Page 1
AFP
X-Ray Film Processors
"Mini-Medical" Series
Installation, Operation,
Service & Parts Manual
250 Clearbrook Rd.
Elmsford, N.Y. 10523
December 15, 2001 0000061122 REV 04
(914) 592-6100
Page 2

WARRANTY
AFP Imaging Corporation warrants to the original purchaser that each newAFP product is free from defects in
workmanship and material for 12 months from date of installation or 18 months from date of sale, whichever occurs first.
If no warranty card is returned to AFP within 30 days of installation, the maximum warranty period will be 13 months from
date of shipment from AFP’s warehouse. In the event any product or component of equipment is replaced by AFP
under this warranty, such item is covered by this same warranty for the remainder of the original period or ninety days
from the date of installation, whichever is longer. AFP’s obligation during this warranty period is expressly limited to repair
or, at its discretion, replacement of non-expendable original equipment or components which it finds defective.
Upon authorization from AFP, a proper party asserting a claim under this warranty shall prepay all transportation costs
and return the equipment to a location specified by AFP. That party shall also bear all reasonable service and labor
charges incident to any warranty claim. This warranty does NOT APPLY (1) to any expendable parts including, but not
limited to lamps, photocells, or consumable supplies (2) to any AFP product or component which has been repaired or altered
with parts or by persons not approved in writing by AFP, provided, however, that such approval is not to be
unreasonably withheld, or (3) to any product on which the serial number or name has been altered, defaced, or
This warranty also shall NOT APPLY to any AFP product whose unsatisfactory performance or condition is due to:
- Instability of sensitized materials or chemical concentrations
and replenishing rates of chemical and wash water immersions or sequences;
- Lack of applied adequate quality production control procedures as
recommended by the sensitized material and chemical suppliers;
- Changes in characteristics or process procedures made by suppliers of
sensitized materials or chemicals after delivery of the AFP product to the purchaser;
removed.
- Lack of sufficient volume of sensitized materials for economical AFP product operation;
- Failure to follow the installation, maintenance, venting, or safety procedures
recommended for AFP product operation;
- Unusual physical or electrical stress;
- Accident, neglect, misuse, failure of electric power, air conditioning,
humidity control, transportation or causes other than ordinary use in the purposes
for which the product was intended;
THE ABOVE EXPRESS LIMITED WARRANTY IS IN LIEU OF ALL OTHER WARRANTIES, EXPRESSED OR
IMPLIED AND THERE ARE NO WARRANTIES BEYOND THOSE STATED IN THIS DOCUMENT. THE IMPLIED
WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE AND ALL OTHER
WARRANTIES, EXPRESSED OR IMPLIED, OR INFERABLE FROM THE COURSE OF DEALING OR USAGE OF
TRADE, ARE EXCLUDED AND SHALL NOT APPLY TO THIS PRODUCT.
THE PROVISIONS FOR REPAIR OR REPLACEMENT OF DEFECTIVE PARTS PROVIDED IN THIS WARRANTY
SHALL BE THE EXCLUSIVE AND SOLE REMEDY OF THE PURCHASER. AFP SHALL NOT BE LIABLE FOR
ANY OTHER DAMAGES (WHETHER IN TORT, DUE TO NEGLIGENCE OR OTHERWISE) INCLUDING BUT NOT
LIMITED TO, LOSS OF LABOR, TIME, MATERIALS, CUSTOMER PROFITS, GOODWILL, OR ANY OTHER
INDIRECT, SPECIAL, INCIDENTAL OR CONSEQUENTIAL DAMAGES IN CONNECTION WITH THE FURNISHING, OPERATION OR FAULTY PERFORMANCE OF THIS PRODUCT. THIS EXCLUSIVE REMEDY SHALL NOT
BE DEEMED TO HAVE FAILED OF ITS ESSENTIAL PURPOSE SO LONG AS AFP IS WILLING AND ABLE TO
REPAIR OR REPLACE DEFECTIVE PARTS IN THE PRESCRIBED MANNER
THIS WRITING CONSTITUTES THE FINAL COMPLETE AND EXCLUSIVE EXPRESSION OF THE TERMS OF
WARRANTY AND REMEDY AS AGREED TO BY THE PARTIES TO THIS SALE. AFP NEITHER AUTHORIZES
NOR ADOPTS ANY STATEMENT MADE BY ANY REPRESENTATIVE WHICH DIFFERS FROM THE TERMS OF
THIS WRITING AND ALL SUCH STATEMENTS ARE SUPERSEDED BY THIS DOCUMENT.
0000061122
AFP IMAGING CORP. 250 Clearbrook Road, Elmsford, N.Y. 10523
Page 3
REVISION RECORD
Title: AFP X-Ray Film Processors "Mini-Medical Series"
Document Number: 0000061122
Revision Effective Date Description
01 August 1, 1992 Initial Release
02 January 1, 1997 Total Publication Revision
03 April 26, 2001 Warranty Page Revised
04 December 15, 2001 Total Publication Revision
Page 4

Page 5

AFP Mini-Medical
X-Ray Film Processor
Table Of Contents
Section 0 - Safety Information
Section 1 - Introduction
Content 1-1
Description 1-1
Operation 1-2
Capabilities 1-2
Transport System 1-2...4
Developer System 1-4
Fixer System 1-4
Developer & Fixer Replenishment 1-5
Anti-Crystallization 1-5
Wash System 1-5
"No Plumbing" System (Optional) 1-5
Dryer System 1-6
Cover Interlock Switches 1-6
General Specifications 1-7...8
Accessories 1-9
Index
Section 2 - Installation
Introduction 2-1
Pre-Installation 2-1
Location 2-1
Dimensions & Weight 2-2
Through-the-Wall Installation 2-2
Ventilation 2-2
Electrical 2-2
Plumbing 2-3
"No Plumbing" System Option 2-3
Installation 2-4
Set Up 2-4
Assemble Stand 2-4
Position Processor 2-4
Connect Replenishment 2-4
Replenish Mode 2-5
Batch Mode 2-6
Connect Plumbing 2-7
"No Plumbing" Option 2-7
Control Panel Position 2-8
I
Mini Med Series0000061122
Page 6

Index
Section 2 - Installation (Continued)
Processor Checkout 2-9
Operational Checkout 2-9...10
Transport Film 2-11
Complete Checkout 2-11
Processor Set Up Checklist 2-12
Operational Checklist 2-13
Notes 2-14
Section 3 - Operation
Controls and Indicators 3-1
User Controls 3-1
Power Switch 3-1
Manual Replenishment Switch 3-2
Power ON LED 3-2
Dev Temp LED 3-2
Wait LED 3-2
Low Dev LED 3-2
Drain Valves 3-2
Overflow Lines 3-2
Top Cover Interlock Switch 3-2
Loading Chemicals 3-3
Daily Start Up 3-4
Processor ON, Fill Wash Tank 3-4
Check Developer and Fixer Levels 3-4
Check Drive 3-4
Processing Film 3-5
Shutdown and Daily Cleaning 3-5
Drain Wash Tank 3-5
Clean Top Cover, Guides & Rollers 3-5
Wipe Off Processor 3-5
Quality Control 3-6
Developer 3-6
Fixer 3-6
Replenishment 3-7
Checklists for Daily Use 3-8
Startup 3-8
Operation 3-8
Shutdown and Daily Cleaning 3-8
Mini Med Series 0000061122
II
Page 7

Section 4 - Maintenance
Maintenance Program 4-1
Maintenance Records 4-1
Cleaning 4-1
Mini-Medical Processor Maintenance Schedule 4-2
Daily 4-2
Weekly 4-2
Monthly 4-2
Yearly 4-2
Mini-Medical Processor Maintenance Log 4-3
Weekly Cleaning 4-4
Monthly Cleaning 4-5
Clean Tanks 4-6
Inspect Processor 4-6
Prepare Fresh Chemicals 4-7
Lubrication 4-7
Annual Maintenance 4-7
Removing Old Lubricants 4-8
Lubrication Points 4-8
Index
Section 5 - Service
Content 5-1
Troubleshooting 5-1
Service Procedures 5-2
Service Procedures Index 5-2
Schematics 5-2
Troubleshooting Processor Problems 5-3...5
Service Procedure 5-1, Main Drive Belt 5-6
Service Procedure 5-1A, Film Sensors/Adjustments 5-7...8
Figure 5-1, Film Sensor Location Configurations 5-8
Service Procedure 5-2, Servicing Circulation Pumps 5-9...11
Figure 5-2, Recirculation Pump Head, Early Style 5-11
Figure 5-3, Recirculation Pump, Later Style 5-11
Service Procedure 5-3, Servicing Replenisher Pumps 5-12
Service Procedure 5-4, Circuit Descriptions &
Developer Temperature Control 5-13
Developer Temperature Calibration 5-14
Dryer Temperature Control 5-14
Over Temperature Protection 5-15
Dryer Temperature Calibration 5-15
Replenishment Operation 5-16
Replenishment Calibration 5-16
Triacs & SCR's 5-17
Outputs 5-17
Fuses 5-17
Calibration Procedures 5-13...17
III
Mini Med Series0000061122
Page 8

Index
Section 5, Service (Cont'd)
Service Procedure 5-5, Theory of Operation 5-18...5-19
Solution Temperature Control 5-18
Dryer Temperature Control 5-18
Solution Level Sensor 5-19
Automatic Shut-Off 5-19
AC Interface Board 5-19
Waveforms & Voltages 5-20...5-21
Schematics & Wiring Diagrams 5-22...5-30
Main Wiring Diagram 5-22
Dryer Rack Wiring Diagram 5-23
AC Interface Board Schematic 5-24
Logic Board Schematic 5-25
Logic Board Layout 5-26
AC Interface Board Layout 5-27
Ready Tone Generator Layouts & Schematics 5-28...5-29
LED Board Layout & Schematic 5-30
Mini Med Series 0000061122
IV
Page 9

Section 6 - Parts
Introduction 6-1
How Parts Are Listed 6-1
When Ordering Parts 6-1
Maintenance Kit 6-1
Documentation 6-1
Part Listings
General Assembly 6-2
Figure 6-1, General Assembly 6-3
Figure 6-2a, Feed Tray & Control Chassis Assembly 6-4
Figure 6-2b, Feed Tray & Control Chassis Assembly 6-5
Figure 6-3, Drive System 6-6
Figure 6-4, Tank/Frame Assembly 6-7
Figure 6-5, Recirculation Pumps 6-8
Recirculation Pump Parts (For P/N 0000021145) 6-8
Wet Rack Assembly 6-9
Figure 6-6, Wet Rack Assembly, Top Roller Group 6-10
Figure 6-7, Wet Racks, Bottom Roller Group 6-11
Figure 6-8, Wet Racks, Film Guide Group 6-12
Figure 6-9, Wash Rack, Squeegee Roller Group 6-13
Dryer Assembly, Front Exit 6-14
Figure 6-10, Dryer Assembly, Full View 6-15
Figure 6-11, Dryer Assembly, Side View 6-15
Dryer Assembly, Rear Exit 6-16
Figure 6-12, Dryer Assembly, Rear Exit, Full View 6-17
Figure 6-13, Dryer Assembly, Rear Exit, Side View 6-17
Plumbing Schematic (Early Style Parts Listing) 6-18
Figure 6-14, Plumbing Schematic (Early Style) 6-19
Plumbing Schematic, (Later Style Parts Listing) 6-20
Figure 6-15, Plumbing Schematic (Later Style) 6-21
Notes 6-22
Index
Section 7 - Accessories
Processor Support Stand 7-1
Support Stand Parts 7-2
Stand Turning Kit 7-3
Mounting Options Utilizing Turning Kit 7-4
Side Plumbing Kit 7-5
Through-the-Wall Installation Kit, Front Exit 7-6
Through-the-Wall Installation Kit, Rear Exit 7-7
Notes 7-8
V
Mini Med Series0000061122
Page 10

Page 11

Section 0
Safety Information
- General Index -
Section 0 - Safety Information
Section 1 - Introduction
Section 2 - Installation
Section 3 - Operation
Section 4 - Maintenance
0000061122
Section 5 - Service
Section 6 - Parts
Section 7 - Accessories
AFP Mini-Medical
X-Ray Film Processors
Page 12

Page 13
IMPORTANT SAFETY INFORMATION
TO REDUCE THE RISK OF INJURY OR ILLNESS, READ, UNDERSTAND, AND HEED THE INFORMATION ON THIS SHEET, ALL PRECAUTIONARY
LABELS ON THE EQUIPMENT, AND ALL INSTRUCTIONS INCLUDED WITH THE EQUIPMENT BEFORE ATTEMPTING INSTALLATION, USE, OR
MAINTENANCE.
WARNING: SERIOUS BODILY INJURY can result from improper handling or usage.
WARNING: NEVER move the equipment without enough help and/or lifting tools.
WARNING: ALWAYS use care when opening the shipping carton. Strapping bands can snap and injure you.
WARNING: NEVER operate the equipment without its protective panels and guards installed. Beware of rotating gears and belts, rollers,
and chains, and keep from becoming entangled in them.
DANGER: POTENTIALLY FATAL VOLTAGES ARE PRESENT IN THIS EQUIPMENT.
CAUTION: NEVER make electrical connections to the equipment unless you are a qualified electrician.
WARNING: ALWAYS route power supply wiring through a nearby disconnect device.
WARNING: NEVER attempt electrical service on the equipment unless you are a qualified electronics technician.
CAUTION: ALWAYS shut off power at the disconnect device before making electrical connections or servicing electrical components.
CAUTION: ALWAYS replace fuses with those of the same type and rating.
WARNING: NEVER touch supply voltages; THEY CAN BE LETHAL.
CAUTION: NEVER operate the equipment until it is reliably electrically grounded, NOT through the water system.
CAUTION: "DEV" indicates "developer solution".
PROCESSORS AND PROCESSOR ACCESSORIES
DANGER! POISON!
OF REACH OF CHILDREN. Always review and follow the hazard warnings and the ventilation, use, and disposal instructions of the chemicals
manufacturer. Install all fluids correctly before operating.
CAUTION: TO AVOID POSSIBLE DRINKING WATER CONTAMINATION, make certain that all plumbing complies with local codes.
WARNING: PROCESSING CHEMICALS CAN CAUSE SEVERE BURNS. Do not get in eyes, on skin, or on clothing. Avoid breathing vapor,
mist or dust, and use only with adequate ventilation. ALWAYS FOLLOW THE SAFETY RECOMMENDATIONS OF THE CHEMICALS
MANUFACTURER.
PROCESSING CHEMICALS MAY BE HARMFUL OR FATAL IF SWALLOWED. KEEP OUT
LITERATURE
The following publications relate to safety in film processing.
PUBLICATION AVAILABLE FROM
ANSI.PH 4.37 Photographic Processing Effluents American National Standards Institute
Technical Data Sheet, Photographic Processing Wastes ( 6 pages) E.I. DuPont DeNemours and Co., Inc.
1430 Broadway
New York, N.Y. 10018
Photo Products Dept.
Wilmington, Delaware 19898
J4: Safe Handling of Photographic Chemicals Eastman Kodak Co.
J28: Disposal of Photographic Processing Effluents and Solutions 343 State Street
J43: A Simple Waste-Treatment System Dept. 412-L
J50: Sampling and Flow-measurement Methods Rochester, N.Y. 14650
J52: Disposal of Small Volumes of Photographic Processing Solutions
K13: Photolab Design
S39: Water Conservation in Photographic Processing
The preceding information is presented as a guide to precautions associated with photographic processing. No claim is made as to the currency, accuracy or
completeness of the listed information. Please do not fail to contact your chemicals supplier to obtain additional advice and assistance.
0000061122
Page 14

Page 15

Section 1
Introduction
- General Index -
Section 0 - Safety Information
Section 1 - Introduction
Section 2 - Installation
Section 3 - Operation
Section 4 - Maintenance
0000061122
Section 5 - Service
Section 6 - Parts
Section 7 - Accessories
AFP Mini-Medical
X-Ray Film Processors
Page 16

