Abbott i-Stat 1 User manual

®
i-STAT
1
System Manual
Rev. Date: 08/14/06 Art: 714336-01E

Patents
CA 1,281,072; CA 1,303,175; CA 1,330,888; CA 2,002,848; CA 2,087,720; CA 2,087,966;
CA 2,175,228; CA 2,193,000; CA 2,194,795; CA 2,221,178; EP 0408575; EP 0412119; EP 0434742; EP
0442969; EP 0505169; EP 0540691; EP 0543916; JP 2113412; JP 2521826; JP 2833809; JP 2948321;
JP 3093274; JP 3105919; JP 3105922; JP 3137612; JP 3269553; JP 3392871; US 4,864,229; US
4,933,048; US 4,954,087; US 5,008,616; US 5,096,669; US 5,112,455; US 5,121,050; US 5,124,661; US
5,200,051; US 5,447,440; US 5,466,575; US 5,514,253; US 5,554,339; US 5,605,664; US 5,609,824; US
5,614,416; US 5,628,961; US 5,789,253; US 5,821,399; US 5,837,446; US 6,030,827; US 6,379,883; US
6,438,498; US 6,750,053; US D332,833; US D337,164
Symbol Technologies Corporation is the owner of US Patent Nos. 4,758,717; 5,130,520; 5,262,628;
5,396,055; 5,532,469.
Trademarks
i-STAT is a registered trademark of Abbott Laboratories. MediSense is a registered trademark of
Abbott Laboratories. Precision and PCx are trademarks of Abbott Laboratories. Windows is a registered
trademark of Microsoft Corporation.
Abbott Point of Care Inc.
104 Windsor Center Drive
East Windsor, NJ 08520 • USA
Tel: (609) 443-9300
Fax: (609) 443-9310
©2006 Abbott Point of Care Inc. All rights reserved. Printed in USA.
Art: 714336-01E Rev. Date: 08/14/06
Emergo Europe
P.O. Box 18510
2502 EM The Hague
The Netherlands
Tel: (31)70 345 8570
Fax: (31)70 346 7299

i-STAT 1 SYSTEM MANUAL CONFIGURATION
Please ensure that the contents of your System Manual are complete and up to date. In the event that your System
Manual does not contain the current configuration, it is recommended that you contact your i-STAT support provider.
As of April 2008, your i-STAT®1 System Manual should be configured with the contents as listed below and in the
order shown.
ITEM Art #
Cover Sheet ..................................................................................... 714336-01E
Configuration Sheet .......................................................................... 714419-01Y
Table of Contents.............................................................................. 714362-01G
Section 1 ........................................................................................... 714363-01K
Section 2 ........................................................................................... 714364-01H
Section 3 ........................................................................................... 714365-01C
Section 4 ........................................................................................... 714366-01B
Section 5 ........................................................................................... 714367-01B
Section 6 ........................................................................................... 714368-01E
Section 7 ........................................................................................... 714369-01D
Section 8 ........................................................................................... 714370-01C
Section 9 ........................................................................................... 714371-01D
Section 10 ......................................................................................... 714372-01G
Section 11 ......................................................................................... 714373-01C
Section 12 ......................................................................................... 714374-01E
Section 13 ......................................................................................... 714375-01C
Section 14 ......................................................................................... 714376-01F
Section 15 ......................................................................................... 714377-01F
Section 16 ......................................................................................... 714378-01C
Section 17 ......................................................................................... 714379-01E
Section 18 ......................................................................................... 714380-01E
Section 19 ......................................................................................... 714381-01E
Section 20 ......................................................................................... 714382-01C
Section 21 ......................................................................................... 714383-01E
Section 22 ......................................................................................... 714384-01D
CTI Sheets
Introduction ....................................................................................... 714258-01K
Sodium.............................................................................................. 714173-01H
Potassium ......................................................................................... 714174-01F
Chloride............................................................................................. 714175-01G
Urea Nitrogen/BUN ........................................................................... 714176-01H
Glucose............................................................................................. 714177-01G
Hematocrit/Hemoglobin..................................................................... 714178-01G
Ionized Calcium................................................................................. 714179-01H
PO
/ sO
.......................................................................................... 714180-01H
2
pH ..................................................................................................... 714181-01J
PCO
Total Carbon Dioxide/TCO2............................................................... 716661-01D
Creatinine.......................................................................................... 714183-01K
Lactate .............................................................................................. 714184-01F
Celite ACT......................................................................................... 714185-01E
Kaolin ACT........................................................................................ 715878-01D
Prothrombin Time PT/INR................................................................. 715236-01F
Cardiac Troponin I............................................................................. 715595-01F
Creatine Kinase MB / CK-MB............................................................ 716675-01B
B-Type Natriuretic Peptide/BNP........................................................ 716969-01A
2
/HCO3/BE/AG........................................................................... 714182-01M
2
Art.: 714419-01Y Rev. Date: 03/03/08

Technical Bulletins
Analyzer Coded Messages ............................................................... 714260-01G
Hematocrit Determination in the i-STAT System
and Comparison to Other Methods. .................................................. 714261-01C
Installation Guide for the Central Data Station to
Receive Data from a Philips Clinical Data Server ............................. 714270-01B
K
EDTA and K3EDTA Customization for
2
Hematocrit on the i-STAT System..................................................... 716240-01B
ACT Test Result Options: Prewarmed vs. Non-Prewarmed
Result Calibration Modes for the i-STAT 1 Analyzer ......................... 715617-01C
Support Services............................................................................... 716144-01G
Using i-STAT
®
Analyzer Customization Features to
Minimize ID Entry Errors. .................................................................. 720654-01A
April 2008 Update to the Central Data Station Version 5 .................. 721106-01A
Art.: 714419-01Y Rev. Date: 03/03/08

Contents
INTRODUCTION .................................................................................................... 1 - 1
This Manual .......................................................................................................................................... 1 - 1
Intended Use ........................................................................................................................................ 1 - 1
Overview of the i-STAT System ............................................................................................................ 1 - 1
Components ........................................................................................................................................ 1 - 2
Selection of Components ....................................................................................................................
Summary of the Procedure .................................................................................................................. 1 - 2
Data Management ............................................................................................................................... 1 - 3
Interfacing ............................................................................................................................................ 1 - 3
Note Regarding System Reliability ...................................................................................................... 1 - 3
Symbols ............................................................................................................................................... 1 - 3
Warranty ............................................................................................................................................... 1 - 7
SyStem ComponentS
i-STAT 1 ANALYZER .............................................................................................. 2 - 1
Introduction .......................................................................................................................................... 2 - 1
Before You Use the Analyzer ............................................................................................................... 2 - 1
Specifications....................................................................................................................................... 2 - 2
Software ............................................................................................................................................... 2 - 3
Power ...................................................................................................................................................2 - 3
Disposable Batteries ............................................................................................................................ 2 - 3
Rechargeable Battery .......................................................................................................................... 2 - 3
Low Battery Warning............................................................................................................................ 2 - 3
Cartridge Port ...................................................................................................................................... 2 - 4
Test Strip Port ...................................................................................................................................... 2 - 4
Infrared Communication Window ........................................................................................................ 2 - 5
Thermal Control ................................................................................................................................... 2 - 5
Barometric Pressure Sensor ................................................................................................................ 2 - 5
Cartridge Test Cycle ............................................................................................................................. 2 - 5
Strip Test Cycle .................................................................................................................................... 2 - 5
Data Entry ............................................................................................................................................2 - 6
Storage of Results ............................................................................................................................... 2 - 6
LCD Display and Backlight .................................................................................................................. 2 - 7
Audible Indicator .................................................................................................................................. 2 - 7
Time Out .............................................................................................................................................. 2 - 7
Keypad ................................................................................................................................................. 2 - 8
i-STAT 1 Menu Tree .............................................................................................................................. 2 - 9
Test Menu ............................................................................................................................................. 2 - 10
Administration Menu ............................................................................................................................2 - 10
Analyzer Status .................................................................................................................................... 2 - 11
Data Review ......................................................................................................................................... 2 - 11
Quality Tests ......................................................................................................................................... 2 - 12
Customization ..................................................................................................................................... 2 - 13
Set Clock ............................................................................................................................................. 2 - 17
Transmit Data ....................................................................................................................................... 2 - 17
Utility .................................................................................................................................................... 2 - 17
Laser Barcode Scanner ....................................................................................................................... 2 - 18
Prompts and Messages ....................................................................................................................... 2 - 19
1 - 2
Art: 714362-01G Rev. Date: 09/13/06 i

