Page 1

The Advanced® Micro-Osmometer
Model 3300
User’s Guide
3305 Rev20 051109
Page 2

Copyright
This user’s guide is copyrighted by Advanced Instruments, Inc. with all
rights reserved. Under copyright laws, this guide may not be reproduced
in any form, in whole or part, without the prior written consent of
Advanced Instruments.
© 2002 Advanced Instruments, Inc.
Windows
®
is a registered trademark of Microsoft Corporation in the
United States and other countries. Intel®is a registered trademark of
Intel Corporation in the United States and other countries. All other
trademarks are the property of Advanced Instruments, Inc.
Advanced Instruments has reviewed this guide thoroughly. All material
contained within is believed reliable, but the accuracy and completeness
are not guaranteed or warranted, and are not intended to be representations or warranties concerning the product described.
Hot-Line®Service
If you have any questions or problems regarding the proper operation of
your instrument, please contact our Hot-Line Service department:
• 800-225-4034 (toll-free within the USA and Canada)
• +US 781-320-9000 (elsewhere)
• 781-320-0811 (fax)
ii
Advanced®Micro-Osmometer Model 3300 User’s Guide
Page 3
Table of Contents
Parts & Accessories v
Calibrators & Standards vii
Safe Use ix
Symbol conventions ix
General cautions x
Foreword: Theory and Technique xiii
Chapter 1 — Installation & Setup 1
Step 1 — Find a location for your instrument 1
Step 2 — Unpack your instrument 1
Step 3 — Obtain additional items 2
Step 4 — Power up your instrument 2
Step 5 — Set your date and time 4
Step 6 — Check your initial calibration 4
Step 7 — Proceed to instrument operation 5
Figure 1: Model 3300 Micro-Osmometer and Accessories 3
Table 1: Model 3300 Micro-Osmometer Packing List 3
Chapter 2 — Instrument Operation 7
Hazardous material cautions 7
Function of major components 7
Sample handling 12
Standards & controls 12
Sample test procedure 13
Sample test errors 14
Recall results 15
Repeatability tips 15
Statistics 16
iii
Page 4

Changing operating settings 17
Using a barcode scanner with the Model 3300 20
Using a printer with the Model 3300 21
Using the Model 3300’s RS-232 port 21
Instrument software update 22
Figure 2: Model 3300 Components and Controls 8
Figure 3: Model 3300 Back Panel 10
Figure 4: Sample Cell Tips and Sample Levels 13
Table 2: Barcode Port Connections 11
Chapter 3 — Calibration 23
Calibration procedure 23
Calibration notes 24
Chapter 4 — Troubleshooting & Service 27
Service & maintenance cautions 27
Obtaining service 29
Troubleshooting checks 30
Internal diagnostics 30
Fuse replacement 34
Chamber cleaning 35
Sample plunger replacement and calibration 36
Figure 5: Fuse Replacement 35
Figure 6: Sample Plunger Replacement and Calibration 37
Appendices
Appendix A: Troubleshooting Table 39
Appendix B: Product Specifications 45
Appendix C: Regulatory Notices 47
Appendix D: Warranty and Warranty Duties 51
Appendix E: Supplemental RS-232 Information 55
Appendix F: Symbol Definitions 57
Appendix G: Service Log 61
Index 63
iv
Advanced®Micro-Osmometer Model 3300 User’s Guide
Page 5

Parts & Accessories
v
To order parts and accessories, contact the Advanced
Instruments Customer Service Department:
• 800-225-4034 (toll-free within the USA and Canada)
• +US 781-320-9000 (elsewhere)
• 781-320-3669 (fax)
PART DESCRIPTION
20-µL Sampler
Replacement Sample Probe
Micro-Sample Test Kit (500 tests)
Sampler Plunger Wires (2)
Thermal Printer with Interface Cable, Operation Manual,
Thermal Paper Roll, and Printer Power Supply (100-120
VAC)
Thermal Printer with Interface Cable, Operation Manual,
Thermal Paper Roll, and Printer Power Supply (230 VAC)
Printer Paper (5 rolls)
User’s Guide
Service Manual
Serial Cable
Software Upgrade*
Barcode Scanner
PART NO.
3M0825
330700
3MA800
3M0828
210555_NA
210555_EU
3D3835
3305
3305SM
RS232-Cable
330990
330016
* Note: 330990 upgrade kit comes with a 3-meter serial cable #330053.
Page 6

vi
Advanced®Micro-Osmometer Model 3300 User’s Guide
Notes:
Page 7
vii
Calibrators & Standards
To order calibrators and standards, contact the Advanced
Instruments Customer Service Department:
• 800-225-4034 (toll-free within the USA and Canada)
• +US 781-320-9000 (elsewhere)
• 781-320-3669 (fax)
DESCRIPTION
ClinitrolTMReference Solution (10 2-mL ampules)
Protinol®3-Level Protein Control Kit (9 3-mL bottles, 3 of
each level)
Renol™ Urine Osmolality Controls (2 levels)
5-Value Osmolality Linearity Set (10 5-mL ampules, 2 of
each value)
50 mOsm/kg Calibration Standard (10 2-mL ampules)
850 mOsm/kg Calibration Standard (10 2-mL ampules)
P ART NO.
3MA029
3MA028
3LA085
3LA028
3MA005
3MA085
Page 8

viii
Advanced®Micro-Osmometer Model 3300 User’s Guide
Notes:
Page 9
Safe Use
To reduce the risk of bodily injury, electric shock, fire, and
damage to your instrument, please read and observe the precautions in this User’s Guide.
• If the product is used in a manner not in accordance with the
equipment design, operating instructions or manufacturer's
recommendations, the operation of the product may be
impaired to the extent that a safety hazard is created.
• Do not attempt to perform electrical work if you are not fully
qualified. This manual is not a substitute for electrical training.
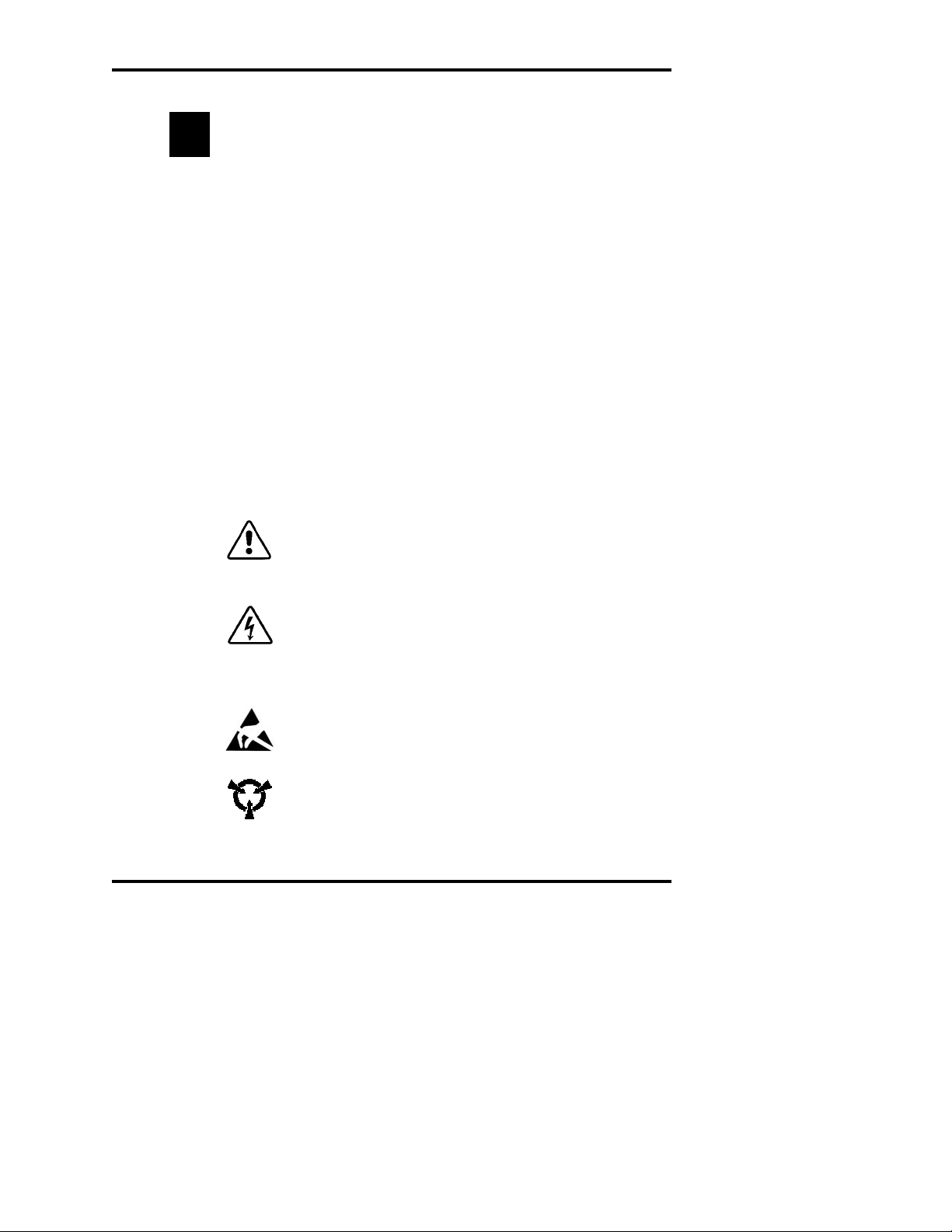
Symbol conventions
The exclamation point within an equilateral triangle is
intended to alert the user to the presence of important operating and maintenance (servicing) instructions in the literature
accompanying this product.
The lightning flash with arrowhead symbol within an equilateral triangle is intended to alert the user to the presence of
uninsulated dangerous voltage within the product's enclosure
that may be of sufficient magnitude to constitute risk of electric shock to persons.
The static symbol within an equilateral triangle is intended to
alert the user to the presence of internal components that
could be damaged by static electricity.
This static symbol is intended to alert the user to the presence of a specific component that could be damaged by static electricity.
ix
Page 10

