Page 1

DiagnostixTMAneroids
®
Pocket, Palm, Clock Type
Use, Care, & Maintenance
1
Page 2

ADC®Aneroid Sphygmomanometer
LATEX
°
F
(manometer, cuff, bladder, bulb and valve)
®
Thank you for choosing an ADC
DIAGNOSTIXTMAneroid Sphygmomanometer. Your new
ADC®DIAGNOSTIXTManeroid is designed to provide unrivaled durability, and unparalleled comfort. Every component has been carefully designed to maximize performance.
This Use, Care, & Maintenance guide refers to ADC model series; 700, 703, 705, 720,
728, 731, 732, 740, 750, 752, 778, and 788.
Device Description and Intended Use
ADC®aneroid sphygmomanometers are used by professional healthcare providers and
individuals trained in the auscultatory blood pressure technique to determine systolic and
diastolic blood pressure in humans.
Size Chart
Contraindications:
Aneroid sphygmomanometers are contraindicated for neonate use. Do not use
with neonatal cuffs or neonate patients.
Review the size chart (right) for proper
age and limb range usage.
Cuff Size
Infant 7I 3.5 to 5.5 9 to 14
Child 9C 5.1 to 7.6 13 to 19.5
Sm. Adult 10SA 7.4 to 10.6 19 to 27
Adult 11A 9 to 15.7 23 to 40
Lg. Adult 12X 13.3 to 19.6 34 to 50
Thigh 13T 15.7 to 25.9 40 to 66
Bariatric 12BX 17.3 to 25.9 44 to 66
Limb Range
Inches CM
Symbol Definitions
The following symbols are associated with your ADC® DiagnostixTMAneroid
Definition
ymbol
S
Authorized European
Represenat
Manufacturer’s I
Temperature Limit
Humidity Limitation
2
Definition
ymbol
S
Important Warning/Caution
N
rubber
C
Conforms to EU Standards
ot
rcumf
i
made w
lat
erence Si
natural
ith
ex
ze
ve’s Information
i
nformation
Page 3