i-STAT CARTRIDGE ............................................................................................... 3 - 1
Contents .............................................................................................................................................. 3 - 1
Standardization and Calibration........................................................................................................... 3 - 3
Packaging ............................................................................................................................................ 3 - 3
Storage Conditions .............................................................................................................................. 3 - 4
Disposal ...............................................................................................................................................3 - 4
PRECISION PCx AND PCx PLUS BLOOD GLUCOSE TEST STRIPS .................4 - 1
ELECTRONIC SIMULATOR ................................................................................... 5 - 1
Internal Simulator ................................................................................................................................. 5 - 1
External Simulator ................................................................................................................................ 5 - 1
Operating Characteristics .................................................................................................................... 5 - 2
Cleaning the Simulator ......................................................................................................................... 5 - 2
i-STAT 1 DOWNLOADER ....................................................................................... 6 - 1
Function ............................................................................................................................................... 6 - 1
Specifications....................................................................................................................................... 6 - 2
Power Supply ....................................................................................................................................... 6 - 2
Downloader/Recharger Indicator LEDs ............................................................................................... 6 - 3
Power Requirements ............................................................................................................................ 6 - 3
Cautions ............................................................................................................................................... 6 - 3
Transmitting Data from Downloader to the Data Manager ..................................................................6 - 4
Transmitting Data from Downloader/Recharger to the Data Manager................................................. 6 - 4
Transmitted Information ....................................................................................................................... 6 - 4
Troubleshooting ................................................................................................................................... 6 - 5
Charging the Rechargeable Battery ..................................................................................................... 6 - 5
Charging Rechareable Battery in the External Recharge Compartment ............................................. 6 - 6
PORTABLE PRINTER .............................................................................................7 - 1
MARTEL Printer ......................................................................................................................................... 7 - 1
Overview .............................................................................................................................................. 7 - 1
Specifications....................................................................................................................................... 7 - 1
Supplies Provided with Printer ............................................................................................................. 7 - 2
Power ................................................................................................................................................... 7 - 2
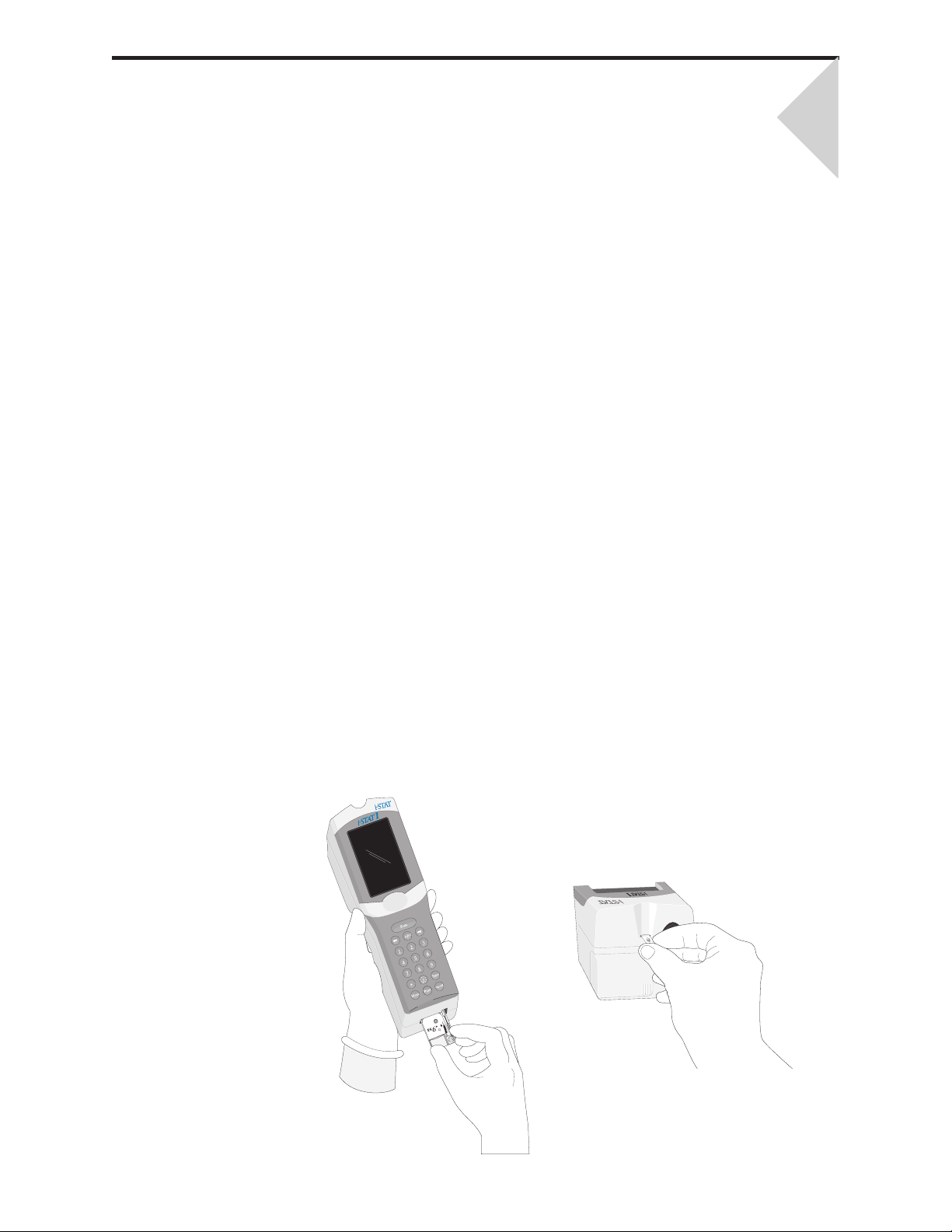
Loading Paper ...................................................................................................................................... 7 - 2
Printing Directly from the Analyzer ....................................................................................................... 7 - 3
Printing Via a Downloader .................................................................................................................... 7 - 3
Printing Many Results .......................................................................................................................... 7 - 4
What is Printed ..................................................................................................................................... 7 - 4
Cautions ............................................................................................................................................... 7 - 4
Troubleshooting ................................................................................................................................... 7 - 5
DATA MANAGEMENT ............................................................................................ 8 - 1
Introduction .......................................................................................................................................... 8 - 1
Components ........................................................................................................................................ 8 - 1
Data Manager ...................................................................................................................................... 8 - 2
i-STAT Central Data Station Version 5 Software .................................................................................. 8 - 2
Downloader and Downloader/Recharger ............................................................................................. 8 - 3
IR Link .................................................................................................................................................. 8 - 3
LIS/HIS Interface .................................................................................................................................. 8 - 3
Standard Data Management Configuration ......................................................................................... 8 - 4
Connecting Components ..................................................................................................................... 8 - 4
CUSTOMIZATION .................................................................................................. 9 - 1
ii Art: 714362-01G Rev. Date: 09/13/06

proCedureS
SAMPLE COLLECTION ......................................................................................... 10 - 1
Specimen Collection ............................................................................................................................ 10 - 1
Venipuncture - General ........................................................................................................................ 10 - 1
Venipuncture - pH, PCO2, Electrolyte, Chemistry, and Hematocrit Tests .......................................... 10 - 2
Venipuncture - Coagulation Tests ........................................................................................................ 10 - 4
Venipuncture - Glucose Test Strip ....................................................................................................... 10 - 4
Arterial Puncture - General .................................................................................................................. 10 - 4
Arterial Puncture - Blood Gas, Electrolyte, Chemistry, and Hematocrit Tests .................................... 10 - 4
Arterial Puncture - Coagulation Tests ................................................................................................. 10 - 6
Arterial Puncture - Glucose Test Strips ............................................................................................... 10 - 6
Indwelling Line ..................................................................................................................................... 10 - 6
Skin Puncture ....................................................................................................................................... 10 - 7
Sample Transfer Devices ..................................................................................................................... 10 - 7
References ........................................................................................................................................... 10 - 8
PROCEDURE FOR HANDLING CARTRIDGES .................................................... 11 - 1
Preparation for Testing ......................................................................................................................... 11 - 1
Filling and Sealing Cartridge Using Transfer Device ............................................................................ 11 - 2
Filling and Sealing PT/INR Cartridges Using Direct Fingerstick Sampling .......................................... 11 - 2
Filling and Closing a cTnI Cartridge Using Transfer Device ................................................................. 11 - 3
Inserting and Removing the Cartridge From the Analyzer ................................................................... 11 - 4
Incorrect Procedure ............................................................................................................................. 11 - 5
PROCEDURE FOR CARTRIDGE TESTING .......................................................... 12 - 1
Cautions ............................................................................................................................................... 12 - 1
Performing Patient Analysis with Cartridge – Information First Disabled ............................................ 12 - 2
Performing Patient Analysis with Cartridge – Information First Enabled ............................................. 12 - 4
Interpretation of Displayed Results ..................................................................................................... 12 - 7
Troubleshooting ................................................................................................................................... 12 - 9
PROCEDURES FOR GLUCOSE TEST STRIP TESTING ..................................... 13 - 1
Operating Guidelines for All Samples .................................................................................................. 13 - 1
Performing Patient Glucose Assay with Test Strip .............................................................................. 13 - 2
Caution ................................................................................................................................................. 13 - 4
Interpretation of Displayed Results ...................................................................................................... 13 - 5
Troubleshooting ................................................................................................................................... 13 - 6
QUALITY CONTROL .............................................................................................. 14 - 1
Overview ............................................................................................................................................. 14 - 1
Quality Control for i-STAT Cartridges and the Analyzer’s Cartridge Test Cycle ................................... 14 - 1
Controls for Blood Gas/Electrolyte/Metabolite Cartridges (Except CHEM8+ Cartridges) ................... 14 - 3
Correction of PO2 at Extreme Altitude................................................................................................. 14 - 5
Controls for CHEM8+ Cartridges ......................................................................................................... 14 - 6
RNA® Medical Hematocrit Control ....................................................................................................... 14 - 8
Meter Trax™ Controls for Hematocrit Sensor...................................................................................... 14 - 9
Controls for ACT Cartridges ................................................................................................................ 14 - 9
Controls for PT/INR Cartridges ............................................................................................................ 14 - 10
Controls for cTnI Cartridges................................................................................................................. 14 - 12
Cliniqa Liquid QC™ Cardiac Marker Controls for i-STAT .................................................................... 14 - 13
i-STAT BNP Controls ............................................................................................................................ 14 - 14
Performing Electronic Simulator Test ................................................................................................... 14 - 15
Procedure for Internal Electronic Simulator ......................................................................................... 14 - 15
Procedure for External Electronic Simulator ........................................................................................ 14 - 15
Troubleshooting Failed Electronic Simulator Test ................................................................................ 14 - 16
Checking the Thermal Probes in the i-STAT Analyzers ........................................................................ 14 - 17
Art: 714362-01G Rev. Date: 09/13/06 iii

