ASP STERRAD 200 User manual

STERRAD® 200 Sterilization System
User’s Guide
REF 99712
99712_01 May 2008

STERRAD® 200 Sterilization System
User’s Guide
REF 99712
33 Technology Drive Irvine, California 92618 USA
1-888-STERRAD (1-888-783-7723)
949-581-5799 www.sterrad.com
For warranty information, please visit our website or contact our Customer Care Center.
For additional copies of this guide, please visit www.e-ifu.com
©2008 Advanced Sterilization Products. All rights reserved. STERRAD® is a registered trademark of Advanced Sterilization Products. Teflon®, Tyvek® and Nylon® are registered trademarks of E.I. du Pont de Nemours and Company. Other products mentioned in this publication are trademarked by their respective corporations. Reproduction, adaptation or translation of this publication without prior written permission is prohibited. Printed in the USA.
Contents |
|
About This Guide |
5 |
Overview .................................................................................................................... |
5 |
Chapter 1. Introduction |
7 |
Overview .................................................................................................................... |
7 |
System Details ............................................................................................................ |
8 |
Chapter 2. For Your Safety |
11 |
Overview .................................................................................................................... |
11 |
Personal Safety and First Aid ..................................................................................... |
11 |
Device Safety.............................................................................................................. |
13 |
Cassette Handling ....................................................................................................... |
14 |
Safe Maintenance ....................................................................................................... |
15 |
Additional Information ............................................................................................... |
15 |
Chapter 3. Preparing Items To Be Sterilized |
17 |
Overview .................................................................................................................... |
17 |
Indications for Use ................................................................................................ |
17 |
How to Determine What Can be Sterilized in the STERRAD® 200 System.............. |
19 |
Items Not Recommended ........................................................................................... |
20 |
Cleaning, Rinsing, and Drying ................................................................................... |
21 |
Guidelines for Wrapping, Packaging, and Loading.................................................... |
23 |
Chapter 4. Day-to-Day Operation |
27 |
Safe Operation ............................................................................................................ |
27 |
Sterilizer Operation..................................................................................................... |
27 |
Display Sequence .................................................................................................. |
29 |
Using the Displays................................................................................................. |
30 |
Preparing the Load...................................................................................................... |
32 |
Biological Indicators ............................................................................................. |
34 |
Chemical Indicators............................................................................................... |
36 |
Preparing the Two-Tier Shelf................................................................................ |
37 |
Creating the Load Item Data List .......................................................................... |
38 |
Transferring the Load into the Sterilizer ............................................................... |
40 |
Inserting a Cassette................................................................................................ |
42 |
Starting a Cycle .......................................................................................................... |
45 |
Entering a Password .............................................................................................. |
46 |
Cycle Notes ........................................................................................................... |
50 |
Watching a Cycle .................................................................................................. |
51 |
Completing a Cycle ............................................................................................... |
53 |
STERRAD® 200 User’s Guide |
3 |
Additional System Information ............................................................................. |
58 |
Unloading and Handling ....................................................................................... |
58 |
Cycle Completion Flow Chart............................................................................... |
61 |
Cassette Control.......................................................................................................... |
62 |
Obtaining a Cycle History .......................................................................................... |
63 |
Administrative Functions............................................................................................ |
66 |
System Setup ......................................................................................................... |
66 |
System Configuration Screen ................................................................................ |
67 |
User/Password Administration Screen .................................................................. |
68 |
Resetting the Date and Time ................................................................................. |
69 |
Network Settings Screen ....................................................................................... |
71 |
Chapter 5. Routine Maintenance |
73 |
Overview .................................................................................................................... |
73 |
Maintaining the Printer............................................................................................... |
74 |
Replacing the Printer Ribbon Cartridge ................................................................ |
74 |
Replacing the Printer Paper................................................................................... |
75 |
Cleaning the Sterilizer ................................................................................................ |
76 |
Chapter 6. Troubleshooting |
77 |
Overview .................................................................................................................... |
77 |
Rebooting the System................................................................................................. |
77 |
Using the Message Table............................................................................................ |
78 |
Appendix A. Specifications |
81 |
4 |
STERRAD® 200 User’s Guide |
About This Guide
Overview
This guide is designed to provide useful and complete information on the day-to- day operation and routine maintenance of the STERRAD® 200 Sterilizer.
The guide is divided into 6 chapters and 1 appendix. These chapters provide information on using the sterilizer, load preparation, routine maintenance, and troubleshooting the system should problems arise. The appendix contains the system specification.
The chapters and the appendix are:
•About This Guide-this section gives you important information on how to get the most use out of this guide.
•Chapter 1. Introduction-the first chapter of the guide has important details about the STERRAD 200 System including the major parts of the sterilizer and STERRAD Process information.
STERRAD® 200 User’s Guide |
5 |

About This Guide
•Chapter 2. For Your Safety-this may be the most important chapter in the guide. You must read it thoroughly, understand the information, and follow all the safety procedures in this chapter. These safety procedures include safe handling of cassettes, safe transfer of sterilized materials using the two-tier shelf and carriage, and first aid information in case of possible hydrogen peroxide exposure.
•Chapter 3. Preparing Items To Be Sterilized-this chapter provides details on preparing items to be sterilized, packaging the load, using the two-tier shelf and carriage to transfer the load to the sterilizer and a brief description of the items that can be sterilized in the STERRAD 200 System.
•Chapter 4. Day-to-Day Operation-this chapter gives you detailed information on how to use the sterilizer. It shows you the typical displays that you will see as you use the sterilizer and the different screens available to the operator and administrator level passwords. It details how to use the touch screens and how to run cycles.