Page 17
Introduction
Content
Description
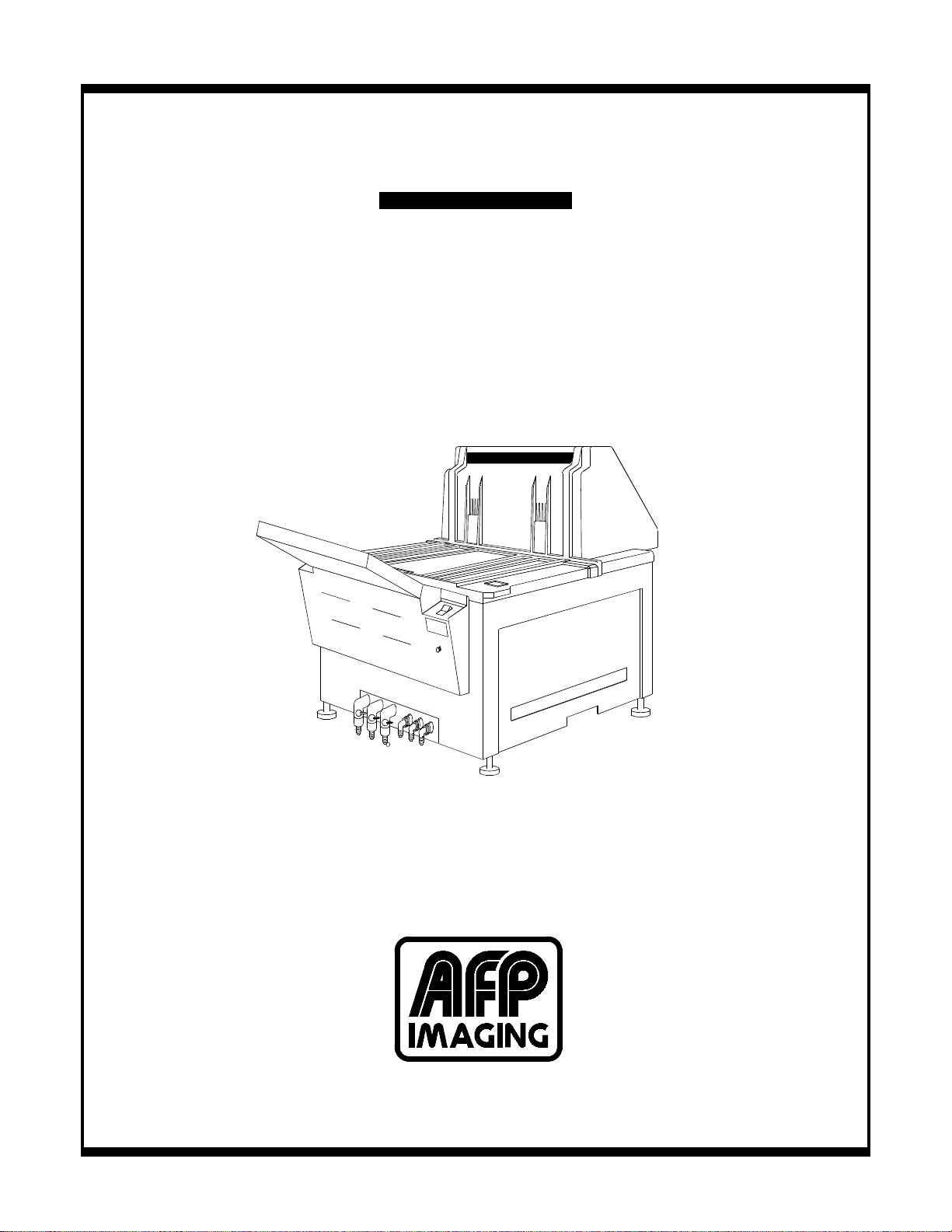
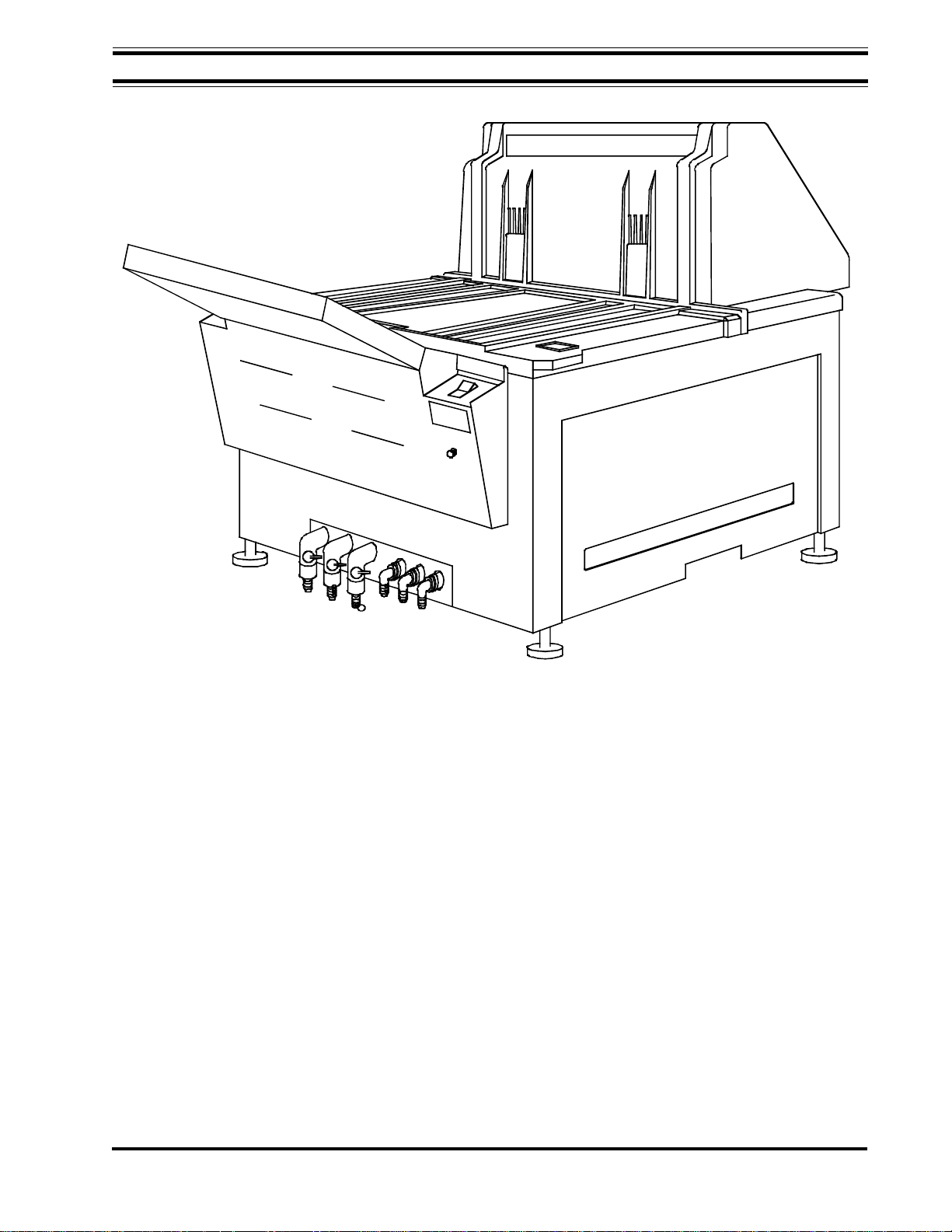
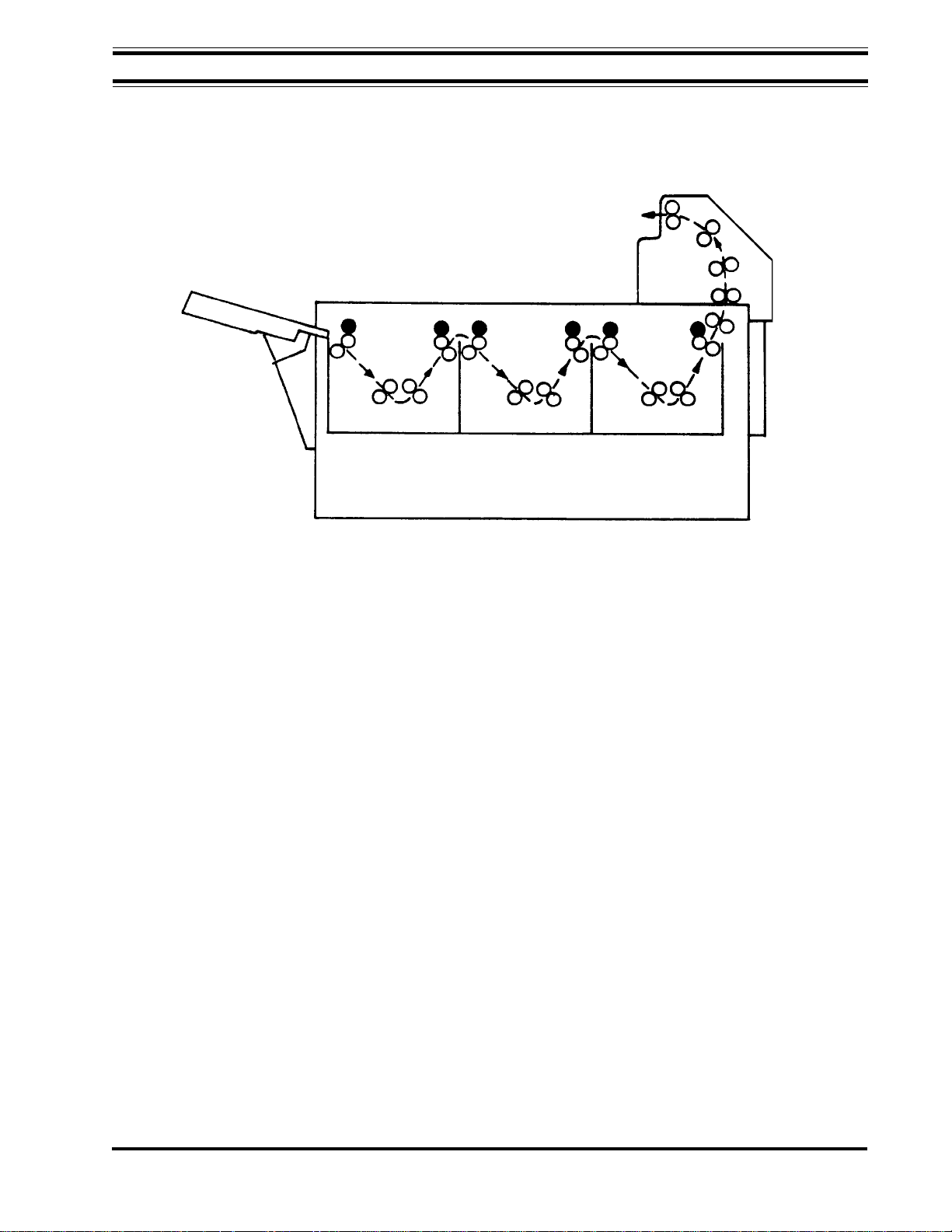
Figure 1-1, AFP Mini-Medical Series X-Ray Film Processor
This manual contains instructions for installing, using and maintaining the three different
models of the AFP Mini-Medical Series of X-Ray film processors. This series includes the
Mini-Medical, the Mini-Medical/90 and the Mini-Medical/EP processors. With the exception of pre-set processing speed and developer temperatures, these three processors are
identical in appearance, operation, maintenance and service. Differences, where existing,
will be noted in the text of this manual.
The Mini-Medical system includes the processor, with daylight tight film feed tray, support
stand, replenishment tanks, necessary hoses and this manual.
Major processor sections and components are shown in Figure 1-1.
0000061122
1-1
Mini Med Series
Page 18

Introduction
Operation
The processor is operated from the control panel. Basic processor functions are described
in the following paragraphs. Figure 1-2 is a diagram of the transport system.
Capabilities
Mini-Medical processors develop, fix, wash and dry exposed RP type medical X-ray films
of all sizes, from 4" X 4" (10 X 10 cm) to 14" X 36" (35 X 91 cm).
Hourly production capacity of 14" X 17" (35 X 45 cm) sheets of film, at the indicated, preset, lead edge in to lead edge out time, is:
Model Lead-to-Lead Productivity
Mini-Medical 130 sec 55 sheets per hour
Mini-Medical/90 90 sec 85 sheets per hour
Mini-Medical/EP 180 sec 42 sheets per hour
Transport System
Four removable roller rack modules transport the material being processed through the
developer, fixer, wash and dryer sections.
The developer, fixer and wash sections make use of “Deep Tank” racks to maintain developing quality and improve productivity. The dryer section includes a long path length
vertical dryer to assure material drying at short developing times, reduce space requirements and return the film to the operator’s position for ease of pick-up.
All racks and rollers in the wet sections and dryer are driven from a common drive shaft by
a fractional horsepower ac motor.
For ease of use, and accuracy of processing, developing times and developing temperatures
are factory set at the following values:
Model Dev. Time Developer Temp.
Mini-Medical 29 sec 90 Degrees f (32 C.)
Mini-Medical/90 22 sec 95 Degrees f (35 C.)
Mini-Medical/EP 44 sec 95 Degrees f (35 C.)
Mini Med Series 0000061122
1-2
Page 19
Transport System, (Cont'd)
Introduction
Figure 1-2, Transport System
Before processing film, the processor must first be turned ON and allowed to bring the
developer up to operating temperature.
As the processor warms up, it runs for one (1) process cycle (approximately 5 minutes) and
then remains in the Stand-By mode. In this mode, only the developer heater, one dryer
blower and the circulation pumps operate.
Film being fed into the processor is detected by a dual film sensor assembly located in the
feed slot. When film is sensed, the Wait lamp will illuminate and stay illuminated until
shortly after the trailing edge of the film has passed the sensor(s).
The above activation of the film feed sensor(s) also places the processor into Process mode,
starting the transport system, the dryer heaters and blowers and, as long as the feed switch
is tripped, operating the replenishment system.
Shortly after the Wait lamp extinguishes, an audible signal will sound, indicating to the
operator that additional film may be fed into the processor.
0000061122
An electronic holding circuit will keep the processor in Process mode for approximately 4
minutes after the feed switch is released. This will allow complete processing of the film,
after which time the processor will return to the Stand-By mode to conserve energy, water
and wear on the processor.
1-3
Mini Med Series
Page 20

Introduction
Transport System,
Cont'd)
Film is pulled into the processor by the input roller set on the developer rack. The film then
passes through the recirculating developer bath. As it leaves the developer, excess chemicals are squeegeed off by the exit rollers. This process is repeated in the fix, wash and dryer
sections.
Processed and dried film is then deposited in the film delivery area on top of the processor.
Developer System
As the film passes through the developer tank, developer is continuously circulated and
agitated around the rollers in the developer rack.
This developer circulation and agitation is provided by the developer chemistry being drawn
down into the developer circulation pump, located in the base of the tank, and then being
pumped back through the side of the tank, at a rate of approx. 2 gallons per minute.
Fixer System
The developer is replenished during operation by chemicals being drawn from the replenishment tank by a pump controlled by the replenishment circuit. This circuit operates the
pump continuously, with the actual output rate, in ml/minute, being electronically controlled by the processor’s circuitry.
Developer heat is provided by a 500 watt heater located in a heat-exchanger below the tank.
Developer temperature is sensed by a temperature sensor, located in the bottom of the developer tank.
Developer temperature is factory set at the values shown on Page 1-2, and may be readjusted by the installing technician to temperature values from ambient to 115 degrees f
(46 C.)
The film is fixed in the fix tank. Fixer is agitated, circulated and replenished in the same
manner as the developer. The fixer is not heated.
Mini Med Series 0000061122
1-4
Page 21

