Page 1

Agilent AdvanceBio Sialic Acid Profiling and Quantitation Kit
User Manual
Page 2
Notices
CAUTION
WARNING
© Agilent Technologies, Inc. 2021
No part of this manual may be reproduced in
any form or by any means (including electronic
storage and retrieval or translation into a foreign language) without prior agreement and
written consent from Agilent Technologies,
Inc. as governed by United States and international copyright laws.
Manual Part Number
5994-2800EN
GS24-SAP
Rev. AA beta
Edition
Second edition, January 2021
Printed in USA
Agilent Technologies, Inc.
Warranty
The material contained in this document is
provided “as is,” and is subject to being
changed, without notice, in future editions.
Further, to the maximum extent permitted by
applicable law, Agilent disclaims all warranties, either express or implied, with regard to
this manual and any information contained
herein, including but not limited to the implied
warranties of merchantability and fitness for
a particular purpose. Agilent shall not be liable for errors or for incidental or consequential damages in connection with the
furnishing, use, or performance of this document or of any information contained herein.
Should Agilent and the user have a separate
written agreement with warranty terms covering the material in this document that conflict with these terms, the warranty terms in
the separate agreement shall control.
Technology Licenses
The hardware and/or software described in this
document are furnished under a license and
may be used or copied only in accordance with
the terms of such license.
Restricted Rights Legend
U.S. Government Restricted Rights. Software
and technical data rights granted to the federal
government include only those rights customarily provided to end user customers. Agilent
provides this customary commercial license in
Software and technical data pursuant to FAR
12.211 (Technical Data) and 12.212 (Computer
Software) and, for the Department of Defense,
DFARS 252.227-7015 (Technical Data -Commercial Items) and DFARS 227.7202-3 (Rights
in Commercial Computer Software or Computer Software Documentation).
Safety Notices
A CAUTION notice denotes a
hazard. It calls attention to an
operating procedure, practice, or the
like that, if not correctly performed
or adhered to, could result in
damage to the product or loss of
important data. Do not proceed
beyond a CAUTION notice until the
indicated conditions are fully
understood and met.
A WARNING notice denotes a
hazard. It calls attention to an
operating procedure, practice, or the
like that, if not correctly performed
or adhered to, could result in
personal injury or death. Do not
proceed beyond a WARNING notice
until the indicated conditions are
fully understood and met.
Page 3

Contents
Introduction 5
Kit Workflow 6
Kit Components 7
Equipment and Reagents Provided By User 8
Sample Considerations 9
Kit Capacity 10
Protocol 11
Getting started 11
Performing the assay 11
Sample Analysis by UHPLC or HPLC 14
Suggested MS conditions for DMB Labeled Sialic Acids 15
FAQs 16
References 18
Technical Assistance 18
Agilent AdvanceBio Sialic Acid Profiling and Quantation Kit User Manual 3
Page 4

