AliveCor AC019 Users Manual

Instructions for Use (IFU)
for
KardiaMobile® (Model AC-019)
19LB01.X13
AliveCor, Inc.
444 Castro Street,
Mountain View, CA 94041, USA
© AliveCor, Inc. 2019
AliveCor is a registered trademark of AliveCor, Inc.

Instructions for Use for KardiaMobile® (Model AC-019)
Table of Contents
Introduction 3
Guide to Parts 3
Warnings 4
Cautions 5
Indications For Use 5
Terms 5
Setting up your KardiaMobile® (Model AC-019) hardware for the first time 6
Recording a Six-Lead ECG 8
Recording a Single-Lead ECG 10
ECG Analysis 12
Heart Rate 13
Previously Recorded ECGs 13
Clinical Testing 14
Environmental Specifications 14
Expected Service Life 14
Maintenance 15
Electromagnetic & Other Interferences 15
FCC Compliance 15
Ingress Protection Marking 16
Applied Parts 16
Troubleshooting 16
Electrical Safety 18
Equipment Symbols 23
Page 2 of 23

Instructions for Use for KardiaMobile® (Model AC-019)
KardiaMobile® (Model AC-019)
Introduction
1. The KardiaMobile® (Model AC-019) System allows you to record a Six-Lead ECG and a
Single-Lead ECG.
a. It is recommended that you record a Six-Lead ECG (which provides more data to
your cardiologist) when possible.
2. The KardiaMobile® (Model AC-019) hardware is used with a user-supplied compatible
smartphone or tablet.
3. The KardiaMobile® (Model AC-019) System consists of:
a. KardiaMobile® (Model AC-019) – a device that has 3 electrodes to sense and
transmit 6-Lead ECG rhythms to the smartphone or tablet and that can optionally
attach to your compatible smartphone with the sold-separately phone clip;
b. Kardia app – SW application installed on a compatible smartphone or tablet used to
collect, view, and save ECG recordings and to wirelessly transmit to the AliveCor
server;
Guide to Parts
TOP VIEW
BOTTOM VIEW
Page 3 of 23

Instructions for Use for KardiaMobile® (Model AC-019)
Warnings
1. AliveCor does not guarantee that you are not experiencing an arrhythmia or other health
conditions when labeling an ECG as normal. You should notify your physician for possible
changes in your health.DO use this device to record heart rate and heart rhythm only.
2. DO NOT use to diagnose heart- related conditions.
3. DO NOT use to self-diagnose heart related conditions. Consult with your physician before
making any medical decision, including altering your use of any drug or treatment.
4. DO NOT continue use until further instructed by a physician if your skin is irritated or
inflamed around the electrode.
5. AliveCor makes no warranty for any data or information that is collected erroneously by the
device, or misuse or malfunction as a result of abuse, accidents, alteration, misuse, neglect,
or failure to maintain the products as instructed. Interpretations made by this device are
potential findings, not a complete diagnosis of cardiac conditions. All interpretations should
be reviewed by a medical professional for clinical decision-making.
6. The device has not been tested for and is not intended for pediatric use.
7. Keep device away from young children. Contents may be harmful if swallowed. Device
contains a coin cell battery that is not accessible during normal use but, if exposed, can be a
choking hazard and may cause severe tissue injury if ingested.
8. DO NOT replace the battery when device is in use.
9. DO NOT use the electrode on a portion of the body with too much body fat, body hair or
very dry skin, a successful recording may not be possible.
10. DO NOT take a recording while driving or during physical activity.
11. DO NOT store in extremely hot, cold, humid, wet, or bright conditions.
12. DO NOT take a recording if electrodes are dirty. Clean them first.
13. DO NOT immerse device or expose device to excessive liquid.
14. DO NOT use while charging your phone.
15. DO NOT drop or bump with excessive force.
16. DO NOT expose to strong electromagnetic fields.
17. DO NOT expose the device to a magnetic resonance (MR) environment.
18. DO NOT use with a cardiac pacemaker, ICDs, or other implanted electronic devices.
19. DO NOT use during cautery and external defibrillation procedures.
20. DO NOT place electrodes in contact with other conductive parts including earth.
21. DO NOT use with un-approved accessories. Use of non-AliveCor approved accessories or
transducers and cables could result in electromagnetic emissions or decreased
electromagnetic immunity of this device and result in improper operation.
