Page 1
Application Note
Molecular Biology and
Biochemical
Using the Agilent BioTek EL406
Combination Washer Dispenser to
Semi-Automate a Competitive ELISA
for Melamine
Semi-automated melamine quantitation
Author
Paul Held, PhD
Agilent Technologies, Inc.
Abstract
Melamine is a nitrogen rich compound normally used as either a flame retardant
or in conjunction with formaldehyde to produce melamine resin, a durable
thermosetting plastic used in the manufacture of countertops, fabrics, and
glues. However, in addition to the normal use of melamine, several illicit uses
for the material have been reported. This application note describes the use of
the AgilentBioTek EL406 combination washer dispenser in conjunction with
AgilentBioTek BioStack and AgilentBioTek liquid handling control (LHC) software to
semi-automate a melamine ELISA.
Page 2
Introduction
Melamine is a nitrogen rich compound that has gained
notoriety as a result of its illicit use as an additive that mimics
protein in foods. The practice of using melamine scrap as
an additive to animal feed and food products to give the
appearance of increased protein content is widespread in
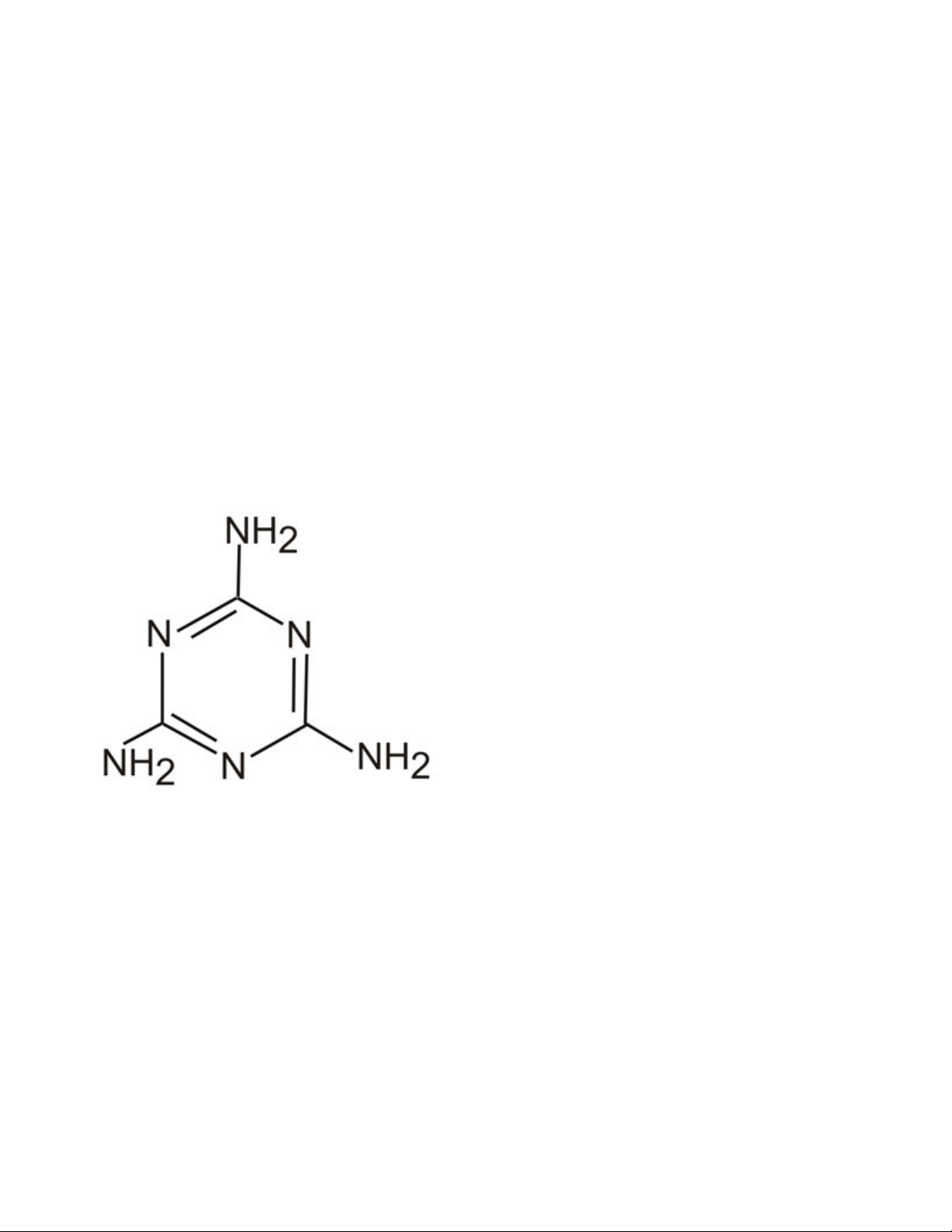
many countries. The basis for this are the presence of three
primary amine groups, which are known to react with biuret
reagent used in many of the commonly used protein assays
(Figure 1). Recently, a scandal in China has implicated over
two dozen companies and numerous individuals of adding
melamine to milk and infant formula, leading to kidney stones
and renal failure, resulting in the deaths of several infants and
the sickening over 53,000 others. Trace amounts have been
reported by the FDA to be in some infant formula products in
the US as well.
Figure 1. Chemical structure of melamine.
