Page 1

Operating Instructions
Soleoline
Soleo Sono
GB
Page 2

Page 3
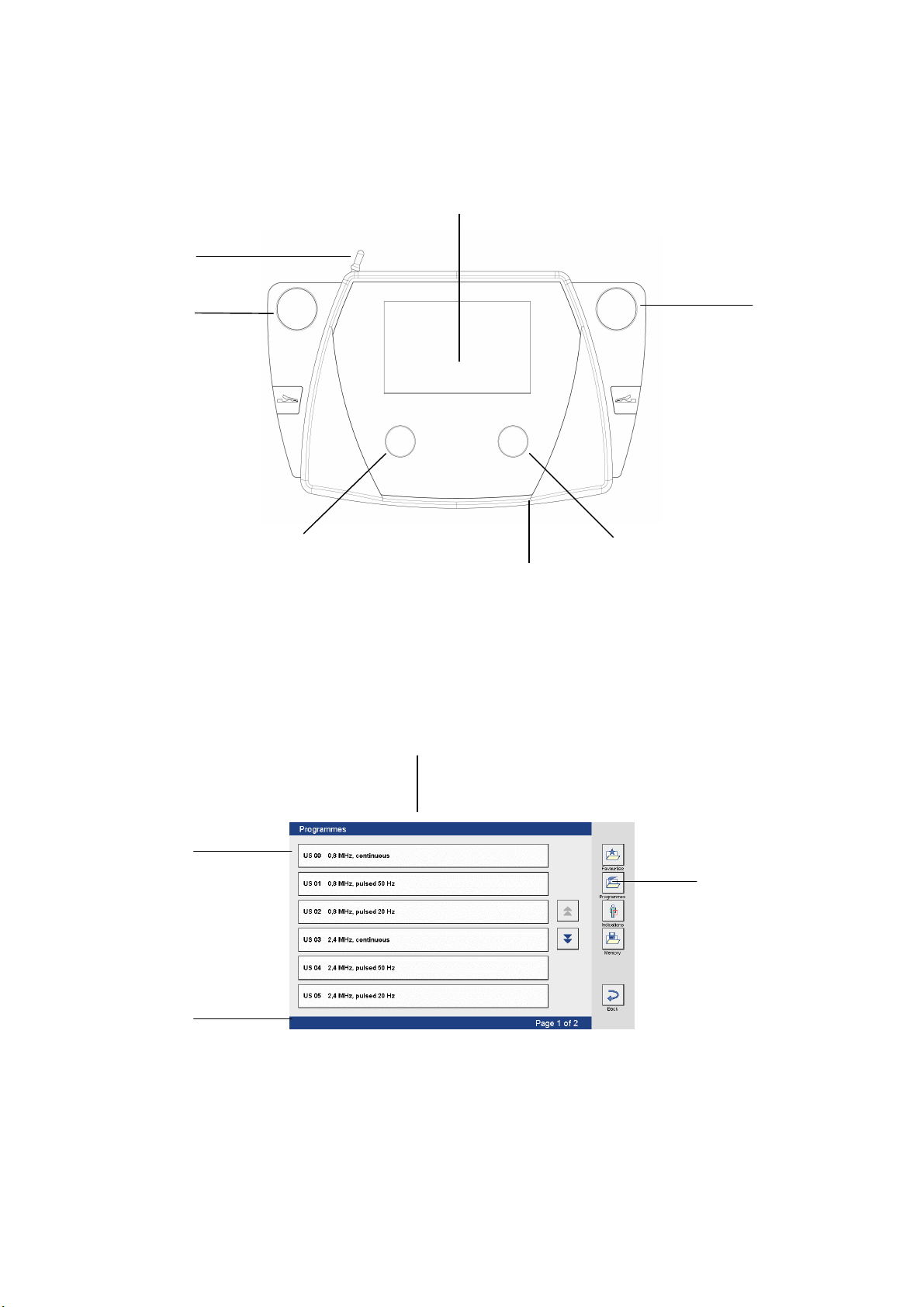
3
Fig. 1
Soleo Sono
Front view of device
4
5
3
1
2
6
Selection and control elements
1 Intensity regulator 4 Screen
2 Frequency ratio regulator 5 Touch pen in holder
3 Slot for ultrasound head 6 SD card slot
Fig. 2
9
10
8
7
Screen readouts
7 Status bar 9 Title bar
8 Navigation bar 10 Buttons on the screen
Page 4
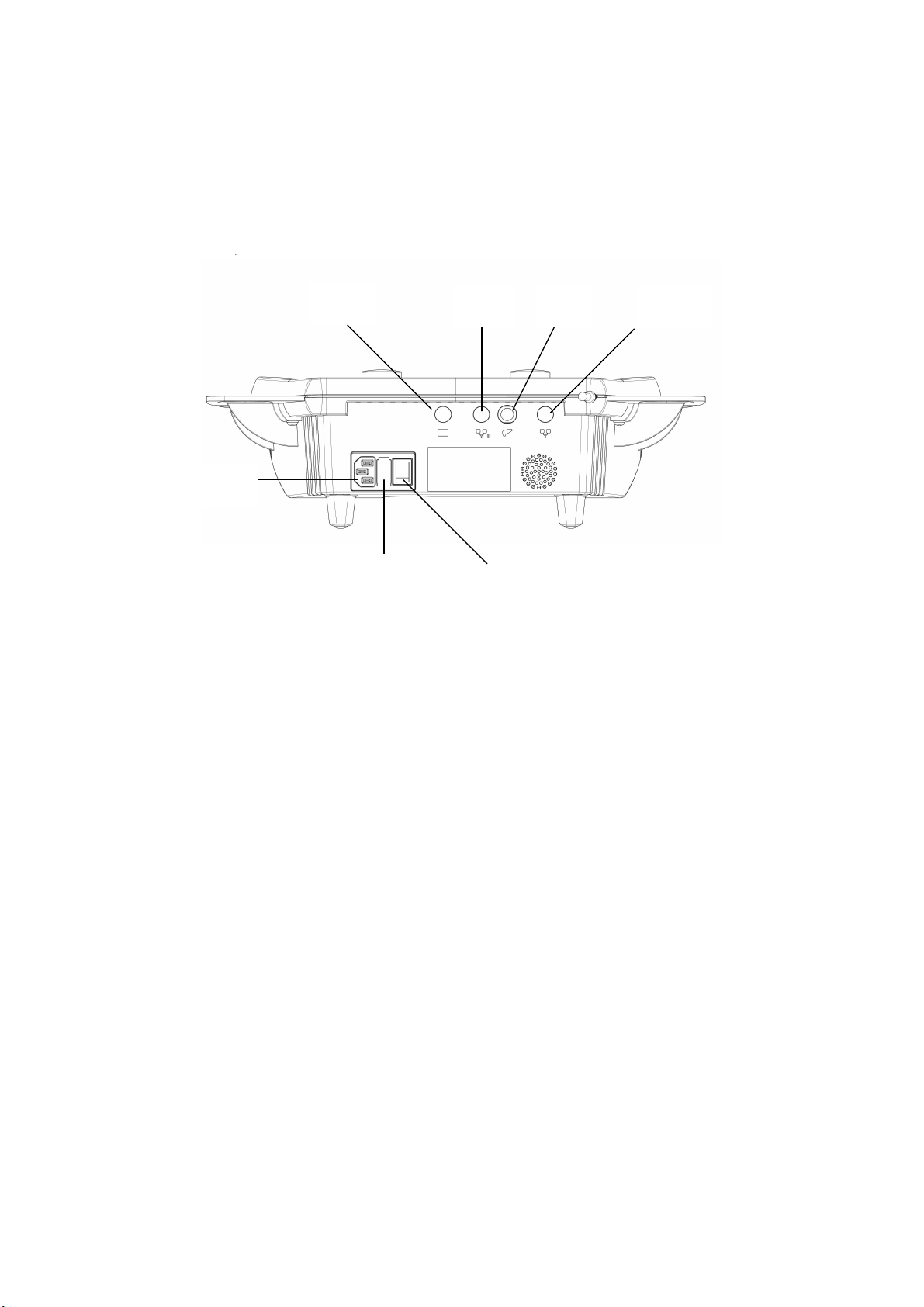
Fig. 3
Soleo Sono
Rear view of device
(15*)
11
Switch and connector sockets
11 Connector for mains cable 13 On/off switch
12 Fuse holder for mains fuse 14 Socket for 0.8/2.4 MHz ultrasound head
Note: Sockets (15*), (16*) and (17*) have no function on Soleo Sono.
12
(16*)
14 (17*)
13
Page 5

