Page 1
Application Note
Cell Analysis
Assessing the Impact of Drug
Treatment on Cardiomyocyte Function
Through combined analysis of contractility, metabolic
flux, and cellular oxygenation
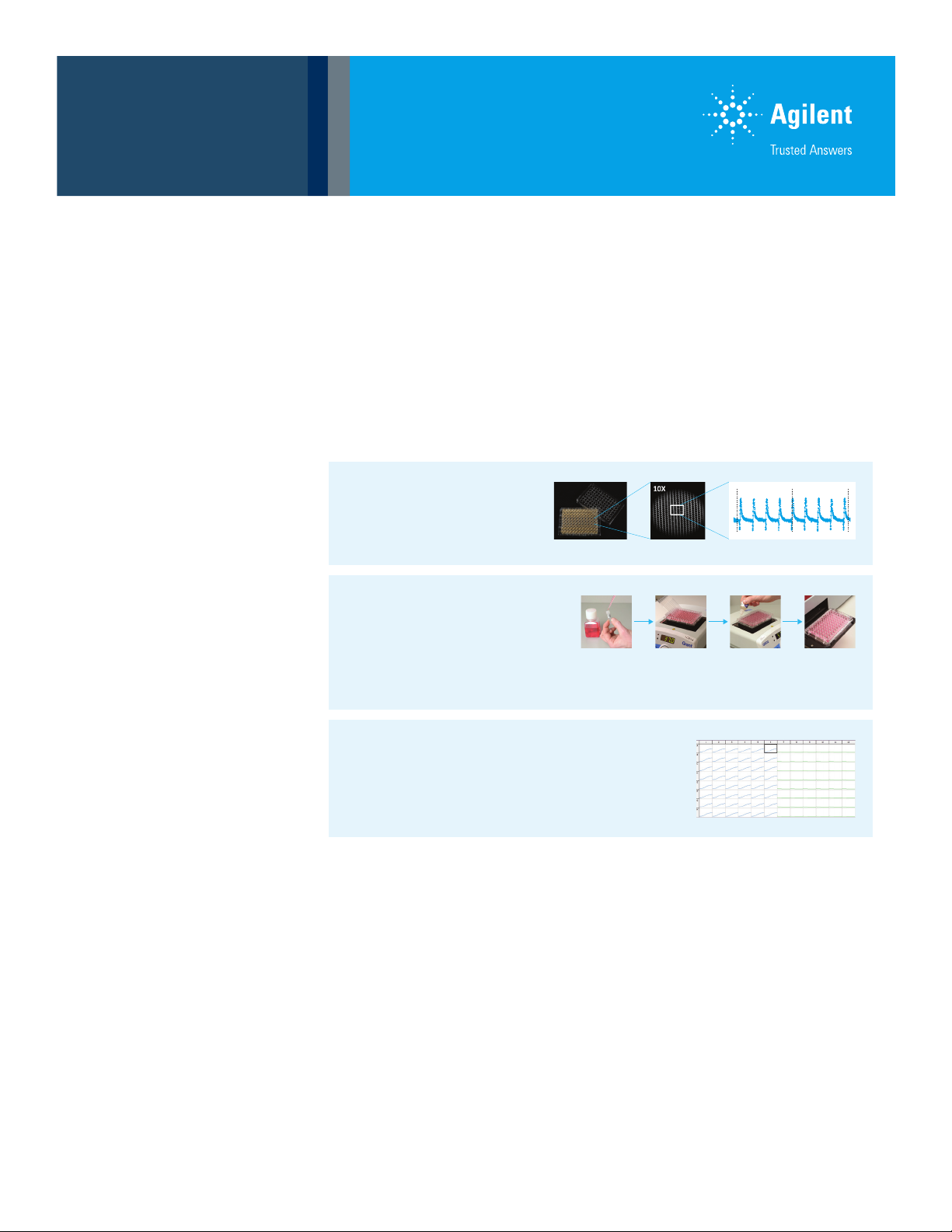
iPS Cardiomyocyte Contractility:
Cells cultured on RTCA E-Plate Cardio 96
Measured on xCELLigence RTCA Cardio
Allows interrogation of Contractility
Authors
Ryan McGarrigle, Conn Carey,
and James Hynes
Agilent Technologies, Inc.