The melamine assay is a competitive ELISA, where
melamine-HRP conjugate competes for binding to the
melamine antibody attached to the wells of the microplate.
Following the completion of the binding reaction, unbound
sample and conjugate is removed by washing. Substrate
reagent is immediately added and the color allowed to
develop. The color-development reaction is terminated by the
addition of stop solution and the absorbance of each well is
determined. Unknown concentrations are then determined
by interpolation from a standard curve generated by running
standards of known melamine concentrations. To easily
compare multiple experiments, the data are expressed as
a ratio to the zero-standard. This ratio is often expressed
asB/Bo.
Materials and methods
The melamine assay used was an ELISA kit from Abraxis
(Warminster, PA) and was performed as described by the
kit instructions. Samples and standards were prepared
offline and 100 µL of each was pipetted manually into the
assay plate. Plates were loaded into the AgilentBioTek
BioStack storage stacker and the assay initiated. Plates were
automatically transferred sequentially to an AgilentBioTek
EL406, where 50 µL of assay conjugate was added using the
peristaltic pump dispenser. The plates were then returned
to the BioStack and restacked to restore their original
order and allowed to complete the 30-minute incubation at
room temperature. After incubation, the plates were again
transferred automatically to the EL406 and washed four
times with 300 µL of washer buffer followed by the addition
of 100 µL of substrate solution using one of the syringe
pump dispensers. Plates were automatically returned to the
BioStack and restacked. The color development continued
for 20 minutes. After color development, the plates were
automatically returned to the EL406. A 100 µL amount of stop
solution was added using the second syringe-pump dispenser
and the plates returned to the BioStack. After restacking, the
absorbance of each well at 450 nm was determined using an
AgilentBioTek Synergy 4 multimode microplate reader.
2
Page 3

This procedure was automated using LHC PC software to
control the plate movement, processing, and incubation steps
(Figure3). Incubation steps were carried out in the BioStack,
while plate processing, such as wash steps, shaking, and
reagent addition was accomplished using the EL406. A
single LHC program file was written to carry out all of the
process steps after the addition of sample to the plate up
to the point of reading the plate. Plates were moved to the
EL406 for liquid handling steps such as wash steps to remove
unbound materials or the addition of reagents (e.g. conjugate,
substrate, and stop solutions). The incubation steps were
carried out using the BioStack as a means to store the plates.
Delay times were calculated such that the process times and
restack procedures were accounted for (Figure 3).
Figure 2. Schematic diagram of the procedural steps of the melamine
ELISA reaction. Processes carried out by the Agilent BioTek EL406 washer
dispenser are indicated in red.
Figure 3. Agilent BioTek LHC process steps for a 3-plate assay batch.
3
Page 4

Results and discussion
The data in Figure 4 demonstrate the efficacy of using the
EL406 to automate the fluid handling tasks of the melamine
ELISA. Unbound material was removed using the washer
function, while the necessary reagents were added using the
three dispensers of the instrument. Using a B/Bo calculation
where the ratio of each standard to that of the zero-standard,
significant changes in signal are observed as a function of
melamine concentration.
Using a 5-parameter logistic fit of the data, unknown sample
concentrations can be determined by interpolation from the
curve. Detection limit calculated by interpolation from the
standard curve of the mean of the zero standards minus
3times its standard deviation, ranged from 10to30 ng/mL in
three different experiments.
The data presented here demonstrate the ability of the
AgilentBioTek EL406 to automate the liquid handling tasks
required to run the melamine ELISA kit from Abraxis. The
resulting data was equivalent to that provided by the kit
insert as example data. EL406 can serve to provide the same
functionality as four different instruments (1 washer and 3
dispensers). In conjunction with the Agilent BioTek BioStack,
the EL406 can be used to automate the liquid handling
processes of thisassay.
The melamine ELISA has the capability to detect melamine
contamination in the parts per billion (PPB) ranges. This is
sufficient for many applications, as the FDA guidelines for
consumption list levels above 1 ppm as being hazardous.
The FDA methods employ LC/MS or GC/MS and have
the advantage of being more sensitive and being able to
detect cyanuric acid concurrently. However, these methods
also require much more expensive instrumentation, more
extensive sample preparation prior to the assay, and a higher
degree of technical training of the technician carrying out the
assay. In many instances, the ELISA method can be used as a
screening tool when there are large numbers of samples and
time to result is critical.
Figure 4. Melamine concentration curve. B/Bo calculations based on
the zero-standard were determined using Agilent BioTek Gen5 data
analysis software from three different plates. The data were plotted using
GraphPad5. Each data point represents the mean and SEM of a total of
72determinations.
Conclusion
Semi-automation of ELISA procedures such as the melamine
assay allow unattended processing of a number of assay
plates. While the assay is not completely “walk-away”, the
time consuming liquid handling steps, such as reagent
addition, washing, and incubation have been automated.
After processing, the plates are read manually using an
absorbance reader. Once the absorbance has been measured,
B/Bo calculations, the generation of a standard curve and
the interpolation of the curve for the determination of
sample concentrations is automatically performed by the
AgilentBioTek Gen5 data analysis software.
www.agilent.com/lifesciences/biotek
DE44173.2272337963
This information is subject to change without notice.
Agilent Technologies, Inc. 2009, 2021
Printed in the USA, February 1, 2021
5994-2689EN
 Loading...
Loading...