Bandelin RK 52, RK 514, RK 510, RK 52 H, RK 512 H User Manual
...
User instructions
36
Ultrasonic baths for aqueous fluids
Valid for:
RK 31, RK 31 H, RK 52, RK 52 H
RK 100, RK 100 H, RK 102 H, RK 103 H, RK 106
RK 156, RK 156 BH
RK 170 H
RK 255, RK 255 H
RK 510, RK 510 H, RK 512 H
RK 514, RK 514 H, RK 514 BH
RK 1028, RK 1028 H, RK 1028 C, RK 1028 CH
RK 1040, RK 1050, RK 1050 CH
1556g GB/2019-02
(
Copyright & limitation of liability
This document may not be reproduced, either in full or in extracts, without prior approval of BANDELIN electronic GmbH & Co. KG, hereinafter referred to as BANDELIN.
The German original is the binding version of this document. Any difference in the translation is not binding and has no legal effect. In case of any discrepancies between the translation and the original of this document, the original version has priority.
BANDELIN accepts no responsibility or liability for damages caused by improper handling or usage contrary to the intended purpose.
The documentation was prepared with great care. Liability for indirect or direct damages arising because of incomplete or erroneous information in this documentation, as well as in its delivery and usage, is excluded.
All images are provided as examples and are not true to size. Decorative elements are not included in the scope of delivery. © 2019
12GmbH & Co. KG, Heinrichstrasse 3–4, Germany, 12207 Berlin, Tel.: +49-30-768 80 - 0, Fax: +49-30-773 46 99, info@bandelin.com
2 / 36 |
1556g GB/2019-02 |
1electronic GmbH & Co. KG |
• Heinrichstraße 3-4 • 12207 Berlin • Deutschland • info@bandelin.com |
General
The device, the accessories and the preparations are to be used in accordance with the operating instructions and/or the product information.
The instructions are part of the scope of delivery and are to be stored in the vicinity of the device for later reference. This also applies if possession of the device is transferred.
Before the device is put into operation, these User Instructions are to be read carefully and completely in order for the user to become familiarised with all functions.
The warnings and safety precautions (chapter 1.5) are always to be followed during use.
The manufacturer will not assume any responsibility for the device's safety or functional ability in the event of improper handling or usage contrary to the intended purpose. In the event of
unauthorised alterations/modifications, both the warranty claim and the CE conformity will expire.
If service is required, please contact the specialist dealer in charge or the manufacturer.
Symbols used:
Symbol |
Significance |
Explanation |
|
|
|
|
|
|
Danger |
Identifies information that could signify a risk to life and limb, |
|
|
especially through electric shock, if not observed. |
||
|
|
||
|
|
|
|
|
|
Identifies information that is to be observed and adhered to |
|
|
|
without fail, to prevent damage to the device and danger to |
|
|
Caution |
the user. |
|
|
|
When device parts are labelled with this symbol, reference |
|
|
|
must be made to the documentation. |
|
|
|
|
|
|
Warning |
Warning |
|
|
|
|
|
|
Important |
Identifies information that is important for execution. |
|
|
|
|
|
|
Note |
Identifies information provided for explanatory purposes. |
|
|
|
|
|
|
Medical note |
Identifies information that is important for medical use. |
|
|
|
|
|
|
Do not grip inside |
For health reasons, touching the oscillating fluid is prohibited. |
|
|
|
|
|
|
Wear ear protectors |
For health reasons, standing for long periods of time in the |
|
|
vicinity of the device without ear protectors is prohibited. |
||
|
|
||
|
|
|
|
|
Operating sequence |
Identifies instructions that are to be followed in the described |
|
instructions |
sequence. |
||
|
|||
|
|
|
1556g GB/2019-02 |
3 / 36 |
1electronic GmbH & Co. KG • Heinrichstraße 3-4 • 12207 Berlin • Deutschland • info@bandelin.com
|
Contents |
|
1 |
Product description ......................................................................................................... |
6 |
1.1 |
Mode of operation .......................................................................................................... |
6 |
1.2 |
Purpose .......................................................................................................................... |
7 |
1.3 |
CE conformity ................................................................................................................. |
7 |
1.4 |
Technical data ................................................................................................................ |
8 |
1.4.1 |
Electromagnetic ambient conditions (EMC) ................................................................... |
9 |
1.5 |
Warnings and safety precautions ................................................................................. |
10 |
2 |
Preparation ................................................................................................................... |
11 |
2.1 |
Scope of delivery .......................................................................................................... |
11 |
2.2 |
Set-up / assembly ......................................................................................................... |
11 |
2.3 |
Start-up ......................................................................................................................... |
11 |
3 |
Operation ...................................................................................................................... |