4 Agilent AdvanceBio Sialic Acid Profiling and Quantation Kit User Manual
Page 5

Introduction
Glycans are carbohydrates composed of monosaccharides arranged into many different possible
oligosaccharide structures based on composition and linkage position. Sialic acid capping at the
nonreducing terminal of N- or O-glycans can serve a key role biological processes and in
mediating the effectiveness of therapeutic glycoproteins.1 The composition of glycans present on
biotherapeutic glycoproteins can affect immunogenicity, pharmacokinetics, and
pharmacodynamics.1 Depending on the molecule and the application, terminal sialic acid may
reduce the rate of clearance, reduce antibody-dependent cellular cytotoxicity (ADCC) activity, or
can be anti-inflammatory.3
N-acetylneuraminic acid (NANA or Neu5Ac) and N-glycolylneuraminic acid (NGNA or Neu5Gc).
Neu5Ac is usually the predominant species, while Neu5Gc is not synthesized by humans and its
presence on biotherapeutics can potentially be immunogenic. Because of this, it is essential to
monitor not only the absolute quantity of sialic acid, but also the levels of different sialic acid
species present in therapeutic glycoproteins.
The AdvanceBio Sialic Acid profiling and quantitation kit represents a sensitive, high-throughput
approach to sialic acid quantitation. Sialic acids are released from glycoproteins using acid
hydrolysis, followed by derivatization with the fluorophore 1,2-diamino
4,5-methylenedioxybenzene (DMB), allowing for separation by reversed-phase (RP) liquid
chromatography with fluorescence detection (FLD).
The workflow offers both qualitative characterization of sialic acid species using a sialic acid
reference panel (SARP), as well as quantitation with picomolar level sensitivity using included
NANA and NGNA quantitative standards. The workflow enables reliable and reproducible
high-throughput profiling and quantitation of sialic acids, providing a broad detection range and
improved sensitivity for molecules with low levels of sialylation versus traditional DMB labeling
workflows such as our GKK-407 kit.
-
5 Two sialic acid species commonly found in biotherapeutics are
Sialic acid species comparison may be made by labeling the included qualitative sialic acid
reference panel (SARP, p/n GKRP-2503), which includes the following sialic acid species: Neu5Gc
(NGNA); Neu5Ac (NANA); Neu5,7Ac2; Neu5Gc,9Ac; Neu5,9Ac2; Neu5,7(8)9Ac3.
Use of the AdvanceBio Sialic Acid profiling and quantitation kit offers several advantages:
• 96-well format
• Broad range of detection of sialic acid levels, from 1 to 2,000 pmol
• Picomolar sensitivity for proteins with low levels of sialylation
• Quantitative NANA and NGNA standards are included
• Workflow may be completed in five hours including incubation periods
• Automation-friendly: please contact us for assistance, we are happy to provide guidance on
how the kit can be implemented on your automation platform of choice.
Agilent AdvanceBio Sialic Acid Profiling and Quantation Kit User Manual 5
Page 6
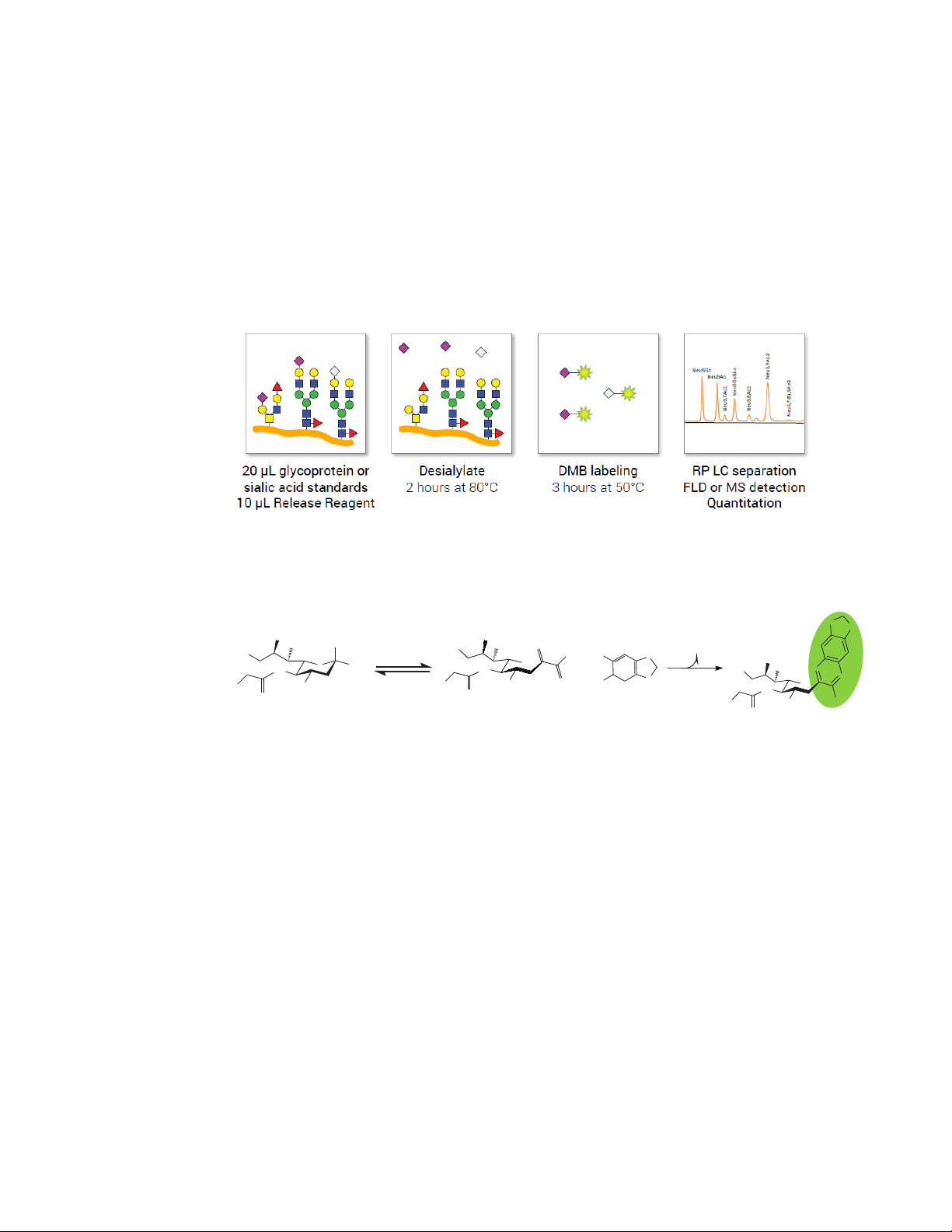
Kit Workflow
Step 1: Release of sialic acid using acid hydrolysis (2 hours)
Sialylated Glycoprotein + acid Sialic Acid + Glycoprotein
Step 2: Labeling of released sialic acid (3 hours)
Samples are now ready for separation by reversed-phase liquid chromatography.
Figure 1. Sialic acid release and DMB labeling workflow.
Neu5Gc
OH
HO
HN
HO
O
OH
OH
O
HO
COOH
Ring
opening
HO
OH
HO
HN
O
O
OH
HO
OH
OH
O
Figure 2. DMB labeling mechanism of sialic acid Neu5Gc.
DMB-labeled
DMB
H
N
2
+
H2N
2 H2O
O
O
Neu5Gc
OH
OH
HO
HN
HO
HO
O
O
O
N
N
OH
OH
6 Agilent AdvanceBio Sialic Acid Profiling and Quantation Kit User Manual
Page 7

