Page 1
i-STAT
Abbott Point of Care Inc.
104 Windsor Center Drive
East Windsor, NJ 08520 • USA
USA Tech Suport: (800) 366-8020
Fax: (609) 443-9310
Emergo Europe
P.O. Box 18510
2502 EM The Hague
The Netherlands
Tel: (31)70 345 8570
Fax: (31)70 346 7299
Art: 714254-01E Rev. Date: 07/18/06
i-STAT®1
User Guide
©2006 Abbott Point of Care Inc. All rights reserved. Printed in USA.
i-STAT is a registered trademark of Abbott Laboratories.
Page 2

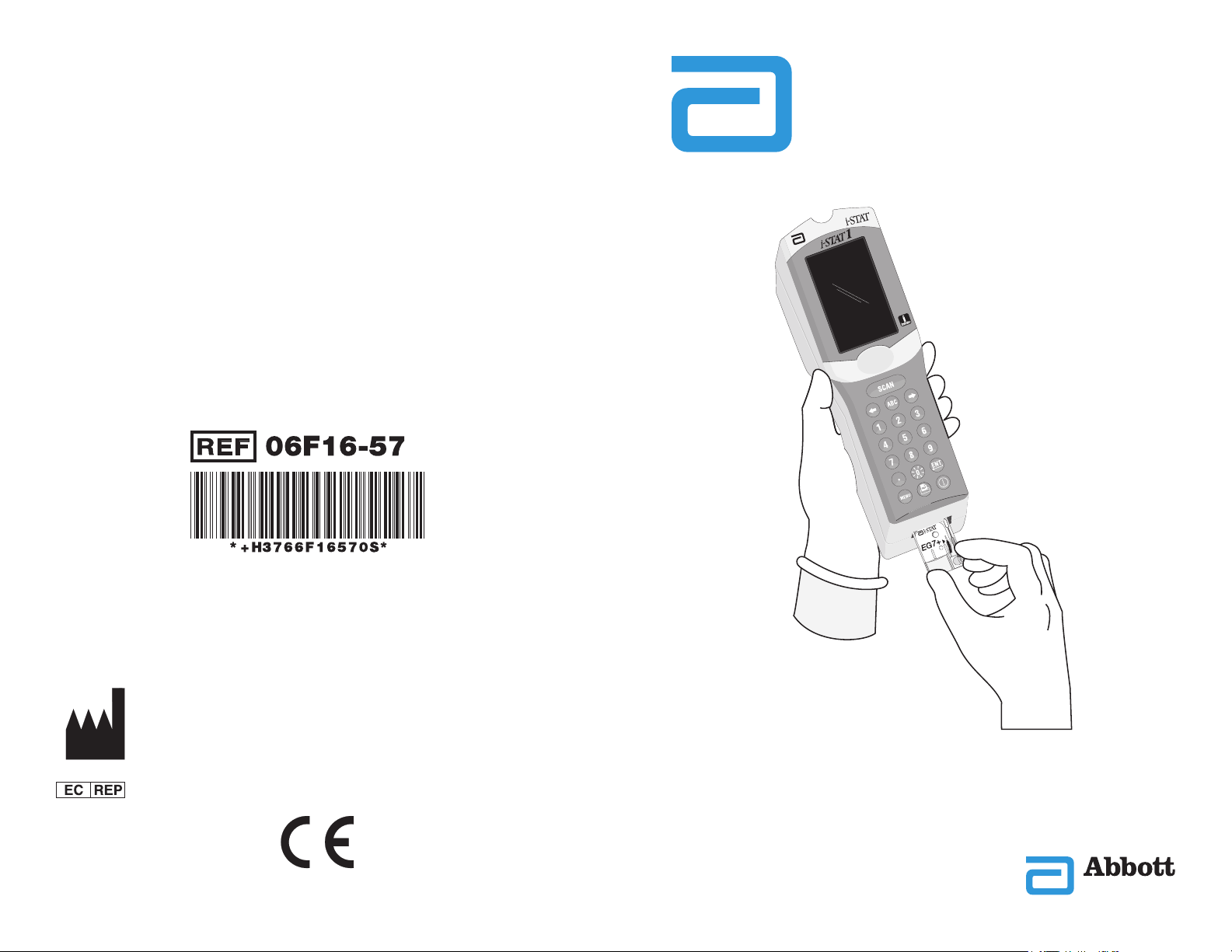
System Components
i-STAT 1 Analyzer
i-STAT cartridge
Foil Packet containing
the MediSense®
Precision PCx™ or
PCx™ Plus Glucose Test
Strip
i-STAT 1
Downloader
i-STAT 1 Downloader/
Recharger
For in vitro diagnostic use.
See System Manual for instructions.