•Chapter 5. Routine Maintenance-the routine maintenance of the STERRAD 200 Sterilizer is very minimal. This chapter shows you how to change the printer paper and ribbon and what steps to follow to keep the sterilizer clean.
•Chapter 6. Troubleshooting-the STERRAD 200 Sterilizer displays a number of messages to tell you what the system status is at any given moment. Many of these messages do not require any action from you. Others require that you call your ASP service representative.
•Appendix A. Specifications-this appendix provides tables detailing the technical data relevant to the STERRAD 200 Sterilizer. Size, electrical requirements, equipment rating and an explanation of the warning symbols used on the sterilizer are some of the information found in this appendix.
6 |
STERRAD® 200 User’s Guide |
Chapter 1.
Introduction
Overview
The STERRAD® 200 Sterilization System is a general purpose, low temperature sterilizer using the STERRAD Process to inactivate microorganisms on a broad range of medical devices and surgical instruments. This sterilizer offers an effective, safe, fast, economical, easy to use, reliable, and flexible sterilization method.
The length of all cycle phases and the setpoints for all critical process parameters are controlled by a microprocessor and software. The system reliably sterilizes various material and load configurations, without leaving toxic residue.
The system consistently provides 10-6 sterility assurance level, as defined by international standards, for clinical use on all allowed substrates within the limits of the claims for materials and geometries.
STERRAD® 200 User’s Guide |
7 |
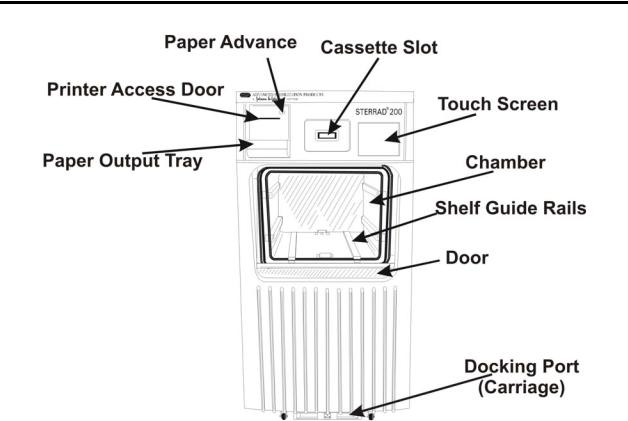
1Introduction
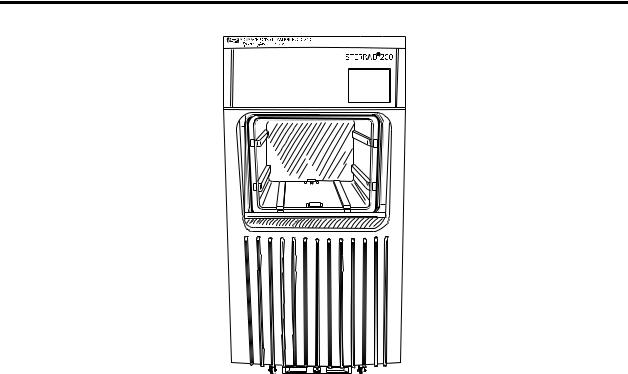
Figure 1. STERRAD® 200 Sterilizer input side on a two door unit, or front side on a one door unit.
System Details
Here are details of the important parts of the STERRAD 200 Sterilizer.
•The items to be sterilized are placed on the two-tier shelf. The two-tier shelf (see the following diagram) has 1 adjustable, removable shelf and a fixed bottom shelf. The packed two-tier shelf is easily moved, using the carriage, into or out of the sterilizer.
8 |
STERRAD® 200 User’s Guide |
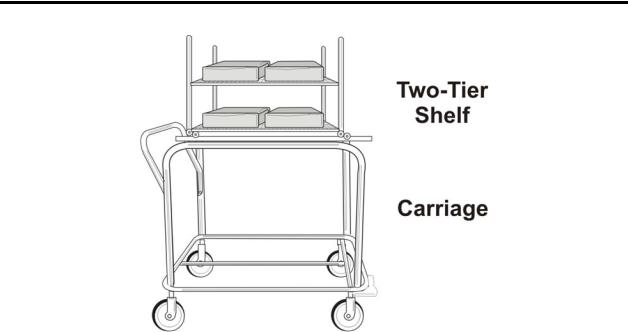
Introduction 1
Figure 2. STERRAD® 200 Two-Tier Shelf and Carriage
•The carriage is used to move the two-tier shelf into position. This carriage is a wheeled unit that locks onto the front of the machine so that you can easily transfer a large load into or out of the sterilizer.
•Your system may have one or two doors. If you have a two-door unit, a door is located on each side of the unit. One side, the input side, is used for moving wrapped items to be sterilized into the sterilizer. The other side, the output side, is used to remove the sterile items from the sterilizer. These sterile items are then usually moved into a sterile storage area. On the one door unit, the door is used for both input and output.
STERRAD® 200 User’s Guide |
9 |
1Introduction
Figure 3. STERRAD® 200 Sterilizer–output side on a two door unit. Note the absence of a cassette slot.
•A touch screen computer display, mounted on the upper right front of the sterilizer, enables you to operate the sterilizer. There is a touch screen on each side of the double door unit.
•A printer, located on the upper left front panel of the unit, delivers printed messages, process parameters, and other information for your permanent records. There is usually only one printer on the unit regardless of the number of doors, however other printer options are available. Contact the ASP Customer Care Center for more details.