12 |
3.1 |
Operating elements ...................................................................................................... |
12 |
3.1.1 |
Ultrasound .................................................................................................................... |
12 |
3.1.2 |
Heating ......................................................................................................................... |
13 |
4 |
Use ............................................................................................................................... |
14 |
4.1 |
Instructions for use ....................................................................................................... |
14 |
4.2 |
General use .................................................................................................................. |
16 |
4.3 |
Treatment of medical and dental instruments .............................................................. |
19 |
4.4 |
Further information ....................................................................................................... |
20 |
4.4.1 |
Degassing .................................................................................................................... |
20 |
4.4.2 |
Disposal of sonication fluids ......................................................................................... |
20 |
5 |
Cleaning and maintenance of the ultrasonic bath ........................................................ |
21 |
5.1 |
Cleaning and care ........................................................................................................ |
21 |
5.2 |
Disinfection for medical applications ............................................................................ |
21 |
5.3 |
Warehousing / storing ................................................................................................... |
21 |
4 / 36 |
1556g GB/2019-02 |
1electronic GmbH & Co. KG |
• Heinrichstraße 3-4 • 12207 Berlin • Deutschland • info@bandelin.com |

6 |
Maintenance and repair ................................................................................................ |
22 |
6.1 |
Maintenance ................................................................................................................. |
22 |
6.2 |
Functional checks ......................................................................................................... |
22 |
6.3 |
Error analysis ............................................................................................................... |
22 |
6.4 |
Repairs and service ...................................................................................................... |
23 |
7 |
Accessories .................................................................................................................. |
24 |
7.1 |
Required accessories ................................................................................................... |
24 |
7.2 |
Preparations ................................................................................................................. |
24 |
8 |
Taking the unit out of service ........................................................................................ |
25 |
Informative appendices
AAccessories
BFoil test
CDosing table
1556g GB/2019-02 |
5 / 36 |
1electronic GmbH & Co. KG • Heinrichstraße 3-4 • 12207 Berlin • Deutschland • info@bandelin.com

1 Product description
Ultrasonic bath of type SONOREX SUPER RK ... .
The exact type specification and serial number are found on the type plate, on the rear side of the ultrasonic bath.
Product features:
• Stainless steel oscillating tank (1) with transducers, ultrasound frequency 35 kHz
• Time switch for 1 - 15 min and continuous operation (2)
• Filling level mark for safe filling (3)
• Compact, easy to clean stainless steel housing (4)
• Rubber feet for safe positioning (5)
• As of type RK 102 H, drain outlet with ball valve (6) for easy discharge of bath liquidand handles (7)
• Depending on model, comes with heating (type "H") or a special oscillating tank (types "C")
|
|
1 |
3 |
1 |
3 |
|
|
|
|
|
7 |
|
|
4 |
|
|
4 |
|
|
|
2 |
|
6 |
|
|
|
|
|
|
|
2 |
SONOREX SUPER RK 31 H |
5 |
5 |
|
|
|
|
SONOREX SUPER RK 102 H
1.1Mode of operation
SONOREX ultrasonic baths use the effect of cavitation. Under their oscillating tank bottoms they contain piezoelectric transducers, the energy of which is transferred to the bath liquid with ultrasound frequency as mechanical oscillations. As a result, microscopically small bubbles are continuously formed in the bath liquid, which release energy upon imploding and generate local micro currents. This process is called cavitation. During cleaning processes, it causes contamination to be regularly "blasted" from the hard surfaces of the objects being treated. At the same time, dirt particles are dispersed and fresh bath liquid flows in. During sonochemical
processes, cavitation may have a catalytic effect, e.g. with the production of stable emulsions or the rapid degasification of fluids with a high gas content.
SONOREX ultrasonic baths are efficiently supported by SweepTec® automatic frequency control. SweepTec® immediately balances load-dependent working point fluctuations to the optimal working point using fast frequency modulation. This produces an especially homogeneous and uniform ultrasound field in the bath volume for constantly reproducible results.