Developer & Fixer
Replenishment
Mini-Medical Series processors are designed to operate in either “Batch” or “Replenishment” mode. As such, replenishment chemicals may be replenished as necessary, with tank
overflow being directed into a drain or collection container for disposal, or recycled until
exhausted, then discarded and fresh chemicals installed. For additional information refer to
the Operation and Maintenance sections of this manual.
Anti-Crystallization
To prevent the build-up of chemicals on the processing rollers, an anti-crystallization or
“Jog” feature is built into all Mini-Medical processors.
This feature automatically runs the drive system at process speed for 20 seconds every 4
minutes, allowing fresh chemistry to be washed over the air-exposed rollers, effectively
preventing crystallization of chemistry on the roller surfaces.
Introduction
Wash System
The film being processed is washed in the wash tank before entering the dryer. The wash
water solenoid is actuated during the processing cycle and refreshes the water in the wash
tank with tempered water from an external source.
“No Plumbing’’
System (Optional)
The available “No Plumbing” wash water recirculation system P/N 9992305003 (115 VAC)
or P/N 9992305004 (230VAC) allows the installation of Mini-Medical processors without
external plumbing connections.
When using this option, wash water is recirculated from a 7 gallon reservoir, to the processor, and then back into the 7 gallon reservoir.
Processing chemistry (developer & fixer) is recirculated through each respective processing tank and replenishment container until its activity level is no longer satisfactory, at
which time it is drained and replaced.
0000061122
1-5
Mini Med Series
Page 22

Introduction
Dryer System
As film passes through the dryer, it is subjected to warm air from two linear infrared quartz
heating elements and a pair of fans.
Upon leaving the dryer the film is deposited in the receiving bin.
Cover Interlock Switch
To prevent accidental injury from moving parts, a mechanical safety switch is interlocked
with the processor’s top cover. If the top cover is removed, the processor automatically
shuts down.
Mini Med Series 0000061122
1-6
Page 23

General Specifications
Films
RP type medical X-Ray films and compatible chemicals designed for RP type processing.
Film Size
Minimum Size: 4" X 4" (10 X 10 cm)
Maximum Size: 14" X 36" (35.6 C 91.4 cm)
Base thicknesses 0.004 - 0.008"
Developing Time
Factory set as follows:
Model Dev. Time Linear Speed
Introduction
Mini-Medical 29 sec 20" per minute
Mini-Medical/90 22 sec 36" per minute
Min-Medical/EP 44 sec 10" per minute
Developer, Fix, & Wash
Systems
Capacity: 1.9 gallons (7.2 L.).
Temperature Control:
Developer: Factory Set as follows:
Mini-Medical 90 Degrees f. (32 C.)
Mini-Medical/90 95 Degrees f. (35 C.)
Mini-Medical/EP 95 Degrees f. (35 C.)
Fixer Ambient
Wash: Controlled by incoming water supply, .25 GPM (.95 LPM) during process and anticrystallization cycle. There is no water flow in standby mode.
Dryer System
Temperature: Factory set at 120 degrees f. (49 C.).
0000061122
1-7
Mini Med Series
Page 24

Introduction
General Specifications,
Continued
Environmental Conditions
Temperature: 40-80 Degrees f.
Humidlty: 40% - 60% RH.
Electrical Requirements
120 VAC, 15 amps, 60 Hz.
230 VAC, 7.5 amps, 50 Hz. (Optional)
Dimensions
Width 22" (56.1 Cm) Stand Only: Width 22.875"
Height: 24.5" (62.2 Cm) Height 29.5"
Length: 33" (84.1 Cm) Length 22.125"
(Including feed tray) Allow 1" (approximately) for leveling
Weight
Approximate Shipping Wt.: 110 lbs.
Approximate Operating Wt.: 160 lbs.
Air Conditioning Heat Load (Approximate)
Total Heat @ 60Hz Process Mode Standby Mode
2800 B.T.U./Hour 1800 B.T.U./Hour
Darkroom venting is required. Use a blower and vent combination that allows for approximately 300 CFM air flow through the darkroom.
Component Power Requirements
Component Amperage @ 115VAC
Solenoid 0.10
Developer Heater 4.55
Recirculation Pumps (2) 0.22 Ea.
Replenisher Pumps (2) 0.35 Ea.
Dryer Lamps (2) 2.17 Ea.
Fan Motors (2) 0.44 Ea.
Drive Motor 0.76
Total: 11.77 Amps
Mini Med Series 0000061122
1-8
Page 25

Accessories
Introduction
Stand Turn Kit (Allows sideways positioning of processor on stand for access to replenisher
containers) P/N 9992305001.
Side Plumbing Kit (Moves plumbing from front to side of processor) P/N 9992305002.
“No Plumbing’ Wash Water Recirculation Kit.
P/N 9992305003 (115 VAC)
P/N 9992305004 (230 VAC)
Specifications are subject to change without prior notice.
0000061122
1-9
Mini Med Series
Page 26

Introduction
Mini Med Series 0000061122
Page 27

Section 2
Installation
- General Index -
Section 0 - Safety Information
Section 1 - Introduction
Section 2 - Installation
Section 3 - Operation
Section 4 - Maintenance
0000061122
Section 5 - Service
Section 6 - Parts
Section 7 - Accessories
AFP Mini-Medical
X-Ray Film Processors
Page 28

Page 29

Attention:
When testing or operating the processor with water (as opposed to chemistryj), there will be a
LOW LEVEL condition in effect which will disable the solution heater. This is due to the fact that water
by itself cannot conduct well enough. To prevent this, add 1-2 tablespoons of salt or a cup of used or
fresh developer to the developer tank.
Mini Med Series
0000061122
Page 30

Page 31

22"
Installation
24"
33"
Introduction
This section includes instructions for Pre-Installation, Installation and Check Out of AFP
Mini-Medical X-Ray film processors.
Pre-Installation
Pre-installation includes instructions for preparing the processor operating site.
Have these operations completed before the scheduled installation date.
If the installing technician is delayed by incomplete site preparation, you may be charged
for costs during the delay.
Location
AFP Mini-Medical Series processors must be operated in a darkroom suitably safelighted
for the film being used.
Figure 2-1, Processor Dimensions
2-1
Mini Med Series0000061122
Page 32

Installation
Dimensions
Weight
Ventilation
Mini-Medical Processors occupy 8.0 square feet (22.5" X 24") (57 X 61 cm.) of floor or
counter space. The processor should be positioned to allow easy access to all sides of the
unit for routine cleaning and preventive maintenance. Drain tubes, leading out of the “front”
of the processor, below the feed tray, must be readily accessible.
The Mini-Medical Processors weigh approx. 110 lbs. when empty, and approximately 160
lbs. when operating.
To support this weight a Processor Stand Assembly is included. Instructions for the assembly of this stand can be found in Section 7, Accessories. If the stand is not used, a sturdy,
stable and level stand, table or counter must be provided.
WARNING: Some processing chemical fumes may irritate eyes and/or respiratory systems
when used in a poorly ventilated area. If the processor is to operate in a confined area,
provide for at least ten complete changes of air per hour.
Provide adequate ventilation for proper machine operation and operator comfort. The processor generates a moderate amount of heat when operating and must not be placed in a
confined space, such as a closet.
For best processing results, relative humldity should be between 40% and 60%.
Electrical
Electrical connections must include a ground and conform to local codes. The processor
plugs into a standard 120 VAC, 60 Hz, 15 amp., 3 wire outlet. As a factory installed option,
Mini-Medical Processors may also be configured for 230 VAC, 50 Hz, operation.
Through-the-Wall
Installation
If your processor is to be Installed through the darkroom wall, refer to Section 7, pages 6
and 7.
Mini Med Series 0000061122
2-2
Page 33

Pre-Installation,
Continued
Plumbing
WARNING: Obey all instructions of the chemicals manufacturer, and follow all recommended safety precautions when handling, using and disposing of chemicals.
The following plumbing requirements are recommended for installation of the Mini- Medical Processors:
1) A flow controlled water source for wash water and for cleaning the processor.
2) A sink, with running tempered water, approximately 12" X 16", for use when cleaning
rack modules.
3) A drain suitable for dumping photographic chemical wastes.
Installation
Caution: In some locales, environmental regulations may require the capturing and safe
disposal of photographic processing wastes other than in the sanitary sewer system.
Check with your local authorities if you are unsure of regulations in your area.
NOTE: The replenisher and drain connections may be run out of the front of the processor
in standard configuration or, using the optional Side Drain Kit P/N 9992305002, these lines
may be routed out either the right or left side of the processor. See Section 7, Accessories
for additional details.
"No Plumbing" System Option
The available “No Plumbing” wash water recirculation system, p/n 9992305003 (115 VAC)
or p/n 9992305004 (230 VAC), allows the installation of Mini-Medical Processors without
the need for an external water supply or drains. If this system is being installed with the
processor, refer to the instructions packed with that unit and to Section 7, Accessories, in
this manual.
This completes the pre-installation preparations you are expected to have completed before
the processor installation date.
2-3
Mini Med Series0000061122
Page 34

Installation
Installation
NOTE: Do not unpack the processor until you have thoroughly inspected the shipping container for evidence of damage. If there is any damage, contact your shipper immediately for
instructions on filing a claim.
Set Up
Unpack the processor and accessory boxes and inspect for any visible shipping damage. As
above, if any damage is discovered after unpacking, contact the shipper immediately for
instructions on filing a claim.
Remove the processor side covers. Remove each of the rack modules and any packing
material from the tanks. Inspect each of the racks for loose parts or screws.
Assemble Stand
Following the instructions in Section 7, Accessories, unpack and assemble the included
processor stand.
Position Processor
Using two people, carefully position the processor on its stand.
Using a level placed across the walls of the processing tanks, adjust the leveling feet until
the processor is level in both directions.
Connect Replenishment
The processor may be set up to operate its replenishment system in either “Replenish” or
“Batch” mode.
In “Replenish” mode the chemicals will be replenished with fresh chemicals from the
replenisher supply and the overflow will be collected for disposal or routed directly to a
drain.
In “Batch “ mode the developer and fixer chemicals will be recycled from the replenisher
supply to the processing tank and back to the replenisher supply.
Mini Med Series 0000061122
2-4
Page 35

Replenish Mode
DEV
FIX
To Repl. Pump (s)
To Drain
In “Replenish” mode, fresh replenisher will be pumped from the replenisher supply to the
processing tank. Excessive chemicals in the processing tank will flow out of the tank at an
overflow port and into either a container for disposal or an appropriate drain line. In this
manner, constant processing chemical strength may be maintained for longer periods of
operation.
To install the processor for “Replenish” mode replenishment operation proceed as follows:
(See Figure 2-2)
1) Attach the red developer replenisher pickup tube from the developer replenisher pump
to the developer replenisher reservoir fitting.
2) Route the red developer drain line and red overflow line from the processor to an overflow container or drain line.
3) Attach the blue fixer replenisher pickup tube from the fixer replenisher pump to the fixer
replenishment reservior fitting.
Installation
4) Route the blue fixer developer drain line and blue overflow line from the processor to the
overflow container or drain line.
Figure 2-2, Replenish Mode Operation
2-5
Mini Med Series0000061122
Page 36

Installation
)
Batch Mode
In “Batch” mode the replenisher will be recycled from the replenisher supply to the processing tank and will then, via the tank overflow port, return to the replenisher supply to be
recycled again. As the chemical's processing strength becomes depleted, the entire batch is
disposed of and new chemistry installed.
To install the processor for “Batch” mode replenishment operation proceed as follows:
(See Figure 2-3)
1) Route the red developer replenisher pickup tube from the developer replenisher pump to
the developer replenishment reservior or to a container of developer replenisher.
2) Route the red developer drain line and red overflow line from the processor to the same
container as the developer replenisher pickup tube.
3) Route the blue fixer replenisher pickup tube from the fixer replenisher pump to the fixer
replenishment reservoir or to a container of fixer replenisher.
4) Route the blue fixer developer drain line and blue overflow line from the processor to the
same container as the fixer replenisher pickup tube.
To Drain
To Repl. Pump (s
DEV
FIX
Figure 2-3, Batch Mode Operation
Mini Med Series 0000061122
2-6
Page 37

Connect Plumbing
Wash water into the processor is controlled by the water solenoid valve. When the processor is in the process mode the solenoid is actuated, allowing wash water to flow into the
bottom of the wash tank. Excess water in the wash tank flows over the overflow port and
down the drain.
To connect the wash water proceed as follows: (See Figures 2-2 and 2-3)
1) Route the supplied reinforced water hose from the output of your water panel to the
fitting on the wash water solenoid under the right side of the processor.
2) Route the clear wash water drain line and the clear overflow line from the utility section
of the processor to the building drain or a suitable overflow container.
CAUTION: If you are draining your processor directly into a sanitary sewer, be certain that
such connections are in accordance with local plumbing codes and comply with all local
and federal EPA anti-pollution requirements.
Installation
DO NOT drain the processor into any drain lines that are made of copper pipe as chemical
reactions will quickly damage the pipes.
"No Plumbing" System Option
The available “No Plumbing” wash water recirculation system, P/N 9992305003 (115 VAC)
or 9992305004 (230), allows the installation of any of the Mini-Medical Series processors
without the need for an external water supply or drains. If this system is being installed
with the processor, refer to the instructions packed with that unit and to Section 7, Accessories, in this manual.
2-7
Mini Med Series0000061122
Page 38

Installation
Control Panel Positioning
In some installations, such as with the right side of the processor against a wall, it may be
difficult for the processor operator to view the LED’s on the Display Panel to the left of the
feed tray.
If this is the case, to make the LED Display Panel more visible, use the following procedure
to reverse the positions of the Power Switch/Circuit Breaker and the LED Display Panel.
1) Disconnect the processor power cable from its outlet.
2) Remove the two screws holding the control chassis panel to the front of the processor.
Carefully lower the panel until it is supported by its restraining straps.
3) Locate the small screw(s) that holds the LED Display Panel and the Power Switch panel
in place. Remove the screws.
4) Carefully unlace the cables for each panel from the retaining clips back as far as the
center of the loom.
5) Re-install the panels in the desired position,taking care to replace the cables through the
retaining clips.
6) Replace the panel securing screws and close the control chassis and secure it with its two
screws.
7) Return the processor to service.
Mini Med Series 0000061122
2-8
Page 39

Processor Checkout
Following set up, inspect the processor as described below to make sure it is ready for use.
WARNING: During this inspection, be sure that the processor power is disconnected at the
wall plug.
Inspect and clean the processor tanks, racks and hoses as described below:
1) Open the drain valves on the front of the processor for the developer, fixer and wash
tanks. Use warm water to rinse each tank clear of dust and debris. Close all drain valves.
2) Shine a light through all hoses to check for foreign matter. To remove anything, disconnect hose at one end, flush with water, and reconnect.
3) Check, and tighten if necessary, loose hose clamps and/or hardware on the processor.
4) Check that processor is level from front-to-rear and side-to-side. Correct as necessary.
Installation
Operational Checkout
Read these instructions completely before starting the processor.
WARNING: Never operate the processor without an electrical ground connection.
1) Close the tank drain valves.
2) If not already done, remove the three racks and set aside.
3) Carefully pour about 1.5 gallons of warm water into each of the solution tanks. Do Not
attempt to fill the tank to the overflow.
Attention:
When testing or operating the processor with water (as opposed to chemistry), there will be
a LOW LEVEL condition in effect which will disable the solution heater. This is due to the
fact that water by itself cannot conduct well enough. To prevent this, add 1-2 tablespoons
of salt or a cup of used or fresh developer to the developer tank
4) Install all three racks in their appropriate tanks.
5) With the Power Switch OFF, plug in the power cord.
2-9
Mini Med Series0000061122
Page 40