This symbol indicates the presence of alternating current (AC).
This symbol indicates the presence of a fuse.
This symbol indicates the presence of protective earth ground.
General cautions
• This product should be operated only with the type of power source
indicated on the product’s electrical ratings label. Refer to the installation instructions included with the product.
• If the power cord provided is replaced for any reason or if an alternate cord is used, the cord must be approved for use in the local
country. The power cord must be approved for the product’s listed
operating voltage and be rated at least 20% greater than the ampere
ratings marked on the product’s electrical ratings label. The cord
end that connects to the product must have an IEC 60320 connector.
• Plug the product into an approved grounded electrical outlet.
• Do not disable the power cord’s grounding plug.
• If an extension cord or power strip is used, make sure that the cord
or strip is rated for the product, and that the total ampere ratings of
all products plugged into the extension cord or strip do not exceed
80% of the cord’s or strip’s rating limit.
• Route power cords so that they will not be walked on, tripped on, or
pinched by items placed upon or against them. Pay particular attention to the plug, electrical outlet, and the point where the cord exits
the product.
• Do not pull on cords and cables. When unplugging cords or cables,
grasp the corresponding connector.
x
Advanced®Micro-Osmometer Model 3300 User’s Guide
Page 11

• Do not install or use this product in any area subject to extreme
short-term temperature variations, or locations that exceed the specified operating environment temperatures.
• Never use this product in a wet area.
• To avoid injury or fire hazard, do not operate this product in an
explosive atmosphere.
• Do not install or use the product on an unstable, non-level work surface.
• Do not operate this product with the covers removed or unsecured.
xi
Safe Use
Page 12

xii
Advanced®Micro-Osmometer Model 3300 User’s Guide
Notes:
Page 13
Foreword
xiii
Intended Use
Advanced®Osmometers use the technique of freezing-point
depression to measure osmolality. Osmolality is the total solute
concentration of an aqueous solution. Osmometers measure the
number of solute particles irrespective of molecular weight or
ionic charge. This information is useful to the following disciplines:
Clinical, emergency and sports medicine
Medical research
Biotechnology and pharmaceutical research and
manufacturing
Food and beverage manufacturing
Environmental research and monitoring
Academic research
Industrial applications
When used by a trained operator in clinical applications, the
osmometer provides results that assist in establishing the proper
diagnoses and treatments for patients with disorders involving
water and electrolyte imbalances. Osmometers will test virtually any biological fluid including, but not limited to, whole
blood, plasma, urine, feces, sweat, and tissue homogenate.
Operation of the instrument is deemed to be low-complexity
under CLIA and FDA guidelines.
Page 14

xiv
Advanced®Micro-Osmometer Model 3300 User’s Guide
Principles of Freezing-Point Osmometry
When a solute is dissolved in a pure solvent, the following changes in
the solution's properties occur:
• the freezing point is depressed,
• boiling point is raised,
• osmotic pressure is increased, and
• vapor pressure is lowered.
These are the so-called "colligative" or concentrative properties of the
solution which, within reasonable limits, change in direct proportion to
the solute concentration; in other words, the number of particles in solution.
Of the colligative properties, measurement of the freezing point allows
the concentration of an aqueous solution to be easily determined with
great precision.
The freezing point of pure H
2
O is precisely +0.010°C. One mole of a
non-dissociating solute such as glucose (where the solute does not dissociate into ionic species, but remains intact), when dissolved in 1 kilogram (kg) of water will depress the freezing point by 1.858°C. This
change is known as the freezing point depression constant for water.
The freezing point depression also depends upon the degree of dissociation of the solute. If the solute is ionic, the freezing point is depressed
by 1.858°C for each ionic species. For example, if one mole of sodium
chloride were to completely dissociate into two ionic species (Na+ and
Cl-) in 1 kg of water, the freezing point would be depressed by
3.716°C. However, dissociation is never complete. Interference
between solute molecules reduces dissociation by a factor called the
osmotic coefficient.
Page 15

xv
In a simple solution such as glucose or sodium chloride in water, the
freezing point can be measured and the unit concentration easily determined from an equation or a reference table. However, the equation is
unique for each solute. In a more complex solution, all ionized and
non-dissociated species contribute to the freezing-point depression and
the concentration of each solute cannot be easily determined.
Each of the colligative properties has a similar problem, and though
each of the colligative properties changes in direct proportion to the
solute concentration, each requires a different mode & unit of measurement. Osmolality is a common unit of concentration measurement that
can be used to relate all the colligative properties to each other, and to
other concentration units. Because of its universality, most osmometry
applications regularly use osmolality, expressed as "mOsm/kg H2O", as
the common unit of concentration rather than applying further conversion factors.
Instrumentation
Advanced Osmometers are devices for the determination of the concentration of solutions by means of freezing-point measurement.
Advanced Osmometers utilize high-precision thermometers to sense the
sample temperature, to control the degree of supercooling and freeze
induction, and to measure the freezing point of the sample. They can
routinely determine differences of ±1 mOsm/kg H
2
O.
Freezing-Point Thermodynamics
The quickest and most precise way to measure the freezing point of a
solution is to supercool it several degrees below its freezing point. It is
unstable in this state, and a mechanical agitation induces crystallization.
The heat of fusion suddenly liberated causes the sample temperature to
rise toward a plateau temperature, where a liquid/solid equilibrium
occurs. The equilibrium temperature is, by definition, the freezing point
Foreword
Page 16

of the solution. Managing the plateau temperature for precise measurement is the basis for several patents issued to Augustus Fiske.
The time over which liquid/solid equilibrium develops and is maintained, is a function of the speed with which the heat-of-fusion is liberated vs. the speed it is transferred away, or absorbed, by the surrounding
environment. This ratio can be slowed and the equilibrium time
stretched, to give a distinct plateau height measurable to 0.001°C.
Sensitive thermistor probes monitor the sample temperature and control
the thermoelectric cooling element. Microprocessor control and automated operation minimize imprecision due to operator technique.
The following Standard Freezing Curve illustrates the temperature of a
sample as it progresses through the freezing cycle and shows the action
of the instrument at each stage of the cycle.
Definitions
Solution: Ahomogeneous mixture of solute and solvent in which the
solvent is usually the major component, and the solute is the minor
component.
Concentration: The ratio of solute to a given amount of solvent
(molal), or ratio of solute to solution (molar).
xvi
Advanced®Micro-Osmometer Model 3300 User’s Guide
Standard Freezing Curve
Page 17
The amount of solute is usually expressed in terms of moles, i.e., gram
molecular weights. One mole = 6.028 x 10
23
molecules (Avogadro's
number). One mole of glucose (180.2 g) and one mole of sodium chloride (58.4 g) each contain Avogadro's number of molecules.
Common units of concentration are:
• Molality: Moles of solute per kilogram of pure solvent.
• Osmolality: Osmols of solute particles per kilogram of pure sol-
vent. As noted above, most ionic solutes do not completely dissociate. Osmolality is a unit of concentration that takes into
account the dissociative effect. Osmolality is usually expressed
in mOsm/kg H
2
O. One milliosmol (mOsm) is 10-3osmols.
Osmolality is defined as:
where:
ø = osmotic coefficient, which accounts for the degree of mole-
cular dissociation.
n = number of particles into which a molecule can dissociate.
C = molal concentration of the solution.
• Molarity: Moles of solute per liter of solution.
• Osmolarity: Osmols of solute particles per liter of solution.
Although molarity and osmolarity may be common units of
measurement in other branches of chemistry, they are not used in
osmometry because the ratio of solute to solution is not linear.
Molality and osmolality are linear, independent of the effect of
temperature and volume displaced by solute. Acalculated conversion between units of molality and molarity is complex and
generally unnecessary when the terms are properly understood.
xvii
Foreword
OHkg
osmol
nCOsmolality
2
==
φ
Page 18

Freezing Point/Melting Point: The temperature at which the liquid
and solid phases of a substance will remain together in equilibrium.
Freezing-Point Depression: When a solute is added to a solvent, the
freezing point of the solvent is lowered. In aqueous solutions, one
mOsm of solute per kilogram of water depresses the freezing point by
1.858 millidegrees Celsius (m°C).
Supercooling: The tendency of a substance to remain in the liquid state
when cooled below its freezing point.
Crystallization Temperature: Aqueous solutions can be induced to
freeze (i.e., crystallize) most reliably when supercooled. When supercooled, agitating the solution (freeze pulse) induces crystal formation.
The crystallization temperature is the temperature at which crystallization is induced. During crystallization, the heat of fusion raises the temperature of the sample to an ice/water freezing-point plateau.
Heat of Fusion: The heat released when the mobile molecules of a liquid are frozen into rigid ice crystals.
Freezing-Point Plateau: The constant temperature maintained during
the time that ice and liquid exist in isothermal equilibrium after crystallization occurs.
xviii
Advanced®Micro-Osmometer Model 3300 User’s Guide
Page 19
1
Installation & Setup
In order to set up your instrument properly, it is important that
you read and follow the steps in this section. Please follow these
steps carefully and be sure to read Chapter 2 — Instrument
Operation before attempting to run tests on your instrument.
Step 1 — Find a location for your instrument
When choosing a location for your new osmometer, be sure to
meet the following criteria.
• Adequate space. The dimensions of the instrument are 13
× 10.5 × 15.5 inches (26.7 × 33 × 39.4 cm). Be sure to keep
your workplace free of debris, especially near the front of
the instrument where proper ventilation is needed.
• Electric outlet availability.Your instrument will need to
operate within five feet of a properly grounded, three-prong
electrical outlet capable of continuously supplying 1 ampere
at 200-250V to 1.25 amperes at 100-130V. If the instrument
is not grounded properly, its operation may be impaired and
a safety hazard may exist. Therefore, be sure to test the outlet and record the results before operating your instrument.
Step 2 — Unpack your instrument
To unpack your osmometer, take the following steps.
a. Carefully unpack your osmometer, accessories and supplies
and inspect them for shipping damage. Use the enclosed
packing list to verify that all items have been received.
1
Page 20

b. Save your osmometer's shipping boxes and packaging material in
case future transport of the instrument becomes necessary.
c. If any item on the packing list appears to be missing from your ship-
ment, please search carefully through and under all packing materials. If the item is not found, notify your receiving department immediately. Advanced Instruments can only be responsible for items
reported missing within 10 days of a shipment’s arrival.
d. If you receive any damaged items, save the cartons and packing
material those items came in for inspection by the insurer. The carrier, dealer, and Advanced Instruments must be notified within 24
hours in order for your warranty and insurance to apply. Have the
transportation company inspect items, fill out a “Report of Concealed
Damage,” and file your claim. Then, notify Advanced Instruments
immediately for repair or replacement.
e. Fill out the postage-paid “(U.S.A. Only)” warranty card enclosed.
Mark the appropriate boxes if you wish to receive additional information. Customers outside of the United States may fax the warranty
card to 781-320-8181.
Step 3 — Obtain additional items
Soft, no-lint, non-ionic paper tissues are needed for wiping the sample
cells prior to testing. Please be sure that you have an adequate supply on
hand before attempting to run tests on your instrument.
Step 4 — Power up your instrument
Turn your instrument on by pushing the rocker-style power switch on
the instrument’s back panel into the on ( | ) position.
Each time your instrument is turned on, it automatically runs a oneminute setup and self-diagnostic program. During these diagnostics, your
display and printer (if in use) display critical instrument information, such
as software version and block and sample probe bin numbers. The first
time you power up your osmometer, record the software revision and
Advanced®Micro-Osmometer Model 3300 User’s Guide
2
Page 21