General Warnings
!
A warning statement in this manual identifies a condition or practice which,
if not corrected or discontinued immediately could lead to patient injury, illness, or death.
WARNING:
!
Do not allow a blood pressure cuff to remain on patient for more than 10
minutes when inflated above 10 mmHg. This may cause patient distress,
disturb blood circulation, and contribute to the injury of peripheral nerves.
!
WARNING:
If luer lock connectors are used in the construction of tubing, there is a
possibility that they might be inadvertently connected to intra-vascular fluid systems,
allowing air to be pumped into a blood vessel. Immediately consult a physician if this occurs.
WARNING: Safety and effectiveness with neonate cuff sizes 1 through 5 is not estab-
!
lished.
!
WARNING: If this equipment is modified, appropriate inspection and testing must be
conducted to ensure its continued safe use.
WARNING: Do not apply cuff to delicate or damaged skin. Check cuff site frequently
!
for irritation.
WARNING: Only use the cuff when the range markings indicated on the cuff show
!
that the proper cuff size is selected, otherwise erroneous readings may result.
WARNING: Allow space between patient and cuff. Two fingers should fit in
!
this space if the cuff is correctly positioned.
WARNING: Do not apply cuff to limbs used for IV infusion.
!
WARNING: Patient should remain still during measurement to avoid erroneous read-
!
ings.
WARNING: When using with an infant or child cuff, extra care must be taken to pre-
!
vent over-inflation. With smaller cuffs (infant or child) the cuff can inflate to over
300mmHg with just two full compressions of the bulb. To prevent discomfort or injury to
the patient and damage to the instrument, bulb should only be partially squeezed, so that
each “stroke” inflates the cuff in 40mmHg to 60mmHg increments until inflated to the desired level.
!
WARNING: This product may contain a chemical known to the state of California to
cause cancer, birth defects, or other reproductive harm.
CAUTION:
!
To obtain the greatest accuracy from your blood pressure instrument, it is
recommended that the instrument be used within a temperature range of 50°F (10°C)
to 104°F (40°C), with a relative humidity range of 15%-85%
(non-condensing).
CAUTION: Extreme altitudes may affect blood pressure readings. Your device has
!
been designed for normal environmental conditions.
3
Page 4
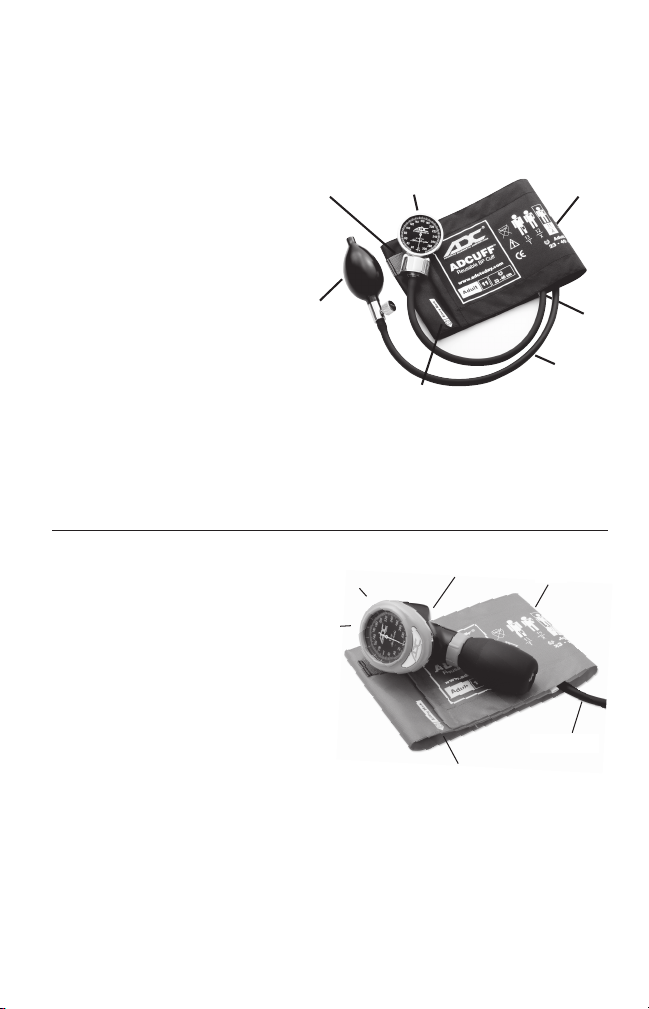
Operation of DiagnostixTMPocket Aneroids
This booklet contains operating and
maintenance information for the DIAG-
TM
NOSTIX
700, 720, 728, and 778 Se-
Hang Tab
ries pocket aneroid sphygmomanometers.
Please read and retain.
Your ADC DIAGNOSTIX
aneroid sphygmomanometer consists of
an aneroid manometer (gauge), com-
TM
brand pocket
Bulb & Valve
plete inflation system (ADCUFFTMcalibrated nylon cuff, latex-free inflation
bladder, squeeze bulb, and the AD-
TM
FLOW
valve), a
zippered carrying case, and operating instructions.
Most models are preassembled and ready for use. In units requiring assembly, the bulb
and valve should connect to the tube closest to the Index Line. The gauge connects to the
remaining tube.
Gauge
Artery Mark
Index Line
Bladder &
Tubing
Cuff
Operation of DiagnostixTMPalm
Connector Port
Trigger Valve
Cuff
Aneroids
This booklet contains operating and
maintenance information for the
DIAGNOSTIX
TM
703, 788 Series,
System 3, Pediatric, General Practice, System 4
and System 5 Multicuff
instruments. Please read and retain.
™
Your DIAGNOSTIX
palm style aneroid
manometer with integral bulb and trigger air release valve
(#804N), ADCUFF™ calibrated cuff with Size Guide™marking system, bladder, luer slip connector (attached to bladder tubing), zippered carrying case, and instruction booklet. ADC®’s Multicuff Systems (System 3, 4, 5, GPK, and PMK) contain the #804N
manometer and a variety of cuffs with single tube bladders, each equipped with a luer slip connector, and a compartmentalized case.
Gauge
Bladder Tubing
Artery Mark
4
Page 5