•There is an optional barcode scanner available for the sterilizer. The barcode scanner is used to conveniently enter bar-coded data from product tags, labels or other items.
•The usable chamber capacity is approximately 150 liters.
10 |
STERRAD® 200 User’s Guide |
Chapter 2.
For Your Safety
Overview
Your safety is of primary concern to ASP. This chapter provides information on safely using the sterilizer. You must read, understand and use the information in this chapter before operating the unit. Also, always pay attention to the warnings, cautions and notes throughout this guide. This information is for your safety and to ensure that you receive the most benefit from the safe operation of your STERRAD® 200 Sterilization System.
Personal Safety and First Aid
♦WARNING! HYDROGEN PEROXIDE IS CORROSIVE
Concentrated hydrogen peroxide is corrosive to skin, eyes, nose, throat, lungs, and the gastrointestinal tract. Always wear chemical resistant latex, PVC (vinyl), or nitrile gloves when removing items from the sterilizer following a cancelled cycle or if any moisture is noted on items in the load following a completed cycle.
♦WARNING! HYDROGEN PEROXIDE IS AN OXIDIZER
Hydrogen peroxide is a strong oxidizing agent and poses a hazard for fire, explosion, or container rupture. Avoid allowing hydrogen peroxide to contact organic materials, including paper, cotton, wood, or lubricants. Do not use or store near heat or open flame. Shoes, clothing, or other combustible material that have come into contact with hydrogen peroxide must be immediately and thoroughly rinsed with water to avoid a potential fire hazard. In case of fire, use only water to extinguish.
STERRAD® 200 User’s Guide |
11 |

2 For Your Safety
♦WARNING! CONCENTRATED HYDROGEN PEROXIDE IS TOXIC
Ingestion of hydrogen peroxide may be life threatening. If swallowed, call a “poison control” center or physician immediately for treatment advice. Have the person drink plenty of water if the person is able to swallow. Do not give anything by mouth to an unconscious person. Do not induce vomiting unless instructed to do so by the poison control center or physician.
♦WARNING! RISK OF EYE INJURY
Direct hydrogen peroxide contact with eyes can cause irreversible tissue damage. If contact with eyes occurs, hold the eyes open and flush with large amounts of water for at least 15-20 minutes. Remove contact lenses, if present, and then continue rinsing the eyes. Consult a physician immediately after flushing the eyes.
♦WARNING! RISK OF RESPIRATORY IRRITATION
Inhalation of hydrogen peroxide mist can cause severe irritation of lungs, throat, and nose. If inhalation occurs, move the person to fresh air. If the person is not breathing, call for emergency medical attention, or an ambulance, then give artificial respiration, preferably mouth-to-mouth, if possible. Consult a physician immediately.
♦WARNING! RISK OF SKIN INJURY
Direct hydrogen peroxide contact with the skin can cause severe irritation. Wear chemical resistant latex, PVC (vinyl) or nitrile gloves when handling used cassettes or ejected cassettes, items from a cancelled cycle, or items that have moisture present after a completed cycle. Immediately take off contaminated clothing and rinse thoroughly with water to avoid potential fire hazard and wash before re-use.
♦WARNING! HEATED STERILIZATION SURFACES.
At the end of a cycle, the interior of the sterilizer may be hot. Do not touch the inside of the chamber or door with your bare or gloved hands. The vaporizer may be hot. Do not touch it with your bare or gloved hands. Allow the sterilizer to cool before touching interior surfaces.
12 |
STERRAD® 200 User’s Guide |

For Your Safety 2
♦WARNING! RISK OF BREATHING DIFFICULTIES
On rare occasions, the outlet filter on the vacuum pump can prematurely fail. If this occurs, you may see mist or what some users have described as “haze” or “smoke” in the room where the sterilizer is operating. The chemical composition of the mist is primarily airborne mineral oil with trace amounts of other compounds. Oil mist exposure may, theoretically, pose an increased risk to people with certain respiratory conditions, such as asthma, and they should take special precautions not to be exposed to the mist. If you observe these conditions, personnel should leave the room as a precaution and discontinue use of the STERRAD System until the system is repaired. Personnel should avoid working in the room until the mist has cleared.
Please note that all STERRAD Sterilizers should be used and installed in a well-ventilated environment (a minimum of 10 air exchanges per hour).
Device Safety
•DO NOT ATTEMPT TO STERILIZE ITEMS OR MATERIALS THAT DO NOT COMPLY WITH THE GUIDELINES SPECIFIED IN THIS GUIDE. In addition, you should read the medical device manufacturer's instructions, or call the ASP Customer Care Center to determine whether an item can be sterilized by this unit. Information may also be obtained from the device manufacturer.
•All items must be cleaned and thoroughly dried before loading into the sterilizer. Loads containing moisture may cause cycle cancellation.
•The chapter on preparing items to be sterilized contains information about the materials and devices that can be processed by the STERRAD 200 Sterilizer.
•Metal objects must not come into contact with the chamber walls, the doors or the electrode. Contact with the walls, doors, or electrode could damage the sterilizer or instruments.
•Do not change the power source without checking the electrical phase rotation. Prior to relocating the STERRAD 200 Sterilizer to a new power source, electrical phase rotation should be checked by a qualified technician. Failure to verify and match phase rotation may cause damage to the sterilizer and void the warranty.
•If you plan to disconnect and store the sterilizer for any length of time, please contact your ASP service representative at 1-888-STERRAD (1-888-783- 7723) for instructions.
STERRAD® 200 User’s Guide |
13 |

2 For Your Safety
•An ASP-approved biological indicator (BI) should be used to monitor the sterilization cycle. Should a cancellation occur when a biological indicator is in the chamber, discard the biological indicator and then use a new biological indicator when re-starting the cycle. Call the ASP Customer Care Center for information on approved biological indicators.
