Page 1
Identification of
Pyridylethylated Cysteine
Residues in Peptide Maps
Using Diode-array Detection
Cysteine and cystine residues are important amino acid residues because they are involved in
the correct formation of the tertiary and quaternary structure of peptides and proteins. The full
biological acitivity relies on correctly synthesized intra- and intermolecular disulfide bridges
between two particular cysteine residues. As a consequence of disulfide bridge formation and
hence a globular structure many proteins are hardly succeptible to proteolytic attack. Therefore,
the protein chemical characterization of these proteins is often not possible without prior
modification of the cysteine/cystine residues.
Column
0.3 ˘ 250 mm Vydac C-18
Mobile phase
A = water, 0.05 % TFA
B = acetonitrile, 0.045 % TFA
Flow rate
4 µl/min after split from 100 µl/min
Gradient
at 0 min 2 % B
at 140 min 45 % B
UV detector
diode-array detector
206 nm, 254 nm, 280 nm
spectra 200–320 nm
500 nl micro flow cell
Temperature
ambient
Conditions
Agilent Technologies
Innovating the HP Way
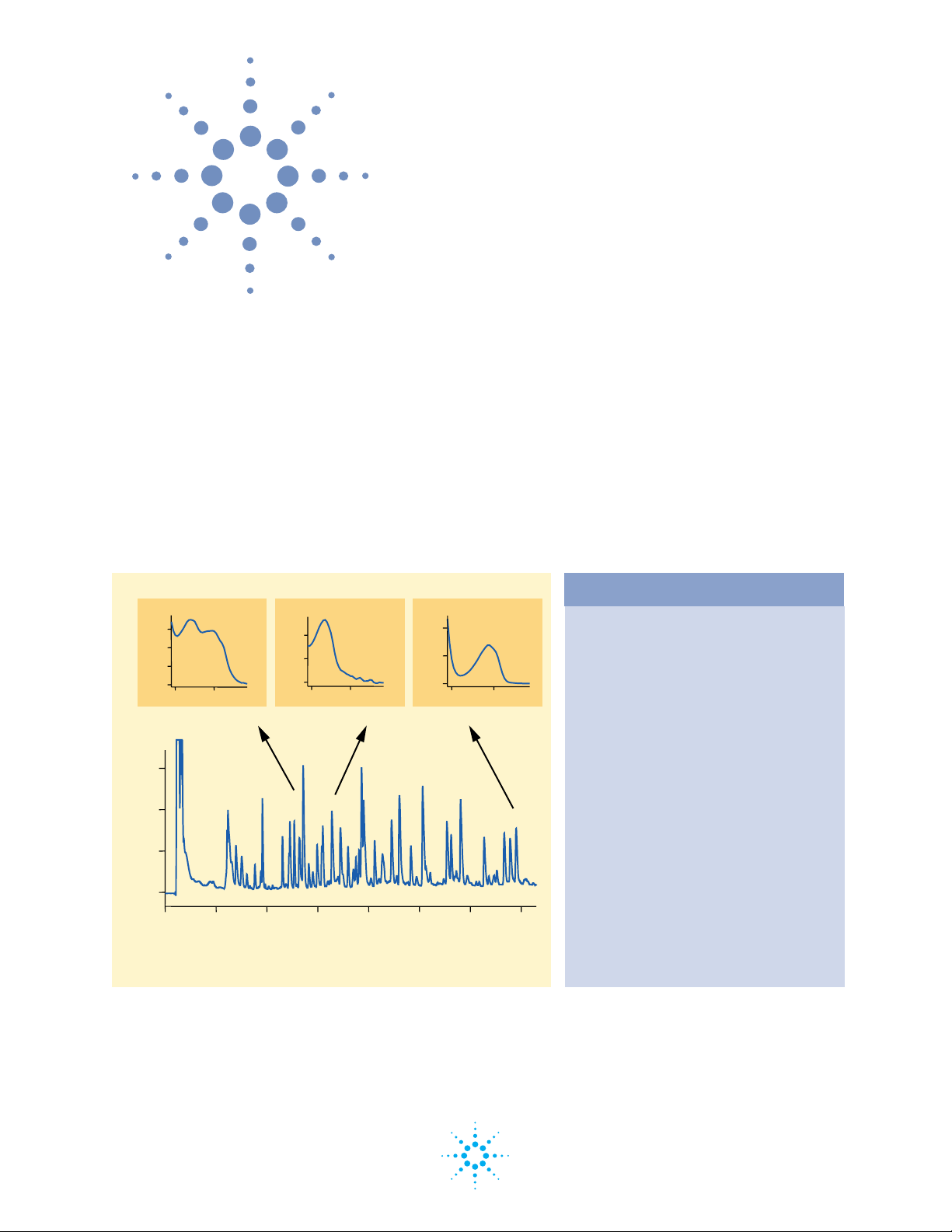
Figure 1
Tryptic digest of a bacterial neurotoxin protein after
reduction and alklation with vinylpyridine
Biopharmaceutical
[mAU]
12
8
4
0
VP-Cys
+Trp
240 280
[nm]
[mAU]
4
2
0
240 280
VP-Cys
[nm]
[mAU]
40
20
0
240
280
Absorbance
[mAU]
120
80
40
0
0 20 40 60 80 100 120 140
Time [min]
Tyr
[nm]
Page 2

Equipment
Agilent 1100 Series
• Binary pump
• Vacuum degasser
• Standard autosampler
• Diode-array detector
micro flow cell
10-mm path length, 500 nl
• Agilent ChemStation
+ 3D software
LC Packings flow splitter,
IC-100-VAR precolumn
microflow processor*
© Copyright 1999 Agilent Technologies
Released 03/99
Publication Number 5968-4739E
HPLC Performance
Typically, reduction with a variety of reagents, such as mercaptoethanol or dithiodithreithol is carried out followed by alkylation using
iodoacetic acid, iodoacetamide or vinylpyridine. Afterwards peptides
are separated by HPLC and identified by Edman sequencing or
MS-MS sequencing.
Prior to sequence analysis peptide maps are routinely separated by
microbore or capillary HPLC. Identification of modified cysteine
residues is best performed using vinylpyridine as alkylation reagent,
since it adds additional spectral characteristics to this residue. As
shown in figure 1, using diode-array detection vinylpyridinilated
cysteine residues can be unambiguously identified in peptides
separated by reversed-phase capillary HPLC by their characteristic
absorption maximum at 254 nm They can thus be easily distinguished
from tyrosine and tryptophan residues which have an absorption
maximum around 280 nm, even if they are present in the same peptide.
With this approach collected peptide fractions can be easily analyzed
further by protein sequencing or alternatively online by
MS-MS sequence analysis.
Agilent Technologies
Innovating the HP Way
*LC Packings,
Baarsjesweg 154
1057 Amsterdam,
The Netherlands
LC Packings
80 Carolina Street,
San Francisco, CA 94103, USA
 Loading...
Loading...