Page 1

!!
!
!
!
!
!
!
!
!
ADC® ADView® 2 Modular Diagnostic
Station
User Manual
2
2
TM
Page 2

93-9005-00 ADView 2 User Manual | 2
September 1, 2016
!
! !
Page 3

Changes
This manual is identified as item number: 93-9005-00. The most recent version is available for download from the
ADC website. Should you notice errors or omissions in this manual, please notify us at:
American Diagnostic Corporation
55 Commerce Drive
Hauppauge, NY 11788
Tel: 1.800.ADC.2670
1.631.273.9600
Fax: 1.631.273.9659
Email: customerservice@adctoday.com
Web: www.adctoday.com
Copyright Information
All content in this manual is the property of American Diagnostic Corporation and is provided solely for purposes of
operation, maintenance or service of the ADView 2. This manual and the ADView 2 described herein are protected
under copyright law under which states they may not be copied, in whole or in part, without written consent of ADC.
The information in this manual is furnished for guidance only, is subject to change without notice, and should not be
construed as a commitment by ADC. ADC assumes no liability for errors or inaccuracies that may appear in this
manual.
© 2016 American Diagnostic Corporation. All rights reserved. ADC, the ADC logo, and ADView are registered
trademarks of American Diagnostic Corporation. All other trademarks are the property of their respective owners.
Page 4

Welcome to the ADC® ADView® 2
Thank you for choosing the ADC® ADView® 2 Modular Diagnostic Station for accurate spot-checking of vital signs.
The ADView 2 is designed to be simple and efficient to use, and features:
• A contemporary, compact design
• An integrated handle for easy mobility
• Automatic BP checks
• Averaging of multiple BP readings
• Optional use of stethoscope for traditional auscultatory BP measurement
• A robust memory
• Connection options for EMR system integration
• Multiple options for pulse oximetry and temperature measurement
ADView 2 Description and Operation
The ADView 2 can perform automatic blood pressure, pulse oximetry and body temperature measurements for
clinical professionals. For measuring blood pressure, a blood pressure cuff is placed around the patient’s nondominant upper arm. The cuff is inflated automatically and blood pressure is determined by the oscillometric
method—which senses pressure waves in the artery when occluded by pressure in the cuff. Measurement of the
frequency of the pressure waves enables heart rate to also be measured. The pulse oximetry function non-invasively
measures the patient’s percent oxygen saturation of arterial hemoglobin using principles of plethysmography via an
SpO2 sensor placed on the patient’s finger. Temperature can be measured using an oral/axillary or a rectal
temperature probe containing a thermistor that generates a voltage based on changes in temperature. These
voltages are recorded by the temperature circuitry. The ADView 2 is a portable device, approximately 205 x 190 x 140
mm in size and weighs approximately 1440 g without battery. Control buttons allow the user to stop/start a BP
measurement, save a set of measurements to memory, change between BP modes, and return to the home screen.
There is also a selection knob that is used to scroll and select different device options. The backlit LCD display shows
the user device status and measurement information. The device uses a microprocessor with software, which is not
accessible to the user. The unit is powered by a single rechargeable lithium-ion battery at the rear of the device. Two
USB-A port connections can be used to connect, an optional printer, barcode scanner or Wi-Fi dongle. There is also
RJ11 Ethernet port for network connectivity, and a mini-USB port used to connect the device to a PC or laptop for
advanced device configuration.
Note: For purposes of this manual, the ADView 2 (Model 9005) may be referred to as “the ADView 2, or “the device.”
Page 5

93-9005-00 ADView 2 User Manual | 5
September 1, 2016
Table of Contents!
Changes!...........................................................................!3!
Copyright!Information!....................................................!3!
Welcome!to!the!ADC®!ADView®!2!.................................!4!
ADView!2!Description!and!Operation!.............................!4!
1.! Safety!Considerations!........................!6!
Intended!Use!...................................................................!6!
Indications!for!Use!..........................................................!6!
User!Responsibility!.........................................................!6!
Possible!Adverse!Reactions!.............................................!7!
Icons,!Symbols!and!Abbreviations !..................................!9!
Commonly!Used!Abbreviations!....................................!10!
2.! Setting!Up!the!ADView!2!..................!10!
Unpacking!the!Monitor!.................................................!10!
Rear!Panel!Configuration!..............................................!11!
Side/Temperature!Panel!Configuration!........................!12!
Side/SpO2!Panel!Configuration!.....................................!12!
Install!Batteries!.............................................................!13!
Battery!Disposal!............................................................!13!
Attach!Connections!to!the!Device!................................!14!
Mounting!Options!.........................................................!14!
System!Configuration!....................................................!14!
3.!Getting!to!Know!the!ADView!2!............!15!
Measurement!Display!...................................................!15!
Control!Panel!................................................................!18!
Monitor!Setup!...............................................................!18!
Visual!Alarms!................................................................!18!
4.!Good!to!Know!Before!You!Begin!.........!19!
Power!Modes!................................................................!19!
Documenting!Measurements!.......................................!19!
Printing!..........................................................................!19!
Saving!Measurements!...................................................!20!
5.!!Using!ADView!2!for!BP!Measurement!21!
Step!1:!Preparing!the!Patient!........................................!21!
Step!2:!Select!Between!Adult!Vs!Pediatric!Mode!.........!21!
Step!3:!Select!Measurement!Mode!..............................!22!
Step!4:!How!to!Measure!BP!in!Each!Mode!...................!24!
Step!5:!Record!Results!..................................................!25!
Step!6:!Prepare!for!New!Patient!...................................!25!
6.!Using!ADView!2!for!Heart!Rate!
Measurement!.........................................!25!
BP!Heart!Rate!Measurement!........................................!25!
SpO2!Heart!Rate!Measurement!....................................!25!
7.!Using!ADView!2!for!Pulse!Oximetry!....!26!
General!Principle!of!Operation:!....................................!26!
System!Description:!......................................................!28!
Taking!SpO2!Measurements:!.........................................!28!
Special!Notes!for!Masimo!SET!SpO2!Module:!...............!28!
Special!Notes!for!ChipOx!SpO2!module:!........................!30!
8.!Using!ADView!2!for!Temperature!
Measurement!........................................!32!
Covidien!Filac!3000!Thermometry!Module!...................!32!
Oral!Temperature!Taking!..............................................!32!
Axillary!Temperature!Taking!.........................................!33!
Rectal!Temperature!Taking!...........................................!33!
Other!Temperature!Settings!.........................................!33!
9.!Using!ADView!2!EMR!and!Memory!
Functions!...............................................!34!
Memory!Mode!..............................................................!34!
EMR!Transmissions!.......................................................!35!
10.!Taking!Care!of!Your!ADView!2!..........!36!
Cleaning!........................................................................!36!
Preventative!Maintenance!............................................!37!
Replacing!and!Disposal!of!the!Battery!..........................!37!
Battery!Disposal!............................................................!37!
Product!Disposal!...........................................................!37!
Cuff!Disposal!.................................................................!37!
Routine!Calibration!.......................................................!37!
11.!Accessories!&!Replacement!Parts!.....!38!
Complete!Modules!(when!purchased!separately)!........!38!
Mounting!Platforms!......................................................!38!
Blood!Pressure!Cuffs!.....................................................!38!
Power!Supplies!..............................................................!39!
Temperature!Accessories!..............................................!39!
SpO2!Accessories!..........................................................!39!
Other!Accessories!.........................................................!39!
12.!Status!Messages!&!Alarms!................!40!
Status!Messages!............................................................!40!
Out!of!Range!Measurements!........................................!42!
Service!Centers!.............................................................!43!
13.!Frequently!Asked!Questions!.............!43!
14.!Technical!Information!......................!44!
EMC!Statement!.............................................................!44!
Specifications,!General!..................................................!48!
Specifications,!Blood!Pressure!Measurement!..............!48!
Notes!on!Blood!Pressure!Data!......................................!49!
Specifications!SP02!Sensor!............................................!49!
Specifications!Temperature!Sensor!..............................!49!
Limited!Warranty!..........................................................!50!
!
Page 6

93-9005-00 ADView 2 User Manual | 6
September 1, 2016
1. Safety Considerations
Intended Use
The ADView 2 is a clinical-grade, automated blood pressure measurement device with optional temperature
and pulse oximetry modules for spot-check vital sign measurements in physician offices, long-term care
facilities, and low-acuity areas in hospitals. The ADView 2 can be used in combination with a clinical IT
network to transfer and store patient measurement data on an EMR system. !
Indications for Use
The ADView 2 is a non-invasive oscillometric spot-check vital signs device. The ADView 2 is capable of measuring
and displaying brachial systolic and diastolic blood pressure, heart rate, percent oxygenated hemoglobin (SpO2) and
body temperature on children 3 years of age to adults. This device is intended for use by a qualified clinician when it
is necessary to take a single or a series of vital signs measurements on a patient. The ADView 2 is only for
measurement, recording and display. It makes no specific diagnoses.
The ADView 2 is intended to be used on adult and pediatric patients using appropriately sized ADC ADView 2 blood
pressure cuffs.
User Responsibility
Your ADView 2 is designed to perform in conformity with the description thereof contained in this operation manual
and accompanying labels and inserts, when assembled, operated, maintained and repaired in accordance with the
instructions provided.
Further, the user of this device bears sole responsibility for any malfunction that results from improper use, faulty
maintenance, improper repair, damage or alteration by anyone other than ADC or authorized service personnel.
Use of ADView 2
Use only ADView 2 blood pressure cuffs supplied by ADC.
Observe the patient carefully during the measurement. Ensure pressure compatibility to all patients. If any
abnormality occurs, either in the unit or the patient, suspend the operation immediately and disconnect the BP cuff,
SpO2 sensor and thermometer (if applicable) from the patient.
Accuracy of any BP reading or oxygen saturation measurement may be affected by the position of the patient, their
physical condition and use outside of the operating instructions detailed in this guide. The interpretation of BP and
oxygen saturation measurements should only be made by a physician.
Safety and effectiveness when used with pregnant women, children under 3 years of age and neonates have not been
established.
Pulse Oximetry (SpO2)
ChipOx SpO2 Module: Use only pulse oximeter sensors supplied by ADC or original Nellcor
TM
pulse oximeter sensors
supplied by Covidien® (except for forehead reflectance sensors).
Masimo® SpO2 Module: Use only original Masimo pulse oximeter sensors and cables.
Check the application site of the SpO2 sensor frequently to confirm proper positioning of the sensor and to check the
circulation and skin sensitivity of the patient.
Page 7

93-9005-00 ADView 2 User Manual | 7
September 1, 2016
Wireless Networking
When connecting this device to a wireless network, it is important to use only the hardware specified by ADC (see
Accessories section for details). Unsupported USB accessories, including unsupported wireless adapters, have been
intentionally disabled and will not function with this device.
Possible Adverse Reactions
In the area of the BP cuff or SpO2 sensor, allergic exanthema (symptomatic eruption) may result, including the
formation of urticaria (allergic reaction including raised edematous patches of skin or mucous membrane and
intense itching) caused by the fabric material of the cuff or sensor.
Following the application of the BP cuff, petechia formation (a minute reddish or purplish spot containing blood that
appears in the skin) or Rumpel-Leede phenomenon (multiple petechia) may appear on the arm, which may lead to
idiopathic-thrombocytopenia (spontaneous persistent decrease in the number of platelets, associated with
hemorrhagic conditions) or phlebitis (inflammation of a vein).
!Warnings and Cautions
!
WARNING: Do not attach the cuff to a limb being used for IV infusions or any other intravascular access, therapy or
an arteriovenous (A-V) shunt. The cuff inflation can temporarily block blood flow, potentially causing harm to the
patient.
WARNING: Pressurization of the cuff can temporarily cause loss of functionality of SpO2 if simultaneously using
device on the same limb.
WARNING: Not designed for neonates.
WARNING: Do not apply the BP cuff to a limb being used for IV infusions as the cuff inflation can temporarily block
the infusion, causing harm to the patient.
WARNING: Check frequently by observing the limb that operation of the AUTOMATED SPHYGMOMANOMETER does
not result in prolonged impairment of the circulation of the patient.
WARNING: The cuff should not be applied over a wound as this can cause further injury.
WARNING: The cuff should not be placed on the arm on the side of a mastectomy. In the case of a double
mastectomy use the side of the least dominant arm.
WARNING: The ADView 2 is NOT defibrillator protected.
WARNING: Do not use in the presence of flammable anesthetics or other flammable substances in combination with
air, oxygen-enriched environments, or nitrous oxide.
WARNING: Do not use the device if it has failed its diagnostics self-test, or if it displays a greater than zero pressure
with no BP cuff attached or a value of oxygen saturation with no SpO2 sensor attached.
WARNING: Do not use if device is dropped and/or damaged. Have a qualified service representative check the unit
before using again.
WARNING: Do not remove unit covers. Doing so may cause electrical shock to the user. The device does not contain
any user serviceable components.
WARNING: Do not immerse the device in any fluid, place fluids on top, or attempt to clean the unit with any liquid
detergents, cleaning agents, or solvents. This may cause an electrical hazard. Refer to the cleaning section of this
guide for instructions on cleaning. If any of these situations apply, please contact ADC.
WARNING: Remove power before servicing device. Failure to remove power could cause electrical shock or death.
WARNING: A pulse oximeter should NOT be used as an apnea monitor.
Page 8

