Page 1
Application Note
Food Testing and
Agriculture
Quantitative Screening of Multiresidue
Veterinary Drugs in Milk and Egg
Using the Agilent 6495C Triple
Quadrupole LC/MS
Authors
Siji Joseph, Aimei Zou,
LimianZhao, Patrick Batoon,
and Chee Sian Gan
Agilent Technologies, Inc.
Abstract
This application note demonstrates the use of the Agilent Comprehensive
Veterinary Drug dMRM Solution for the screening of 210 target residues in milk and
eggmatrices. The workflow specifies conditions for chromatographic separation,
MS detection, and data processing, using a slightly modified sample preparation
procedure. Workflow performance was assessed based on limit of detection (LOD),
limit of quantitation (LOQ), calibration curve linearity, accuracy, precision, recovery,
and repeatability. Over 93% of veterinary drugs showed LOD of ≤1 μg/kg in milk
samples. Calibration curves for all targets ranged from the LOQ to 100 μg/kg with
a coefficient of correlation (R2) ≥0.99. Target peak area response (%RSD) was
<15%, and retention time (RT) %RSD was <0.5%. Method accuracy values, based on
matrix-matched calibration were within 87 to 117%. The average recovery of 95%
of targets was within 60 to 120%, with repeatability %RSD of ≤15%. Both milk and
egg matrices showed similar quantitative results. Injection-to-injection robustness
results demonstrated excellent target peak area and RT reproducibility across
400injections, confirming the workflow capability for routine multiresidue screening
with large-scale sample sets.
Page 2

Introduction
The Agilent Comprehensive Veterinary
Drug dMRM Solution is an end-to-end
workflow solution for targeted screening
or quantitation of 210 veterinary drug
residues in animal origin matrices,
which accelerates and simplifies routine
laboratory testing. The solution includes
comprehensive sample preparation,
chromatographic separations,
optimized MS detection method
conditions, data analysis methods, and
reporting templates for 210 veterinary
drugs in various food matrices. The
Comprehensive Veterinary Drug dMRM
Solution minimizes method development
time and combines multiple food
matrix analyses into one easy-to-follow
protocol. Agilent MassHunter data
acquisition software, together with
the dMRM database offers easy
customization of dMRM submethods
based on preferred target list or
regulation, as determined by the user.
The solution is available and has been
verified with two mass spectrometers
(Agilent 6470 triple quadrupole LC/MS
and the Agilent 6495C triple quadrupole
LC/MS) to address diverse sensitivity
demands based on the choice of sample
matrix and specific regulations that
varyglobally.
The solution was originally developed
for the quantitative screening of
210multiclass veterinary drugs in
chick, beef, and pork.1 It was then
demonstrated to be effective for
seafood using salmon and shrimp as
example matrices.2 This study further
demonstrates the applicability for
milk and eggs using the 6495C triple
quadrupole LC/MS. For the 210 target
analytes screened in this study, 103
of them had maximum residue limits
(MRL) established in milk regulated
by the AOAC3—with an additional
16targets regulated by US FDA-CFR4,
US FSIS5, or EU6 regulations/guidelines.
The MRL values are typically lower in
milk compared to meat and seafood,
thus requiring a higher MS detection
sensitivity. Additionally, the high fat
and protein content in milk demands
effective sample preparation and a
sensitive detector to monitor trace levels
of drug residues. Compared to milk, the
number of MRL-established targets for
the egg matrix is fewer and the residue
limits are more relaxed.
Experimental
Standards and reagents
Veterinary drug standards were
purchased from Sigma-Aldrich (St. Louis,
MO, USA), Toronto Research Chemicals
(Ontario, Canada), and Alta Scientific
(Tianjin, China). Agilent LC/MS-grade
acetonitrile (ACN, partnumber
5191-4496), methanol (MeOH,
partnumber 5191-4497), and water
(part number 5191-4498) were used
for the study. All other solvents used
were HPLC-grade from Sigma-Aldrich.
LC/MS additives for mobile phases were
also purchased from Sigma-Aldrich.
Individual stock solutions of veterinary
drugs were prepared from powdered or
liquid veterinary drug standards at 1,000
or 2,000 µg/mL using an appropriate
solvent (MeOH, dimethyl sulfoxide
(DMSO), ACN, or water or solvent
mixture). A few stock standard solutions
(100 µg/mL) were obtained from the
suppliers listed above.
A comprehensive standard mix
(1µg/mL of each target analyte in
50/50 ACN/water) was prepared from
individual stock solutions and used for
this experiment.
Sample preparation
Milk and egg samples were purchased
from a local grocery. For the analysis
of milk, a 2.0 ±0.1 mL portion of milk
was transferred in a 50 mL conical
polypropylene tube. For the analysis
of egg, a 2.0 ±0.1 g portion of the
homogenized sample was weighed in a
50 mL conical polypropylene tube. If not
analyzed immediately, the samples were
stored at –20 °C.
Sample preparation was performed
as per the procedure defined in the
Comprehensive Veterinary Drug
dMRM Solution (G5368AA) using
solvent extraction followed by
Agilent Captiva EMR—Lipid cleanup
(partnumber5190-1003), aided by
the Agilent positive pressure manifold
processor (PPM-48, part number
5191-4101).7 The sample preparation
procedure is summarized in Figure1. The
aqueous extraction step was modified
to adjust the target dilution due to
increased water content in milk andegg.
The following deviations from the
protocol defined in the Comprehensive
Veterinary Drug dMRM Solution
are recommended for the aqueous
extraction step:
– Milk: Concentration of EDTA solution:
1 M, volume added: 200 µL.
– Egg: Concentration of EDTA solution:
0.1M (same as workflow guide),
volume added: 1 mL
Matrix-spiked (pre-extraction) QC
samples were prepared by spiking the
appropriate veterinary standard solution
into the milk and egg matrices at various
levels: 1 μg/kg for low-range QC (LQC),
10 μg/kg for mid-range QC (MQC), and
25 μg/kg for high-range QC (HQC),
respectively. An additional QC level lower
than the LQC of 0.1 μg/kg (LLQC) was
included in the milk analysis to verify
the analytical characteristics of a few
targets, and to meet the very low MRL
requirement. After spiking standards, the
samples were vortexed for 30seconds,
then equilibrated for 15 to 20 minutes
to allow the spiked standards to
infiltrate the sample matrix before
sampleextraction.
2
Page 3

Matrix-matched calibration standards
Two 50 mL
tubes 2.0 g
sample
for QC samples
Blank matrix eluent
Matrix-matched (postextraction)
calibration standards were prepared
as per the workflow protocol by
spiking appropriate standards into the
blank matrix extract.7 The targeted
concentrations of matrix-matched
calibration levels were 0.1, 0.25, 0.5, 1.0,
2.5, 5.0, 10.0, 25.0, 50.0, and 100.0 μg/kg
(10 levels). An additional matrix-matched
calibration level of 0.05μg/kg was
added for milk analysis for similar
consideration of few targets with very
low MRL requirement. Considering the
10x dilution factor introduced during
sample preparation, the actual spiking
concentrations of postextraction
calibration standards were 0.005, 0.01,
0.025, 0.05, 0.10, 0.25, 0.5, 1.0, 2.5, 5.0,
and 10.0 μg/L (ppb) in the milk blank
matrix extract.
Neat standards at 2.5 μg/L in a 50/50
ratio of ACN/water was used to
evaluate matrix effects by comparing
the responses in the corresponding
postextraction-spiked calibration
standards.
Chromatographic separation was
performed using an Agilent InfinityLab
Poroshell 120 EC-C18 column
(partnumber 695575-302) installed on
an Agilent 1290 Infinity II LC, including
Agilent 1290 Infinity II flexible pump
(G7104C), Agilent 1290 Infinity II
multisampler (G7167A), and Agilent1290
Infinity II multicolumn thermostat
(G7116A).
Mobile phase A was water with 4.5 mM
ammonium formate, 0.5 mM ammonium
fluoride, and 0.1% formic acid; mobile
phase B was 50/50 ACN/MeOH with
4.5 mM ammonium formate, 0.5 mM
ammonium fluoride, and 0.1% formic
acid. The LC system was equipped with
a 20 µL injection loop and multiwash
capability. Please see the workflow guide
included with the Agilent Comprehensive
Veterinary Drug dMRM Solution
(G5368AA) for additional details.