Cell Metabolism:
Cells cultured on E-Plate Cardio 96
Measured on TRF Fluorescence Plate Reader
Agilent assays monitor mitochondrial function
(MitoXpress Xtra), glycolytic flux (pH-Xtra) and
cellular oxygenation (MitoXpress Intra).
Workflow Integration:
Allows measurement on E-Plates such that
metabolism and contractility can be measured
sequentially on the same test plate.
Abstract
In this application note, we demonstrate the feasibility of combining
microelectrode-based iPS cardiomyocyte contractility measurements with a
microplate-based bioenergetics assessment to better characterize cellular
responses to drug treatment. Contractility was assessed on 96-well E-Plate
Cardio96 using the Agilent xCELLigence RTCA Cardio system while cell metabolism
was measured on the same E-plate using a multiplexed fluorometric measurement
of O2 consumption with Agilent MitoXpress Xtra, glycolytic flux with Agilent pH Xtra,
and cellular oxygenation using Agilent MitoXpress Intra.
Page 2
Introduction
Cardiotoxicity and related cardiac impairment remain one
of the main reasons for both drug withdrawal1 and FDA
black box warning2 and are a significant cause of compound
attrition in preclinical development. In vitro assays are capable
of better characterizing cardiac response to drug treatments
and are therefore of significant importance to better predict
such adverse effects in vivo.
Cardiac tissue requires an uninterrupted supply of respiratory
substrates to meet the very high ATP demand imposed by
continuous beating. Over 95% of this ATP is generated by
oxidative phosphorylation (OXPHOS) with the necessary
mitochondrial network taking up approximately one-third
of cardiomyocyte cell volume. Energy starvation and
mitochondrial dysfunction are therefore significant factors
in the progression of cardiotoxicity and so detection of such
metabolic dysfunction is an important aspect of cardiotoxicity
screening. This detection is best achieved by monitoring the
two main ATP generating processes, OXPHOS andglycolysis.
In vivo, the most important respiratory substrates for
ATP production are pyruvate and fatty acyl CoA, however,
cardiomyocyte metabolism is particularly adaptable
and substrates such as amino acids, lactate, and ketone
bodies can also be used. Examples of this adaptability
include hypoxia inducible factor (HIF) mediated metabolic
responses to hypoxia and ischemia and the shift from fatty
acid oxidation (FAO) to glucose metabolism that occurs
in hypertrophic cardiac tissue. These adaptions highlight
the importance of information on substrate preference
and oxygenation when designing and interpreting in vitro
cardiomyocyteanalyses.
As cardiac contraction is the main ATP consumer, the