Installation
Operational Checkout
Continued
Caution: Never attempt to operate the processor without liquid in the tanks.
6) Switch the Power Switch to ON.
7) The transport system will run at Process speed for the duration of one processing cycle,
the recirculation pumps will operate and the dryer heaters and fans will be activated.
NOTE: If either of the recirculation pumps do not prime, squeeze the rubber elbow on the
inlet side of the pump(s) to purge the air from the line.
8) Inspect all rack modules to verify that they are turning freely.
9) Carefully inspect the underside of the processor for any signs of leakage. Correct if
necessary.
10) Operate the Manual Replenishment switch to run the replenishment pumps until the
developer and fixer tanks are full of water to the overflow port.
11) Activate the Film Feed switch with a piece of film. The Wait light will turn ON and
every few seconds the replenishment pumps will cycle. Remove the film from the sensor.
In a few seconds the Wait light will go out and the beeper will sound.
12) When the Dev Temp lamp turns OFF, check the temperature of the developer with a
metal stem or digital thermometer*. If it is not correct for the film you will be using, adjust
the temperature as outlined in Section 5, Service.
*Warning: Do not use a mercury thermometer. If a mercury thermometer breaks, it will
contaminate the machine
Mini Med Series 0000061122
2-10
Page 41

Transport Film
Transport several pieces of film of your usual size(s) through the processor. Inspect and, if
necessary, correct for the following:
1) Film Feed switch operation. The Wait lamp should stay on continuously until a few
seconds after the trailing edge of the material being processed is clear of the Film Feed
switch.
As the Wait light turns off, an audible beeper will sound indicating it is safe to feed in
another piece of material.
The processor will remain in the process mode for approximately 5 minutes after the film
feed switch is released.
2) Drift or Skewing. The film should feed through the processor in a straight line. If it
drifts, skews or wrinkles, check the racks for proper seating or loose assembly screws. Be
certain you are feeding the film in straight before checking racks.
Installation
3) Drying. Be sure the dryer is operating properly. Film processed in water alone may still
be slightly tacky or damp when exiting the processor.
Complete Checkout
1) Turn the Power Switch OFF. Unplug the power cord.
2) Drain each of the processing tanks and the replenishment containers for the developer
and fix replenishment systems. Close all drain valves.
3) Wipe any excess water from the racks and tanks.
The processor is now ready to be charged with fresh chemistry as instructed in Section 3,
OPERATION.
2-11
Mini Med Series0000061122
Page 42

Installation
Processor Set Up Checklist
1) Uncrate processor. Inspect for shipping damage.
2) Assemble processor stand.
3) Set processor on stand.
4) Level processor.
5) Inspect tank and racks for loose parts.
6) Install replenisher system, replenish or batch mode.
7) Connect wash water system and drain.
8) Rinse out wet tanks, inspect recirculation lines.
9) Perform Operational Checkout.
Figure 2-4, Setup Checklist
Mini Med Series 0000061122
2-12
Page 43

Operational Checklist
l) Close drain valves.
2) Remove racks.
3) Partially fill tanks with warm water.
4) Replace racks.
5) Plug in processor, apply Power.
6) Inspect transport drive system.
7) Check recirculation plumbing for leaks.
8) Top off tanks using Manual Replenishment switch.
Installation
9) Check Film Feed switch and “Beeper” operation.
10) Check temperature control systems.
11) Check developing time.
12) Check for dryer heat and operation of both fans.
13) Check transport of material.
14) Drain Processor.
15) Charge with fresh chemistry.
Figure 2-5, Operational Checklist
2-13
Mini Med Series0000061122
Page 44

Installation
Notes:
Mini Med Series 0000061122
2-14
Page 45

Section 3
Operation
- General Index -
Section 0 - Safety Information
Section 1 - Introduction
Section 2 - Installation
Section 3 - Operation
Section 4 - Maintenance
0000061122
Section 5 - Service
Section 6 - Parts
Section 7 - Accessories
AFP Mini-Medical
X-Ray Film Processors
Page 46

Page 47

Operation
9
3
4
5
6
NOTE: For operator convenience, the
LED Display Panel and the Power Switch
Panel may be switched, right to left,
during installation of the processor.
See Page 2-8 for details.
8
7
1
2
Controls and Indicators
All of the user controls and indicators for operation of Mini-Medical Processors are
located on the front of the processor. These controls are described below and on the
following page.
User Controls
1. Power Switch
OFF All power to processor is OFF.
ON Processor is ON, (runs one approximate 5 minute process cycle initially),
then reverts to standby mode. Circulation pumps, developer heater and air
circulation fan are ON. Transport, replenishment system and dryer will
operate when Film Feed switch is activated. Power On lamp will light.
CENTER This switch also serves as the circuit breaker for the processor. If tripped to
the center position reset to OFF, then turn ON. If the switch trips again, the
processor probably needs a service call. Do not use the processor if it trips
off repeatedly.
Figure 3-1, User Controls
0000061122
3-1
Mini Med Series
Page 48

Operation
User Controls,
Continued
2. Manual Replenishment Switch
3. Power ON LED
4. Dev Temp LED
5. Wait LED
Provides for manual operation of replenishment pumps. Use to “top off” tanks or to
turn over chemistry when activity levels have dropped.
Lights when Power Switch is ON.
Lights when developer heater is ON. Wait for light to cycle OFF before first use each
day.
Illuminates when Film Feed switch is activated. To prevent fogging of film, wait until
lamp goes OFF or beeper sounds before opening feed tray.
6. Low Dev LED
Lamp ON indicates that developer is too low for safe operation. To prevent damage to
the processor, the developer heat function is turned OFF when a low level condition
exists.
7 Drain Valves
Drains the Developer, Fixer, Wash tank and recirculation pumps.
8. Overflow Lines
Drain lines from developer, fix & wash overflow ports.
9 Top Cover Interlock Switch
To prevent accidental injury from moving parts, a safety switch is interlocked with the
processor’s top cover. If the top cover is removed, the processor automatically shuts
down. This interlock may be overridden for service use only by using the service hold
down tool included in the Maintenance Kit provided with each unit.
Mini Med Series
Caution: DO NOT attempt to process film in this unit when the interlock is overridden.
3-2
0000061122
Page 49

Loading Chemicals
Always begin with a clean processor. The processor should have been cleaned in the
normal course of installation or maintenance.
With the developer and/or fixer tank cleaned and drained, add processing chemicals as
described below:
WARNING: Read and heed safety precautions given by your chemical manufacturer
in mixing, using and disposing of processing solutions.
To prevent chemical splashing and the risk of contamination follow these instructions
carefully.
1) Close the tank drain valves at the front of the processor.
2) If not already done, remove the three racks and set aside.
3) Cover the developer tank with a sheet of newspaper to protect it from accidental
splashes of fixer.
Operation
4) Carefully pour about 1.5 gallons of fixer working solution into the fix tank. Do Not
attempt to fill the tank to the overflow.
5) Cover the fixer tank with a sheet of newspaper to protect it from accidental splashes
of developer.
6) Carefully pour about 1.5 gallons of developer working solution into the developer
tank. Do Not attempt to fill the tank to the overflow.
7) Carefully pour about 1.5 gallons of warm water into the wash tank. Do Not attempt
to fill the tank to the overflow.
8) Replace the racks. Lower them into the tanks slowly to prevent splashing. Check for
correct seating on the locating pins and driveshaft.
9) Attach the replenisher hoses to the fittings on the replenisher supply containers.
10) Turn the Power Switch-ON. Operate the Manual Replenishment switch to run the
replenishment pumps until the developer and fixer are seen in the overflow drain tubes.
0000061122
3-3
Mini Med Series
Page 50

Operation
Daily Start Up
Processor ON,
Fill Wash Tank
The daily start up procedure is as follows:
1) Close the wash tank drain valve.
2) Switch the Power Switch to the ON position. The processor will start in the process
mode and run for approximately 5 minutes, filling the wash tank. If, at the end of the
process cycle, the wash water has not reached the overflow port, trip the Manual Replenishment switch to initiate another processing cycle.
3) Allow the developer to warm up to operating temperature (Dev Temp light will
cycle OFF). Check for leaks around all hose fittings.
Caution: Always inspect to see that all drain tubes are properly positioned and draining
correctly. All drain tubes must be routed in a continuously downward direction, without dips or loops that can cause airlocks.
Caution: A kink or twist in a drain tube can cause a serious chemical or water spill in
the processor.
Check Developer
and Fixer Levels
If not previously done, check the developer and fix tanks to see that they contain adequate solution. Prepare fresh replenisher if necessary and using the Manual Replenishment switch, top off each tank with chemistry to the overflow port.
Check Drive
With the Power Switch in the ON position, check all turning drive gears to see that they
mesh properly and turn without binding. Make sure the transport rollers are turning
freely, without interference or binding.
Mini Med Series
3-4
0000061122
Page 51

Processing Film
Feed film into the processor. As it actuates the feed sensor, the WAIT lamp on the
control panel will light.
If you turn the room lights on after feeding film, to prevent fogging the end of your
film, do not open the daylight cover until after the Wait light goes out or the beeper
sounds.
After the processor completes its processing cycle it will automatically return to Stand-
By mode.
Shutdown and Daily Cleaning
Basic care of the processor goes hand-in-hand with its operation. Following each day’s
work, allow 15 minutes to clean the processor as described below.
Drain Wash Tank
Operation
Open the wash tank drain valve and allow the wash water to drain. Rinse out the wash
tank with fresh warm water, then close the drain valve.
Clean Top Cover,
Guides & Rollers
Using a separate wet cloth for developer and fixer, wipe the exposed rollers on each
rack.
Caution: Do not use the same cloth for fixer and developer racks. Fixer may contami-
nate the developer.
Wipe off Processor
Thoroughly wipe the inside and outside surfaces of the top cover and side panels with
a damp cloth. Replace the top cover, leaving a slight opening over the drive shaft to
prevent condensation of chemistry vapors.
0000061122
3-5
Mini Med Series
Page 52

Operation
Quality Control
Developer
Fixer
A good quality control program is essential to the production of quality radiographs.
It is recommended that a quality control program for your processor be established and
maintained to assure the quality of your output.
Following are some suggestions for those areas that should be monitored. Contact your
film and chemistry technical representative for additional information and assistance.
Developer activity can be monitored by use of pre-exposed control strips, available
from your film supplier, or by careful monitoring of your production work.
Exhausted fixer will usually result in dark streaks in your film’s emulsion that may
appear immediately after processing or may not appear until hours or even days after
processing.
Exhausted fixer can also contribute to transport problems such as jams and will frequently prevent proper drying from taking place, resulting in sticky film surfaces.
The general health of your fixer can be determined by monitoring the pH of the chemistry.
When pH is too high, films may jam in the wash tank and the dryer. To determine pH,
immerse pH test strips, available from your dealer, in the fixer and read its pH value
from the resultant color change on the strip. If the pH rises toward the chemical
manufacturer’s recommended upper limit, dump the old fixer and replace with fresh
chemicals.
NOTE: Only terminal-type silver recovery systems are recommended for use with this
processor. Do not try to re-use fixer after silver has been removed.
Mini Med Series
3-6
0000061122
Page 53

Replenishment
Operation
Replenishment in Mini-Medical Processors consists of “topping” off the developer and
fixer tanks with fresh working solution at the start of each shift and automatic replen-
ishment by the replenishment system.
Automatic replenishment is accomplished by the film tripping one or both of the Film
Feed Switches which, in turn, actuates the electronic replenishment circuitry. Depend-
ing on technician set adjustments, the developer and fixer pumps will cycle on and off
during film feeding to replenish the working solution in the developer and fixer tanks.
Manual Replenishment may be required for one of three reasons. They are:
1) To top off the tanks at start-up.
2) To restore chemical strength after several days of shut down.
3) To compensate for a basic under-replenishment condition. (See NOTE below)
NOTE: Chemistry requirements vary by the type of work and average size of films you
are processing. If you find that you must frequently use the Manual Replenishment
switch to add fresh chemistry, it is recommended that you have your technician adjust
the replenishment control circuits as required to allow for adequate automatic replen-
ishment.
0000061122
3-7
Mini Med Series
Page 54

Operation
Checklists for Daily Use
Startup
1. Check solution levels.
a) Top off processing tanks and fill wash tank.
2. Power Switch to ON, check drive gears for meshing;
allow 15-30 minutes warm up.
3. Clean feed tray, receiving bin.
4. Check developer activity, fixer pH.
Operation
1. Feed material, trip Film Feed switch to start processing cycle.
2. Wait until Wait light goes out or beeper sounds before opening film feed tray.
Shutdown and Daily Cleaning
1. Switch off power.
2. Drain and rinse wash tank, close valve.
3. Clean:
a) roller surfaces, rack and tank area
b) splashes from inside top cover.
4. Wipe outside surfaces of the processor, inside surfaces of feed tray & cover box
and both side panels.
5. Leave top cover slightly open to prevent condensation.
NOTE: Duplicate these checklists and post them near the processor.
Mini Med Series
3-8
0000061122
Page 55

Section 4
Maintenance
- General Index -
Section 0 - Safety Information
Section 1 - Introduction
Section 2 - Installation
Section 3 - Operation
Section 4 - Maintenance
0000061122
Section 5 - Service
Section 6 - Parts
Section 7 - Accessories
AFP Mini-Medical
X-Ray Film Processors
Page 56

Page 57

Maintenance Program
Maintenance of Mini-Medical Processors consists of cleaning and adjustment operations,
routinely performed, to keep the processor operating correctly. Early in the life of your
processor set up a maintenance program, with specific people responsible for performing
each maintenance task.
Maintenance Records
Good preventive maintenance is essential to assure a long and trouble free life for your
processor. Keeping on-going records of maintenance will help assure that the work is
performed when scheduled.
Figure 4-1 is a Maintenance Schedule that lists tasks to be performed at prescribed maintenance intervals.
Figure 4-2 is a Maintenance Log for keeping monthly records of maintenance performed.
Make additional copies and post near the processor.
Maintenance
Cleaning
Cleaning is the most important form of maintenance. If chemicals are allowed to accumulate on processor parts they can cause corrosion or other damage which may seriously
affect production and output quality.
Perform daily cleaning, as outlined on the Maintenance Schedule, Figure 4-1, as part of the
shutdown procedure.
Weekly cleaning, described below, should take about thirty minutes following the last shutdown and daily cleaning.
Do not replace items removed for daily cleaning until after weekly cleanup has been completed.
Caution: Never use harsh abrasive material to clean racks or processing tanks. Never
use scrub pads such as "Scotchbrite" on rollers.
0000061122
4-1
Mini Med Series
Page 58

Maintenance
Daily Clean:
Developer Rollers
Top Covers, Side Panels
Feed Tray, Receiving Bin
Check:
Chemical Levels
Replenisher Levels
Weekly Clean:
Developer Rack
Fix Rack
Wash Rack
Wash Tank
Tank Exteriors
Mini-Medical Processor
Maintenance Schedule
Monthly Clean:
Developer Tank, Circulation &
Replenishment System
Fixer Tank, Circulation &
Replenishment System
Wash Tank, Drain & Overflow System
Check:
Hose Clamps & Plumbing
Rack Bearings
Lubrication Points
Yearly Clean:
Developer & Fixer Circulation Pumps
Check:
Drive Belt
Drive Motor Brushes
Lubrication Points
Mini Med Series
Figure 4-1, Maintenance Schedule
4-2
0000061122
Page 59