Procedure to Check Ambient Temperature .......................................................................................... 14 - 19
Performing Control Test on Cartridge .................................................................................................. 14 - 19
Troubleshooting Out-of-Range Control Results on Cartridge ............................................................. 14 - 21
Controls for Glucose Test Strip ............................................................................................................ 14 - 21
Recommendations for Glucose Test Strips ......................................................................................... 14 - 22
Performing Control Test with Glucose Strip ......................................................................................... 14 - 22
Troubleshooting Out-of-Range Results on Test Strips ........................................................................ 14 - 24
Quality Control Log Sheets .................................................................................................................. 14 - 25
CALIBRATION VERIFICATION .............................................................................. 15 - 1
Calibration Verification for
Blood Gas/Electrolyte/Metabolite Cartridges (Except CHEM8+ Cartridges) ....................................... 15 - 1
i-STAT Calibration Verification Set ....................................................................................................... 15 - 2
i-STAT CHEM8+ Calibration Verification Set ........................................................................................ 15 - 3
i-STAT Cardiac Marker Calibration Verification Set .............................................................................. 15 - 5
i-STAT BNP Calibration Verification Set ............................................................................................... 15 - 6
RNA® Medical Hematocrit Calibration Verification Controls ................................................................ 15 - 7
Verification Procedure for Hematocrit .................................................................................................. 15 - 8
Verification Procedure for ACT ............................................................................................................. 15 - 9
Procedure for Cartridges ..................................................................................................................... 15 - 10
Troubleshooting Cartridge Tests .......................................................................................................... 15 - 11
Linearity/Calibration Verification Test for Test Strips ............................................................................ 15 - 12
Troubleshooting Strip Tests.................................................................................................................. 15 - 13
PROFICIENCY or EXTERNAL QUALITY CONTROL TESTING ........................... 16 - 1
Purpose ................................................................................................................................................ 16 - 1
General Procedure ............................................................................................................................... 16 - 1
Procedure For Cartridges .................................................................................................................... 16 - 1
Troubleshooting ................................................................................................................................... 16 - 3
Procedure for Test Strips ..................................................................................................................... 16 - 4
Care and Software updateS
ROUTINE CARE of the ANALYZER and DOWNLOADER ................................... 17 - 1
Drying a Wet Analyzer or Downloader ................................................................................................ 17 - 1
Cleaning the Analyzer and Downloader ............................................................................................... 17 - 1
Removing and Replacing Disposable Batteries ................................................................................... 17 - 2
Removing and Replacing the Rechargeable Battery ........................................................................... 17 - 3
UPDATING THE SOFTWARE ................................................................................. 18 - 1
CLEW .................................................................................................................................................. 18 - 1
Application Software ............................................................................................................................ 18 - 1
Software Updates ................................................................................................................................ 18 - 1
Updating Analyzer Software: JAMMLITE Utility ................................................................................... 18 - 2
Troubleshooting ................................................................................................................................... 18 - 8
Analyzer-to-Analyzer Software Updates .............................................................................................. 18 - 9
Performing a JAMS and CLEW update on an i-STAT 1 Analyzer using
the Customization Workspace ............................................................................................................ 18 - 10
troubleShooting the analyzer
TROUBLESHOOTING THE ANALYZER ................................................................ 19 - 1
Introduction .......................................................................................................................................... 19 - 1
Information Needed ............................................................................................................................. 19 - 1
Startup Messages ................................................................................................................................ 19 - 2
iv Art: 714362-01G Rev. Date: 09/13/06

Test Cycle Messages and Quality Check Codes ................................................................................. 19 - 3
Glucose Strip Errors ............................................................................................................................. 19 - 6
No Display ............................................................................................................................................ 19 - 7
“Cartridge Locked” Not Removed ....................................................................................................... 19 - 7
theory
THEORY .................................................................................................................. 20 - 1
Analyzer Functions ............................................................................................................................... 20 - 1
Electrochemical Measurements ........................................................................................................... 20 - 3
Determination of Test Results .............................................................................................................. 20 - 4
Determination of Cell Concentration .................................................................................................... 20 - 5
CPB ...................................................................................................................................................... 20 - 5
Determination of Coagulation Endpoints ............................................................................................. 20 - 7
Quality Control and the i-STAT System ................................................................................................ 20 - 7
Quality Control and the i-STAT Coagulation Tests ............................................................................... 20 - 13
downloader programming
DOWNLOADER PROGRAMMING AND WIRING ................................................. 21 - 1
Programming the Network Downloaders ............................................................................................. 21 - 1
Wiring the Downloaders ....................................................................................................................... 21 - 6
Central data Station
CENTRAL DATA STATION ..................................................................................... 22 - 1
i-STAT License Agreement and Warranty for Central Data Station Program ....................................... 22 - 1
Installation Of The Central Data Station............................................................................................... 22 - 3
General Procedures and Conventions ................................................................................................. 22 - 5
Customization of the Central Data Station .......................................................................................... 22 - 9
Interface Program Customization ........................................................................................................ 22 - 15
Overview of the Central Data Station Program .................................................................................... 22 - 17
Administration Tools ............................................................................................................................. 22 - 19
Instrument and Location Workspace ................................................................................................... 22 - 19
Operator Workspace ............................................................................................................................ 22 - 25
Operator List Import ............................................................................................................................. 22 - 31
Database Maintenance ........................................................................................................................ 22 - 33
Inventory Workspace ........................................................................................................................... 22 - 37
Customization Workspace ................................................................................................................... 22 - 43
User Administration Workspace .......................................................................................................... 22 - 51
Password Management ....................................................................................................................... 22 - 53
Data Viewers ........................................................................................................................................ 22 - 55
Data Export .......................................................................................................................................... 22 - 62
Monitors ............................................................................................................................................... 22 - 63
Reports ................................................................................................................................................ 22 - 65
System ................................................................................................................................................. 22 - 68
Windows Operating System and Language Support .......................................................................... 22 - 69
Art: 714362-01G Rev. Date: 09/13/06 v

