipment Required. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-4
Initial Setup. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-4
Downstream Occlusion Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-5
Upstream Occlusion Calibration. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-6
Air Sensor Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-8
Downstream Occlusion Calibration Pressure Test . . . . . . . . . . . . . . . . . 7-9
Calibration Data Sheet . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-11
Overview
Baxter Healthcare Corporation recommends that these test and calibration procedures be
performed on an annual basis and when any electronic or mechanical parts have been
removed and/or replaced in the pump.
To perform these procedures, first refer to the disassembly procedures in Chapter 6 and
disassemble the pump. If your facility is not equipped to perform the procedures in this
chapter of t he man ua l, ca ll a B axt er r ep res en ta tive for the procedure required to return the
pump for service or repair.
It is recommended that the results of the tests and calibration be recorded on a copy of the
Calibration Data Sheet provided at the end of this chapter. A copy of the Calibration Data
Sheet should be kept as a preventive maintenance record for each pump.
Record the pump Product Code and Serial Number on the Calibration Data Sheet. If the
Mechanism Assembly or MPU PCBA are replaced, enter the serial numbers of the new
parts.
07-19-A8-092 Ipump Pain Management System Service Manual 7-1
Page 84

7 - Internal Tests & Pump Calibration
3V Backup Battery Test
Battery Load Test
This test MUST be performed before the NO LOAD test.
1. Create a
Figure 7-1. Connect the Load Circuit to a Digital Multi-Meter (DMM), and set the
meter to an “ohms” reading. Without connecting to the battery, the resistance value
must read between 6.46 K and 7.14 K. If the value is outside this range, the Load Circuit must be reworked or replaced.
2. Set the DMM to an appropriate voltage range (>5V DC).
3. With the Load Circuit connected to the DMM, touch the DMM test leads
to the appropriate (+ and -) terminals on the battery and hold for 4 seconds.
4. Note the 4-second voltage value, remove the test leads, and then record the voltage
reading with a 3 decimal place accuracy (#.###), as the “V Load” reading
on the Calibration Data Sheet.
“Load Circuit” using a 6.8K Ohm resistor with the parameters shown in
Figure 7-1. Battery Load Circuit
Figure 7-2. 3V Backup Battery
7-2 Ipump Pain Management System Service Manual 07-19-A8-092
Page 85

7 - Internal Tests & Pump Calibration
Battery No Load Test
NOTE: For this test, do not use the Load Circuit created for the previous procedure.
1. Using a DMM set to an appropriate voltage range (>5V DC), touch the DMM test
leads to the appropriate (+ and -) terminals on the Battery.
2. Record the voltage reading with a 3 decimal place accuracy (#.###), as the “V No
Load” reading on the Calibration Data Sheet.
3. Calculate the value of “V No Load - V Load” and record this voltage on the Calibra-
tion Data Sheet.
4. If the “V No Load” voltage is greater than or equal to 3.000 VDC, record “PASS” on
the Calibration Data Sheet and proceed to the next step.
If the “V No Load” voltage is less than 3.000 VDC, record “FAIL” and replace the 3V
battery using the appropriate disassembly and reassembly instructions found in Chapter 6 of this manual.
NOTE: After replacement, test the new Backup Battery, and use a new Calibration Data
Sheet.
5. If the calculated “V No Load - V Load” value is less than or equal to 0.050 volts,
record “PASS” on the Calibration Data Sheet.
If the calculated “V No Load - V Load” value is greater than 0.050 volts, record
“FAIL” and replace the 3V Battery using the appropriate disassembly and reassembly
instructions found in Chapter 6 of this manual.
NOTE: After replacement, retest the new Backup Battery, and use a new Calibration Data
Sheet.
07-19-A8-092 Ipump Pain Management System Service Manual 7-3
Page 86