Contents
Soleo Sono
Front view of device
Selection and control elements/screen readouts
Rear view of device
Switch and connector sockets
Page
1. Soleoline – Summary / Soleo Sono 1
2. Fitting the cables, Starting the system 2
3. Configuration
3.1 General 3
3.2 Ultrasound therapy 9
3.3 Attendance/Maintenance 10
4. Quick operating instructions 11
4.1 Water bath treatment 15
5. General instructions – SD card 17
6. Description of the selection buttons 18
7. Screenshot of the therapy screen 22
8. Description of the screen elements and the buttons
8.1 Screen elements 23
8.2 Buttons 26
9. Indications menu 29
10. Saving a modified programme 32
10.1 Favourites 34
10.2 Memory 35
11. Retrieving and editing favourites and memory
11.1 Retrieving favourites 36
11.2 Editing favourites 37
11.3 Retrieving and editing memory 39
Page 6

Contents
Page
12. Indications 40
13. Contraindications 41
14. Explanation of symbols 42
15. Warnings 43
16. Technical Information
16.1 General 44
16.2 Specific 45
17. Cleaning, Disinfection 46
18. CE Marking 47
19. Contents on delivery, accessories 48
20. Safety and maintenance 49
21. Functional test 50
22. Error messages, Troubleshooting, Disposal 51
23. Manufacturer's EMC declaration 52
Valid for the Soleo Sono device.
These Operating Instructions are an integral part of the device.
They must be stored with the device and kept accessible at all times for anyone authorised to operate this
device.
These Operating Instructions are valid from 01.01.10.
Page 7

Soleoline – Summary
Soleo Sono
What is Soleoline?
Note: The operation of Soleo SonoStim and Soleo Galva is described in a separate
What are the
advantages of
Soleoline?
What does the Soleo
Sono do?
An ultramodern and innovative range of products with 3 different devices
available.
Soleo Sono
An ultramodern and innovative ultrasound therapy device.
Soleo SonoStim
An ultramodern and innovative combination device for electrotherapy and
ultrasound therapy with the option of attaching a vacuum unit.
Soleo Galva
An ultramodern and innovative electrotherapy device with the option of
attaching a vacuum unit.
set of operating instructions.
A clear contemporary colour screen showing all parameters necessary for
therapy as well as modern touch control.
Individual programme start configuration and clear, simple menu navigation
make operation of the device easy and comfortable for users.
The compact design saves room in the practice and is highly suited for use in
house visits.
Delivers therapeutic ultrasound via a modern variable frequency ultrasound
head.
1
Innovations in Soleo
Sono?
Note: The device should only be used by medical specialists (e.g., doctors,
SonoSwing, the innovation in the field of ultrasound therapy:
• a single ultrasound head with two frequencies 0.8 MHz and 2.4 MHz
• freely selectable penetration depths using percentage adjustment of the
frequency ratios.
therapists and health paraprofessionals).
1
Page 8

