Page 1

Data recording in pharmaceutical processes
Improve your process monitoring effectiveness using ABB’s
RVG200 data recorder
A complete view of your
pharmaceutical process data
at your fingertips
Introduction
ABB’s RVG200 next generation paperless data recorder offers
a host of additional features and functions, making it ideal for
data recording in pharmaceutical manufacturing applications.
How can the RVG200 help?
Touchscreen technology for easy operation
Featuring touchscreen technology, the RVG200 makes it easier
than ever to find the data you need. Intuitive icon-based menus
and a ‘swipeable’ interface enable data to be quickly located
and viewed in a variety of formats, including individual or
grouped data in chart, bar graph or digital indicator displays.
Extensive security for optimum data protection
The RVG200 features a comprehensive range of security
measures which enable it to comply with the Food and Drug
Administration’s (FDA) stringent 21 CFR Part 11 criteria for
acceptance of electronic process records. These measures
include encrypted data files, multi-user password protection
and automatic audit trail generation.
With the RVG200, you also have the option of a specific
validation template, which helps to simplify the process of
satisfying the overall installation and operation requirements of
21 CFR Part 11.
Page 2
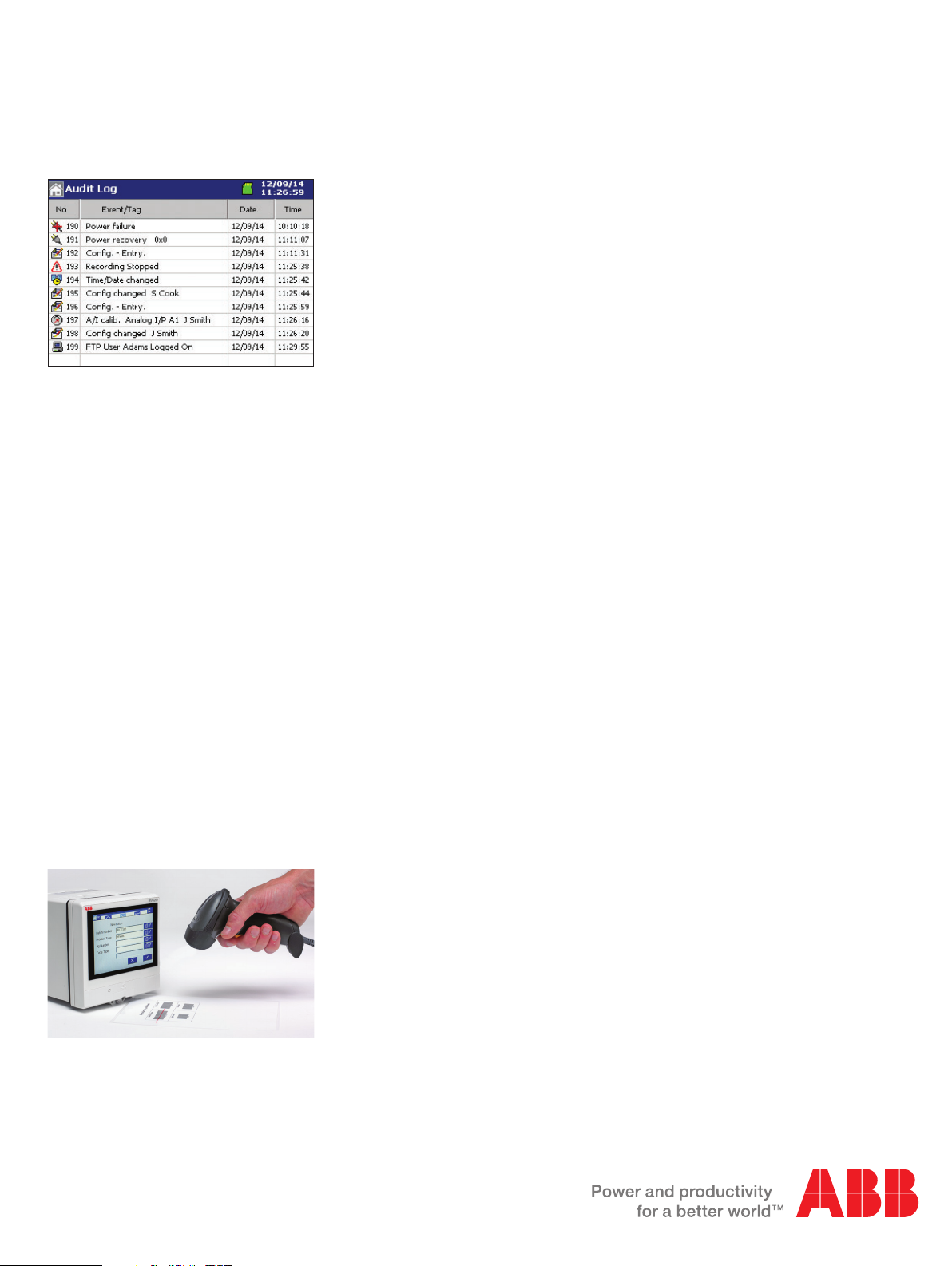
Audit log for maximum traceability
The RVG200’s audit log gives you a complete history of
your process. The log automatically stores details of all
process events together with details of time, date and, where
applicable, the details of any configuration or calibration
changes, together with details of who made them.
When viewed alongside process data, the audit log provides
comprehensive evidence of the validity of the process data
and the recorder on which is was created.
F0 calculation for enhanced sterilisation efficiency
When compared to a basic time based system, the cycle time
of a sterilisation process can be substantially reduced by the
use of F0 calculation. By accounting for the time a process
spends below and above the specified sterilising temperature,
F0 calculation can improve efficiency whilst guaranteeing
sterilisation effectiveness. An increase in the sterilisation
temperature of just one degree from 121ºC to 122ºC, for
example, can reduce the time needed to kill bacteria by 26%.
The use of the RVG200’s F0 calculation during a sterilisation
cycle will take into account the heating and cooling phases,
benefitting almost all sterilisation processes.
Pharmaceutical xxxx 2013
Batch recording for fast and detailed batch data
The RVG200’s batch recording option provides a simple
means of recording when a particular batch is started or
finished.
This data can be reviewed using ABB’s DataManagerPro
analysis software. Providing a quick and simple way of
retrieving batch data, DataManagerPro lets you retrieve data
by entering the batch number, searching by type or product or
by specifying the time and date of processing.
Barcode scanner for fast and accurate batch
information entry
With the RVG200, you now have the option of connecting
a barcode scanner into the device’s front or rear USB,
providing a fast and more accurate alternative to manually
keying in batch information.
Batch information can be scanned directly from product
paperwork, eliminating the scope for human error.
Find out more
For more information about the benefits of using the RVG200
in your heat treatment process, please contact your local ABB
sales representative or visit www.abb.com/recorders.
ABB Limited
Howard Road
St. Neots
Cambridgeshire PE19 8EU
UK
Tel: +44 (0)1480 475321
Fax: +44 (0)1480 217948
 Loading...
Loading...