Page 1

YSI 2900 Ser ies
Biochemistry Analyzers
Operations and Maintenance Manual
Page 2

Table of Content s
Introduction .................................................................................................................................... 5
1.
Description ................................................................................................................................. 5 1.1
User Features ............................................................................................................................ 5 1.2
Models ....................................................................................................................................... 5 1.3
2900 Series General Specifications .......................................................................................... 6 1.4
2960 Online Monitor Specifications ........................................................................................... 6 1.5
Monitor ................................................................................................................................ 6
1.5.1
1.5.2 Analog/Control .................................................................................................................... 7
2. Safety ............................................................................................................................................... 7
Important Safety Instructions ..................................................................................................... 7 2.1
Explanation of Symbols ............................................................................................................. 8 2.2
Getting Started ............................................................................................................................... 9
3.
Unpacking .................................................................................................................................. 9 3.1
Warranty Card ......................................................................................................................... 10 3.2
What You Need ....................................................................................................................... 10 3.3
Major Components .................................................................................................................. 10 3.4
Basic Setup ................................................................................................................................... 12
4.
Install Bottle Racks .................................................................................................................. 13 4.1
Right Side .......................................................................................................................... 13
4.1.1
4.1.2 Left Side (2950 models only) ............................................................................................ 14
Connect Printer ........................................................................................................................ 15 4.2
Connect AC Power .................................................................................................................. 15 4.3
Connect 2960 Online Monitor .................................................................................................. 16 4.4
Align Sipper ............................................................................................................................. 16 4.5
Configure Instrument Chemistries ........................................................................................... 19 4.6
Assign Chemistries to Probes ........................................................................................... 19
4.6.1
4.6.2 Assign Reagents ............................................................................................................... 22
Prepare and Install Buffer Solutions ........................................................................................ 24 4.7
Prepare Buffer ................................................................................................................... 25
4.7.1
4.7.2 Install Buffer Solution(s) .................................................................................................... 25
Install Calibrator Solution(s) .................................................................................................... 25 4.8
Install Membranes and ISEs .................................................................................................... 26 4.9
Enzyme Membranes ......................................................................................................... 26
4.9.1
4.9.2 Ion Selective Electrodes.................................................................................................... 27
Prime the Fluid System ........................................................................................................... 29 4.10
Check Probe Currents ............................................................................................................. 30 4.11
Biosensor Probes ....................................................................................................... 30
4.11.1
4.11.2 ISE Probes.................................................................................................................. 30
Enable 21 CFR Part 11 Mode ................................................................................................. 30 4.12
Running the Instrument ............................................................................................................... 30
5.
Perform Daily Operational Checks .......................................................................................... 30 5.1
Enzyme Membrane Integrity Test ..................................................................................... 31
5.1.1
5.1.2 Linearity Test ..................................................................................................................... 33
5.1.3 Results .............................................................................................................................. 33
Sample Preparation ................................................................................................................. 35 5.2
Run Batch ................................................................................................................................ 35 5.3
1
Page 3