6 / 36 |
1556g GB/2019-02 |
1electronic GmbH & Co. KG |
• Heinrichstraße 3-4 • 12207 Berlin • Deutschland • info@bandelin.com |

1.2Purpose
General application
SONOREX ultrasonic baths are intended for the sonication of aqueous fluids. They work on the basis of low-frequency ultrasound and can be used in versatile ways. Their main application is gentle and intensive cleaning of objects of diverse shapes, types and sizes. Alternatively, chemical processes can be favourably supported and accelerated in an ultrasonic bath, e.g. when preparing or treating samples.
Sonication is always carried out in connection with a suitable preparation that is added to the bath liquid. In order to use the device as intended, a basket or another inset beaker, into which objects are placed during sonication, is also required. Only in this manner is the optimum diffusion of the ultrasound guaranteed.
The ultrasonic bath is operated from the front. The operation is usually carried out on a table.
Ultrasound treatment of medical instruments
SONOREX ultrasonic baths are used to treat medical instruments:
a)during manual treatment
b)before machine treatment
c)after machine treatment
In this connection, they are to be used together with suitable, non-fixative disinfection and/or detergent preparations, in order to support or expedite their effect. Pursuant to section 2, para. 1 and section 3, paras. 1, 9 and 10 of the Medical Devices Act (MPG), the ultrasonic bath thus becomes a medical device as an accessory to the preparations, and is to be treated as one. This includes preand post-processing steps for the medical instruments, e.g. the observance of KRINKO/1 recommendations in "Hygiene requirements for the treatment of medical products" and other applicable domestic regulations. Additional information in this respect can be found in chapter 4.3.
1.3CE conformity
SONOREX ultrasonic baths are declared as medical products/2 and satisfy the CE marking criteria for the European
-"Medical Device" directive
-"Low-voltage directive"
-"Electromagnetic compatibility" directive
-WEEE - Directive
in their currently valid versions.
A declaration of conformity can be requested from the manufacturer by providing the serial number.
/1 In Germany: Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut (RKI) und des Bundesinstitutes für Arzneimittel und Medizinprodukte (BfArM); Bundesgesundheitsblatt - 2012•55:1244-1310.
/2 Exception: see "Technical data" overview
1556g GB/2019-02 |
7 / 36 |
1electronic GmbH & Co. KG • Heinrichstraße 3-4 • 12207 Berlin • Deutschland • info@bandelin.com
1.4Technical data
SONOREX ultrasonic baths are interference-free and CE-marked.
Safety: EN 61010-1,
EMC: EN 61326-1
Mains supply: |
|
230 V~ (± 10 %) 50/60 Hz, (115 V upon request), |
|
|
|
|
||||||||||
|
|
|
|
mains cable length 2 m |
|
|
|
|
|
|
|
|
|
|||
Protection class: |
Class I |
|
|
|
|
|
|
|
|
|
|
|
||||
Frequency |
|
35 kHz |
|
|
|
|
|
|
|
|
|
|
|
|||
Oscillating tank: |
Stainless steel |
|
|
|
|
|
|
|
|
|
|
|||||
Serial number (SN): |
See type label |
|
|
|
|
|
|
|
|
|
|
|||||
Degree of protection: |
IP 32 according to DIN 60529 |
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
Protected against access by |
Protected from |
|||||||
Ambient conditions according to EN 61 010-1 |
|
instruments to dangerous |
dripping water |
|||||||||||||
Overvoltage category: |
|
|
|
II |
components, protected against solid up to 15° from its |
|||||||||||
|
|
|
foreign bodies with a diameter of |
vertical axis |
||||||||||||
Degree of contamination: |
|
|
2 |
|
|
2.5 mm or larger |
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
||||||
Permissible ambient temperature: |
|
5 to 40 °C |
|
|
|
|
|
|
|
|
||||||
Permissible relative humidity up to 31 °C |
80 % |
|
|
|
|
|
|