Connecting Gauge to Cuff
Connector
To connect the cuff to the gauge insert the luer slip connector
at the end of the bladder tube into the receptacle on the top of the gauge
with a firm twist until secure. To remove, reverse procedure.
NOTE: To avoid damage to the instrument, do not force connection into port.
Using the Trigger Valve
Deflation: Gently squeeze the bottom of the trigger to deflate the cuff. Altering
the pressure affects the deflation rate.
Rapid Exhaust: To exhaust remaining air after measurement is completed,
squeeze trigger until it clicks and locks in place.
Caution: Never force trigger.
Operation of DiagnostixTMClock Aneroids
This booklet contains operating and maintenance
information for the DIAGNOSTIX™750W series,
Wall Mount, 752M series, Mobile and 750D series, Desktop Aneroid Sphygmomanometers.
Please read and retain.
Your DIAGNOSTIX
™
750/752 consists of a large
“clockface” aneroid with easy-to-read 6” dial, an
integral swivel bracket/cuff storage compartment
(wall series only), complete inflation system
(which includes the ADCUFF
™
nylon cuff with Size
Guide™marking system, latex-free inflation bladder, bulb, and the ADFLOW™valve), 8 foot length
Valve
Bulb
coiled tubing, luer connectors, mounting hardware, and operating instructions. The DIAGNOSTIX™ 752M is mounted on a height adjustable,
Spider-Leg
To assemble the inflation system, connect coiled tubing to air inlet nipple at bottom of gauge. Insert
male luer adapter on bladder tubing into female receptacle at free end of coiled tubing. Store folded inflation system in swivel bracket behind gauge on 750W and 750D or in integral basket on 752M.
™
5 leg mobile stand. The DIAGNOSTIX™ 750D is mounted on a desktop stand.
Cuff
Manometer
Coiled Tubing
5
Page 6

AdcuffTMIntended Use:
Index Line
T
M
1
3
T
1
2
X
1
0
SA9C7I
1
1
A
Adult - 11
23-40 cm
11
T
M
23-40 cm
Reusable BP Cuff
11
Index Line
ADC®blood pressure cuffs are noninvasive and are intended for use with manual and automated noninvasive blood pressure measurement devices.
®
’s Size Guide™ marking system assures use of correct cuff size and proper cuff
ADC
alignment. Printed Index and Range markings and applicable limb range (in cm) allow
easy identification of the correct cuff size. An artery mark printed on both sides indicates
bladder midpoint for correct cuff positioning. A convenient nylon gauge hang tab permits
flexible use with either pocket aneroids or mercury manometers. Hook and loop adhesive
surface provides a snug, infinitely variable fit and is designed to withstand a minimum of
30,000 open/close cycles.
Gauge Hang
Tab
Size Icons
Index Line Artery Mark
Range Lines
6
Hook & Loop
Closure Adhesive
Bladder FlapArtery Mark
Page 7

