Page 1
Conditioning Solution
For Fluoro Silicone Acrylate and Silicone Acrylate Rigid Gas Permeable Contact Lenses
Description: A sterile, aqueous buffered, solution containing a cellulosic viscosifier, cationic
cellulose derivative polymer and polyvinyl alcohol as wetting and cushioning agents;
preserved with chlorhexidine gluconate (0.006%), and edetate disodium (0.05%).
Actions: Boston® Conditioning Solution contains a wetting agent that coats the surface of
your lenses to make them smooth, wet and comfortable between your lenses and your eyes.
Indications: For wetting, disinfecting and soaking (after cleaning and rinsing) of fluoro
silicone acrylate and silicone acrylate rigid gas permeable contact lenses.
Contraindications: Do not use this solution if you are allergic to any ingredient in this
product. Not For Use With Soft (Hydrophilic) Contact Lenses.
Warnings: To avoid contamination, never touch dropper tip of the container with your
hands or to any surface. Keep bottle tightly closed when not in use.
Contact lenses and solutions, if not handled properly, can become contaminated by common
bacteria known to cause ocular infections. Serious damage to the eye and loss of vision may
result from using contaminated solutions or from wearing contaminated or damaged contact
lenses.
PROBLEMS WITH CONTACT LENSES AND LENS CARE PRODUCTS COULD RESULT IN SERIOUS
INJURY TO YOUR EYES. It is essential that you follow your eye care professional’s directions
and all labeling instructions for proper use of your lenses and lens care products.
EYE PROBLEMS, INCLUDING CORNEAL ULCERS CAN DEVELOP RAPIDLY AND LEAD TO LOSS OF
VISION. THEREFORE, IF YOU EXPERIENCE EYE DISCOMFORT, EXCESSIVE TEARING, VISION
CHANGES, OR REDNESS OF THE EYES, IMMEDIATELY REMOVE YOUR LENSES AND PROMPTLY
CONTACT YOUR EYE CARE PRACTITIONER.
Precautions: To ensure proper hygiene and handling of contact lenses and contact lens
solutions, always follow label directions carefully.
• Never reuse this solution.
• Always use fresh solution for soaking and storing lenses.
• Always wash your hands before handling your lenses.
• Store solutions at room temperature. Avoid freezing.
• After inserting your lenses, always thoroughly clean the interior of the
lens case with hot water and air dry after each use.
• Use before expiration date on the container.
• NEVER SOAK LENSES IN ANY CLEANER.
• ALWAYS RINSE LENSES THOROUGHLY WITH FRESH TAP WATER AFTER CLEANING.
• KEEP OUT OF REACH OF CHILDREN.
Adverse Effects: The following problems may occur:
• Eyes stinging, burning, itching (irritation)
• Excessive watering (tearing) of the eyes
• Unusual eye secretions
• Redness of the eyes
• Reduced sharpness of vision (visual quality)
• Blurred vision
• Sensitivity to light (photophobia)
• Dry eyes
If you notice any of the above conditions, immediately remove and examine the lenses. If
the problem stops and the lenses appear to be undamaged, thoroughly clean, rinse and
disinfect the lenses and reinsert. If the problem continues or if a lens appears to be
damaged, DO NOT REINSERT. Contact your eye care professional immediately. If any of the
above problems occur, a serious condition such as infection, corneal ulcer, neovascularization
or iritis may be present. Seek immediate professional identification of the problem and
treatment to avoid serious eye damage.