Kit Components
Table 1 shows the Sialic Acid profiling and quantitation kit components.
Table 1 Kit Components
Module Component Units Storage
AdvanceBio Sialic Acid
profiling and
quantitation kit
GS24-SAP
100 µM N-acetylneuraminic acid (NANA, NeuAc) Sialic
Acid Standard, 200 µL
100 µM N-glycolylneuraminic acid (NGNA, NeuGc) Sialic
Acid Standard, 200 µL
Sialic Acid Reference Panel, lyophilized 1 –20 °C
Vial A: Labeling diluent, 300 µL 1 –20 °C
Vial B: Reductant 2 –20 °C
Vial C: DMB dye 2 –20 °C
Vial D: Release reagent, 300 µL 1 –20 °C
Cap strips 6 –20 °C to RT
96-well reaction plate 1 –20 °C to RT
1–20 °C
1–20 °C
Agilent AdvanceBio Sialic Acid Profiling and Quantation Kit User Manual 7
Page 8

Equipment and Reagents Provided By User
• Thermocycler (preferred), or heater with PCR block insert with lid capable of 50 °C and 80 °C
incubation and standard heavy-duty aluminum foil such as Reynolds Wrap Heavy Duty or
equivalent.
• HPLC or UHPLC with fluorescence detection (373 nm excitation, 448 nm emission)
• Agilent InfinityLab Poroshell 120 EC-C18, 2.1 × 75 mm column, p/n 697775-902 or equivalent
(for further information see “Sample Analysis by UHPLC or HPLC” on page 14.
8 Agilent AdvanceBio Sialic Acid Profiling and Quantation Kit User Manual
Page 9

Sample Considerations
• Samples that can be measured by the kit include glycoproteins, glycopeptides, glycolipids,
polysialic acids, serum, plasma, tissue, or whole cells.
• The dynamic range of this assay is 1 to 2,000 pmol sialic acid per well. Sample concentration
may need to be adjusted to assure the signal falls within the range.
• Samples can be concentrated by drying and reconstituting in a smaller volume of DI water
before use. Sample can be dried directly in the analysis wells or prepared in a separate tube.
If you have questions on the kit protocol, please contact Agilent at:
www.agilent.com/en/contact-us/page or advancebio.glycan@agilent.com
Agilent AdvanceBio Sialic Acid Profiling and Quantation Kit User Manual 9
Page 10