Cartridge and teSt information
Cartridge and Test Information
Sodium
Potassium
Chloride
BUN/Urea
Glucose
Hematocrit/Hemoglobin
Ionized Calcium
PO
2
pH
PCO
2
Total Carbon Dioxide/TCO
Creatinine
Lactate
Celite ACT
Kaolin ACT
Prothrombin Time PT/INR
Cardiac Troponin I
Creatine Kinase MB/CK-MB
2
B-Type Natriuretic Peptide/BNP
teChniCal bulletinS
vi Art: 714362-01G Rev. Date: 09/13/06
INTRODUCTION
1
This Manual
Intended Use
Overview of the
i-STAT System
This manual describes the i-STAT1 Analyzer and the Central Data Station software.
Related sections are grouped behind tabs.
The i-STAT Portable Clinical Analyzer and the Philips Medical Systems Blood
Analysis Module (formerly supplied by Agilent Technologies, and Hewlett-Packard,
Inc., for the Viridia CMS Patient Monitors) are described in a separate manual.
The i-STAT 1 Analyzer is intended for use with i-STAT cartridges for in vitro
quantification of various analytes in whole blood and with the Abbott MediSense
Precision PCx Blood Glucose Test Strip for the in vitro quantification of glucose
in whole blood. Analyzers, cartridges, and test strips should be used by healthcare
professionals trained and certified to use the system and should be used according
to the facility’s policies and procedures.
In the USA, for the purpose of CLIA compliance, the i-STAT CHEM8+ cartridge is
categorized as Waived. All other i-STAT cartridges are categorized as moderate
complexity.
The i-STAT System incorporates a comprehensive group of components needed
to perform blood analysis at the point of care. A portable handheld analyzer, a
cartridge with the required tests, and 2-3 drops of blood will allow the caregiver
to view quantitative test results for blood gas, chemistry and coagulation tests in
approximately two minutes. Glucose results are available from the Precision PCx
Blood Glucose Test Strip in as little as 20 seconds on the handheld analyzer.
Portable printers and infrared communication devices allow all patient information
obtained at the bedside to be printed on demand and transmitted to centralized
information systems for record keeping and billing.
The Central Data Station program provides system management tools including
real-time monitoring of testing and operator competency.
Art: 714363-01K Rev. Date: 03/03/08 1-1

Components
The i-STAT System consists of:
i-STAT Cartridges
Abbott MediSense Precision PCx and PCx Plus Blood Glucose Test
Strips
i-STAT 1 Analyzer
i-STAT Portable Clinical Analyzer
Philips Medical Systems Blood Analysis Module (used in conjunction
with a patient monitor)
Portable Printer
Quality Assurance Materials
• Electronic Simulator
• Control Solutions
• Calibration Verification Set (for cartridges)
• Linearity Assessment Kit (for test strips)
Data Management System
• i-STAT 1 Downloader
• i-STAT 1 Downloader/Recharger
• IR Link for Portable Clinical Analyzer
•
Data Manager
Central Data Station (data management software for cartridges)
QC Manager (data management software for test strips)
Selection of
Components
Summary of the
Procedure
Data Manager Printer
LIS/HIS Interface Software
The selection of system components is dependent on factors unique to each
facility such as:
Types of tests to be performed
Number of testing sites
Number of tests per site
System administration requirements
To perform cartridge testing, the operator fills a cartridge with sample, seals the
cartridge with its snap closure, and inserts the cartridge into the analyzer. Inserting the
cartridge activates the analyzer. Alternatively, the cartridge test cycle can be initiated
from the keypad/menu system. The unit-use cartridge contains all components to
perform one or more tests including: calibrating solution, sample handling system,
sensors and reagents. The analyzer automatically controls all steps in the testing cycle,
which may include: fluid movement, reagent mixing, calibration and thermal control.
Quality checks are performed continuously throughout the test cycle. Operator and
patient IDs and patient chart information can be entered. When the test cycle is
completed, results are displayed and the test record is stored. To perform glucose
test strip testing, the operator selects a the glucose strip option from the menu, scans
the test strip barcode, inserts the test strip into the analyzer test strip port and applies
sample to the test strip. This degree of automation, along with the ability to test fresh
whole blood, eliminates many sources of error as well as time-consuming and costly
steps inherent in other methods.
1-2 Art: 714363-01K Rev. Date: 03/03/08
Data Management
Test records can be transmitted to the Data Manager where they can be printed
and/or transmitted to the Laboratory Information System or Hospital Information
System. An optional portable printer enables the operator to print results at the
point of care.
Interfacing
The Data Manager can be interfaced to a Laboratory Information System (LIS)
or Hospital Information System (HIS) to automate billing and patient record
keeping.
Note Regarding
System Reliability
The i-STAT System automatically runs a comprehensive set of quality checks of
analyzer and cartridge performance each time a sample is tested. This internal quality
system will suppress results if the analyzer or cartridge does not meet certain internal
specifications (see Quality Control section in System Manual for detailed information).
To minimize the probability of delivering a result with medically significant error the
internal specifications are very stringent. It is typical for the system to suppress a
very small percentage of results in normal operation given the stringency of these
specifications. If however the analyzer or cartridges have been compromised,
results may be persistently suppressed, and one or the other must be replaced to
restore normal operating conditions. Where unavailability of results while awaiting
replacement of analyzers or cartridges is unacceptable, Abbott Point of Care Inc.
recommends maintaining both a backup i-STAT System analyzer and cartridges
from an alternate lot number.
Symbols Symbols can be helpful in reducing the necessity for translating important information
into multiple languages, particularly where space is limited. The following symbols
may be found on components of the i-STAT System.
Symbol Definition
Attention: See instructions for use.
Caution: Risk of electrical shock.
Laser radiation hazard symbol.
Biological Risks.
Temperature limitations. The upper and lower limits for storage are adjacent to
upper and lower arms.
Upper limit of temperature.
The upper limit for storage is adjacent to the upper arm
Use by or expiration date.
An expiration date expressed as YYYY-MM-DD means the last day the product can be
used.
An expiration date expressed as YYYY-MM means the product cannot be used past the
last day of the month specified.
Art: 714363-01K Rev. Date: 03/03/08 1-3
Symbol Definition
Manufacturer's lot number or batch code. The lot number or batch will appear
adjacent to this symbol.
Catalog number, list number, or reference number. The number adjacent to this
symbol is used to reorder the product.
Serial number. The serial number will appear adjacent to this symbol.
MN
Model number. The model number will appear adjacent to this symbol.
Date of manufacture.
Manufacturer
In vitro diagnostic medical device.
Authorized Representative for Regulatory Affairs in the European
Community.
Contains sufficient for < n > tests.
Direct Current (DC)
Alternating Current (AC)
Class II Construction
Consult instructions for use or see System Manual for instructions.
Control
Signifies that the product bearing the ETL Listed mark complies with both U.S. and
Canadian product safety standards:
UL 61010A-1
CAN/CSA C22.2 No. 1010.1-92
i/immuno: Cartridges bearing this symbol must be run on i-STAT analyzers that also
bear this symbol.
Battery: i-STAT 1 Analyzer low battery icon (flashes on lower left side of display
screen).
Separate waste collection for this electrical/electronic item indicated.
1-4 Art: 714363-01K Rev. Date: 03/03/08
Symbol Definition
Separate waste collection for this electrical/electronic item indicated; Equipment
manufactured / put on the market after 13 August 2005; Indicates compliance with
Article 10(3) of Directive 2002/96/EC (WEEE) for the European Union (EU).
BODxxxx-xx Born On Date: the label BODxxxx-xx defines the year and month of manufacture.
Do not reuse.
This symbol is used for compliance with the China RoHS regulation(s). It indicates
in years the Environmentally Friendly Use Period (EFUP) for the labeled electronic
medical device product
As the Martel Printer is incapable of printing the ↑ or ↓ symbols, this symbol
<< >>
appears on the Martel printout next to results which are outside the action range
limits.
Symbol The following symbols are used on the i-STAT 1 keypad.
SCAN
ABC
MENU
Symbol
DIS
Key used to scan information into the analyzer.
Key used to enter letters.
Key used to enter information.
Key used to access the analyzer's menu.
Key used to print a test record.
Key used to turn the analyzer off and on.
The following symbols are used on the i-STAT Portable Clinical Analyzer Keypad
Key used to activate the display.
ENT
PRT
CLR
Art: 714363-01K Rev. Date: 03/03/08 1-5
Key used to enter information.
Key used to print a test record.
Key used to clear an incorrect entry.
Symbol
The following symbols are used on i-STAT Value Assignment Sheets
Mean
R
Symbol TEST
Na
K
Cl
Glu
Lac
Crea
pH
PCO
PO
2
iCa
Range
Sodium
Potassium
Chloride
Glucose
Lactate
Creatinine
pH
2
Partial pressure of carbon dioxide.
Partial pressure of oxygen.
Ionized Calcium
BUN/UREA
Hct
ACTc
Celite ACT
ACTk
Kaolin ACT
PT/INR
Hb
TCO2
HCO3
BE (b&ecf)
AnGap
sO2
cTnI
CK-MB
Urea nitrogen/Urea
Hematocrit
Activated Clotting Time with Celite® activator.
Activated Clotting Time with Kaolin activator.
Prothrombin Time / International Normalized Ratio
Hemoglobin
Total carbon dioxide concentration.
Bicarbonate
Base excess (b for blood, ecf for extra cellular fluid)
Anion Gap
Oxygen saturation
Cardiac Troponin I
Creatine Kinase MB Isoenzyme
BNP
1-6 Art: 714363-01K Rev. Date: 03/03/08
B-type Natriuretic Peptide