Fitting the cables
Starting the system
Note: On the connector cable for the ultrasound head there is a green arrow as a
guide for correct connection with the device.
Connecting the
ultrasound head
Connecting the
mains cable
Switching on the
device
Note: All buttons, menus and submenus are activated directly on the screen by
When connecting the ultrasound head, ensure that the green arrow is pointing
left when being plugged in.
Connect the ultrasound head to the appropriate socket (14).
Plug the mains cable into the appropriate socket (11) on the device and then
plug into the mains socket.
Switch on the device using the rocker switch (13).
touching it or using the touch pen.
2
2
Page 9
Configuration
3.1 General
Note: Changes to the default settings can only be made from the start screen.
Start screen After switching on the device and the self-test, the start screen opens.
Selecting
configuration
3
Press the button to open the configuration menu.
3
Page 10
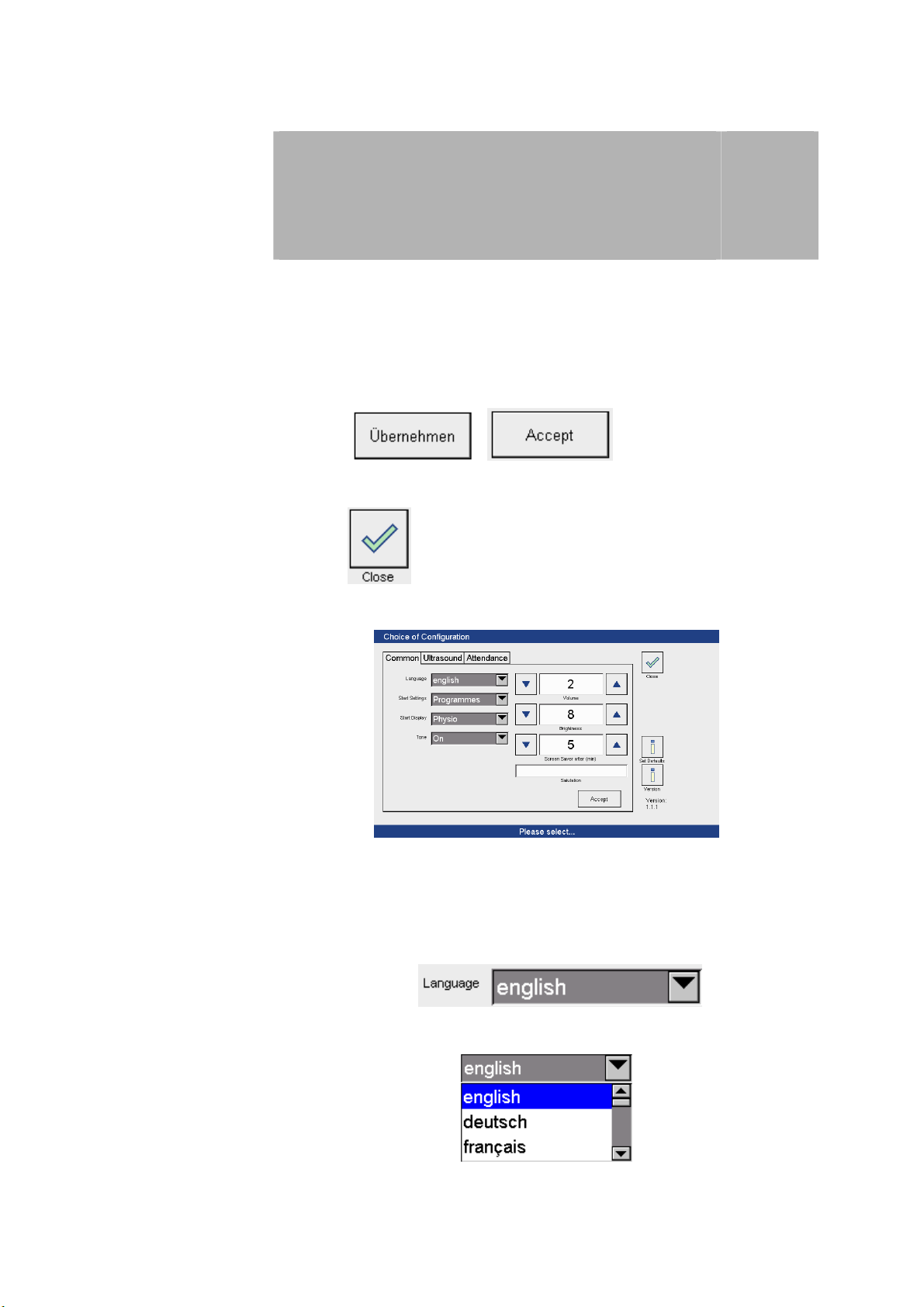
Configuration
3.1 General
Configuration menu In the configuration menu the factory settings can be changed and individually
set.
After activation of the configuration menu the ‘Choice of Configuration’ screen
is active.
Saving settings
Closing the
configuration menu
General settings
Press the / button to save the new
settings.
Press the button to return to the start screen.
3
The setting options are outlined below.
In the factory, the default settings are pre-programmed as shown on the
screen.
Language
Press the arrow key to open the
drop-down menu to select the language.
The language is selected by pressing on the appropriate row.
4
Page 11
Configuration
3.1 General
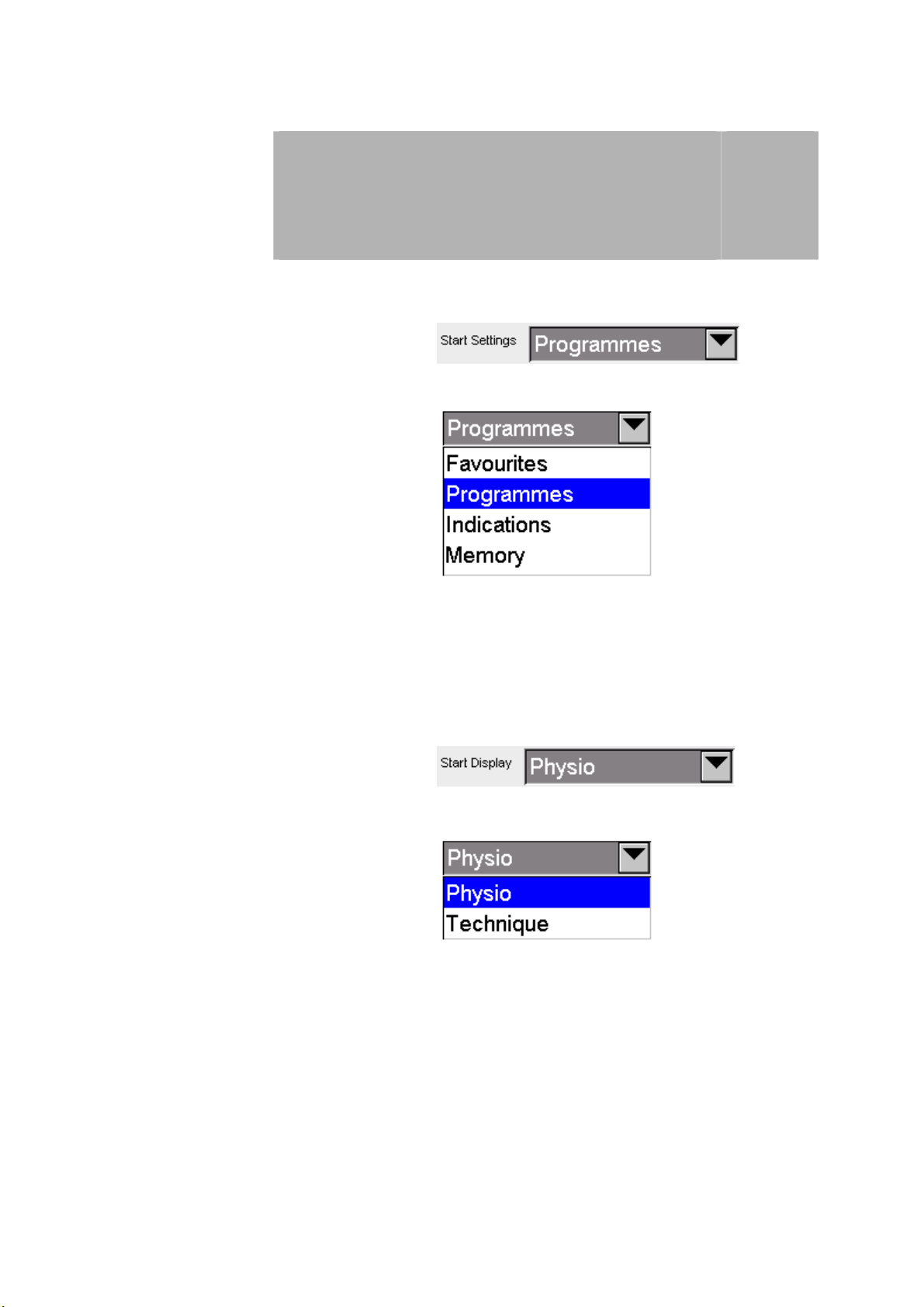
Start settings Configuration options for the programme start settings:
Press the arrow button
to open the drop-down menu to select the programme start configurations.
The selection is made by pressing on the appropriate row.
Start screen Option to choose between 2 start screens:
3
Press the arrow button