Mini-Medical Processor
Maintenance Log
Maintenance
DAY
MONTH
INITIAL UPON COMPLETION
CLEAN DEV. ROLLERS
RECORD
CLEAN TOP COVERS
CLEAN SQUEEGEE ROLLERS
CLEAN FEED TRAY
CLEAN RECEIVING BIN
CHECK CHEMICAL LEVELS
DAILY
CLEAN DEV. TRANSPORT
CLEAN FIX TRANSPORT
CHANGE WASH WATER
CLEAN WASH TRANSPORT
1
2
3
4
5
6
7
DATEHRS
MONTHLY MAINTENANCE
8
9
10
11
12
13
14
15
16
17
YEARLY MAINTENANCE
18
19
20
21
22
23
LUBRICATION
24
25
26
27
28
29
30
31
Note: If Processor is run 80 hours or more a week, perform maintenance twice as often.
WEEKLY
CLEAN WASH TANK
CLEAN TANK EXTERIORS
CHANGE DEVELOPER
CHANGE FIXER
OPERATION
CLEAN DEVELOPER SYSTEM
CLEAN FIXER SYSTEM
CLEAN WASH SYSTEM
CHECK HOSE CLAMPS
CHECK LUBRICATION DIAGRAM
CLEAN FIX & WASH PUMPS
CHECK DRIVE BELT
CHECK MOTOR BRUSHES
CHECK LUBRICATION DIAGRAM
DRIVE SHAFT BRGS (MONTHLY)
DRIVE SHAFT GEARS (MONTHLY)
DRYER TRANSPORT GEARS (MONTHLY)
1. Duplicate this copy to provide a supply of log sheets.
2. Perform operations as instructed in User's Manual.
3. List operating hours at each operation and initial.
4. Retain completed log sheets for continuing history.
* Where applicable
NOTES
INIT
*
0000061122
Figure 4-2, Maintenance Log
4-3
Mini Med Series
Page 60

Maintenance
Weekly Cleaning
1) Remove the developer, fixer and wash racks. To prevent chemical contamination, wash
off each rack separately with clean, lint-free cloths and warm water. Clean each roller over
its entire surface. Use isopropyl alcohol if necessary to remove traces of adhesives.
NOTE: Soft scrub pads, such as nylon net over sponge, work well on rollers. Metallic, or
non-metallic, scrub pads such as "Scotchbrite", must not be used on rollers as they will
damage the roller surface.
2) Inspect each rack thoroughly. Verify that the rollers turn freely and that all guides and
baffles are properly in place. Carefully set each rack aside to drain and dry while you are
cleaning the rest of the processor.
3) Clean the outside surfaces of the processing tank, using warm water with a sponge or
non-metallic scrub pad.
Caution: Never use steel wool on any part of the processor as its residue may cause
rust to form on the metallic parts of the processor.
4) Clean the dryer rollers and rack parts with a damp cloth and wipe dry.
5) Replace all removed racks and other parts.
Mini Med Series
4-4
0000061122
Page 61

Monthly Cleaning
As film is processed, by-products are released into the developer, fix and wash systems.
These must be removed by regular cleaning. Every month, schedule two hours of processor
downtime to thoroughly clean the developer, fixer and wash systems.
NOTE: This cleaning will replace the scheduled Weekly Cleaning due on the same date.
1) Open the drain valves, drain and dispose of the used developer, fixer and wash water.
Allow the tanks and recirculation systems to drain completely.
Caution: When filling or rinsing the processor tanks, use water no hotter than 120
degrees F (54 degrees C).
2) Bring a small bucket or container of warm water to the processor and place the red and
blue intake hoses going to the replenisher pumps into the container. Press and hold the
manual replenishment switch (located on the upper right hand front corner of the machine)
until the water comes out clear. Remove the intake hoses from the container and press and
hold the switch again until the pumps and lines are purged. Replace the hoses to their
original chemistry containers.
Maintenance
3) Rinse out each tank and then close the drain valves and fill the wash tank with warm
water.
4) Systems Cleaning.
The use of Developer Systems and Fixer Systems Cleaners are recommended for cleaning
the developer and fixer system. Carefully follow the manufacturer’s instructions and precautions. Dissolve any powdered chemicals in water before adding to the tank. Be sure to
accomplish the neutralizing and rinsing steps recommended by the systems cleaner manufacturer.
WARNING: Beware of all rotating gears, shafts and drive belts when operating the
processor with its access panels removed.
5) After the developer and fixer systems are thoroughly cleaned, neutralized and rinsed, fill
each tank with fresh warm water and install the racks. Switch processor ON and allow the
transport and recirculation systems to run for about 15 minutes as a final rinse.
(Continued on Next Page)
0000061122
4-5
Mini Med Series
Page 62

Maintenance
Monthly Cleaning,
Continued
Systems cleaning will remove most, if not all, of the chemical residue from the transport
racks. For additional cleaning and inspection, proceed as outlined below:
A) Remove the rack from the tank.
B) Clean the developer and fix racks without disassembling them.
C) Appropriate Systems Cleaner may be used to remove stubborn deposits. Never use
“Scotchbrite” type pads on roller surfaces. Rinse the rack thoroughly after it has been
cleaned.
D) Inspect all rack end plates for wear. Be sure the rollers turn freely.
Bearing wear differs according to the solution in which the rack is used. Since bearings
tend to wear more quickly in the fixer solution, the fixer rack end plates should be checked
more frequently for wear.
Clean Tanks
Inspect the empty processing tank for foreign matter and, if necessary, use a soft scrub pad
or brush and warm water to clean the tank interior. Flush the tank with warm water and
drain.
Inspect Processor
Check the hose clamps on the developer, fixer and wash pumps and the base of each pump
for leaks. Secure as necessary.
Caution: Do not over-tighten clamps. This can cause leakage or damage to the pump
heads.
(Continued on Next Page)
Mini Med Series
4-6
0000061122
Page 63

Prepare Fresh Chemicals
When the developer and fixer systems are clean, prepare and load fresh chemicals in accordance with instructions in Section 3, Operation, and the manufacturer’s instructions and
precautions.
Monthly Lubrication
Refer to Figure 4-2, Maintenance Log and Figure 4-3, Lubrication Points and lubricate as
indicated. Be sure to clean off all old lubricants and any excessive new lubricants.
Annual Maintenance
Once a year, following a routine monthly cleaning, perform the following tasks on the
processor:
1) Inspect the drive gears on each rack and replace any gears that are excessively worn or
damaged.
Maintenance
2) Refer to Service Procedure 5-1. Inspect, and adjust or replace if necessary, the main
drive belt.
3) Refer to Service Procedure 5-2. Inspect and clean the fixer circulation pump. The
developer pump is usually cleaned adequately by systems cleaning and does not require
additional servicing.
4) Refer to Service Procedure 5-3. Inspect and clean the developer and fixer replenishment
pumps.
5) Refer to Figure 4-2, Maintenance Log and Figure 4-3, Lubrication Points and lubricate
as indicated.
Be sure to clean off all old lubricants and any excessive new lubricants.
0000061122
4-7
Mini Med Series
Page 64

Maintenance
Removing Old Lubricants
Dust and dirt, mixed with oil or grease, can prevent fresh lubricants from reaching the
surfaces that need it most.
Before applying new lubricant, clean accumulations of grease from the gears. Hold a rag
alongside each gear, and brush debris from the gear onto the rag. Clean as much of every
drive gear as possible, then run the processor transport system very briefly, and clean again
until all portions of the gears have been exposed for cleaning.
After the drive gears have been cleaned, lubricate them as indicated in Figure 4-3.
Lubrication Points
Location Interval Lubricant
Drive Shaft Bearings & Worm Gears Monthly Oil/Teflon Oiler
Dryer Gears Monthly Oil/Teflon Oiler
NOTE:
DO NOT lubricate any gears or other parts that come in contact with solutions or water.
Figure 4-3, Lubrication Points
Mini Med Series
4-8
0000061122
Page 65

Section 5
Service
- General Index -
Section 0 - Safety Information
Section 1 - Introduction
Section 2 - Installation
Section 3 - Operation
Section 4 - Maintenance
0000061122
Section 5 - Service
Section 6 - Parts
Section 7 - Accessories
AFP Mini-Medical
X-Ray Film Processors
Page 66

Page 67

Content
Service
This section contains information on trouble-shooting and repairing AFP Mini-Medical
Series Processors.
Always consult the Troubleshooting Chart before attempting service or repair, or before
calling a service representative.
Even if you do not plan to service the processor yourself, the chart will help you explain
the problem to a service representative.
WARNING: Be extremely careful when trouble-shooting or servicing the processor
with the power on. Dangerous, potentially lethal, electrical voltages are present at
several points.
Following the Troubleshooting Charts are instructions for performing adjustment and
repair procedures that may be required to keep the processor functioning.
Also in this section is a description of the control electronics in the processor, with
applicable schematics and a wiring diagram. These will enable users who are trained
and equipped for electronics trouble-shooting to trace failures in the electronics.
NOTE: The circuit cards in this processor are not considered field repairable and in the
event of a component failure, should be replaced.
Attempting to repair them could invalidate any remaining warranty, or may cancel the
exchange credit value that some cards may have.
Troubleshooting
The Troubleshooting Charts are divided into three columns. To use either chart, find
on the left, under symptom, a problem that sounds like yours. In the middle column, in
diminishing order of likelihood, are the Probable Causes for such a symptom. The
right-hand column, Remedy, provides corrective action(s) for each probable cause.
(Continued on Next Page)
0000061122
5-1
Mini Med Series
Page 68

Service
Service Procedures
Following the Troubleshooting Charts are service procedures for repair and maintenance
of the processor.
Below is an index to those procedures:
Procedure Title
5-1 Inspecting, Adjusting & Changing the Main Drive Belt
5-lA Film Sensors and Adjustments
5-2 Servicing Circulation Pumps
5-3 Servicing Replenisher Pumps
5-4 Calibration Procedures
5-5 Circuit Descriptions
Schematics
The following schematics are included for servicing AFP Mini Medical Series X-Ray
film processors.
Figure 5-1 AC Interconnect Diagram
Figure 5-2 Dryer Rack Wiring Diagram
Figure 5-3 AC Interface Board
Figure 5-4 AC Interface Board Schematic
Figure 5-5 Logic Board Schematic
Figure 5-6 Logic Board Layout
Figure 5-7 Ready Tone Generator Schematic
Mini Med Series
5-2
0000061122
Page 69
Troubleshooting
Processor Problems
Symptom Probable Cause Remedy
Service
1. Developing time not
constant.
2. Solution temperature
too high.
3. Solution temperature
too low.
A. Excessive load on drive motor.
B. Solution levels low.
A. Temperature control setting
moved.
B. Shorted heater triac.
C. Defective temperature sensor.
(Open)
D. Logic failure.
A. Heater failed.
B. Heater triac failed. (Open)
C. Temperature control setting
moved.
D. Shorted temperature sensor.
E. Logic failure.
A. Check that racks are
seated and turn freely.
B. Add chemicals as
required.
A. Restore correct setting.
B. Replace heater triac.
C. Replace temperature
sensor.
D. Replace logic board.
A. Replace heater.
B. Replace heater triac.
C. Restore correct setting.
D. Replace sensor.
E. Replace logic board.
4. Dryer temperature
too low.
5. Dryer temperature
too high.
6. Film jams.
0000061122
A. Failed heating element.
B. Open overtemp switch on
dryer.
C. Shorted temperature sensor.
A. Open temperature sensor.
B. Blower failure.
C. Logic failure.
A. Fi lm not fe d in squarely.
B. Improper fixing, fixer too old,
pH too high, or improperly
mixed fixer or fixer replenisher.
5-3
A. Replace element.
B. Will reset when cool.
Inspect for cause ; fan not
running, dirt build-up, etc.
C. Replace sensor
A. Replace sensor.
B. Replace blower.
C. Replace logic board.
A. Feed film in carefully,
leading edge parallel to
rollers.
B. Check pH. If pH is above
5.0, dump and mix fresh.
Follow the mfg's
instructions exactly.
Mini Med Series
Page 70

Service
Troubleshooting
Processor Problems,
Continued
Symptom Probable Cause Remedy
7. Films overlap or
become skewed
during transport.
8. Film is tacky, wet or
curled when leaving
dryer.
9. Dirt particles on film.
A. Bound rollers.
B. Rack end plate bearings worn.
C. Missing rack springs.
A. Improper fixing.
B. Dryer temperature too low.
A. Foreign particles in dryer or on
squeegee rollers.
B. Algae deposits on film.
C. Foreign particles on squeegee
A. Clean each roller;
check for causes of
binding.
B. Replace bearings.
C. Replace springs.
A. See 5B.
B. Check dryer for
proper operation.
A. Run several outdated
sheets of unexposed
film.
B. Clean wash tanks &
racks with nylon
scrub brush and
warm water. Drain
wash tank each
night.
C. Clean squeegee
roller(s).
10. Scratches on film
emulsion.
Mini Med Series
A. Dirt on feed rollers
B. Chemicals crystallized on
underside of top film guides.
C. Dirt or silver accumulation on
rollers.
D. Roller in rack not turning.
E. Dirty feed tray surface.
5-4
A. Clean feed rollers.
B. Clean film guides.
C. Clean rollers using a
nylon scrub pad and
warm water, or
developer systems
cleaner for developer
racks/fixer systems
cleaner for fix racks.
D. Check all rollers for
operation; repair as
required.
E. Clean feed tray.
0000061122
Page 71

Troubleshooting
Processor Problems,
Continued
Symptom Probable Cause Remedy
Service
11. Increase in image
12. Decrease in image
density.
A. Film is over exposed.
B. Developer temperature too high.
C. Excessive developing time due
to mechanical binding.
D. New developer improperly mixed.
.
A. Film is under exposed.
B. Developer under replenished
or exha usted.
C. Developer temperature too low.
D . Developer time too short.
E. New developer improperly
mixed.
A. Coordinate exposure
with developing time.
B. Have service technician
troubleshoot developer
temperature.
C. See symptom 1.
D. Dump and mix fresh,
following mfg's
instructions exactly.
A. Coordinate exposure
B. Change developer.
Check replenishment
rates.
C. See Symptom 3.
D. Check developer for
low level.
E. Dump developer and
mix fresh.
13. Over replenishing.
14. No functions
0000061122
A. Shorted film presence switch.
B. Shorted manual replenishment
switch.
C. Pendulum magnet weak or
stuck in the up position.
D. Logic failure
A. Interupter switch not activated.
B. Interupter switch defective.
A. Replace switch.
B. Replace switch.
C. Clean or replace
pendulum.
D. Replace logic board.
A. Put top cover in place.
B. Replace switch.
Note: The interupter switch may be bypassed for troubleshooting purposes only by
either utilizing the hold down tool (P/N 0000021801) to keep the switch activated.
Jumping the pins on J-14 on the logic board is an alternate method.
5-5
Mini Med Series
Page 72

Service
Service Procedure 5-1
Inspecting, Adjusting & Changing
the Main Drive Belt
The main drive belt requires only minimal maintenance and normally lasts many years
with normal use.
Inspection
The belt should be inspected yearly. Replacement is indicated if any of the following
conditions are found:
1) Excessive slack that cannot be corrected.
2) Frayed or badly worn edges.
3) Missing or damaged drive lugs on the belt surface.
Adjustment
CAUTION: Overtightening the belt will cause the belt to jump on the wormshaft
pulley and will cause the saddle bearing nearest the pulley to become excessively
hot.
Adjusting slots to tension the drive belt are provided in the motor mounting plate.
Adjust in the following manner:
1) Unplug the processor from its power outlet.
2) Loosen the four motor mounting plate screws. These are accessible on the back
3) Slide the motor until the belt has approximately 1/2" of play. With no racks in
4) Tighten the four screws.
5) If most of the slack cannot be removed, the belt is worn excessively and should
Replacement
The following procedure is to be followed when replacing a worn drive belt:
1) Unplug the processor from its power outlet.
2) Remove all four racks (dev, fix, wash & dryer) and carefully set aside.
3) Loosen the four drive motor mounting plate attaching screws on the rear of the
4) Remove the cotter pin holding the drive belt pulley to the driveshaft.
5) Slide the driveshaft pulley forward far enough to allow removal of the belt
6) Install the new belt in the reverse order.
7) Adjust the new belt as described above.
of the processor, below the dryer.
place, you should be able to rotate the driveshaft with your fingers approximately 1/8" back and forth.
be replaced.
processor. Move the motor to remove the drive belt.
around the end of the driveshaft.
Mini Med Series
5-6
0000061122
Page 73