Warranty
Abbott Point of Care Inc. warrants this medical product (excluding disposable or
consumable supplies) against defects in materials and workmanship for one year
from the date of shipment. If Abbott Point of Care Inc. receives notice of such
defects during the warranty period, Abbott Point of Care Inc. shall, at its option,
either repair or replace products which prove to be defective. With respect to
software or firmware, if Abbott Point of Care Inc. receives notice of defects in
these products during the warranty period, Abbott Point of Care Inc. shall repair or
replace software media and firmware which does not execute their programming
instructions due to such defects. Abbott Point of Care Inc. does not warrant that the
operating of the software, firmware or hardware shall be uninterrupted or error free.
If Abbott Point of Care Inc. is unable, within a reasonable time, to repair or replace
any product to a condition as warranted, Buyer shall be entitled to a refund of the
purchase price upon return of the product to Abbott Point of Care Inc..
Note: Warranty rights may vary from state to state, province to province and
country to country.
Limitations of
Warranty
Selling or Leasing the
i-STAT System
The foregoing warranty shall not apply to defects resulting from:
1 Improper or inadequate maintenance by Buyer or an unauthorized
person,
2
Using accessories and/or consumables that are not approved by Abbott
Point of Care Inc.,
3 Buyer-supplied software or interfacing,
4 Unauthorized repairs, modifications, misuse, or damage caused by
disposable batteries, or rechargeable batteries not supplied by Abbott
Point of Care Inc..
5 Operating outside of the environmental specifications of the product, or
6 Improper site preparation or maintenance.
THE WARRANTY SET FORTH ABOVE IS EXCLUSIVE AND NO OTHER WARRANTY,
WHETHER WRITTEN OR ORAL, IS EXPRESSED OR IMPLIED. ABBOTT
SPECIFICALLY DISCLAIMS THE IMPLIED WARRANTIES OR MERCHANTABILITY
AND FITNESS FOR A PARTICULAR PURPOSE.
If you sell an i-STAT analyzer, please notify Abbott Point of Care Inc. so that
we can enter the new owner into our software update database. If you rent an
i-STAT analyzer and do not intend to provide software updates to the leaser, please
notify Abbott Point of Care Inc. so that we can enter the leaser into our software
database.
Art: 714363-01K Rev. Date: 03/03/08 1-7

INTRODUCTION
The i-STAT 1 Analyzer is used in conjunction with i-STAT cartridges for the
simultaneous quantitative determination of specific analytes in whole blood and with
the MediSense Precision PCx and PCx Plus Glucose Test Strips for the quantitative
measurement of glucose in whole blood.
Refer to the Cartridge and Test Information section of this manual for information
on analytes that can be measured using i-STAT cartridges.
BEFORE YOU USE THE ANALYZER
i-STAT 1 ANALYZER
2
Install Batteries
Check Date and
Time
Check Software
Customization
Two disposable lithium batteries are supplied with the analyzer. See the Care of the
Analyzer section in this manual for the procedure to install the disposable batteries.
If a rechargeable battery is to be used, the disposable batteries can be used while
the rechargeable battery pack is charged in the Downloader/Recharger. Charge
rechargeable batteries fully before use. See the i-STAT 1 Downloader section for
this procedure. When using a rechargeable battery, store the disposable battery
carrier for possible future use.
Press the On/Off key and check that the date and time at the top of the display are
correct. To change the date and time, see Administration Menu in this section.
Caution
or replaced will have standard CLEW and application software. If a different
CLEW and/or application software is in use in your facility, it must be installed
in new, repaired or replaced analyzers before they are put into use. Check the
Analyzer Status page for the installed CLEW and application software. See under
“Standardization and Calibration” in section 3 of this manual for an explanation
of CLEW.
Analyzers can be customized for many site-specific testing requirements. See the
Customization section for a list of customizable parameters and their default values.
To change the customization profile via the analyzer keypad see “Customization”
under “Administration” in this section of the manual. To change the customization
profile via the Central Data Station, see the “Customization Workspace” in the
Central Data section of this manual.
: New analyzers or analyzers that have been repaired and returned
Caution: New analyzers or analyzers that have been repaired and returned or
replaced will have the factory default settings in the customization profile, as
indicated by the DEFAULT0 on the Analyzer Status page. If analyzers in your facility
do not use the default customization profile, the appropriate customization profile
should be installed before a new, repaired or replaced analyzer is put into use.
The i-STAT 1 Analyzer is shipped with the glucose test strip functionality disabled.
The glucose test strip functionality can be enabled through the Customization
function on the Central Data Station or analyzer.
Older i-STAT 1 analyzers may have the test strip port blocked. The test strip port
can be unlocked as follows. A small flat-head screwdriver is needed to remove
the plug.
Art: 714364-01H Rev. Date: 03/03/08 2-1
1 Hold the analyzer with the test strip port facing you and the display
facing up.
2 Hold the screwdriver with the blade horizontal. Carefully insert the blade
into the horizontal gap under the plug.
3 Pry up gently until the plug pops free. Take care not to force the
screwdriver into the port.
4 Remove the screwdriver and then remove the plug. The plug can be
replaced if necessary.
Perform Quality
Check
DESCRIPTION
Specifications
Use the Electronic Simulator to verify the cartridge-reading performance of new
or repaired analyzers.
Use QC protocols to verify the test strip-reading performance of new or repaired
analyzers.
DIMENSIONS Width 7.68 cm (3.035 in.)
Length 23.48 cm (9.245 in.)
Depth 7.24 cm (2.85 in.)
WEIGHT With rechargeable battery 650 grams (22.9
oz.)
With disposable battery 635 grams (22.4 oz.)
POWER Two 9-volt lithium batteries, or rechargeable
battery.
CALIBRATION
MEMORY/CLOCK BACKUP
POWER
DISPLAY
COMMUNICATION LINK Infrared light-emitting diode (LED)
OPERATING TEMPERATURE
TRANSPORT
TEMPERATURE
RELATIVE HUMIDITY
BAROMETRIC PRESSURE 300-1000 mmHg
LASER SCANNER
Factory: electronic, mechanical, thermal,
pressure
Lithium Battery
Dot matrix supertwist liquid crystal
15-40°C (59-104°F) for Medisense strip
testing
16-30°C (61-86°F) for i-STAT cartridge testing
-10-46°C (14-115°F)
90% (maximum) non-condensing
Complies with U.S. 21 CFR 1040.10 and
1040.11 except for deviations pursuant to
laser Notice No. 50, dated July 26, 2001.
EN 60825-1:1994 + A1:2002 + A2:2001
IEC 60825-1:1993 + A1:1997 + A2:2001
2-2 Art: 714364-01H Rev. Date: 03/03/08