to open the drop-down menu to select the start screen.
The selection is made by pressing on the appropriate row.
5
Page 12
Configuration
3.1 General
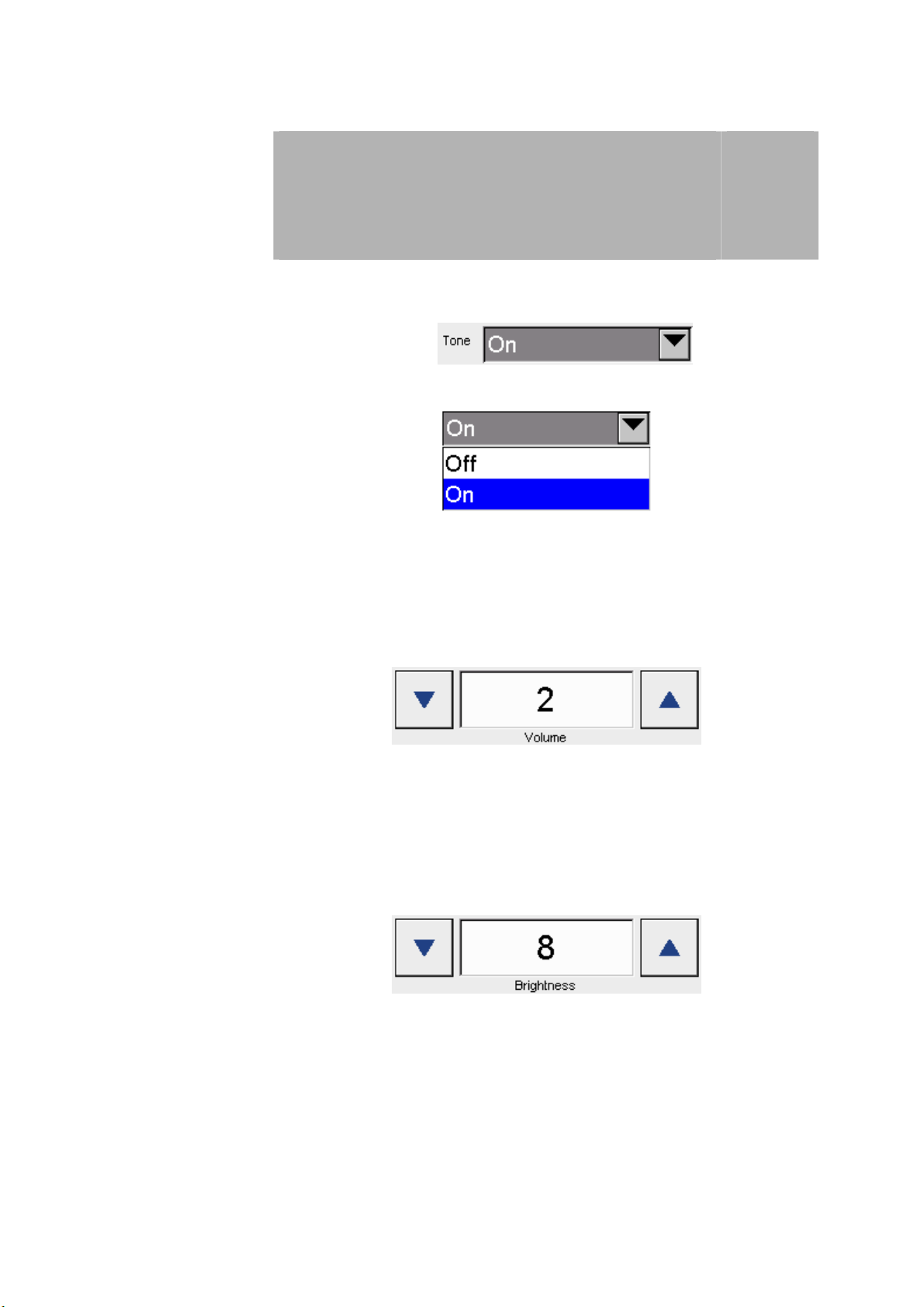
Sounds To switch the signal sound on and off when activating the control fields:
Press the arrow button
to open the drop-down menu to switch sounds on and off.
The selection is made by pressing on the appropriate row.
Volume Option to adjust the volume in steps from 1 to 4:
3
Press the arrow button
to open the window to adjust the volume.
The volume is adjusted using both arrow keys.
Brightness Option to adjust the screen brightness in steps from 0 to 10:
The brightness is adjusted using both arrow keys.
6
Page 13
Configuration
3.1 General
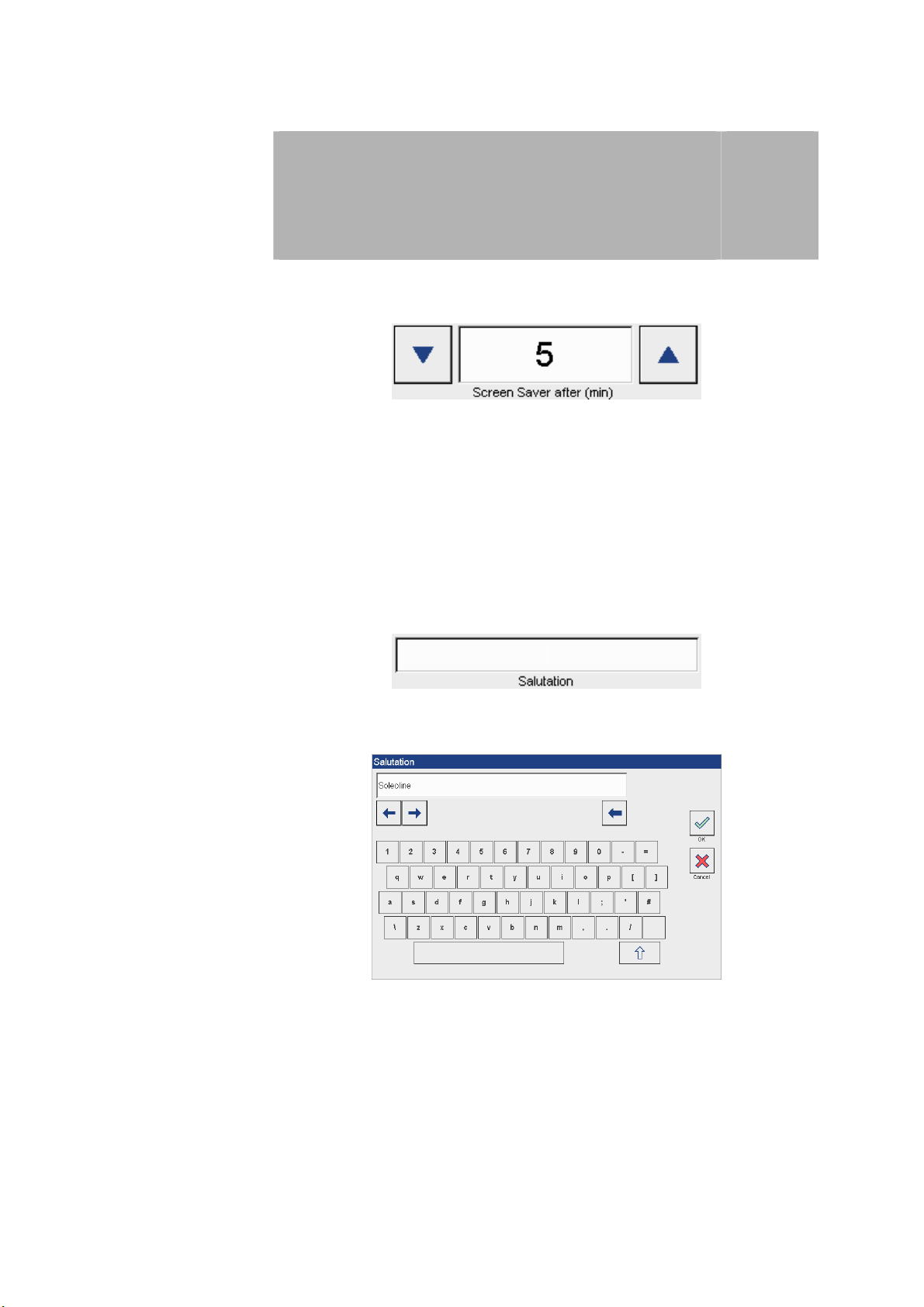
Screen saver Option to configure the start of the screen saver after 0 to 20 minutes:
The start time is adjusted using both arrow keys.
Note: While therapy is running, the screen saver function is deactivated.
Welcome message Option to configure an individual welcome message.
Press the field
3
to open the screen keyboard to enter a welcome message.
7
Page 14
Setting defaults
Version
Configuration
3.1 General
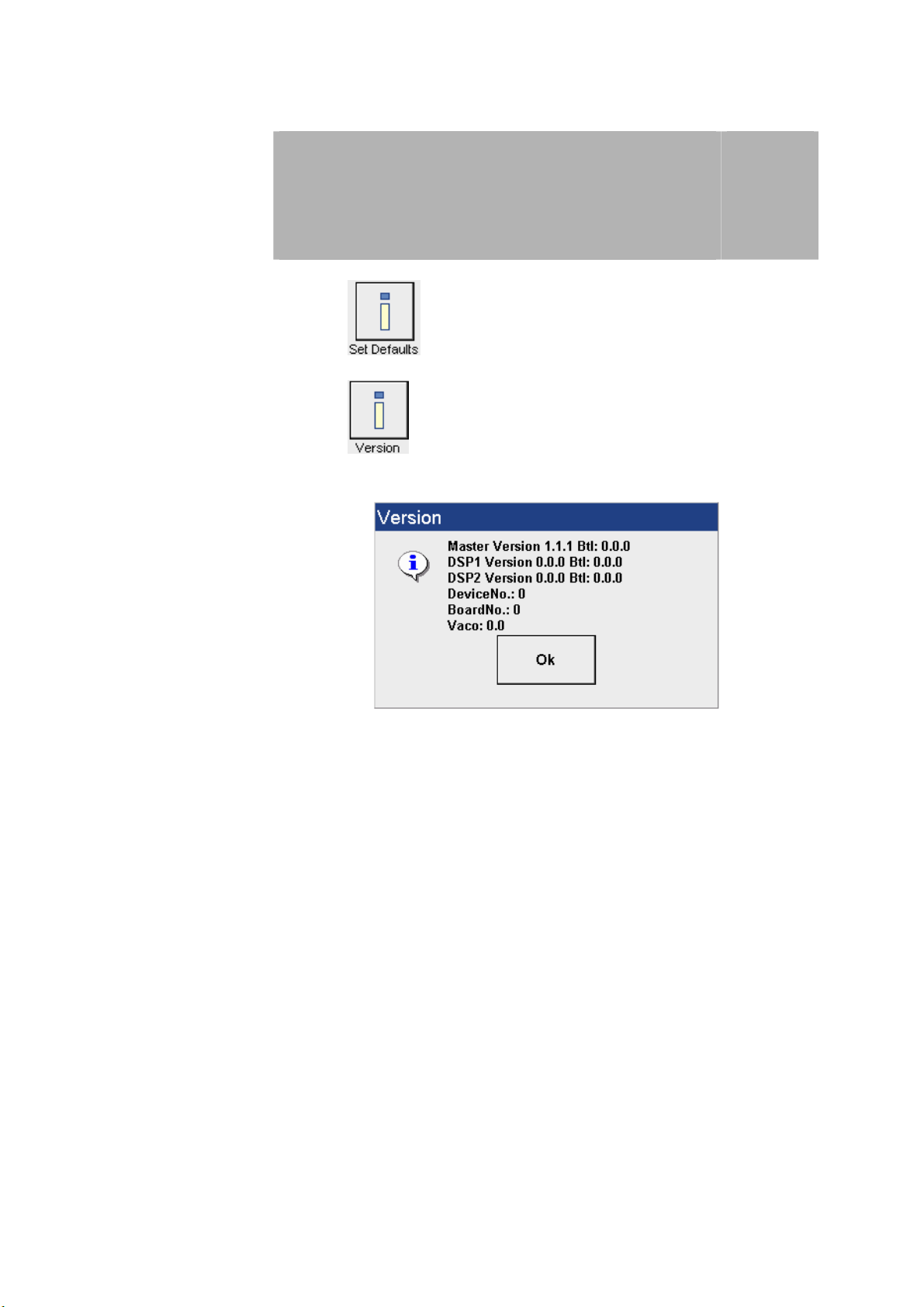
Press the button to reset the factory standard settings.
Press the button to open the window with information about the
current software version.
3
Press the OK button to close the window.
8
Page 15
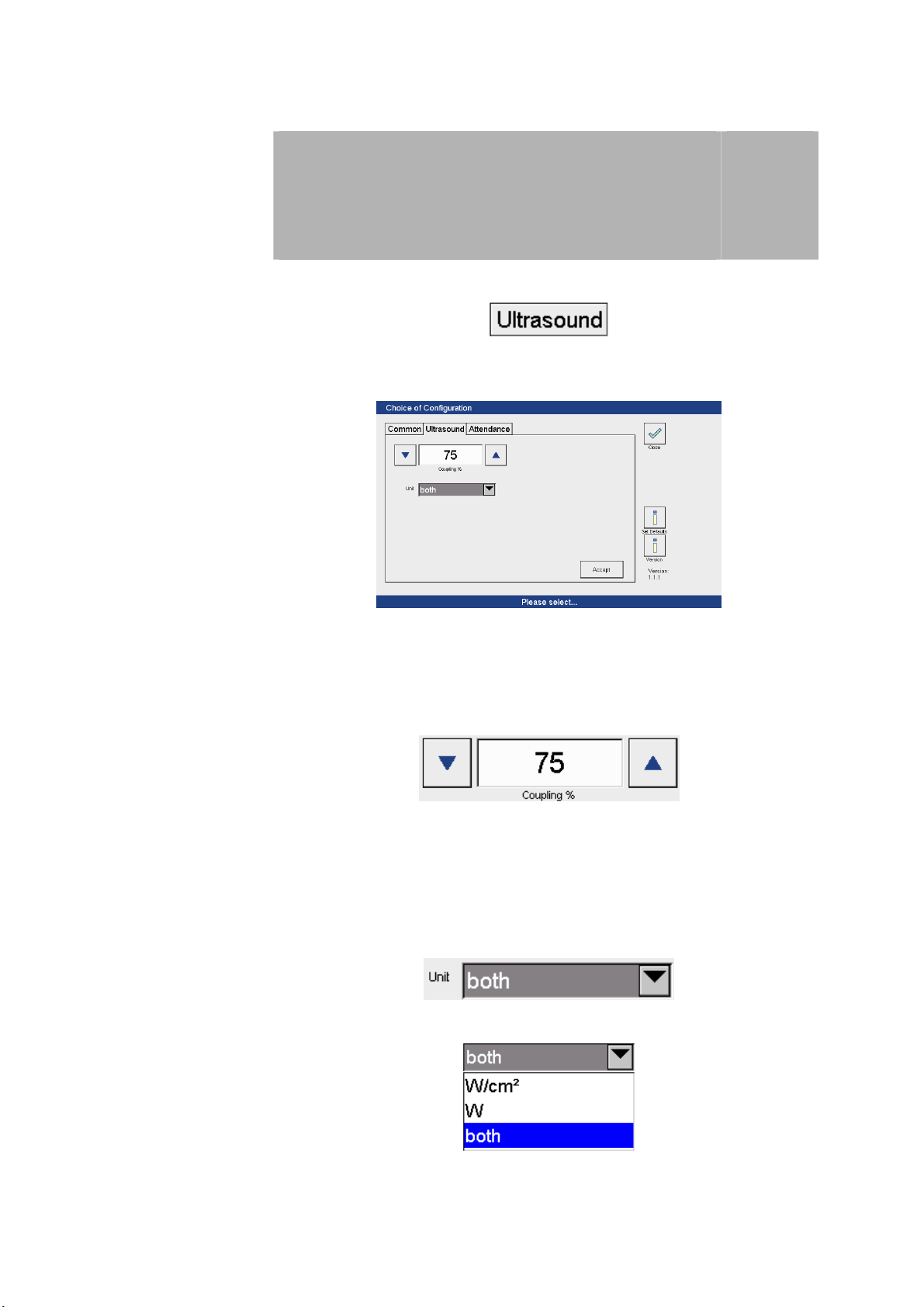
Configuration
3.2 Ultrasound therapy
Ultrasound therapy
settings
Activate the menu
to open the ‘Ultrasound therapy configuration’ screen.
3
The settings required for therapy can be adjusted here.
Coupling signal Option to adjust (50 to 95%) the threshold for coupling:
The threshold is adjusted using both arrow keys.
Units Option to configure the units for intensity in a bar graph:
Press the arrow button
to open the drop-down menu to select the desired units for power.
The selection is made by pressing on the appropriate row.
9
Page 16