Intended Use: The i-STAT 1 Analyzer is intended for use with i-STAT cartridges
for the in vitro quantification of various analytes in whole blood and with the
Abbott Medisense® Precision PCx™ and PCx™ Plus Blood Glucose Test Strips for
the in vitro quantification of glucose in whole blood.
Electronic
Simulator
Martel Printer
Page 3
Message Cause Action
Strip Error A wet strip inserted, strip
No display Disposable batteries dead
Cartridge Locked
does not disappear after test cycle
completed
pulled out during testing
cycle, temperature of
analyzer exceeds operation
temperature for strip testing
or rechargeable battery fully
discharged. Keypad not
responding. Start switch
broken.
Dead battery(s). Mechanical
problem.
Repeat test using proper procedure.
Check Analyzer Status under the
Administration Menu for analyzer
temperature.
Change disposable batteries or
recharge battery. If still no display,
call Support Services.
Wait until analyzer turns off. Turn
analyzer on. If resets, remove
cartridge. If not, change or recharge
battery(s) and turn analyzer on.
Blood Collection
Acceptable Samples for Cartridges
Arterial: Plain syringe, heparinized syringe labeled for analytes to be tested
and filled to capacity, or syringe with minimum volume of heparin to prevent
clotting (10 U/mL of blood). For ionized calcium, use balanced heparin
syringes. Mix heparinized syringes by rolling between palms for at least
5 seconds in 2 directions, then invert the syringe repeatedly for at least 5
seconds. Test for lactate immediately. Samples for pH, PCO2, PO
and ionized calcium should be tested within 10 minutes. Test for other
analytes within 30 minutes.
• Avoid drawing air into syringes for blood gas and ionized calcium tests.
• If not tested immediately, remix and discard 2 drops of blood before
filling cartridge.
• Do not use iced samples.
Venous: Collection tube with lithium or sodium heparin filled to capacity
and mixed by gentle inversion at least 10 times. Test within 10 minutes.
• Do not leave tourniquet on for more than 2 minutes.
• Do not draw above an I.V.
Skin puncture: Lithium heparin capillary tubes for testing all analytes but
ionized calcium. For all analytes including ionized calcium, use plain or
balanced heparin capillary tubes. Test immediately.
• Allow alcohol to dry over puncture site before collecting sample.
• Do not “milk” finger or heel while collecting sample.
Coagulation Tests:
• The ACT test can be performed using venous or arterial samples, while
the PT/INR test can be performed using capillary or venous samples.
• Use plain plastic syringes or plastic evacuated tubes with no
anticoagulant, activators, or serum separators.
• Test sample immediately upon draw.
• For venipuncture, some experts recommend drawing and discarding
a sample of at least 1 mL prior to drawing samples for coagulation
testing.
• If a second measurement is needed, draw a fresh sample.
• For In-dwelling line testing for ACT:
a. Fluid drip through the line must be discontinued.
b. Withdraw 2 mL of blood into a syringe and discard it.
c. Withdraw the sample into a fresh plastic syringe with no
anticoagulant, and test immediately.
2 ,
TCO
2
Page 4
• For extracorporeal line testing for ACT:
a. Flush the extracorporeal blood access line by withdrawing 5 mL of
blood into a syringe and discard the syringe.
b. Withdraw the sample into a fresh plastic syringe with no
anticoagulant, and test immediately.
• For skin puncture testing for PT/INR, see the section on "Patient Test
Procedures".
CHEM8+ Cartridges
• CHEM8+ cartridges require the use of:
a. whole blood collected in non-heparinized capillary tubes,
evacuated tubes, or syringes, as long as sample is tested
immediately upon draw,
b. heparinized whole blood collected in balanced heparin syringes or
capillary tubes, or
c. heparinized whole blood collected in evacuated tubes containing
lithium or sodium heparin, as long as the tubes are filled to
capacity.
Troponin I/cTnI and CK-MB Tests
• cTnI and CK-MB cartridges require the use of either:
a. heparinized whole blood or plasma samples collected in syringes
or evacuated tubes containing lithium or sodium heparin, or
b. non-heparinized whole blood or plasma samples tested within one
minute of drawing from a patient into a plastic syringe or plastic
evacuated tube containing no additives.
• The use of whole blood or plasma samples containing other
anticoagulants such as EDTA, oxalate, and citrate will cause
deactivation of the alkaline phosphatase, resulting in decreased cTnI or
CK-MB readings.
• Capillary tubes and direct skin punctures (e.g. fingersticks) should not
be used with the cTnI or CK-MB cartridge.
• Samples should not be used unless the blood collection tube is filled at
least half full.
BNP Tests
• BNP cartridges require the use of EDTA whole blood or plasma
samples collected in plastic syringes or evacuated tubes containing
EDTA.
• The use of whole blood or plasma samples containing other
anticoagulants such as oxalate and citrate is not reccommended.