7
The “6495 Veterinary Drug
Comprehensive” method included in the
Comprehensive Veterinary Drug dMRM
Solution for the 6495C triple quadrupole
LC/MS (G6495C) was used directly for
acquisition. The 6495C LC/MS triple
quadrupole with an Agilent Jet Stream
(AJS) ion source was operated in
dynamic MRM (dMRM) mode. Autotune
was performed in unit resolution with
report m/z below 100 mode enabled.
MassHunter acquisition software version
10.0 was used for data acquisition,
and MassHunter quantitative analysis
software version 10.0 was used to
process the data.
Results and discussion
Workflow performance in milk
Chromatographic separation using the
Agilent InfinityLab Poroshell EC-C18
column resulted in good separation and
RT distribution of 210 veterinary drugs
within a 13-minute elution window.
Target-specific MRM transitions
included in the dynamic MRM method
helped to meet regulatory requirements
for compound identification and
confirmation. The default dynamic
MRM method utilized a cycle time of
750ms, and dwell times for each dMRM
transition ranged from 7 to 370ms,
offering more than 10 data points
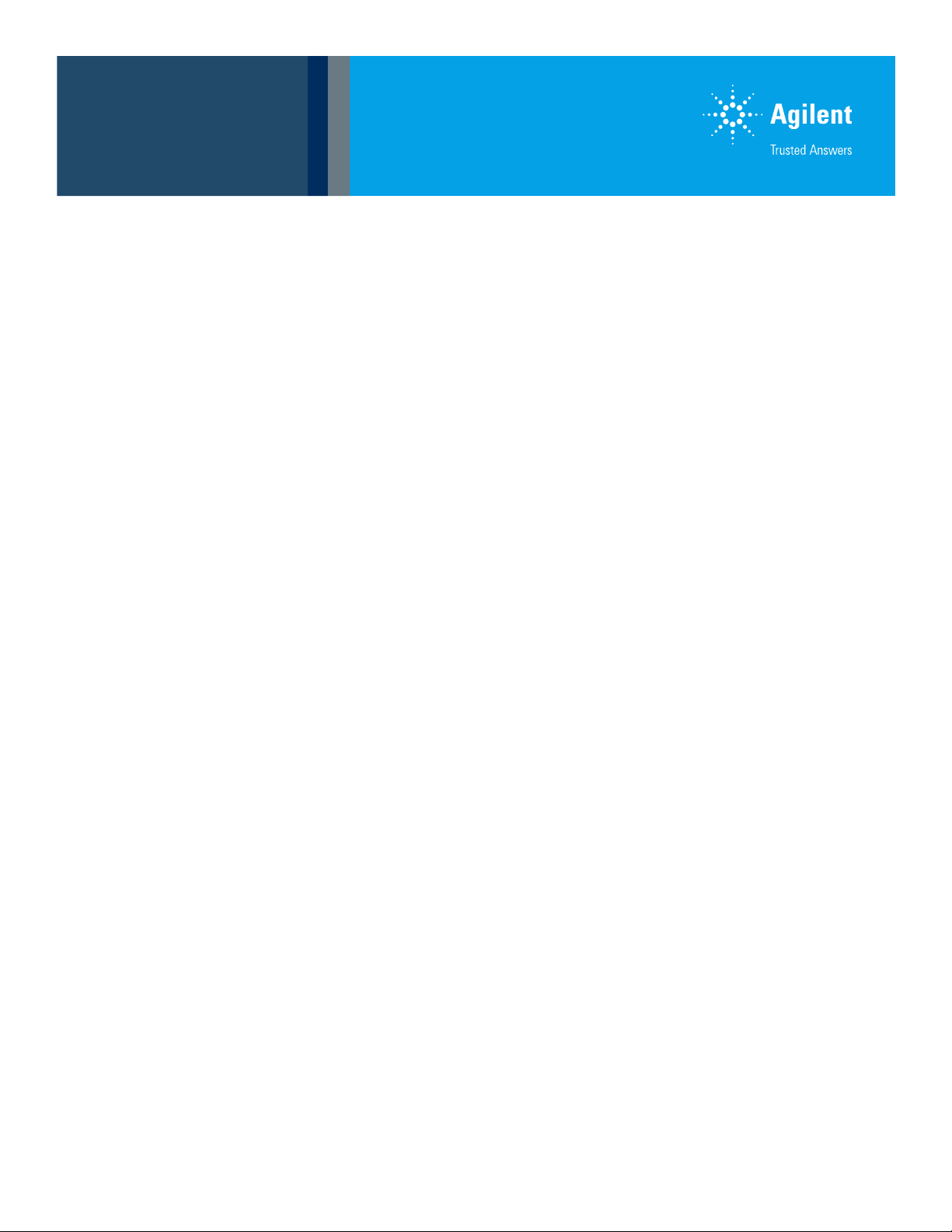
across any given peaks. Figure2 shows
a representative MRM chromatogram
for all 210 veterinary drug targets,
postextraction spiked at 1.0 μg/L in the
milk blank matrix extract. Considering
the dilution factor during sample
preparation was 10x, this 1.0 μg/L
postextraction spike was equivalent to a
10 μg/kg spike in milk. The symmetrically
sharp peaks demonstrate the efficient
chromatographic separation of targets
within the elution window. Table 1 lists
the name, chemical class, CAS number,
and RT of all 210 targets covered in
thiswork.
1
2
1
Figure 1. Sample extraction procedure using solvent extraction followed with Agilent Captiva EMR—Lipid cleanup.
Blank matrix
(no spike)
2
Pre-extraction spike
2-Step solvent
extraction
Centrifuge
Sample cleanup using
Agilent Captiva EMR—Lipid 3 mL
cartridges on Agilent PPM-48
Homogenized
eluents
for matrix blank
and post-extraction
spike calibration levels
LC/MS analysis using
Agilent 6495C TQ
Pre-extraction spike
eluents as QC samples
3
Page 4
×10
5
Acquisition time (min)
Counts
Acquisition time (min)
Counts
2.0
1.5
1.0
0.5
0
1 2 3 4 5 6 7 8 9 10 11 12
Figure 2. Representative MRM chromatogram of 210 veterinary drug targets postextraction spiked at 1.0 μg/L in the milk
blank matrix extract using the Agilent 6495C triple quadrupole LC/MS).
From the AOAC MRL established
list, the early eluted analytes,
including amoxicillin, baquiloprim,
deacetylcefapirin, diminazene, imidocarb,
norgestomet, sulfaguanidine, and
tilmicosin showed split peaks due
to solvent effects. The “spectrum
summation” integrator algorithm was
used to reliably and automatically
integrate these targets for consistent RT,
and thus eliminated the need for manual
reintegrations.8 The peak shape for these
targets can be improved by converting
samples into a higher aqueous mixture
prior to LC/TQ injection.
LOD, LOQ and calibration
curvelinearity
LOD and LOQ were established using
various low level matrix-matched
calibration standards.