3
Figure 1: Model 3300 Micro-Osmometer and Accessories
Quantity
Part No. Description
1 3300 The Advanced®Model 3300 Micro-Osmometer
1 pack 3MA029 Clinitrol
™
290 mOsm/kg Reference Solution
(ten 2 mL ampules)
1 pack 3MA005 50 mOsm/kg Calibration Standard (ten 2 mL
ampules)
1 pack 3MA085 850 mOsm/kg Calibration Standard (ten 2 mL
ampules)
1 kit 3MA800 Micro-Sample Kit: includes 500 disposable
sample cells (tips) and 500 chamber cleaners
2 3D3185 Operator/Supervisor Keys
1 3MO825 20-µL Sampler
1 Power Cord (as specified)
1 90P01 Advanced
®
User Information CD-ROM
1 3305 User’s Guide
1 3305-6 Warranty Card
1 3D3P021 Customer Satisfaction Card
Table 1: Model 3300 Micro-Osmometer Packing List
Installation & Setup
Page 22
block and sample probe bin numbers in the Service Log at the end of this
user’s guide. Maintaining a record of this information will facilitate any
necessary service.
When the self-diagnostics have been completed and the instrument is
ready for testing, the display reads “Osmometer Ready”.
NOTE To avoid any misunderstanding that might arise due to an
unfamiliar display language, all displayed messages may be
changed from English to several other languages. (For language selection instructions, see Chapter 2.)
CAUTION If a power interruption occurs, turn the instrument off at
once. Leave it turned off for at least 5 seconds after power
has been restored (even if power restoration is immediate).
Step 5 — Set your date and time
If you wish, you may now change your date and time settings by using
the instructions found on page 17 of this user ’s guide.
Step 6 — Check your initial calibration
Your instrument has been carefully calibrated by the manufacturer, but
to verify that this calibration is accurate within your operating environment, run tests on the 290 mOsm/kg reference solution and/or Protinol
®
biological controls before testing samples. The number and type of tests
that you run should be determined by your own laboratory’s standard
protocol. Use the operating technique described in Chapter 2 when running these controls.
If you determine that the initial calibration is incorrect, re-calibrate as
described in Chapter 3.
NOTE If your Model 3300 has just been moved from a different loca-
tion, it should be allowed to warm up for 20 to 30 minutes
before you run calibration verification tests.
4
Advanced®Micro-Osmometer Model 3300 User’s Guide
Page 23

Step 7 — Proceed to instrument operation
If you have followed the steps outlined in this chapter, your instrument
is ready for use. To learn how to operate your instrument, read the next
chapter, “Instrument Operation”. We strongly recommend that you read
the entire second chapter before attempting to operate your osmometer.
5
Installation & Setup
Page 24

6
Advanced®Micro-Osmometer Model 3300 User’s Guide
Notes:
Page 25
2
Instrument Operation
In order to run your instrument properly, it is important that
you read and adhere to the instructions in this section. For
information on calibration, see Chapter 3 — Calibration.
Hazardous material cautions
• WARNING: Handle all biohazardous materials according to
established good laboratory practices and follow your institution’s exposure control plan. Persons handling human blood
and body fluid samples must be trained in blood-borne hazards and observe universal precautions. Universal precautions
is an approach to infection control, where all human blood and
body fluids are treated as if known to be infectious. Use personal protective equipment such as gloves, gowns, etc., to prevent exposure. Store biohazardous materials in regulated waste
containers and dispose of these materials in a safe and acceptable manner that is in compliance with all country, state and
local requirements.
• If a biohazardous material is spilled on or inside the equip-
ment, decontaminate the equipment using a 1% bleach solution, or as outlined by those policies and procedures established within your institution.
• To avoid injury or fire hazard, do not operate this product in
an explosive atmosphere.
Function of major components
The major components of the Model 3300 Micro-Osmometer
are an operating cradle; a sample port to precisely position
each sample for the osmolality test; a high-precision thermistor
7
Page 26

Advanced®Micro-Osmometer Model 3300 User’s Guide
probe; measurement and control circuitry; and a message display
panel. A sample handling pipette is also used in the testing process.
The function of many of these parts is described below.
Electronic circuits (inside)
The main circuitry is contained on two printed circuit boards in a motherboard/daughterboard configuration. More in-depth technical details
are available in the unit service manual, sold separately.
Processor Board: The processor board is the smaller of the two printed
circuit boards and contains the Intel 80C186EB central processor, two
flash EPROM’s, RAM, Realtime clock, watchdog circuit, and glue
logic.
8
Figure 2: Model 3300 Components and Controls
Display
Panel
Multifunction
Soft keys
Operating
Cradle
Sampler
Cooling
Chamber
(inside)
Chamber
Cleaners
Sample
Tips
Page 27

Application Board: The application board contains the circuits for controlling and interfacing with the other subsystems such as the keypad,
display, cooling assembly, RS-232, printer and barcode ports, etc. The
application board also contains two 20-bit A/D converters for reading
the sample and block probe thermistors.
Sampler (Figure 2)
The sampler is a specialized pipette which contains and positions a very
specific volume of sample for each test. The sampler consists of the
pipette body and disposable plastic sample tip that must be replaced
after each test.
Operating cradle (Figure 2)
The operating cradle is a sliding mechanism that guides and introduces
the sampler to the freezing chamber.
Cooling chamber & measurement and control circuits:
A high-reliability thermoelectric module controls cooling chamber temperature, while a thermistor sample probe measures the dynamic temperature of a sample. Test results, based on the freezing point of the
sample, are automatically sent to the instrument’s display.
Display panel (Figure 2)
The display panel displays:
• Test status and results.
• Status messages when the instrument is turned on or when functions are
selected.
• Pertinent error messages when fault conditions occur. (Messages longer
than 24 characters wrap to the second line.)
Multifunction soft keys (Figure 2)
Other than starting osmolality tests, which are initiated by the operating
cradle, all operator communication to the Model 3300 is accomplished by
means of the two multifunction soft keys.
9
Instrument Operation
Page 28

Advanced®Micro-Osmometer Model 3300 User’s Guide
Power module (Figure 3)
The power module on the rear of the instrument contains the following
components.
• Power switch
The rocker-style power switch controls the power to the instrument.
• Power cord connector
The power cord connector accommodates a power cord suitable for the
power available.
• Fuse holder & fuses
The fuse holder contains the instrument’s necessary fuses. For instructions on replacing fuses, see Chapter 4.
Printer port (Figure 3)
The printer port on the rear panel allows you to use a printer in conjunction with this instrument. For instructions on using a printer with this
instrument, see “Using a printer with the Model 3300” in this chapter.
RS-232 port (Figure 3)
The RS-232 port allows you to output your instrument’s data/messages to
an external device, such as a computer, and to upgrade your instrument
software. For more detailed information dealing with the RS-232 port,
please read the sections titled “Using the Model 3300’s RS-232 Port” and
“Instrument Software Update”, found later in this chapter.
10
Figure 3: Model 3300 Back Panel
Page 29

Barcode port (Figure 3)
A D-type, 15-pin barcode port is provided in the back of the 3300 for
connecting and providing power to such a device. For proper operation,
the barcode port requires a 1200 bps, RS232-level signal providing
asynchronous serial data containing 1 start bit, 8 data bits, 1 stop bit and
no parity.
A suitable RS232 barcode scanner is available from Advanced
Instruments, Inc. To interface with the 3300, the barcode scanner must
be programmed as follows, referring to the scanner users guide.
10 msec Intercharacter Delay
Disable Line Feed Suffix
No Parity
1200 BAUD Rate
8 Data Bits
Supervisor/operator keyswitch (Figure 3)
The supervisor/operator keyswitch allows certain instrument functions to
be locked. The Supervisor keyswitch position is required for re-calibration
and for access to the following diagnostic menu items: Set Probe Bin #s,
Set Serial Rate, Serial Comm Set and Select Language.
If a restricted function is attempted while Supervisor/Operator keyswitch
is in the operator position, a “Supervisor Key Needed” message is displayed and may be cleared by pressing both function buttons simultaneously before selecting any other function.
To utilize the supervisor/operator keyswitch you must use the key supplied with your instrument.
+5V DC 1 to reader
receive data 10 from reader
gnd/earth 9 common
Signal Pin Direction
Table 2: Barcode Port Connections
11
Instrument Operation
Page 30