• When drawn into an evacuated tube containing EDTA, samples should
not be used unless the blood collection tube is filled at least half full.
• Capillary tubes and direct skin punctures (e.g. fingersticks) should not
be used with the BNP cartridge.
Quality Check Messages and Codes
Message Cause Action
Date Invalid, Check
Clock
Dead Batteries,
Replace Batteries
Temperature Out of
Range, Check Status
page
Invalid or Expired
CLEW
Analyzer Interrupted,
Use Another
Cartridge
Cartridge Error Usually problem with sample
Cartridge Preburst Calibrant pack burst before
Unable to Position
Sample
Sample Positioned
Short of Fill Mark
Sample Positioned
Beyond Fill Mark
Test Cancelled by
Operator
Cartridge Type Not
Recognized
Analyzer Error, Use
Electronic Simulator
Analyzer Error, See
Manual
Date outside six month
lifetime of software.
Insufficient power to
complete a test cylce.
Temperature outside
operating range of 16 to
30 °C.
Software expired or corrupt. Verify that the analyzer’s date is
Last cartridge run not
completed.
or cartridge filling.
cartridge inserted into
analyzer.
Cartridge not sealed. Clot in
sample. Aberrant cartridge.
Cartridge underfilled. Use another cartridge - fill to Fill
Cartridge overfilled. Use another cartridge - do not fill
User did not respond to
mandatory prompt before
analyzer time out.
Software does not recognize
cartridge.
Analyzer detects problem
from which it is likely to
recover.
Analyzer detects problem
from which it may not
recover.
Select 5-Clock Set from
Administration Menu. (Password
protected.)
Replace disposable batteries or
recharge the rechargeable battery.
Check analyzer temperature by
pressing 1 for Analyzer Status under
the Administration Menu. Move
analyzer to warmer area if below
operating range or to cooler area if
above the range.
correct. Change software if expired.
Update again if not expired.
Check the battery pack inserted
properly. Check for Low Battery
startup warning.
Use another cartridge. If same code
repeats more than twice, try another
analyzer.
Use another cartridge - do not press
on center of cartridge. Check that
cartridges have not been frozen.
Use another cartridge.
Mark.
beyond Fill Mark.
No action required.
Update software. Check to see if
cartridges are expired.
Insert the Electronic Simulator. If
PASS, continue to use analyzer.
Insert Electronic Simulator. If PASS,
insert a cartridge with sample
or control. If the code does not
reappear, continue to use analyzer.
Page 5

Troubleshooting
Unexpected Results
When results do not reflect the patient’s condition, repeat the test using a fresh
cartridge and sample. If results are still suspect, test the lot of cartridges in
use with i-STAT control solutions. If the controls are in range, there may be an
interfering substance in the sample. Check the Cartridge and Test Information
sheets for the test in question. Test by another method to verify the result. If the
controls are out of range there may be a problem with the cartridge lot number.
Use another lot number or repeat the test using another method, and refer to
Support Services Information in the Troubleshooting section of the i-STAT 1
System Manual.
Startup Messages
The analyzer performs self-checks when it is turned on. If a condition that
should be corrected in the near future, but that will not affect results, is
detected, a warning is displayed. The operator presses the 1 key to access the
Test Menu. The analyzer can be customized to lock out the operator until the
corrective action is taken.
Message Action
Electronic Simulator Test Required Insert Electronic Simulator.
PCx Glucose Strip Control Required Test controls.
Stored Memory Low Place analyzer in Downloader.
Stored Memory Full Place analyzer in Downloader.
Upload Required Place analyzer in Downloader.
Battery Low Replace batteries or recharge battery.
CLEW Expiring, Update Required Upgrade software.
Acceptable Samples for PCx and PCx Plus Glucose Test Strips
Arterial and venous: Syringe or tube with lithium heparin, sodium heparin or
EDTA. Test within 30 minutes.
Skin puncture: direct application of sample to test strip or capillary tube
with lithium heparin, sodium heparin or EDTA. Test immediately.
Limitations
Interfering substances in the patient’s sample may cause an increase or
decrease in a result. Refer to the Cartridge and Test Information Sheets and
Technical Bulletins for substances and/or conditions that may interfere with
cartridge tests and to section 13 of the i-STAT 1 System Manual for causes of
lower or higher than expected glucose results when using the tests strips.
Patient Test Procedures
Cartridge Test Procedure
1. Remove cartridge from pouch. Handle a cartridge by its edges. Avoid
touching the contact pads or exerting pressure over center of cartridge.
2. Following thorough mixing of the sample, direct
syringe tip, pipette tip or capillary tube into the
sample well. Dispense sample until it reaches
the fill mark on the cartridge.
3. Fold the snap cover over the sample well until it
snaps into place. Press on round tab, not over
sample well.