Kit Capacity
The kit contains sufficient reagents for 24 data points (two sets of 12 reactions).
For routine use, NGNA and NANA standards can be combined (1:1) and used neat to form a single
1,000 pmol standard mixture. Alternatively, standards can be used individually as 2,000 pmol
standards. Dilutions with DI water can be prepared to generate a standard curve. When using a
single point standard or NGNA/NANA standard mix, the recommendation is triplicates with
duplicate blank and optional Sialic Acid Reference Panel.
Table 2 Kit Capacity for a 24-well run using a single level NGNA/NANA standard mixture and triplicate or duplicate
sample analysis.
Standard
NGNA/NANA
Total Data
Kit
GS24-SAP 24 3 1 2 18 6 9
Points
mix (triplicate
analysis
Sialic Acid
Reference
Panel
Blank
(Duplicate
Analysis)
Data Points
Remaining for
Samples
No. Samples
(Triplicate
Analyses)
No. Samples
(Duplicate
Analyses)
10 Agilent AdvanceBio Sialic Acid Profiling and Quantation Kit User Manual
Page 11

NOTE
NOTE
WARNING
WARNING
Protocol
Getting started
1 Prepare heater:
a For thermocycler, program a heating cycle to heat to 80 °C for two hours and then to cool
and hold at 20 °C. Thermocycler lid should be set between 80 °C and 100 °C.
b For heat block, set heater containing heat block to 80 °C. Heavy-duty aluminum foil will be
required to insulate samples during incubation. If using a heat block, cover capped tube
strips with foil entirely, press down foil around the capped tubes and heat block to tightly
seal the tubes, and enclose heat block with a lid.
c When using a dry block heater, it is important to control both well-to-well temperature
variation and reagent evaporation/condensation in the well caps. Depending on the block
design, interior wells of the heat block may give more consistent temperatures.
2 Remove the kit from –20 °C storage and allow to equilibrate to room temperature. Have items
on hand:
• 96-well break-away plate
Reactions can be carried out in 0.2 mL flip top PCR tubes if desired.
• Cap strips
• Appropriate pipettors and pipette tips
• DI water
Performing the assay
Sialic acid release
1 Add 20 µL of sample glycoprotein per well to the 96-well break-away plate.
Sample dilution may be required prior to assay. For monoclonal antibodies with low levels of
sialylation, up to 20 mg/mL may be used. For highly sialylated proteins, a concentration of
0.25 mg/mL may be appropriate. Target range for sialic acid should be 10 to 2,000 pmol per
well.
Samples should be handled in a fume hood.
Wear appropriate PPE when handling the Release Reagent, Vial D, which is corrosive.
Agilent AdvanceBio Sialic Acid Profiling and Quantation Kit User Manual 11
2 Allow Release Reagent (Vial D) to equilibrate to room temperature. Following equilibration,
vortex Vial D before use.
3 Add 10 µL of Release Reagent (Vial D) each well containing glycoprotein. Mix by pipette, or by
vortex (if vortexing, spin down/tap to collect samples at the bottom of the well before next
step).
Page 12