coupling of contractility to ATP production, and by extension,
mitochondrial activity, is critically important to normal
cardiomyocyte function, particularly as the mitochondrial
reticulum also regulates intracellular calcium homeostasis
and a multitude of critical signally pathways. The ability to
relate cardiomyocyte beating to alter metabolic activity would
therefore be of significant utility.
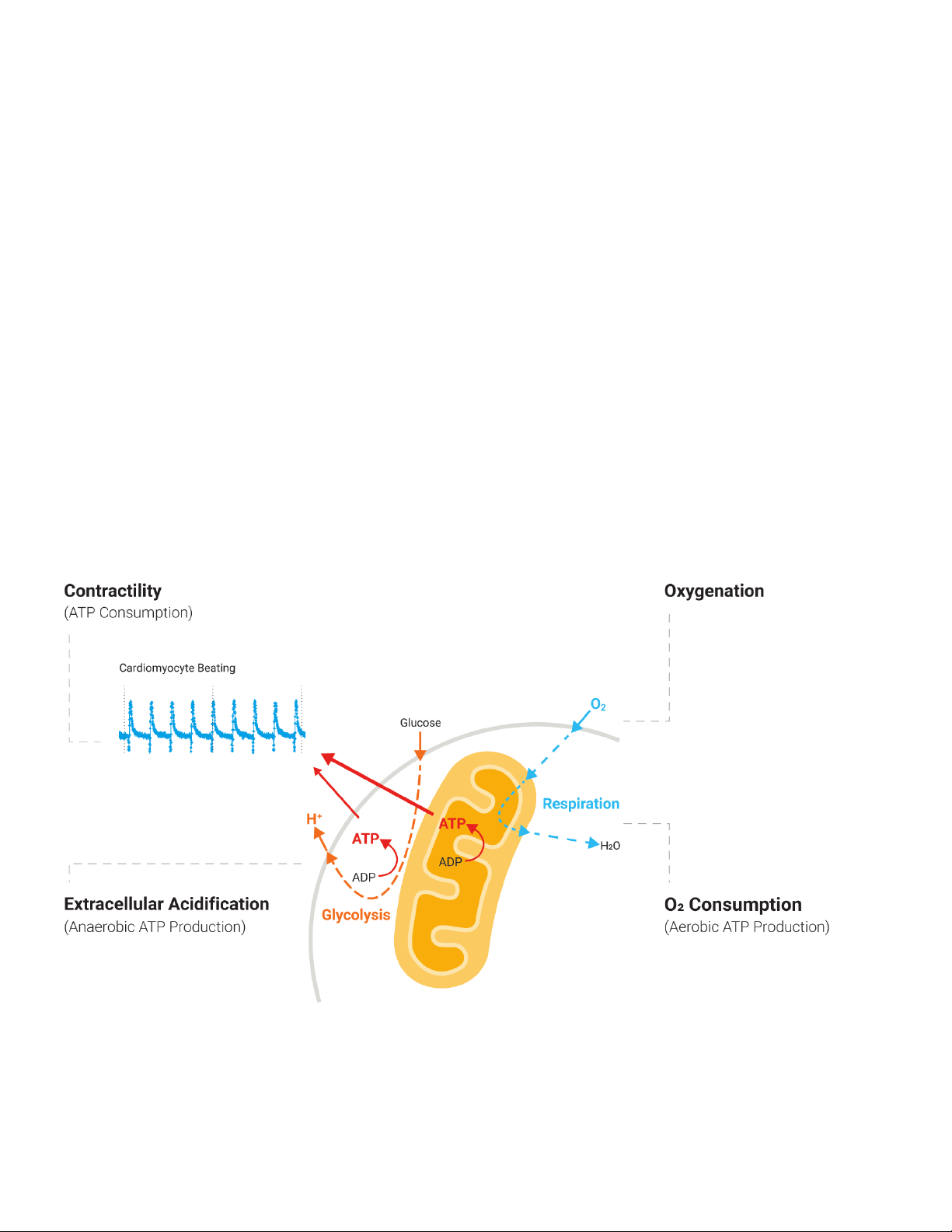
Figure 1. A simplified schematic of the inter-relationship between cardiomyocyte metabolism and beating activity. OXPHOS produces most of the ATP needed,
with pyruvate and Acyl CoA being the main respirator y substrates. By measuring beating, OXPHOS (via O2 consumption), glycolytic flux (viaextracellular
acidification), and cellular oxygenation a more complete picture of cardiomyocyte function can be established.
2
Page 3

Mitochondrial dysfunction and contractility
Metabolism Testing
Contractility is measured by culturing iPS cardiomyocytes
on E-Plate Cardio 96 and measuring them on the Agilent
xCELLigence RTCA Cardio system in real time. The E-Plate
has interdigitated impedance (IMP) microelectrode arrays
on the bottom of each well. IMP electrodes measure
cellular impedance, which is affected by the number of cells
covering the electrode, the morphology of the cells, and
the degree of the cell attachment. The fast sampling rate
of IMP measurement (12.9ms/77 Hz) allows capturing
temporal rhythmic changes in cell morphology and degree
of cell attachment to the plate associated with contraction
of cardiomyocytes. Therefore, the Cardio system is used to
predict drug-induced proarrhythmia, contractile liability, and
chronic toxicity of drugs under development.
Cell metabolism is measured using the Agilent MitoXpress
Xtra oxygen consumption assay to assess mitochondrial
function and the Agilent pH-Xtra glycolysis assay, which
uses extracellular acidification (ECA) to assess glycolytic
function. Soluble metabolic sensor reagents show a change
in fluorescence signal in response to changes in oxygen or
acidification as a result of energy production. Both reagents
can be measured using dual-read TR-F (time resolved
fluorescence) detection.3 This allows measurement on
E-Plates such that, if necessary, metabolism and contractility
can be measured sequentially on the same test plate.
Furthermore, cellular oxygenation measurements with the
Agilent MitoXpress Intra intracellular oxygen assay can
be conducted between xCELLigence RTCA time points (in
parallel but on plate reader platform) ifdesired.
Results and discussion
iPS cardiomyocytes maintain beat rates in the presence of
mitochondrial inhibitors.