93-9005-00 ADView 2 User Manual | 8
September 1, 2016
WARNING: Pulse rate measurement is based on the optical detection of a peripheral flow pulse and therefore may
not detect certain arrhythmias. The pulse oximeter should not be used as a replacement or substitute for ECG-based
arrhythmia analysis.
WARNING: Do not use the device or any of its accessories during magnetic resonance imaging (MRI) scanning.
Induced current could potentially cause burns.
WARNING: Tissue damage can be caused by incorrect application or use of an SpO2 sensor, for example by wrapping
the sensor too tightly. Inspect the sensor site as directed in the sensor Directions for Use to ensure skin integrity and
correct positioning and adhesion of the sensor.
WARNING: Do not use high frequency surgical equipment with the ADView 2 as this may cause loss of stored data
WARNING: No modification of this equipment is allowed.
WARNING: Federal (U.S.) law restricts this device to sale by or on the order of a physician.
CAUTION: A compressed or kinked connection hose may cause continuous cuff pressure resulting in blood flow
interference and potentially harmful injury to the patient.
CAUTION: Check calibration of this device annually.
CAUTION: Calibration should be done by a biomedical technician or other authorized personnel.
CAUTION: Never knowingly use a defective device.
CAUTION: Immediately replace parts that are broken, worn, missing, incomplete, damaged or contaminated.
CAUTION: Contact the nearest ADC-approved service center should repair or replacement become necessary. A list
of approved service centers appears in the guide or on our website at www.adctoday.com.
CAUTION: The reliability of the device depends upon conformance with the operation and service instructions, as
detailed in this manual.
CAUTION: Only replace battery with same type and model number.
CAUTION: To avoid the risk of electrical shock, this equipment must be only connected to supply mains with
protective earth.
CAUTION: Do not connect the device to equipment that does not meet EN60601-1. When the device is attached to a
patient, the device’s communication ports must only be connected to equipment that meets EN60601-1 standard.
CAUTION: Use only ADC-branded cuffs approved for use on the ADView 2 device.
CAUTION: Use only Masimo oximetry sensors for SpO2 measurements with the Masimo SpO2 module. Other oxygen
transducers (sensors) may cause improper performance.
CAUTION: Do not use damaged SpO2 or temperature sensors. Do not use an SpO2 sensor with exposed optical
components.
CAUTION: Do not immerse the SpO2 or temperature sensors in water, solvents, or cleaning solutions (the sensors and
connectors are not waterproof). Do not sterilize by irradiation, steam, or ethylene oxide. See the cleaning instructions
in the Sensor Directions for Use.
CAUTION: Do not use damaged patient cables. Do not immerse the patient cables in water, solvents, or cleaning
solutions (the patient cable connectors are not waterproof). Do not sterilize by irradiation, steam, or ethylene oxide.
See the cleaning instructions in the Patient Cable Directions for Use.
CAUTION: Do not position the device so that it is difficult to access and remove the power cord from the electrical
supply. The AC power cord is the means of disconnection to the supply mains.
Page 9

93-9005-00 ADView 2 User Manual | 9
September 1, 2016
Icons, Symbols and Abbreviations
Icons and Symbols
The following icons and symbols are used in this guide, on the ADView 2 equipment, and in packaging.
!
!
Warning message
!
Caution message
!
Manufacturer
!
Manufacture date
!
Authorized representative in the European Community
!
Catalog number
!
Serial number
!
Batch or lot code
!
Fragile, handle with care
!
Keep dry
!
Temperature limit
!
Humidity limitation
!
Consult instructions for use
!
!
Refer to instruction manual/booklet
!
Type B
!
Type BF applied part. Part is isolated from earth ground.
!
Indicates that the device contains materials which may be
hazardous to human health.
!
CE mark: Product meets the Medical Device Directive and is CE
marked to indicate conformance
!
SpO2 sensor. Type BF applied part
!
USB-A or USB-B
!
Warning: Federal (U.S.) law restricts this device to sale by or on
the order of a licensed health care practitioner.
!
Device includes RF transmitter.
!
Indicates the arm circumference which is appropriate for the cuff
!
Cuff index marker
Page 10