4. Insert cartridge into cartridge port. For ACT
and PT/INR cartridges, keep analyzer on a
level surface with the display facing up during
testing. Do not attempt to remove cartridge
while Cartridge Locked message is displayed.
5. Scan or enter operator ID. Repeat if prompted.
6. Scan or enter patient ID. Repeat if prompted.
7. Select tests to be reported if prompted.
8. Enter sample type and blood gas parameters on chart page if applicable.
9. View results on analyzer’s display.
10. Enter Comment Code if prompted.
11. Remove cartridge after Cartridge Locked message disappears. The
analyzer is ready for the next test immediately.
Page 6

Cartridge Test Procedure - Information First
Charging Rechargeable Battery in External Recharge Compartment
1. Press the On/Off ( ) key to turn analyzer on.
2. Press 2 for i-STAT Cartridge from the Test Menu.
3. Scan or enter operator ID. Repeat if prompted.
4. Scan or enter patient ID. Repeat if prompted.
5. Remove cartridge from pouch. Handle a cartridge by its edges. Avoid
touching the contact pads or exerting pressure
over center of cartridge.
6. Following thorough mixing of the sample, direct
syringe tip, pipette tip or capillary tube into the
sample well. Dispense sample until it reaches the
fill mark on the cartridge.
7. Fold the snap cover over the sample well until it
snaps into place. Press on round tab, not over
sample well.
8. Insert cartridge into cartridge port. For ACT and
PT/INR cartridges, keep analyzer on a level surface
with the display facing up during testing.
9. Select tests to be reported if prompted.
10. Enter sample type and blood gas parameters on chart page if applicable.
11. View results on analyzer’s display.
12. Enter Comment Code if prompted.
13. Remove cartridge after Cartridge Locked message disappears. The analyzer
is ready for the next test immediately.
PT/INR Cartridge Test Procedure
Caution
The i-STAT PT/INR cartridge is designed to accept a sample between 20 and
45 microliters. A single drop of blood from either a finger puncture or as formed
at the tip of a syringe will typically be within this range. If a larger volume is
delivered to the sample well, use caution when closing the cartridge as excess
blood may be expelled from the cartridge.
Skin Punctures
1. Remove cartridge from foil pouch and place the cartridge on a flat surface.
2. Prepare lancet device and set aside until needed.
3. Clean and prepare the finger to be sampled. Allow finger to dry thoroughly
before sampling.
Placing a rechargeable battery into the recharging compartment will
automatically initiate trickle recharging. The indicator light near the recharging
compartment will be green when a rechargeable battery is placed in the
compartment.
Step Action
1 The battery pack has two labels: one for orientation in the analyzer and
one for orientation in the Downloader/Recharger. With the label with the
Downloader facing up and the electrical contact end of the pack facing the
contacts in the battery compartment, insert the pack into the compartment
as shown on the label.
2 To remove the battery after it is charged, back the pack out of the
compartment.
Full recharge from a discharged state takes approximately 40 hours.
Caution
If you are using rechargeable batteries, use only rechargeable batteries and
recharging equipment supplied by your i-STAT distributor. Other batteries and
rechargers may affect test results and pose other hazards to operators and
patients.
Cleaning the Analyzer and Downloader
Clean the display and case with a gauze pad moistened with a mild nonabrasive cleaner, detergent, soap and water, alcohol or 10% bleach solution.
Rinse with another pad moistened with water and dry.
Replacing Paper in the Martel Printer
1. Squeeze the front and back of the paper cup to open.
2. Remove remaining paper by pressing the Paper feed button. Do not
pull paper through printer mechanism.
3. Reel off a few centimeters of paper from the new paper roll and check
that the end has a clean straight edge.
4. Slide the leading edge of the paper through the paper entry slot until
you feel resistance. Paper feeds from underneath the roll.
5. Press the Paper Feed button and feed the paper through the printer
mechanism.
6. Keep the Paper Feed button depressed until the paper passes through
the paper exit slot.
7. Sit the new paper roll in the paper cup and close the lid.
Page 7

Procedure
1. Turn the analyzer on.
2. Press the 3 key for PCx Glucose Strip.
3. Press the 2 key for Control.
4. Scan or enter Operator ID.
5. Scan or enter Low Level Control lot number.
6. Scan or enter test strip lot number.
7. Open foil test strip packet and remove test strip.
8. Insert into test strip port.
9. Apply control solution.
10. Enter chart page information if applicable.
11. View results on analyzer’s display. Enter Comment Code if applicable.
12. Remove strip.
13. Press 1 for Test Options and 1 for Next Level if testing another level of
control.
Hardware Procedures
Replacing Batteries
1. Slide the battery compartment door off.
2. Tilt the analyzer slightly to slide out the battery carrier.
3. Remove the old batteries from the carrier and replace
with 2 new 9V lithium batteries.