Advanced®Micro-Osmometer Model 3300 User’s Guide
Sample handling
The sampling system kit supplied with your Model 3300 contains a special sampler and a starter supply of disposable sample cells and chamber cleaners. Each kit contains re-order information.
Be sure to replace the sampler plunger with each new test kit (500 samples). For instructions on this procedure, see “Sample plunger replacement and verification” in Chapter 4.
The Model 3300 measures the osmolality of a sample inside the
sample cell while on the pipette. This feature requires sample
cells specially designed to match both the sampler and the
Model 3300. Do not use any other sample-handling system
with your Model 3300 osmometer, and do not use the Model
3300 sampling system with any other laboratory procedure.
Standards & controls
Advanced Instruments offers and recommends the use of specific standards and controls with your Model 3300 osmometer. Each type may be
used to assess a different aspect of your instrument’s performance. For
information on obtaining these standards and controls, contact
Advanced Instruments or an authorized representative.
Clinitrol 290 Reference Solution (Part No. 3MA029)
We recommend that you run samples of Clinitrol 290 Reference
Solution daily to check your instrument operation and confirm your calibration. You should also run the Clinitrol 290 Reference Solution when
you receive erratic results. Doing so will allow you to verify proper
operation or recognize and diagnose problems promptly.
Protinol(Part No. 3MA028)
Advanced Protinol protein-based controls, at 240, 280 and 320 mOsm
(±5 mOsm), provide control values that closely bracket those of most
serum samples. These controls are formulated to mimic the freezing
characteristics of actual serum samples, and they can be used to verify
12
Page 31

the precision and reliability of your osmometer results. Protinol should
be used once every shift.
Advanced Five-Level Linearity Set (Part No. 3LA028)
The Advanced Linearity Set contains five controls (100, 500, 900, 1500,
and 2000 mOsm). Use this set to verify or establish the reportable range
of patient test results.
Sample test procedure
Use the following procedure to run sample tests.
1. Snap a sample tip into place on the sampler. The sample cell must be
straight and firmly seated. Be careful not to crack the sample tip.
2. Depress the sampler ’s plunger and insert the sample tip at least
¼ inch (6 mm) below the surface of the fluid to be tested. Gently
release the plunger to load a 20-µL sample.
3. Visually inspect the sample. If there are any large voids or bubbles in
the sample, expel the sample and load a bubble-free sample.
4. Blot the sides of the loaded sample cell with a soft, no-lint, non-ionic
paper tissue to remove any clinging droplets. Then blot the end of
the cell tip to remove any fluid protruding beyond the tip. Be careful
not to wick out any of the sample. The meniscus remaining may be
13
Instrument Operation
Figure 4: Sample Cell Tips and Sample Levels
Page 32

slightly concave, but the sample must be slightly longer than it is
wide. (See figure 4.)
5. Remove the chamber cleaner from the sample port and discard.
6. Holding the sampler by the barrel and letting the filled sample tip
follow the guide groove into the sample port, rest the sampler within
the operating cradle and beneath the cradle top.
7. To start the test, push the entire operating cradle down until it reaches a positive stop. Your instrument will run the test for approximately one minute and display the result in the format “Osmolality xxx
mOsm”.
8. Record the results and raise the operating cradle to a positive stop.
9. Remove the sampler from the operating cradle.
10.Insert a clean, dry chamber cleaner into the sample port and rotate it
four or five times in both a clockwise and counterclockwise direction. Withdraw chamber cleaner and insert the opposite end. Rotate
the chamber cleaner in the same manner and leave it in the sample
port until your next test.
11.Remove the used sample tip from the sampler by pressing firmly
enough on the sampler plunger to dislodge the tip. Discard the used
sample tip.
12.Blot the Teflon plunger tip with a soft, no-lint, non-ionic paper tissue. Be careful not to dislodge the tip.
13.To run additional tests, repeat this procedure beginning with step 1.
Sample test errors
Occasionally a test will not run to completion and your instrument will
display an error message. Refer to the Troubleshooting Table appendix
at the end of this user’s guide for an explanation of a particular message.
Advanced®Micro-Osmometer Model 3300 User’s Guide
14
Page 33

Recall results
The recall results feature of the Model 3300 allows you display or print
the results of your last 30 tests. If you need to see any or all of these
results, take the following steps.
1. At “Osmometer Ready”, the two multifunction soft keys are labeled
[CALIB] and [DIAG], respectively. Press the soft key labeled
[DIAG] to enter the diagnostic test menu.
2. While you are within the diagnostic test menu, the soft key labels will
change to [START] and [INDEX]. Use the [INDEX] keypad to cycle
through your choices until you see “1. Recall Results”.
15
Instrument Operation
Repeatability Tips
• Treat all samples, as well as standards and reference solutions, uni-
formly before the test.
• Microsamples are more susceptible to contamination and evapora-
tion than larger samples. Avoid leaving sample containers open.
Cold samples are susceptible to condensation; warmer samples are
susceptible to evaporation.
• Use only the Advanced Model 3300 sampling system. Each system
comes with specific operating instructions and re-order information.
• If an occasional sample produces irregular results, discard obvious-
ly discrepant readings as long as the instrument has been producing
accurate readings repeatedly. Repeat the sample in question.
• For repeat runs, use additional samples from the same source.
• Keep the cooling chamber clean between tests. Never inject any-
thing into the cooling chamber.
Page 34

Advanced®Micro-Osmometer Model 3300 User’s Guide
3. Press [STAR T] to enter the recall results mode. Your instrument will
display the data of the most recent test result.
4. Press the right soft key to see the result of the next-to-last test result.
5. Continue in this manner until you have recalled the necessary results.
6. To return to the diagnostic menu, press the two soft keys simultaneously .
7. To return to “Osmometer Ready”, again press the two function keypads simultaneously.
Statistics
The statistics option allows you to recall the stored results from a selected
last number of tests stored in the recall buffer and calculate their average,
standard deviation and coefficient of variance. If any test errors are stored
in the range you select, they will be filtered out of the calculations. To use
this option, follow this procedure:
1. At “Osmometer Ready”, the two multifunction soft keys are
labeled [CALIB] and [DIAG], respectively. Press the soft key
labeled [DIAG] to enter the diagnostic test menu.
2. While you are within the diagnostic test menu, the soft key labels
will change to [START] and [INDEX]. Use the [INDEX] soft key
to cycle through your choices until you see “2. Statistics”.
3. Press [START] to enter the statistics mode. Your instrument will dis-
play the number of tests to be considered when computing statistics.
4. Press the right [INDEX] soft key to index to the number of results
that you wish to consider.
NOTE If you request calculations for more samples than are stored
in the recall buffer, you will receive incorrect results.
5. Press the left [START] soft key to display and print (if using exter-
nal printer or serial port) the average of the selected number of last
results.
16
Page 35

6. Press the left [START] soft key again to display and print the stan-
dard deviation of the selected number of last results.
7. Press the left [START] soft key a third time to display and print the
coefficient of variance of the selected number of last results.
8. To return to the diagnostic menu, press the two multifunction soft
keys simultaneously.
9. To return to “Osmometer Ready”, again press the two function keypads simultaneously.
Changing operating settings
Date and time
To reset the date and time, take the following steps:
1. At “Osmometer Ready”, the two multifunction soft keys are labeled
[CALIB] and [DIAG], respectively. Press the soft key labeled
[DIAG] to enter the diagnostic test menu.
2. While you are within the diagnostic test menu, the soft key labels
will change to [START] and [INDEX]. Use the [INDEX] soft key
to cycle through your choices until you see “13. Set Date/Time”.
3. Press [START] to enter the Set Date/Time mode. Your instrument
will display the current date and time.
4. Press the left soft key to select the date or the right soft key to select
the time.
5. If you choose to set the date:
a) Select between DD/MM/YYYY and MM/DD/YYYY formats.
Your instrument will display the current date in the format you
selected and a blinking cursor element.
b) Use the left soft key to increment the number at the cursor and
the right soft key to change between digits in the date until the
correct date is displayed.
17
Instrument Operation
Page 36

c) Press both soft key buttons to save the new date setting and
return to the diagnostic test menu.
6. If you choose to set the time
a) Select between AM/PM and 24-hour formats. After you select a
display format, your instrument will display the current time in
the selected format.
b) Use the right keypad to toggle between hours and minutes and
the left keypad to change the number of hours or minutes displayed.
c) Press both soft key buttons to save the new time setting and
return to the diagnostic test menu.
Set Probe Bin #'s
To reset the block and sample probe bin numbers, take the following
steps:
1. At “Osmometer Ready”, the two multifunction soft keys are labeled
[CALIB] and [DIAG], respectively. Press the soft key labeled
[DIAG] to enter the diagnostic test menu.
2. While you are within the diagnostic test menu, the soft key labels
will change to [START] and [INDEX]. Use the [INDEX] soft key
to cycle through your choices until you see “3. Set Probe Bin #’s”.
3. Press [START] to enter the selection menu.
4. Press the right [INDEX] soft key to advance the probe bin #, or
press the left [NEXT] soft key to advance to the next probe bin #.
Be sure to record the new bin settings in the Service Log at the back
of this user's guide.
5. Press [NEXT] soft key to advance to the save options.
6. Select [YES] to save the new setting, or [NO] if no change was
made.
Advanced®Micro-Osmometer Model 3300 User’s Guide
18
Page 37

Data Capture
To change the RS-232 output between standard mode and capture mode,
take the following steps:
NOTE With Capture selected, freezing curve output data will be
presented to the serial port in real time. This data is updated
every 50 msec after crossing 0ºC, and may be captured with
any RS-232 compatible computer program for use in plotting freezing curves.
In Standard mode, ony the final result and some status messages will be presented to the serial port.
1. At “Osmometer Ready”, the two multifunction soft keys are labeled
[CALIB] and [DIAG], respectively. Press the soft key labeled
[DIAG] to enter the diagnostic test menu.
2. While you are within the diagnostic test menu, the soft key labels
will change to [START] and [INDEX]. Use the [INDEX] soft key
to cycle through your choices until you see “12. Data Capture”.
3. Press [START] to enter the selection menu.
4. Select the soft key for the mode you want to use.
5. Press the two soft keys simultaneously to lock in your selection.
6. Press the two soft keys again simultaneously to return to
“Osmometer Ready”.
Language
To reset the display language, take the following steps:
1. At “Osmometer Ready”, the two multifunction soft keys are
labeled [CALIB] and [DIAG], respectively. Press the soft key
labeled [DIAG] to enter the diagnostic test menu.
2. While you are within the diagnostic test menu, the soft key labels
will change to [START] and [INDEX]. Use the [INDEX] keypad to
cycle through your choices until you see “14. Select Language”.
19
Instrument Operation
Page 38