93-9005-00 ADView 2 User Manual | 10
September 1, 2016
!
Artery marker indicating proper placement – Arrow and symbol
should be placed over the brachial artery.
!
Cuff range indication
!
Device is not made with natural rubber latex
!
Device is not made with PVC
!
!
Class II equipment
IPX1!
Protection against vertically falling drops of water
!
Expiration date
!
Single use only
Commonly Used Abbreviations
BP Blood Pressure
BPM Beats Per Minute
EMR Electronic Medical Record system
K-sounds Korotkoff sounds
MAP Mean Arterial Pressure (Not available in the U.S.)
DIA Diastolic BP
NIBP Non-Invasive Blood Pressure
SpO2 Percent Oxygen Saturation of Arterial Blood (hemoglobin)
SYS Systolic BP
2. Setting Up the ADView 2
Your ADView 2 comes ready to use. The battery and any optional modules purchased were installed prior to shipping.
Unpacking the Monitor
As you unpack your ADView 2, check to make sure you have all the proper components.
Refer to the separate packing label stating which components you received based on the options you ordered with
your device.
Page 11
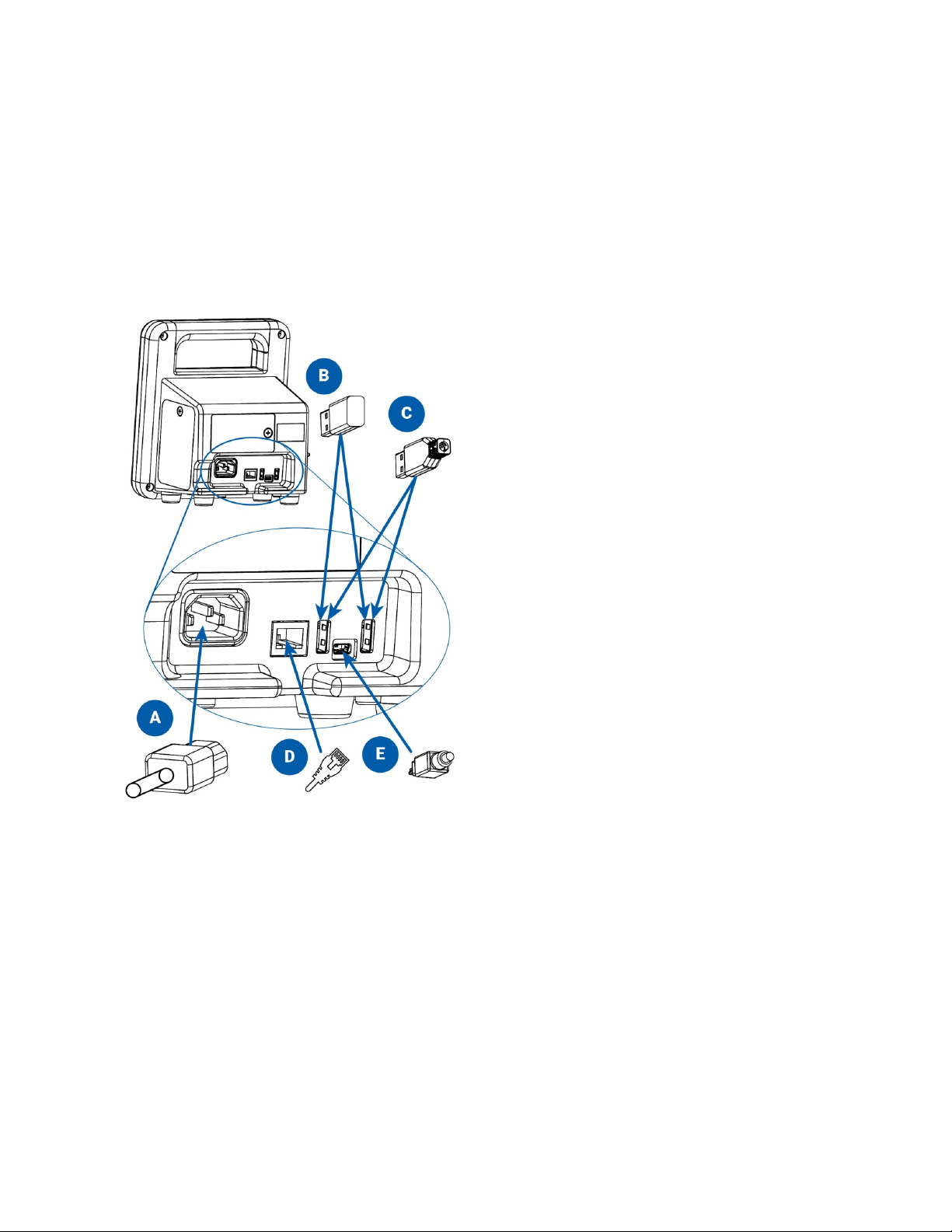
93-9005-00 ADView 2 User Manual | 11
September 1, 2016
Rear Panel Configuration
ADView 2 connections on the back of the device:
!
A. AC power cord
B. Wi-Fi USB dongle (optional)
C. Printer or barcode scanner USB cable (optional)
D. Ethernet cable (optional)
E. Mini USB cable to connect to PC or laptop
Page 12
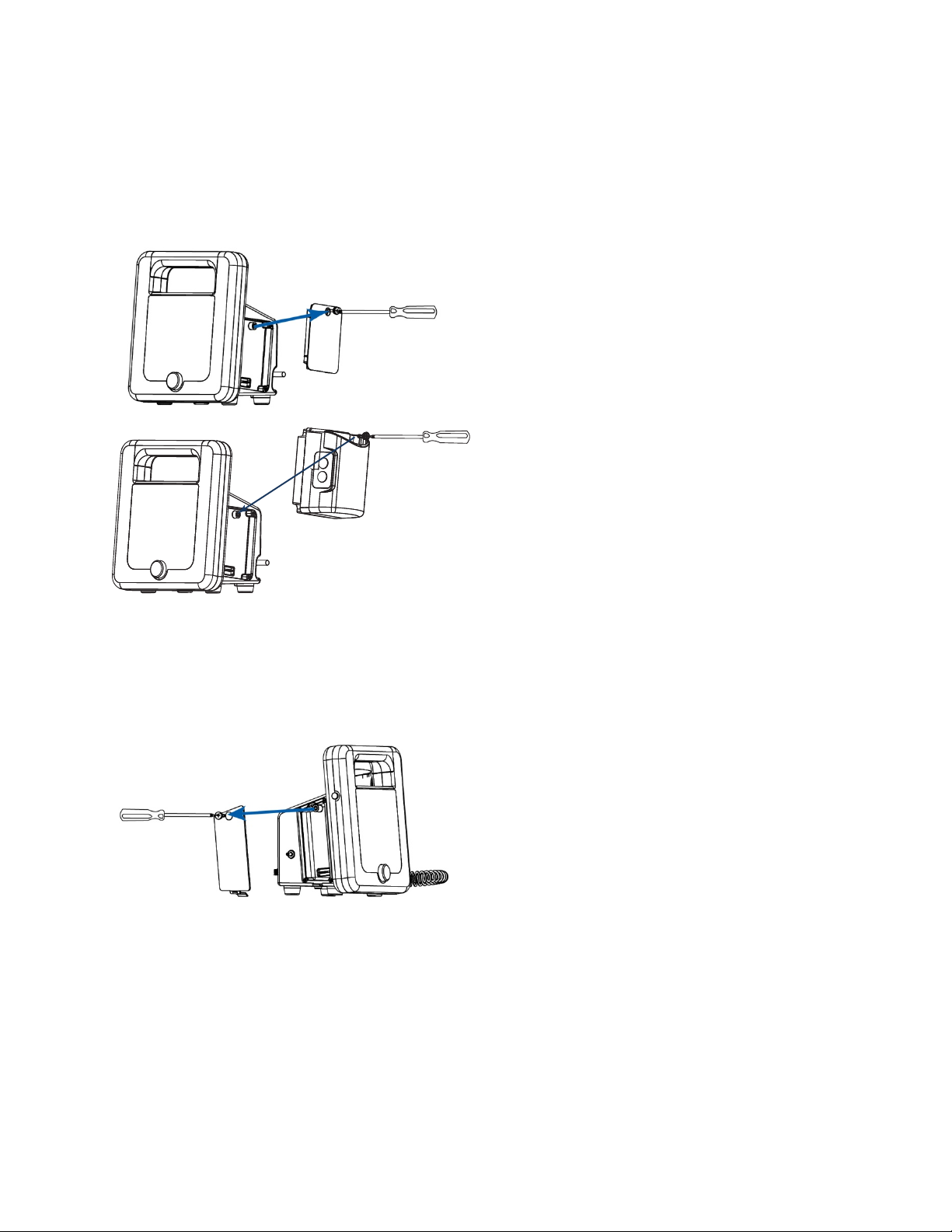
93-9005-00 ADView 2 User Manual | 12
September 1, 2016
Side/Temperature Panel Configuration
Optional modules ordered at the time of original purchase are installed prior to shipping. Modules can also be
ordered later and installed in the field. To install the Covidien Filac™ temperature module, use the included Phillips
head screwdriver to remove the panel cover on the right side of the unit (the right side when the screen is facing the
user). Attach the module and fasten in place. Insert the probe/well assembly into the top of the module.
Side/SpO2 Panel Configuration
Optional modules ordered at the time of original purchase are installed prior to shipping. Modules can also be
ordered later and installed in the field. To install an SpO2 module, use the included Phillips head screwdriver to
remove the cover on the left side of the unit (the left side when the screen is facing the user). Attach the module and
fasten in place.
Filac
Page 13
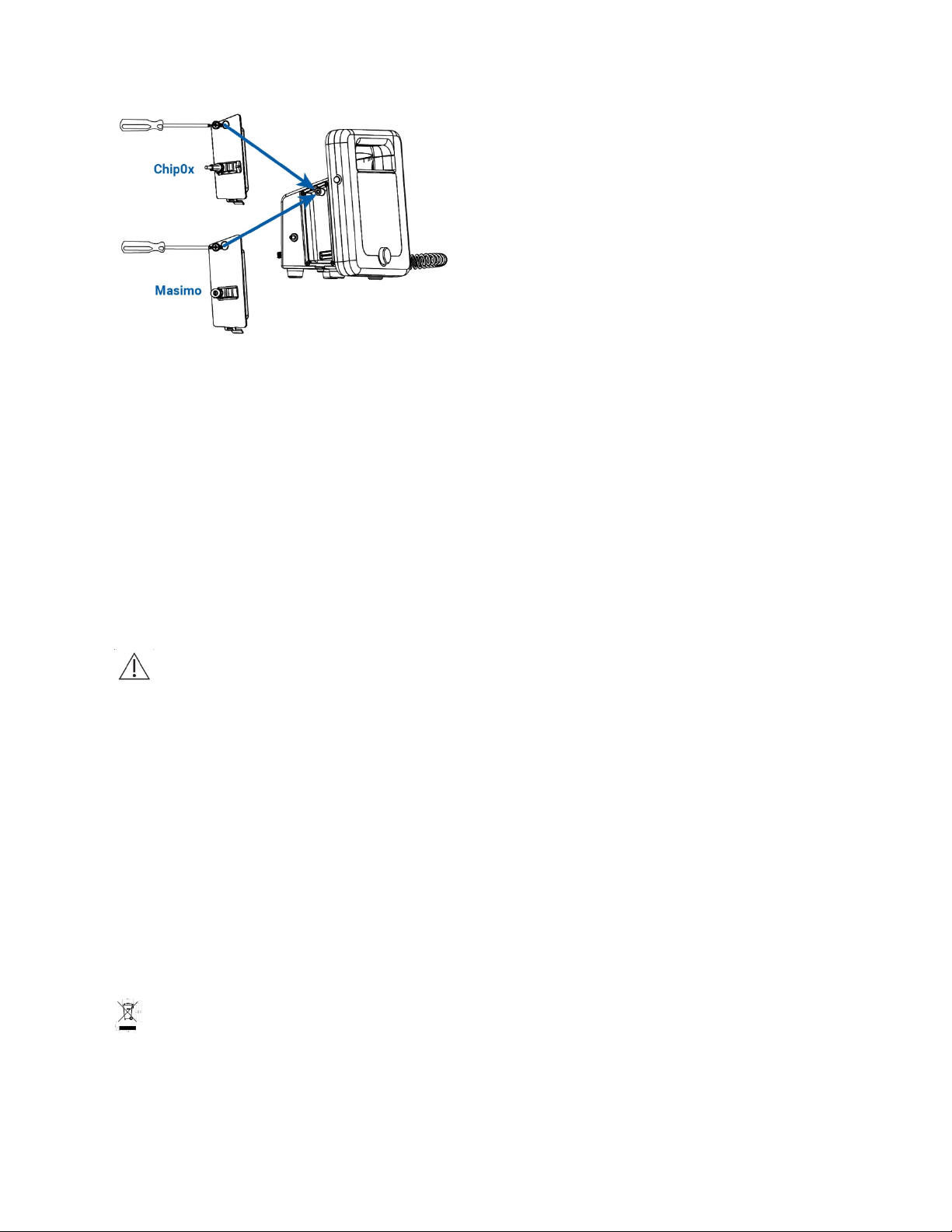
93-9005-00 ADView 2 User Manual | 13
September 1, 2016
For ChipOx:
Attach the ChipOx SpO2 sensor to the connector on the module while the retention clip is held upwards. Once the
sensor/cable connector is inserted, push the clip downward over the connector to hold it securely in the module. For
easy insertion, ensure the cable plug is parallel to the device.
For Masimo:
Attach the Masimo extension cable to the connector on the module, making sure that the connectors lock together.
Then, attach the Masimo SpO2 sensor to the other end of the extension cable making sure that the retaining clip is in
place.
Install Batteries
The rechargeable lithium-ion battery is installed prior to shipping. Please allow 8 to 12 hours for charging before
using your unit. All segments of the Battery Symbol will be lit when the device is fully charged.
CAUTION: Fire, explosion and severe burn hazard. Replace only with ADC part number9005BAT.
If not installed in the device, the battery shall be kept away from heat, fire or other high temperature environments.
Keep the battery in a dry place stored at room temperature.
Do not disassemble, attempt to repair or use the battery for any other device or for any other purpose.
Do not place near any metal or use metal to shield the battery from physical damage as this may cause battery
overheating and/or a fire risk.
Do not short across the contacts of the battery or attempt to discharge the battery by shorting as a risk of fire or
explosion may result.
Do not expose or immerse the battery in water or attempt to clean with any cleaning agents. Only wipe battery with a
damp cloth if necessary.
Wash the affected area if electrolyte spills on skin or clothes. Leaking electrolyte may cause discomfort to the skin. If
it gets into the eyes, do not rob the eyes. Flush eyes immediately with water, and seek medical attention.
Battery Disposal
The ADView 2 has a lithium-ion battery that contains materials which may be hazardous to human health. Do
NOT dispose of battery in domestic waste! Instead, please dispose of in an environmentally responsible way, or
return the battery to ADC. A prepaid return label can be obtained. Please see our website for more information.
Page 14

93-9005-00 ADView 2 User Manual | 14
September 1, 2016
Attach Connections to the Device
After connecting the Power Cable, plug into an available AC power outlet to charge batteries.
Connect the BP cable, SpO2 sensor cable, and temperature sensor (if option is included) to the device.
Connect the barcode scanner and/or printer to the device if these options are included.
Use the Power Button on the left side of the ADView 2 to turn it on.
NOTE: The blue LED around the Selection Knob will be lit whenever the device is powered on.
Mounting Options
The ADView 2 is designed to be used on a tabletop or mounted to a wall or mobile stand. Your device may be
delivered with the appropriate mounting kit, depending on the configuration your facility ordered. Please see the
separate mounting hardware instructions.
System Configuration
The ADView 2 can be used out of the box with the factory settings.
Changes to the factory settings can be made using the Advanced Configuration Application, which can be accessed
by connecting the unit to a Windows computing device. This method can be used to customize certain default
settings, like choosing either BP or Spo2 as the pulse rate source. Advanced configuration can also be used by
qualified technicians to connect to a network or EMR system.
Please see the separate Advanced Configuration Application guide for more information.
Page 15
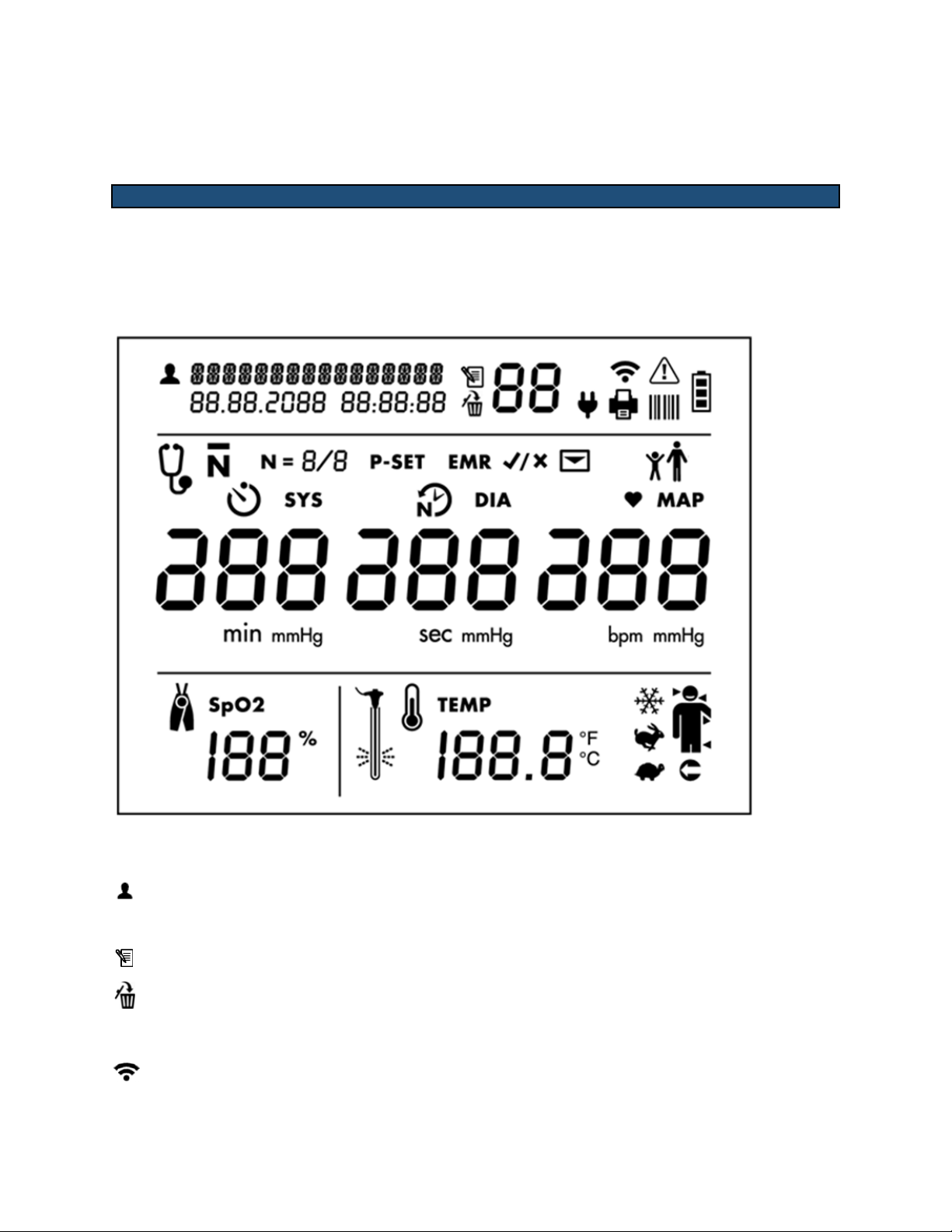
93-9005-00 ADView 2 User Manual | 15
September 1, 2016
3. Getting to Know the ADView 2
Measurement Display
!
!
Patient Identification and Date/Time Stamp
Patient ID
Memory Mode Information
Memory icon
Memory Delete icon
Connections to Ancillary Devices and Networks
Wi-Fi radio on (segments will illuminate in series)
Page 16