Configuration
3.3 Maintenance
Maintenance
Activate the menu
to open the ‘Maintenance’ screen.
3
/
The maintenance programmes are protected by passwords. Use the keyboard
to insert the password.
To open the update menu, enter the password ‘armin’.
Software can be updated using the updates menu.
You will receive the latest information about updating software when an update
is planned.
For Servicing other passwords are necessary. Servicing is permissible by
Zimmer technicians or technicians trained by Zimmer only.
10
Page 17

Starting the
programme
Soleo Sono
programmes
Quick operating instructions
Press the
is selected here.
button to open the ‘Programme’ screen. The programme
4
There are 9 different programmes available in Soleo Sono.
11
Page 18

Quick operating instructions
Selecting the
programme
Therapy screen After selecting the ultrasound therapy programme, the therapy screen opens.
Select the desired ultrasound therapy programme by pressing on the
appropriate row (here US 01).
4
Note: Check that the information shown on the Parameter button (here 5 cm2)
matches the ultrasound head connected before starting therapy.
12
Page 19

Quick operating instructions
Setting intensity Adjust the intensity using the intensity controller on the left.
Note: Change the percentage frequency ratio using the right hand regulator.
Starting therapy
Press the button to start the therapy.
4
The display in the bottom status bar changes from ‘Ready’ to ‘Active’ with the
start of therapy and the ‘Start’ button changes to ‘Stop’. The dose set is shown
in the bar graph and the therapy time counts down in seconds. The coupling
display is active.
13
Page 20

Quick operating instructions
Ending therapy At the end of the therapy time an acoustic signal indicates that the therapy has
ended and the clock is reset to 00:00. The intensity automatically returns to
zero, the bar graph display disappears and the coupling display is inactive. The
display in the bottom status bar changes from ‘Active’ to ‘Ready’.
The therapy time is automatically reset and the ‘Stop’ button changes to ‘Start’
at the end of the therapy.
4
14
Page 21

Quick operating instructions
4.1 Water bath treatment
Note:
Implementation
If the ultrasound therapy is done in a water bath, the ultrasound head
temperature monitoring must be changed before starting the therapy.
Press the Pulse
button to open the ‘Ultrasound Parameters’ window.
4
Pressing ‘Water bath’
button
By pressing the ‘Water bath’
button and confirming with OK, the ultrasound head temperature monitoring is
modified for therapy in a water bath.
15
Page 22