22. DO NOT use adjacent to or stacked with other equipment because it could result in
improper operation.
23. DO NOT use portable RF communications equipment (including peripherals such as
antenna cables and external antennas) closer than 30 cm (12 inches) to any part of the
KardiaMobile® (Model AC-019) System. Otherwise, degradation of the performance of the
KardiaMobile® (Model AC-019) System could result.
Page 4 of 23

Instructions for Use for KardiaMobile® (Model AC-019)
Cautions
1. Detection of possible Atrial Fibrillation (AF) in your EKG results are not to be used for
diagnosis. If you are experiencing any concerning symptoms, contact your physician.
2. Result of "Bradycardia" or "Tachycardia" are designations of heart rate, not a clinical
diagnosis of an arrhythmia. Please consult with your physician should you receive
consistent identifications of "Bradycardia" or "Tachycardia".
3. “Unreadable” EKG results determines that you didn't have proper EKG recording for
analysis. You may try to re-record your EKG.
Indications For Use
The KardiaMobile® (Model AC-019) System is intended to record, store and transfer one- and
two-channel electrocardiogram (ECG) rhythms. In single channel mode, the KardiaMobile®
(Model AC-019) System can record Lead-I. In two channel mode, the KardiaMobile® (Model
AC-019) System can record Lead-I and Lead-II simultaneously and derive Lead-III and unipolar
limb leads aVR, aVF and aVL. The KardiaMobile® (Model AC-019) System also displays ECG
rhythms and output of ECG analysis from AliveCor’s KardiaAI platform including detecting the
presence of normal sinus rhythm, atrial fibrillation, bradycardia, tachycardia, and others. The
KardiaMobile® (Model AC-019) System is intended for use by healthcare professionals, patients
with known or suspected heart conditions and health conscious individuals. The device has not
been tested and is not intended for pediatric use.
Terms
ECG: Also known as an electrocardiogram, an ECG is a test that detects and records the
strength and timing of the electrical activity in your heart. This information is recorded on a
graph that shows each phase of the electrical signal as it travels through your heart.
Single-Lead ECG: When the 2 top electrodes (Left Hand Electrode and Right Hand Electrode)
are used, the Kardia app will display a Single-Lead ECG that is comparable to Lead I on
standard ECG machines.
Six-Lead ECG: When all 3 electrodes (Left Hand Electrode, Right Hand Electrode, and Left Leg
Electrode) are used, the Kardia app will display a Six-lead ECG that is comparable to Leads I, II,
III, aVF, aVL, and aVR on standard ECG machines. It is recommended that you record a SixLead ECG (which provides more data to your cardiologist) when possible.
Page 5 of 23
Instructions for Use for KardiaMobile® (Model AC-019)
Setting up your KardiaMobile® (Model AC-019)
hardware for the first time
1. Remove KardiaMobile® (Model AC-019) from packaging.
2. Setup KardiaMobile® (Model AC-019)
○ Download the Kardia app from the App Store or Google Play on your compatible iOS
or Android device (check for compatibility at www.alivecor.com/compatibility).
○ Make sure you have Bluetooth turned on your smartphone (or tablet).
3. Tap on your home screen to launch the Kardia app and tap Create Account.
○ Follow the onscreen instructions to create an account.