1,2
The
signal-to-noise ratio (S/N) was calculated
using the peak height for signal and an
auto-RMS algorithm for noise, included
in the MassHunter quantitative analysis
software. The method sensitivity using
the 6495C LC/TQ system offered a
LOD ≤1 μg/kg for over 93% of analytes
tested in both milk and egg. The low
detection limits achieved allowed the
high sensitivity demand for screening
trace level veterinary drug residues in
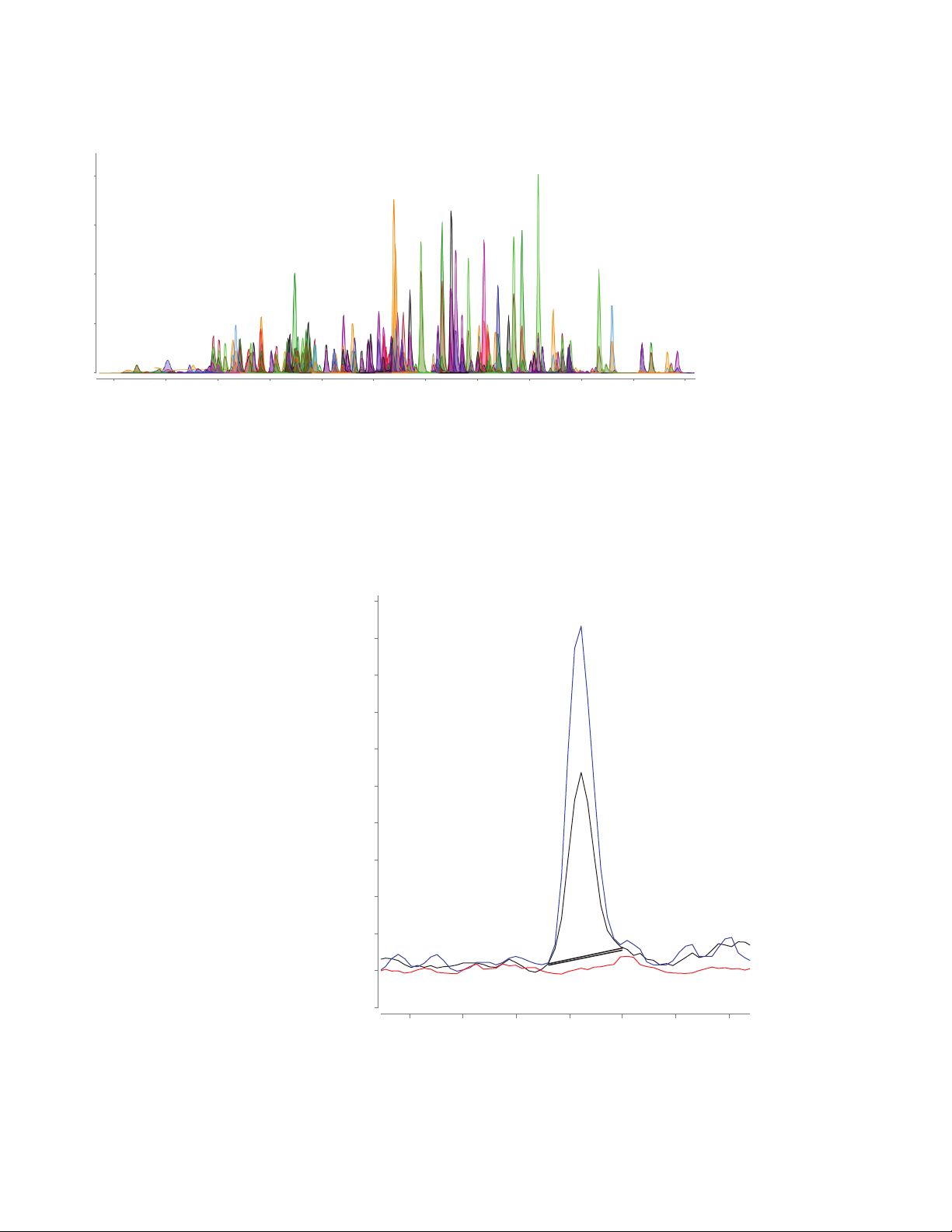
milk. As an example, AOAC regulated
MRL of 0.05μg/kg for clenbuterol in milk.
The 6495C TQ-based workflow provided
a clean, symmetrical peak with S/N of
calibration level, thus enabling confident
target identification and quantitation
(Figure 3).
32 at the 0.05 μg/kg matrix-matched
2
×10
RT: 5.42 (min)
5
4
3
2
1
0
5.1 5.2 5.3 5.4 5.5 5.6 5.7
Figure 3. MRM chromatogram of clenbuterol (MRM 277.1 & 202.9)
postextraction-spiked at 0.005 μg/L (black trace) and 0.01 μg/L (blue trace) in
the milk matrix extract, overlaid with matrix blank (red trace). The defined LOD
of clenbuterol is 0.05 μg/kg (S/N: 32) and LOQ is 0.1 μg/kg (S/N: 76).
4
Page 5
A calibration curve for each target
Acquisition time (min)
Counts
was generated using matrix-matched
calibration standards at levels
ranging from the defined LOQ to
the highest-spiked level. The linear
regression was used with ignored
origin and 1/x or 1/x2 weight. All targets
met the calibration curve linearity
requirement of R2 ≥0.99. The LOD, LOQ,
and calibration curve data of all targets in
the milk are shown in Table1.
Instrument method precision
andaccuracy
Precision was determined by calculating
the %RSD of the target response and
RT using triplicate injections of the
matrix-matched calibration levels.
The average accuracy value for each
matrix-matched calibration level was also
calculated from the triplicateinjections.
Good precision and accuracy values
were obtained for all targets in milk.
Target response %RSD for all targets in
the milk matrix at 10 μg/kg was <15%,
and the RT %RSD of all targets were
within 0.5%.9 The accuracy values of
all targets at 10 µg/kg within a range
of 87 to 117%. These results confirm
the reproducibility of chromatographic
separation and MS detection.
average recovery was calculated from
duplicate injections of four technical
preparations. The intrabatch recovery
repeatability was measured as %RSD
of recovery, calculated using four
technical preparations of matrix-spiked
QCsamples.
The results showed that recoveries of
about 93% of MRL-established targets
reached the acceptable range of 60
to 120% with an excellent intrabatch
RSD ≤20%.9 Recoveries of the
remaining seven targets, baquiloprim,
chlortetracycline, deacetylcefapirin,
diclofenac, imidocarb, oxytetracycline,
and trichlorfon [DEP], were within
a range of 30 to <60% or >120 to
124%. However, for these targets, the
workflow still provided good recovery
repeatability values within a %RSD of
2
×10
7
6
5
RT: 5.40 (min)
9%, demonstrating consistent extraction
behavior. These results confirmed
the entire workflow reproducibility
using Captiva EMR—Lipid sample
extraction and cleanup protocol in the
6495-TQ-based instrument detection.
The recovery and repeatability results
of all 210 targets are included in Table 1
(see Appendix).
The workflow performance combined
with the 6495C LC/TQ detection helped
confident recovery and repeatability
assessment at trace levels in milk.
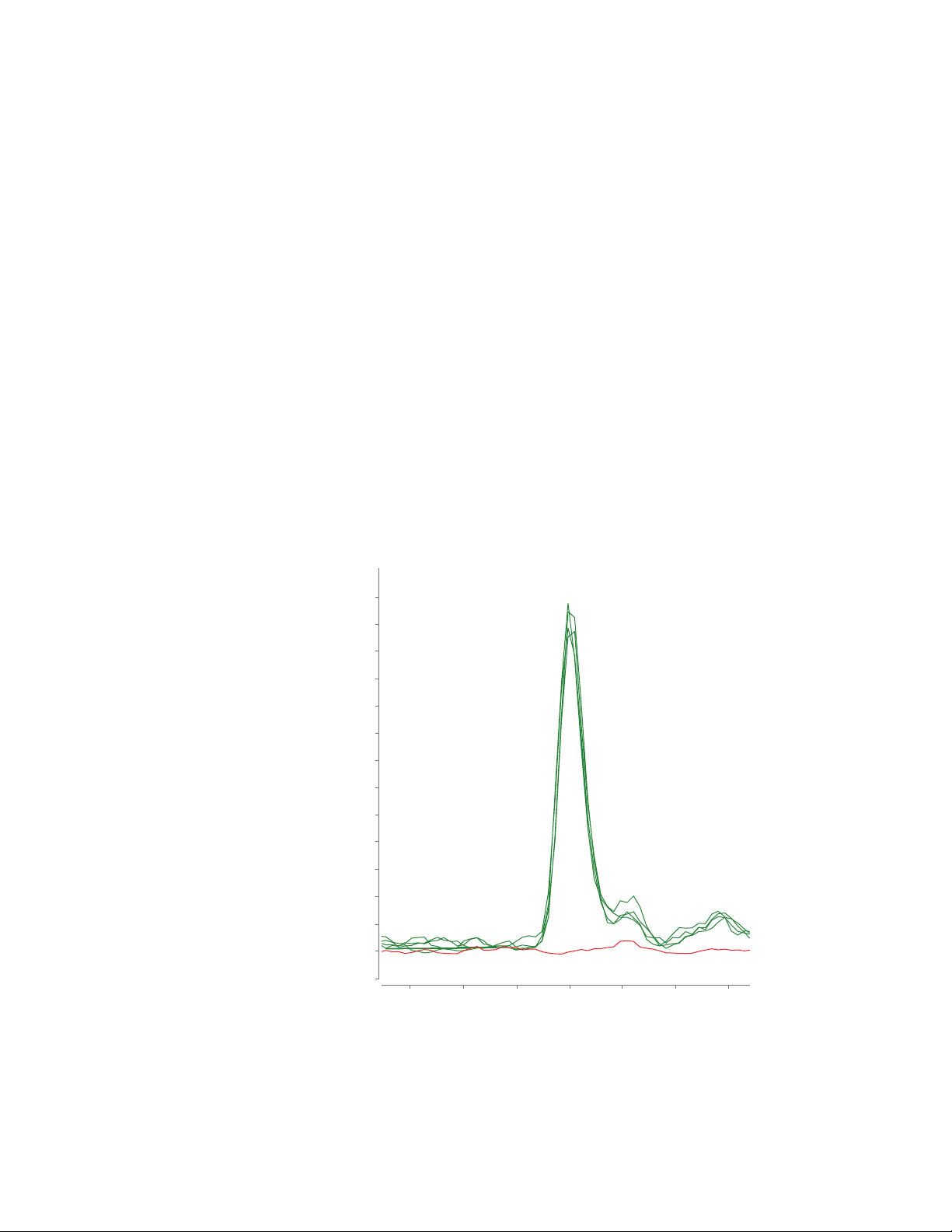
Figure 4 shows an example of workflow
recovery and repeatability for clenbuterol
at 0.1 μg/kg in milk. The average
recovery of this target using the LLQC
sample is 118% with good recovery
repeatability of %RSD <5%.