4. Insert the carrier back into the compartment – label
facing up and electrical contacts first.
5. Slide the battery compartment door into place.
4. Prick the bottom side of the fingertip with the lancet device.
5. Gently squeeze the finger, developing a hanging drop of blood and perform
the test with the first sample of blood. Avoid strong repetitive pressure
("milking") as it may cause hemolysis or tissue fluid contamination of the
specimen.
6. Touch the drop of blood against the bottom of the sample well. Once in
contact with the sample well, the blood will be drawn into the cartridge.
7. Apply sample until it reaches the fill mark
indicated on the cartridge.
8. Fold the sample closure over the sample well.
9. Press the rounded end of the closure until it
snaps into place.
Note: To further simplify the sample application into the test cartridge, it is
possible to bring the cartridge to the finger for easier application. Do
ensure that the instrument remains on a flat vibration-free surface for
testing.
cTnI, CK-MB, and BNP Cartridge Test Procedures
The i-STAT cTnI, CK-MB, and BNP cartridges can only be used with the i-STAT
1 Analyzer bearing the symbol. The analysis time for the cTnI and BNP
cartridges is 10 minutes. The anlysis time for CK-MB cartridges is 5 minutes.
Before testing cTnI, CK-MB, or BNP cartridges on the i-STAT 1 Analyzer, the
analyzer must be customized through the Central Data Station (CDS) or through
the analyzer’s Customization menu for the following option(s):
1. Cartridge Information First Required AND Cartridge Lot Number Required,
or
2. Cartridge Barcode Required
Charging the Rechargeable Battery
Placing an analyzer in a Downloader/Recharger will automatically initiate
recharging of the rechargeable battery. The indicator light on top of the
Downloader/Recharger will be green (trickle charge), red (fast charge), or blinking
red (fast charge pending) when an analyzer with a rechargeable battery is placed
in the Downloader/Recharger.
No damage will be caused if an analyzer with disposable batteries installed is
placed in the Downloader/Recharger.
Information First Customization Test Procedure
1. Press the (On/Off) key to turn analyzer on.
2. Press 2 for i-STAT Cartridge from the Test Menu.
3. Scan or Enter Operator ID. Repeat if prompted.
4. Scan or Enter Patient ID. Repeat if prompted.
5. Scan Cartridge Lot number from the cartridge portion pack.
6. Remove cartridge from portion pack. Handle the cartridge by its edges.
Avoid touching the contact pads or exerting pressure over the center of the
cartridge.
Page 8

7. Following thorough mixing of the sample, discard 1 drop from the delivery
device to clear unseen bubbles. Hang drop(s) slightly larger than round
"target well". Touch the drop to the well allowing cartridge to draw sample
in. DO NOT load catridge with a needle. Confirm sample volume lines up
with top of "RED FILL LINE" diagram.
8. Close the cTnI, CK-MB, or BNP cartridge:
a. First anchor the cartridge in place by using the thumb and index finger
of one hand to grasp the cartridge from its side edges away from the
sample inlet.
b. Use the thumb of the other hand to slide the plastic closure clip to the
right until it locks into place over the sample well. Note: When sliding
the closure clip, the index finger of that same hand should not be
placed directly across from the thumb, as this could result in the sample
being pushed onto the user’s glove. This index finger should be placed
just above the position of the sliding clip during closure or not at all.
9. Insert cartridge into cartridge port. Grasp the catridge "slide cover"
between your first finger and thumb, using the thumb recess. Hold the
analyzer in place with one hand. With the other gently guide the cartridge
into the analyzer, releasing the cartridge only after it is fully inserted.
The analyzer must remain on a level surface with the display facing up
during testing. Motion of the analyzer during testing can increase the
frequency of suppressed results or quality check codes.
10. Select tests to be reported, if prompted.
11. Enter sample type on chart page, if applicable.
12. View results on analyzer’s display.
13. Remove cartridge after Cartridge Locked message disappears. The
analyzer is ready for the next test immediately.
Cartridge
Check temperature strip enclosed with each shipment of cartridges. If the
windows are clear or if the A or B windows are blue, or the 1 or 2 windows
are red, the cartridges should be accepted. If the C or D windows are
blue, or the 3 or 4 windows are red contact Support Services (see System
Manual).
Verify the integrity of a new shipment of cartridges, on receipt, by analyzing
2 levels of i-STAT controls and RNA Medical or Hematronix Meter Trax
controls for hematocrit using any analyzer that has passed the Electronic
Simulator test and a representative sample of the lot(s) of cartridges
received. Use expected values published in the fluids’ package inserts to
verify the integrity of the cartridges.
Verify that the storage conditions listed above have been maintained.
Procedure
1. Turn the analyzer on and press the Menu key to access the Administration
Menu.