Cassette Handling
•STERRAD 200 CASSETTES CONTAIN CONCENTRATED HYDROGEN PEROXIDE, A STRONG OXIDIZER. CONCENTRATED HYDROGEN PEROXIDE IS CORROSIVE TO SKIN, EYES, NOSE, THROAT, LUNGS, AND GASTROINTESTINAL TRACT. Direct contact with the skin can cause severe irritation. If skin contact occurs, immediately flush with large amounts of water. If symptoms are severe or persist, consult a physician immediately. Direct contact with eyes can cause irreversible tissue damage. If eye contact occurs, immediately flush with large amounts of water and immediately consult a physician. Inhalation of mist can cause severe irritation of lungs, throat, and nose. If inhalation occurs, move to fresh air and consult a physician immediately. Ingestion can produce corrosion that may be life threatening. If swallowed, drink plenty of water immediately to dilute. Do not induce vomiting. Consult a physician.
•Do not remove the plastic wrapper from the cassette package if the indicator strip is red. Red indicates that the cassette might have been damaged. Call the ASP Customer Care Center for more information.
•Do not remove used cassettes from the protective cardboard sleeve. Dispose of the cassette inside the protective sleeve according to your facility’s procedures.
•If it is necessary to handle a used cassette that is not in the cardboard sleeve, wear chemical resistant latex, PVC (vinyl) or nitrile gloves. Do not touch your gloved hands to your face or eyes.
14 |
STERRAD® 200 User’s Guide |

For Your Safety 2
Safe Maintenance
•WARNING! HYDROGEN PEROXIDE MAY BE PRESENT
If white residue is visible on the load; this may be residue from the hydrogen peroxide stabilizer. Wear chemical resistant latex, PVC (vinyl), or nitrile gloves when removing a load with visible white residue. White residue can be minimized by making sure regular Planned Maintenance (PM) procedures are performed on your sterilizer. The sterilizer will inform you when Planned Maintenance is due. Please schedule your PM service in a timely manner.
•Repairs and adjustments should only be attempted by experienced technicians who are fully trained to maintain and repair the STERRAD 200 Sterilizer.
•Use of unauthorized parts for maintenance or repair could cause personal injury, result in costly damage or unit malfunction, and void the warranty.
•Do not clean the chamber door area with abrasives. The sterilization chamber uses an O-ring vacuum seal to maintain a vacuum in the chamber. Never use rough cleaning tools, such as a wire brush or steel wool, on the door housing or chamber assembly. This could damage the seal.
Additional Information
•The information contained in this chapter is repeated where appropriate throughout this guide for your safety and use. This information is subsequently labeled: WARNINGS, Cautions or Notes as appropriate.
•WARNINGS are shown in the text in all bold upper case letters. They indicate events or conditions that can result in serious injury or death.
•Cautions are shown in the text in bold letters. They indicate events or conditions that can result in damage to equipment.
• Notes are shown in the text with a check mark . They highlight specific information about the proper use and maintenance of the STERRAD 200 Sterilizer.
STERRAD® 200 User’s Guide |
15 |
Chapter 3.
Preparing Items To Be
Sterilized
Overview
The STERRAD® 200 Sterilizer can process many of the items you commonly sterilize as well as instruments that are sensitive to heat and moisture. However, there are a few important exceptions. Please review the “How to Determine What Can be Sterilized in the STERRAD 200 System” foldout page contained in this chapter. It contains details on recommended materials and lumen sizes.
Indications for Use
The STERRAD 200 Sterilizer is designed for sterilization of both metal and nonmetal medical devices at low temperatures. Because the cycle operates within a dry environment and at low temperatures, it is especially suitable for instruments sensitive to heat and moisture. The sections that follow contain more information on the types of items and materials that can be sterilized in the STERRAD 200 Sterilizer.
•The STERRAD 200 Sterilizer can sterilize instruments having diffusionrestricted spaces, such as the hinged portion of forceps and scissors.
•Teflon® or polyethylene lumened instruments with inside diameters of 6 mm or larger and lengths of 310 mm or shorter can be processed in the STERRAD 200 Sterilizer.
•Medical devices with only a single stainless steel lumen with:
STERRAD® 200 User’s Guide |
17 |

3Preparing Items To Be Sterilized
»an inside diameter of 1 mm or larger and a length of 125 mm or shorter,*
»an inside diameter of 2 mm or larger and a length of 250 mm or shorter,*
»an inside diameter of 3 mm or larger and a length of 400 mm or shorter. can be processed in the STERRAD 200 Sterilizer.
*Validation testing for this lumen size was conducted using a maximum of 12 lumens per load. Your loads should not exceed the maximum number of lumens validated by this testing.
Note: The validation studies for this sterilizer were performed using the STERRAD cycle consisting of a 6-minute, 45second injection phase followed by a 2-minute diffusion phase, and a 2-minute plasma phase. The validation studies were performed using a validation load consisting of four instrument trays, each weighing 9.12 lbs. (the total weight of the load was 36.48 lbs.).
Note: Determination of true length is based upon actual instruments identified as intended for reprocessing and meet all other criteria for processing in the STERRAD 200 Sterilizer.
WARNING! DO NOT ATTEMPT TO STERILIZE ITEMS OR MATERIALS THAT DO NOT COMPLY WITH THE GUIDELINES SPECIFIED IN THIS GUIDE. IN ADDITION, YOU SHOULD READ THE MEDICAL DEVICE MANUFACTURER’S INSTRUCTIONS, OR CALL THE ASP CUSTOMER CARE CENTER TO DETERMINE WHETHER AN ITEM CAN BE STERILIZED BY THIS UNIT.