Measurement Procedure
Index Line
1. Patient Position
The patient should sit or lie comfortably. The arm should be fully supported on a flat
surface at heart level. (If the arm’s position varies, or is not level with the heart, measurement values obtained will not be consistent with the patient’s true blood pressure.) When
seated, the patient should have their back and arm supported, and their legs should not be
crossed. The patient should relax prior to measurement comfortably for five (5) minutes
and should refrain from talking or moving during measurement. Observer should view
manometer in a direct line to avoid “Parallax error”.
2. Apply the cuff
TM
ADCUFF
signed to promote the precisely accurate
determination of blood pressure. Index
and range markings ensure use of the correct cuff size. The artery mark indicates
proper cuff positioning.
Place the cuff over the bare upper arm
with the artery mark positioned directly
over the brachial artery. The bottom edge of the cuff should be positioned approximately
(1”) one inch (2-3cm) above the antecubital fold. Wrap the end of the cuff not containing
the bladder around the arm snugly and smoothly and engage adhesive strips (Figure 1).
To verify a correct fit, check that the Index Line falls between the two Range Lines (Figure
2).
nylon cuffs are specially de-
Select Smaller Cuff
Left Arm
Tube
(Figure 1)
Correct Cuff Size
Select Larger Cuff
Index Line Artery Mark
Range Lines
7
(Figure 2)
Page 8

3. Inflate the cuff
Close the ADFLOWTMvalve by turning thumbscrew clockwise.
Palpate the radial artery while inflating the cuff. Be sure to inflate cuff quickly by squeezing
bulb rapidly.
Inflate cuff 20-30 mmHg above the point at which the radial pulse disappears.
NOTE: Cuff pressure range is 0 mmHg to 300 mmHg.
4. Position the Stethoscope
Position the chestpiece in the antecubital space below the cuff, distal to the brachium. Do
not place chestpiece underneath the cuff, as this impedes accurate measurement. Use the
bell side of a combination stethoscope for clearest detection of the low pitched Korotkoff
(pulse) sounds.
5. Deflate the cuff
Open the valve to deflate the cuff gradually at a rate of 2-3 mmHg per second.
6. Measurement
Record the onset of Korotkoff sounds as the systolic pressure, and the disappearance of
these sounds as diastolic pressure. (Some healthcare professionals recommend recording
diastolic 1 and diastolic 2. Diastolic one occurs at phase 4).
NOTE: It is recommended that K4 be used in children aged 3 to 12, and K5 should be
used for pregnant female patients unless sounds are audible with the cuff deflated, in
which case K4 should be used. K5 should be used for all other adult patients.
After measurement is completed, open valve fully to release any remaining air in the cuff.
Remove cuff.
8
Page 9

Care and Maintenance
STORAGE:
Pocket Gauge: After measurement, fully exhaust cuff then wrap cuff around gauge and
bulb and store in zippered carrying case.
Palm Gauge: After measurement, fully exhaust cuff and store in zippered carrying case.
If unit will not be used for an extended period of time, leave the trigger valve in the full exhaust position.
Clock Gauge: After measurement, fully exhaust cuff then wrap cuff around bulb and store
in storage compartment.
NOTE: This product will maintain the safety and
performance characteristics specified at temperatures ranging from 50°F to 104°F (10°C to 40°C) at a relative humidity level of 15% to 85%. This device can safely be stored at
temperatures ranging from -4°F (-20°C) to 131°F (55°C)
with a relative humidity of 90%.
Manometer: Your ADC DIAGNOSTIX
TM
brand pocket
aneroid gauge (#800 or #802), palm aneroid (804N) or clock aneroid (805) requires minimal care and maintenance.
The manometer may be cleaned with a soft cloth but should not be dismantled under any
circumstances.
Gauge accuracy can be checked visually; simply be certain the needle rests within the
printed oval when the unit is fully deflated (Figure 3).
Should the indicator needle of the manometer rest outside of this calibration mark, then
the manometer must be re-calibrated to within ±3 mmHg when compared to a reference
device that has been certified to national or international measurement standards. A
manometer whose indicator needle is resting outside of this mark, is NOT acceptable for
use.
NOTE: Store gauge with
valve in full exhaust position.
(Figure 3)
In the event that the gauge is ever in need of calibration, simply return to ADC. Damaged
or broken parts will be replaced as needed at a minimum charge. Refer to the warranty for
specific details of warranty coverage.
The manufacturer recommends a calibration check every 2 years.
9
Page 10