Target recovery and
intrabatchrepeatability
The impact of sample preparation on
target recovery was assessed using
matrix-spiked QC samples. Each QC
level was prepared with four technical
preparations and was injected for
instrument analysis in duplicates. An
appropriate level of matrix-spiked QC
sample based on MRL was selected
to evaluate target-specific recovery
and repeatability. Recovery was
calculated using target response in
matrix-spiked QCs, and measured
response using matrix-matched
calibration curve equations. The
4
3
2
1
0
5.1 5.2 5.3 5.4 5.5 5.6 5.7
Figure 4. MRM chromatograms of clenbuterol (MRM 277.1 & 202.9) using
four technical preparations of LLQC samples in milk (green traces) overlaid
with matrix blank (red trace).
5
Page 6

Matrix effect assessment
Matrix effect (ME) is an important
parameter for method sensitivity and
reliability assessments. ME is defined as
the ratio of analyte area response (I) in
matrix-matched samples with those in
the corresponding neat standards (see
Equation1). The closer the ME value is to
100%, the less the matrix effect presents;
results lower than 100% indicate matrix
suppression, while results >100%
indicate potential enhancement.
Equation 1.
I
matrix
I
solvent
× 100
ME =
In this study, ME was investigated
using target response from
postextraction-spiked calibration
levels at 2.5 μg/L in blank matrix
extract, compared with corresponding
neatstandards.
In the milk matrix, within a total of
103 MRL established analytes, >95%
of targets showed an ME of >75%,
indicating negligible matrix suppression.
Four targets (amoxicillin, cefalonium,
nafcillin, and sulfamethizole) resulted in
an ME of 50 to 75%, indicating moderate
ion suppression. Target deacetylcefapirin
showed an ME of 48%, indicating
significant ion suppression.
Method verification in egg matrix
The method sensitivity in the egg was
similar to that of the milk matrix. Linear
matrix-matched calibration curves
ranging from LOQ to 100 μg/kg were
demonstrated with R2 ≥ 0.99. Instrument
method precision (%RSD) for target
responses and RTs were <15% and
<0.5%, respectively. Instrument method
accuracy for the matrix-matched
calibration level at 10.0 μg/kg were within
80 to 113% (n = 3). Recoveries of over
94% targets in the egg matrix were within
60 to 120% acceptance criteria, and
recovery repeatability with %RSD values
≤15%. Targets amprolium, baquiloprim,
chlortetracycline, deacetylcefapirin,
doxycycline, erythromycin,
oxytetracycline, tetracycline, and
rafoxanide showed <60% recoveries, but
recovery repeatability values were within
an acceptable limit of <15%RSD. Severe
ion suppression and poor recovery
(<20%) was observed for the dipyrone
hydrate-metabolite, however, no MRL is
established for this veterinary drug in
theegg.
AOAC MRL-based residue screening
in milk and egg
The MRL values of 103 AOAC-listed
targets range from 0.05 to
200μg/kg in milk.3 The method
sensitivity in milk using the 6495C
LC/TQ enabled confident screening of
all targets, except for diclofenac and
norgestomet. Table 1 summarizes the
MRL requirement and observed results
for all targets. Among the comprehensive
target list, 22 have MRL established for
egg under AOAC guidelines, and the
values range from 0.7 to 4,000μg/kg.3
The method sensitivity easily met
the screening requirement for all
MRL-established targets in egg per the
AOAC guidelines.
High blank contribution was observed
for the analysis of chlorhexidine,
clindamycin, progesterone, and
gonadotropin in both milk and egg,
indicating the potential positive
incurrence in the used sample
matrix. Trace residues of ethopabate,
oxibendazole, piperonyl butoxide
ammonia, and tripelennamine
affected the LOQ determination in
milk. Alternatively, the residues from
imidocarb, oxyphenbutazone, piperonyl
butoxide ammonia, and testosterone
affected the LOQ determination in egg.
Method robustness
The method robustness was assessed
by 400 continuous injections of
AgilentVeterinary Drug System Suitability
test mix (part number5799-0015)
postspiked in milk matrix. Peak
responses and RT consistency were
monitored for all 25 targets over time.
The 25 veterinary drug targets are from
10 different chemical classes, with a
broad range of molecular weight, eluted
evenly across the elution window, and
cover both positive and negative polarity
ionization. The dMRM peak area %RSD
and RT %RSD of all 25 targets were
calculated from the 400 injections of
1.0 μg/L postspiked milk blank matrix
extract. The data acquisition was
continuous, and the instrument was
operated without readjusting any tune
parameters. The entire run lasted for
>120 hours.
The elution profile using the InfinityLab
Poroshell column was extremely
consistent over 400 injections. A good
response reproducibility with %RSD
<4.0% and RT %RSD of <0.2% were
observed for all 25 targets. The response
reproducibility of all 25 standards
over 400 injections is summarized in
Figure 5, and an overlay of five total
ion chromatograms (TIC) of Agilent
Veterinary Drug System Suitability test
mix MRM (spread across 400injections)
are shown in Figure 6. The innovative ion
transfer optics design of Agilenttriple
quadrupole mass spectrometers
minimizes the source contamination
from the matrix, thus providing a robust
analytical platform for the confident
analysis of trace veterinary drug residues
(Figure 7). The sample preparation
procedure here provided efficient
sample matrix cleanup, greatly reduced
the matrix residue accumulation on
the ion source interface, and provided
extended column lifetime and detection
consistency. The method robustness,
calculated from a 5-day continuous data
acquisition, confirmed the sustainable
performance of the LC/TQ workflow for
day-to-day operations.
6
Page 7

500,000
600,000
400
MRM peak area
Injection number
Acquisition time (min)
Counts
400,000
300,000
200,000
100,000
0
050 100 150 200 250 300 350
Figure 5. The response reproducibility of 25 targets included in the Agilent Veterinary Drug System Suitability test mix over 400 continuous injections.
Concentration: postspiked at 1.0 μg/L in milk blank matrix extract (equivalent to a 10 μg/kg matrix spike in milk). Please refer to Table 1 for the list of 25 targets in
the Veterinary Drug System Suitability test mix.