To assess the effects of metabolism on beat rate,
cardiomyocytes were treated with mitochondrial modulators
on an E-Plate. Beat rates were assessed 0.5 and 24 hours
post-treatment (Figure 2A). Interestingly 1 µM FCCP
influenced the beat rate at both time points suggesting that
cardiomyocytes cannot recover following mitochondrial
uncoupling (Figure 2A). Lower concentrations did not reduce
the beat rate.
AB
0.5 h post treatment
FCCPAntimycinRotenone
Figure 2. The impact of mitochondrial impairment on cardiomyocyte beating. Beating is maintained in the presence of mitochondrial inhibitors through increased
glycolytic ATP supply. 30 s xCELLigence traces at 0.5 and 24 hours post-treatment (A). O2 consumption, extracellular acidification, and ATP were measured at
fixed concentrations (B). O2 consumption, extracellular acidification dose responses for antimycin (C)and FCCP (D). Data presented relative to untreated control.
Contractility Testing
30s
24 h post-treatment
30s
Vehicle
1 µM
0.1 µM
0.01 µM
Vehicle
10 µM
1 µM
0.1 µM
Vehicle
1 µM
100 nM
10 nM
C
600
500
400
300
% Effect
200
100
0
DMSO Antimycin ARotenoneFCCP
350
O2Consumption
300
Glycolytic Flux (ECA)
Baseline
250
200
150
% Effect
100
50
0
00.5 1
Antimycin (µM)
O2Consumption
Glycolytic Flux (ECA)
AT P
(1 µM) (1 µM) (1 µM) (2.5 µM)
900
800
700
600
500
400
300
200
100
O2Consumption
Glycolytic Flux (ECA)
Baseline
0
0510
FCCP (µM)
3
Page 4

Inhibitory concentrations of antimycin A and rotenone (1 µM)
Compound treatment
Antimycin (1µM)
Reduction in
intr
driven by
respiration
Fu
depletion
caused by
Isoproterenol
treatment
MitoXpress Xtra (µs)
Time (min)
A
Isoproterenol (1µM)
did not have a significant impact on beat rates at both time
points (Figure 2A). This suggests that cardiomyocytes can
still generate ATP. High concentrations of antimycin A did
reduce beat rates after 24 hours.
Measuring oxygen consumption rates using MitoXpress
Xtra confirmed that antimycin A and rotenone decrease
mitochondrial respiration as oxygen consumption decreases
acutely upon treatment (Figure 2B). FCCP was shown to
increase oxygen consumption but as mitochondria are
uncoupled, they are unable to generate ATP (Figure 2B).
Analysis of the extracellular acidification using pH-Xtra
glycolysis assay shows that when mitochondria are inhibited
or uncoupled, glycolysis is increased (Figure 2B). There is
a clear concentration-dependent increase in acidification
(Figure 2C) suggesting that ATP depletion is ameliorated
through increased glycolysis in cardiomyocytes.
Together, this suggests that increased glycolysis supplies
the cells with enough ATP to facilitate cardiomyocyte
beating despite the lack of mitochondrial ATP from
oxidative phosphorylation. This is consistent with previous
observations on specific cell lines.4
Conversely, cells treated with isoproterenol were shown
to have an increased beat rate, and therefore oxygen
consumption experience as low as 6%oxygen as a result of
the increased oxygen consumption. This causes a significant
but temporary reduction in oxygen availability with values of
~6% observed for >15 minutes despite cells being cultured
and measured at 21% O2.
Untreated
Isoproterenol (1µM)
B
34
32
30
28
26
24
22
20
30 40 50 60
70 80 90
Untreated
Antimycin A
Cell metabolism is tightly coupled to contractile activity
The β-adrenoreceptor agonist, isoproterenol is used for the
treatment of bradycardia (slow heart rate). Figure 3A shows
beat rate traces of cardiomyocytes using the xCELLigence
RTCA, treatment with isoproterenol increased the beat rate
by ~45% compared to control 30 minutes post drug addition
(Figure 3A). Isoproterenol also caused a similar increase in
oxygen consumption (Figure3B).
These data suggest that when the beat rate is elevated,
the increased ATP demand is met by increasing aerobic
ATP production through mitochondrial respiration
(Figure3B). An antimycin A control was included to measure
non-mitochondrial oxygen consumption. Acidification rates
did not increase (data not shown) suggesting that OXPHOS
rather than glycolysis is supplying the additional ATP required.