93-9005-00 ADView 2 User Manual | 16
September 1, 2016
Wi-Fi radio connected (segments will be illuminated continuously)
AC power connected
Printer connected and powered on
Barcode scanner connected and powered on
Error Alert Symbol
WARNING: User must take immediate action (see additional information in Section 12)
Battery Level Indicator
Battery symbol (all bars illuminated indicate a full charge)
Blood Pressure Measurement Mode Selection Icons
Manual Mode (for auscultatory readings)
Averaging Mode
Number of measurements taken in averaging mode
Maximum pressure setting for Manual Mode
Time before first measurement in minutes (Illuminates when Averaging Mode is selected)
Time between measurements in seconds (Illuminates when Averaging Mode is selected)
Blood Pressure Results (DIA and SYS)
DIA and mmHg: Diastolic blood pressure
SYS and mmHg: Systolic blood pressure
EMR Transmission Icons
EMR icon prompts the user to confirm whether or not to send measurement data as message to EMR
system. (Only shown if EMR connectivity has been set up via Configuration Application.)
Message icon (Indicates if EMR messaging is successful)
If successful, the icon will flash on and off with check mark.
If not successful, it will flash on and off with X.
Adult or Child Patient Selection
Press and hold toggle button for 4 seconds, until selection knob starts flashing. Release toggle button, and use the
selection knob to select the icon needed. Selected icon will blink after 1 second. Press the selection knob to confirm
the selection.
Adult BP Mode icon. This icon is illuminated when the Adult BP mode is selected. The ADView 2 will remain in the
selected patient mode until a new mode is set.
Page 17

93-9005-00 ADView 2 User Manual | 17
September 1, 2016
Pediatric BP Mode icon. This icon is illuminated when the Pediatric BP mode is selected. The ADView 2 will
remain in the selected patient mode until a new mode is set.
Heart Rate and MAP Measurements
Measured in BPM (beats per minute) is illuminated when a heart rate is shown in the heart rate display.
MAP Measured in mmHg. Illuminated when MAP functionality is enabled and a MAP value is shown. NOTE: This is a
factory setting. MAP is not available in the U.S.
Pulse Oximetry Measurement Display
Expressed in % of arterial oxyhemoglobin
Temperature Measurement Settings and Results Display
Body temperature in either Fahrenheit or Celsius (user selectable)
Cold Mode
Predictive Mode
Direct Mode
Human Thermometry Mode. Oral (arrow on right side of head), Axillary (arrow under arm), or Rectal (arrow
pointing to rectum).
Apply temp probe cover
Page 18

93-9005-00 ADView 2 User Manual | 18
September 1, 2016
Control Panel
Memory Button
Press this button to enter Memory Mode and to store, view or delete saved measurements.
Save Current Reading: A quick press gives the user an option to save the patients reading, suggested to be done after
each reading.
Access Previous Reading: A long press (holding the button down for 4 seconds) gives the user the option to access
the bank of prior readings. Please see Section 4 to read more about saving and accessing saved readings.
Toggle Mode Button
A quick press gives the user an option to switch between the three BP measurements modes:
Single Measurement, Averaging Measurement and Manual Mode.
A long press (holding the button down for four seconds) gives the user the option to switch between pediatric or
adult mode. More information on switching from adult to pediatric mode can be found in Section 5.
Selection Knob
This round knob rotates clockwise and counterclockwise and can be pressed in to select different options on the
ADView 2. This button can be used to change parameters and to set the intended parameter.
Start/Stop Button
Press this button to start or stop a BP measurement.
Home Button
Pressing this button returns you to the single measurement mode.
Monitor Setup
Press the power button to start Initial Power-up. The display will gradually illuminate over a 3-second period. If this is
the first time the device has been turned on since it left the factory or since the device was set to factory defaults, the
user will be prompted to set the time and date in the following format: MM.DD.YYYY HH:MM:SS. The “MM” portion
of the field will flash first, prompting you to set the month. Turn the Selection Knob to select the appropriate month
and then press the knob in. The next field, “DD,” will flash, prompting you to select the day, and so on, until you have
set “SS” for seconds.
After setting the date and time, the device is ready to use! It is possible to make additional configuration changes to
the ADView 2 using the Advanced Configuration Application.
Visual Alarms
When an error occurs, the Warning icon will blink, in addition to the relevant parameter experiencing the error.
Page 19

93-9005-00 ADView 2 User Manual | 19
September 1, 2016
Blood pressure error is displaced in the BP heart rate field.
Temperature error will displace in the lower right hand.
SpO2 error will displace in lower left side of the screen.
Battery symbol will flash if error is related to battery.
Depending on the out-of-range value, the display will indicate whether the value is out-of-range (hi) or out-of-range
(lo). If the out-of-range parameter is the systolic or diastolic pressure (or both), and MAP is enabled, MAP is not
displayed for that measurement. NOTE: MAP is not available in the U.S.
See Section 12, “Status Messages and Alarms,” for more details.
4. Good to Know Before You Begin
Power Modes
Initial Power-up occurs the first time the device is turned on after being received from the factory, after the device
has been reset to factory defaults or after the battery has been changed. See “Monitor Setup” for information about
Initial Power-up.
Nominal Power-up refers to every time the device is turned on after Initial Power-up. Simply press the power button
and the display will illuminate.
NOTE: The blue LED around the Selection Knob will be lit whenever the device is powered on.
Power-down occurs when you press the power button when the device is powered up. During power-down, all LCD
segments and icons illuminate for 1 second and then the entire display gradually darkens from normal brightness to
black and then one beep sounds. At this point, the device enters Sleep Mode, a low-power state, and is considered
off.
Automatic Power-down occurs when the device has not been used (i.e., no measurements taken or buttons pressed)
for 1 hour. The device will automatically execute the Power-down sequence and enter Sleep Mode.
Documenting Measurements
Before you start taking measurements, it’s important to document the patient ID per your facility’s procedures.
With a Barcode Scanner
If your ADView 2 is connected to a barcode scanner, you can scan your patient’s barcode ID and it will show at the top
left of your screen. Then, the patient ID will remain attached to all measurements taken, saved in memory, and/or
sent to your facility’s EMR.
Without a Barcode Scanner
If you don’t require a barcode scanner, all measurement results will be displayed on the main screen and can be
saved to the device’s memory, written down in a chart, printed out (with optional thermal printer) or captured in
another way that meets your facility’s documentation procedures.
Printing
The ADView 2 can be connected to an optional thermal printer to easily print out measurement results. See Section
11 for ordering information.
Page 20

93-9005-00 ADView 2 User Manual | 20
September 1, 2016
After connecting the optional thermal printer (see Section 2 and Section 3), ensure that the green LED on the top of
the printer is lit and unblinking before printing.
Reset Print
To print the information currently displayed on the ADView 2 screen, press the print button on the top of the printer
(see diagram above).
Saving Measurements
With EMR Connectivity
Technicians may have already connected the ADView 2 to your facility’s EMR system. If so, you can easily send
measurements directly to the EMR.
With the patient’s current measurements displayed on the screen, press the Memory button . The EMR icon
and Check Mark icon will begin flashing.
Press the Selection Knob to send the data to the EMR. If the measurements are delivered to the EMR successfully,
you will see the EMR Message icon and the Check Mark icon blink four times.
To store the displayed measurements in the device memory without sending to the EMR:
Press the Memory Button ,
While the EMR and Check Icons are flashing, turn the Selection Knob until the X icon is displayed and then press
the Selection Knob. The measurements will be saved to the device memory only.
Without EMR Connectivity
The ADView 2 does not have to be connected to an EMR system to save measurements. You can save them to the
device’s own memory. With the patient’s current measurements displayed on the screen:
Press the Memory button .
All of the measurement values on the screen, the Check icon and the Memory icon will flash on and off 2
times.
The display will then return to a blank screen/shows null values, but the Check Mark icon will flash 2 more times,
this verifies the measurements are successfully stored to the device’s memory.
Note: The ADView 2 does not autosave. The user must save the reading to memory after every reading.
To view results stored in memory, press the Memory Button for more than four seconds. You will see the
most recently saved measurements displayed on the screen, and can turn the Selection Knob counterclockwise to
see older measurements and then clockwise to see newer measurements.
Page 21

93-9005-00 ADView 2 User Manual | 21
September 1, 2016
TIP: When you send results to memory, make note of the “Memory Location” number to keep track of an individual
patient’s measurements. Then later on, you can match the Memory Locator number with a patient’s name if you pull
up or print out results for documentation.
The memory will hold up to 99 records. At 100 readings the device will continue to save, but will save by overwriting
the oldest records.
5. Using ADView 2 for BP Measurement
The ADView 2 provides flexibility to allow you to measure BP using several different modes, depending on your
facility’s preferred procedure, the patient’s condition or other care considerations.
Step 1: Preparing the Patient
Patient Position
According to AHA guidelines, the patient should be seated for at least five minutes before taking a blood pressure
reading. The patient should be seated with feet flat on the floor and back supported. The patient’s upper arm should
be fully supported and resting at heart level. Encourage the patient to relax, and to be still and quiet.
Select and position BP cuff
Selecting the wrong size cuff will produce an inaccurate blood pressure measurement. When wrapped around the
patient’s arm, the Cuff Index Line should fall within the Range Markers printed on the cuff. If not, select a larger or
smaller cuff to ensure optimal BP measurement accuracy. The Artery Marker printed on the cuff must point to the
brachial artery, located inside the patient’s upper arm, between bicep and tricep. Wrap the cuff snugly around the
patient’s upper arm, so that the bottom edge of the cuff is approximately 1 inch above elbow joint.
!
Step 2: Select Between Adult Vs Pediatric Mode
The ADView 2 offers two different measurement modes for two very different types of patients: Adults and Pediatric
patients (children between the ages of 3 and 12).
To switch between Adult and Pediatric modes:
: Adult BP Mode icon : Pediatric BP Mode icon
Hold the Toggle Mode button down for 4 seconds until the adult and pediatric icons start blinking.
Page 22

93-9005-00 ADView 2 User Manual | 22
September 1, 2016
Turn the Selection Knob in either direction to select the desired icon
After making your selection, wait 2 seconds. The selected icon will be the one flashing.
Once you have selected the intended mode, press the Selection Knob to finalize this selection.
Note: Adult and Pediatric mode can be used in conjunction with any type of measurement mode.
Step 3: Select Measurement Mode
The ADView 2 offers three different ways to measure BP.
Single Measurement Mode: You can take a single, automatic measurement.
Averaging Measurement Mode: Automatically take and average up to 5 multiple BP measurements.
Manual Mode. Allows you to verify an automated oscillometric BP reading with a manual auscultatory measurement.
Note: Use of Manual Mode may be required to meet certain clinical trial protocols. It also can be used to verify a BP
reading if the automated BP results diverge significantly from a patient’s prior history, or if you as the clinician deem
it necessary. Manual Mode mimics a traditional sphygmomanometer and does not measure Systolic or Diastolic
blood pressure.
Note: Averaging Measurement Mode can only be used for Automatic BP Measurements. It cannot be used to
automatically average measurements taken in Manual Mode.
!
Single Measurement Mode
This is the device’s default mode. The ADView 2 will be in this mode each time it is powered up.
Pressing the Home button will also revert to single measurement mode.
!
Example of center display when Single Measurement Mode is selected
Note: If you see the Auscultatory SphygMode Icon or the Averaging Mode Icon , press the Mode Toggle Button
in succession until these icons no longer show to return to Single Measurement Mode.
Page 23