Advanced®Micro-Osmometer Model 3300 User’s Guide
3. Press [START] to enter the Select Language mode. Your instrument
will display the currently set language.
4. Press the left soft key to switch between languages.
5. To lock in the selected language and return to the diagnostic menu,
press the two soft keys simultaneously.
6. Press the two soft keys again simultaneously to return to
“Osmometer Ready”.
Using a barcode scanner (Part # 330016) with the
Model 3300
The barcode option provides a means of entering an identification code
with an internally decoding barcode scanner connected to the barcode
port. To interface with the Model 3300, the barcode scanner must be
programmed as follows, referring to the scanner users guide:
10 msec Intercharacter Delay
Disable Line Feed Suffix
No Parity
1200 BAUD Rate
8 Data Bits
When the barcode scanner (part # 330016) is connected to the Model
3300, the instrument operator may scan an identifying barcode before
introducing a sample.
NOTE The barcode configuration may work with many different
scanners, but not all are recommended and supported by
Advanced Instruments. For information on which scanners are
supported by Advanced Instruments, please contact Advanced
Instruments customer service department.
NOTE To avoid possible damage to your instrument or the barcode
scanner, turn off the instrument power before connecting to
the barcode port.
20
Page 39

If you introduce a sample without a prior barcode scan, the test results
will display without further identification. If you scan a barcode, the
Model 3300 will display “Successful Scan [Cancel]” on the first line
and the decoded barcode on the second line. During that display, the
instrument operator may introduce a sample for test and identification
with the decoded barcode or may cancel and either re-scan or introduce
a sample without barcode identification.
Using a printer with the Model 3300
The 25-pin output printer port on the back of the Model 3300 allows the
use of most standard printers. For information on ordering a compatible
printer, contact the Customer Service Department at Advanced
Instruments. See page v for ordering information.
The use of a printer cable and/or printer power cable other than supplied
by Advanced Instruments may result in excessive electromagnetic noise
and unintended interference with other devices.
Using the Model 3300’s RS-232 port
The RS-232 port allows you to output to an external device, such as a
computer, and to update your instrument software. Almost every item of
information displayed by your instrument is also transmitted over the
RS-232 port, including test results, all error messages, and most display
data from the diagnostic menu.
The default serial data rate for communications is 9600 bps (bits per
second), though you may alternatively select 1200, 2400, 4800, and
19200 bps.
The DB-9 RS-232 port on your instrument conforms to the DTE EIA232 standard and can reliably communicate over shielded cable up to 10
meters in length, depending on the baud rate you use.
Note Your instrument is only designed to support unidirectional com-
munication with an external device. At this time, there is no protocol for bidirectional communication.
21
Instrument Operation
Page 40

For a sample RS-232 Port Setup, please see the Supplemental RS-232
Information Appendix at the end of this user’s guide.
Instrument software update
Your instrument software contained in two flash EPROMs on the main
processor board may be updated via the RS-232 port. Such software
updates are made available as new features and other improvements are
added during the life cycle of the instrument. For information on the
availability of software updates contact Advanced Instruments, Inc. or
an authorized distributor (see Chapter 4, page 29). For directions on
performing the RS-232 serial port software upgrade consult the documentation supplied with the upgrade package.
Advanced®Micro-Osmometer Model 3300 User’s Guide
22
Page 41

3
Calibration
This chapter describes the procedure for calibrating your
instrument. If you have questions or problems regarding the
calibration procedure, please consult Chapter 4 —
Troubleshooting & Service.
Calibration procedure
Calibration of the Model 3300 is a simple procedure that
requires no adjustment of the instrument by the user. Simply
run the menu-driven calibration program requiring the testing
of standards at each of two calibration points. If the repeatability is acceptable on these calibration points, the instrument
automatically performs internal calibration.
The calibration procedure is as follows. Note that maintaining
and loading accurate, uncontaminated standard samples is
extremely important.
1. At “Osmometer Ready,” press the left soft key, labeled
[CALIB] to initiate the calibration process. The calibration
program will prompt you to run a sample of the 50 mOsm
calibration standard.
2. Run the 50 mOsm calibration standard as you would an
actual sample (see Chapter 2 — Operation). When the
instrument completes each test and reports the results (not
necessarily the exact standard value), raise the operating cradle, remove the sampler, and clean the chamber as recommended in Chapter 2.
23
Page 42

3. The calibration program will prompt you for a second 50 mOsm calibration standard. Run the second 50 mOsm calibration standard.
When the test finishes, your instrument will display a result and
prompt you for an 850 mOsm calibration standard.
4. Run your first 850 mOsm calibration standard as described above.
The program will report a result and request another 850 mOsm calibration standard. Continue in this manner until the instrument display reads “Calibration complete”. The program can require anywhere between three and six 850 standard samples, depending on the
repeatability of the results.
5. Verify the calibration by running a Clinitrol 290 reference solution
before running unknown samples.
If the instrument display shows “Calibration Not Complete” after six
samples at either calibration level, the calibration procedure has failed
and you should carefully repeat the procedure. If you experience
“Calibration Not Complete” more than once, you have a repeatability
problem and should consult Chapter 4 for troubleshooting and service
information.
Calibration notes
• The Model 3300 will retain its previous calibration data until it completes a re-calibration and the display reads “Calibration
Complete”.
• If the instrument has calibration information in memory and you
have not changed any probes, the first test result displayed should be
close to the nominal value of the standard loaded. If the instrument
has no calibration information in memory or a probe has been
changed, the test result displayed may be far from the nominal value
of the standard loaded. If the displayed values repeat consistently,
the calibration will automatically adjust when the calibration test
sequence is complete.
Advanced®Micro-Osmometer Model 3300 User’s Guide
24
Page 43

• You may terminate the calibration procedure at any time by raising
the operating cradle and pressing [CANCEL]. The Model 3300 will
display “Test Canceled”, beep once, then display “Calib. Canceled”
and beep again. The existing calibration will not be affected.
• If you accidentally press a function soft key during a series of regu-
lar tests, the same procedure will terminate the calibrate mode without affecting the calibration and normal testing may be continued.
25
Calibration
Page 44

Advanced®Micro-Osmometer Model 3300 User’s Guide
26
Notes:
Page 45

4
Troubleshooting & Service
This chapter contains very basic information to help you solve
problems that might arise with your osmometer. Please read all
instructions very carefully, and if a solution cannot be found in
this guide, contact Advanced Instruments for Hot-Line Service.
Service & maintenance cautions
• Do not perform any service or maintenance yourself, except
as detailed in the User’s Guide.
• Unplug the power cord prior to opening or removing covers,
or else you may be exposed to electric shock, excessive temperatures, or mechanical hazards.
• Performing service or maintenance not detailed in the User’s
Guide, with or without a Service Manual, should only be
done by a qualified service technician.
• Never restrict airflow into or out of the product. Occasion-
ally, check the air vents for blockage.
• Wipe the exterior of the product with a soft, damp cloth as
needed. Using cleaning products other than those specified,
may discolor or damage the finish.
• If the product requires service for any of the following rea-
sons, unplug the product from the electrical outlet and refer
service to a qualified service technician.
- The power cord, extension cord, power strip, or power
input module is damaged.
27
Page 46

Advanced®Micro-Osmometer Model 3300 User’s Guide
- Liquid has been spilled into the interior of the product.
- A foreign object has fallen into the product.
- The product has been dropped or damaged by a falling object.
- There are noticeable signs of overheating or a burning odor.
- The product does not operate normally when you follow the oper-
ating procedures.
- The main supply fuse(s) or any internal fuse(s) continually fail.
• A discharge of static electricity from contact with the human body or
other conductor may damage system boards or static sensitive
devices. Never perform internal maintenance without following recommended static protection procedures.
• The product is equipped with operator accessible fuses. If a fuse
blows, it may be due to a power surge or failure of a component.
Replace the fuse only once. If the fuse blows a second time, it is
probably caused by failure of a component part. If this occurs, refer
service to qualified service personnel. Always replace the fuse with
one of the same rating, voltage, and type. Never replace the fuse
with one of a higher current rating.
• When servicing the product, use only factory-specified parts.
• WARNING: When returning this product for service, or shipping
this product to a second location, remove all hazardous
specimens and decontaminate the product before packaging for shipment. If the product cannot be decontaminated, consult with your shipping agent on appropriate packaging and marking.
28
Page 47

Obtaining service
Before contacting Advanced Instruments for Hot-Line®Service, be sure
to read through this user's guide for instructions on routine adjustments,
instrument care and troubleshooting. If this information does not solve
your problem, call the appropriate number below.
• 800-225-4034 (toll-free within the USA and Canada)
• +US 781-320-9000 (elsewhere)
• 781-320-0811 (fax)
If you purchased your instrument outside of the U.S. or Canada, please
contact your Advanced Instruments authorized dealer for service or
repair.
When contacting our service personnel, please have the model and serial numbers from the label on the back of your instrument, your user’s
guide or service manual, and the symptoms of your problem ready. You
should use a telephone as close to your instrument as possible to facilitate making recommended diagnostic checks. If you need to order parts
or service, a purchase order from your purchasing agent will be necessary.
After Hot-Line diagnosis, your service technician may assist you in
making minor repairs over the phone, dispatch a local service representative, or have you ship your instrument for factory repair.
If you need to return an instrument for repair or replacement:
• Notify our service department to obtain an RMA.
• Be sure to telephone Advanced Instruments before shipping to avoid
any delays.
• Carefully pack and send everything except your instrument’s supply
items.
• Be sure to prepay for any shipment to the factory. Advanced
Instruments cannot accept collect shipments without prior factory
approval. Please insure the shipment or accept the damage risk.
29
Troubleshooting & Service
Page 48

Troubleshooting checks
Check operational requirements. If you are experiencing difficulties
with your instrument, first carefully review the operational requirements
listed in the product specifications and the recommended setup and
operating procedures.
Check fuses. You will find the power switch and fuse holder beside
the power cord connector on the back panel of the instrument. Switch
the power switch to the off position and disconnect the power cord.
Use a small flat-bladed screwdriver or similar tool to pry out the fuse
holder. Visually check for a blown fuse. If there is any doubt, test the
fuses with a continuity checker or ohmmeter or simply replace them
with new fuses.
Check error messages. The software of your instrument is designed
in such a way that any incomplete task will be associated with an error
message, many self-explanatory, that will help you discover the source
of your problem. You can find all error messages and descriptions of
what they mean in Appendix B.
Internal diagnostics
The diagnostic menu allows any of a series of tests to be run to check
one functional subsystem of the instrument, or to perform some necessary adjustment or set-up.
At “Osmometer Ready”, the function keypads are respectively labeled
[CALIB] and [DIAG] with the supervisor/operator keyswitch in the
Supervisor position.
1. If necessary, press both soft keys simultaneously to display [DIAG].
2. When [DIAG] is displayed, press the function button under [DIAG].
When you press the [DIAG] soft key, “1. Recall Results” and the
choice of [START] or [INDEX] is displayed.
3. While any diagnostic menu item is being displayed, use either the
Advanced®Micro-Osmometer Model 3300 User’s Guide
30
Page 49

