
High Sensitivity RNA ScreenTape Assay for TapeStation
Systems
Quick Guide
The Agilent 4150 (G2992AA) and 4200 (G2991AA and G2991BA) TapeStation systems are automated platforms for
scalable, flexible, fast, and reliable electrophoresis of nucleic acids.
This Quick Guide is intended for use with the Agilent 4150 and 4200 TapeStation systems only. A Quick Guide specific
for use with the Agilent 2200 TapeStation system is available online.
The High Sensitivity RNA ScreenTape assay is designed for analyzing and assessing integrity of eukaryotic and
prokaryotic total RNA.
Specifications
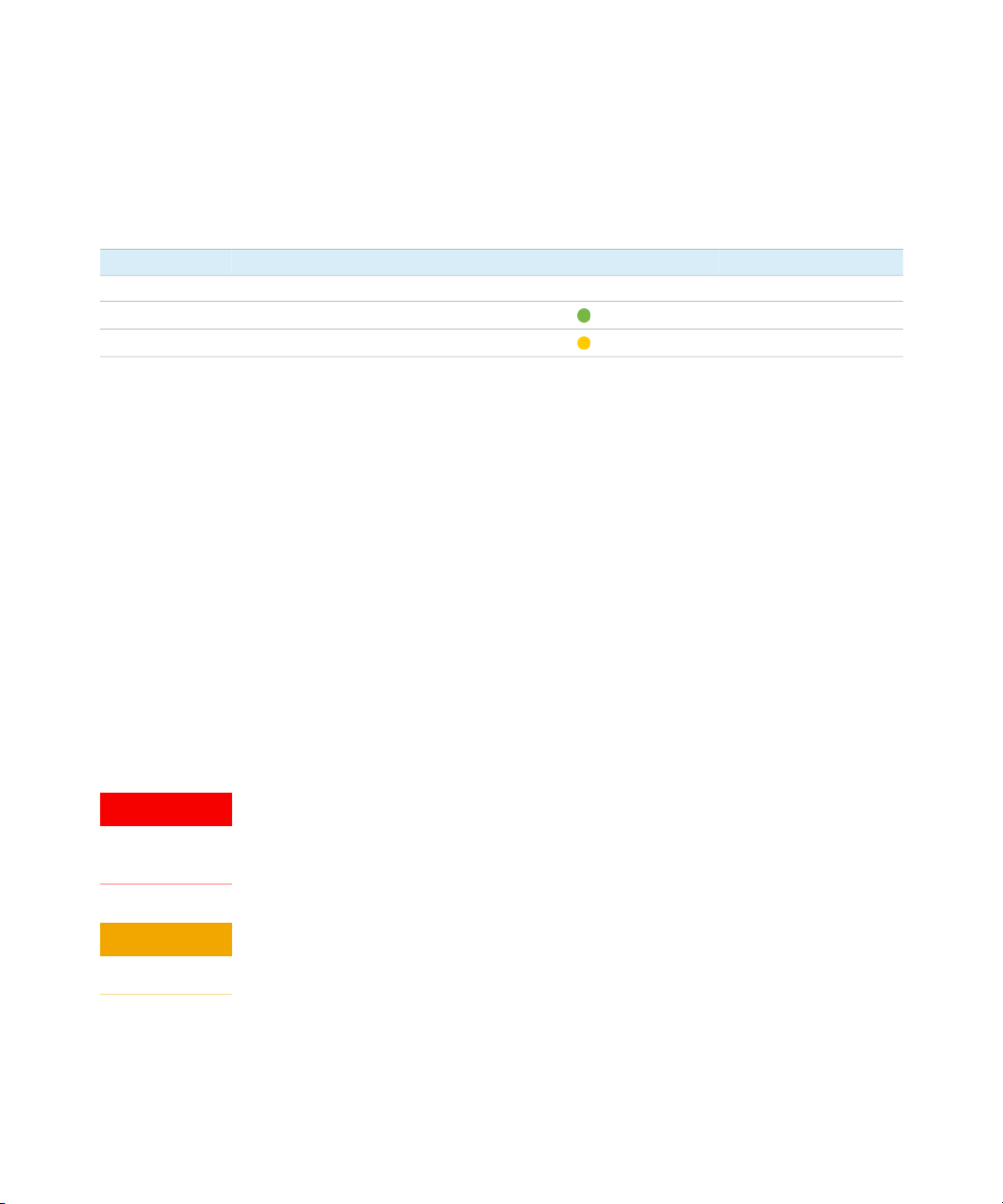
Analytical specifications High Sensitivity RNA ScreenTape assay
Sensitivity
Quantitative precision 15 % CV
Quantitative accuracy ±30 %
Quantitative range 500 – 10000 pg/µL
RIN
Maximum buffer concentration in
sample
Physical specifications
Analysis time 16 samples: <35 min, 96 samples: <180 min
Samples per consumable 16
Sample volume required 2 µL
Kit stability 4 months
Kit size 112 samples
1
2
1
e
functional range
Signal-to-noise >3 (single peak)
RINe – RNA integrity number equivalent
2
100 pg/µL
1000 – 25000 pg/µL
10 mM Tris, 1 mM EDTA
Agilent High Sensitivity RNA ScreenTape Quick Guide for TapeStation Systems
High Sensitivity RNA ScreenTape Assay for TapeStation Systems
Storage Conditions
• Store High Sensitivity RNA sample buffer and ScreenTape devices: 2 – 8 °C (36 – 46 °F).
• Store High Sensitivity RNA ladder at -20 °C to -5 °C (-4 °F to 23 °F).
• Store partially used ScreenTape devices upright at 2 – 8 °C (36 – 46 °F) for a maximum of 2 weeks.
• Never freeze ScreenTape devices. Discard any accidentally frozen ScreenTape devices.
Kit Components
Part Number Name Color Amount