5
×10
2.4
2.2
2.0
1.8
1.6
1.4
1.2
1.0
0.8
0.6
0.4
0.2
0
TIC MRM Injection 400
TIC MRM Injection 300
TIC MRM Injection 200
TIC MRM Injection 100
TIC MRM Injection 001
3.5 4.0 4.5 5.0 5.5 6.0 6.5 7.0 7.5 8.0 8.5 9.0 9.5 10.0
Figure 6. Overlay of five selected system suitability mix TIC MRM chromatograms, spread across 400 continuous replicate injections demonstrating the target
elution consistency. Concentration: postextraction spiked at 1.0 μg/L in milk blank matrix extract, LC separation column: Agilent InfinityLab Poroshell 120 EC-C18
(part number 695575-302). (offset X, Y-axis values, injections: 1, 100, 200, 300, and 400).
7
Page 8

Conclusion
A
B
This study demonstrates the applicability
of the Agilent Comprehensive Veterinary
Drug dMRM Solution for the screening
and quantitation of 210 multiclass
veterinary drug residues in milk and egg
matrices. The workflow-recommended
sample preparation protocol, using
solvent extraction followed by Agilent
Captiva EMR—Lipid cleanup, was shown
to be efficient for target extraction
and matrix cleanup from milk and
egg. The workflow performance was
characterized by good results in terms
of linearity, accuracy, recovery, and
repeatability, allowing sensitive detection
of multiclass veterinary drug residues.
The Agilent 6495C triple quadrupole
LC/MS-based workflow provided
sub-1µg/kg (ppb) LODs for most
analytes, and exceeded the sensitivity
requirements set by global regulatory
Figure 7. The Agilent Jet Stream technology ion source (AJS) of
the Agilent6495C triple quadrupole LC/MS before (A) and after
(B)400continuous injections of milk matrix.
agencies for screening trace veterinary
residues in complex matrices like milk
and eggs. The results demonstrated the
method reliability for routine screening
of over 98% of AOAC-listed veterinary
drug targets from the milk matrix, and
100% of AOAC-listed targets from
the egg matrix. The robustness of
400continuous injections confirmed the
method consistency and reliability, with
minimized sample residue accumulation
on the ion source interface.
8
Page 9

References
1. Siji Joseph et al. An End-To-End
Workflow for Quantitative Screening
of Multiclass, Multiresidue Veterinary
Drugs in Meat Using the Agilent
6470 Triple Quadrupole LC/MS,
Agilent Technologies application note,
publication number 5994-1932EN.
2. Siji Joseph et al. Quantitative
Screening of Multiresidue Veterinary
Drugs in Seafood Using the Agilent
6470 Triple Quadrupole LC/MS,
Agilent Technologies application note,
publication number 5994-2832EN.
3. Screening and identification method
for regulated veterinary drug residues
in food, AOAC guidelines, Version 7,
June 20, 2018.
4. The United States, Code of Federal
Regulations (CFR) - Title 21,
Tolerance of Residues in New Animal
Drugs in Food, Part 556, volume 6,
April 1, 2019.
5. The United States, Chemical
contaminants of public health
concern used by the Food Safety and
Inspection Service (FSIS), 2017.
6. Pharmacologically active substances
and their classification regarding
maximum residue limits (MRL),
Official Journal of the European
Union, Commission Regulation (EU)
No 37/2010.
7. G5368AA Comprehensive Veterinary
Drug dMRM Solution, Agilent
Technologies workflow guide,
D0002979.
8. Steven J. Lehotay, Utility of the
Summation Chromatographic Peak
Integration Function to Avoid Manual
Reintegrations in the Analysis of
Targeted Analytes, LCGC North
America June 2017, 35(6), 391.
9. Guidelines for Standard Method
Performance Requirements, AOAC
Official Methods of Analysis (2016)
Appendix F.
Appendix
Table 1. Target screening results using milk matrix based on AOAC guidelines. The results were generated based on the Agilent 1290 Infinity II LC and
Agilent 6495C triple quadrupole LC/MS systems. Note that these compounds may be obtained from Agilent, and those highlighted in bold are included
in the Agilent Veterinary Drug System Suitability test mix (part number 5799-0015).
Linear
No. Compound Name
2, 4, 6-triamino-pyrimidine-5-carbonitrile 1.62 Insecticide 465531-97-9 N/A 2.5 5 to 100 105 9
1
2,4-DMA [Amitraz Metabolite] 4.45 Insecticide 33089-74-6 10 0.5 1 to 100 108 3
2
2-Quinoxalinecarboxylic acid [QCA] 4.20 Quinoxalines 879-65-2 N/A 2.5 5 to 100 108 4
3
4-epi-oxytetracycline 4.36 Antibiotic/Tetracycline 14206-58-7 100 0.25 0.5 to 100 84 5
4
4-epi-tetracycline 4.28 Antibiotic/Tetracycline 79-85-6 100 0.25 0.5 to 100 82 4
5
5-Hydroxy thiabendazole 3.59 Anthelmintic/Benzimidazoles 948-71-0 50 0.1 0.25 to 100 110 2
6
5-Hydroxyflunixin 8.36 NSAIDs 75369-61-8 2 0.05 0.1 to 100 112* 10*
7
Acepromazine 7.44 Tranquilizer 61-00-7 N/A 0.05 0.1 to 100 100 2
8
Acetyl isovaleryl tylosin [Tylvalosin] 8.80 Antibiotic/Macrolides 63409-12-1 N/A 0.5 1 to 100 106 5
9
Albendazole 8.09 Anthelmintic/Benzimidazoles 54965-21-8 100 0.05 0.1 to 100 110 2
10
Albendazole sulfone 6.22 Anthelmintic/Benzimidazoles 75184-71-3 100 0.1 0.25 to 100 118 2
11
Albendazole sulfoxide 5.62 Anthelmintic/Benzimidazoles 54029-12-8 100 0.1 0.25 to 100 115 3
12
Albendazole-2-aminosulfone 3.81 Anthelmintic/Benzimidazoles 80983-34-2 100 0.25 0.5 to 100 105 2
13
Alpha Zearalanol 8.33 Hormones 26538-44-3 N/A 1 2.5 to 100 110 4
14
Altrenogest 9.05 Hormones 850-52-2 N/A 0.1 0.25 to 100 112 2
15
Aminoflubendazole 6.19 Anthelmintic/Benzimidazoles 82050-13-3 10 0.05 0.1 to 100 112 2
16
Amoxicillin 2.76 Antibiotic/Beta-lactam 26787-78-0 4 1 2.5 to 100 110* 7*