18 |
STERRAD® 200 User’s Guide |

Preparing Items To Be Sterilized 3
How to Determine What Can be Sterilized in the STERRAD® 200 System
The following page is a chart that unfolds to show you detailed lists of recommended items, materials, and some typical devices that can be sterilized in the STERRAD 200 Sterilizer. You should refer to it whenever you need materials information. Be sure to check with the medical device manufacturer’s instruction before loading any item into the STERRAD 200 Sterilizer.
♦Important! Stainless steel, polyethylene, and Teflon® are the only types of lumens that can be sterilized in the STERRAD 200 Sterilizer.
Note: There are a wide variety of materials and devices that can be sterilized in the STERRAD 200 Sterilizer. As more manufacturers complete testing of their products with the STERRAD 200 Sterilizer, the range of recommended and/or compatible items grows. ASP maintains updated information and we are happy to share it with you. Please contact the ASP Customer Care Center for an up-to-date list of recommended materials, devices and/or device manufacturer information. Call 1-888-STERRAD (1-888- 783-7723).
STERRAD® 200 User’s Guide |
19 |
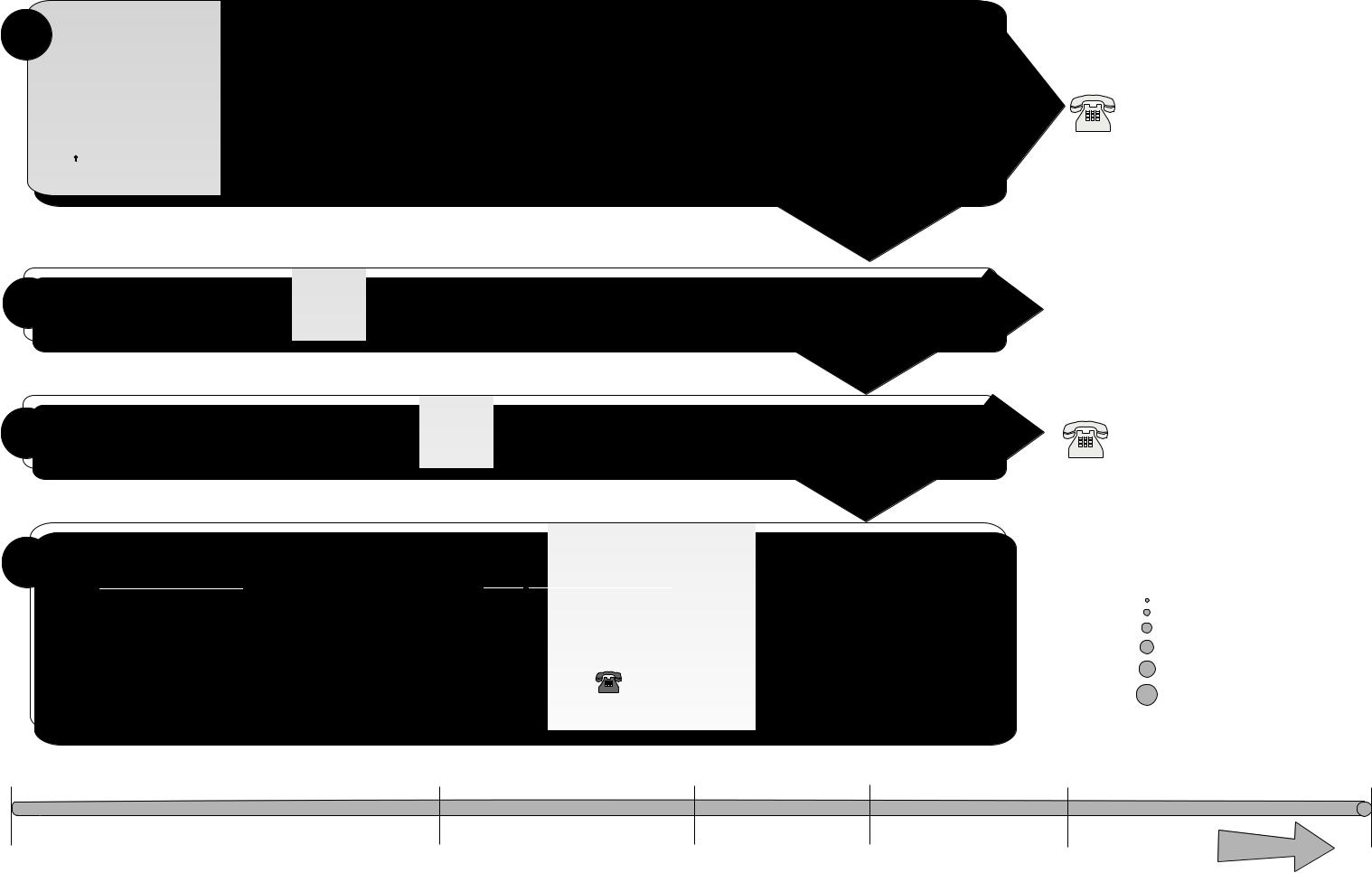
How To Determine What Can Be Sterilized In The STERRAD® 200 Sterilizer
1 |
Is The Reprocessable Medical Device Made Of The Following Materials?** |
|||||
** This list of materials does not apply to trays and containers or other packaging materials. Please refer to the STERRAD® 200 User’s Guide for information on appropriate packaging |
||||||
|
||||||
|
materials for use in the STERRAD System. |
|
|
|
|
|
|
- Aluminum |
- KRATONTM Polymers |
|
- Polyetherimide (ULTEM® Polymers) |
- Polyurethane |
|
|
- Brass |
- Neoprene |
|
- Polymethyl methacrylate (PMMA) |
- Polyvinyl chloride (PVC) |
|
|
- Delrin® acetal resin (polyacetal) |
- Non-mated Nylon® (polyamide) |
- Polyphenylene sulfone (Radel®) |
- Silicone elastomers |
||
|
- Ethylvinyl acetate (EVA) |
- Polycarbonate |
|
- Polypropylene |
- Stainless steel |
|
|
- Glass |
- Polyethylene |
|
- Polystyrene |
- Teflon® (polytetrafluoroethylene) |
|
|
May have limited life after repeated sterilization. |
|
|
|
- Titanium |
|
|
|
|
|
|
||
|
Delrin®, Nylon®, and Teflon® are registered trademarks of the DuPont Corporation. |
ULTEM® Polymers is a registered trademark of the GE Company. |
|
|||
|
KRATON™ Polymers is a trademark of KRATON Polymers U.S. L.L.C. |
Contact ASP or the device manufacturer to determine the compatibility of any device manufactured from PMMA. |
||||
|
|
|
||||
No/Don’t |
Please call the device manufacturer for |
|
|
Know |
information on how to properly sterilize |
|
this device. |
2
3
4
0 mm
Yes
Does The Reprocessable Medical Device Have A Lumen? |
No |
Yes
Is The Lumen Made Of Stainless Steel, Or Teflon®? |