[START] soft key to start the displayed test; the [INDEX] soft key to
display the next menu item; or both soft keys simultaneously to exit
from the menu.
4. If you choose to press the [START] soft key, execution of the cho-
sen function may require the use of either of the soft keys for further
selections, as designated below. To end any function and return to
the menu, press both soft keys and release them simultaneously.
5. You may then use [INDEX] to index to another item on the menu or
press both soft keys and release them simultaneously to exit from the
diagnostic menu to “Osmometer Ready”.
Any instructions necessary for the menu function started are provided
on the display. Adescription of each menu item follows:
A/D Test
This set of tests may be used to test the block probe, the sample-cooling
assembly, or the sample probe.
On entry, the A/D tests display the target sample cooling block temperature, the channel being read, and the current channel reading or duty
cycle. The display is in the form, “off Blk NNNN.NN ohm”, where
“off” indicates that a target temperature has not yet been selected, “Blk”
indicates that the block probe channel is being tested, “NNNN.NN” is a
numeric readout of the probe and “ohms” indicates the units of the readout. These readings are updated continuously.
Pressing the left function soft key sequentially changes the target cooling block temperature from “off” to “+1”°C, to “0”°C, to “-1”°C, to
“-2”°C, to “-4”°C, to “-8”°C, to “-12”°C, to “off”, etc.
Pressing the right function soft key sequentially changes the channel
and readout units from probe resistance in ohms, to probe temperature
in tenths of a degree Celsius, to cooling-block duty cycle (“NN%” on)
to probe resistance in ohms, etc.
31
Troubleshooting & Service
Page 50

CAUTION The temperature values displayed are based on the block
probe resistance which the instrument is configured for.
Thus, if the block probe bin # has been incorrectly set,
both the displayed temperatures and resistances will be
incorrect, as well as the actual temperature of the cooling
block.
Press both soft keys and release them simultaneously to exit to the
menu.
Probe Bin Test
This test is used to determine the resistance of the sample probe at a
specific temperature. It is essentially the same as any other sample test
except that the sample probe resistance and correct bin # are determined
and displayed in place of the sample osmolality. Use the following procedure to run the test.
1. On entry, the probe bin test will display “Probe Bin Test [YES]
Ready? [CANCEL]”.
2. Press [YES] to start the test. When [YES] is pressed, the display
changes to: “Insert 50 mOsm Sample [CANCEL]”.
3. To continue, load a sample from a freshly-opened ampule of
Advanced 50-mOsm standard and run the test using the sample test
procedure detailed in Chapter 2.
4. “Cooling Sample” is displayed (as in a normal test), then the sample
probe resistance in ohms is dynamically displayed as the sample is
cooled. At the end of each test, the final display will be the test
result in ohms and the sample probe bin # (e.g., “5801.02 Ohms Bin
6”).
5. Record your results in the Service Log before running another test or
canceling to the diagnostic menu.
6. Raise the operating cradle to complete the test.
7. When the operating cradle is raised, “[YES] Ready? [CANCEL]”
Advanced®Micro-Osmometer Model 3300 User’s Guide
32
Page 51

provides the opportunity to either run another 50-mOsm probe test
or finish (cancel) the procedure. Run two more bin tests and average
the results of the three to determine your bin number.
8. Press both soft keys and release them simultaneously (or press the
[CANCEL] soft key) to exit to the menu.
Barcode Test
This test performs a continuous check of the barcode port.
1. Press the START key to enter the test.
2. At “[YES] Ready? [CANCEL]” you may press YES once the bar-
code scanner is connected, or press CANCEL to return to the main
menu.
After you press YES you may begin scanning barcodes. If your code
will not scan, check the manufacturers user’s guide to verify correct
setup of the scanner for your symbologies.
3. Press CANCEL or both keys to exit to the test menu.
Display/Print Test
This menu item is a simple check of the display, serial port and printer
(if connected). On entry, a single line of characters will be displayed
and sent to the serial and parallel ports. The characters displayed have
been chosen to exercise every dot in the character matrix. This makes it
possible to distinguish any dots that no longer work (on the printer, if
connected, as well as on the display).
Press both soft keys and release them simultaneously to exit to the
menu.
Beeper Test
This menu item exercises the beeper. On entry, this test displays “[ON]
Beeper [OFF]”. Press the [ON] soft key to cause the beeper to beep
repeatedly; press the [OFF] soft key to stop the beeper.
Press both soft keys and release them simultaneously to exit to the
menu.
33
Troubleshooting & Service
Page 52

Advanced®Micro-Osmometer Model 3300 User’s Guide
Solenoid Test
This test is used to exercise the freeze pulse solenoid.
On entry into this test, press the [IMPACT] soft key for a single impact
or the [BUZZ] soft key for a set of repeated impacts. If either or both
of these do not occur when the appropriate soft key has been pressed,
contact Advanced Instruments for Hot-Line Service.
Press both soft keys and release them simultaneously to exit to the
menu.
Key Test
This menu item allows individually testing the switches in the soft keys.
On entry, this test displays [PRESS] over each soft key. When each
soft key is pressed the beeper should sound and an asterisk should
appear above the pressed soft key to indicate proper operation.
Event Record
On entry, this menu item enables downloading the internally stored
event record to both the serial and parallel ports. When ready, press
[YES] to download.
Press [CANCEL] (or both soft keys and release them simultaneously)
to exit to the menu.
Toggle the [INDEX] soft key to select another menu item or press both
soft keys and release them simultaneously to exit from the diagnostic
menu. As necessary on exit from the diagnostic menu, the power-up
tests will automatically begin and should finish with the display,
“Osmometer Ready”.
Fuse replacement
If you determine that your instrument is not functioning because of
blown fuses, you will need to replace the fuses using the following procedure.
1. Switch the power switch to the off position and disconnect the power
cord.
34
Page 53

2. Use a small flat-bladed screwdriver or similar tool to pry open the
fuse holder door. Remove the fuse holder located inside.
3. Double-check the values marked on the fuses. The Model 3300 will
automatically adjust for voltages between 100VAC and 250VAC but
appropriately rated fuses must be installed. Use 5 × 20mm, 250V,
time delay (Type T) fuses. For 100-130V operation, use 1.25-Amp
fuses; for 200-250V operation use 1-Amp fuses.
4. Re-install the fuse holder into the back of the instrument and close
the fuse holder door.
5. Re-connect the power cord and switch the power switch to the on
position. The instrument should start up as normal.
Chamber cleaning
The cooling chamber and probe are easy to keep clean and dry by faithfully following the operating instructions for cleaning the freezing
chamber after each test. If traces of standards or biological samples are
left in the sample chamber, however, the task will be more difficult and
damp cleaning will probably be required. Two indicators that damp
cleaning may be required are:
• The instrument has been in use but no clean, dry chamber cleaner is
found in the sample port and successive results on aliquots of the
same sample indicate chamber contamination (the first aliquot read-
35
Troubleshooting & Service
Figure 5: Fuse Replacement
FUSE HOLDER
Page 54

Advanced®Micro-Osmometer Model 3300 User’s Guide
ing very high and subsequent readings progressively lower).
•“Sample Pre-freeze” errors begin to occur quite frequently.
When indicated, the cooling chamber may be damp cleaned as follows.
1. Dampen (do not saturate) the end of a chamber cleaner with 70% isopropanol solution.
2. Firmly insert the dampened chamber cleaner all the way into the sample port, rotate it four or five times (clockwise and counter-clockwise)
and withdraw.
3. Repeat with a dry chamber cleaner. Insert and leave a clean, dry
chamber cleaner in the sample port until the next sample is to be
tested.
Sampler plunger replacement and verification
To ensure proper instrument operation, you should replace the plunger
tip of the sampler every 500 tests (or every package of sample cells).
To replace the plunger tip, use the following procedure.
1. Unscrew the calibration gauge and key.
2. Rotate the sampler shaft until the calibration setscrew appears
beneath the access hole in the side of the sampler body.
3. Place the key end of the calibration gauge in the access hole and turn
counter-clockwise to loosen the setscrew.
4. Carefully remove the old sampler plunger wire.
5. Place a sample tip on the sampler to help you place new wire correctly.
6. Slip the sampler plunger wire into the sample cell so the Teflon
plunger tip protrudes about 1/16” or 1.6 mm from the end of the
sample cell.
7. Using the key end of the calibration gauge, push the plunger into the
36
Page 55

sampler as far as it will
go.
8. Tighten the calibration
setscrew with the calibration gauge.
9. Screw the calibration
gauge and key back into
the top of the sampler.
Your 20-µL sampler is now
calibrated and ready to use.
For verification that the wire
is calibrated correctly, use
the following procedure.
1. Place a new sample tip on
the sampler
2. Unscrew the calibration
gauge and key.
3. Insert the key end of the
calibration gauge into the
sample tip.
4. Visually inspect the position of the end of the
sampler plunger tip and
the end of the calibration
key. There should be no
gap between the two.
5. If necessary, reset the
sampler plunger wire as described above.
37
Figure 6: Sample Plunger Replacement
and Calibration
Troubleshooting & Service
Page 56