2. Press the 3 key for Quality Tests.
3. Press the 1 key for Control.
4. Press the 1 key for i-STAT Cartridge.
5. Scan or enter Operator ID.
6. Enter the control lot number.
7. Enter the cartridge lot number.
8. Fill a cartridge with the control and close the cover.
9. Insert the cartridge into the cartridge port.
10. Enter chart page information if applicable.
11. View results on analyzer’s display.
12. Remove and discard cartridge when Cartridge Locked message disappears.
13. Press the 1 key for Test Options on the results page and press 1 for Next
Level if testing another level of control.
Test Strip
Analyze Low and High Precision Control Solutions when a new lot number
of test strips is opened.
Analyze the Low and High Precision Control Solutions using the test strip lot
number in use on a daily basis.
Analyze control solutions when test strip glucose result is questioned, when
diabetes medication plan is adjusted, when strips have been exposed to
temperatures outside the storage conditions.
Control ranges are programmed into the analyzer when the test strip lot
number is scanned or entered into the analyzer and control results will be
displayed with the acceptable ranges as well as Pass or Fail.
Page 9

Analyzer
Storage/Transport temperature: -10 to 46°C (14-115°F)
The analyzer’s operating temperature range is 16 to 30 °C (61-86°F).
Store analyzers near the testing location or in an area close to the
temperature of the testing area. Do not store analyzers near equipment that
gives off heat or in direct sunlight.
Quality Assurance
Analyzer
Electronic Simulator
Perform an electronic check on each analyzer in use once a day with either the
internal or external Electronic Simulator or as needed for regulatory compliance.
The internal simulator check is initiated, every 24 hours or according to a
customized schedule, when a cartridge is inserted into the cartridge port. If the
internal simulator result is PASS, the cartridge test proceeds and the simulator
results are stored. If FAIL is displayed for the internal simulator, reinsert
the cartridge or use an external simulator. The external simulator check is
performed as follows:
1. Turn the analyzer on.
2. Press the Menu key to access the Administration Menu.
3. Press the 3 key for Quality Tests.
4. Press the 4 key for Simulator.
5. Scan or enter Operator ID.
6. Enter the Simulator ID (serial number).
7. Insert the simulator into the cartridge port.
8. View results on analyzer’s screen.
9. If PASS is displayed, continue to use the analyzer.
10. If FAIL is displayed for the external simulator, reinsert the simulator.
If FAIL is displayed a second time, do not use the analyzer and contact your
Support Services reprersentative.
Thermal Probes and Room Temperature Checks
See System Manual for these quality assurance procedures that are performed
once or twice per year.
Non-Information First Customization Test Procedure
1. Remove cartridge from portion pack. Do not immediately dispose of the
portion pack, as the cartridge lot number listed on it will be scanned into the
analyzer during the testing procedure. Handle the cartridge from its edges.
Avoid touching the contact pads or exerting pressure over the center of the
cartridge.
2. Following thorough mixing of the sample, discard 1 drop from the delivery
device to clear unseen bubbles. Hang drop(s) slightly larger than round
"target well". Touch the drop to the well allowing cartridge to draw sample
in. DO NOT load catridge with a needle. Confirm sample volume lines up
with top of "RED FILL LINE" diagram.
3. Close the cTnI, CK-MB, or BNP cartridge:
a. first anchor the cartridge in place by using the thumb and index finger
of one hand to grasp the cartridge from its side edges away from the
sample inlet.
b. use the thumb of the other hand to slide the plastic closure clip to the
right until it locks into place over the sample well. Note: When sliding
the closure clip, the index finger of that same hand should not be
placed directly across from the thumb, as this could result in the sample
being pushed onto the user’s glove. This index finger should be placed
just above the position of the sliding clip during closure or not used at
all.
4. Insert the cartridge into the cartridge port. Grasp the catridge "slide cover"
between your first finger and thumb, using the thumb recess. Hold the
analyzer in place with one hand. With the other gently guide the cartridge
into the analyzer, releasing the cartridge only after it is fully inserted.
5. Scan the Cartridge Lot number from the cartridge portion pack.
6. Scan or Enter Operator ID Repeat if prompted.
7. Scan or Enter Patient ID. Repeat if prompted.
The analyzer must remain on a level surface with the display facing up
during testing. Motion of the analyzer during testing can increase the
frequency of suppressed results or quality check codes.
8. Select tests to be reported, if prompted.
9. Enter sample type on chart page if applicable.
10. View results on analyzer’s display.
Page 10

PCx Glucose Test Strip Procedure
1. Press the
2. Press 3 for PCx Glucose Strip.
3. Press 1 for Patient.
4. Scan or enter operator ID. Repeat if prompted.
5. Scan or enter patient ID. Repeat if prompted.
6. Scan or enter test strip lot number.
7. Press 1 for Arterial/Capillary or 2 for Venous sample
if prompted.