No/Don’t |
|
Know |
Yes
Proceed With Processing If The Lumen Conforms To The Dimensions Listed Below
Stainless Steel Lumen |
|
® |
|
|
Teflon /Polyethylene Lumens |
||
|
|
__ |
|
Inside Diameter |
Length |
Inside Diameter |
Length |
1 mm or larger |
125 mm or shorter* |
6 mm or larger |
310 mm or shorter |
2 mm or larger |
250 mm or shorter* |
|
|
3 mm or larger |
400 mm or shorter |
|
|
|
|
If the lumens do not conform to these dimensions, please call the device |
|
|
|
|
|
manufacturer for information on how to properly sterilize these devices. |
* Validation testing for this lumen size was conducted using a maximum of 12 lumens per load. |
Lumens not conforming to these dimensions should not be processed in |
|
Your loads should not exceed the maximum number of lumens validated by this testing. |
the STERRAD 200 Sterilizer. |
|
Proceed with
Processing.
Please call the device manufacturer for information on how to properly sterilize this device.
Inside Lumen
Diameters
1 mm (0.039 inches)
2 mm (0.078 inches)
3 mm (0.118 inches)
4 mm (0.157 inches)
5 mm (0.196 inches)
6 mm (0.236 inches)
125 mm |
200 mm |
250 mm |
310 mm |
Lumen Lengths 400 mm |
More Information
Measurements are approximate and are for reference only.
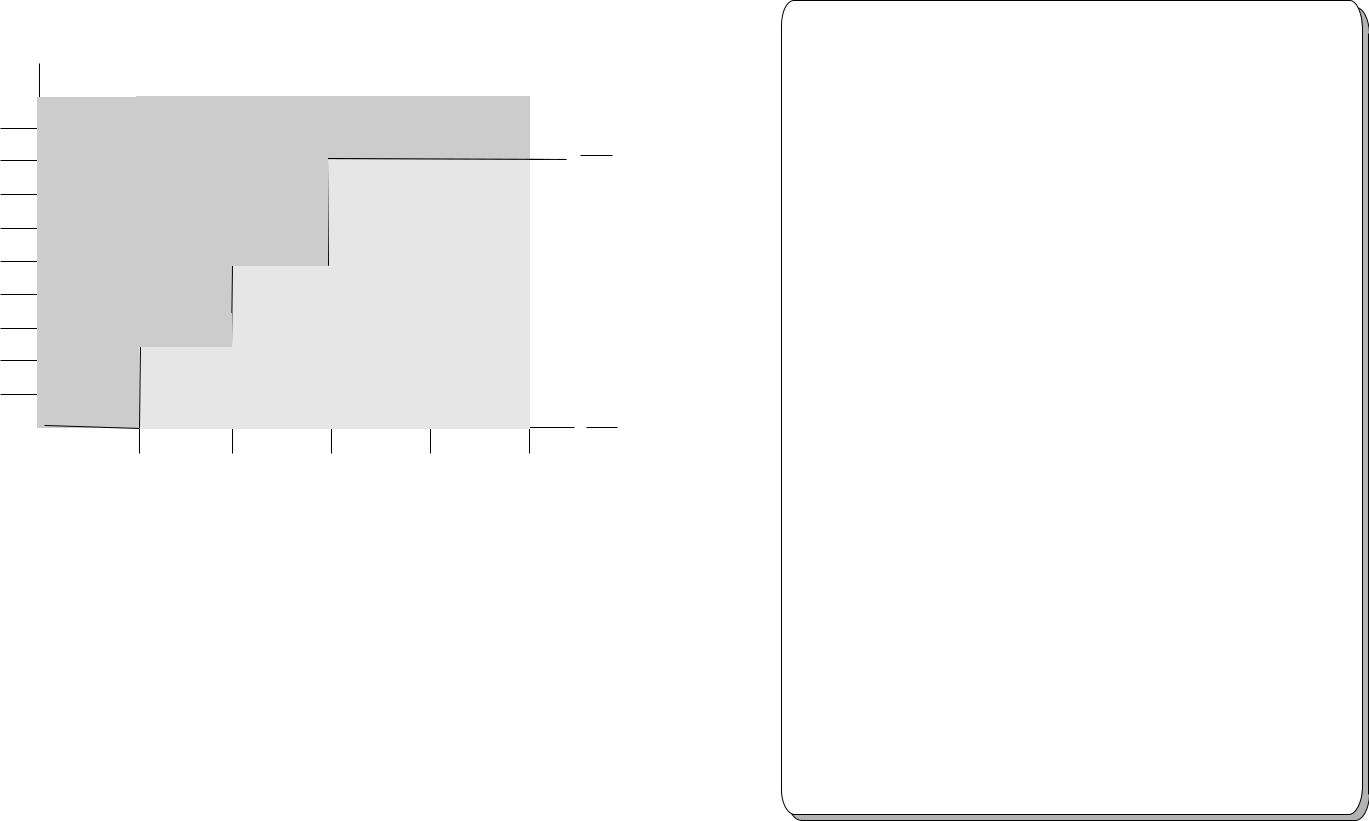
Lengths
500 mm
450 mm
400 mm
350 mm
300 mm
250 mm
200 mm
150 mm
100 mm
50 mm
Processing Stainless Steel Lumens
in the STERRAD® 200 Sterilizer
Cannot be processed in the STERRAD® 200 Sterilizer
400 mm |
~ ~ |
|
250 mm
Can Be Processed In
the STERRAD® 200 Sterilizer
125 mm
~ ~
1mm 2 mm 3 mm 4 mm 5 mm
Inside Diameter
Typical Devices Sterilized in the STERRAD® 200 Sterilizer*
-Stereotactic equipment
-Defibrillator paddles
-Electrocautery instruments
-Esophageal dilators
-Cranial pressure transducer cables
-Metal instruments
-Patient lead cables
-Endoscopic instruments
-Rigid endoscopes
-Laryngoscope blades
-Trocar sheaths
-Cryoprobes
-Surgical power equipment and batteries
-Fiberoptic light cables
-Laser hand-pieces, fibers, and accessories
-Ophthalmic lenses (diagnostic, magnifying)
-Pigmentation hand-pieces
-Dopplers
-Shaver hand-pieces
-Radiation therapy equipment
-Ultrasound probes
-Video cameras and couplers
-Resectoscope/working elements and sheaths
If you have questions about whether your particular device can be sterilized in the STERRAD Sterilizer, please call the device manufacturer or call ASP at 1-888-STERRAD.
Visit our website at www.sterrad.com.
* Any devices processed in the STERRAD 200 Sterilizer must be within the claim limits of the sterilizer.

3Preparing Items To Be Sterilized
Items Not Recommended
♦Items made with copper or copper alloys (such as Monel), should not be used. Please contact the ASP Customer Care Center at 1-888-STERRAD for more information.
♦Instrument mats other than STERRAD Instrument Mats.