7 - Internal Tests & Pump Calibration
Calibration Procedure
This procedure must be performed on a pump whenever the MPU Circuit Board or the
Mechanism Assembly is replaced. If a failure is encountered during any portion of this
procedure, the MPU Circuit Board and/or the Mechanism Assembly should be repaired or
replaced. Pumps that do not meet all the requirements of this calibration procedure MUST
NOT BE USED.
NOTE: It is recommended that this calibration procedure be performed in the sequence
provided. All potentiometers on the Secondary PCB will be adjusted.
Equipment Required
• Two Digital Voltmeters [DVM] with a minimum of 3 ½ digit auto-ranging and
1 megohm (or greater) input impedance on the DC volts range
• One set of mini-grabber clip test leads for each DVM
• One primed administration set
• One [empty tube] administration set
• One small electronic adjustment tool
• One AC Adapter (optional)
• Red GLPT (insulating varnish)
• Five small mini-clip jumpers
•One
• One low point upstream calibration gauge (p/n B069290001)
• One high point upstream calibration gauge (p/n B069290000)
Ipump device calibration gauge (p/n AS4AL4002)
Initial Setup
1. Install a fresh 9-volt battery in the pump. (As an option, the AC Adapter may be used.)
2. If necessary, follow the procedures in Chapter 6 of this manual to open the pump case
and access the component side of the Secondary PCB.
3. Connect DVM1- (common) to DVM2- (common) with a banana lead. Use the
following test leads from the DVMs when required: DVM1+, DVM1-, & DVM2+
4. Turn on both DVMs and set them to read DC volts.
7-4 Ipump Pain Management System Service Manual 07-19-A8-092
Page 87

7 - Internal Tests & Pump Calibration
Downstream Occlusion Calibration
IMPORTANT: Do not turn the pump on!
1. Refer to Figure 7-3. Open the Tubing Door, insert the Calibration Gauge
(p/n AS3AL4002) into the Tubing Channel, then close the Tubing Door.
Figure 7-3. Calibration Gauge
2. Refer to Figure 7-4. Connect one mini-clip jumper from the black test post at J17 to
the orange test post at J20.
3. Connect the DVM1+ test lead to the red test post at J15, and connect the DVM1- test
lead to the black test post at J17.
Figure 7-4. Downstream Occlusion Calibration
4. Adjust potentiometer P1 for an initial DVM1 reading of
1.50 to 2.50 VDC (target
1.90 to 2.10 VDC).
07-19-A8-092 Ipump Pain Management System Service Manual 7-5
Page 88

7 - Internal Tests & Pump Calibration
5. Open the Tubing Door and remove the Calibration Gauge, being careful not to
damage the thin gauge spring.
6. With the Tubing Door open, record the DVM1 voltage with a three decimal place
accuracy (#.###) as the "Downstream Open Without Set Value" on the Calibration
Data Sheet.
7. Refer to Figure 7-5. Ensure the two brown pumping fingers adjacent to the upstream
and downstream actuators are retracted fully into the mech anism. This may be accomplished by pressing on the other fingers until the brown fingers are retracted.
8. Load an empty tubing set and close the Tubing Door.
9. With the Tubing Door closed on the set, record the DVM1 voltage with a three decimal place accuracy (#.###) as the "Downstream Closed on Set Value" on the Calibration Data Sheet.
10. Compare the "Downstream Open Without Set Value" and the "Downstream Closed on
Set Value" readings. The difference between the two must be greater than
(
0.116 VDC). Record the results on the Calibration Data Sheet.
NOTE: If the difference is less than 0.116 VDC, the Mechanism Assembly must be
repaired or replaced.
116 mVDC
11. Open the Tubing Door and remove the set.
Figure 7-5. Brown Pumping Fingers
Upstream Occlusion Calibration
IMPORTANT: Do not turn the pump on!
1. Refer to Figure 7-5. Ensure the two brown pumping fingers adjacent to the upstream
and downstream actuators are retracted fully into the mech anism. This may be accomplished by pressing on the other fingers until the brown fingers are retracted.
2. Insert the
High Point Upstream Calibration Gauge (p/n B069290000) into the
Tubing Channel, then close the Tubing Door.
7-6 Ipump Pain Management System Service Manual 07-19-A8-092
Page 89

7 - Internal Tests & Pump Calibration
Figure 7-6. Upstream Occlusion Calibration
3. Move the DVM1+ test lead to the white test post at J16, and leave the DVM1- test lead
on the black test post at J17.
4. Adjust potentiometer P2 for a DVM1 reading of
2.20 to 2.25 VDC. Record the results
on the Calibration Data Sheet.
NOTE: Always adjust potentiometer reading UP to the correct voltage. If the voltage read-
ing is above the target voltage, adjust the potentiometer to reduce the value to
below the target voltage then slowly adjust up to the target voltage.
5. Remove the High Point Upstream Calibration Gauge and replace it with the Low
Point Upstream Calibration Gauge (p/n B069290001), then close the Tubing Door.
6. Adjust potentiometer P4 for a DVM1 reading of
1.10 to 1.15 VDC. Record the results
on the Calibration Data Sheet.
NOTE: Always adjust potentiometer reading UP to the correct voltage. If the voltage read-
ing is above the target voltage, reduce the value to below the target voltage then
slowly adjust up to the target voltage.
7. Repeat this procedure starting at step 2 until both target voltages can be attained with-
out adjustments. Record the final readings on the Calibration Data Sheet.
8. Open the Tubing Door and remove the
Low Point Upstream Calibration Gauge
from the Tubing Channel.
9. With the Tubing Door open, record the DVM1 voltage with a three decimal place
accuracy (#.###) as the "Upstream Open Without Set Value" on the Calibration Data
Sheet.
10. Ensure that the two brown pumping fingers adjacent to the upstream and downstream
actuators are retracted fully into the mechanism.
11. Load an empty tubing set and close the Tubing Door.
07-19-A8-092 Ipump Pain Management System Service Manual 7-7
Page 90