93-9005-00 ADView 2 User Manual | 23
September 1, 2016
Averaging Measurement Mode
!
Select Mode
Set # of Measurements to Average
Set Time Between Measurements
Press the Mode Toggle Button
until you see the
Averaging Measurement Mode
Icon . . From single
measurement mode, that will only
be one button press.
The Number of Measurements Icon
will flash as will the Selection
Knob. To keep the default of 3
measurements, press Knob. Or turn
Knob to choose a different number
and press Knob to select.
Default values for Time Before First
Measurement and Time
Between Measurements will
flash in sequence as the user is
prompted to set each value. To keep
defaults, press Knob. Or turn Knob to
choose new values and press Knob
to select.
Example of Averaging
Measurement Mode setup screen
with default values.
You can select 2 to 5 measurements
to average*. In this example, 4
measurements are set to be
averaged.
On left is Time Before First Reading
will start. Select 0-5 min. Default = 0.
On right is Time Between
Measurements. Select 15-120 sec.
Default = 15 sec.
*Note: In averaging mode, you are unable to see individual readings. Averaged Results will be displayed at the end of
all the readings. By default, all measurements taken (from 2 to 5) will be included in the averaging calculation. Using
the Advanced Configuration Application, the device can be programmed to always throw out the first measurement
taken and not include it in the averaging calculation.
**Note: All values selected during the Averaging Measurement Mode will be displayed the next time that this mode is
used, so that the same customized averaging protocol can be set and used each time that Averaging Measurement
Mode is used.
Page 24

93-9005-00 ADView 2 User Manual | 24
September 1, 2016
Manual Mode
!
Example shows Manual Mode setup display. Three-digit number is the target cuff inflation pressure setting. The
default is 160 mmHg.
Select Mode
Choose a Pressure Value
Press the Mode Toggle Button until
you see the Manual Mode Icon .
From single measurement mode, that will be two
button presses.
The pressure setting icon and Selection Knob will
flash. To keep the default maximum cuff inflation setting of
160 mmHG, press the Knob. Or choose another pressure level
between 100-280 mmHG and press the Knob to select. The
device will remember the pressure setting and display the
same pressure setting the next time SphygMode is used.
Step 4: How to Measure BP in Each Mode
When in the appropriate home screen (see above)
Single Measurement Mode
Averaging Measurement Mode
Manual Mode
Ensure values are cleared from
display except for SYS value
representing current pressure in
cuff.
Press Start/Stop Button
.
Inflate/deflate cycle proceeds
automatically until results are
displayed.
A long beep will sound to indicate
measurement is complete.
When selecting this mode the
will flash to allow the user to decide
how many reading they would like
averaged.
Select number of readings by
rotating the Selection Knob and
then pushing the knob in.
The minute number will flash and
the user can select how many
minutes until the first reading starts
by rotating the Selection Knob and
pushing in.
The user can then select the time
between readings by rotating the
Selection Knob and pushing in.
The timer will then count down the
seconds until the first reading.
After all measurements are taken
and averaged, results will display.
A long beep will sound to indicate
measurement is complete.
Put stethoscope over artery.
Press Start/Stop to
inflate cuff automatically. Similar to
an analog sphyg gauge, the display
shows current pressure in cuff.
When cuff reaches max pressure,
deflation will begin automatically.
Listen for K-sounds while watching
displayed pressure. Note SYS and
DIA.
Press Start/Stop again to
quickly dump remaining pressure
from the cuff.
Page 25

93-9005-00 ADView 2 User Manual | 25
September 1, 2016
Example of SYS, DIA and HR* after
BP measurement in Single
Measurement Mode.
Example of SYS, DIA and HR* after
all measurements have been
averaged.
Example of Manual Mode display
with cuff pressure at zero. P-set
value is default 160 mmHg.
*Note: Pulse rate source, whether BP or SpO2, can be selected at any time using the Advanced Configuration
Application. The default setting uses BP as HR source. If used outside of the United States, the device also can
be configured to measure MAP using the Configuration Application. If set up to measure MAP, the MAP and HR
measurements will alternate in 3-second intervals. NOTE: MAP is not available in the U.S.
Step 5: Record Results!
When the measurement cycle is complete, values will be displayed for systolic and diastolic pressure, plus heart rate
if BP is selected as the pulse rate source. Manually record, print and/or digitally save your measurements according
to your healthcare facility’s preferred process. To send results to an EMR or to the device memory, follow the steps in
Section 4.
Step 6: Prepare for New Patient
After all measurements have been recorded or captured, remove the cuff from the patient and clean it according to
your facility’s requirements. This disinfecting and storage step applies to BP cuffs as well as other optional
accessories whose use will be covered in the following sections.
Pressing the Memory Button will clear all patient and measurement data from the display, in addition to
saving the patient and measurement information in memory. It is recommended that the display is cleared before
taking readings on a new patient.
6. Using ADView 2 for Heart Rate Measurement
There are two ways to measure heart rate with the ADView 2.
BP Heart Rate Measurement
With this method, HR is captured automatically during BP measurement.
SpO2 Heart Rate Measurement
If you have the optional pulse oximetry module on your device, you can measure HR through the SpO2 sensor. See
next section (section 7) for more on use of this option.
The ADView 2 uses BP as the default HR source. HR source can be selected using the Advanced Configuration
Application.
Page 26

93-9005-00 ADView 2 User Manual | 26
September 1, 2016
7. Using ADView 2 for Pulse Oximetry
An optional SpO2 module allows you to measure the oxygen saturation of arterial bloodflow and display the reading
on the ADView 2. There are two different types of optional SpO2 modules: ChipOx and Masimo SET.
General Principle of Operation:
The pulse oximeter directs red and infrared light into a capillary bed and measures the change in light absorption
during the pulsatile cycle. Red and infrared light-emitting diodes (LEDs) in oximetry sensors serve as the light
sources, and a photodiode serves as the photo detector.
Traditional pulse oximetry assumes that all pulsations in the light absorbance signal are caused by oscillations in the
arterial blood volume.
The traditional pulse oximeter calculates the ratio of pulsatile absorbance (AC) to the mean absorbance (DC) at each
of two wavelengths (typically one visible, the other infrared). The oximeter then calculates the ratio of these two
arterial absorbance signals. This value is used to find the saturation SpO2 in a standard table built into the oximeter
software. The values in the table are based upon human blood studies against a laboratory co-oximeter on healthy
adult volunteers in induced hypoxia studies. The resulting value is reported as oxygen saturation level (%).
!
WARNING: Pulse oximeter probes and sensors are designed for use with specific pulse oximeter devices. Only use
probes and extension/adapter cables that are specified for each SpO2 module.
WARNING: The responsible organization and/or operator must verify the compatibility of the oximeter, oximeter
probe, and extension/adapter cable before use. Otherwise, patient injury can result.
WARNING: Misapplication of a pulse oximeter probe with excessive pressure for prolonged periods can induce
pressure injury.
CAUTION: Inaccurate measurements may be caused by:
• Incorrect sensor application or use. Proper sensor use and placement is critical for good performance.
• Significant levels of dysfunctional hemoglobins (e.g., carboxyhemoglobin or methemoglobin)
• Intravascular dyes such as indocyanine green or methylene blue.
• Dyes or any substance containing dyes that affect light absorption may cause erroneous readings.
• Some nail polish colors (particularly dark shades) or artificial fingernails may reduce light transmission and
affect pulse oximetry accuracy. Remove any nail polish or artificial fingernails before using the SpO2 sensor.
• Exposure to excessive illumination, such as surgical lamps (especially ones with a xenon light source),
bilirubin lamps, fluorescent lights, infrared heating lamps, or direct sunlight (exposure to excessive
illumination can be corrected by covering the sensor with a dark or opaque material)
• Excessive patient movement.
• Abnormal pulse rhythm.
Page 27

93-9005-00 ADView 2 User Manual | 27
September 1, 2016
• Placement of a sensor on an extremity with a blood pressure cuff, arterial catheter, or intravascular line.
Always place the SpO2 sensor on the arm without a blood pressure cuff, arterial catheter, or intravascular
line.
CAUTION: Pulse rate measurement is based on the optical detection of a peripheral flow pulse and therefore may not
detect certain arrhythmias. The pulse oximeter should not be used as a replacement or substitute for ECG based
arrhythmia analysis.
CAUTION: SpO2 is empirically calibrated to functional arterial oxygen saturation in healthy adult volunteers with
normal levels of carboxyhemoglobin (COHb) and methemoglobin (MetHb). A pulse oximeter cannot measure
elevated levels of COHb or MetHb. Increases in either COHb or MetHb will affect the accuracy of the SpO2
measurement.
CAUTION: COHb levels above normal tend to increase the level of SpO2. The level of increase is approximately equal
to the amount of COHb that is present.
CAUTION: High levels of COHb may occur with a seemingly normal SpO2. When elevated levels of COHb are
suspected, laboratory analysis (CO-Oximetry) of a blood sample should be performed.
CAUTION: For increased MetHb, the SpO2 may be decreased by levels of MetHb of up to approximately 10% to 15%.
At higher levels of MetHb, the SpO2 may tend to read in the low to mid 80s. When elevated levels of MetHb are
suspected, laboratory analysis (CO-Oximetry) of a blood sample should be performed.
CAUTION: Venous congestion may cause under reading of actual arterial oxygen saturation. Therefore, assure proper
venous outflow from monitored site. Sensor should not be below heart level (e.g., sensor on hand of a patient in a bed
with arm dangling to the floor).
CAUTION: Venous pulsations may cause erroneous low readings (e.g., tricuspid value regurgitation).
CAUTION: The pulsations from intra-aortic balloon support can be additive to the pulse rate on the oximeter pulse
rate display. Be sure to verify patient's pulse rate.
CAUTION: Elevated levels of Total Bilirubin may lead to inaccurate SpO2, measurements.
CAUTION: With very low perfusion at the monitored site, the readings may read lower than core arterial oxygen
saturation.
CAUTION: Do not immerse the sensor or patient cable in water or, solvents, or cleaning solutions (the sensors and
connectors are not waterproof).
CAUTION: Loss of pulse signal can occur in any of the following situations:
• The sensor is too tight.
• There is excessive illumination from light sources such as a surgical lamp, a bilirubin lamp, or sunlight.
• A blood pressure cuff is inflated on the same extremity as the one with an SpO2 sensor attached.
• The patient has hypotension, severe vasoconstriction, severe anemia, or hypothermia.
• There is arterial occlusion proximal to the sensor.
• The patient is in cardiac arrest or is in shock.
NOTE: See SP02 Sensor Specifications Table on page 45, the range of the peak wavelengths and maximum optical
output power of the light emitted by the pulse oximeter probe, which can be especially useful to clinicians.
NOTE: This device does not include an alarm system that detects an SpO2 or pulse rate physiological alarm
condition. This device is not designed for long-term monitoring and should only be used for spot-check
measurements.
Page 28

93-9005-00 ADView 2 User Manual | 28
September 1, 2016
System Description:
The ADView 2 spot check device with pulse oximetry module consists of a base ADView 2 device for the display of
SpO2 measurement data and other user information, either a ChipOx or Masimo SET SpO2 module which is attached
to the ADView 2 base unit as described in Section 2, and the specific reusable pulse oximeter probe and/or cable that
comes packaged with each module. Device setup is described in more detail in Section 2. Both the Masimo SET and
ChipOx SpO2 modules are calibrated to display functional oxygen saturation (SpO2).
Taking SpO2 Measurements:
1. Insert a finger (preferably the index, middle or ring finger) into the SpO2 sensor until the end of the finger reaches
the finger stop. Do not use the thumb.
2. The fingernail should face the side with the red light. Make sure that long fingernails do not interfere with proper
finger position.
An SpO2 reading will be displayed after a few seconds. Remove the finger from the SpO2 sensor, and the last
measurement will be displayed and flashed.
4. If the sensor is not removed from the finger, SpO2 readings will be automatically stopped after 10 minutes of
continuous measurement, and the last measurement will be displayed and flashed.
5. To start a new measurement, re-apply the SpO2 sensor to the finger as described in Step 1.
NOTE: If the signal quality from the sensor drops below 90%, no measurement will be displayed in the SpO2 section
, and the percent symbol will begin flashing. Check sensor for correct placement and usage.
Special Notes for Masimo SET SpO2 Module:
NOTE: Possession or purchase of this device does not convey any express or implied license to use the device with
unauthorized sensors or cables which would, alone or in combination with this device, fall within the scope of one or
more of the patents relating to this device.
NOTE: If the accuracy of any measurement does not seem reasonable, first check the patient’s vital signs by alternate
means and the check the MS board pulse oximeter for proper functioning.
NOTE: A functional tester cannot be used to assess the accuracy of a Masimo pulse oximeter sensor or pulse
oximeter. Using Clinical Dynamics SmartSat SpO2 simulator, the device can display the entire range of SpO2 values.
Masimo Sensors:
Before use, carefully read the M-LNCSTM DCI/DCIP sensor directions for use, which includes cleaning and disinfection
instructions.
Masimo M-LNCST sensors are provided non-sterile.
Additional Masimo Information:
In addition to the General Principles of Operation mentioned above, the Masimo SET MS board pulse oximeter uses
the following principle:
The MS board decomposes the ratio of pulsatile absorbance (AC) to the mean absorbance (DC) at each of two
wavelengths, 660 nm and 905 nm (S(660) and S(905)) into an arterial signal plus a noise component and calculates
the ratio of the arterial signals without the noise:
S(660) = S1 + N1
S(905) = S2 + N2
R = S1/S2
Page 29