Service Procedure 5-lA
Film Sensors and Adjustments
Mini-Medical processors up to and including S/N MM1899-90
Film sensing for Mini-Medical processors up to and including S/N MM1899-90 was
accomplished by mounting a magnetic reed switch assembly in the center of the upper
edge inside the molded feed tray adapter.
When film is not present in the feed tray, a magnetic pendulum assembly is in close
proximity to the reed switch, wired in a normally closed configuration. This means that
it is held open by the magnet when film is not present. When film is inserted in the feed
slot, the pendulum is pushed upward and away from the reed switch, causing it to close.
This puts a short across pins 1 and 2 of J13 on the ready tone generator board, putting
the machine into the process mode. When the pendulum returns to its position of rest,
the reed switch opens initiating timers for the beeper, wait light and cycle time.
Approximately 8 seconds after the reed switch opens the beeper should sound and the
wait light should go out.
If a machine fails to go into standby, the magnet may have weakened, or the switch may
be stuck in the closed position; or the switch may be adjusted too far away from the
pendulum to activate the switch.
Service
First, unplug J13 from the ready tone generator board. If the wait light goes out and the
beeper sounds after approximately 8 seconds, the switch is either shorted or the magnet
is failing to activate it.
To test the switch, first remove the switch from the machine. Use a magnet to activate
the switch while looking across the switch with a meter set on Ohms. If the switch fails
to open it should be replaced. If it does open, try adjusting it as close to the pendulum
assembly as possible. If this doesn’t help replace the pendulum assy.
If the machine remains in the process mode after unplugging the switch from the J13
connector on the ready tone board, unplug the ready tone board from J13 on the main
logic board. If the wait light goes out after approximately 8 seconds, the ready tone
generator board should be replaced. If unplugging the ready tone board does not clear
up the problem, a rare problem on the logic board itself is indicated.
Mini-Medical processors S/N MM1900-90 and above
Starting with S/N MM-1900-90, Mini-Medical processors feature a new style of film
sensing. This is in the form of two hall-effect magnetic digital sensors (P/N 21878)
located approximately three inches in from the inner right and left sides of the feed tray.
There are now two magnetic pendulum assemblies (P/N 21893), located above the feed
tray, that are used in conjunction with these sensors.
Activation of either switch will put the machine into the process mode. The purpose of
the dual switch arrangement is to accommodate smaller size films. The feed tray assembly
has changed to P/N 21876. (The part numbers given above are for 1 each of the parts.)
0000061122
5-7
Mini Med Series
Page 74

Service
Service Procedure 5-1 A
Film Sensors/Adjustments, Cont'd
Film sensor location configuration (See figure 5-1)
Film Sensor Adjustment: These sensors are adjusted at the factory and normally do
not need adjustment. However, should adjustment be required, follow this procedure:
1) Loosen the screw on each small bracket securing the sensors to the molded feed tray
adapter; the sensors may be moved up or down.
2) Locate each sensor upward close enough to the magnet opposing it so that the
machine remains in standby. Raising either magnet by inserting a film should put the
machine into the process mode. If this does not occur in either case, lower the sensor in
question until it does.
Another Method of Adjustment: Since the two Hall-Effect switches are wired in
parallel, simply short out one and position the other until it activates (allow 10 seconds
after repositioning the switch for the beeper to sound and the wait light to go off).
The easiest way to short out one switch while adjusting the other is to open the front
electrical panel and locate the beeper board. It is the smaller of the three boards, and is
located in the upper middle section of the electrical panel. Each switch is connected to
the board by means of a plug at the end of a 3 wire ribbon cable.
Unplug one switch from the board and jump the second and third pins (count left to
right) together. This completes the circuit for this switch. Now you can adjust the
position of the other one. Once this is done, plug the other switch back into the board
and adjust its position. There is no need to unplug the other one while doing this since
you have already repositioned it. The addition of some silicone caulking to each switch
will help assure that they both stay in position.
Mini Med Series
Figure 5-1, Film Sensor Location Configurations
5-8
0000061122
Page 75

Service Procedure 5-2
Servicing Circulation Pumps
The circulation pumps are easily removed for cleaning and/or servicing. For the early
style pump (Figure 5-2, Serial #'s below 8724), proceed as follows:
Removal
1) Unplug the processor from its power outlet.
2) Drain the tank from which the pump is to be removed.
3) Disconnect the pump power cable from the junction box.
4) Disconnect the outlet hose from the pump body.
5) Remove the drain filter disk from the bottom of the processing tank to expose the
four pump mounting screws.
6) Carefully remove the four screws and lower the pump.
NOTE: There are five “O” rings between the bottom of the tank and the mounting
surface of the pump (see Figure 5-2). Be sure to locate and save all 5 “O” rings when
removing the pump.
Service
Disassembly & Cleaning
Developer recirculation pumps are usually cleaned adequately by systems cleaning and
do not require disassembly.
Fix and wash pumps should be disassembled for cleaning at least once a year to remove
hardened residue build up.
Disassemble and clean as follows:
1) Remove the four pump head assembly screws, (Figure 5-2).
2) Gently separate the pump head assembly. Locate and save the internal “O” ring seal
inside the pump head.
3) Remove the impeller from the pump head and thoroughly clean all pump head parts
in warm water.
Assembly
1) Reassemble the pump in reverse order. Lubricate the internal “O” ring with a silicone
base lubricant before installing. Do not over tighten the self-tapping assembly screws.
Installation
1) Carefully install the pump in reverse order of removal. Lubricate the 5 “O” rings
that seal the pump to the tank base with a silicone base lubricant before installing.
2) Test the pump for operation with water before installing chemistry.
0000061122
5-9
Mini Med Series
Page 76

Service
Service Procedure 5-2
(Cont'd)
Servicing Circulation Pumps (Cont'd)
The circulation pumps are easily removed for cleaning and/or servicing. For the later
style pump (Figure 5-3, Serial #'s starting at 8724 and above), proceed as follows:
Removal
1) Unplug the processor from its power outlet.
2) Drain the tank from which the pump is to be removed.
3) Disconnect the pump power cable from the plug channel.
4) Disconnect the inlet elbow and output hose from the pump body.
5) Loosen the two (2) screws securing the pump mounting feet to the bracket. Slide
the pump mounting feet out from under the tabs provided on the bracket and remove.
Disassembly & Cleaning
Assembly
Installation
Developer recirculation pumps are usually cleaned adequately by systems cleaning and
do not require disassembly.
Fix and wash pumps should be disassembled for cleaning at least once a year to remove
hardened residue build up.
Disassemble and clean as follows:
1) Remove the four pump head assembly screws, (Figure 5-3). Be careful to note the
orientation of the outlet nozzle for reassembly.
2) Gently separate the pump head assembly. Locate and save the internal “O” ring
seal inside the pump head.
3) Remove the impeller from the pump head, taking care to not lose the small green
spacer located on the inside of the indentation of the impeller and thoroughly clean
all pump head parts in warm water.
1) Reassemble the pump in reverse order. Lubricate the internal “O” ring with a
silicone base lubricant before installing. Take care to not lose the lock washer and
flat washer provided with each screw and do not over tighten the screws.
Mini Med Series
1) Carefully install the pump in reverse order of removal. Lubricate the 5 “O” rings
that seal the pump to the tank base with a silicone base lubricant before installing.
2) Test the pump for operation with water before installing chemistry.
5-10
0000061122
Page 77

Figure 5-2, Recirculation Pump Head, Early Style (Up to S/N 8723)
Service
Top ViewFront View
Figure 5-3, Recirculation Pump, Later Style (For S/N's 8724 and higher)
0000061122
5-11
Mini Med Series
Page 78

Service
Service Procedure 5-3
Cleaning and Servicing
Replenisher Pumps
Mini-Medical Processors utilize two high reliability, low maintenance oscillating pumps
to deliver replenishment to the developer and fix tanks. The following steps will correct
most problems that may occur.
NOTE: These pumps operate on half-wave DC. Attempting to operate them on full
wave AC (without a blocking diode in place) will lead to overheating and failure of the
pump solenoid.
If the pumps run but deliver little or no chemistry:
1) Inspect replenishment lines between replenisher tanks and pump inputs for kinks or
other damage. If installed, disassemble and clean in-line filter(s). Check all hose clamps
for security.
2) Replenishment lines should not be lengthened, nor should the tanks be positioned
such that more than 36" of chemical lift is required.
3) Check valves may be obstructed with debris or crystallized chemistry.
A) If pump will move any chemistry, try cleaning valves in place by placing
pickup tube in a container of warm water, not over 120 degrees F (49 C) and
activatlng the pumps wlth the Manual Replenishment switch. The warm
water will usually remove hardened chemical residue.
B) Stubborn deposits and larger pieces of foreign material may require that
the pump be disassembled for cleaning. Figure 5-4, below, details
disassembly of the pumps.
Clean the valves, using hot water if necessary to remove any chemical
residue. Older valves may require replacement.
When assembling, take care to install the pump valves exactly as they were
removed.
Mini Med Series
Figure 5-4, Disassembly of Replenisher Pump
5-12
0000061122
Page 79

Service Procedure 5-4
Circuit Descriptions & Calibration Procedures
WARNING: The following calibration adjustments are made on the Logic Board.
Since potentially fatal voltages are present and easily contacted, these adjustments
must be performed only by qualified service technicians.
Access to the circuit cards may be gained by removing the two mounting screws from
the underside of the feed tray, then lowering the control box door assembly.
All calibrations are done on the Logic Control Board located on the right side of the
opened control box door.
Developer Temperature Control
The developer is heated by a single 500 watt heating element located under the developer
tank in a heater exchange assembly.
Service
Developer temperature is controlled by an IC temperature sensor mounted on the bottom
of the developer tank. This sensor is a constant current generator who’s output is directly
proportional to the temperature of the tank.
The temperature control is a proportional controller that allows full power to be sent to
the heating element during warm-up, when the processor is first turned ON. This will
be indicated by the DEV TEMP light on the control panel or LED L-2 on the logic
board being ON.
When the temperature in the tank is within 2 degrees f (1.1 C.) of the desired temperature,
the controller will start cycling the power to the heater to prevent temperature over run.
At this point the LEDs’ mentioned above will blink, indicating that the temperature is
very near its set point.
The temperature controller signals the triac switch Q2 through opto-isolator U-2 on the
AC interface board. The triac switches line voltage to the heater.
The desired temperature set point is adjusted with trim pot R-20 on the logic board.
NOTE: Clockwise rotation increases temperature. See Calibration Procedure below.
In the unlikely event of an electronic failure, providing continuous full power to the
heating element, an overtemp safety thermostat located on the heater exchange assembly
will open, thus disabling heat. In addition, a protective internal fusible link is present in
the heating element as an added safety feature. When an open heating element is found,
the operation of the triac should also be checked. (The heating element has a nominal
resistance value of approximately 25 ohms).
0000061122
Note that the temperature controller is disabled if a low level condition exists in the
developer tank.
5-13
Mini Med Series
Page 80

Service
Service Procedure 5-4
Circuit Descriptions &
Calibration Procedures,
(Cont'd)
Developer Temperature Calibration
1. Verify that there is enough conductive liquid in the developer tank to cover the low
level sensor, located in the front wall of the tank. (About 1.5 gallons [6 L.])
2. Turn the processor ON.
3. Rotate trim pot R-20 on the Logic Board several turns clockwise to force the heater
ON. The DEV HEAT LED and LED L-2 must be on steady, if not, continue turning R20 clockwise until both LEDs’ are ON steady.
4. Place a known accurate metal stem thermometer into the developer solution.
CAUTION: Do Not use a glass/mercury thermometer in the processor. If a glass
thermometer should break, the resultant mercury leakage would permanently
contaminate the processor.
5. As the temperature of the solution reaches the desired value, slowly adjust trim pot
R-20 counterclockwise until LED L-2 starts to blink. When the desired temperature is
reached the LED should flash ON only occasionally to indicate temperature is being
maintained.
Dryer Temperature Control
The dryer uses two 275 watt infra-red lamps (heaters), and airflow provided by 2 fans,
to dry the film as it passes through the dryer section.
A thermistor in the dryer section is used as a temperature sensor. Control is provided
by the logic board. LED L-1 on the logic board will light when the dryer is calling for
heat.
The temperature controller signals the Triac switch Q-1 through opto-isolator U-l on
the AC interface board. The triac switches line voltage to the heaters.
Trim pot R-21 on the logic board adjusts the set temperature of the dryer.
NOTE: Counterclockwise rotation of the trim pot increases the set temperature of the
dryer.
Mini Med Series
The upper dryer blower is controlled by opto-isolator U-3 and triac Q-3. The lower, or
back blower is on continuously
5-14
0000061122
Page 81

Service Procedure 5-4
Circuit Descriptions &
Calibration Procedures,
(Cont'd)
Over Temperature Protection
The dryer is equipped with over temperature protection in the form of a safety switch
located on one of the lamp reflectors. If temperature inside the dryer exceeds 155 f (68
C), the safety will open and remain open until the temperature drops below 105 f (41
C).
If this should happen the dryer should be checked for dirt build up, nonoperating fans
or shorted triac.
CAUTION: In no event should the processor ever be operated with this safety
device shorted or bypassed. Severe equipment and/or personal injury could occur.
Service
Dryer Temperature Calibration
Dryer temperature should be checked by inspecting the film being processed. If the
film is exiting the dryer damp, temperature may need to be increased. If the processed
film shows heat marks (wavy horizontal lines across the film) the dryer temperature
should be reduced.
1. Inspect a piece of processed film.
NOTE: All covers and panels must be on the processor when checking operation of the
dryer.
2. Adjust trim pot R-21 as follows to adjust dryer operating temperature:
Counterclockwise = Increase Dryer Temperature
Clockwise = Decrease Dryer Temperature
3. Process another piece of film and inspect for proper drying.
0000061122
5-15
Mini Med Series
Page 82

Service
Service Procedure 5-4
Circuit Descriptions &
Calibration Procedures,
(Cont'd)
Replenishment Operation
The replenishment system operates in two modes. The first is by the film feed sensor
being tripped, indicating that material is being processed. This is called automatic
replenishment. The other method is via the manual replenishment button located at the
front of the processor, known appropriately as manual replenishment.
Two trim pots on the logic board allow the adjustment of cycle length for the developer
and fixer replenishment pumps, thus allowing adjustment for differing processing loads,
materials and other variable conditions.
Before attempting adjustment of the replenishment system, verify that the replenisher
lines are clear and not bent or kinked. If replenishment seems to be below earlier
performance, clean the check valves as outlined in Service Procedure 5-3 before
proceeding.
Replenishment Calibration
The following outlines basic calibration for the replenishment system. Depending on
the customer’s materials, film sizes and production, it may be necessary to make further
adjustments to the replenishment rate(s).
1. Turn the processor ON.
2. Lift the developer or fixer “J-Hook” out of the processing tank far enough to hang it
into a graduated container.
3. Press the manual replenishment button until all air is purged out of the replenishment
line.
4. Empty the graduated container.
5. Feed in a piece of 14" X 17" film and measure the amount of replenishment delivered
into the container. The recommended starting point amounts are:
Developer = 110 ml
Fixer = 120 ml
If these amounts are not delivered adjust the appropriate trim pot(s) shown below until
the 1 minute delivery amount for each pump is as recommended or as required for your
application. NOTE: Counterclockwise rotation increases output per minute.
Mini Med Series
Developer = R-47
Fixer = R-28
5-16
0000061122
Page 83