7 - Internal Tests & Pump Calibration
12. With the Tubing Door closed on the set, record the DVM1 voltage with a three decimal place accuracy (#.###) as the "Upstream Closed on Set Value" on the Calibration
Data Sheet.
13. Compare the "Upstream Open Without Set Value" and the "Upstream Closed on Set
Value" readings. The difference between the two must be greater than
(
0.150 VDC). Record the results on the Calibration Data Sheet.
NOTE: If the difference is less than 0.150 VDC, the Mechanism Assembly must be
repaired or replaced.
14. Open the Tubing Door and remove the set.
15. Move the DVM1+ test lead to J15. With no calibration gauge installed, the voltage on
DVM1 must read less than
NOTE: If the reading is not less than 1.7 VDC, the Mechanism Assembly must be
repaired or replaced.
1.7 VDC. Record the results on the Ca li bra tion Data S hee t.
16. Remove the test leads from the pump.
Air Sensor Calibration
150 mVDC
IMPORTANT: Do not turn the pump on!
1. Refer to Figure 7-7. Connect one mini-clip jumper from the black test post at J17 to
the orange test post at J21.
Figure 7-7. Air Sensor Calibration
NOTE: For this process, the two DVMs will be used as follows:
• DVM1 = Air Sensor calibration value
• DVM2 = Air Sensor output voltage
2. Refer to Figure 7-7. Connect the DVM test leads as follows:
• DVM1- (common) to DVM2- (common)
• DVM1 + Test Post J19 (Red)
7-8 Ipump Pain Management System Service Manual 07-19-A8-092
Page 91

7 - Internal Tests & Pump Calibration
• DVM1 - Test Post J17 (Black)
• DVM2 + Test Post J24 (Yellow)
NOTE: Prior to performing Air Sensor calibration, make sure the Mechanism Assembly
tubing channel, and the tubing segment are clean and dry.
3. Turn on both DVMs and set them to read DC volts.
4. Ensure a 9-volt battery is installed in the pump.
5. Insert the air filled [empt y] tubi ng set into the pump, then ful ly cl ose t he tubi ng cove r.
IMPORTANT: Do not turn the pump on!
6. With t he small adjustme nt tool, rotate potentiometer P3 c ounterclockwise until DVM1
indicates a voltage value that is less than
0.120 VDC. If DVM1 is already less than
0.120 VDC, no counterclockwise adjustment of P3 is required.
7. With the small adjustment tool, slowly turn potentiometer P3 clockwise. DVM2 will
jump from a low level (
DVM2 changes to a high level (
<0.1 VDC) to a high level (>4.9 VDC). When the reading on
>4.9 VDC), continue to slowly rotate potentiometer
P3 clockwise. The reading on DVM2 will suddenly drop back to a low level
(
<0.1 VDC) reading.
8. At this point, record the DVM1 voltage reading on the Calibration Da ta Sheet.
9. Verify that DVM2 reading is a low level (
<0.1 VDC) and is stable (not varying), then
mark PASS or FAIL on the Calibration Data Sheet. (It may be necessary to slowly
readjust potentiometer P3 until a stable reading is observed.)
10. Add
0.120 VDC to the recorded DVM1 voltage and record the calculated voltage level
on the Calibration Data Sheet.
11. Using the small adjustment tool, adjust potentiometer P3 so that the DVM1 reading is
within
± 0.010 volts of the calculated voltage level. Record the DVM1 reading on the
Calibration Data Sheet.
12. At this time, with the empty tubing set installed, DVM2 must indicate a voltage level
<0.1 VDC. Record the reading on the Calibration Data Sheet.
13. Open the Tubing Door, remove the empty tubing segment, and insert a primed tubing
set. Ensure that the set is properly primed (i.e., no air pockets or bubbles in the tubing
channel area), then close the Tubing Door.
14. With a primed set installed, DVM2 must indicate a voltage level
>4.9 VDC. Record
the reading on the Calibration Data Sheet.
15. Remove the test leads and tubing set from the pump.
Downstream Occlusion Calibration Pressure Test
1. Close-up the pump and install a fluid-filled tubing set into the pump.
2. Connect the set to a pressure gauge with a minimum range of
0 – 30 psi.
3. Program the Pump as follows:
• Mode = PCA
• Units = mL
07-19-A8-092 Ipump Pain Management System Service Manual 7-9
Page 92