Advanced®Micro-Osmometer Model 3300 User’s Guide
38
Notes:
Page 57

39
Troubleshooting Table
Appendix A
Abrupt loss of power
No response when
sampler is lowered
into operating position
Results not repeatable
(too scattered)
“Block Probe Failure”
If you lose power to your instrument, we
recommend that you check that your outlet
is providing the correct amount of power.
Check that your cord is firmly plugged into
both the instrument and the outlet. Visually
inspect the fuses, and change as necessary.
If there is no response when you lower the
operating cradle into operating position,
there could be a problem with the internal
switch that initiates the test. Try turning the
instrument on and then off. If the problem
persists, please consult an Advanced
Instruments Model 3300 Service Manual or
contact Advanced Instruments for Hot-Line
Service.
Most often, poor repeatability comes as a
result of poor technique or not following
procedure. Be sure all sample tips are clean,
as well as the sampler itself. If the sampler
plunger wire has not been changed in 500
tests, change it as described in Chapter 4. If
the sampler is not properly calibrated, recalibrate it using the procedure described in
Chapter 4.
This message indicates a problem with your
block probe. Contact Advanced Instruments
for Hot-Line Service.
Problem/Message
Explanation
Page 58

Advanced®Micro-Osmometer Model 3300 User’s Guide
40
“Block Probe Open?”
“Calibration out of
Range; Repeat Calib”
“Cooling System
Error”
“Event Record Lost”
“Fan Driver Failure”
“Key Input Timeout”
“Lift Operating
Cradle”
This message indicates a problem with your
block probe. Check the block probe bin
number and change if necessary. If this does
not solve your problem, contact Advanced
Instruments for Hot-Line Service.
This message indicates that your calibration
could not be accepted because the results
were not consistent enough. Retry the calibration, paying particular attention to technique.
This message indicates that your cooling
system is not functioning properly. Contact
Advanced Instruments for Hot-Line Service.
This message indicates that the event record
stored in memory has been corrupted. Try
restarting your instrument. If this does not
solve your problem, Contact Advanced
Instruments to obtain Hot-Line Service.
Try restarting your instrument. If this does
not solve your problem, contact Advanced
Instruments for Hot-Line Service.
This message is caused either by a button
being pressed continuously or a keypad
malfunction. Restart your instrument, and if
the problem persists, contact Advanced
Instruments for Hot-Line Service.
Raise the operating cradle to a positive stop.
Restart your instrument if necessary. If this
does not solve your problem, contact
Advanced Instruments for Hot-Line Service.
Page 59

41
Troubleshooting Table
“Lift Operating
Cradle”
“Clean Sample
Chamber”
“No Plateau
Detected...”
“Parameter RAM
Failed or New
Software Version”
“Recalibration
Needed”
“Reset Probe Config.”
“Result = 0, Bad
Calibration?”
This message, along with audible beeps, indicates the test sample was left in the sample
chamber for an extended period. Lift cradle,
remove sample, and clean chamber. The final
result will appear only for 10 seconds once
you lift the cradle.
Your instrument was unable to detect a freezing plateau, and was therefore unable to give
a result. Check your technique and try again.
If the message persists, obtain Hot-Line
Service.
This informative message tells you that a new
software version has been installed since you
last powered the instrument or that the information stored in parameter RAM has been
corrupted. The instrument will reload factory
defaults..
This message indicates that you need to recalibrate your instrument. Re-calibrate closely
following the instructions in Chapter 3. If the
error message repeats after successful re-calibration, obtain Hot-Line Service.
Most likely, this message indicates that you
should reset your block and sample probe bin
numbers as described in Chapter 4. If this does
not solve the problem, contact Advanced
Instruments for Hot-Line Service.
Unless you are running a sample of distilled
water or some other fluid with an osmolality of
zero, you should not see this message. If you
were expecting a sample to test above zero,
then you may need to re-calibrate your instrument.
Page 60

Advanced®Micro-Osmometer Model 3300 User’s Guide
42
“ROM Serial Number
Error”
“Sample Freeze
Error...”
“Sample Pre-freeze...”
“Sample Probe
Failure”
“Sample Probe Open?”
Try restarting your instrument. If this does
not solve your problem, contact Advanced
Instruments for Hot-Line Service.
This message can be displayed for a number
of reasons. Check your technique and the
condition of your sampler. If neither of these
is the source of the problem, you should
check your sample and block probe numbers.
If this does not solve your problem, contact
Advanced Instruments for Hot-Line Service.
A sample pre freeze message usually
appears as a result of an unclean freezing
chamber. Clean your chamber as described
in Chapter 4, and be sure that the chamber
is cleaned after each test. If this does not
solve your problem, you should check the
probe bin number and obtain service if necessary .
Switch the instrument off, then on. Check
the sample probe by running the A/D tests.
If the error message does not persist and
other error messages are not displayed,
ignore this message. Otherwise, contact
Advanced Instruments for Hot-Line Service.
Switch the instrument off, then on. Check
the sample probe by running the A/D tests.
If the error message does not persist and
other error messages are not displayed,
ignore this message. Otherwise, contact
Advanced Instruments for Hot-Line Service.
Page 61

43
Troubleshooting Table
“Set Probe Bin #'s”
“Sample Did Not
Freeze...”
“Solenoid Driver
Failure”
“Standards Reversed?
Please Repeat...”
“System Error: Reqs”
“System Error: Prim”
“System Error: Comm”
“System Error: Unkn”
“System Error: Trap”
Your instrument will not function unless
there are bin numbers entered for both the
block and sample probes. To set these numbers, see instructions in Chapter 4.
If you heard an impact while the sample
was attempting to run, the fault most likely
lies with the sample preparation or the
block probe. Run another test, paying close
attention to technique. If your problem is
not resolved, check and correct if necessary
the block and probe bin numbers.If you
heard no impact, the problem most likely
lies with the solenoid impactor. Retry the
test, and, if necessary, contact Advanced
Instruments for Hot-Line Service.
This message indicates a problem with your
solenoid impactor. Try restarting your
instrument. If this does not solve your problem, contact Advanced Instruments for HotLine Service.
This message will appear during the calibration procedure if the instrument detects
that the low and high calibration standards
may have been mixed up and were entered
in the wrong order. Retry the calibration,
being sure that the standards are correct.
Each of these error messages indicate a different system error. Try restarting your instrument. If this does not solve your problem, contact Advanced Instruments for Hot-Line
Service.
Page 62

Advanced®Micro-Osmometer Model 3300 User’s Guide
44
“T E Driver Failure”
“Test Time-out Error”
This error message indicates a problem with
your thermoelectrics. Try restarting your
instrument. If this does not solve your problem, contact Advanced Instruments for HotLine Service.
This message indicates that your instrument
was unable to complete the test in the allotted time. Be sure that your operator technique is sound and retry the test. If the
problem persists, check your block probe
number. If you need more assistance call for
Hot-Line Service.
Page 63

45
Product Specifications
Appendix B
Electrical
Power Requirement: 100 to 130 VAC (50-60 Hz) or 200 to 250 VAC (50-60 Hz)
Fuses (2): 250V time delay (Type T): 1.25-Amp (100-130 VAC);
1-Amp (200-250 VAC)
Power Consumption: 60 Watts
Memory Backup: Integral lithium cell; 10-year life (typical);
(not user-replaceable)
Sample Volume: 20 µL
Sample Capacity: Single sample
Readout: 2 line by 24-character vacuum fluorescent display
Units: mOsm/kg H
2
O
Range: 0 to 2000 mOsm/kg H
2
O
Resolution: 1 mOsm/kg H2O
Communications: DTE EIA-232/V.24 (RS-232); serial port, parallel printer
port and optional barcode scanner
Performance at
Reference Conditions
1
Linearity: Less than ±1% from a straight line between 0 and 850
mOsm/kg H2O; less than ±1.5% above 850 mOsm/kg H2O
Repeatability
0 to 400
mOsm: ±2 mOsm/kg (1 S.D.)
400 to 2000
mOsm: ±0.5% (1 S.D.)
Drift: Less than 1 mOsm/kg H2O per month
Performance Over
Operating Conditions
Temperature Less than 1 mOsm/kg H2O for every 5°C (9°F) ambient
Effects: temperature change
1
Reference Conditions: 20°C to 25°C (68°F to 77°F); 40% to 60% Relative Humidity; tolerances of reference
or calibration solutions excluded.
Page 64

Advanced®Micro-Osmometer Model 3300 User’s Guide
46
Operating Conditions
Temperature: 18°C to 35°C (64°F to 95°F)
Humidity: 5 to 80% relative humidity (non-condensing)
Storage Temp.: -40°C to +45°C (-40°F to +113°F)
Start-up Time: 30 seconds from power-on
Test Time: 60 seconds
Dimensions inches centimeters
Width: 10.5 26.7
Depth: 13.0 33.0
Height: 15.5 39.4
Weight pounds kilograms
Net: 16.0 7.3
Shipping: 25.0 11.4
Warranty: One-year limited warranty on workmanship and all parts
except glass, plastic, and parts warranted by their makers
Certification:
Refer to Regulatory Notices (Appendix C) for applicable
standards
Installation Class: I
Over-Voltage
Category: II
Pollution Degree: 2
Moisture Protection: IPX0 (ordinary)
Page 65

47
• This product has been designed and manufactured in accordance
with U.S., Canadian, and European regulatory requirements as outlined below. Modifications made to this product that are not
expressly approved in writing by the manufacturer will void the
user’s authority to operate this product, previously issued factory
approvals, and the user’s rights under the warranty.
• The distributor or dealer may have applied additional local, national,
or international approvals to this product. Consult the distributor or
dealer for more information and documentation.
• Connections to this product must be made with shielded cables. Use
of non-shielded cables may violate RFI/EMI limits.
Symbol Conventions
This symbol indicates conformity to relevant European
directives.
This symbol indicates the product was tested to conform to
relevant Canadian and U.S. safety standards by Intertek
Testing Services NA, Inc. The ETL mark is approved in the
United States as a Nationally Recognized Testing Lab
(NRTL) by OSHA, and in Canada by the Standards Council
of Canada.
In Vitro Diagnostic Medical Device complying with EU
Directive 98/79/EC.
Regulatory Notices
Appendix C
Page 66