Cuff Cleaning and Disinfecting
LOT 0 00000 000
Hauppauge, NY
ADC
NOTE: Use one or more of the following methods and allow to air dry:
• Wipe with mild detergent and water solution (1:9 solution). Rinse.
• Wipe with Enzol per manufacturer’s instructions. Rinse.
• Wipe with .5% bleach and water solution. Rinse
• Wipe with 70% isopropyl alcohol.
• Launder with mild detergent in warm water, normal wash cycle.
Remove bladder first. Cuff is compatible with 5 wash cycles.
Low Level Disinfection
Prepare Enzol enzymatic detergent according to the manufacturer’s instructions. Spray detergent solution liberally onto cuff and use a sterile brush to agitate the detergent solution
over entire cuff surface for five minutes. Rinse continuously with distilled water for five minutes. To disinfect, first follow the cleaning steps above, then spray cuff with 10% bleach
solution until saturated, agitate with a sterile brush over entire cuff surface for five minutes.
Rinse continuously with distilled water for five minutes. Wipe off excess water with sterile
cloth and allow cuff to air dry.
CAUTION: Do not iron cuff.
!
!
CAUTION: Do not heat or steam sterilize cuff.
Manometer Quality Control
A Serial number and Lot number are
automatically assigned to every aneroid
during manufacturing, ensuring every
item is "controlled".
(Figure 4)
The Serial Number can be located on the
faceplate of each aneroid (Figure 4).
The Lot number is located on the outside
label of the manometer
packaging (Figure 5).
10
Lot Number
(Figure 5)
Page 11

Standards
ANSI/AAMI/ISO 81060-1:2007
EN/ISO 81060-1:2012
Disposal
When your sphygmomanometer has reached its end of life, please be sure to dispose of it
in accordance with all regional and national environmental regulations. Devices that have
become contaminated should be disposed of in accordance with all local ordinances and
regulations.
Warranty
American Diagnostic Corporation’s (ADC®) warranty service extends to the original retail
purchaser only and commences from the date of delivery. ADC warrants its products against
defects in materials and workmanship under normal use and service as follows:
• Your DIAGNOSTIX™ manometer is warranted for life. The manometer is
warranted to remain accurate to +/-3mmHg (or the prevailing standard) over its full
range when compared to a reference standard for life.
• Inflation system components (cuff, bladder, tubing, bulb, valves, connectors) are
warranted for three years.
What is Covered: Replacement of parts, and labor.
What is Not Covered: Transportation charges to ADC
®
. Damages caused by abuse,
misuse, accident, or negligence. Incidental, special, or consequential damages. Some
states do not allow the exclusion or limitation of incidental, special, or consequential
damages, so this limitation may not apply to you.
To Obtain Warranty Service: Send item(s) postage paid to ADC
®
, Attn: Repair Dept.,
55 Commerce Dr., Hauppauge, NY 11788. Please include your name and address, phone
no., proof of purchase, and a brief note explaining the problem.
Implied Warranty: Any implied warranty shall be limited in duration to the terms of
this warranty and in no case beyond the original selling price (except where prohibited
by law). This warranty gives you specific legal rights and you may have other rights
which vary from state to state.
11
Page 12

To register your product visit us at
www.adctoday.com/register
FOR QUESTIONS, COMMENTS,
OR SUGGESTIONS CALL TOLL FREE:
1-800-ADC-2670
OR VISIT
www.adctoday.com/feedback
ADC
55 Commerce Drive
Hauppauge, NY 11788
U.S.A.
ADC (UK) Ltd.
Unit 6, PO14 1TH
United Kingdom
IB p/n 9350N-00 rev 7 Printed in the U.S.A.
Assembled, inspected, and
packaged in the U.S.A.
tel: 631-273-9600
toll free: 1-800-232-2670
fax: 631-273-9659
www.adctoday.com
info@adctoday.com
0197
 Loading...
Loading...