7 - Internal Tests & Pump Calibration
• Set fluid volume = 0100 mL
4. Program the Pump as Follows:
•PCA DOSE = 1.0 mL
• DELAY = 3 minutes
• 1 HR LIMIT = 10.0 mL
• BOLUS = 05.0 mL
5. Start the bolus infusion and let the pump run until it goes into a downstream occlusion
alarm.
6. Observe the Occlusion Pressure Value and record the reading on the Calibration Data
Sheet.
• If the reading is between
15 and 29 psi, record the value on the Calibration Data
Sheet.
• If the reading is outside these limits, repeat the Downstream Occlusion
Calibration and this pressure test.
- If the pressure was high, adjust the voltage higher.
- If the pressure was low, adjust the voltage lower.
Continue this loop until the downstream occlusion value is
22 ± 7 psi.
7. Disconnect the test apparatus from the pump.
8. Place a small amount of GLPT (insulating varnish) on the adjustment screws of potentiometers P1, P2, P3, and P4 to secure them in place.
Pump Calibration is now complete. Ensure the Calibration Data Sheet is
properly reviewed and signed, then refer to Chapter 6 and perform the
required internal inspection steps as you reassemble the pump.
7-10 Ipump Pain Management System Service Manual 07-19-A8-092
Page 93

7 - Internal Tests & Pump Calibration
Calibration Data Sheet
Record the results of the Ipump device calibration here. This sheet may be reproduced.
Product Code: 2L3107 [ ] 2L3107R [ ] 2L3107K [ ] PUMP SERIAL NUMBER: ________________________
If the Mechanism Assembly or the MPU PCBA is replaced, enter the Serial Numbers of these replacement parts.
Mechanism Assembly Serial Number: ___________________________________
MPU PCBA Serial Number: ____________________________________
PROCEDURE STEPS READING N/A PASS FAIL
3V Backup Battery Test
LOAD TEST,
NO LOAD TEST,
Calculated
Downstream Open Without Set Value
Downstream Closed On Set Value
Difference between Open and Closed values (
“V LOAD” reading (VDC)
“V NO LOAD” reading (VDC)
“V NO LOAD – V LOAD” value (VDC)
Downstream Occlusion Calibration
>0.116 VDC
[ ]
[ ] [ ] [ ]
[ ] [ ] [ ]
)
Upstream Occlusion Calibration
2.20 ≤ P2 voltage ≤ 2.25 VDC (Final Reading)
1.10 ≤ P4 voltage ≤ 1.15 VDC (Final Reading)
Voltages meet above conditions w/o adjustment
Upstream Open Without Set Value
Upstream Closed On Set Value
Difference between Open and Closed values (
Voltage <1.7 VDC
Record DVM1 reading
DVM2 readin g < 0.1 (and stable)
Calculated Sum of DVM1 + 0.120 VDC
DVM1 readin g = ± 0.010 VDC
DVM2 readin g < 0.1 VDC
DVM2 readin g > 4.9 VDC
Downstream Occlusion Calibration Pressure Test
The Pressure Reading is 15 psi to 29 psi
>0.150 VDC
Air Sensor Calibration
[ ] [ ] [ ]
[ ] [ ] [ ]
N/A
)
N/A
N/A
N/A
[ ] [ ] [ ]
[ ] [ ] [ ]
[ ] [ ] [ ]
[ ] [ ] [ ]
[ ]
[ ] [ ] [ ]
[ ] [ ] [ ]
[ ] [ ] [ ]
[ ] [ ] [ ]
Signature: ______________________________________________________DATE: ____________
Reviewed by: ____________________________________________________DATE: ____________
07-19-A8-092 Ipump Pain Management System Service Manual 7-11
Page 94

7 - Internal Tests & Pump Calibration
7-12 Ipump Pain Management System Service Manual 07-19-A8-092
Page 95