Quick operating instructions
4.1 Water bath treatment
If at the end of the therapy an additional treatment with the same ultrasound
programme will be carried out, after adjusting the intensity the following
message appears:
If at the end of therapy the programme will be changed, the function of the
water bath button is automatically deactivated.
Continuing therapy
in the water bath
Press the button
4
Ending therapy in the
water bath
Note: Once therapy in the water bath is complete, the temperature of the ultrasound
Press the button
head may be too high for treatment outside the water bath.
This is shown in the status bar by the message ‘Ultrasound head temperature
adjustment’. The ultrasound head cannot be used while the temperature is
adjusting. Once the temperature adjustment of the ultrasound head is
complete, the message disappears and the therapy can be continued.
16
Page 23

General instructions
SD card
SD card User-defined settings and the indications list are saved on the SD card.
Note: If the SD card is not inserted, the message ‘No SD card found’ appears when
the Indications, Favourites and Memory buttons are pressed.
Deactivate the message as described in Section 22.
5
17
Page 24

Description of the selection buttons
Note: The following descriptions are all based on the factory settings.
Configuration
Start
Favourites
Programmes
Press the button to open the settings menu.
The options are described in detail in Section 3.
Press the button to open the start screen from the Programme window.
Press the button
• to open Favourites for editing
• to add the programme to Favourites in memory mode.
Press the button to open the Programmes window.
6
18
Page 25

Indications
Memory
Saving
Description of the selection buttons
Press the button to open the indications menu.
Press the button
• to open the memory list for editing
• to add the programme to the memory list in memory mode.
Press the button to open the screen to save a programme.
The ‘Save’ button can only be pressed from the therapy screen.
6
Back
Editing
Press the button to go back one screen.
Press the button
• to open the ‘Memory’ screen to edit the memory list
• to open the ‘Favourites’ screen to edit Favourites
19
Page 26

Moving
Moving
Deleting
Scrolling forwards
Scrolling backwards
Description of the selection buttons
Press the button to move the order of the list upwards by one position.
Press the button to move the order of the list downwards by one position.
Press the button to delete the selected programme from the list.
Press the button to scroll one page down the list.
Press the button to scroll one page up the list.
6
20
Page 27

Close
Cancel
OK
Description of the selection buttons
Press the button to close the Favourites and Memory programmes.
Press the button to reject the changes made.
Press the button to confirm the changes in the selected list.
6
OK
The changes are applied by pressing the button.
21
Page 28

Screenshot of the therapy screen
7
22
Page 29

Title bar
Status bar
Parameters
Description of the screen elements
and buttons
8.1 Screen elements
The title bar shows the current ultrasound therapy programme.
The status bar shows information on the current status of the therapy. If the
therapy is not active, it shows the word ‘Ready’. During therapy, it shows the
word ‘Active’.
8
Pulse
Shows the active ultrasound head.
Shows the operating mode selected.
23
Page 30

Description of the screen elements
8.
and buttons
8.1 Screen elements
Operating mode
Pulse ratio
Pulse frequency
Depth effect The bar graph shows the current frequency ratio of 0.8 MHz (800 kHz) to 2.4
Graphic view of the operating mode
continuous
or
pulsed
Pulsed operating mode showing the pulse ratio (1:2 here) and the pulse
frequency (50 Hz here).
MHz as a percentage.
In this example: 25% 2.4 MHz
75% 0.8 MHz (800 kHz)
For further explanation please consult the Therapy Manual.
Adjusting frequency
ratio
Adjust the percentage frequency ratios of 2.4 MHz to 0.8 MHz using the right
hand regulator.
24
Page 31

Coupling
Important:
Description of the screen elements
and buttons
8.1 Screen elements
The coupling is shown digitally as a percentage.
The most beneficial coupling value is 100%.
If the coupling is below the preset value (factory setting 75%), an acoustic
signal sounds and the therapy time is paused.
In this case:
Set a new coupling value and when the coupling is sufficient the therapy will
continue.
8
Bar graph
Shows the currently specified intensity.
25
Page 32

Description of the screen elements
and buttons
8.2 Buttons
Note:
Note: Only the parameters that are shown in the pulse window can be changed.
Activating the Pulse
button
If the Pulse button is activated during therapy, the therapy will be interrupted,
the intensity returns to zero and the therapy time is set to the standard value.
When the ‘Pulse’ button is activated, the ‘Ultrasound Parameters’ window is
opened.
8
Ultrasound parameters
The ultrasound mode can be selected here and the ultrasound head can be
switched to therapy in a water bath.
26
Page 33

Description of the screen elements
and buttons
8.2 Buttons
Selecting the mode Press the two arrow buttons
to select the desired mode.
Modes:
• continuous
• pulsed with duty cycles of 1:2, 1:3, 1:5 and 1:10
Selecting the
frequency
Press the two arrow buttons
8
to select the desired frequency.
Frequencies: 20 Hz, 50 Hz and 100 Hz.
Water bath Press the
button to switch the ultrasound head temperature monitoring to therapy in a
water bath.
27
Page 34

Description of the screen elements
and buttons
8.2 Buttons
Saving Press the
button to save the modified parameters.
Cancelling
changes
Press the
button to reject changes made.
8
28
Page 35

Indications menu
The Indications menu helps you to select the therapy.
Indications
Press the
button to open the ‘Indications’ menu.
9
Selecting by body
region
Select a body region by touching a blue circle.
29
Page 36

Selecting by
symptoms
Indications menu
9
After selecting the desired body region (shoulder in this example) the
Indications window opens showing various symptoms in the shoulder region.
Select the symptoms by clicking on the relevant row (shoulder-arm syndrome
in this example).
Selecting by
detailed symptoms
After selecting the symptoms another window opens showing detailed
symptoms.
Select the detailed symptoms by clicking on the relevant row (painful
tendinous insertion sites and tendons in this example).
30
Page 37

Indications menu
Therapy information After selecting the detailed symptoms another window opens showing
detailed therapy information and a suggested programme.
Selecting the therapy
programme
Press the
9
button to open the therapy screen with the programme.
31
Page 38

Saving a modified programme
As described in the previous sections, the programme parameters can be
separately modified and saved.
Selecting the
programme
Changing the
parameters
The changes are displayed on the screen.
10
32
Page 39

Opening the memory
list
Saving a modified programme
Press the
button to open the screen to enter the programme name.
10
Naming the
programme
Note: When entering a custom programme name, the name in the input field must
Two options are available for naming a programme.
1. Accept the programme name in the input field.
2. Enter a custom programme name. If using a custom name, use the
keyboard to enter the programme name.
first be deleted.
33
Page 40

Saving a programme in
Favourites
Saving a modified programme
10.1 Favourites
Press the
button to open Favourites.
10
Adding the programme
to Favourites
Press the
button to add the programme to Favourites.
The programme is automatically saved in the first free space in the list.
F: Favourites
00: Programme number in the list
34
Page 41

Saving a programme in
the Memory
Saving a modified programme
10.2 Memory
Press the
button to open the Memory.
10
Adding the programme
to the Memory
Press the
button to add the programme to the Memory.
The programme is automatically saved in the first free space in the list.
S: Memory
00: Programme number in the list
35
Page 42

Retrieving and editing favourites and
memory
11.1 Retrieving favourites
The individual saved programs are listed in Favourites.
From here they can be:
1. retrieved for therapy or
2. edited (sequence changed or deleted).
Selecting Favourites
Press the
button to open Favourites.
11
Selecting therapy Select the desired programme by pressing on the appropriate row.
36
Page 43

Editing Favourites
Retrieving and editing favourites and
memory
11.2 Editing favourites
Press the
button to open the ‘Edit Favourites’ screen.
11
Selecting the
programme
Changing sequence Activate the appropriate arrow keys to move the programme up or down in
Select the programme to be edited by clicking on the appropriate row.
the sequence.
37
Page 44

Deleting a programme
Retrieving and editing favourites and
memory
11.2 Editing favourites
Pressing the
button triggers the security question ‘Delete’.
11
Press the
button to delete the programme.
Press the
button to keep the programme.
38
Page 45