93-9005-00 ADView 2 User Manual | 29
September 1, 2016
Again, R is the ratio of two arterial pulse-added absorbance signals and its value is used to find the saturation SpO2 in
an empirically derived equation into the oximeter’s software. The values in the empirically derived equation are based
upon human blood studies against a laboratory co-oximeter on healthy adult volunteers in induced hypoxia studies.
The above equations are combined and a noise reference (N’) is determined:
N’ = S(660) - S(905) x R
If there is no noise N’ = 0: then S(660) = S(905) x R which is the same relationship for the traditional pulse oximeter.
The equation for the noise reference is based on the value of R, the value being sought to determine the SpO2. The
MS board software sweeps through possible values of R that correspond to SpO2 values between 1% and 100% and
generates an N’ value for each of these R-values. The S(660) and S(905) signals are processed with each possible N’
noise reference through an adaptive correlation canceler (ACC) which yields an output power for each possible value
of R (i.e., each possible SpO2 from 1% to 100%). The result is a Discrete Saturation Transform (DST™) plot of relative
output power versus possible SpO2 value as shown in the following figure where R corresponds to SpO2 = 97%:
The DST plot has two peaks: the peak corresponding to the higher saturation is selected as the SpO2 value. This
entire sequence is repeated once every two seconds on the most recent four seconds of raw data. The MS board
SpO2 therefore corresponds to a running average of arterial hemoglobin saturation that is updated every two
seconds.
Masimo Graphical Plot:
Below is a graphical plot of discreet A
RMS
values measured with Masimo SET Oximetry in a clinical study using
DCI/DCIP pulse oximetry sensors:
Page 30

93-9005-00 ADView 2 User Manual | 30
September 1, 2016
The tested device meets the stated accuracy specification of RMS Error = 2.00% for the range 70% to 100% SaO2. The
accuracy is not specifed below 70% SaO2. In addition, here are detailed measured A
RMS
values over the specified
discreet ranges:
Range
A
RMS
90-100%
0.60%
80-90%
0.54%
70-80%
0.67%
Special Notes for ChipOx SpO2 module:
NOTE: Use only SpO2 sensors from ADC approved for use on the ADView 2 ChipOx SpO2 module.
NOTE: A functional tester cannot be used to assess the accuracy of a ChipOx pulse oximeter sensor or pulse
oximeter. Using FLUKE Biomedical Index 2 SpO2 simulator, the device can display the entire range of SpO2 values.
ChipOx Sensors:
Before using, carefully read the ChipOx sensor directions for use, which includes cleaning and disinfection
instructions.
ChipOx sensors are provided non-sterile.
ChipOx Graphical Plot:
Below is a graphical plot of discreet A
RMS
values measured with ChipOx Oximetry in a clinical study using ChipOx
pulse oximetry sensors:
Page 31

93-9005-00 ADView 2 User Manual | 31
September 1, 2016
The tested device meets the stated accuracy specification of RMS Error = 1.89% for the range 70% to 100% SaO2. The
accuracy is not specifed below 70% SaO2. In addition, here are detailed measured A
RMS
values over the specified
discreet ranges:
Range
A
RMS
90-100%
1.54%
80-90%
2.10%
70-80%
2.56%
Page 32

93-9005-00 ADView 2 User Manual | 32
September 1, 2016
8. Using ADView 2 for Temperature Measurement
The ADView 2 has several options for measuring a patient’s body temperature.
Covidien Filac 3000 Thermometry Module
Your device may come equipped with a Covidien Filac 3000 digital thermometer capable of taking an oral, axillary
(under arm) or rectal temperature reading. Your thermometer should come with a blue isolation chamber/probe unit
for taking oral and axillary temperature or with a red isolation chamber/probe unit for taking rectal temperature.
NOTE: Always apply a new probe cover before taking temperature. Do not reuse probe cover. Use only probe covers
designed for use with Filac 3000 probes. Using the incorrect probe cover can severely damage the probe and cause
measurement inaccuracies.
NOTE: To change between Celsius and Fahrenheit, press the °C/°F button on the temperature module. Press again as
needed.
NOTE: After removing the probe from the isolation chamber, the Install/Remove Probe Cover icon will flash to
remind you to apply a new probe cover. A new probe cover must be applied in order to take a measurement.
After you withdraw the probe from the isolation chamber and apply a probe cover, the thermometer begins working
automatically. You will see the Human Thermometry Mode icon illuminated on your display with the appropriate
arrow illuminated (e.g., pointing to the mouth on right side of head for oral temperature taking). Press the Site button
on your thermometer unit to switch between Oral and Axillary mode.
Oral Temperature Taking
Insert the probe tip under the tongue on one side or the other. Ask the patient to close his mouth. Hold the probe in
place until there is a long beep and the temperature reading displays.
!
Page 33

93-9005-00 ADView 2 User Manual | 33
September 1, 2016
Axillary Temperature Taking
With the patient’s arm uplifted, place the probe tip into the patient’s armpit, directly on the skin. Ask the patient to
lower his arm and hold still. Hold the probe perpendicular to the arm until there is a long beep and the temperature
reading displays.
!
Rectal Temperature Taking
Apply lubricant to the probe cover and insert it gently into the patient’s rectum only one-half inch to three-fourth inch
(12 mm to 19 mm) for adults or one-fourth to one-half inch (6 mm to 13 mm) for children. Hold the probe still until
there is a long beep and the temperature reading displays.
NOTE: If the temp probe is returned to the probe well before the “long beep” is heard, no temperature measurement
will be displayed.
NOTE: Be sure to eject the used probe cover into an approved bio-hazard container before attempting to place the
probe back in the isolation chamber.
Other Temperature Settings
Your device’s thermometer operates in several different modes.
Quick Mode is an oral predictive measurement mode that provides a fast reading. Quick Mode allows you to rapidly
identify patients with normal body temperatures. If the patient temperature is outside of the “normal” range, the
thermometer will automatically switch into its standard predictive mode in order to provide a more accurate reading.
Quick Mode is indicated by a rabbit icon on the display . Quick Mode is not available when in Cold Mode or in
Direct Mode.
The thermometer normally operates in predictive mode to provide fast and accurate temperature measurements.
However, in instances when no measurement site is detected or the temperature does not stabilize, the thermometer
will automatically switch to Direct Mode . Additionally, the thermometer will automatically switch into Direct
Mode if the ambient temperature is greater than 35°C (95°F). If this happens, you will hear two short beeps and see
the Direct Mode turtle icon appear . This icon will be continuously displayed whenever the thermometer is
functioning in Direct Mode. The Direct Mode auto feature is always functional for both the red or blue isolation
chamber/probe combinations.
Cold Mode can be selected if a patient’s body temperature is expected to be lower than normal, such as when he is
coming out of surgery. Cold Mode is activated by pressing the Site Selection button and °C/°F button simultaneously
on the temp module. When selected, the Cold Mode snowflake icon is displayed, and the probe will preheat to
33°C (91°F). The accuracy and measurement time of Cold Mode measurements are equivalent to standard prediction
measurements at the respective body sites.
Page 34

93-9005-00 ADView 2 User Manual | 34
September 1, 2016
9. Using ADView 2 EMR and Memory Functions
Section 4, “Good to Know Before You Begin,” provides some basic information about the ADView 2’s EMR and
memory functions. Here are more details about how these work.
Memory Mode
When you power up the unit, the memory identifier appears at the top of the display beside the Memory Icon .
This is the number that will be associated with the next set of measurement results that are sent to the device
memory.
Press the Memory Button on the front of the device to save results to memory. The Memory Icon, Check
Mark icon and all values that are displayed on the screen (at the time the button is pressed) will flash before the
results are saved. The following values can be saved to memory.
Parameter Name
Field Type
Patient ID
Alphanumeric Text
Time Stamp
Numeric, HH:MM:SS
Date Stamp
Numeric, MM.DD.YYYY or DD.MM.YYYY
Memory Location
Numeric
Systolic BP
Numeric
Diastolic BP
Numeric
BP Type
Text: AVG or SM
Pulse Rate
Numeric
Pulse Rate Source
Text: BP or SpO2
Mean Arterial Pressure
Numeric
SpO2
Numeric
Temperature
Numeric
Page 35

93-9005-00 ADView 2 User Manual | 35
September 1, 2016
Temperature U/M
Text: C or F
Temperature Site
Text: ORL, AXL, RCT
!
NOTE: If a parameter has no data showing at the time the memory button is pressed, then all values for that
parameter will be stored as ‘null.’
To recall measurements from memory:
Press and hold the Memory Button for four seconds on the front of the device .
You will then be able to use the Selection knob to scroll through prior readings!
Press the Memory Button or the Home Button to exit memory mode.
Clearing Measurements from Memory
To clear a single measurement from memory
Press the Selection Knob while in memory mode. The Memory Delete icon and the Check icon will flash, along
with the Memory Identifier number.
Ensure you are viewing the measurement you wish to delete.
Press the Selection Knob to delete that measurement. Or rotate the knob clockwise until the X icon flashes and
press the Selection Knob to cancel the deletion.
To clear all measurement values from memory:
While in Memory Mode, press and hold the Memory Button for less than three seconds. While holding, press the
Selection Knob.
The Memory Delete icon and Check icon will flash in sync with the Selection Knob. All other values on the
screen will be blank
Press the Selection Knob again to delete all measurements, or rotate the knob clockwise until the X icon flashes
and press the Selection Knob to cancel the deletion.
EMR Transmissions
Section 4 covers the basics of sending measurements to EMR. Here are more details.
Validating Patient ID with EMR
Scan the patient’s barcode ID with your barcode scanner. If your ADView 2 is connected to an EMR, a verification
query will automatically be sent to the EMR to confirm the validity of the patient ID. If the EMR responds that the
patient ID is valid, then the patient ID icon, field values and Check Icon will flash four times.
If the EMR responds that the Patient ID is invalid, then the Patient icon field, field values and X icon will flash six times
and a message will appear in the Patient ID field indicating that the Patient ID entered is not valid.
NOTE: The Patient ID must be validated by the EMR before any vital signs measurements can be sent to the EMR.
Sending Measurements to EMR
Sending measurements to an EMR will work only if your ADView 2 has been configured to connect to an EMR. After
scanning a valid Patient ID, and taking vital signs measurements, press the Memory Button . The EMR icon
Page 36