5067-5579 High Sensitivity RNA ScreenTape 7 ScreenTape devices
5067-5580 High Sensitivity RNA ScreenTape Sample Buffer 1 vial, 250 µL
5067-5581 High Sensitivity RNA ScreenTape Ladder 1 vial, 10 µL
Limited Use Label License
Some products within this assay contain SYBR™ Gold, which is licensed from Life Technologies Corporation for use in
research and development only. SYBR™ is a registered trademark of Life Technologies Corporation.
For Research Use Only
Not for use in Diagnostic Procedures.
Additional Material Required for Analysis with the TapeStation Systems
• Loading tips (5067-5598, 1 pk or 5067-5599, 10 pk)
• Optical Tube 8x Strip (401428) and Optical Tube Cap 8x Strip (401425)
• Vortex mixer IKA MS3 with 96-well sample plate adapter
• 96-well sample plates (5042-8502) and 96-well Plate Foil Seal (5067-5154) (4200 TapeStation systems only)
Additional Equipment Required (Not Supplied)
• Volumetric micropipettes for handling volumes from 1 to 15 µL
• Centrifuges for tube strips and 96-well sample plates
• Heating block or PCR cycler compatible with 200 µL tube strip vials and full skirted 96-well sample plates
WARN I NG
CA UTI ON
2
Toxic agents
Refer to product material safety datasheets for further information.
When working with the ScreenTape assay follow the appropriate safety procedures such as
wearing safety goggles, laboratory gloves and protective clothing.
Damage to the TapeStation systems
Only use the recommended consumables and reagents with the TapeStation systems.

High Sensitivity RNA ScreenTape Assay for TapeStation Systems
Essential Measurement Practices
Read about good measurement practices in the Agilent Information Center and/or in the System Manual.