Advanced®Micro-Osmometer Model 3300 User’s Guide
48
Regulatory
approval type Description
U.S. Safety This product has been listed by ETL testing laborato-
ries as being in compliance with the requirements of
UL 3101-1, 1st Edition, "Electrical Equipment for
Laboratory Use; Part 1: General Requirements". The
"US" in the lower right of the ETL mark demonstrates
this listing.
Canadian Safety This product has been listed by ETL testing laborato-
ries as being in compliance with the requirements of
CAN/CSA C22.2 No.1010.1-92, "Safety Requirements
for Electrical Equipment for Measurement, Control
and Laboratory Use; General Requirements"; Including
Amendment Two. The "C" in the lower left of the ETL
mark demonstrates this listing.
EC Declaration of This product meets the intent of Directive 89/336/EEC
Conformity - EMC for Electromagnetic Compatibility. Compliance was
demonstrated using the following standards, as listed in
the Official Journal of the European Communities:
Consult the Declaration of Conformance certificate
shipped with the product for the latest update.
· EN 61326: 1998, Group 1, Class B, "Electrical
Equipment for Measurement, Control, and
Laboratory Use".
EC Declaration of: This product meets the intent of Directive 73/23/EEC,
Conformity - Low the Low Voltage Directive. Compliance was demonVoltage strated using the following standards as listed in the
Official Journal of the European Communities: Consult
the Declaration of Conformance certificate shipped
with the product (if required) for the latest update.
· EN 61010-1 (1993), "Safety Requirements for
Electrical Equipment for Measurement,Control and
Laboratory Use - General Requirements"; Including
Amendment Two (1995).
Page 67

49
Regulatory Notices
Regulatory
approval type Description
EC Declaration of This product meets the intent of Directive 98/79/EC
Conformity - IVD for In Vitro Diagnostic Medical Devices. Consult the
Declaration of Conformance certificate shipped with
the product (if required) for the latest update.
Page 68

50
Advanced®Micro-Osmometer Model 3300 User’s Guide
Notes:
Page 69

51
Warranty & Warranty Duties
Appendix D
By accepting and operating this instrument, the user and Advanced Instruments
agree to the following responsibilities
which constitute contractual warranties
and conditions between the seller and the
user for the maximum benefit and usefulness of the instrument.
ADVANCED INSTRUMENTS
WARRANTS THAT IT:
1. Has produced equipment equal to or
exceeding that of any competitive
product in the same price range in
standards of design, material and
workmanship.
Page 70

Advanced®Micro-Osmometer Model 3300 User’s Guide
52
2. Knows of no defects in design or materials which may cause bodily injury.
3. Will endeavor to advise the user of
changes or improvements in the instrument as they are developed, so that the
user may take steps to improve the
safety and performance of his equipment throughout its useful life.
4. Will replace or repair equipment
according to the guarantee on the
attached warranty.
5. Will cooperate closely in common
defense of any accident involving this
equipment, or third-party suit against
the user or operating personnel, if
advised immediately by the user of the
occurrence of any accident.
THE USER WARRANTS THAT:
1. The instrument will be used reasonably.
2. The instrument will be regularly maintained according to this manual,
including a log of all service, tests and
repairs performed on the equipment,
and records will be kept of all requests
for repair made to Advanced
Instruments where such repairs were
beyond the ability of local service personnel.
3. The instrument will not be altered
without written approval from
Advanced Instruments.
4. Advanced Instruments will be notified
immediately if any injury occurs in any
association with the instrument and will
be allowed prompt and thorough exami-
nation of the instrument in question.
5. Advanced Instruments will be held
harmless in cases of injury arising (see
definitions below):
a. Beyond the useful life of the
equipment.
b. From unreasonable use.
c. When Advanced Instruments is not
immediately notified of said
injury.
d. From interpretation of results.
DEFINITIONS
1. "Useful life" is:
a. The same as the depreciation life in
the Internal Revenue Service guide-
lines, whether or not the user actual-
ly depreciates the instrument, but not
to exceed 10 years from date of
delivery to the user.
b. Only during the time the equipment
has been maintained on a regular
basis as prescribed by Advanced
Instruments. If the user is in an
area which has no local service,
Advanced Instruments may require
a local service person (understood
to mean the person actually per-
forming the "hands-on" service of
the equipment) to attend and pass a
reasonable maintenance and repair
course.
c. Only during the time when the user
has not altered the equipment in
any way without written approval
from Advanced Instruments.
Page 71

53
Warranty & Warranty Duties
d. Only during the time when the user
has not loaned, leased or resold the
equipment to any third party.
2. "Reasonable use" is use:
a. According to the instructions sup-
plied by Advanced Instruments
(assuming English-reading personnel or supervision). If neither the
supervisor nor the operator reads
English, the user agrees to obtain
accurate translations of the instrument labels, instructions, user's
guides and/or manuals provided.
b. Under direct, on-the-job supervi-
sion of the supervisor or other professional in charge.
c. In which there are no known
defects or uncorrected repairs.
d. Only for the purpose stated in the
instructions provided with the
instrument.
e. In which the equipment has been
maintained according to the
instructions provided.
3. "Immediate notification" is:
a. Recognition that time is of the
essence when any accident, malpractice or product liability arises
which involves Advanced
Instruments equipment.
b. Notification to Advanced
Instruments immediately (the same
day, if possible) in the event of
injury to any person in circumstances involving Advanced
Instruments equipment in which
Advanced Instruments might be
named as a defendant in any form
of litigation.
c. Allowing Advanced Instruments
or its representatives, immediate,
full, and thorough examination of
Advanced Instruments equipment,
and all records pertaining to such
equipment.
Page 72

Advanced®Micro-Osmometer Model 3300 User’s Guide
54
Notes:
Page 73

The DB-9 RS-232 port on your instrument conforms to the DTE EIA232 standard and can reliably communicate over shielded cable up to 10
meters in length, depending on the baud rate you use. Almost every
item of information displayed by your instrument is also transmitted
over the RS-232 port, including test results, all error messages, and
most display data from the diagnostic menu.
Data is transmitted asynchronously as 1 start bit, 8 data bits and 1 stop
bit, with no parity. Each message transmitted from the communication
port is terminated by the sequence, Carriage Return (0D Hex), Line
Feed (0A Hex). Note that your instrument is only capable of outputting
information. At this time, there is no protocol for talking to the instrument.
Sample RS-232 Setup
As a typical example of a communications program setup, the following
instructions will describe the process necessary for using your instru-
ment in conjunction with Hyperterminal for Windows
®
95 or later operating systems and a null modem cable, available from Advanced
Instruments, as shown in the diagram below. You can use this procedure
to be sure that your instrument and cable are operating correctly.
1. Create a Hyperterminal connection using the Hyperterminal pro-
gram.
2. While working within that connection, choose the Properties selec-
tion from the File menu.
55
Supplemental RS-232
Information
Appendix E
Page 74

3. Select the Configure button.
4. Choose the following settings for the Configure menu and click on
the OK button.
Bits per second See user’s guide.
Data bits 8
Parity None
Stop bits 1
Flow control None
5. Select the Settings menu.
6. Chose TTY for the Emulation option.
7. Select ASCII setup.
8. Set the Sending option to Echo Typed Characters Locally.
9. Set the Receiving option to Wrap Lines that Exceed Terminal Width.
You should at this point see all instrument output in the Hyperterminal
window of your computer screen.
Advanced®Micro-Osmometer Model 3300 User’s Guide
56
Null Modem Cable
Shield connects to
metalized connector
housing at both ends.
Page 75

57
Symbol Definitions
Appendix F
On-Off
Feed
Interrupt
Test
Start
Stop
Record Review
Setup
Calibration
Cancel; Delete
Functional Arrow
Printer
Enter
RS232
Bar Code
Attention
Caution Hot Surface
Dangerous Voltage
Lifting Hazard
Calibrator
Page 76

58
Advanced®Micro-Osmometer Model 3300 User’s Guide
Content
Control
Negative Control
Positive Control
Flammable
Fragile
Irritant
Keep Dry
Date Manufactured
Sterile
Non-Sterile
Serial Number
Solution
Do Not Open Top
Handle With Care
Toxic
Use Blade To Open
Do Not Re-Use
For In Vitro Diagnostic
Use
European Conformity
Temperature Limit
See Instructions
Lot Number
Use By; Expiration
Date
Authorized
Representative
Part Number
Page 77

59
Symbol Definitions
Sufficient for [x]
Tests
Open Here
Low Fluid Level
Keep Hands Clear
Manufacturer
Latex-Free
Diluent
See Instructions for
Temperature Guidelines
Potential Puncture
Hazard
x
Page 78

60
Advanced®Micro-Osmometer Model 3300 User’s Guide
Notes:
Page 79

Model: 3300
Serial Number:
Software Revision:
Sample Probe Number:
Block Probe Number:
61
Service Log
Appendix G
Date
Problem/Symptom
Action
Page 80

Advanced®Micro-Osmometer Model 3300 User’s Guide
62
Date Problem/Symptom
Action
Page 81

Index
63
B
barcode port 11
barcode scanner 11, 20
barcode test 33
beeper test 33
C
calibration 23, 36
calibration gauge 36
calibration setscrew 37
calibration standard 23
chamber cleaning 35
Clinitrol 290 Reference
Solution 12
concentrative properties 35
controls 12
crystallization
temperature xviii
D
data capture 19
date 17
diagnostic test menu 15
diagnostics 30
display/print test 33
E
error messages 30, 39
errors 14
event record 34
F
freezing point xiv, xv
freezing point depression xiv
freezing-point plateau xviii
fuse replacement 34
fuses 10, 30
H
heat of fusion xv, xviii
Hyperterminal 55
I
installation 1
K
key test 34
L
language 19
linearity set 13
Page 82

M
melting point xviii
molality xvii
molarity xvii
multifunction soft keys 9
O
operating cradle 9
operational requirements 30
osmolality xv, xvii
osmolarity xvii
osmometry xiv
P
pipette, See sampler
plunger tip 37
power switch 10
printer v, 21
printer port 10
probe bin test 32
Protinol 12
R
recall results 15
repeatability 15
RS-232 port 10, 21, 55
S
sample freeze error 42
sample plunger 36
sample pre-freeze 42
sample preparation 12
sample probe 9
sample test procedure 13
sampler 9
select language 19
service 27
service log 61
set date/time 17
set probe bin #'s 18
settings 17
solenoid test 34
specifications 39
standards 12
statistics 16
supercooling xv, xviii
supervisor/operator
keyswitch 11
T
test time-out error 44
time (setting) 17
troubleshooting 27, 39
U
unpacking 1
W
warranty 51
64
Advanced®Micro-Osmometer Model 3300 User’s Guide
 Loading...
Loading...