|
|
|||||||
Permissible relative humidity up to 40 °C |
50 % |
|
|
|
|
|
|
|
|
|||||||
No dewing. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Only for indoor operation. |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operat- |
|
Ultrasonic peak |
|
|
|
|
Current |
|
Current |
|
|
|
Order |
|
Interior dimensions |
Outlet |
power* / Ultra- |
|
Weight |
Heating |
|
consump- |
|
consump- |
|
|||
Bath type |
|
ing |
|
|
|
|
||||||||||
No. |
|
(L × W × D) |
(valve) |
sonic nominal |
|
(net) |
power |
|
tion |
|
tion |
|
||||
|
|
volume |
|
|
|
|
||||||||||
|
|
|
|
|
|
|
output |
|
|
|
|
(230 V) |
|
(115 V) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
mm |
|
l |
|
|
W / Weff |
|
kg |
W |
|
A |
|
A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 31 |
329 |
|
190 × 85 |
× 60 |
0.6 |
- |
|
160 / 40 |
|
2.2 |
- |
|
0.2 |
|
0.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 31 H |
7523 |
|
190 × 85 |
× 60 |
0.6 |
- |
|
160 / 40 |
|
2.3 |
70 |
|
0.5 |
|
1.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 52 |
311 |
|
150 × 140 |
× 100 |
1.2 |
- |
|
240 / 60 |
|
2.4 |
- |
|
0.3 |
|
0.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 52 H |
164 |
|
150 × 140 |
× 100 |
1.2 |
- |
|
240 / 60 |
|
2.6 |
140 |
|
0.9 |
|
1.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 100 |
301 |
|
240 × 140 |
× 100 |
2.0 |
- |
|
320 / 80 |
|
3.2 |
- |
|
0.4 |
|
0.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 100 H |
312 |
|
240 × 140 |
× 100 |
2.0 |
- |
|
320 / 80 |
|
3.4 |
140 |
|
1.0 |
|
2.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 102 H |
303 |
|
240 × 140 |
× 100 |
2.0 |
G ¼ |
|
480 / 120 |
|
4.1 |
140 |
|
1.2 |
|
2.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 103 H |
326 |
|
240 × 140 |
× 150 |
2.5 |
G ¼ |
|
560 / 140 |
|
4.3 |
200 |
|
1.5 |
|
3.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 106 |
306 |
|
Ø 240 × 130 |
4.0 |
G ¼ |
|
480 / 120 |
|
5.2 |
- |
|
0.6 |
|
1.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 156 |
305 |
|
500 × 140 |
× 100 |
4.0 |
G ¼ |
|
640 / 160 |
|
6.0 |
- |
|
0.7 |
|
1.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 156 BH |
646 |
|
500 × 140 |
× 150 |
6.0 |
G ¼ |
|
860 / 215 |
|
7.3 |
600 |
|
3.6 |
|
7.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
RK 170 H |
7506 |
|
1000 × 200 × 200 |
26.0 |
G ½ |
1520 / 380 |
|
26.2 |
1600 |
|
8.7 |
|
17.3 |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 255 |
3066 |
|
300 × 150 |
× 150 |
3.8 |
G ¼ |
|
640 / 160 |
|
4.8 |
- |
|
0.7 |
|
1.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 255 H |
316 |
|
300 × 150 |
× 150 |
3.8 |
G ¼ |
|
640 / 160 |
|
5.0 |
280 |
|
2.0 |
|
3.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 510 |
327 |
|
300 × 240 |
× 150 |
6.6 |
G ½ |
|
640 / 160 |
|
7.2 |
- |
|
0.7 |
|
1.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 510 H |
321 |
|
300 × 240 |
× 150 |
6.6 |
G ½ |
|
640 / 160 |
|
7.4 |
400 |
|
2.5 |
|
4.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8 / 36 |
1556g GB/2019-02 |
1electronic GmbH & Co. KG |
• Heinrichstraße 3-4 • 12207 Berlin • Deutschland • info@bandelin.com |

|
|
|
|
|
Operat- |
|
|
Ultrasonic peak |
|
|
|
Current |
Current |
|
Order |
|
Interior dimensions |
|
Outlet |
power* / Ultra- |
Weight |
Heating |
|
consump- |
consump- |
||
Bath type |
|
ing |
|
|
|||||||||
No. |
|
(L × W × D) |
|
(valve) |
sonic nominal |
(net) |
power |
|
tion |
tion |
|||
|
|
volume |
|
|
|||||||||
|
|
|
|
|
|
|
output |
|
|
|
(230 V) |
(115 V) |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
mm |
l |
|
|
W / Weff |
kg |
W |
|
A |
A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 512 H |
795 |
|
300 |
× 240 × 200 |
8.7 |
|
G ½ |
860 / 215 |
8.3 |
400 |
|
2.7 |
5.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 514 |