Environmental
conditions
Working with RNA
samples
Steps before sample
preparation
Pipetting practice • Pipette reagents carefully against the side of the 96-well sample plate or sample tube
Mixing and
centrifugation
recommendations
• Ambient operating temperature: 14 – 30 °C (57 – 86 °F)
• Keep reagents during sample preparation at room temperature
• Wear gloves all the time
• Thaw RNA samples and ladder on ice and keep them on ice during sample preparation
• Ensure that all working areas and plastic ware are RNase free
• Allow Sample Buffer to equilibrate at room temperature for 30 min prior to use
• Vortex each vial and briefly spin down
• Flick ScreenTape device to eliminate bubbles in the buffer chamber
• Ensure that no sample or Sample Buffer remains within or on the outside of the tip
• Care must be taken due to viscosity of the Sample Buffer
• Apply foil seal to 96-well sample plate or cap the tube strips prior to mixing and centrifugation
• Centrifuge to collect liquid at the base; then vortex using the IKA MS3 vortexer and adaptor at
2000 rpm for 1 min
• Briefly centrifuge and visually confirm that all liquid is collected at the bottom of the 96-well
sample plate or tube strips and any air bubble is removed
• Run samples immediately after preparation
Ladder Considerations
• Ladder is exclusively loaded from location A1 on the tube strip holder.
• Always use a complete tube strip when running ladder or samples from the tube strip holder.
• For best sizing and molarity quantification results, a ladder per analysis is recommended. Alternatively, an
electronic ladder is available, which can be selected in the Agilent TapeStation Controller software.
3

High Sensitivity RNA ScreenTape Assay for TapeStation Systems
Sample
Buffer
Flick
Samples, tips, and ScreenTape device
RNA
1 µL
2 µL
Vortex 1 min
Spin down 1 min Spin down 1 min
High Sensitivity
RNA
Heat 72 °C, 3 min
Cool 2 min on ice
Samples:
High Sensitivity RNA ScreenTape Assay Operating Procedure
1 Allow High Sensitivity RNA Sample Buffer to equilibrate at room temperature for 30 minutes.
2 Thaw High Sensitivity RNA Ladder and total RNA samples on ice.
3 Launch the Agilent TapeStation Controller software.
4 Flick the High Sensitivity RNA ScreenTape device and insert it into the ScreenTape nest of the TapeStation
instrument.
5 Select required sample positions in the TapeStation Controller software.
6 The required consumables (tips, further ScreenTape devices) are displayed in the TapeStation Controller software.
7 Vortex reagents and samples. Spin down before use.
8 Prepare diluted Ladder solution by adding 10 µL RNase free water to the High Sensitivity RNA Ladder vial and mix
thoroughly. Pipette 1 µL High Sensitivity RNA Sample Buffer ( ) and 2 µL diluted High Sensitivity RNA Ladder ( )
at position A1 in a tube strip.
9 For each sample, pipette 1 µL High Sensitivity RNA Sample Buffer ( ) and 2 µL RNA sample in a tube strip or
96-well sample plate
10 Apply caps to tube strips and/or foil seals to 96-well sample plates.
11 Mix liquids using the IKA MS3 vortexer at 2000 rpm for 1 min.
12 Spin down samples and ladder for 1 min.
13 Samples and ladder denaturation:
a Heat samples and ladder at 72 °C (162 °F) for 3 min.
b Place samples and ladder on ice for 2 min.
c Spin down sample and ladder for 1 min.
1
.
Sample Analysis
1 Load samples into the TapeStation instrument. Place ladder in position A1 on tube strip holder.
2 Carefully remove caps of tube strips. Visually confirm that liquid is positioned at the bottom.
3 Click Start.
4 The TapeStation Analysis software opens automatically after the run and displays results.
Technical Support and Further Information
For technical support, please visit www.agilent.com/chem/contactus. Visit Agilent Technologies’ web site. It offers
useful information, support and current developments about the products and technology:
www.agilent.com/genomics/tapestation.
1
Agilent 4200 TapeStation system only
www.agilent.com
Agilent Technologies Inc. 2018-2021
Printed in Germany, Edition: 01/2021
*G2991-90121*
Part No: G2991-90121 Rev. B
Document No: SD-UF0000089 Rev. B
 Loading...
Loading...