Software
All analyzer functions are controlled by application software that can be updated
as additional tests and features are developed. Coefficients used to maintain the
accuracy of cartridge results over time are programmed into the analyzer via CLEW
software updates every four months. See under “Standardization and Calibration”
in section 3 of this manual for an explanation of CLEW.
Note: Calibration information for the glucose test strips is included in the barcode
on the foil packet in which each test strip is packaged. The analyzer requires
that this information be scanned or entered via the keypad before the test
strip can be inserted into the analyzer.
Power
Battery
Compartment
Disposable Batteries
Rechargeable
Battery
There are two power options for the analyzer: disposable and rechargeable. The
analyzer is shipped with two disposable 9-volt lithium batteries and a battery carrier.
Lithium batteries may be ordered from i-STAT or obtained locally. ULTRALIFE
lithium batteries (ULTRALIFE Batteries, Inc., Newark, NY, USA) are recommended.
Only i-STAT rechargeable batteries may be used.
The battery compartment is located at the display end of the analyzer next to
the laser barcode scanner window. The procedure for changing disposable and
rechargeable batteries can be found in the Routine Care of the Analyzer and
Downloader section of this manual.
The analyzer requires two 9-volt lithium batteries. The lifetime for a set of batteries
is mainly dependent on the mix of cartridges in use. Cartridges that require thermal
control consume more energy because of heating. Coagulation and immunoassay
cartridges consume more energy because of the longer test cycle. A minimum
of 400 thermally controlled cartridge uses, about 100 coagulation cartridges, 50
immunoassay cartridges, or about 1,000 glucose test strips can be expected before
replacement is necessary. Backlighting, if used continuously, may reduce battery
life up to 50%. Extensive laser scanning will affect battery life slightly.
The lithium batteries should be removed from the analyzer when long periods, such
as six months, of no use are anticipated.
The analyzer can be powered by a nickel-metal-hydride rechargeable battery. The
battery capacity for one full charge is 30% (minimum) of the capacity of one set of
disposable lithium batteries (see above). If the analyzer is not in use, batteries will
lose approximately 10-30% of their charge over 30 days if not recharged.
Store rechargeable batteries in a cool dry place when not in use.
The battery recharges when the analyzer is placed in a Downloader/Recharger.
The battery pack can be removed from the analyzer and placed in the separate
recharging compartment on the Downloader/Recharger. Full recharge from a
discharged state takes approximately 40 hours. The analyzer will display “Low
Battery” when battery recharge is needed.
Caution: Do not sho rt circui t, inc iner ate or mutilate the recharegable
batteries.
Low Battery
Warning
The analyzer will display “Low Battery” when the On/Off key is pressed. Additionally,
a flashing battery icon will display on the results screens, as well as the Test Menu
and Administration Menu screens when battery replacement is needed. Data is
not lost when batteries are fully discharged.
Art: 714364-01H Rev. Date: 03/03/08 2-3
Additional Power
A lithium battery inside the analyzer maintains the clock/calendar and customization
profile. This battery should last seven years.
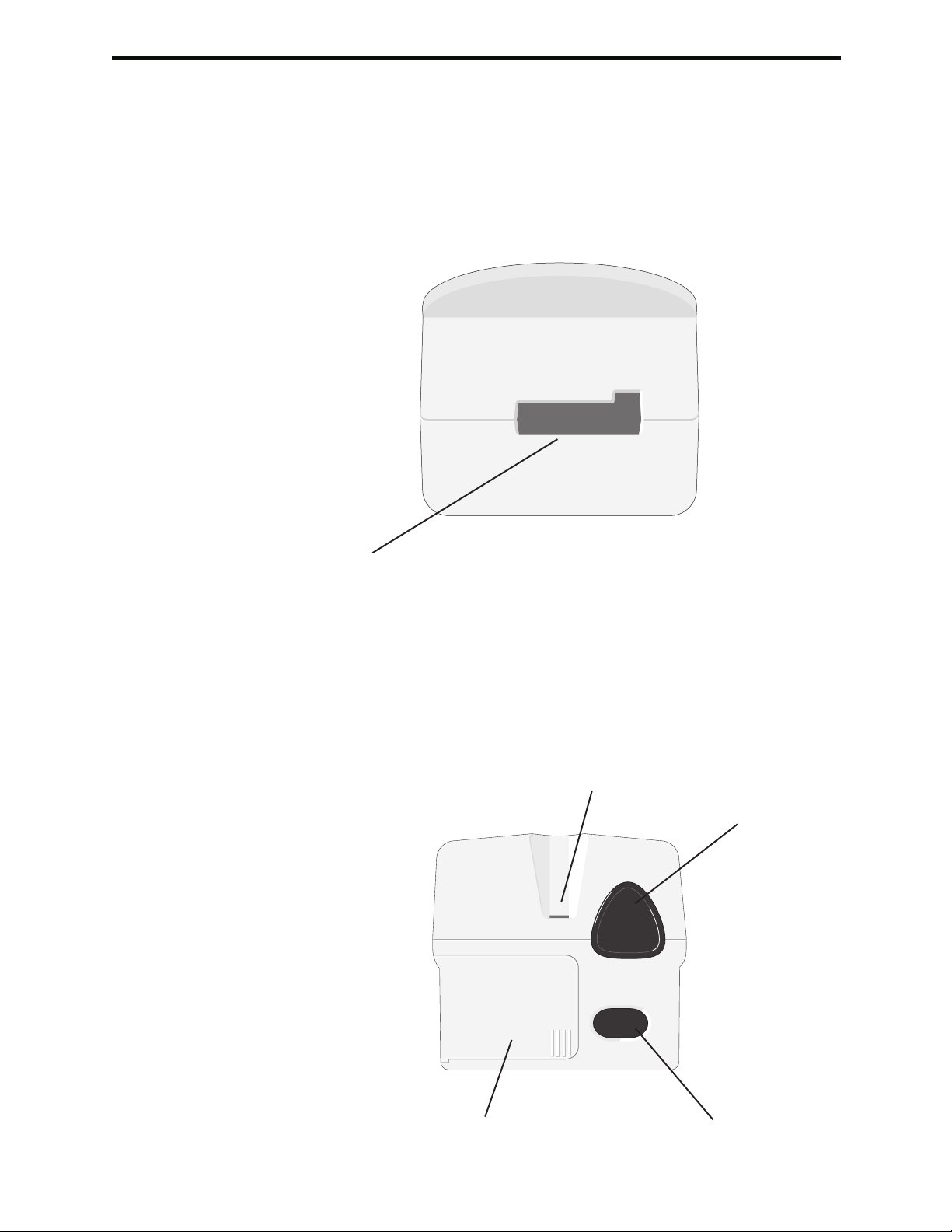
Cartridge Port
Cartridges and the Electronic Simulator are inserted into the analyzer through the
cartridge port on the keypad end of the analyzer. Unless the analyzer is customized
to require information input before a test, inserting a cartridge or Electronic Simulator
initiates the test cycle (i.e., the analyzer does not need to be turned on first). The
cartridge and test strip ports cannot be used simultaneously.
i-STAT Cartridge Port
Test Strip Port
Precision PCx and PCx Plus Blood Glucose Test Strips are inserted into the analyzer
through the test strip port on the display end of the analyzer when prompted by
the analyzer.
Precision PCx and PCx Plus
Glucose Test Strip Port
Infrared Communication
Window
Battery Compartment Laser Barcode Scanner
Window
2-4 Art: 714364-01H Rev. Date: 03/03/08

Infrared
Communication
Window
The Infrared Communication Window provides the analyzer with two-way
communication to the Central Data Station via a Downloader, allows analyzerto-analyzer software updates, and allows analyzer-to-printer communication for
printing.
Thermal Control
Barometric Pressure
Sensor
Cartridge Test Cycle
The analyzer contains a thermal control subsystem of thermistors and heating
contact wires that controls the temperature of the sensors and fluids that come
into contact with the sensors to 37°C. This subsystem is activated automatically
when a cartridge containing tests which require thermal control at 37°C is inserted
into the analyzer.
The analyzer contains a solid-state barometric pressure sensor, which determines
the ambient atmospheric pressure used for the PO2 sensor calibration.
An operator starts a cartridge test cycle either by inserting a cartridge into the
analyzer or by selecting the i-STAT Cartridge option from the Test or Quality Tests
Menu.
The analyzer:
makes electrical contact with the cartridge
identifies the cartridge type
releases calibration fluid to the sensors (when applicable)
mixes sample and reagent (when applicable)
measures barometric pressure
heats the sensors to 37°C (when required by the test )
measures electrical signals generated by the sensors and calibration
fluid (when applicable)
displaces the calibrant solution with sample (when applicable)
measures electrical signals generated by the sensors and sample
accepts the operator and patient IDs scanned or entered by the
operator
accepts chart page information
calculates and displays results
stores results
Strip Test Cycle
An operator starts a test strip test cycle by selecting the PCx Glucose Strip option
from the Test or Quality Tests Menu.
The analyzer:
accepts test strip lot data
prompts the operator to insert the test strip
prompts the operator to apply the sample
measures electrical signals generated by the glucose sensor and sample
accepts the operator and patient IDs scanned or entered by the
operator
accepts chart page information entered by the operator
calculates and displays results
stores results
Art: 714364-01H Rev. Date: 03/03/08 2-5