5.3.1
Create Batches ................................................................................................................. 35
5.3.2 Load Samples ................................................................................................................... 37
5.3.3 Start ................................................................................................................................... 39
5.3.4 Status ................................................................................................................................ 39
Run Stat ................................................................................................................................... 39 5.4
Results ..................................................................................................................................... 42 5.5
Online Monitor and Control......................................................................................................... 42
6.
2960 Installation....................................................................................................................... 43 6.1
Align Sipper ............................................................................................................................. 46 6.2
Sample Interface...................................................................................................................... 46 6.3
Sterilization ........................................................................................................................ 47
6.3.1
Electrical Interface ................................................................................................................... 47 6.4
Analog Outputs ................................................................................................................. 47
6.4.1
6.4.2 Pump Control Outputs....................................................................................................... 48
6.4.3 Auxiliary Connector / Signal List ....................................................................................... 48
Monitor Setup .......................................................................................................................... 48 6.5
Name ................................................................................................................................. 49
6.5.1
6.5.2 Flow Rate and Purge Time ............................................................................................... 50
6.5.3 Antiseptic ........................................................................................................................... 50
6.5.4 Filtrate Pump ..................................................................................................................... 51
6.5.5 Module............................................................................................................................... 51
Start Monitor ............................................................................................................................ 56 6.6
Manual Sample during Monitor Sess ion ........................................................................... 57
6.6.1
Stop Monitor ............................................................................................................................ 58 6.7
Control Setup ........................................................................................................................... 58 6.8
Control Type ...................................................................................................................... 58
6.8.1
Print Monitor Configuration ...................................................................................................... 67 6.9
System Configuration .................................................................................................................. 68
7.
Settings .................................................................................................................................... 68 7.1
System .............................................................................................................................. 68
7.1.1
7.1.2 Fluid Detection (Bottles) .................................................................................................... 70
7.1.3 Display............................................................................................................................... 71
7.1.4 Auto-Cal Settings .............................................................................................................. 73
7.1.5 Scheduler .......................................................................................................................... 74
7.1.6 21 CFR Part 11 ................................................................................................................. 76
7.1.7 Date/Time .......................................................................................................................... 81
Service ..................................................................................................................................... 82 7.2
Sipper ................................................................................................................................ 82
7.2.1
7.2.2 Pumps ............................................................................................................................... 85
7.2.3 Modules ............................................................................................................................. 86
7.2.4 Stirbar ................................................................................................................................ 87
7.2.5 Monitor .............................................................................................................................. 89
Data ......................................................................................................................................... 90 7.3
Plate .................................................................................................................................. 90
7.3.1
7.3.2 Monitor .............................................................................................................................. 92
7.3.3 Calibration ......................................................................................................................... 94
Help ......................................................................................................................................... 95 7.4
About ................................................................................................................................. 95
7.4.1
7.4.2 Software ............................................................................................................................ 96
7.4.3 FAQ ................................................................................................................................... 97
7.4.4 Training ............................................................................................................................. 97
2
Page 4

Ethernet Port ............................................................................................................................ 98 7.5
LAN (Shared Network Connection) ................................................................................... 98
7.5.1
7.5.2 Router (Private Network Connection) ............................................................................... 98
7.5.3 Accessing Stored Data...................................................................................................... 99
8. Chemistry Setup ......................................................................................................................... 101
Sample Volume ..................................................................................................................... 101 8.1
Measurement Parameter Information .................................................................................... 101 8.2
Choline ............................................................................................................................ 102
8.2.1
8.2.2 Ethanol/Ethanol-HC ........................................................................................................ 103
8.2.3 Galactose ........................................................................................................................ 104
8.2.4 D-Glucose (Dextrose) ..................................................................................................... 105
8.2.5 L-Glutamate (L-Glutamic Acid) ....................................................................................... 106
8.2.6 L-Glutamine ..................................................................................................................... 107
8.2.7 Glycerol ........................................................................................................................... 108
8.2.8 Hydrogen Peroxide ......................................................................................................... 109
8.2.9 L-Lactate ......................................................................................................................... 110
8.2.10 Lactose ..................................................................................................................... 111
8.2.11 Methanol ................................................................................................................... 112
8.2.12 Sucrose ..................................................................................................................... 113
8.2.13 Xylose ....................................................................................................................... 114
8.2.14 Simultaneous Ammonium and Potassium ................................................................ 115
8.2.15 Simultaneous Glucose and L-Lactate ...................................................................... 116
8.2.16 Simultaneous Glucose and Sucrose ........................................................................ 117
8.2.17 Simultaneous Glucose and Xylose ........................................................................... 118
8.2.18 Simultaneous L-Glutamate and L-Glutamine ........................................................... 119
9. Operational Checks and Maintenance ..................................................................................... 119
Cleaning, Disinfecting, and Dec ontaminating ........................................................................ 119 9.1
Touch Panel .................................................................................................................... 119
9.1.1
9.1.2 Decontamination Procedures .......................................................................................... 119
Daily Maintenance ................................................................................................................. 120 9.2
Empty the Waste Bottle(s) .............................................................................................. 120
9.2.1
9.2.2 Check the Calibrator Bottle(s) ......................................................................................... 120
9.2.3 Check the Buffer Bottle(s) ............................................................................................... 120
9.2.4 Check for Leaks .............................................................................................................. 120
9.2.5 Clean up Spills ................................................................................................................ 120
9.2.6 Daily Operational Checks................................................................................................ 120
Monthly Maintenance ............................................................................................................ 120 9.3
Calibration Pumping System Maintenance ..................................................................... 120
9.3.1
Preventive Maintenance – 6 months or 1000 Hours ............................................................. 121 9.4
Sample Module Cleaning ................................................................................................ 121
9.4.1
9.4.2 Waste Module Cleaning .................................................................................................. 122
9.4.3 Enzyme Probe Cleaning ................................................................................................. 122
9.4.4 ISE Cleaning ................................................................................................................... 123
9.4.5 Sipper pump Seal Replacement ..................................................................................... 123
9.4.6 Bottle Tubing ................................................................................................................... 125
9.4.7 Pump Tubing Replacement ............................................................................................ 127
9.4.8 Install Waste Modules ..................................................................................................... 130
9.4.9 Waste Tubing .................................................................................................................. 130
9.4.10 Install Sample Modules ............................................................................................ 131
9.4.11 Sipper Replacement ................................................................................................. 131
9.4.12 Calibrate Sipper ........................................................................................................ 132
9.4.13 Install Bottles ............................................................................................................ 132
9.4.14 Install Membranes and ISEs..................................................................................... 132
9.4.15 Prime Fluid System .................................................................................................. 132
3
Page 5