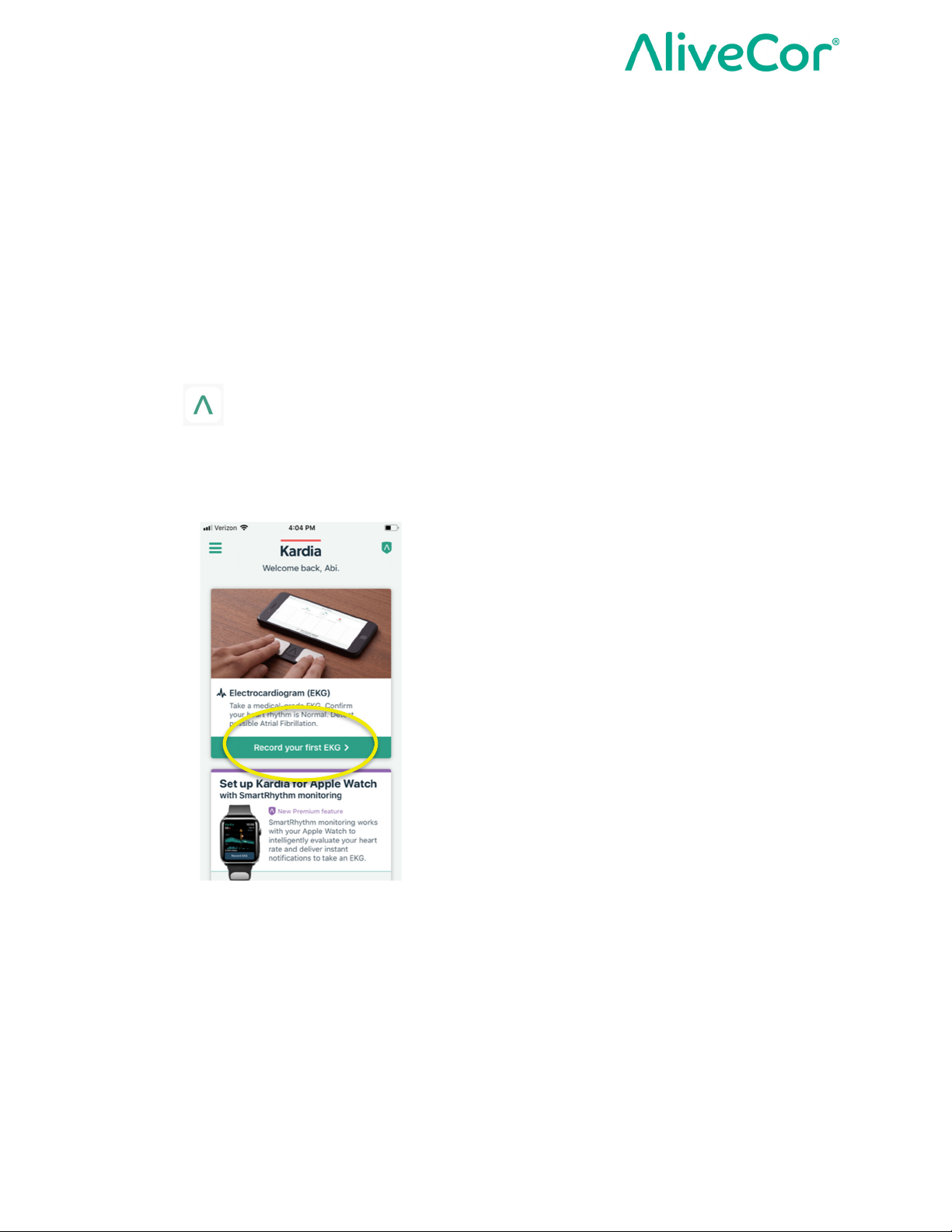
4. From the Kardia app home screen, tap Record your first ECG.
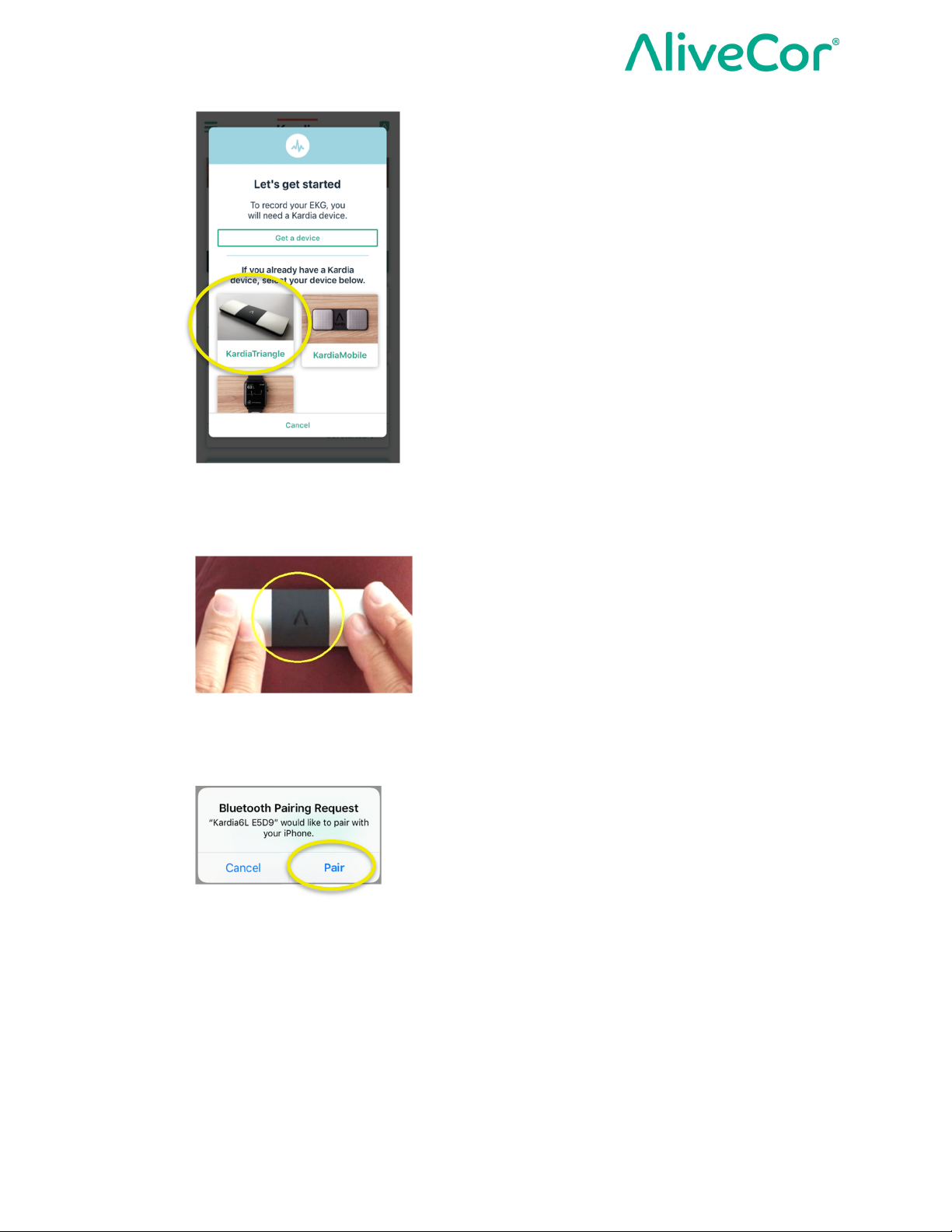
5. Select KardiaMobile® (Model AC-019) as your device
Page 6 of 23
Instructions for Use for KardiaMobile® (Model AC-019)
6. Tap lightly on the 2 top electrodes (Left Hand Electrode and Right Hand Electrode) for 5
seconds to wake up your KardiaMobile® (Model AC-019) hardware and initialize Bluetooth
pairing.
7. Select Pair to pair the KardiaMobile® (Model AC-019 hardware with your phone via
Bluetooth.
Page 7 of 23
 Loading...
Loading...