Service Procedure 5-4
Circuit Descriptions &
Calibration Procedures,
(Cont'd)
Triacs & SCR’s
There are 5 Triac’s and 2 SCR’s on the AC interface board. Their functions are:
Ql Controls dryer heaters (lamps).
Q2 Controls developer heater.
Q3 Controls dryer blower.
Q4 Controls drive motor and water solenoid.
Q5 Not used on thls processor.
Q6 Controls fixer replenishment pump.
Q7 Controls developer replenishment pump.
Outputs
Service
The output of the AC interface board to the main harness is via connector J1 on the
following pins:
Pin 1 Dryer lamps (heaters).
Pin 2 Developer heater.
Pin 3 Drive motor, wash water replenishment pump or solenoid.
Pin 4 Dryer blower.
Pin 5 Not used.
Pin 6 Fixer replenishment pump.
Pln 7 Developer replenishment pump.
Fuses
The following fuses on the AC interface board protect the indicated circuits:
Fuse Type/Rating Protects
F1 3AGC 1 amp Developer & fixer replenishment pumps.
F4 3AGC 5 amp Developer heater.
F5 3AGC 8 amp Dryer lamps, 115V(heaters).
F5 3AGC 5 amp Dryer lamps, 220V(heaters).
F6 3AGC 5 amp Drive motor, wash water solenoid.
F7 3AGC 5 amp Dryer Blower
0000061122
5-17
Mini Med Series
Page 84

Service
Service Procedure 5-5
Theory of Operation
Solution Temperature Control
Temperature in the developer tank is sensed by an integrated circuit temperature sensor
which generates a current that is directly proportional to temperature.
The signal from temperature sensor is sent to I.C. U2, an LM 3900 quad currentdifferencing amp. The amplifiers in this package amplify differences in current at its
inputs. Two of the amplifiers are connected as comparators comparing the current
generated by the temperature sensor with the current set by the front panel temperature
control. R20 sets the maximum temperature at which the front panel control can be set.
R20 is preset at the factory for approximatly 110°F maximum temperature.
When replacing the Logic board or when replacing U2 or the temperature sensor, R20
must be re-calibrated (see calibration Procedures). The other two amplifiers in U2 are
used as a triangle wave signal which is mixed with the current from the controls. This
gives proportional temperature control supplying less heat as the temperature approaches
the set temperature, and automatically adjusts the amount of heat supplied to maintain
temperature as closely as possible. The action of the temperature control is indicated
by the LED lamp on the front panel and L2 on the Logic Board. When the lamp is on,
the heater is activated.
As the tank temperature approaches the set temperature, the LED will begin blinking
and will remain on for less time as the temperature comes closer to the set temperature.
Drive to the heater is disabled if the solution level in the developer tank drops below the
level sensor probe.
Dryer Temperature Control
Temperature in the dryer transport is sensed by an integrated circuit temperature sensor
which generates a current that is directly proportional to temperature.
The signal from the temperature sensor is sent to I.C. U2, an LM 3900 quad currentdifferencing amp. The amplifiers in this package amplify differences in current at its
inputs. Two of the amplifiers are connected as comparators comparing the current
generated by the temperature sensor with the current set on the board. R21 sets the
temperature for the dryer. R21 is preset at the factory for 135°F maximum temperature.
When replacing the Logic board or when replacing U2 or the temperature sensor, R21
must be re-calibrated (see calibration Procedures). The other two amplifiers in U2 are
used as a triangle wave signal which is mixed with the current from the controls. This
gives proportional temperature control supplying less heat as the temperature approaches
the set temperature, and automatically adjusts the amount of heat supplied to maintain
temperature as closely as possible. The action of the temperature control is indicated
by the LED L1 on the logic board. When the lamp is on, the heater is activated.
Mini Med Series
As the dryer temperature approaches the set temperature, the LED L1 will begin blinking
and will remain on for less time as the temperature comes closer to the set temperature.
5-18
0000061122
Page 85

Service Procedure 5-5
Theory of Operation
(Cont'd)
Solution Level Sensor
A metal screw located in the developer tank detects when the solution level becomes so
low that there is a danger of the heater becoming dry. If the developer level drops
below the end of the metal screw, the red Low Solution light will turn on to warn the
operator. When the red light is on, the heater is turned off to prevent tank damage. If an
attempt is made to run the processor, it will turn on as usual but the red Low Solution
light will begin to blink, drawing attention to the problem. When the solution level in
the tank is again at the proper level, the red light will go out and heating will automatically
resumes.
Automatic Shut-Off
The processor is preset at the factory to shut off and go into a standby mode approximately
5 minutes after the sensor(s) in the film entry lip is released by the trailing edge of the
film.
Service
This time interval is controlled by adjusting Pot R29. R29 adjusts the frequency of an
oscillator in U6. The output of the oscillator is sent to U5 which divides the frequency
by 16,384. The oscillator is adjusted so that after division, one pulse is output by U5
after approximately 4 minutes. When the sensor is triggered by film presence, it resets
U5 to zero and holds it there. When the sensor is released, U5 again functions and
outputs its pulse after approximately 5 minutes. The output of U5 goes to a flip flop
(U4) which turns off all machine functions except solution temperature control,
recirculation pumps and one (1) dryer blower.
When the reset pushbutton is depressed, the timer is disabled and all machine functions
run continuously.
AC Interface Board
This board connects the low voltage signals from the main logic board and uses them to
switch the various AC components in the machine on and off.
The low level signals are interfaced by six optically isolated TRIAC TRIGGERS (UlU7). These contain an LED which is optically coupled to a small TRIAC Trigger. The
LED in the Trigger is connected in series with the appropriate control panel LED which
indicates whether each component is operating. If either the trigger LED is bad or the
control panel LED is bad, the circuit will not operate.
0000061122
5-19
Mini Med Series
Page 86

Service
Waveforms & Voltages
1. Pin 4 of U2 on Logic Board
3V
2V
1V
2.7V
1.7V
0V
1.121s
1.134s
0
2. Pin 9 of U2 on Logic Board
11V
0V
15ms
1 Pulse
3. Pin 5 of I.C. U2 on Logic Board
11.25 V
Note: Duty cycle = 34%
0V
1.13s
7 Pulse Bursts
8. Pin 7 of I.C. U5 on Logic Board
12 V
Note: Duty cycle = 50%
0V
600ms
4. Pin 12 of I.C. U4 on Logic Board = 2.4VDC
5. Pin 13 of I.C. U4 on Logic Board = 0VDC
6. Pin 11 of I.C. U4 on Logic Board = 11.9VDC
7. Pin 5 of I.C. U1 on Logic Board = 11.38VDC
Mini Med Series
5-20
9. Pin 18 of I.C U7 on Logic Board = 10 VDC
10. Pin 15 of I.C. U7 on Logic Board = 0VDC
9.5V
Note: Duty cycle = 12%
Pulse present only while
Reset Pushbutton is
depressed.
0V
550ms
0000061122
Page 87

11. Pin 11 of I.C. U6 on Logic Board = 0VDC
550ms
Note: Same as #10, but
inverted. Duty cycle = 88%
Pulse present only while
Reset Pushbutton is
depressed.
0V
9.5V
12. Pin 17 of I.C. U7 on Logic Board = Same as #11 only while
Reset Pushbutton is depressed. 5 second duration
13. Pin 14 of I.C. U7 on Logic Board = Same as #10
14. Pin 10 of I.C. U6 on Logic Board = Same as #'s 11&12
15. Pin 8 of I.C. U6 on Logic Board
10V
5V
8.25V
4.7 Sec
10V
572 ms
36 ms
16. Pin 13 of I.C. U6 on Logic Board
1V
11.88V
41 Sec
Ready Tone Generator Board
Middle pin of J13 = Low (Gnd)
when no film is present
= High (12V)
when film is present
Middle pin of J12 = Low (Gnd)
when no film is present
= High (12V)
when film is present
Output of either Q1 or Q2 = 12V
when either input goes high
Pin 5 of I.C. U1 = Output of Q1
or Q2 Inverted.
Pin 6 of I.C U2 = high (12V) normally
Low pulse when film exits
12V
0V
35 ms
Waveforms & Voltages
(Cont'd)
Service
0000061122
5-21
Mini Med Series
Page 88

Service
Main Wiring Diagram
G R N / G N D
W H T / N E U T
1 1 5 V A C / 2 3 0 V A C
P O W E R S O U R C E
J 1 2J 1 1J 1 0
D R I V E
M O T O RS O L E N O IDD E V
1 / 2 A 2 A
U P P E R
D R Y E R F A N
2 7 5 W E A
O V E R
D R Y E R
I R L A M P S ( 2 )
T E M P
J 9
B L K
B L K / H O T
V L T
232
2
1
1
J 1 2
J 1 4
M A I N H A R N E S S P C B P / N 3 5 3 1 6
T B 1
L O G I C B O A R D
6 C H A N N E L C O N T R O L L E R
R 2 1
R 2 0
1 2 3 4 5 6 7
D E V T E M P
D R Y T E M P
G R Y
2
1
1
J 9
J 1 3
2 3 0 V A C P / N 3 5 3 0 4
1 1 5 V A C P / N 3 5 3 0 2
R 4 7
R 2 8
D E V R E P L S E T
J 8
1 2 31 2 3 4 5 6 7 8
B L U
G R N
J 2 0
2 1
J 7J 4
R 2 9
J 5
1 2
1 2
T I M I N G
F I X R E P L S E T
B L K
M A I N
R E P L .
S W I T C H
C O V E R
S W I T C H
S W I T C H
L E V E L
S E N S O R
D R Y
S E N S O RS E N S O R
J 8J 6J 5
L O W E R
D R Y E R F A N
J 1 3
1 2 3
1 2 3 4 5 6 7 8
P O W E R P C B O A R D P / N 3 5 2 3 3
BROYGBV
J 7
J 2
1
J 1
D R Y E R H E A T
F U N C T I O N
T R I A C Q 1
C O N T R O L E R
J 2
L E D P C B
P / N 3 5 2 9 9
2
34567
D E V E L O P E R H E A T
D R I V E M T R . W A S H S O L .
T R I A C Q 4
T R I A C Q 2
D R Y E R F A N S
T R I A C Q 3
J 1 4
1 2 3
R E A D Y T O N E
2345678
J 2
P O W E R B O A R D
P / N 3 5 2 3 3
1 AF 3
F 7 5 A
F I X , D E V R E P L .
A U X A C
G E N E R A T O R
F A N S
F I X
R E P L E N W A S H
F I X
R E C I R C
1
J 4J 3J 2
D E V
H E A T E R
O V E R
T E M P
D E V
R E P L E N
1 / 2 A 1 / 2 A 5 0 0 W 1 / 2 A 1 / 2 A 1 / 2 A 1 / 2 A
R E C I R C
B R N
O R N
B L U
G R N
W H T
G R Y
B L K
Y E L
R E D
P N K
V L T
P N K
R E D
G R Y
G R N
B L U
O R N
B R N
B L K
V L T
Y E L
B L K
W H T
J 1
8 7 6 5 4 3 2 1
J 3
4 3 2 1
F 1 1 A
O R B
3 5 3 0 6 - 1
8 A - 1 1 5 V A C
5 A - 2 3 0 V A C
F 4 5 A
F 5
F 6 3 A
D E V H E A T
D R Y E R
P U M P , F A N / M O T O R
D E V
R E E D S W I T C H E S
G
G R Y
V L T
B L U
J 9 / J 2 0
G R N
1 2 3 4
8N O N E
A U X . A C N O T U S E D
F I X E R R E P L E N I S H
D E V E L O P E R R E P L E N IS H
N O N E
S C R Q 7
S C R Q 6
Q 5
1
2
34567
-
Mini Med Series
Figure 5-5, Main Wiring Diagram
5-22
0000061122
Page 89

Dryer Rack Wiring Diagram
H E A T E R S
H E A T
S E N S O R
O V E R T E M P
S A F T Y
Service
F A N
1 2 3 4 5 6 7 8
B L K
W H T
2 2 A W G
R I B B O N
L O G I C P C
B O A R D
J 5
B L K
W H T
2 2 A W G
R I B B O N
4
5
B R N
D R Y E R
H E A T
1
B
R
N
8 7 6 5 4 3 2 1
G R N
O R N
W H T
D R Y E R
F A N
2
3
G
W
R
H
N
T
1
2
3
O
G
W
R
R
H
N
N
T
2
6
3
1
B R O W N 1
O R A N G E 2
W H I T E 3
4 B L A C K 2 2 A W G
5 W H I T E 2 2 A W G
6 G R E E N
0000061122
Figure 5-6, Dryer Rack Wiring Diagram
5-23
Mini Med Series
Page 90

Service
AC Interface Board Schematic
SCR
S4004
A
C
GATE
TRIAC
SC146D
MT2
MT1
GATE
GATE
ANODE
CATHODE
MT1
MT2
GATE
Mini Med Series
Figure 5-7, AC Interface Board Schematic
5-24
0000061122
Page 91

Service
Q 1 7
Q 1 6
4
3
1 1
1 0
8
9
1 2
1 3
126
5
D E V T E M P
O T H E R W I S E S P E C I F I E D
S P E C I F I E D
U N L E S S O T H E R W I S E
A L L R E S I S T O R S A R E
I N O H M S U N L E S S
A L L C A P A C I T O R S
A R E I N u f
3 0 K
J U M P E R F R O M
1 N 3 0 6 4 O R 1 N 9 1 4
L O G I C T O J O G
B O A R D
D R Y E R P R O B E
D E V P R O B E
+
-
A D 5 9 0
+
-
6 . 8 K
R 5 0
J 5
R 2 3
2 7 K
R 1 8
U 3 M C I 4 0 8 1
I
U 6 , U 6 A C D 4 0 9 3 B C N
U 7 U L N 2 8 0 4 A
V R 1 , V R 2 L M 7 8 1 2 C T
U 4 C D 4 0 0 1
U 5 M C 1 4 0 2 0
T O L E D B O A R D
L
O
E
V
SHN
I
U 3
U 3
1 0 K
U 2 L M 3 9 0 0
U 1 N E 5 5 6
J 8
L O W L E V E L
P R O B E
5
9
R 2 1
1 4
U 2
1
7
+ 1 2
6
C 8 0
2 0 K
R 2 0
1 3
1 0 0 K
R 2 2
+
U 2
-
8
1 3
1 2
R 2 5
1 0 K
R 2 6
5
6
1 1
U 4
3
9
8
M
T
D
R93
J 2 0
L O W L E V E L
L E D
P
E
R
Y
1 0 u f / 2 0
C 1 7 A
+ 1 2
C R 2 7
WOT
NRTCO
R
D
D
ERL
N
A
D FEV
MOT
A
I
PELRE
D FEV
I
X
R24
83R
1
4
0
4
RR R
3
4
4
4
R
U 7
7 5 0
4
R 2 7
1 6
8
1 1
U 7
3 3 0
R 4 8
1 0
1 27
U 7
7
A D 5 9 0
6 . 8 K
R 4 9
J 4
+ 1 2
F 1
T B 1
M O V
6
5
1
6
7 8 1 2
+ 1 2 J 1 3
V R 2
2
1 0
1 2
1 M
R 1 3
1 M
R 1 9
4 7 0 K
R 1 4
U 2
+
1 1
-
1 0 0 K
R 1 7
1 0 u f / 2 0 V
1 N 4 0 0 2
C 3 A
R 1 5
1 5 0 K
1 M
R 1 2
3
U 2
+
-
4
4 7 0 u f / 3 5 V
C 1 7
1 3
6
U 7
3
2 N 4 4 0 1
Q 2
2
U 3
1
J U M P E R
V R 1
7 8 1 2
C R 8
1 N 4 0 0 2
C R 9
1 4 A - 1 0 R - 2 8
4
3
1
9
1 2
1 0
7
S W I T C H
C 3 2
2 2 0 0 u f
212
1
J 1 4
T O P
C O V E R S A F E T Y
T O J 1 F R O M U 6
+ 1 2 L O G I C
1 0 u f / 2 0 V
1 0 u f / 2 0 V
3
C 5
3
C 4
G N D
R I B B O N C A B L E
S R 1 6
2 0 K
N O T U S E D
J 7
C R 1 6
C R 1 7
R 3 2
1 0 K
J O G / F L O O D
J O G O N L Y
G E N E R A T O R
R E A D Y T O N E
1
+ 1 2
1 m
D E V R E P L
R 4 7
1 0 0
M A N U A L
R E P L E N I S H M E N T
J 1 2
S W I T C H
12
S R 1 7
2 0 K
J 1 3
2 N 2 9 0 7
R 1 0
1 7
U 7
2
C R 1 8
1 4
R 4 5
3 . 3 K
U 7
5
P O W E R O N
J 9
2
3
12
L E D
C R 2 5
R 1 1
3 9 K
R 3 7
1 u f
U 6
4
1 0 0
6
5
U 6
C R 1 9
1 0
8
C 1 2
9
C 3
J 1 . 1
1 m
F I X R E P L
R 2 8
C R 2 4
6
R 3 1
2 N 2 9 0 7
1 8
U 7
1
C R 2 0
1 5
3 . 3 K
U 7
4
2 2 0 K
R 4 6
8
U 4
U 4
4
3
5
2
1
C R 2 3
U 4
1 0
9
8
7
1 1
U 5
3 1 6
U 6 A
J 1 . 7
J 1 . 2
J 1
U 6
C R 2 1
R 3 6
1 0 0
1 1
C 1 6
1 u f
1 3
1 2
1
1 u f
C 7
R I B B O N C A B L E
C 8
1 u f
F R E Q . A D J .
U 6
R 3 5
3 6 K
1 0
3
2
5 0 0 K
R 2 9
1 0 u f
T O
+ 1 2
3
J 1 . 1 2
C R 2
J 1 . 5
J 1 . 6
J 1 . 3
J 1 . 4
J 1 . 1 1
J 1 . 9
J 1 . 1 0
C R 1
U 6 A
U 6 A
J 1 . 1 3
6
U 1
4
U 6 A
J 1 . 8 J 1 . 1 4
1 M
C 2
. 0 1 u f
2
1 M
R 1 A
1
9
7
1 1
U 1
1 0
1 4
1 0 0 u f / 2 0 V
C 1
R 1
1 0 0 u f
2 0 V
1 0 0 K
. 0 1 u f
C 4
1 2
C 5
1 3
8
R 3
R 2
1 M
2 . 2 M
R 2 A
L 1
L 2 L 3 L 4 L 5 L 6
N O T E : R 3 8 - R 4 4
= 7 5 0
9
+ 1 2
1
2
3
+ 1 2
R 5 2
1 m
+
+
+
7
1 4
+
+
+
9
+
1
2
1
2
+ 1 2
+ 1 2
1 2 3
1 2
1 2 3 4 5 6 7 8
+ 1 2
+ 1 2
7 8 1 2
Logic Board Schematic
Figure 5-8, Logic Board Schematic
0000061122
5-25
Mini Med Series
Page 92