NOTE
NOTE
WARNING
4 Tightly seal with cap strip and incubate at 80 °C for two hours in a thermocycler followed by
cooling and hold step at 20 °C. If using a heat block, tightly cover capped tube strips with foil to
reduce condensation and enclose heat block with lid and incubate for two hours at 80 °C.
5 If using a heat block, remove from heat after incubation and allow to cool down to room
temperature for at least two minutes. Spin down/tap to collect samples at the bottom of the
well before uncapping.
Standards and controls
Duplicate or triplicate wells for each quantitative standard are recommended. NGNA and
NANA standard can be combined in a single well. A standard curve can be performed using
dilution of the standards with DI water. Sialic Acid Reference Panel can be run in singlicate.
1 The NGNA and NANA standards are supplied at a concentration of 100 µM. To create a
2,000 pmol/well standard, use 20 µL of each standard directly without dilution.
2 To create a combined 1,000 pmol/well NGNA + 1,000 pmol/well NANA standard add 10 µL of
each standard to a single well.
3 A standard curve can be prepared from either the individual or combined standards by diluting
with DI water. For example, prepare a 4x dilution of the 2,000 pmol/well standard to make a
500 pmol/well standard by diluting 20 µL of standard with 60 µL of DI water and mixing by
pipette. Repeat the dilution with 20 µL of the 500 pmol/well standard plus 60 µL of DI water to
make a 125 pmol/well standard. Prepare additional dilutions as desired.
4 Prepare Sialic Acid Reference Panel (SARP) by diluting lyophilized material with 50 µL of DI
water. Use 20 µL of this solution for each assay and freeze remainder for future use.
5 Add 20 µL of standards, SARP, and blanks to empty wells on the cooled PCR strips containing
glycoproteins following Sialic Acid Release reaction.
6 Add 10 µL of release reagent (vial D) to all wells containing standards, SARP, and blanks to
bring to a total volume of 30 µL and mix using pipette tip.
Prepare DMB labeling mixture
Labeling mixture should be used within three hours of preparation.
1 Transfer 140 µL of Labeling diluent (Vial A) to Reductant (Vial B) and mix thoroughly by pipette.
2 Using the same pipette tip, transfer the entire contents of the reconstituted Reductant (Vial B)
to the DMB Dye (Vial C) and mix. The labeling mix is now ready for use and is stable for three
hours at room temperature.
DMB labeling reaction
1 Using fresh tips, add 10 µL of DMB Labeling mixture (steps 3 and 4) to each well containing
samples, standards, controls, and blanks. Mix with pipette tip after each addition.
2 Tightly seal with cap strip and incubate at 50 °C for three hours in a thermocycler. If using a
heat block, tightly cover capped tube strips with foil to reduce condensation and enclose heat
block with a lid.
12 Agilent AdvanceBio Sialic Acid Profiling and Quantation Kit User Manual
3 Following incubation, allow samples to cool for at least five minutes. Spin down/tap to collect
samples at the bottom of the well before uncapping.
Samples should be handled in a fume hood.
Page 13

NOTE
4 Uncap and bring the final volume of samples to 200 µL (40 µL sample + 160 µL of DI water)
and mix well with the same tips.
5 Samples are now ready for analysis by HPLC or UHPLC. Analyze samples immediately or
store in the dark at 4 °C for up to three days.
Some glycoproteins or their formulations may form precipitates during this reaction. Samples
may be filtered using optional Agilent PVDF membrane filter plate (part number 200981-100)
or equivalent (order separately).
Agilent AdvanceBio Sialic Acid Profiling and Quantation Kit User Manual 13
Page 14

NOTE
Sample Analysis by UHPLC or HPLC
The preferred method of analysis for DMB-labeled sialic acids is reversed-phase liquid
chromatography with isocratic elution, coupled to fluorescence detection.
Recommended column: Agilent InfinityLab Poroshell 120 EC-C18, 2.1 × 75 mm, 2.7 µm (part
number 697775-902).
Mobile Phase A: Methanol:Acetonitrile:Water (4:8:88)
Mobile Phase B: Acetonitrile
We recommend that mobile phase A is prepared as follows. In a 1 L graduated cylinder, add
~500 mL of water and a stir bar. Add 40 mL of methanol and 80 mL of acetonitrile while
stirring. Bring the final volume to 1 L with water.
A suggested method is shown in Tab l e 3 . Pressure should be less than 400 bar using this
method.
Needle Wash: 50% Acetonitrile in water
Table 3 Suggested LC method for separation of DMB labeled sialic acid samples.
Parameter Value
Instrument Agilent 1290 Infinity II LC System
Column Agilent InfinityLab PoroShell 120 EC-C18, 2.1 × 75 mm, 2.7 µm (part number
Column Temp 30 °C
Mobile Phase A) methanol: acetonitrile:water (4:8:88)
Gradient Program Time %A %b Flow rate
Injection Volume 10 µL (1 to 20 µL acceptable)
Detection Agilent 1260 Infinity II FLD λ
697775-902)
B) acetonitrile
(min) (mL/min)
0.00 100 0 0.4 Isocratic
6.00 100 0 0.4 Elution
6.25 20 80 0.4
7.30 20 80 0.4 Wash
7.50 100 0 0.4 Re-equilibration
10.00 100 0 0.4
373 nm, λEm 448 nm
Ex
DMB may also be detected using UV at 370 nm.7 Increase injection volume to 20 µL for increased
sensitivity.
14 Agilent AdvanceBio Sialic Acid Profiling and Quantation Kit User Manual
Page 15