93-9005-00 ADView 2 User Manual | 36
September 1, 2016
and Check Mark Icon will start flashing. Press the Selection Knob to send the data to the EMR. If the
measurements are delivered to the EMR successfully, you will see the EMR Message Icon and the Check Mark
Icon flash four times.
If the EMR has a problem receiving the measurements, the EMR Message icon and X icon will flash eight
times and you will hear 4 short beeps. You will see a message indicating the nature of the problem, such as:
“Rejected” or “Rejected for an error.” This message occurs when the device is connected to the network but the EMR
rejects the message. A corresponding message will appear in the Patient ID field during a three-second period when
the Message Icon and X icon flash on and off.
“No response (time out).” This message occurs when the device is connected to the network but the EMR is not
responding. It also can occur if network connectivity is lost. If there is a network connection, the ADView 2 will
continue to try to transmit the message every 10 seconds. A message in the Patient ID field will alert you that another
attempt to send the message will be made in 10 seconds. If network connectivity is lost, you will see a corresponding
message in the Patient ID field, and the device will continue trying to re-establish a connection and send the
measurements at intervals set during EMR setup. If problems persist, you may wish to contact your facility’s
information technology department. As a backup, the patient’s measurements are saved in the device memory when
the memory button was pressed, and can be retrieved using the procedure detailed in the beginning of Section 9.
Note: After the memory button is pressed, all values to be stored to memory will flash off and on twice, along with the
check mark icon, memory icon and memory location identifier. After flashing, the display will revert to null values for
the patient ID and all measurement data, and the check mark icon will flash off and on twice more.
10. Taking Care of Your ADView 2
Cleaning
CAUTION: The ADView 2 is not sterilizable. Do not immerse the monitor in any fluid or attempt to clean with
any liquid detergents, cleaning agents, or solvents.
Dampen a soft cloth with mild medical grade disinfectant and wipe the device to remove surface dust and dirt. Dry
surface thoroughly before use.
SpO2 Sensors and Thermometers
CAUTION: Never immerse sensors, clips or thermometers in fluids. Do not pour or spray any liquids onto the
sensor or thermometers. Caustic or abrasive cleaners will cause permanent damage. Do not open the case of the
Sp02 sensor finger clip sensor more than 45° or the case will be damaged.
Clean the Sp02 sensor and thermometers with a soft cloth dampened with a mild medical grade disinfectant or
isopropyl alcohol. Remove all tape residues. Allow the sensor and thermometer to dry thoroughly before reusing.
Page 37

93-9005-00 ADView 2 User Manual | 37
September 1, 2016
Preventative Maintenance
System Self Checks
The ADView 2 performs a range of system checks during normal operation. If the device detects a problem, it will
display an error code.Replaceable Parts
On a routine basis, inspect the monitor, cuffs, and hoses for cracks, fraying, or kinks. Immediately replace any
damaged part. Refer to the list of Accessories & Replacement Parts in this guide.
Replacing and Disposal of the Battery
CAUTION: Fire, explosion and severe burn hazard. Replace only with ADC part number 9005BAT.
When the battery no longer charges or it needs excessive recharging, it may need to be replaced. See the “Installing
Battery” section above for proper installation and precautions to be taken when installing the rechargeable battery.
Please dispose of the old battery per the instructions below.
Battery Disposal
The ADView 2 device contains a lithium-ion battery that contains materials which may be hazardous to human
health. Do NOT dispose of battery in domestic waste! Instead, please dispose of in an environmentally responsible
way, or return the battery to ADC. A prepaid return label can be obtained. Please see our website for more
information.
Product Disposal
Do not dispose of this product as unsorted municipal waste. Prepare this product for reuse or separate
collection as specified by Directive 2002/96/EC of the European Parliament and the Council of the European Union on
Waste Electronic and Electrical Equipment (WEEE).
Cuff Disposal
Do not return used cuffs. Used blood pressure cuffs may be contaminated medical waste and should be dealt
with in accordance to your local regulations.
Routine Calibration
Have the ADView 2 blood pressure function checked every two years to verify the accuracy of the pressure
transducers and indicators. Please have this done by a biomedical technician or ADC Customer Service.
CAUTION: Calibration should be done by a biomedical technician or other person familiar with the ADView 2
device.
If a biomedical technician performs this function, they should contact ADC for instructions to access “Verify
Calibration.” Instructions are also available in the ADView 2 Advanced Configuration manual (ADC Part 93-9005AC-
00).
American Diagnostic Corporation
55 Commerce Drive
Hauppauge, NY 11788
Tel: 1.800.ADC.2670
Page 38

93-9005-00 ADView 2 User Manual | 38
September 1, 2016
1.631.273.9600
Fax: 1.631.273.9659
Email: customerservice@adctoday.com
Web: www.adctoday.com
11. Accessories & Replacement Parts
Contact your ADC sales representative to purchase the following accessories or items:
Complete Modules (when purchased separately)
Part Number
Description
9005TO
Temperature Module, Oral/Axillary
9005TR
Temperature Module, Rectal
9005S
SpO2 ChipOx Module
9005SM
SpO2 Masimo Module
Mounting Platforms
Part Number
Description
9005D
Desktop Caddy*
9005W
Wall Mount with Basket
9005M
Mobile Stand with Basket
9005PSHELF
Printer Shelf for Mobile Stand
*Base unit is self-standing. Add the optional Desk Caddy to keep cuffs and cords organized.
Blood Pressure Cuffs
Part Number
Description
9005-9CGR-1MB
Child, Green (12-19cm)
9005-10SARB-1MB
Small Adult, Royal Blue (17-25cm)
9005-11AN-1MB
Adult, Navy (23-33cm)
9005-11APN-1MB
Adult Plus, Navy (28-40cm)
9005-12XBD-1MB
Large Adult, Burgundy (31-40cm)
Page 39

93-9005-00 ADView 2 User Manual | 39
September 1, 2016
9005-12XPBD-1MB
Large Adult Plus, Burgundy (40-55cm)
9005-13TBR-1MB
Thigh, Brown (38-50cm)
9005CKP
Pediatric Cuff Kit (Child, Small Adult, Adult)
9005CKA
Adult Cuff Kit (Adult, Large Adult)
9005CK
Five Cuff Kit (Child, Small Adult, Adult, Large Adult, Thigh)
*All cuffs equipped with male bayonet connectors.
Power Supplies
Part Number
Description
9005BAT
Rechargeable lithium-ion battery
9005PC
AC Power Cord, U.S.
9000PCEU
AC Power Cord, Europe
9000PCUK
AC Power Cord, U.K.
9000PCAU
AC Power Cord, Australia
Temperature Accessories
Part Number
Description
9000TP
Thermometer Probe Covers (20/Box, 25 Boxes/Case)
9000TP-5000
Thermometer Probe Covers (5000/Case)
9000TOP
Oral/Axillary Temperature Probe
9005TWB
Oral/Axillary Temperature Well
9005TOK
Oral/Axillary Temperature Kit (Probe, Well, Probe Covers)
9000TRP
Rectal Temperature Probe
9005TWR
Rectal Temperature Well
9005TRK
Rectal Temperature Kit (Probe, Well, Probe Covers)
SpO2 Accessories
Part Number
Description
9005SP
SpO2 ChipOx Finger Sensor, Adult
9005SPDYS
SpO2 ChipOx Finger Sensor, Pediatric
Other Accessories
Part Number
Description
Page 40

93-9005-00 ADView 2 User Manual | 40
September 1, 2016
9005USB
Mini USB to USB Cable
9005DON
USB Wi-Fi Dongle
9005P
Thermal Printer with Paper
9005PAPER
Thermal Printer Paper
9005SCAN
Barcode Scanner
12. Status Messages & Alarms!
If the ADView 2 has a problem taking a measurement, you may see a code on the display screen.
The icon will blink with the measurement display result and code.
Below are common error codes. Take action as directed on the screen, or as suggested in the table below.
Status Messages
Code Displayed
Action User Should Take
Blood Pressure:
1-2, 4, 87 & 88
Check that the cuff is in the correct position.
Check that the cuff is properly tightened and that the tubing is properly connected.
Check that there is no excessive clothing between the arm and cuff.
Check that the cuff applied is the correct size.
The patient may have been moving too much.
Take another BP reading.
Page 41

93-9005-00 ADView 2 User Manual | 41
September 1, 2016
3
No action possible. Values are outside the reportable range.
85 & 89
Check that the hose has no sharp bends and is not pinched.
Check that the patient is not lying on the cuff.
Check that the cuff is in the correct position.
Take another BP reading.
86
If cancelled reading was unintended, allow cuff to deflate & restart reading.
If cancelled reading was intended, no action needed.
90, 91, 97-107
Power off and repower on the ADView 2.
If issue persists, call SMI Customer Service. Service Required
Temperature:
505 & 506
Make sure patient has not had any warm beverages recently (oral), and then retry
measurement.
501-504, 507-514,
516-517
Remove and then replace the probe in the well.
Retry reading.
Power off and repower on the ADView 2.
Retry reading. If issue persists, call customer service.
520-524 & 526
Power off and repower on the ADView 2.
Retry reading.
If issue persists, replace the probe. Retry reading.
If issue persists, replace module or call Customer Service.
530
Use in normal room temperature environment suitable to the device.
SpO2:
121-124, 133-136,
150-156
Remove sensor from the patient and ensure proper positioning. Attempt new measurement
ensuring proper protocol as described in Section 7.
Unplug sensor and reconnect.
If issue persists, power off and repower on the ADView 2.
Retry reading. If issue persists, call Customer Service.
126-132
137-149 & 157-192
Power off and repower on the ADView 2.
If issue persists, replace module or call Customer Service
Electronic Medical Records (EMR):
601-610 & 612,614
Check EMR configuration, wireless strength, and proximity. Call your biomedical engineer or
IT support.
611
Ensure proper barcode and patient ID scanned.
Page 42

93-9005-00 ADView 2 User Manual | 42
September 1, 2016
613
Multiple patients have the same patient ID.
ADView 2 System:
300-302
Power off and repower on the ADView 2.
341-344
Power off and repower on the ADView 2.
Return for service.
Powering Device:
201
Run only from A/C power source.
Have battery service performed at earliest convenience.
202
Power off and repower on the ADView 2.
If repower attempt does not work, plug in power source and power off and repower on
ADView 2 again.
Retry reading. If issue persists, call Customer Service.
203-211
Clear the error and power off and repower on the ADView 2.
Replace battery.
212-214
Call Customer Service.
215
Charge battery.
Barcode Scanner:
701-706
Check and ensure proper barcode and patient ID was scanned
Rescan patient ID
Disconnect and reconnect cable from USB port on ADView 2.
Replace barcode scanner
Printing:
381
Close printer door.
382
Replace paper.
383- 387 & 388-390
Check cables. Reset printer. Replace printer.
Out of Range Measurements
The ADView 2 can measure BP that falls within the following ranges.
Systolic: Adult 40-260 mmHg, Pediatric 40-230 mmHg
Diastolic: Adult 20-200 mmHg, Pediatric 20-160 mmHg
Heart Rate: 30 - 220 BPM
The ADView 2 will display temperature and SpO2 within the following ranges.
Page 43

93-9005-00 ADView 2 User Manual | 43
September 1, 2016
Temperature: 30.1 to 42.9 °C (86.2 – 109.2 °F)
SpO2: 70 – 100%
If measurement is above or below specified ranges:
Single Measurement Mode: The highest or lowest possible threshold range will blink in the appropriate BP
measurement field.
Averaging Mode: will blink in addition to the status code “3” in the blood pressure measurement display.
Temperature: Depending on the out-of-range value, the display will indicate whether the value is “Hi” or “Lo.”
SP02: Results falling below the measurement range will display “Lo” in the results field.
Service Centers
American Diagnostic Corporation
55 Commerce Drive
Hauppauge, NY 11788
Tel: 1.800.ADC.2670
1.631.273.9600
Fax: 1.631.273.9659
Email: customerservice@adctoday.com
Web: www.adctoday.com
13. Frequently Asked Questions
What is the expected battery life for the ADView 2?
When batteries are fully charged, the ADView 2 is designed to run on internal battery power for 200 measurement
cycles (BP, temperature and SpO2 measurements) before requiring recharge. When only the bottom segment of the
battery symbol is illuminated, there is between 11 percent and 40 percent battery power remaining.
Will the device automatically power down?
When no measurements have been taken or buttons pressed for one hour, the device will automatically power down
and enter a low-power state. This is considered “off.”
What is the significance of different beeps I hear during operation?
One long beep (approximately 3 seconds): Successful end of BP measurement.
Page 44