277 |
|
325 |
× 300 × 150 |
9.0 |
|
G ½ |
860 / 215 |
8.8 |
- |
|
1.0 |
1.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 514 H |
207 |
|
325 |
× 300 × 150 |
9.0 |
|
G ½ |
860 / 215 |
8.8 |
600 |
|
3.6 |
7.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 514 BH |
263 |
|
325 |
× 300 × 200 |
12.5 |
|
G ½ |
860 / 215 |
9.8 |
600 |
|
3.6 |
7.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 1028 |
322 |
|
500 |
× 300 × 200 |
19.0 |
|
G ½ |
1200 / 300 |
14.0 |
- |
|
1.4 |
2.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 1028 C |
661 |
|
500 |
× 300 × 300 |
30.0 |
|
G ½ |
2000 / 500 |
24.5 |
- |
|
2.2 |
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 1028 H |
324 |
|
500 |
× 300 × 200 |
19.0 |
|
G ½ |
1200 / 300 |
14.7 |
1300 |
|
7.0 |
14.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 1028 CH |
143 |
|
500 |
× 300 × 300 |
30.0 |
|
G ½ |
1200 / 300 |
23.4 |
1450 |
|
7.7 |
15.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 1040 |
319 |
|
Ø 500 × 195 |
28.0 |
|
G ½ |
1520 / 380 |
19.4 |
- |
|
1.7 |
3.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 1050 |
323 |
|
600 × 500 × 200 |
41.0 |
|
G ½ |
2400 / 600 |
30.0 |
- |
|
2.7 |
5.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RK 1050 CH |
184 |
|
600 × 500 × 300 |
60.0 |
|
G ½ |
2400 / 600 |
36.0 |
1950 |
|
11.1 |
17.9 |
|
|
|
|
|
|
|
|
|
|
|
||||
/* In order to improve the effect the ultrasound is being modulated whereby a 4-fold ultrasonic nominal output value is yielded as |
|||||||||||||
ultrasonic peak power. |
|
|
|
|
|
|
|
|
|
|
|
||
Specifications for use as a medical device |
|
|
|
|
|
|
|||||||
Name: |
|
|
|
|
|
Ultrasonic bath |
|
|
|
|
|||
UMDNS nomenclature (ECRI / DIMDI): |
|
14-263 |
|
|
|
|
|
||||||
Purpose: |
|
|
|
|
|
See chapter 1.2. |
|
|
|
|
|||
Classification (Medical Device |
|
|
|
|
|
|
|
|
|
||||
Directive 93/42/EWG, Appendix IX): |
|
Class I; active, non-invasive, |
|
|
|||||||||
|
|
|
|
|
|
|
|
non-implantable medical device |
|
||||
Type, model, serial number, year of manufacture: See type plate on the rear side for information
The ultrasonic bath has been inspected pursuant to norms currently in effect and is to be installed and put into operation pursuant to EMC directions.
Specifications pursuant to the Medical Devices Operator Ordinance (MPBetreibV):
Startup on location, functional check |
|
and personnel training (section 4): |
not required |
Technical safety controls, (STK, section 11): |
no specifications |
Technical measurement controls, (MTK, section 14): |
not applicable |
1.4.1Electromagnetic ambient conditions (EMC)
The device was tested to DIN EN 61326-1 for electromagnetic compatibility (EMC) and complies with the requirements for class B devices according to EN 55011.
It is suitable for use in facilities and areas which are directly connected to a public low-voltage supply network, e.g. medical laboratory facilities.
It may generate radio interferences or disrupt the operation of devices nearby. It may be necessary to take remedial measures such as realigning the device or a reconfiguring the ultrasonic bath or the shield.
1556g GB/2019-02 |
9 / 36 |
1electronic GmbH & Co. KG • Heinrichstraße 3-4 • 12207 Berlin • Deutschland • info@bandelin.com

During operation, portable or mobile HF communication systems in the vicinity of the ultrasonic bath should be turned off - their operation may be disrupted.
1.5Warnings and safety precautions
General
•Keep the ultrasonic bath out of the reach of children and persons who have not been instructed in its operation by reference to these instructions.
•We will not offer a guarantee for damages to the ultrasonic bath or oscillating tank, or to the objects to be treated, as a result of use of inadequate disinfection agents or detergents.