Service
D
Logic Board Layout
YELLOW WIRE
SINGLE ULTRAVIOLET
WIRE TO HARNESS BOARD
WHITE WIRE
TO HARNESS
SINGLE BLACK
WIRE TO HARNESS
TO MAIN HARNESS BOARD (BK3)
SINGLE BLACK WIRE TO TERM 1 OF AC BOARD
C1
R8
C12
C14
U6
CR24
+
VR1
L6
R35
CR23
CR9
CR8
C32
C8
R29
C5
C4
VR2
R10
CR25
J13
C6
C9
R11
C7
TO INTERRUPTER
SWITCH
J14
TO MAIN SWITCH
J12
(Two individual
Yellow & Orange
Wires - NOT ribbon)
TO READY TONE
GENERATOR BOAR
J9
TO LED BOARD
(Purple/Grey
Ribbon)
2
1
J1
U6A
R17
R15
R19
R14
+
R20
-
R49
R13
R18
J4
L2
345
JOG ONLY
JOG/FLOOD
C80
U2
R21
R23
J5
C4A
C5A
C33
C3A
R22
R50
R3
R2
R2A
R12
-
+
U1
R52
C15
U3
JUMPER
35302
ONLY
L1
67
C3R1C2
R1A
CR1
LOGIC BOARD AFP 35302
R40
R41
R38
R39
R42
L3
C17A
J7
J20
TB1
FUSE 1/4 AMP
U7
R43
R44
L4
R26
J8
R46
R25
T1
CR27
R45
R27
R42
R30
L5
MOV
Q2
R32
U4
R37
R36
C17
CR18
CR20
CR19
C16
CR21
R47
R28
C10
R31
U5
DEV TEMP
SENSOR
Mini Med Series
DRYER TEMP
SENSOR
LED
LEVEL SENSOR
BOARD
TO LED BOARD
(Blue/Green Ribbon)
Figure 5-9, Logic Board Layout
5-26
0000061122
Page 93

AC Interface Board Layout
E
B L A C K W I R E J U M P E R F R O M
T B 1 - 7 L O G I C P C B
3
4
J 3
F 1
F 3
F 4
F 5
F I X , D E V R E P L
F I X H E A T / F A N S
F 7
F I X H E A T / F A N S
D E V H E A T
1 A
1 A
5 A
5 A
5 A - 8 A
1
2
C R 1
R 1
R 3
Q 2
S C 1 4 6 M
Q 6
S 6 0 1 0
F 1
Service
B L U E
P I N K
Q 7
S 6 0 1 0
R 8
U 6
U 3
. 1 1 0 0 0 V
R 9
F 1
U 7
U 2
C 1
8 7
1
2
3
4
5
6
7
8
564
J 2
U 1
R 4
Q 1
S C 1 4 6 M
R E D
G R A Y
3
R 6
B R O W N
O R A N G
2
1
J 1
T P 1
T P 2
T P 3
T P 4
T P G R N
U 4
. 1 1 0 0 0 V
C 2
F 6
0000061122
D R Y E R
R E C I R C W A S H R E P L F A N S
M O T O R W A S H R E P L .
3 A
S C 1 4 6 M
Q 3
R 2
Figure 5-10, AC Interface Board Layout
5-27
R 1 0
S C 1 4 6 M
Q 4
R 5
Mini Med Series
Page 94

Service
Ready Tone Generator Layout & Schematic
R8
VCC
R7
C8
J13
J14
C4
R6
1K
+
B1
BEEPER
C7
100K
10uf
+
68K
C6
100uf
+
.1uf
5
2191213
THR2
THR1
OUT2
OUT1
U2
NE556
1uf
+
DCG1
VCC
14
TRG1
CNT1
RST1
6
348
C3
.1uf
TRG2
GND
CNT2
RST2 DCG2
11
10
C5
.1uf
-
FROM J13 LOGIC PCB
ORN RED BRN
VCC
GND
R5
10K
Q2
2N2222
R4
10K
CR1
1N4004
R1
390
R2
C2 1uf
+
R3
1K
2N2222
Q1
R11
10K
U1
R10
10K
390
123
1N437
6
Q3
+
C1
33uf
R9
5.1K
5
4
2N2222
R12
10K
R13
10K
Mini Med Series
J13
TO FILM
SENSOR
Figure 5-11, Ready Tone Generator Layout & Schematic
(For Serial #'s 1900 And Higher)
5-28
J14
TO FILM
SENSOR
0000061122
Page 95
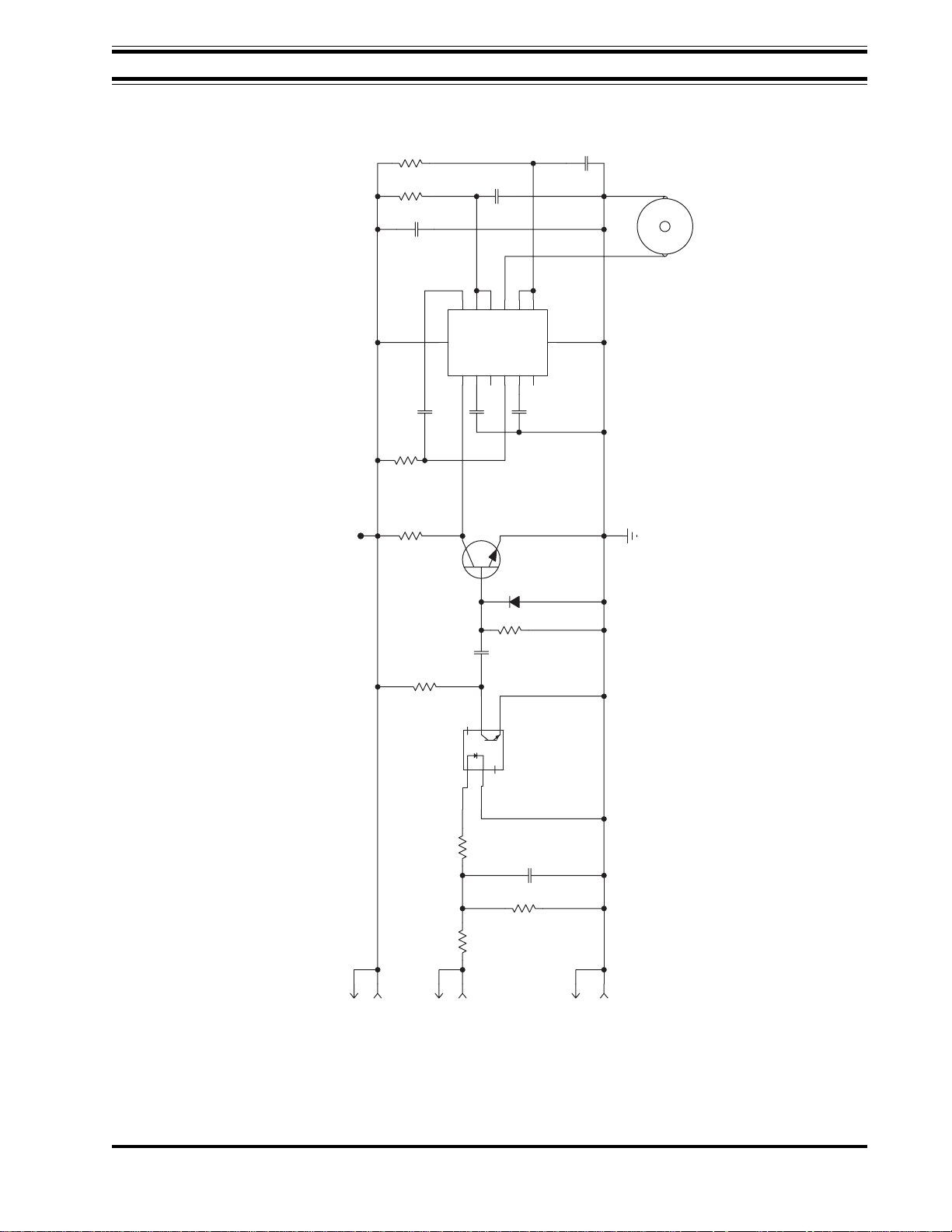
Service
K
R8
100
100uf
60K
R7
C6
+
C5
.1uf
1
9
5
2
12
13
THR2
THR1
OUT2
OUT1
DCG1
14
R6
+12
VCC
U2
NE556
TRG1
CNT1
RST1
TRG2
3
4811
2N2222A
CNT2
10
1uf
C5
6
1uf
+
C4 1uf
1K
10K
R5
C3
Q2
RST2 DCG2
GND
C7
10uf
+
-
BEEPER
+
7
1N4004
CR1
R4
10K
1uf
C2
R3
1K
U1
4N37
+
4
5
6
U1
1N437
1
2
3
R2
390
33uf
C1
+
R9
R1
390
PJ13
Pin #1
Pin #1
Pin #2
Pin #2
50K
Pin #3
Pin #3
0000061122
Figure 5-12, Ready Tone Generator Schematic
(For Serial #'s Below 1900)
5-29
Mini Med Series
Page 96

Service
LED Board Layout & Schematic
R2
750
R1
750
POWER
DEV
WAIT
LED PCB MINI MED
BROWN
RED
ORANGE
YELLOW
GREEN
BLUE
VIOLET
GRAY
Mini Med Series
LOW LEVEL
(TO J9 & J20)
Figure 5-13, LED Board Layout & Schematic
5-30
0000061122
Page 97

Section 6
Parts
- General Index -
Section 0 - Safety Information
Section 1 - Introduction
Section 2 - Installation
Section 3 - Operation
Section 4 - Maintenance
0000061122
Section 5 - Service
Section 6 - Parts
Section 7 - Accessories
AFP Mini-Medical
X-Ray Film Processors
Page 98

Page 99

Introduction
This section is an illustrated catalog of repair and replacement parts for the Mini-Medical
Series Processors. Illustrations show the locations of replaceable parts and corresponding lists give the part number, description and quantity per assembly for each.
How Parts Are Listed
Most parts shown in the illustrations are identified with reference numbers which are
repeated on the accompanying parts list(s). The list(s) will include the part number,
description and quantity of the parts.
Attaching parts, when listed, are shown below the listing for that part or assembly.
When Ordering Parts
Give the part number, description and quantity required for each part. Give also the
model and serial number of the processor for which the parts are needed.
Parts
Maintenance Kit
The following items are found in the Maintenance Kit (P/N 9992302604) included
with each Mini-Medical Series processor.
Item
1
2
3
4
5
6
7
8
9
10
11
12
13
14
Part Number
0000021306-K
0000021467
0000021346
568-007004-1
0000047802
0000021085
883-055-55
0000084407
800-089210
0000021658
0000041406
0000046285-C
0000046251-C
0000021801
Description
Worm Gear
Drive Gear, 24P, 24T
Retaining Ring, 1/4 Noryl
Washer, Retaining, .640 Dia
Spring, Extension, Racks
Gear, Idler, W4P 18T, 3/4 PD
Oiler, 1/4 Ounce
Beaker, Graduated, Tri
Scrub Brush
Key, Side Plate, Double Hole
Clamp, Hose, Worm Gear, 3/8-7
Tee, 1/2" X 1/2" X 1/2" Barb
Elbow, !/2" Barb X 1/2" Barb
Service Tool, Hold Down
No.
Used
1
1
3
3
4
1
1
2
2
1
8
2
1
1
Documentation
0000061122
Mini Medical Series Manual, P/N 0000061122
6-1
Mini Med Series
Page 100

Parts
General Assembly
Ite m
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
Part Number
0000021257-2
0000021421-1
See Pgs 4 & 5
0000021700
0000021701
568-007074
0000022120
N/A
0000021845
0000021846
N/A
0000021881
0000022003
See Pgs 4 & 5
0000021442
568-007064-1
0000037112
0000037283
0000044755
0000037051
0000046251-C
0000047833
0000087222
0000087220
0000046191-E
0000046291-B
0000046300-A
9526062640
9527062640
9528062640
0000032679
0000021795
000-09000-AA-H
000-05400-AM-H
9992305005
Description
Ta n k / F r a m e U n i t
Side Cover
Cover, Elect. Door
Panel, Drain
Clamp, Panel, Drain
Tank, 7 Gal. Replenisher
Dip Tube Assy, Repl. Tank
N/A
Front Top Cover
Rear Top Cover
N/A
Rear Top Cover (Rear Exit - NotShown)
Foot, Molded
Film Feed Tray
Feed Tray Cover
Floating Lid
Manual Repleni shme nt S witch
Circuit Breaker, 15 Amp
Leveling Foot
Interrupter Switch, Rear
Elbow, 1/2"
Spring, Compression
B all Valve, Drain (Gray W/Blue Hand le )
Ball Valve, Drain (Black older style)
Nipple, 1/4 X 2 In Toe
Wash Water Hose
Hose Gasket
Tubing, 1/2" I.D. X 11/16" O.D. Clear
Tubing, 1/2" I.D. X 11/16" O.D. Red
Tubing, 1/2" I.D. X 11/16" O.D. Blue
Cover, interrupter switch, harness
Plate, interrupter switch
Flat washer, switch
Mounting screws, switch
Replenisher Tank Set, 7 Gal.
No.
Used
1
2
1
1
1
2
2
N/A
1
1
N/A
1
4
1
1
1
1
1
4
1
3
4
3
3
3
1
2
4 ft.
4 ft.
4 ft.
1
1
1
1
1
Mini Med Series
6-2
0000061122
 Loading...
Loading...