8 - Electronic Assembly Drawings
In this section Page
Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-1
Interconnecting Wiring Diagram . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-1
Keypad Cable & Motor Connectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-2
Mechanism Assembly Flex-Circuit Connector. . . . . . . . . . . . . . . . . . . . . . . 8-2
MPU PCBA Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-3
Direction & Drive Motor Module PCBA (DDMM) Daughter Board . . . . . 8-4
Overview
This chapter contains the interconnecting wiring diagram, the electrical connector pinouts,
and the circuit board assembly drawings for the pump.
Interconnecting Wiring Diagram
Keypad
J8 and J9
(see below)
Pumping
Mechanism
(see below)
Battery Contacts
J10
10 Pi n
Direction and
Drive Motor
Module
6 Pin 6 Pin
J6
J14
(DDMM)
J2
J1
15 Pin
LCD Board
16 Pi n at J 23
(B ackli ght)
2 Pin 16 Pin
MPU
Board
2 Pin
Reed Switch
J1
(on rear case)
2 Pi n
2 Pi n
5 Pi n
AC
Connector
J4
2 Pi n
AC Adaptor
Accessory
PCA Cable
Accessory
J3
Printer Adaptor
Accessory
J5
Figure 8-1. interconnecting Wiring Diagram
07-19-A8-092 Ipump Pain Management System Service Manual 8-1
Page 96

8 - Electronic Assembly Drawings
Keypad Cable & Motor Connectors
Figure 8-2. Keypad Cable & Motor Connectors Pinouts
Mechanism Assembly Flex Circuit Connector
Pin No. Feature
1XMIT LO
2XMIT HI
3 NOT CONNECTED
4 NOT CONNECTED
5RCVR LO
6 RCVR HI
7DOWNSTREAM -
8DOWNSTREAM +
9 UPSTREAM +
10 UPSTREAM -
11 OCCLUSION V+
12 OCCLUSION V-
13 PUMP COVER
14 COMMON
15 REED SWITCH ENC2
Table 8-1. Mechanism Assembly Flex-Circuit Connector Pinouts
8-2 Ipump Pain Management System Service Manual 07-19-A8-092
Page 97

MPU PCBA Assembly
8 - Electronic Assembly Drawings
Primary PCB
Secondary PCB
Connector PCB
Figure 8-3. MPU PCBA Assembly
07-19-A8-092 Ipump Pain Management System Service Manual 8-3
Page 98

8 - Electronic Assembly Drawings
Direction & Drive Motor Module PCBA (DDMM) Daughter Board
Figure 8-4. DIRECTION AND DRIVE MOTOR MODULE PCBA
(Daughter Board)
8-4 Ipump Pain Management System Service Manual 07-19-A8-092
Page 99

In this section Page
Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-1
Assembly Parts Lists. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-2
Alphabetical Parts Lists . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-7
Numerical Parts Lists . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-9
Overview
This chapter contains a listing of the repair parts available for the pump. Three listings are
provided.
9 - Repair Parts
Bag Cover Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-2
Rear Case Assembly. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-3
Pump Mechanism & Battery Compartment Assemblies . . . . . . . . . . . . 9-4
MPU Board Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-5
LCD Circuit Board & Front Case Assemblies . . . . . . . . . . . . . . . . . . . . 9-6
• Assembly Parts List
• Alphabetical Parts List
• Numerical Parts List
The Alphabetical and Numerical lists are cross-referenced to the assembly-specific parts
list by both figure and index number.
07-19-A8-092 Ipump Pain Management System Service Manual 9-1
Page 100

9 - Repair Parts
Assembly Parts List
Bag Cover Assembly
Figure
ID
500 mL Bag Cover Assembly
250 mL Extended Bag Cover Assembly
A1
A2 #6 x 5/16" Self-Tap Screw 5101180 2
A3 2-56 x 1/4" Pan Head Screw 5101101 3
A4 Mounting Plate 6465644 1
A5 Hinge Cover 4909620001 1
250 mL Extended Bag Cover Assembly, Amber
250 mL Bag Cover Assembly
100 mL Bag Cover Assembly
(Each of the above parts include items A2 and A4)
or
or
or
or
Part Description
Baxter Part
Number
2L3221
2L3217
2L3261
2L3220
2L3218
Assembly
Table 9-1. Bag Cover Assembly
Qty per
1
Figure 9-1. Bag Cover Assembly
9-2 Ipump Pain Management System Service Manual 07-19-A8-092
 Loading...
Loading...