•Keep the surface of the ultrasonic bath and operating elements clean and dry.
•Do not expose the ultrasonic bath to corroding influences.
•Move the ultrasonic bath only when it is empty.
•Empty the ultrasonic bath only while turned off.
•Ultrasonic baths adhere to prescribed EMC limit values, such that it can be assumed that the electromagnetic radiation emanating from the units is harmless to humans. A binding statement for wearers of implants can only be made at the place of work and together with the implant manufacturer, however. In case of doubt, information regarding the allowable electromagnetic exposure level is to be obtained from the implant manufacturer.
Operation
•Observe ambient and set-up conditions, see chapter 1.4.
•Only plug in the ultrasonic bath to an outlet with a grounded socket.
•Do not operate the ultrasonic bath without fluids.
•Do not stand or lay any objects on the tank bottom, accessories must be used, see chapter 7.
•Do not immerse any parts of the body (e.g. hands, feet) or living beings (animals or plants) into the tank; in particular, do not immerse them in the ultrasonic fluid during ultrasound operation. Danger: Ultrasounds have a cell-destroying effect.
•In the event of continuous activity within a 2 m radius, adequate hearing protection must be used. Danger: Hearing disturbances during operation when not wearing hearing protection - the typical ultrasound cavitation noise can be very uncomfortable.
•When preheating the bath liquid, stir at least every 15 min. or switch on the ultrasound. Danger:
Scalding due to retardation of boiling.
•Do not operate the ultrasonic bath while unattended.
Advice for the medical field
•The ultrasonic bath is exclusively intended for use by medical skilled personnel.
•When handling contaminated instruments, relevant personnel protection regulations are to be observed.
•When treating instruments, the instructions of the instrument manufacturer are to be followed.
•Ultrasound cleaning is especially suited for instruments made of stainless steel and hard plastics. Do not treat lenses, camera systems or light cables with ultrasound.
10 / 36 |
1556g GB/2019-02 |
1electronic GmbH & Co. KG |
• Heinrichstraße 3-4 • 12207 Berlin • Deutschland • info@bandelin.com |

Damages
•If damage to the ultrasonic bath is detected, do not connect the ultrasonic bath to the mains.
•In the event of defects, disconnect the power plug immediately.
•Repairs are only to be conducted by authorised skilled personnel or by the manufacturer.
•Defective parts may only be replaced with original SONOREX parts.
2 Preparation
Carefully unpack the ultrasonic bath and accessories and inspect them for completeness or possible transportation damages. If any damages or defects are found, these are to be immediately notified in writing to the transportation company and to the supplier.
Before startup, the ultrasonic bath is to be left to stand at its operating location for 2 hours so that it may adapt to the ambient conditions.
2.1Scope of delivery
1 Ultrasonic bath, optionally with heating - see delivery note
1Ball valve (brass, galvanized) with hose, as of type RK 102 H, packaged separately with sealing tape and assembly instructions
1 Instruction manual
Additional accessories according to order - see delivery note
2.2Set-up / assembly
•Place the ultrasonic bath atop a firm, level and dry surface. In doing so
-observe the maximum weight of the ultrasonic bath, including fluid. Net weight see technical data chapter 1.4.
-do not block the air supply below the ultrasonic bath.
-guard against moisture and wetness - risk of electric shock.
•In the case of ultrasonic baths with a drain outlet, mount the ball valve, hose socket and hose, which are included in the delivery, pursuant to the enclosed assembly instructions.
2.3Start-up
Thoroughly rinse the ultrasonic bath's oscillating tank with water before its first use.
Verify that the control buttons are in the "off" position, i.e. the switch indicator above or the toggle switch is set to "0", then connect the ultrasonic bath to the mains (grounded socket).
Conduct function test - briefly plug in the ultrasound (maximum of 1 to 2 seconds), a hissing noise should be heard. Set to "0" once again.
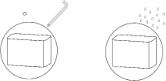
It is recommended that a foil test be conducted as part of quality assurance prior to the first use.
This test is to be saved for later comparison, see appendix for information.
If applicable, hang accessories in the ultrasonic bath and place lid on top.
1556g GB/2019-02 |
11 / 36 |
1electronic GmbH & Co. KG • Heinrichstraße 3-4 • 12207 Berlin • Deutschland • info@bandelin.com
 Loading...
Loading...