Data Entry
Data that can be scanned into the analyzer or entered via the keypad include:
Operator ID
Patient ID, Proficiency ID, or Simulator ID
Cartridge and Strip Lot Number
Control Lot Number
Cal Ver Kit Lot Number
Comment codes for patient and control
results
Chart Page
Sample Type
Patient Temperature - The analyzer
will interpret numbers between 50.0
and 110.0 as degrees Fahrenheit
and between 10.0 and 45.0 as
degrees centigrade. When a patient
temperature is entered, blood gas
results will be displayed at both 37°C and the patient's temperature.
FIO2
Free Fields: three fields, up to 9 characters each
See the Customization section in this manual for barcode formats recognized by
the analyzer.
Storage of Results The analyzer automatically stores up to 5,000 test records. A test record consists
of:
a set of results
the date and time the test was performed
the cartridge type
all information entered by barcode scanner or keypad including:
Operator and Patient IDs
Lot numbers for controls, cartridges and test strips
Chart page data
Serial number of the Electronic Simulator
the serial number of the analyzer
the number of times the analyzer has been used
the software and CLEW versions installed in the analyzer
the name of the analyzer’s customization profile
Quality Check Codes, which may appear during the test cycle indicating a problem
with the sample, calibration, sensors, mechanical or electrical functions of the analyzer,
are also stored.
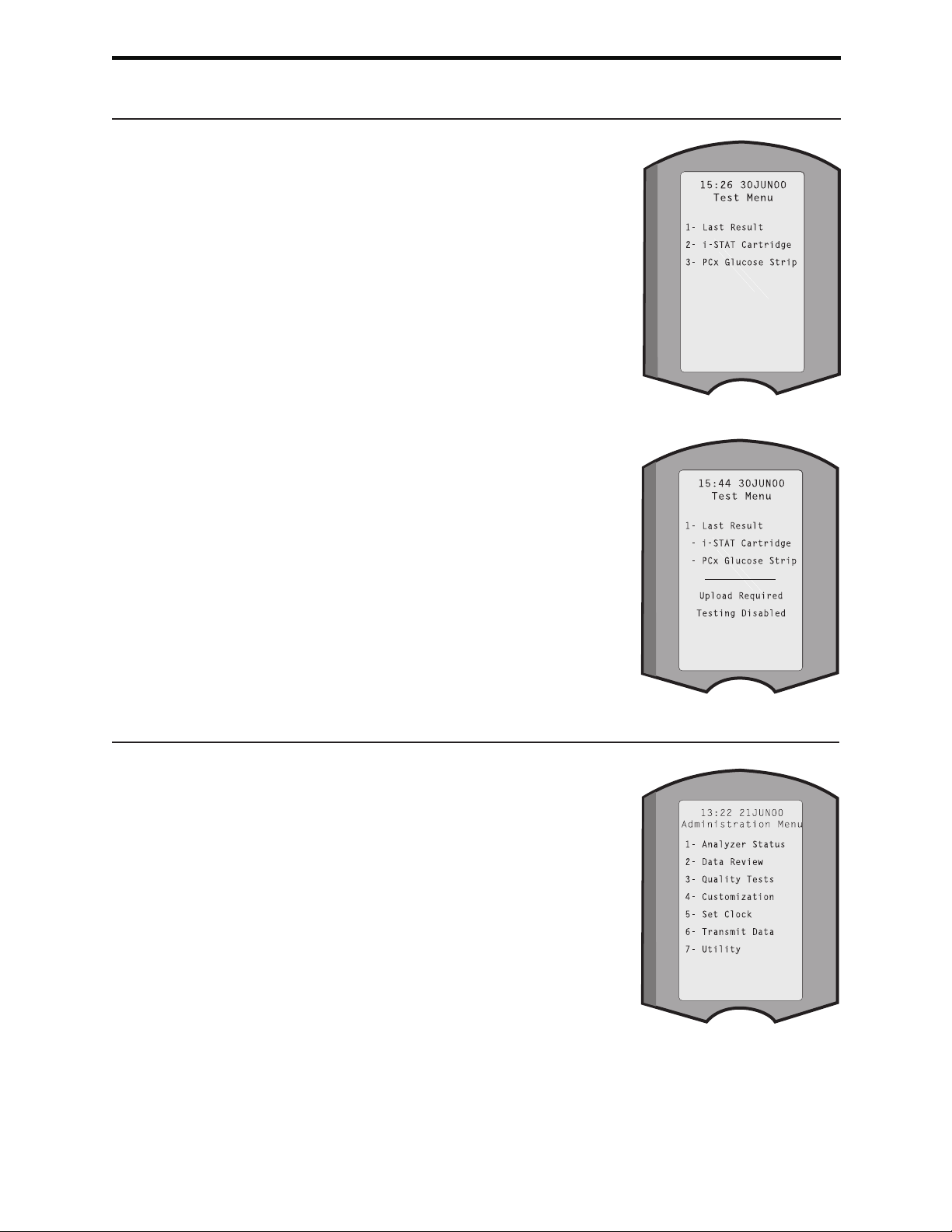
The Analyzer Status option under the Administration Menu lists the number of stored
records as “Total” and “Unsent” records. Test records are stored as “Unsent” until
the analyzer uploads data to the Central Data Station at which time the records are
marked as sent. The analyzer can be customized to display a Memory Full prompt or
to disable testing until data is transmitted to the Central Data Station. Otherwise, the
oldest data is overwritten when the memory becomes full. Stored test records can be
reviewed through the Data Review option on the Administration Menu screen described
later in this section.
2-6 Art: 714364-01H Rev. Date: 03/03/08