Changes in cellular oxygenation were measured using
MitoXpress Intra. Figure 4 demonstrates that untreated
cardiomyocytes under these conditions experience ~14%
oxygen, ~7% less than ambient oxygen due to respiration
and other non-mitochondrial background oxygen-consuming
processes. When cells are treated with antimycin A,
experienced oxygen increases to around ambient levels
(~21%) as aerobic ATP production has been inhibited.
Figure 3. Impact of isoproterenol on cardiomyocyte beat rate measured
on an Agilent xCELLigence RTCA Cardio system (A) and cardiomyocyte
metabolism (B) measured on an advanced TR-F detection compatible
fluorescence plate reader. Increased oxygen consumption caused more
rapid oxygendepletion.
% O
2
20
acellular O2
rther O2
Figure 4. Impact of isoproterenol on cardiomyocyte oxygenation
measured using advanced TR-F detection fluorescence plate reader with
atmosphericcontrol.
18
16
14
12
10
8
6
10
20 30 40 50 60
Time
Untreated
Isoproterenol (1µM)
4
Page 5

Contractility can be perturbed using several compounds
11
AB
(ECA)
AB
such as nifedipine or E-4031. Nifedipine is used to treat
and manage angina, high blood pressure, and several other
conditions, it acts as an L-type Ca2+ channel antagonist.
Figure5A demonstrates the dose-dependent effects of
nifedipine on contractile force, while Figure 5B illustrates a
dose-dependent decrease in cardiomyocyte O2 consumption.
Extracellular acidification was also reduced (data not shown).
The hERG channel inhibitor E-4031 causes an irregular beat
rate pattern (Figure 6A), which also causes a decrease in
oxygen consumption and a minor decrease in acidification
rates (Figure 6B). Suggesting that with a decrease in
ATP demand the cell responds by decreasing both ATP
generatingpathways.
20s
Figure 5. The impact of nifedipine on the beat rate (A) and metabolism
(B). Beating was measured 30minutes post-treatment. A range of
concentrations from 10 nM to 1 µM were assayed. Metabolism data
presented as oxygen consumption rate as a percentage of untreated control.
40
s
Figure 6. The impact of E-4031 on the beat rate (A) and metabolism (B).
Beating was measured 30minutes post-treatment. A single concentration
1µM of E-4031 was used. Metabolism data presented as oxygen
consumption rate and ECA as a percentage of untreated control.
Nifedipine
Vehicle
10 nM
25 nM
50 nM
100 nM
0.25 µM
0.5 µM
1.0 µM
E-4031
Vehicle
1.0 µM
120
100
80
60
40
% Effect
20
0
0.01
100
80
60
40
% Effect
20
0
O2Consumption
00
[Nifedipine] (nM)
Glycolytic Flux
Materials and methods
Cell culture
Induced pluripotent stem cells cardiomyocytes were supplied
by NCARDIA. Cells were plated onto fibronectin-coated
E-Plate Cardio 96 and placed in culture for 2to3 days,
performing media changes as per the manufacturer’s
instructions. Cells were plated at 4 to 5 ×104 cells/well for
pH-Xtra and MitoXpress-Xtra assays.
Oxygen consumption assay
Fresh media containing the MitoXpress Xtra reagent,
150µL/well was added before measurement. Compounds
were added directly, then all wells were sealed with
prewarmed HS oil. Plates were measured kinetically for
2.5to3.0hours at 37 °C (Ex 380 nm, Em 650 nm, and
Advanced dual-read TR-F plate readerdetection)
Glycolysis assay
The sample plate is placed in CO2 free incubator 3 hours
before measurement, to remove CO2. Samples were washed
three times using respiration buffer (1 mM phosphate)
prepared using the buffer tablet provided. 150 µL of
respiration buffer containing the pH-Xtra reagent was added
to sample wells. Compounds were added directly, and the
plate was measured kinetically for 2.5hours at 37 °C (Ex
380 nm, Em 615nm, and Advanced dual-read TR-F plate
readerdetection).
Cellular oxygenation assay
Cells were loaded with MitoXpress-Intra reagent
overnight (14hours) in a E-Plate Cardio 96 the day before
measurement. Cells were washed twice and 150 µL of fresh
media was added. The plate was measured kinetically at
37°C. (Ex380nm, Em 650 nm, and Advanced dual-read TR-F
plate reader detection).