93-9005-00 ADView 2 User Manual | 44
September 1, 2016
Three long beeps: Severe hardware error.
One short beep (1 second): Power up or Power down complete.
Four short beeps: There has been an error.
If you abort a BP measurement by pressing the Start/Stop button, you will hear one short beep. Then, after the
pressure has been completely dumped from the cuff, you will hear one long beep.
Can pulse oximetry, temperature measurement, a barcode scanner or printer be added later too?
Yes, you can add vital signs modules and accessories to the ADView 2 at any time.
Can EMR connectivity be established at a later date if not enabled as part of initial configuration?
Yes, EMR settings can be accessed via the Advanced Configuration Application at any time.
Why is no heart rate data showing on the display?
If the device is configured to use the Sp02 sensor as the pulse rate source, and the sensor is not attached to the
patient, no heart rate data will show on the display. Only dashes will appear, or MAP results if MAP measurement is
enabled. Note that MAP is not available in the United States.
How many measurements can be stored in the ADView 2 memory?
The ADView 2 can store 99 unique sets of measurements. After the 99th measurement has been saved, the next
measurement will have the Memory Location Identifier label “01” and will overwrite the most recent measurement
(measurement 99). After all results are cleared from the memory, the Memory Location Identifier also will return to
“01.”
14. Technical Information!
EMC Statement
This equipment needs special precautions regarding EMC and needs to be installed and put into service according to
the EMC information provided in this document. This equipment has been tested and found to comply with the limits
for medical devices to IEC60601-1-2: 2007. These limits are designed to provide reasonable protection against
harmful interference in a typical medical installation. This equipment generates, uses, and can radiate radio
frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference
to other devices in the vicinity. However, there is no guarantee that interference will not occur in a particular
installation. If this equipment does cause harmful interference to other devices, which can be determined by turning
the equipment off and on, the user is encouraged to try to correct the interference by one or more of the following
measures:
Reorient or relocate the receiving device.
Increase the separation between the equipment.
Connect the equipment into an outlet on a circuit different from which the other device(s) are connected.
Consult the manufacturer or field service technician for help.
Portable and mobile RF communications equipment can affect Medical Electrical Equipment.
WARNING: Use of accessories, transducers, and cables other than those specified may result in increased emissions
or decreased immunity of the ADView 2.
Page 45

93-9005-00 ADView 2 User Manual | 45
September 1, 2016
WARNING: The ADView 2 should not be used adjacent to or stacked with other equipment. If adjacent or stacked use
is necessary, the ADView 2 should be observed to verify normal operation in the configuration in which it will be used.
Guidance and manufacturer’s declaration – electromagnetic emissions
The ADView 2 is intended for use in the electromagnetic environment specified below. The customer or the user of
the ADView 2 should assure that it is used in such an environment.
Emissions
Compliance
Electromagnetic environment-- guidance
RF emissions
CISPR 11
Group 1
The ADView 2 uses RF energy only for its internal function. Therefore,
its RF emissions are very low and are not likely to cause any
interference in nearby electronic equipment.
RF emissions
CISPR 11
Class B
The ADView 2 is suitable for use in all establishments, including
domestic establishments and those directly connected to the public
low-voltage power supply network that supplies buildings used for
domestic purposes.
Harmonic
emissions IEC
61000-3-2
Class A
Voltage
fluctuations/flicker
emissions IEC
61000-3-3
Complies
Guidance and manufacturer’s declaration – electromagnetic immunity
The ADView 2 is intended for use in the electromagnetic environment specified below. The customer or the user of
the ADView 2 should assure that it is used in such an environment. When the product is in an environment with
static, the product may show error SpO2 reading, it will recover automatically when the static is gone. Though it
may exhibit DEGRADATION of performance (e.g., deviation from MANUFACTURER’S specifications) that does not
affect BASIC SAFETY or ESSENTIAL PERFORMANCE.
Immunity test
IEC 60601
test level
Compliance level
Electromagnetic environment
--guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
±6 kV contact
±8 kV air
±4 kV contact
±4 kV air
Floors should be wood, concrete or
ceramic tile. If floors are covered with
synthetic material, the relative humidity
should be at least 40%.
Electrical fast
transient/burst
IEC 61000-4-4
±2 kV for power
supply lines and
patient coupled
lines
±2 kV for power
supply lines and patient
coupled lines
Mains power quality should be that of a
typical commercial or hospital
environment.
Surge
IEC 61000-4-5
±1 kV line(s) and
neutral
±1 kV line(s) and neutral
Mains power quality should be that of a
typical commercial or hospital
environment.
Page 46

93-9005-00 ADView 2 User Manual | 46
September 1, 2016
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
<5 % UT
(>95 % dip in UT)
for 0,5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
<5 % UT
(>95 % dip in UT)
for 5s
<5 % UT
(>95 % dip in UT)
for 0,5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles)
<5 % UT
(>95 % dip in UT)
for 5s
Mains power quality should be that of a
typical commercial or hospital
environment. If a dip or an interruption of
mains power occurs, the current of the
ADView 2 may be dropped off from
normal level, it may be necessary to use
uninterruptible power supply or a battery.
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
3 A/m
3A/m
Power frequency magnetic fields should
be at levels characteristic of a typical
location in a typical commercial or
hospital environment.
NOTE: UT is the A.C. mains voltage prior to application of the test level
In the event of an error, the device will auto-recover within 5 seconds.
Immunity test
IEC 60601
test level
Compliance
level
Electromagnetic environment – guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to
80 MHz
3 V/m
80 MHz to
2.5 GHz
3 Vrms
3 V/m
Portable and mobile RF communications equipment should
be used no closer to any part of the ADView 2, including
cables, than the recommended separation distance
calculated from the equation applicable to the frequency of
the transmitter.
Recommended separation distance
where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation
Distance in metres (m).
Field strengths from fixed RF transmitters, as determined by
an electromagnetic site survey, should be less than the
Page 47

93-9005-00 ADView 2 User Manual | 47
September 1, 2016
compliance level in each frequency range.
Interference may occur in the vicinity of equipment
marked with the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption
and reflection from structures, objects and people.
a. Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land
mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with
accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey
should be considered. If the measured field strength in the location in which the ADView 2 is used exceeds the
applicable RF compliance level above, the ADView 2 should be observed to verify normal operation. If abnormal
performance is observed, additional measures may be necessary, such as re-orienting or relocating the ADView 2.
b. Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
!
Recommended separation distances between portable and mobile RF communications equipment and the ADView
2
The ADView 2 is intended for use in an electromagnetic environment in which radiated RF disturbances are
controlled. The customer or the user of the ADView 2 can help prevent electromagnetic interference by maintaining
a minimum distance between portable and mobile RF communications equipment (transmitters) and the ADView 2
as recommended below, according to the maximum output power of the communications equipment.
Rated maximum output
power
of transmitter
W
Separation distance according to frequency of transmitter
m
150 kHz to 80 MHz
80 MHz to 800 MHz
800 MHz to 2.5 GHz
0.01
0.12
0.12
0.23
0.1
0.38
0.38
0.73 1 1.2
1.2
2.3
10
3.8
3.8
7.3
100
12
12
23
Page 48

93-9005-00 ADView 2 User Manual | 48
September 1, 2016
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in
metres (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the
maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption
and reflection from structures, objects and people.
Specifications, General!
Conditions for
Use:
Operating: 10°C (50°F) to 40°C (104°F) 15 – 90% RH non-condensing - 700 kPa - 1060 kPa.
Operating the monitor in an environment at maximum temperature can produce temperatures
exceeding 41°C (41.6°C highest recorded) on a patient applied part. It is up to the operator to
determine if this temperature is too high based upon the condition of a patient and, if so, to
ensure the ambient temperature of the environment is 38°C or below.
Storage:
-20°C (-4°F) to 55°C (131°F) 15 – 90% RH non-condensing - 500 kPa - 1060 kPa. Performance
can be affected if, used or stored outside the specified temperature, humidity, or altitude listed
in the ranges above.
Power:
Internal power supply. Input: 100-240 VAC @ 1.5A max, 50-60 Hz. Output +9VDC @ 5A IEC 320
type input connector.
Calibration:
The accuracy of cuff - pressure transducers/indicators should be verified bi-annually.
Safety
Systems:
Independent hardware over-pressure circuit and redundant software overpressure algorithm to
limit cuff pressure to less than 300 mmHg (+20/-10mmHg). Independent hardware timing
circuit and redundant software timer algorithm to limit the duration of a blood pressure cycle to
less than 180 seconds.
Dimensions:
Size (without thermometer option): 8” x 6.75’’ x 5.2’’)
Standards:
ANSI/AAMI ES60601-1:2005/(R)2012 and A1:2012, C1:2009/(R)2012 and A2:2010/(R)2012, EN
60601-1:2006/A1:2013, IEC 60601-1-2: 2007 EMC, IEC 80601-2-30: 2013, ISO 80601-2-61: 2011,
ISO 15223-1:2012, ISO 10993-1, 2009, ISO 10993-5, 2009, EN ISO 10993-10, 2010, ISO 810602:2013, EN 50419: 2006, EN ISO 14971:2009, CSA C22.2 No. 60601-1, EN ISO 81060-1: 2012,
IEC 60601-1-6: 2013
Classifications:
Equipment Classification: Class IIa per MDD, Class II (Electrical Shock), Continuous mode of
operation, CE
Ingress of
liquid
Ingress Protection: IPX1 for BP and SpO2 modules.
IPX0 for temperature modules (no protection of ingress to liquids).
Weight
0.6 lbs (284 gm)
Specifications, Blood Pressure Measurement
Measurement:
Oscillometric with step deflation
Range:
Pressure: Diastolic: 20-200 mmHg (adult), 20-160
mmHg (pediatric). Systolic: 40-260 mmHg (adult), 40230 mmHg (pediatric)
Heart Rate: 30-220 BPM (beats per
minute)
Accuracy (BP):
Meets or exceeds ANSI/AAMI/ISO 81060-2:2013 standard for noninvasive accuracy (±5mmHg
Page 49

93-9005-00 ADView 2 User Manual | 49
September 1, 2016
mean error with 8mmHg standard deviation).
!
Notes on Blood Pressure Data
Any blood pressure reading can be affected by the measurement site, the position of the patient, exercise, or the
patient’s physiologic condition. Environmental or operational factors which can affect the performance of the device
and/or its blood pressure reading are pacemakers and common arrhythmias such as atrial or ventricular premature
beats or atrial fibrillation, arterial sclerosis, poor perfusion, diabetes, age, pregnancy, pre-clampsia, renal diseases,
patient motion, trembling, and shivering.
Specifications SP02 Sensor
Function
ChipOx
Masimo M-LNCS DCI
Saturation Normal (no motion)
± 2%
(70-100%A
rms
)
± 2%
(70-100%A
rms
)
Saturation Motion
N/A
± 3%
(70-100%A
rms
)
Low Perfusion
N/A
SpO2 +/-2%
Pulse Heart rate
20-300 BPM: ±3 BPM
± 3% BPM
Pulse Heart rate w/motion
20-300 BPM: ±3 BPM
± 5% BPM
Wavelength & Output Power
RED: 660nm @ 3.5-4.5mW
IR: 905nm @ 3.5-4.5mW
RED: 660nm @ ≤ 15mW
IR: 905nm @ ≤ 15mW
For more detailed information, see the instructions for use included with each device.
Specifications Temperature Sensor
Temperature accuracy
30.1 to 42.9 °C (86.2 – 109.2 °F)
Quick Mode (Oral): ± 0.3°C
Std. Mode (Axillary/Rectal): ± 0.1°C
Direct Mode: ± 0.1°C
Response Time
3-10 sec (Oral Quick Mode)
6-10 (Oral Standard Mode)
8-12 sec.(Axillary Mode)
10-14 sec.(Rectal Mode)
60-120 sec.(Direct Mode all sites)
Resolution
0.1°C
Page 50

93-9005-00 ADView 2 User Manual | 50
September 1, 2016
Limited Warranty
ADC. provides to the original purchaser the following limited warranty from date of invoice.
Main unit, SpO2 modules, temp modules
3 years
Blood Pressure Cuffs
2 years
Desktop Caddy, Wall Mount, Mobile Stand
2 years
Accessories (SpO2 sensors, temp probes, etc.)
90 days
ADC warrants each instrument to be free from defects in material and workmanship. Liability under this warranty
covers servicing of the instrument when returned from the customer’s facility within the United States prepaid to the
factory. ADC will repair any component(s) or part(s) that it finds to be defective during the period of this limited
warranty. Should a defect become apparent, the original purchaser should first notify ADC of the suspected defect.
The device should be carefully packaged and shipped prepaid to:
American Diagnostic Corporation
55 Commerce Drive
Hauppauge, NY 11788
Tel: 1.800.ADC.2670
1.631.273.9600
Fax: 1.631.273.9659
The device will be repaired in the shortest possible time and returned prepaid by the same shipping method as
received by the factory. This limited warranty is void if the device has been damaged by accident, misuse, negligence,
or act of God, or serviced by any person not authorized by ADC. This limited warranty contains the entire obligation of
ADC and no other warranties expressed, implied, or statutory are given. No representative or employee of ADC is
authorized to assume any further liability or grant any further warranties except as herein.
Page 51

93-9005-00 ADView 2 User Manual | 51
September 1, 2016
 Loading...
Loading...