♦Instrument trays other than STERRAD Instrument Trays or APTIMAX® Instrument Trays.
♦Any item that is not completely dry.
♦Items or materials that absorb liquids.
♦Items made of materials that contain cellulose, such as cotton, paper or cardboard, linens, huck towels, gauze sponges, or any item containing wood pulp.
♦Paper instrument count sheets or lot stickers.
♦Liquids and powders.
♦Items with mated, Nylon® surfaces.
♦Single use items for which the manufacturer does not recommend resterilization.
♦Implants for which the manufacturer has not specifically recommended sterilization in the STERRAD 200 Sterilizer.
♦Instruments and devices that cannot withstand a vacuum and are labeled for gravity steam sterilization methods only.
♦Items whose design permits the surfaces to collapse onto each other unless some method is used to keep the surfaces separated.
♦Devices with internal parts, such as sealed bearings, that cannot be immersed may present difficulties in cleaning and should not be processed in the STERRAD 200 Sterilizer.
20 |
STERRAD® 200 User’s Guide |

Preparing Items To Be Sterilized 3
Cleaning, Rinsing, and Drying
Cleaning and sterilization are two separate processes. Proper cleaning of instruments and devices is a critical and necessary step prior to sterilization.
♦All items including trays must be thoroughly cleaned, rinsed, and dried before loading into the sterilizer.
♦Carefully inspect all instruments and devices for cleanliness and dryness prior to packaging. If visible soil is present, the item must be re-cleaned and dried prior to sterilization. If moisture is present, dry the item thoroughly prior to sterilization.
♦Carefully inspect all instruments and devices for flaws or damage prior to packaging. Devices and instruments with flaws or damage should be replaced or repaired before using.
Note: Periodic careful inspection of the items after repeated exposure to disinfectant/cleaner/sterilant is necessary, due to the potential damaging effects of the chemical agents on the items.
Cleaning is necessary to remove organic and inorganic soil and debris from equipment. This process also removes many microorganisms from the surface of the items. Sterilization then inactivates all remaining spores and live microorganisms.
♦Clean your devices according to the medical device manufacturers' instructions. You must remove all blood, tissue, and soil from items using appropriate detergents, cleansers and/or methods.
♦Rinse items thoroughly to remove detergent or cleanser residue. Use treated water that is of a quality that ensures hard water stains do not occur. Failure to remove all organic materials or detergents may result in the formation of light-colored residue on the devices. If residue is visible, you should clean, rinse, dry, and resterilize the device prior to use.