Selecting Memory
Retrieving and editing favourites and
memory
11.3 Retrieving and editing memory
Pressing the
button to open the Memory.
11
Selecting therapy Select the desired programme by pressing on the appropriate row.
Editing Memory
Note:
The following steps to edit the Memory correspond exactly to those used to
edit Favourites that were described in detail in the preceding section.
39
Page 46

Indications for
orthopaedics, surgery,
traumatology,
rheumatology
Other indications
Indications
12
• Vertebral pain syndrome, e.g., cervical syndrome
• Anyklosing spondylitis
(only during inflammation-free periods)
• Joint diseases
• Rheumatoid arthritis
(if heat treatment is indicated)
• Joint degeneration
• Periarthropathy
• Epicondylopathy
• Tendinosis, periostitis, heel spurs
• Achillodynia
• Scars, contractures, Dupuytren’s contracture
• Posttraumatic disorders
• Fractures
(particularly with delayed callus formation)
• Bronchial asthma
• Rhinopathy
• Persistent cervical spine complaints after whiplash injuries with repetitive
blocking
• Headaches
• Earaches
• Postherpetic neuralgia
• Functional disorders of the stomach and duodenum
• Pelvipathy
• Functional complaints of the pelvis minor
40
Page 47

General
contraindications
Contraindications
• Diseases in which heat should not be applied, e.g., acute inflammatory
diseases
• Diseases in which mechanical forces are contraindicated,
e.g., phlebothrombosis, haemorrhagic diathesis
• Do not apply ultrasound higher than C3
• Do not apply ultrasound to parenchymatous or heat-sensitive organs
(testes, eyes, pregnant uterus, liver, kidney, etc.)
• Anaesthetised skin areas
• Temperature regulation disorders
• After treatment with ionising radiation
• Epiphyseal plates and lines
• Tumours
• Do not use over electronic pacemakers
Metal implants and endoprostheses
There are no longer any concerns about dynamic ultrasound application in
low doses.
13
41
Page 48

Explanation of symbols
!
14
In the Operating Instructions, this symbol stands for danger.
Follow directions in the Operating Instructions at all times.
In the Operating Instructions this symbol stands for
‘Caution’ with regard to possible material damage.
Follow Operating Instructions
Manufacturer
Serial number
Type BF (as per IEC 601-1):
Degree of protection against electric shock.
Use on hearts is prohibited.
The device is Class II (IEC) or double-insulated.
42
Page 49

Warnings
Do not conduct ultrasound therapy on patients with implants or any other
implanted electronic device unless the risk has been assessed and found to
be negligible.
Patients must not be connected to a radio-frequency surgical device at the
same time. This may result in burns.
Operation of the ultrasound device in the vicinity (e.g., within 1 m) of strong
electromagnetic fields (e.g., tomographs, X-ray or diathermy devices) may
interfere with the operation of the device. Please maintain a safe distance of
several metres.
Handle the ultrasound head carefully as rough treatment may alter its
properties. Do not bring the ultrasound head into contact with sharp or
pointed objects as the aluminium head is easily scratched.
Disinfect the ultrasound head with standard disinfectants after use.
The use of coupling agents other than the special ultrasound gel Sono plus
may damage the ultrasound head.
We recommend Sono plus by Zimmer MedizinSysteme GmbH.
This device is exclusively for use by qualified medical personnel. This device
may cause malfunctioning in or may interfere with the operation of devices in
its vicinity. It may be necessary to take action to avoid interference such as
using a different alignment, moving the device or shielding it.
15
43
Page 50

Technical Information
16.1 General
Operating voltage 100–240 V, 220 V / 50/ 60 Hz
Power consumption max. 60 VA
Protection class II
Mains fuse 2 x 3.15 A T
Applied part Type BF
Dimensions 322 mm x 234 mm x 130 mm
Weight 2.1 kg
Transport in original packaging only
Operational
environment
Storage +10°C to + 50°C, 10% to 90% rel. humidity,
+10°C to + 40°C, 30% to 75% rel. humidity,
700–1060 hPa
700–1060 hPa
16
44
Page 51

Technical Information
16.2 Specific
Ultrasound heads
Frequency 800 kHz (0.8 MHz) and 2.4 MHz
Small ultrasound head 1 cm² , ERA = 1.1 cm² at 800 kHz (0.8 MHz), 0.5 cm² at 2.4 MHz
Maximum output 1 W at 800 kHz (0.8 MHz), 0.5 W at 2.4 MHz
Intensity steps 0.1 to 1 W/cm² eff. in steps of 0.1 W / cm²
Large ultrasound head 5 cm² , ERA = 2.3 cm² at 800 kHz (0.8 MHz), 4 cm² at 2.4 MHz
Maximum output 7 W at 800 kHz (0.8 MHz), 10 W at 2.4 MHz
Intensity steps 0.1 to 3 W/cm² eff. in steps of 0.1 W / cm²
Accuracy < ± 20 %
(This value represents the legally permissible value required
by law, and not the actual accuracy level for each device)
Ultrasound modes 1. Continuous ultrasound
2. Pulsed ultrasound, adjustable pulse frequencies:
20 Hz, 50 Hz, 100 Hz
Duty factor: 1 : 1, 1 : 2, 1 : 3, 1 : 5, 1 : 10
Replacement parts Ultrasound heads are factory-calibrated and can be easily replaced.
16
45
Page 52

Cleaning
Disinfection
Housing Clean housing with standard alcohol-free plastic cleaner.
Disinfect the housing with standard alcohol-free disinfectant suitable for
plastic.
Screen Clean the screen with standard alcohol-free plastic cleaner.
Disinfect the screen with standard alcohol-free disinfectant suitable for
plastic.
Ultrasound heads Clean the ultrasound heads with tap water.
Disinfect the ultrasound heads with standard alcohol-free disinfectant suitable
for plastic.
!
Do not use cleaning agents containing alcohol.
17
46
Page 53

CE Marking
The products bear the CE marking
in accordance with EU Medical Devices Directive 93/42/EEC and meets the
essential requirements of Annex I to this Directive.
The product Soleo Sono is rated in Class IIb according to Annex IX of the
Directive.
Soleo Sono has been developed, manufactured and tested under the ISO
13485 quality management system.
18
47
Page 54

Contents on delivery
Accessories
Contents on delivery Soleo Sono
1 variable frequency ultrasound head 0.8 and 2.4 MHz, ø 28 mm
1 storage tray, right
1 storage tray, left
1 mains cable
2 touch pens
Accessories
Item No.
4100 Variable frequency ultrasound head 0.8 and 2.4 MHz, ø 28 mm
4101 Variable frequency ultrasound head 0.8 and 2.4 MHz, ø 13 mm
118 Mains cable
65910320 Storage tray, right
65910310 Storage tray, left
6 Sono plus, 1 bottle
19
48
Page 55

Safety and maintenance
Soleo Sono is produced in accordance with the safety requirements of EN
60601-1.
Zimmer MedizinSysteme as the manufacturer can be responsible for the
safety and reliability only in the following circumstances:
• if the device is operated from an approved earthed wall socket and the
electrical installation conforms to DIN VDE 0100 Part 710
• if the device is operated in accordance with the Operating Instructions
• if extensions, reconfigurations or modifications are implemented only by
persons authorised by Zimmer MedizinSysteme
• users must ensure that the device is operating correctly and is in good
repair before using it
• before every use check the ultrasound head, cables and connectors for
damage (such as cracks) that could adversely affect the safety of the
device
• the device must be operated by appropriately trained personnel only
• disconnect the device from the power supply immediately if it is exposed
to liquids
• the unit is not designed for use in explosive or inflammable environments
The device does not contain any parts that must be maintained or repaired by
the operator.
Technical safety
checks
General note Store the operating instructions so they are accessible to operators of the
Soleo Sono does not require technical safety checks.
National laws and regulations must be observed when installing and
operating Soleo Sono.
device at all times. Access must be available for inspection authorities at any
time.
20
49
Page 56