Suggested MS conditions for DMB Labeled Sialic Acids
Agilent Jet Stream ESI source, any MS positive mode, sheath gas 400 °C at 12 L/min, dry gas
350 °C at 11 L/min, nebulizer pressure 15 psig, Vcap 1400 V, Nozzle 1800 V, Fragmentor 120 V,
m/z range 400 to 1,000.
Table 4 6545XT Q-TOF parameters for analysis of DMB labeled sialic acids.
6545XT Q-TOF
Source Dual AJS ESI
Gas Temperature 350 ° C
Drying Gas Flow 11 L/min
Nebulizer 15 psi
Sheath Gas Temperature 400 °C
Sheath Gas Flow 12 L/min
Vcap 1,400 V
Nozzle Voltage 1,800 V
Fragmentor 120 V
Skimmer 65 V
Mass Range m/z 400 to 1,000
Scan Rate 1 spectra/sec
Acquisition Mode High resolution (4 GHz)
Figure 3. Example separation of sialic acid reference panel (SARP) using the AdvanceBio PoroShell
EX-120 C18 column, 2.1 × 75 mm, 2.7 µm, using the conditions listed in Tab l e 3 .
Detection is by A) fluorescence using parameters in Table 3 and B) MS using parameters in Tab l e 4 .
Agilent AdvanceBio Sialic Acid Profiling and Quantation Kit User Manual 15
Page 16

NOTE
FAQs
Q. How much glycoprotein sample do I need to use with the kit?
A. This will depend on the sialylation level of your glycoprotein, which in turn will depend on the
number of N- and O-linked glycosylation sites and the relative amount of sialic acid capping at the
nonreducing terminal of the glycans. Samples such as monoclonal IgGs generally have a low level
of sialylation, while Fc fusion proteins and fetuin have a much higher level.
Table 5 shows examples for starting amounts of glycoproteins for use with the kit for
fluorescence detection to allow signal to be within the range of the standard curve. More protein
should be used if using absorbance detection. The optimal amount of starting glycoprotein should
be determined by the user, depending on the level of sialylation and the method of detection used.
Table 5 Examples of starting concentrations and amounts of glycoprotein used with GS24-SAP
(fluorescence detection).
Concentration
Glycoprotein
Fetuin 0.25 20 5 48 104
MabThera 10 20 200 145 1,379
Enbrel 0.25 20 5 150 33
Cetuximab 2 20 40 115 348
NISTmAb 10 20 200 150 1,333
EPO alfa 1 20 20 30.4 658
(mg/ml)
Sample Volume
(µL)
Sample Mass
(µg)
Mol wt (kDa) pmol Protein
Q. What is the source of the Neu5Ac and Neu5Gc Sialic Acid Standard used in the kit?
A. The standards are quantitatively prepared from N-Acetylneuraminic acid and
N-glycolylneuraminic acid, United States Pharmacopeia (USP) Reference Standard using
calibrated, NIST-traceable lab equipment.
Q. Which species of sialic acid should I expect to see in NISTmAb?
A. NISTmAb contains only Neu5Gc (NGNA) and does not contain Neu5Ac (NANA) in any
detectable quantity. This is described in application note 5994-2352EN. While some studies on
the NISTmAb8 have reported the presence of Neu5Ac, the absence of Neu5Ac was confirmed
through in-depth analysis by full MS, CID MS2, and SID.9
NISTmAb material is available from Agilent in convenient 25 µL aliquots (part number
5191-5744) and in a 4 × 25 µL pack (part number 5191-5745).
Q. Can I use a 96-well plate instead of the 8-tube strips included in this kit?
A. Yes, the kit is compatible with other polypropylene 96-well PCR plates with a working volume of
200 µL (300 µL total volume) and matching caps (i.e. 96-well semi-skirted PCR plate part number
4ti-0770 and cap strips part number 4ti-0755 from Brooks Life Sciences)
16 Agilent AdvanceBio Sialic Acid Profiling and Quantation Kit User Manual
Page 17