8. Open foil packet, remove test strip and insert into
analyzer test strip port with black contact bars
facing up and forward.
9. Apply drop of blood to target area of test strip.
Cover the entire area. Do not touch the test strip
after sample is applied. (If test fails to start after
second drop applied or if more than 30 seconds
have passed, discard test strip and repeat the test.)
10. Enter chart page information if applicable.
11. View results on analyzer’s display.
12. Enter Comment Code if applicable.
13. Remove and discard test strip.
• Do not handle test strip with wet or dirty hands.
• Do not scan the barcode of another test strip.
• Do not use test strips that are wet, scratched or damaged in any way.
• Do not re-use test strips.
Scanning
Laser Radiation – Do not stare into beam. Class 2 laser product.
Laser Diode 650 nm Maximum Output 1.0 mW.
1 Press and hold down the Scan key to start the barcode scanner. The
analyzer emits a visible red beam.
2 Position the analyzer and barcode so the beam forms a red line that spans
the entire barcode. Increasing distance between the barcode and analyzer
lengthens the red line. The analyzer does not need to touch the barcode.
3 When the analyzer accepts the barcode, it will beep in acknowledgement
and automatically turn off the beam. The beam will also turn off after 3-4
seconds.
(On/Off) key to turn the analyzer on.
Printing more than one result
1. Turn the analyzer on.
2. Press the Menu key.
3. Press 2 for Data Review.
4. Press 7 for List.
5. Scroll through the test records using the ← and → keys.
6. Press the numbered key for the test record(s). (Press the numbered key
again to deselect a record.)
7. Align analyzer and printer IR window or place in Downloader or
Downloader/Recharger attached to printer. Press the Print key.
Transmitting Results
1. Place analyzer in Downloader or Downloader/Recharger.
2. Do not move analyzer until Communication in Progress
message disappears.
Storage Conditions and Preparation for Use
Cartridges
Store at temperatures between 2 and 8 °C (35-46°F). Do not use after
expiration date on cartridge pouch and box.
Equilibrate a single cartridge for 5 minutes or a box of cartridges for 1 hour
at room temperature before opening pouches.
Store cartridges for 2 weeks at room temperature. Mark the cartridge box
or cartridge pouches with the room temperature expiration date. Do not
expose to temperatures above 30 °C (86°F). Do not return cartridges to the
refrigerator after room temperature equilibration.
Use cartridge immediately after opening pouch. If the pouch has been
punctured, the cartridge should not be used.
PCx and PCx Plus Glucose Test Strips
Store at temperatures between 4 and 30°C (39-86°F). Do not freeze. Keep
out of direct sunlight.
Do not use after expiration date on the barcode label.
Do not cut the test strip in half or attempt to use only a portion of it.
After opening foil packet, use test strip promptly.
Page 11

Test Flags and Operator Action
Martel Printer IR LED
***: Results that are not reportable due to sensor errors or interfering
substances. Draw a fresh sample and repeat test. If results are flagged
again, send sample to the lab.
< , > and < >: Results that are below or above the reportable range or
dependant on results that are outside the reportable range. Send sample to
the lab if necessary.
↑ and ↓: Results that are above or below the action range. Follow facility
procedure for samples with critical values.
Printing Test Results
Without Downloader or Downloader/Recharger
1. Turn printer on if green power light is not on.
2. Align IR windows of analyzer and printer.
3. Display results.
4. Press the Print key.
5. Do not move analyzer or printer until
printing is complete.
6. If printer is not powered from a wall
outlet, turn printer off.
4 View the data that was scanned by the analyzer and verify that it is correct.
5 Release the Scan key.
Note: If the Scan key is released as soon as the beep is heard, the next
prompt will be displayed and the information scanned will not be able
to be viewed.
Reviewing Test Results
The 0 key can be used to backlight the display
to view results in dim lighting. (The backlight
turns off after 90 seconds or when the 0 key is
pressed again.)
Test results are displayed numerically and with
bar graphs. Tick marks indicate the reference
ranges on the bar graphs. (Blood gases and
their associated calculated values are not
displayed with bar graphs and reference ranges.)
Test results are displayed for 2 minutes or a
customized time. To recall the last set of results
to the screen, turn the analyzer on and press 1
for Last Result.
To review results from the same patient, when
results are displayed, press 1 for Test Options
and then 3 for History. Scroll through test
records using the 1 and 2 keys.
To review another patient’s results, turn the
analyzer on and press the Menu key followed
by the 2 key for Data Review and the 1 key for
patient. Scan or enter the Patient’s ID number.
Use the 1 and 2 keys to scroll through the test
records. Or, press the Menu key followed by the
7 key for List. Select the test record(s) to be reviewed and press the Enter
key.