Functional test
21
Soleo Sono runs a self-test that checks all internal components after it is
switched on.
An error message is shown in case of faults.
An extended functional test can also be run as described below.
These tests should be run once a month or if there is any doubt about the
operational reliability of the device.
Select ultrasound head and cover the ultrasound head with gel. The coupling
display must show over 90% at low power and at the start of therapy.
Run the test consecutively using both ultrasound heads.
Then clean ultrasound heads.
50
Page 57

Error messages
Troubleshooting
Disposal
No SD card found
If the SD card is not inserted, the message ‘No SD card found’ appears when
the Indications, Favourites and Memory buttons are pressed.
Insert the card and confirm with OK.
Error The screen shows:
22
In some cases the error can be cleared after switching the device off, waiting
five seconds and switching it on again.
If this does not work, contact customer service.
Customer service is reached through your authorised sales representative or
by contacting the head office in Neu-Ulm.
The device must be sent to the factory in the original packaging only.
Head office Zimmer MedizinSysteme GmbH
Junkersstraße 9
D-89231 Neu-Ulm
Tel. +49 731. 9761-291
Fax +49 731. 9761-299
www.zimmer.de
Disposal The device must be disposed of by an approved disposal company and must
not be discarded with household or special waste.
51
Page 58

Manufacturer's EMC declaration
Medical electrical devices such as Soleo Sono are subject to special precautions with regard to
electromagnetic compatibility (EMC) and must be installed and commissioned in accordance with the
EMC advice given in the operating instructions and accompanying documents.
Portable and mobile RF communications devices (such as mobile telephones) may interfere with
medical electrical devices.
Soleo Sono should only be operated with the original mains cable specified in the list of contents
delivered. The use of accessories other than those specified may result in increased emissions or
decreased immunity of the device.
Guidance and manufacturer's declaration – electromagnetic emissions
The Soleo Sono device is intended for use in one of the electromagnetic environments specified below. The
customer or the user of the Soleo Sono should assure that it is used in such an environment.
23.
Emissions tests
RF emissions CISPR 11
RF emissions CISPR 11
Harmonic emissions IEC 61000-3-2
Voltage fluctuation emissions and flicker
IEC 61000-3-3
Table 201 as per EN 60601-1-2:2006-10
Compliance
Group 2
Class B
Class A
Complies
Electromagnetic environment – guidance
The Soleo Sono device must emit
electromagnetic energy in order to perform its
intended function. Nearby electronic
equipment may be affected.
The Soleo Sono device is suitable for use in
all establishments, including domestic
establishments and those directly connected
to the public low-voltage power supply
network that supplies buildings used for
domestic purposes.
The device should not be used adjacent to or stacked with other equipment. If adjacent or stacked use
is necessary, the device should be monitored to verify normal operation in the configuration in which it
will be used.
52
Page 59

Manufacturer's EMC declaration
23
Guidance and manufacturer's declaration – electromagnetic immunity
The Soleo Sono device is intended for use in one of the electromagnetic environments specified below. The customer or the
user of the Soleo Sono device should ensure that it is used in such an environment.
Immunity tests
Electrostatic discharge
(ESD)
IEC 61000-4-2
Electrical fast
transient/burst
IEC 61000-4-4
Surge
IEC 6100-4-5
IEC 60601 test level Compliance level Electromagnetic environment - Guidance
± 6 kV contact
± 8 kV air
± 2 kV for mains power
lines
± 1 kV for input/output
lines
± 1 kV differential
mode
± 2 kV common mode
± 6 kV contact
± 8 kV air
± 2 kV for mains
power lines
Not applicable
± 1 kV differential
mode
± 2 kV common
mode
Floors should be wood, concrete or ceramic
tile. If floors are covered with synthetic
material, the relative humidity should be at
least 30%.
The quality of the supply voltage should
correspond to a typical business or hospital
environment.
The quality of the supply voltage should
correspond to a typical business or hospital
environment.
The quality of the supply
voltage should correspond
to a typical business or
hospital environment.
Power frequency (50/60
Hz) magnetic field
IEC 61000-4-8
Note: UT is the AC mains voltage prior to application of the test level.
Table 202 as per EN 60601-1-2:2006-10
<5% UT
(>95% dip in UT for 0.5
cycle)
40% UT
(>60% dip in UT for 5
cycles)
70% UT
(>30% dip in UT for 25
cycles)
<5% UT
(>95% dip in UT for 5
sec)
3 A/m
<5% UT
(>95% dip in UT for
0.5 cycle)
40% UT
(>60% dip in UT for 5
cycles)
70% UT
(>30% dip in UT for
25 cycles)
<5% UT
(>95% dip in UT for 5
sec)
3 A/m
Mains power quality should be that of a typical
commercial or hospital environment. If the
user of the Soleo Sono requires continued
operation during power mains interruptions, it
is recommended that the Soleo Sono be
powered from an uninterruptible power
source.
Power frequency magnetic fields should be at
levels characteristic of a typical location in a
typical commercial or hospital environment.
53
Page 60

Manufacturer's EMC declaration
23
Significant performance characteristics of the Soleo Sono are: interference-free delivery of ultrasound,
as well as interference-free operation of all functions with the parameters set.
Guidance and manufacturer's declaration – electromagnetic immunity
The Soleo Sono device is intended for use in one of the electromagnetic environments specified below. The customer or the
user of the Soleo Sono device should ensure that it is used in such an environment.
Immunity
tests
Conducted
RF
IEC
61000-4-6
Radiated
RF
IEC
61000-4-3
IEC 60601 test
level
3 V
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
rms
Compliance level
3 V
rms
150 kHz to 80 MHz
10 V/m
80 MHz to 2.5 GHz
Electromagnetic environment - Guidance
Portable and mobile RF communications equipment
should be used no closer to any part of Soleo Sono,
including cables, than the recommended separation
distance calculated from the equation applicable to the
frequency of the transmitter.
Recommended separation distance:
d= 1.17 √P
d= 0.35 √P for 80 MHz to 800 MHz
d= 0.7 √P for 800 MHz to 2.5 GHz
where P is the maximum output power rating of the
transmitter in Watts (W) according to the transmitter
manufacturer and d is the recommended separation
distance in metres (m).
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site surveya, should
be less than the compliance level in each frequency
rangeb.
Interference may occur in the vicinity of equipment
which is marked with the following symbol:
NOTE 1 At 80 Hz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
54
Page 61

Manufacturer's EMC declaration
23
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land
mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast, cannot be predicted theoretically with
accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be
considered. If the measured field strength in the location where the Soleo Sono device is used exceeds the applicable RF
compliance level above, the Soleo Sono device should be observed to verify normal operation. If abnormal performance is
observed, additional measures may be necessary such as re-orienting or relocating the Soleo Sono device.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
Recommended separation distances between portable and mobile RF communications equipment and the Soleo
Sono device
The Soleo Sono device is intended for use in an environment in which radiated RF disturbances are controlled. The
customer or user of the Soleo Sono device can help prevent electromagnetic interference by maintaining a minimum
distance between portable and mobile RF communications equipment (transmitters) and the device, as recommended
below, according to the maximum output power of the communications equipment.
Rated maximum output power of transmitter
W
0.01 0.12 0.04 0.07
0.1 0.37 0.11 0.22
1 1.17 0.35 0.7
10 3.70 1.11 2.21
100 11.67 3.5 7.0
For transmitters rated at a maximum output power which is not listed above, the recommended separation distance d in
metres (m) can be determined using the equation applicable to the frequency of the transmitter, where P is the maximum
output power rating of the transmitter in Watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
Separation distance according to frequency of transmitter
m
150 kHz to 80 MHz
d= 1.17 √P
80 MHz to 800 MHz
d= 0.35 √P
800 MHz to 2.5 GHz
d= 0.7 √P
We reserve the right to make changes.
55
Page 62

Soleoline
Operating Instructions
PL 10 101 394 GB / Version 2 / 03/2011 / Right of modifications reserved
Zimmer MedizinSysteme GmbH
Junkersstraße 9
D-89231 Neu-Ulm
Tel. +49 731. 97 61-291
Fax +49 731. 97 61-299
export@zimmer.de
www.zimmer.de
 Loading...
Loading...