17
Ampicillin 4.00 Antibiotic/Beta-lactam 69-53-4 4 0.5 1 to 100 115* 7*
18
Amprolium 1.22 Antimicrobial 13082-85-4 N/A 1 2.5 to 100 9 4
19
Azaperone 5.87 Tranquilizer 1649-18-9 N/A 0.1 0.25 to 100 96 3
20
RT
(min)
Functional Use/
Chemical Class CAS Number
AOAC3
MRL
(µg/kg)
(µg/kg)
LOD
Calibration
Curve Range
(μg/kg) with
R2 >0.99
MQC
Recovery
(%)
MQC
RSD
(%)
9
Page 10

Linear
Calibration
No. Compound Name
Azithromycin 6.27 Antibiotic/Macrolides 83905-01-5 N/A 0.1 0.25 to 100 76 3
21
Baquiloprim 2.74 Antimicrobial 102280-35-3 30 0.25 0.5 to 100 33 4
22
Betamethasone 7.83 Growth promoters/Corticosteroids 378-44-9 0.3 0.25 0.5 to 100 119* 3*
23
Cabergoline 4.72 Dopamine receptor 81409-90-7 0.1 0.05 0.1 to 100 111• 15•
24
Carazolol 6.16 Tranquilizer 57775-29-8 1 0.05 0.1 to 100 119* 5*
25
Carbadox 4.47 Antimicrobial 6804-07-5 N/A 0.25 0.5 to 100 113 3
26
Carprofen 9.08 NSAIDs 53716-49-7 N/A 1 2.5 to 100 106 3
27
Cefalexin 4.00 Antibiotic/Beta-lactam 15686-71-2 100 2.5 5 to 100 87 8
28
Cefalonium 3.98 Antibiotic/Beta-lactam 5575-21-3 20 1 2.5 to 100 73 8
29
Cefapirin 3.28 Antibiotic/Beta-lactam 21593-23-7 60 0.1 0.25 to 100 81 4
30
Cefazolin 4.39 Antibiotic/Beta-lactam 25953-19-9 50 1 2.5 to 100 112 2
31
Cefoperazone 5.21 Antibiotic/Beta-lactam 62893-19-0 50 1 2.5 to 100 114 8
32
Cefquinome 3.75 Antibiotic/Beta-lactam 84957-30-2 20 0.5 1 to 100 63 4
33
Ceftiofur 6.35 Antibiotic/Beta-lactam 80370-57-6 100 0.5 1 to 100 117 3
34
Cefuroxime 4.47 Antibiotic/Beta-lactam 55268-75-2 N/A 2.5 5 to 100 112 6
35
Chloramphenicol 6.34 Antibiotic/Amphenicols 56-75-7 N/A 0.5 1 to 100 113 5
36
Chlorhexidine 7.20 Antimicrobial 55-56-1 N/A 5 10 to 100 95 4
37
Chlormadinone 9.51 Hormones 1961-77-9 2.5 0.5 1 to 100 114* 6*
38
Chlorpromazine 8.16 Tranquilizer 50-53-3 N/A 0.05 0.1 to 100 97 2
39
Chlortetracycline 6.04 Antibiotic/Tetracycline 57-62-5 100 0.25 0.5 to 100 42 9
40
Ciprofloxacin 4.52 Antibiotic/Quinolones 85721-33-1 N/A 0.1 0.25 to 100 95 3
41
Clenbuterol 5.41 Growth promoters/Beta-agonists 37148-27-9 0.05 0.05 0.1 to 100 118• 5•
42
Clindamycin 6.55 Antibiotic/Macrolides 18323-44-9 N/A 10 25 to 100 106# 1#
43
Clopidol 3.61 Coccidiostats 2971-90-6 20 0.25 0.5 to 100 113 2
44
Closantel 10.60 Anthelmintic 57808-65-8 45 0.25 0.5 to 100 93 3
45
Colchicine 6.78 NSAIDs 64-86-8 N/A 0.1 0.25 to 100 105 4
46
Cotinine 2.23 Insecticide 486-56-6 N/A 0.1 0.25 to 100 90 3
47
Coumaphos 9.64 Anthelmintic 56-72-4 N/A 0.5 1 to 100 108 5
48
Cyromazine 2.52 Anthelmintic 66215-27-8 N/A 1 2.5 to 100 94 7
49
Danofloxacin 4.73 Antibiotic/Quinolones 112398-08-0 30 0.05 0.1 to 100 95 2
50
Dapson 4.76 Antibiotic/Sulfonamides 80-08-0 N/A 0.05 0.1 to 100 117 2
51
Dapson N-Acetyl 5.51 Antibiotic/Sulfonamides 565-20-8 N/A 0.25 0.5 to 100 115 3
52
Deacetylcefapirin 2.37 Antibiotic/Beta-lactam 104557-24-6 60 1 2.5 to 100 41 8
53
Diaveridine 3.83 Antimicrobial 5355-16-8 N/A 0.05 0.1 to 100 111 2
54
Diazinon 9.71 Insecticide 333-41-5 20 0.1 0.25 to 100 108 2
55
Diclofenac 9.21 NSAIDs 15307-86-5 0.1 0.25 0.5 to 100 123* 8*
56
Dicloxacillin 8.18 Antibiotic/Beta-lactam 3116-76-5 30 2.5 5 to 100 118 7
57
Dicyclanil 2.98 Insecticide 112636-83-6 N/A 0.25 0.5 to 100 108 1
58
Difloxacin 5.39 Antibiotic/Quinolones 98106-17-3 N/A 0.1 0.25 to 100 106 4
59
Diflubenzuron 9.18 Insecticide 35367-38-5 N/A 0.5 1 to 100 108 5
60
Dimetridazole 3.74 Coccidiostats 551-92-8 N/A 2.5 5 to 100 108 2
61
Diminazene 3.06 Coccidiostats 536-71-0 150 2.5 5 to 100 60 4
62
Dinitolmide [Zoalene] 5.66 Coccidiostats 148-01-6 N/A 0.5 1 to 100 115 3
63
Dipyrone hydrate- metabolite
64
[4-Methylaminoantipyrine]
(min)
3.40 NSAIDs 519-98-2 N/A 0.25 0.5 to 100 54 7
RT
Functional Use/
Chemical Class CAS Number
MRL
(µg/kg)
(µg/kg)
AOAC3
LOD
Curve Range
(μg/kg) with
R2 >0.99
MQC
Recovery
(%)
MQC
RSD
(%)
10
Page 11

Linear
Calibration
No. Compound Name
Doxycycline 6.36 Antibiotic/Tetracycline 564-25-0 N/A 0.25 0.5 to 100 32 4
65
Emamectin B1a benzoate 10.18 Anthelmintic/Avermectins 121124-29-6 N/A 0.25 0.5 to 100 89 3
66
Emamectin B1b benzoate 9.99 Anthelmintic/Avermectins 121424-52-0 N/A 0.5 1 to 100 95 5
67
Enrofloxacin 4.85 Antibiotic/Quinolones 93106-60-6 100 0.05 0.1 to 100 102 2
68
Erythromycin 7.52 Antibiotic/Macrolides 114-07-8 N/A 0.5 1 to 100 97 13
69
Ethopabate 6.68 Coccidiostats 59-06-3 N/A 0.1 0.25 to 100 115 3
70
Famphur 8.25 Insecticide 52-85-7 N/A 0.25 0.5 to 100 116 7
71
Febantel 9.22 Anthelmintic/Benzimidazoles 58306-30-2 10 0.1 0.25 to 100 115 4
72
Fenbendazole 8.67 Anthelmintic/Benzimidazoles 43210-67-9 10 0.05 0.1 to 100 112 2
73
Fenbendazole Sulfoxide [Oxfendazole] 6.53 Anthelmintic/Benzimidazoles 53716-50-0 10 0.1 0.25 to 100 118 3
74
Firocoxib 8.04 NSAIDs 189954-96-9 N/A 2.5 5 to 100 111 14
75
Florfenicol 5.64 Antibiotic/Amphenicols 73231-34-2 N/A 0.25 0.5 to 100 115 4
76
Fluazuron 10.24 Insecticide 86811-58-7 N/A 0.25 0.5 to 100 108 6
77
Flubendazole 7.80 Anthelmintic/Benzimidazoles 31430-15-6 10 0.05 0.1 to 100 115 2
78
Flugestone acetate 8.42 Hormones 2529-45-5 1 0.5 1 to 100 120* 4*
79
Flumequine 7.47 Antibiotic/Quinolones 42835-25-6 50 0.05 0.1 to 100 119 2
80
Flunixin 8.83 NSAID’s 38677-85-9 N/A 0.05 0.1 to 100 117 2
81
Fluralaner 9.95 Insecticide 864731-61-3 N/A 0.5 1 to 100 111 6
82
Furazolidone 4.77 Antimicrobial/Furans 67-45-8 N/A 1 2.5 to 100 118 5
83
Gamithromycin 6.56 Antibiotic/Aminoglycosides 145435-72-9 N/A 0.1 0.25 to 100 76 4