DIRECTIONS FOR USE
To ensure proper disinfecting, all steps listed below must be followed:
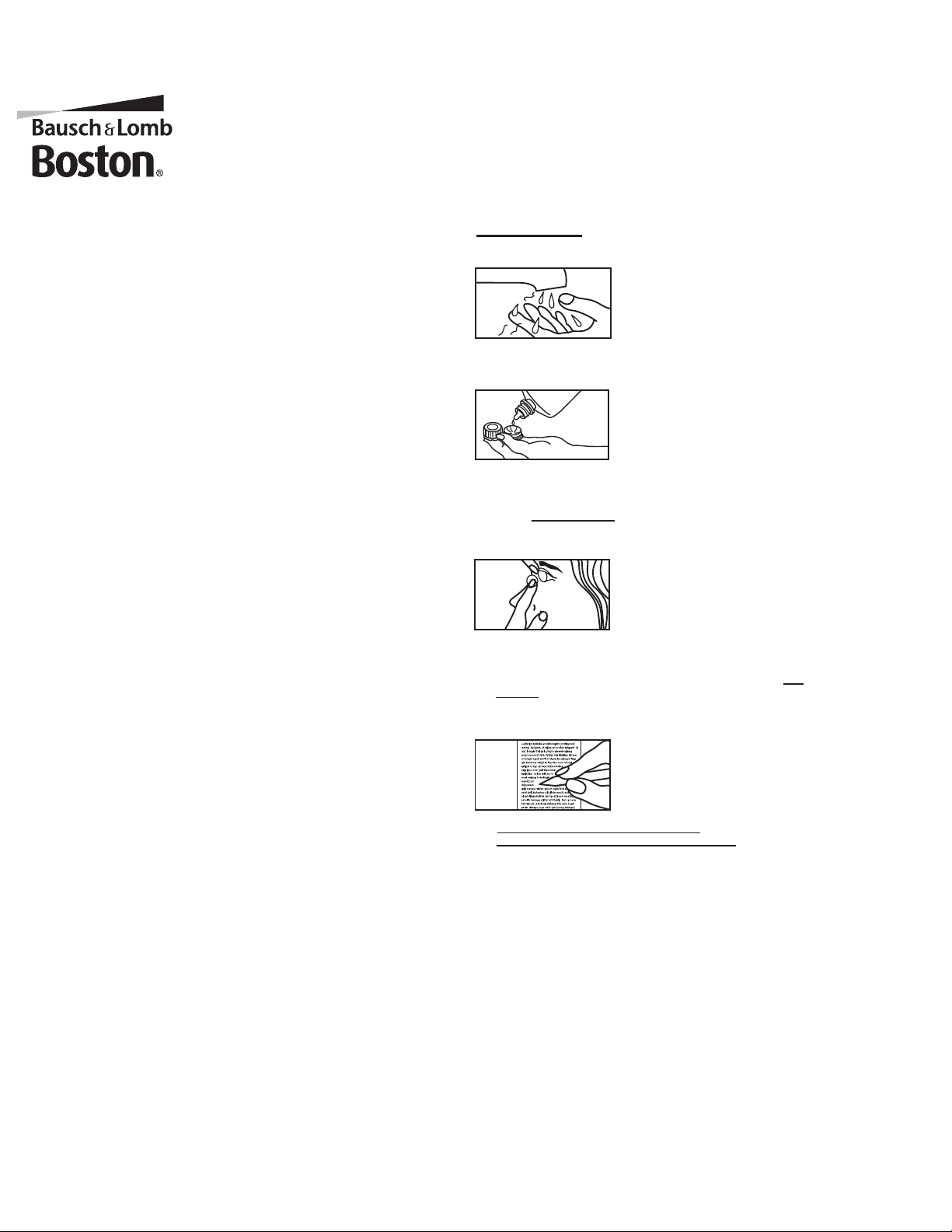
1.
Wash your hands with mild soap. (Caution - pump soaps may contain oil-based
suspension agents.)
2.
After cleaning with Boston® Cleaner and rinsing thoroughly with fresh tap water,
place lenses in empty lens case and fill to top ridges with fresh Boston Conditioning
Solution. Soak lenses for at least four (4) hours (or overnight) before wearing.
Always use fresh solution for soaking and storing lenses.
3.
After removing lenses from the lens case, apply fresh Boston Conditioning Solution
to wet lenses for additional cushioning, if desired, and insert.
4.
Thoroughly clean interior of lens case with hot water and air dry after
each use.
5.
Discard solution 28 days after opening.
Record date opened in space provided on bottle label.
•
Lens cases should be replaced monthly to avoid potential microbial
contamination and ocular irritation.
How Supplied: Boston Conditioning Solution is supplied sterile in 3.5 fluid ounce (105mL) bottles. All
retail Boston® Solutions are packed in unit cartons. Bottles are sealed with an imprinted neckband and
marked with a lot number and expiration date.
Do not use if imprinted neckband on bottle is broken or missing.
®/TM denote trademarks of Bausch & Lomb Incorporated
©Bausch & Lomb Incorporated
Manufactured by: Bausch & Lomb
Rochester, NY 14609. Made in USA.
Questions or Comments:
1-800-333-4730
www.bausch.com
 Loading...
Loading...