With Downloader or Downloader/Recharger
1. Place analyzer in Downloader or Downloader/Rechrager that is wired to the
printer.
2. Display results.
3. Press the Print key.
4. Do not move analyzer or printer until printing is complete.
Page 12

Reportable and Reference Range
Measured:
Test Units
Sodium/Na mmol/L (mEq/L) 100 – 180 138 – 146 138 – 146
Potassium/K mmol/L (mEq/L) 2.0 – 9.0 3.5 – 4.9 3.5 – 4.9
Chloride/Cl mmol/L (mEq/L) 65 – 140 98 – 109 98 – 109
Glucose/Glu mmol/L
Lactate/Lac mmol/L
Creatinine/Crea mg/dL
pH 6.5 – 8.2 7.35 – 7.45 7.31 – 7.41
PCO
2
TCO
2
(on the CHEM8+ cartridge
only)
PO
2
Ionized Calcium/iCa mmol/L
Urea Nitrogen/BUN
Urea
Hematocrit/Hct %PCV
Celite Activated
Clotting Time /
Celite
ACT
The range from 80 - 1000 seconds has been verified through method comparison studies.
Kaolin Activated
Clotting Time /
Kaolin
ACT
The range from 77 - 1000 seconds has been verified through method comparison studies.
mg/dL
g/L
mg/dL
µmol/L
mmHg
kPa
mmol/L (mEq/L) 5-50 23 – 27 24 – 29
mmHg
kPa
mg/dL
mg/dL
mmol/L
mg/dL
Fraction
seconds 50 – 1000 74 – 125 (Prewrm)
seconds 50 – 1000 74 – 137 (Prewrm)
Reportable
Range
1.1 – 38.9
20 – 700
0.20 – 7.00
0.30 – 20.00
2.7 – 180.2
0.2 – 20.0
18 – 1768
5 – 130
0.67 – 17.33
5 – 800
0.7 – 106.6
0.25 – 2.50
1.0 – 10.0
3 – 140
1 – 50
6 – 300
10 – 75
0.10 – 0.75
Reference Range
(arterial) (venous)
3.9 – 5.8
70 – 105
0.70 – 1.05
0.36 – 1.25
3.2 – 11.3
0.6 – 1.3
53 – 115
35 – 45
4.67 – 6.00
80 – 105
10.7 – 14.0
1.12 – 1.32
4.5 – 5.3
8 – 26
2.9 – 9.4
17 – 56
38 – 51
0.38 – 0.51
84 – 139 (Nonwrm)
82 – 152 (Nonwrm)
74 – 125 (Prewrm)
84 – 139 (Nonwrm)
74 – 137 (Prewrm)
82 – 152 (Nonwrm)
3.9 – 5.8
70 – 105
0.70 – 1.05
0.90 – 1.70
8.1 – 15.3
0.6 – 1.3
53 – 115
41 – 51
5.47 – 6.80
1.12 – 1.32
4.5 – 5.3
8 – 26
2.9 – 9.4
17 – 56
38 – 51
0.38 – 0.51
Test Units
Prothrombin Time
/ PT
Performance characteristics have not been established for INRs above 6.0.
Troponin I / cTnI ng/mL (µg/L) 0.00 – 50.00 0.00 – 0.03*
Performance characteristics have not been established for cTnI values above 35.00 ng/mL.
* Represents the 0 to 97.5% range of results.
** Represents the 0 to 99% range of results.
Creatine Kinase MB
/ CK-MB
***Represents the 0 to 95% range of results.
B-Type Natriuretic
Peptide / BNP
# Represents the 0 to 95% range of results.
Celite is a registered trademark of Celite Corporation, Santa Barbara, CA., for its diatomaceous earth products.
INR 0.9 – 8.0
ng/mL (µg/L) 0.0 – 150.0 0.0 – 3.5***
pg/mL (ng/L) 15 – 5000 <15 – 50#
Reportable
Range
Reference Range
(arterial) (venous)
0.00 – 0.08**
Calculated:
Reportable
Test Units
Hemoglobin/Hb g/dL
TCO
2
(on all cartridges but
the CHEM8+)
HCO
3
BE mmol/L (mEq/L) (-30) – (+30) (-2) – (+3) (-2) – (+3)
Anion Gap/
AnGap
sO
2
g/L
mmol/L
mmol/L (mEq/L) 5-50 23 – 27 24 – 29
mmol/L (mEq/L) 1.0 – 85.0 22 – 26 23 – 28
mmol/L (mEq/L) (-10) – (+99) 10 – 20 10 – 20
% 0 – 100 95 – 98
Range Reference Range
(arterial) (venous)
3.4 – 25.5
34 – 255
2.1 – 15.8
12 – 17
120 – 170
7 – 11
12 – 17
120 – 170
7 – 11
 Loading...
Loading...