84
Gonadotropin 7.65 Hormones 33515-09-2 N/A 5 10 to 100 116 7
85
Halofuginone 6.55 Coccidiostats 55837-20-2 N/A 0.25 0.5 to 100 102 5
86
Haloperidol 7.21 Tranquilizer 52-86-8 N/A 0.05 0.1 to 100 116 2
87
Haloxon 8.65 Anthelmintic 321-55-1 N/A 2.5 5 to 100 112 12
88
Imidocarb 3.32 Coccidiostats 27885-92-3 50 1 2.5 to 100 55 5
89
Ipronidazole 6.13 Anthelmintic/Nitroimidazoles 14885-29-1 N/A 1 2.5 to 100 115 3
90
Ipronidazole-OH 4.93 Anthelmintic/Nitroimidazoles 35175-14-5 N/A 0.25 0.5 to 100 114 3
91
Isometamidium 6.09 Anthelmintic 20438-03-3 100 1 2.5 to 100 73 4
92
Josamycin 8.32 Antibiotic/Macrolides 16846-24-5 N/A 0.25 0.5 to 100 110 4
93
Ketamine 4.86 Anesthetic 6740-88-1 N/A 0.05 0.1 to 100 107 2
94
Ketoprofen 8.28 NSAIDs 22071-15-4 50 0.5 1 to 100 118 5
95
Kitasamycin A5 [Leucomycin A5] 7.79 Antibiotic/Aminoglycosides 18361-45-0 N/A 0.25 0.5 to 100 108 4
96
Lasalocid A 11.13 Coccidiostats 25999-31-9 N/A 0.05 0.1 to 100 106 2
97
Leuco Crystal violet 10.44 Fungicides and Dyes 603-48-5 N/A 0.25 0.5 to 100 79 3
98
Leucomalachite green 10.55 Fungicides and Dyes 129-73-7 N/A 0.05 0.1 to 100 96 2
99
Levamisole 3.67 Anthelmintic 14769-73-4 N/A 0.1 0.25 to 100 106 2
100
Lincomycin 3.81 Antibiotic/Aminoglycosides 154-21-2 150 0.05 0.1 to 100 79 3
101
Lufenuron 10.16 Insecticide 103055-07-8 N/A 1 2.5 to 100 113 5
102
Maduramicin Ammonium 11.69 Coccidiostats 79356-08-4 N/A 0.1 0.25 to 100 84 2
103
Malachite green 8.31 Fungicides and Dyes 10309-95-2 N/A 0.05 0.1 to 100 81 2
104
Malathion 9.00 Insecticide 121-75-5 N/A 0.1 0.25 to 100 117 3
105
Marbofloxacin 4.10 Antibiotic/Quinolones 115550-35-1 75 0.1 0.25 to 100 97 2
106
Mebendazole 7.55 Anthelmintic/Benzimidazoles 31431-39-7 N/A 0.05 0.1 to 100 114 2
107
Mefenamic acid 9.75 Anti-inflammatory 61-68-7 N/A 0.1 0.25 to 100 120 3
108
(min)
RT
Functional Use/
Chemical Class CAS Number
MRL
(µg/kg)
(µg/kg)
AOAC3
LOD
Curve Range
(μg/kg) with
R2 >0.99
MQC
Recovery
(%)
MQC
RSD
(%)
11
Page 12
Linear
Calibration
No. Compound Name
Megestrol acetate 9.49 Hormones 595-33-5 N/A 0.1 0.25 to 100 111 3
109
Melengestrol acetate 9.61 Hormones 2919-66-6 N/A 0.1 0.25 to 100 111 4
110
Meloxicam 8.17 NSAIDs 71125-38-7 15 0.05 0.1 to 100 120 2
111
Methylprednisolone 7.86 Growth promoters/Corticosteroids 83-43-2 2 0.25 0.5 to 100 117* 4*
112
Metoserpate 6.66 Tranquilizer 1178-28-5 N/A 0.1 0.25 to 100 113 3
113
Metronidazole 3.28 Anthelmintic/Nitroimidazoles 443-48-1 N/A 0.1 0.25 to 100 116 2
114
Metronidazole-OH 2.84 Anthelmintic/Nitroimidazoles 4812-40-2 N/A 0.25 0.5 to 100 118 2
115
Monensin 11.30 Coccidiostats 17090-79-8 2 0.1 0.25 to 100 98* 5*
116
Monepantel 9.52 Anthelmintic 851976-50-6 N/A 0.1 0.25 to 100 120 3
117
Morantel tartrate 5.39 Anthelmintic 20574-50-9 50 0.1 0.25 to 100 107 2
118
Moxidectin 11.09 Anthelmintic/Avermectins 113507-06-5 40 0.25 0.5 to 100 115 5
119
Nafcillin 8.10 Antibiotic/Beta-lactam 147-52-4 30 0.25 0.5 to 100 100 4
120
Nalidixic acid 7.29 Antibiotic 389-08-2 N/A 0.05 0.1 to 100 117 2
121
Narasin 11.80 Coccidiostats 55134-13-9 N/A 0.1 0.25 to 100 69 7
122
Neo-Spiramycin 5.75 Antibiotic/Macrolides 70253-62-2 200 0.25 0.5 to 100 70 2
123
Nequinate 9.43 Anthelmintic 13997-19-8 N/A 0.05 0.1 to 100 112 3
124
Netobimin 7.11 Anthelmintic 88255-01-0 100 1 2.5 to 100 108 7
125
Nicarbazine 8.84 Coccidiostats 587-90-6 N/A 0.1 0.25 to 100 116 3
126
Nicotine 1.54 Anti-herbivore 54-11-5 N/A 5 10 to 100 67 8
127
Niflumic Acid 9.14 Anti-inflammatory 4394-00-7 N/A 0.05 0.1 to 100 117 2
128
Nitroxynil 6.77 Anthelmintic 1689-89-0 N/A 0.5 1 to 100 114 5
129
Norfloxacin 4.38 Antibiotic/Quinolones 70458-96-7 N/A 0.1 0.25 to 100 95 2
130
Norgestomet 9.44 Hormones 472-54-8 0.12 1 2.5 to 100 117 3
131
Novobiocin 9.82 Antibiotic 303-81-1 50 0.25 0.5 to 100 120 4
132
Olaquindox 3.03 Growth promoters/Anabolic steroids 23696-28-8 N/A 0.25 0.5 to 100 104 2
133
Oleandomycin 7.13 Antibiotic/Aminoglycosides 3922-90-5 50 0.1 0.25 to 100 112 3
134
Orbifloxacin 5.07 Antibiotic/Quinolones 113617-63-3 20 0.1 0.25 to 100 115 3
135
Ormetoprim 4.49 Antibiotic 6981-18-6 N/A 0.1 0.25 to 100 112 2
136
Oxacillin 7.56 Antibiotic/Beta-lactam 66-79-5 30 1 2.5 to 100 114 9
137
Oxibendazole 6.89 Anthelmintic/Benzimidazoles 20559-55-1 50 0.1 0.25 to 100 116 2
138
Oxolinic acid 6.37 Antibiotic/Quinolones 14698-29-4 N/A 0.25 0.5 to 100 112 2
139
Oxyclozanide 9.56 Anthelmintic 2277-92-1 10 0.25 0.5 to 100 112 4
140
Oxyphenbutazone 8.16 NSAIDs 129-20-4 N/A 0.25 0.5 to 100 105 6
141
Oxytetracycline 4.54 Antibiotic/Tetracycline 79-57-2 100 0.25 0.5 to 100 46 9
142
Penicillin G 7.00 Antibiotic/Beta-lactam 61-33-6 N/A 0.5 1 to 100 90 7
143
Penicillin V [Phenoxymethylpenicillin] 7.41 Antibiotic/Beta-lactam 87-08-1 N/A 1 2.5 to 100 117 2
144
Phenylbutazone 9.09 NSAIDs 50-33-9 N/A 0.25 0.5 to 100 106 3
145
Phosalone 9.77 Insecticide 2310-17-0 N/A 0.5 1 to 100 113 9
146
Phoxim 9.70 Insecticide 14816-18-3 N/A 1 2.5 to 100 110 10
147
Piperonyl butoxide Ammonia 10.31 Insecticide 51-03-6 50 0.1 0.25 to 100 116 1
148
Pirlimycin 6.10 Antibiotic/Aminoglycosides 79548-73-5 100 1 2.5 to 100 96 4
149
Praziquantel 8.57 Anthelmintic 55268-74-1 N/A 0.25 0.5 to 100 116 2
150
Prednisolone 7.29 Growth promoters/Corticosteroids 50-24-8 6 0.1 0.25 to 100 120* 2*
151
Prednisone 7.13 Growth promoters/Corticosteroids 53-03-2 N/A 0.25 0.5 to 100 117 3
152
(min)
RT
Functional Use/