STERRAD® 200 User’s Guide |
21 |

3Preparing Items To Be Sterilized
♦Dry all items thoroughly. An acceptable method for drying is to blow compressed gas through the lumen until no moisture exits the distal end of the device. Please ensure that any method used to dry the devices is in accordance with the manufacturers' instructions for use or contact the device manufacturer to obtain appropriate and safe procedures. It is necessary to remove moisture from all parts of the items. Only dry items should be loaded into the sterilization chamber to prevent cycle cancellation.
WARNING! POSSIBLE RESIDUAL HYDROGEN PEROXIDE CONTACT! FAILURE TO ENSURE THAT INSTRUMENTS ARE COMPLETELY DRY BEFORE THEY ARE PROCESSED IN THE STERRAD® STERILIZER MAY RESULT IN RESIDUAL HYDROGEN PEROXIDE BEING PRESENT ON THE OUTER SURFACE OF THE LOAD. THIS MAY CAUSE CONTACT BURNS WHEN THE SURFACE OF THE LOAD IS HANDLED.
♦Some complex reusable medical devices may require disassembly for proper cleaning and sterilization. It is very important that you follow the device
manufacturers' recommendations concerning cleaning and sterilization. In the absence of STERRAD® System-specific instructions, please contact the relevant medical device manufacturer.
WARNING! POSSIBLE NON-STERILE DEVICE! LOADS CONTAINING MOISTURE MAY RESULT IN EITHER A NON-STERILE DEVICE OR CYCLE CANCELLATION. WEAR CHEMICAL RESISTANT LATEX, PVC (VINYL), OR NITRILE GLOVES WHEN HANDLING ITEMS FROM ANY LOAD CONTAINING MOISTURE.
22 |
STERRAD® 200 User’s Guide |
Preparing Items To Be Sterilized 3
Guidelines for Wrapping, Packaging, and Loading
Proper preparation of trays, pouches, and instruments can minimize or prevent cycle cancellation and positive BI results due to load-related problems.
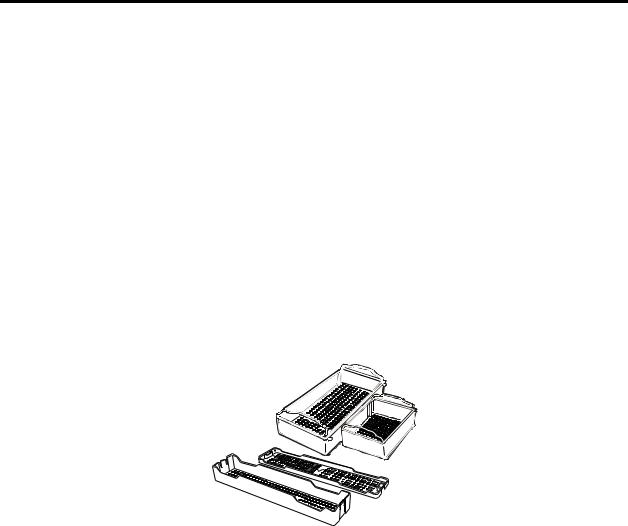
♦Only STERRAD and APTIMAX® Instrument Trays and STERRAD
Accessories are recommended for use in the STERRAD 200 Sterilizer. STERRAD and APTIMAX® Instrument Trays are specially designed to allow diffusion of hydrogen peroxide and the plasma around all the items in the load. The trays should only be padded with STERRAD Instrument Tray Mats or polypropylene sterilization wrap. Do NOT use linen, cellulosic, or any materials shown in the “Items Not Recommended” list. Follow the instructions for use included with the STERRAD Instrument Tray Mats to determine the number of mats that can be used at one time in the chamber.
Figure 4. Use only STERRAD® Instrument Trays and
APTIMAX® Instrument Trays
♦Improper loading of the sterilizer may result in cycle cancellations and/or positive biological indicator results.
♦Configure loads with a combination of metal and nonmetal items, and wrapped trays and pouches to maximize the efficiency of the cycle.
♦Do not use foam pads in instrument trays. They may absorb the hydrogen peroxide.
♦Do not use any wraps or packaging that are not approved by ASP and listed in the previous section on “items not recommended.”
STERRAD® 200 User’s Guide |
23 |

3Preparing Items To Be Sterilized
♦Use only STERRAD 200 Sterilizer compatible polypropylene sterilization wrap and Tyvek® pouches. Do not use paper pouches or sterilization wraps containing cellulose or cotton.
♦Place STERRAD Chemical Indicator Strips inside trays and Tyvek® pouches.
♦Secure all wraps with STERRAD SealSure® Chemical Indicator Tape.
♦Arrange items to ensure that the hydrogen peroxide and plasma can contact all surfaces.
♦Place peel pouches loosely on edge, if possible. Arrange them so that the transparent side of a pouch faces the opaque side of the next pouch. Do not stack pouches on top of each other.
♦Do not allow any item to touch the walls of the sterilization chamber, doors, or electrode.
Note: Do not stack instruments inside the trays. Do not stack trays. Do not stack trays within trays. Do not wrap instruments within a wrapped tray.
Note: If you are using rigid containers cleared by the FDA for use in the STERRAD System, follow the same procedures that are recommended for use with the STERRAD or APTIMAX® Instrument Trays. Do not stack instruments inside the containers. Do not stack containers. Do not stack containers within containers. Do not wrap instruments within the containers.
Caution: Metal objects must not come into contact with the walls of the sterilization chamber, door, or electrode. Contact with the walls, door, or electrode can interrupt the plasma phase of the process, cause a cycle cancellation, and/or damage the item or the sterilizer.
24 |
STERRAD® 200 User’s Guide |
 Loading...
Loading...