Contractile assay
iPS-cardiomyocytes were plated on 96 well E-Plates and
impedance measurements were recorded at selected time
points (60 seconds sweep at a sampling rate of 77 Hz). Drug
treatment was initiated once the culture showed 40to60
synchronic beats/min. The data were normalized to baseline.
5
Page 6

Conclusion
The combination of Agilent MitoXpress Xtra, MitoXpress Intra,
and pH-Xtra metabolic assays with the xCELLigence RTCA
Cardio system and E-Plate Cardio 96 enabled the sequential
measurement of metabolism and contractility from the
same sample using the same plate. Using the dual-read TR-F
measurement approach on conventional TR-F plate readers
informs on oxygen consumption and ECA. The combined
use of microplate-based contractility and metabolism
measurements has been demonstrated to generate a
more complete picture of cardiomyocyte response to drug
treatment and allows the delineation of inter-relationships
between cardiomyocyte beating and the underlying
bioenergetic processes. This multiparametric workflow helps
to improve data density per well of sample.
Complete impairment of OXPHOS through treatment with
electron transport inhibitors did not immediately impair
cardiomyocyte beating. Increased ECA suggests that ATP
supply is maintained through increased glycolytic flux
allowing beating to continue for >24hours post-treatment.
The β-adrenoreceptor agonist isoproterenol increased beat
rate and caused a significant increase in O2 consumption
but little change in ECA. This suggests that increased ATP
demand is being met through OXPHOS rather than glycolysis.
The L-type Ca2+ channel antagonist nifedipine reduced
contractile force and caused a dose-dependent reduction
in both oxygen consumption and ECA, indicative of reduced
OXPHOS and glycolytic activity in response to treatment.
This combined analysis of critical cardiomyocyte functions
therefore delivers a more holistic and informative in vitro
cardiotoxicity screen in that it related cellular function to
the metabolic activity driving that function. In so doing,
it provides additional mechanistic information as to the
cause of observed alterations in cardiomyocyte metabolism
orcontractility.
These highly informative workflows allow users to interrogate
metabolic modulators of cardiomyocyte function. As better
in vitro cardiac models are developed, knowing the metabolic
phenotype is essential to ensure that assays appropriately
reflect mature cardiomyocyte biology. Reliance on glycolysis
or OXPHOS shapes how these cells will respond to drugs
and how they will survive in environments that they can be
exposed to such as nutrient deprivation or hypoxia. These
workflows allow for assessment of contractility followed by
metabolic interrogation with the same biomaterial without
having to re-plate or potentially differentiate additional
cardiomyocytes for parallel measurements. This saves on
cell consumption while improving data density and delivering
multiparameter outputs from single samples. The flexibility of
these workflows makes them well-positioned to characterize
both metabolism and cardiomyocyte function under a range
of conditions including drug screening, nutrient deprivation,
hypoxia, and ischemia/reperfusion. Integrating these Agilent
Cell Analysis technologies offers a complete solution for
assessing cardio-metabolism.
References
1. Lawrence, C. L. et al. In Vitro Models of Proarrhythmia. Br.
J. Pharmacol. 2008 Aug, 154(7), 1516–2
2. Dykens J. A.; Will Y. The Significance of Mitochondrial
Toxicity Testing in Drug Development. Drug Discov. Today
2007 Sep, 12(17–18), 777–85.
3. Hynes J. et al. A High-Throughput Dual Parameter Assay
for Assessing Drug-Induced Mitochondrial Dysfunction
Provides Additional Predictivity Over Two Established
Mitochondrial Toxicity Assays. Toxicol. In Vitro 2013 Mar,
27(2), 560–9.
4. Marroquin L. D. et al. Circumventing the Crabtree Effect:
Replacing Media Glucose with Galactose Increases
Susceptibility of Hepg2 Cells to Mitochondrial Toxicants.
Toxicol. Sci. 2007 Jun, 97(2), 539–47.
www.agilent.com/chem
For Research Use Only. Not for use in diagnostic procedures.
DE44202.2461458333
This information is subject to change without notice.
© Agilent Technologies, Inc. 2021
Printed in the USA, February 1, 2021
5994-2987EN
 Loading...
Loading...