Q. Is there a path to automation for this kit?
A. Yes, the kit is automation friendly. For best results a thermocycler is recommended for
incubations. Plate sealing can be accomplished either through use of an automated plate sealer
(Agilent PlateLoc Thermal Microplate Sealer or equivalent) or using an auto sealing lid (BioRad
part number MSL2022 or equivalent) For further assistance, please contact us.
Q. What if I see a change in the baseline during LC separation, or a change in retention
times of sialic acid species?
A. We recommend you remake the solvent page 12, as changes can result due to evaporation.
The organics (acetonitrile and methanol) evaporate as the mobile phase sits on the instrument
over a couple of days.
Q. What if I see unexpectedly high signal?
A. The protein concentration may be too high. This is dependent on the level of sialylation of the
glycoprotein. We recommend diluting the sample to assess what dilution should be used to fall
within range of the assay.
Alternatively, the sample may have endogenous sialic acid and/or α-keto acids that contribute to
higher readings. Include a negative control (all reactants except the Vial D Release reagent);
subtract the measured value from the sample.
Q. What if I see unexpectedly low signal?
A. The protein concentration may be too low. This is dependent on the level of sialylation of the
glycoprotein. The sample may not be sialylated, or the level of sialylation is below the sensitivity of
the assay. We recommend drying down the samples and resuspending in a lower volume before
starting the assay or concentrating by another method such as molecular weight cutoff filter.
Alternatively, the sample may have lost sialic acid before analysis. Avoid prolonged exposure of
sialylated glycoproteins in aqueous solutions to low pH and/or elevated temperature. In general,
glycans in solution should be kept in the 5.0 to 8.5 pH range at temperatures below 30 °C.
Q. The pressure when using a C18 column for separation is increasing over time, and
retention times are changing. What do I do to address this?
A. This may happen if you are using proteins at high concentration. We suggest filtering the
DMB-labeled sialic acids before separation using the Agilent PVDF membrane filter plate (part
number 200981-100) or equivalent. Use of a PVDF membrane for filtration been shown not to
interfere with this assay since the labeled sialic acids do not interact with this material.
Agilent AdvanceBio Sialic Acid Profiling and Quantation Kit User Manual 17
Page 18

References
1 Varki, A. Sialic acids in human health and disease. Trends Mol Med. 2008, 14(8), 351-360.
2 Liu, L. Antibody Glycosylation and its Impact on the Pharmacokinetics and
3 Li, Y. et al. Sialylation on O-glycans protects platelets from clearance by liver Kupffer cells. Proc
4 Scallon, B. J. et al. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can
5 Kaneko, Y. et al. Anti-inflammatory Activity of Immunoglobulin G Resulting from Fc Sialylation.
6 Reuter, G. and R. Schauer. Determination of Sialic Acids. In Meth Enzymol 230 Academic
7 Zhou, Z et al. Quantifying the Efficiency of N-Phenyl-D-mannosamine to Metabolically Engineer
Pharmacodynamics of Monoclonal Antibodies and Fc-Fusion Proteins. J. Pharm. Sci. 2015,
104(6), 1866-1884.
Natl Acad Sci USA. 2017, 114(31), 8360-8365.
adversely impact functionality. Mol Immunol. 2007, 44(7), 1524-1534.
Science. 2006, 313, 670-673.
Press, New York, pp. 168 199 (1994).
Sialic Acid on Cancer Cell Surface. J Carbohydr Chem. 2014, 33(7-8), 395-407.
8 De Leoz, MLA et al. NIST Interlaboratory Study on Glycosylation Analysis of Monoclonal
Antibodies: Comparison of Results from Diverse Analytical Methods. Molecular & Cellular
Proteomics. 2020, 19(1), 11-30.
9 Zhao, J et al. Analysis of NIST Monoclonal Antibody Reference Material Glycosylation Using
the LC–MS/MS-Based Glycoproteomic Approach. Journal of Proteome Research 2021, 20 (1),
818-830.
Technical Assistance
Agilent is committed to developing rapid, automatable methods for glycan analysis. We value
customer opinions, and encourage you to contact us with your suggestions about product
performance or new applications and techniques. You can also call us to discuss products in
development.
If you have questions or comments, please contact us at www.agilent.com/en/contact-us/page
or advancebio.glycan@agilent.com.
18 Agilent AdvanceBio Sialic Acid Profiling and Quantation Kit User Manual
Page 19

Agilent AdvanceBio Sialic Acid Profiling and Quantation Kit User Manual 19
Page 20

www.agilent.com
Agilent Technologies, Inc. 2021
Second edition, January 2021
*5994-2800EN*
5994-2800EN
GS24-SAP
Rev. AA
 Loading...
Loading...