LCD Display and
Backlight
Test results, operator prompts and other messages are displayed on the analyzer’s
LCD Screen. The backlight for the display is turned on and off by pressing the 0
key for one second. The backlight will automatically turn off after ninety seconds
and when the analyzer powers down or is turned off. The backlight cannot be
turned on while data entry screens are displayed.
Audible Indicator
Time Out
The analyzer will beep to indicate:
whenever a key is pressed.
a successful barcode entry.
sample detection on glucose test strip
tests.
results are ready.
a Quality Check Message is displayed.
The analyzer can be customized to disable
beeping when a key is pressed or results or
messages are displayed.
The analyzer automatically turns off after a
certain period of inactivity.
Results displayed: Results are displayed for 2 minutes before the
analyzer turns off provided that a mandatory Comment Code prompt is not
displayed. This Inactivity Time Out default time can be increased using
Customization.
If a mandatory Comment Code prompt is displayed, the analyzer will turn
off after 15 minutes or after the Inactivity Time Out, whichever is greater. In
the case of a missed required Comment Code, results will be stored and “_
_ _” will be entered as the Comment Code.
Prompting for mandatory data when results are ready for display:
The analyzer will turn off after 15 minutes or after the Inactivity Time Out,
whichever is greater, if there is no response to a mandatory data prompt.
A mandatory data prompt is a prompt for information that must be entered
before pending results are displayed.
In the case of a missed mandatory data prompt, results will not be stored
and the test record will state “Test Cancelled by Operator.”
Waiting for insertion of cartridge: After the prompt “Insert Cartridge”
is displayed, the analyzer will wait 15 minutes for the operator to insert a
cartridge unless the analyzer is in the Proficiency path, in which case the
analyzer will wait 5 minutes. If a cartridge is not inserted, the analyzer will
turn off. This timeout cannot be customized.
Waiting for insertion of test strip: After the prompt “Insert Strip” is
displayed, the analyzer will wait 2 minutes for the operator to insert a test
strip. If a test strip is not inserted, the analyzer will turn off. This timeout
cannot be customized.
Other: The analyzer will turn off after 2 minutes of inactivity (no keys pressed)
in all other circumstances.
Art: 714364-01H Rev. Date: 03/03/08 2-7
Keypad There are 19 keys located directly below the display. When using the keypad to enter
information, the number of dashes in the data entry line will indicate how many characters
can be entered on the line. The dash where the next entry will be placed will flash.
Key Function
Activates the barcode scanner. Information that can be entered
SCAN
ABC
0 – 9
•
into the analyzer via the scanner includes: operator ID, patient ID,
control, cartridge and test strip lot number, patient chart data and
comment codes.
Used to move the cursor on the Set Clock screen and to move
up and down the alphabet when the ABC key is pressed. The
(right arrow) key is used as a page key to move from one
screen to the next. When Patient ID Recall is enabled, the
key will recall the last patient ID when the analyzer is prompting
for Patient ID. The (left arrow) key is used to backspace and
clear keypad entries, and to move backward through the screens
within a menu.
Used to enter alpha characters on data entry screens. When
the ABC key is pressed the letter A is entered. The arrow keys
are used to move up and down the alphabet. To enter a second
letter, press the ABC key once to move to the next position and
again to enter an A. To enter a number after a letter, press a
numbered key. To erase a letter, press the ABC key to move to
the next position, then use the key to backspace and clear the
letter.
Used to enter digits on data entry screens and to select menu
options and stored records.
Enters a decimal point or a comma separator according to the
analyzer’s Customization Profile.
Used to turn the screen backlight on and off.
Enter Used to respond to a prompt to complete an action, such as
entering an operator or patient ID via the keypad.
MENU
Print Used to print either directly to the portable printer or to the
On/Off
2-8 Art: 714364-01H Rev. Date: 03/03/08
Used to return to the previous menu and switch between the Test
and Administration Menus.
portable printer attached to a Downloader.
Turns the analyzer on or off. When the analyzer is on, the On/Off
key must be pressed for a second to turn the analyzer off. This
key is inactive when a test is in progress and when the analyzer
is prompting for mandatory data.
i-STAT 1 Menu Tree
There are two main menus: The Test Menu and the Administration Menu. If the
glucose test strip function is disabled, test strip options will not be displayed.
Test Menu Administration Menu
1- Last Result 1. Analyzer Status Temp
2- i-STAT Cartridge Pressure
3- PCx Glucose Strip 1- Patient Battery
2- Control Uses
Serial
CLEW
Version
Custom
Stored Records
2- Data Review 1-Patient
2-Control 1- i-STAT Cartridge
2- PCx Glucose Strip
3- All
3-Proficiency 1- i-STAT Cartridge
2- PCx Glucose Strip
3- All
4-Cal Ver 1- i-STAT Cartridge
2- PCx Glucose Strip
3- All
5- Simulator
6- All
7- List
3-Quality Tests 1-Control 1- i-STAT Cartridge
2- PCx Glucose Strip
2- Proficiency 1- i-STAT Cartridge
2- PCx Glucose Strip
3- Cal Ver 1- i-STAT Cartridge
2- PCx Glucose Strip
4- Simulator
4- Customization 1- View
2-Change 1- Analyzer
2- ID Entry
3- Patient Tests
4- QC Tests
5- Results
6- Password
7- Restore Factory
Settings
5- Set Clock
6- Transmit Data 1- Most Recent
2- This Month
3- Last Month
4- All
7-Utility 1- Send Software
2- Clear Memory
Art: 714364-01H Rev. Date: 03/03/08 2-9
TEST MENU
The Test Menu is displayed when the analyzer is
turned on using the On/Off key.
The options are:
1 - Last Result
2 - i-STAT Cartridge
3 - PCx Glucose Strip
1 - Patient
2 - Control
Options 2 and 3 are used for testing patient
samples. For the glucose test strip, controls can
also be tested from the Test Menu. Testing controls
from the Test Menu rather than option 3 – Quality
Tests under the Administration Menu may be more
convenient for end users who test glucose test strip
control samples on a daily basis.
Note: If the analyzer is customized with test strip
testing disabled, test strip options will not
be displayed. If the analyzer is customized
to disable testing under certain conditions,
the disabled option will be listed without
its number so that it cannot be selected.
ADMINISTRATION MENU
Overview
The Administration Menu is accessed by pressing
the Menu key from the Test Menu screen. The
options are:
1 - Analyzer Status
2 - Data Review
3 - Quality Tests
4 - Customization
5 - Set Clock
6 - Transmit Data
7 - Utility
2-10 Art: 714364-01H Rev. Date: 03/03/08
Analyzer Status
The Analyzer Status screen contains information about the condition or “status” of
the analyzer. Fresh readings are made whenever this option is selected.
Temp Room temperature.
Pressure Barometric pressure.
Battery Battery voltage.
Uses Total number of cartridge,
simulator and test strip
test cycles, whether or not
results reported.
Serial Serial number of the
analyzer.
CLEW Version of standardization
data installed in the analyzer.
Version Version of application
software installed in the
analyzer.
Custom Customization profile name.
Stored Records Total: The number of test records in the analyzer’s
memory. The maximum storage capacity is 5,000
test records, which include records with results and
Quality Check Codes for patients and controls both
liquid and electronic.
Unsent: The number of test records that have not
been transmitted to the Central Data Station.
Data Review
The Data Review function allows the operator to review stored results by the
categories listed below. The number of test records stored is indicated at the
bottom center of the screen as x/y where x is the record on the screen and y is
the total number of stored records in the selected category. The 1 and 2 keys are
used to scroll through the stored records as indicated on the bottom right and left
of the screen. The most recent test record is always in the first position. The right
arrow key is used to page through the screens of the displayed record.
1 - Patient The records for a patient
are recalled by scanning or
entering via the keypad the
Patient ID. If no Patient ID is
entered, all patient tests are
recalled.
2 - Control 1 – i-STAT Cartridge
2 – PCx Glucose Strip
3 - All
3 - Proficiency 1 – i-STAT Cartridge
2 – PCx Glucose Strip
3 - All
4 - Cal Ver 1 – i-STAT Cartridge
2 – PCx Glucose Strip
3 – All
5 - Simulator All external and internal Electronic Simulator records.
6 - All All test records in the analyzer’s memory.
Art: 714364-01H Rev. Date: 03/03/08 2-11
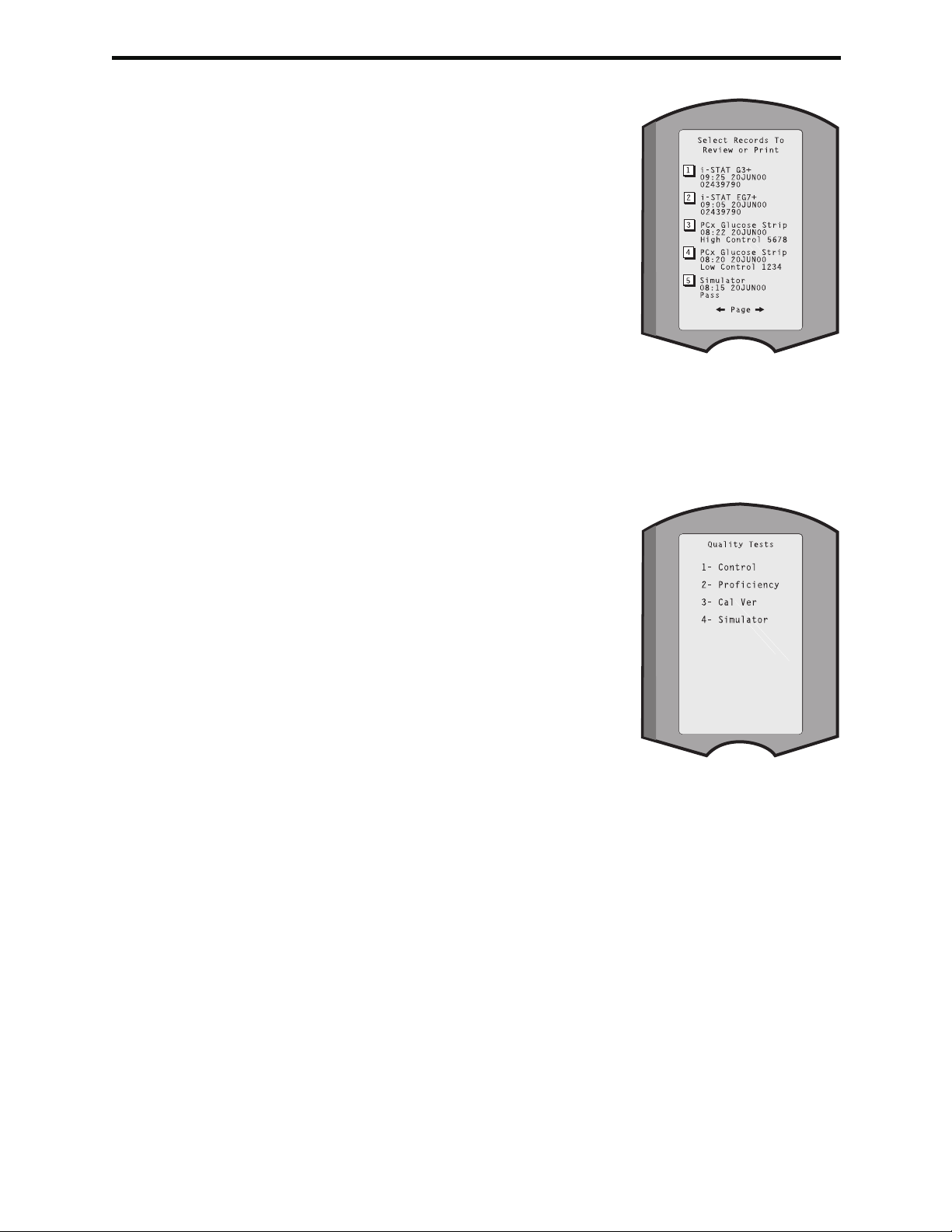
7 - List Records are listed with
Cartridge type or PCx Glucose
Strip, date and time of test,
and patient ID, control lot,
proficiency ID, or Cal Ver lot
and test level as applicable.
Any number of test records
can be selected for viewing
or printing using the number
keys. Pressing the number
key corresponding to a record
selects a record; pressing the
number key a second time
deselects the record.
To view one or more records,
select the records and press
the Enter key. To print records, select the records and
press the Print key.
Quality Tests
Non patient tests can be initiated from the Quality Tests menu. Options are:
1 - Control
2 - Proficiency (external quality control)
3 - Cal Ver (Calibration Verification for
cartridges and Linearity Test
for test strips)
4 - Simulator (cartridge-reading function
only)
When options 1 - 3 are selected, the operator
further selects:
1 – i-STAT Cartridge
2 – PCx Glucose Strip
When testing is initiated from one of these options,
the analyzer prompts the operator to scan or
enter the Operator ID; the Control Lot Number,
Proficiency ID, Cal Ver Kit Lot Number, or Simulator ID as applicable; and the
Cartridge Lot Number or Test Strip Lot Number as applicable.
When the Quality Tests option is used, results can be reviewed according to the
corresponding options under the Data Review option.
Note: The Test Menu option for test strips includes both a Patient and Control
option. Results for test strip controls run from the Test and Quality Tests menus
are stored together.
2-12 Art: 714364-01H Rev. Date: 03/03/08
 Loading...
Loading...