Chemical Class CAS Number
MRL
(µg/kg)
(µg/kg)
AOAC3
LOD
Curve Range
(μg/kg) with
R2 >0.99
MQC
Recovery
(%)
MQC
RSD
(%)
12
Page 13

Linear
Calibration
No. Compound Name
Progesterone 9.60 Hormones 57-83-0 N/A 10 25 to 100 117# 4#
153
Propionylpromazin 8.00 Antiemetic 3568-24-9 N/A 0.05 0.1 to 100 98 2
154
Propyphenazone 7.68 NSAIDs 479-92-5 N/A 0.05 0.1 to 100 118 3
155
Pyrantel 4.29 Anthelmintic 15686-83-6 N/A 0.1 0.25 to 100 105 2
156
Pyrimethamine 6.31 Antimicrobial 58-14-0 N/A 0.05 0.1 to 100 112 2
157
Ractopamine 4.66 Growth promoters/Beta-agonists 97825-25-7 N/A 0.1 0.25 to 100 117 1
158
Rafoxanide 11.11 Anthelmintic 22662-39-1 10 0.25 0.5 to 100 70 4
159
Rifaximin 9.07 Antibiotic 80621-81-4 60 0.1 0.25 to 100 104 4
160
Robenidine 8.58 Coccidiostats 25875-51-8 N/A 0.25 0.5 to 100 101 4
161
Ronidazole 3.40 Anthelmintic/Nitroimidazoles 7681-76-7 N/A 0.1 0.25 to 100 119 2
162
Salbutamol [Albuterol] 3.03 Growth promoters/Beta-agonists 18559-94-9 N/A 0.05 0.1 to 100 105 2
163
Salinomycin 11.62 Coccidiostats 53003-10-4 N/A 0.1 0.25 to 100 77 6
164
Sarafloxacin 5.39 Antibiotic/Quinolones 98105-99-8 N/A 0.1 0.25 to 100 107 3
165
Spiramycin I 6.13 Antibiotic/Macrolides 24916-50-5 200 0.25 0.5 to 100 82 2
166
Sulfabenzamide 6.07 Antibiotic/Sulfonamides 127-71-9 100 0.05 0.1 to 100 120 2
167
Sulfacetamide 3.13 Antibiotic/Sulfonamides 144-80-9 100 0.1 0.25 to 100 115 2
168
Sulfachloropyridazine 5.25 Antibiotic/Sulfonamides 80-32-0 100 0.1 0.25 to 100 116 2
169
Sulfaclozine 6.30 Antibiotic/Sulfonamides 102-65-8 100 0.25 0.5 to 100 116 4
170
Sulfadiazine [Silvadene] 3.42 Antibiotic/Sulfonamides 68-35-9 100 0.1 0.25 to 100 120 2
171
Sulfadimethoxine 6.44 Antibiotic/Sulfonamides 122-11-2 100 0.05 0.1 to 100 119 2
172
Sulfadimidine [Sulfamethazine] 4.62 Antibiotic/Sulfonamides 57-68-1 100 0.1 0.25 to 100 114 2
173
Sulfadoxine 5.58 Antibiotic/Sulfonamides 2447-57-6 100 0.05 0.1 to 100 119 2
174
Sulfaethoxypyridazine 5.93 Antibiotic/Sulfonamides 963-14-4 100 0.05 0.1 to 100 117 1
175
Sulfaguanidine 1.72 Antibiotic/Sulfonamides 57-67-0 100 0.25 0.5 to 100 107 1
176
Sulfamerazine 4.02 Antibiotic/Sulfonamides 127-79-7 100 0.1 0.25 to 100 118 1
177
Sulfameter [sulfamethoxydiazine] 4.48 Antibiotic/Sulfonamides 651-06-9 100 0.1 0.25 to 100 113 2
178
Sulfamethizole 4.50 Antibiotic/Sulfonamides 144-82-1 100 0.1 0.25 to 100 115 3
179
Sulfamethoxazole 5.47 Antibiotic/Sulfonamides 723-46-6 100 0.1 0.25 to 100 116 2
180
Sulfamethoxypyridazine 4.68 Antibiotic/Sulfonamides 80-35-3 100 0.1 0.25 to 100 114 1
181
Sulfamonomethoxine 5.23 Antibiotic/Sulfonamides 1220-83-3 100 0.1 0.25 to 100 118 2
182
Sulfamoxole 4.31 Antibiotic/Sulfonamides 729-99-7 100 0.05 0.1 to 100 112 2
183
Sulfanitran 7.33 Antibiotic/Sulfonamides 122-16-7 100 1 2.5 to 100 115 7
184
Sulfaphenazole 6.34 Antibiotic/Sulfonamides 526-08-9 100 0.1 0.25 to 100 115 3
185
Sulfapyridine 3.83 Antibiotic/Sulfonamides 144-83-2 100 0.1 0.25 to 100 115 1
186
Sulfaquinoxaline 6.51 Antibiotic/Sulfonamides 59-40-5 100 0.1 0.25 to 100 119 3
187
Sulfathiazole 3.62 Antibiotic/Sulfonamides 72-14-0 100 0.1 0.25 to 100 115 2
188
Sulfisomidine 3.34 Antibiotic/Sulfonamides 515-64-0 100 0.05 0.1 to 100 111 1
189
Sulfisoxazole 5.76 Antibiotic/Sulfonamides 127-69-5 100 0.1 0.25 to 100 113 3
190
Sulindac 8.03 Antibiotic/Sulfonamides 38194-50-2 100 0.1 0.25 to 100 117 3
191
Teflubenzuron 10.08 Insecticide 83121-18-0 N/A 0.5 1 to 100 117 3
192
Testosterone 8.56 Growth promoters/Anabolic steroids 58-22-0 N/A 0.1 0.25 to 100 119 4
193
Tetracycline 4.78 Antibiotic/Tetracycline 60-54-8 100 0.25 0.5 to 100 64 4
194
Thiabendazole 4.34 Anthelmintic/Benzimidazoles 148-79-8 50 0.05 0.1 to 100 112 2
195
Thiamphenicol 4.33 Antibiotic/Amphenicols 15318-45-3 50 0.25 0.5 to 100 120 3
196
(min)
RT
Functional Use/
Chemical Class CAS Number
MRL
(µg/kg)
(µg/kg)
AOAC3
LOD
Curve Range
(μg/kg) with
R2 >0.99
MQC
Recovery
(%)
MQC
RSD
(%)
13
Page 14

Linear
Calibration
No. Compound Name
Tiamulin 7.68 Antibiotic 55297-95-5 N/A 0.05 0.1 to 100 114 2
197
Tilmicosin 6.87 Antibiotic/Macrolides 108050-54-0 50 1 2.5 to 100 90 7
198
Tolfenamic acid 9.94 NSAIDs 13710-19-5 50 1 2.5 to 100 114 8
199
Trenbolone 7.98 Growth promoters/Anabolic steroids 10161-33-8 N/A 0.25 0.5 to 100 111 4
200
Trichlorfon [DEP] 5.29 Tranquilizer 52-68-6 50 0.5 1 to 100 124 3
201
Triclabendazole 9.74 Anthelmintic/Benzimidazoles 68786-66-3 10 0.1 0.25 to 100 113 2
202
Trimethoprim 4.12 Antibiotic 738-70-5 50 0.1 0.25 to 100 108 3
203
Tripelennamine 6.39 Anthelmintic 91-81-6 20 0.05 0.1 to 100 105 2
204
Tylosin 7.64 Antibiotic/Macrolides 1401-69-0 50 0.5 1 to 100 109 5
205
Valnemulin 8.39 Antibiotic 101312-92-9 N/A 0.1 0.25 to 100 113 3
206
Vedaprofen 9.14 NSAIDs 71109-09-6 N/A 0.1 0.25 to 100 114 2
207
Virginiamycin M1 8.21 Antibiotic/Macrolides 21411-53-0 N/A 0.1 0.25 to 100 114 5
208
Xylazine 5.24 Tranquilizer 7361-61-7 N/A 0.1 0.25 to 100 109 2
209
Zilpaterol 2.98 Growth promoters/Beta-agonists 119520-05-7 N/A 0.1 0.25 to 100 96 3
210
(min)
RT
Functional Use/
Chemical Class CAS Number
MRL
(µg/kg)
(µg/kg)
AOAC3
LOD
Curve Range
(μg/kg) with
R2 >0.99
• Data using LLQC, * Data using LQC, # Data using HQC
MQC
Recovery
(%)
MQC
RSD
(%)
www.agilent.com/chem
DE44270.4828125
This information is subject to change without notice.
© Agilent Technologies, Inc. 2021
Printed in the USA, March 15, 2021
5994-3124EN
 Loading...
Loading...