Fuse Replacement................................................................................................................. 133 9.5
Fuse Requirements ......................................................................................................... 133
9.5.1
2960 Maintenance ................................................................................................................. 133 9.6
Tubing Replacement ....................................................................................................... 133
9.6.1
9.6.2 Monitor Sample Cup ....................................................................................................... 135
10. Storage ........................................................................................................................................ 136
Instrument Storage ................................................................................................................ 136 10.1
Enzyme Membrane Storage .................................................................................................. 136 10.2
ISE Storage ........................................................................................................................... 136 10.3
Reference ISE .......................................................................................................... 136
10.3.1
10.3.2 Ammonium/Potassium ISE ....................................................................................... 136
Instrument Handling/Transport .............................................................................................. 136 10.4
Troubleshooting ......................................................................................................................... 136
11.
Printout Information ............................................................................................................... 138 11.1
Enzyme Sensors ...................................................................................................... 138
11.1.1
11.1.2 Ion Selective Electr odes ........................................................................................... 140
Troubleshooting Chart ........................................................................................................... 143 11.2
2960 Online Monitor ................................................................................................. 147
11.2.1
12. Principles of Operation .............................................................................................................. 149
Enzyme Sensor Technology .................................................................................................. 149 12.1
Ion Selective Electrode .......................................................................................................... 150 12.2
Measurement Methodology ................................................................................................... 150 12.3
Baseline Stability ................................................................................................................... 151 12.4
Calibration .............................................................................................................................. 151 12.5
Linearity ................................................................................................................................. 151 12.6
Temperature Compensation .................................................................................................. 152 12.7
Level Sensing ........................................................................................................................ 152 12.8
Warranty and Repair .................................................................................................................. 152
13.
Limitation of Warranty ............................................................................................................ 152 13.1
Shipping Instructions ................................................................................................ 152
13.1.1
13.1.2 Cleaning Instructions ................................................................................................ 153
YSI Factory Authorized Service C ent ers ............................................................................... 154 13.2
Notices ........................................................................................................................................ 155
14.
Declaration of Conformity ...................................................................................................... 155 14.1
Declaration of Conformity - Australian and New Zealand Electromagnetic Compatibility .... 156 14.2
Radio and Television Interference Notice ............................................................................. 157 14.3
Appendix A – Software Flowchart ............................................................................................ 158
15.
16. Appendix B – Concentration Unit Conversion ........................................................................ 159
Linearity Test. Concentration Unit Conversion ...................................................................... 160 16.1
FCN Membrane Integrity Test. Concentration Unit Conversion ............................................ 161 16.2
Appendix C - Line Power Cord and Plug Wiring ..................................................................... 162
17.
18. Appendix D - Reagents and Accessori es ................................................................................ 163
4
Page 6

1. Introduction
Ammonium
D-Glucose (Dextrose)
Potassium
Hydrogen Peroxide1
L-Lactate
Sucrose
L-Glutamate
Ethanol
L-Glutamine
Glycerol
Ethanol-HC
Methanol
Xylose
Galactose1
Choline
Lactose
Slim modular design
Easily expand analytes or chemistries
Proprietary enzyme electrode
Fast, accurate, and analyte-specific results
Uses biological separation technology
No hazardous chromatography solvents to dispose
Icon-driven user interface with touchscreen
Easy to learn
Data download options
Save data on a USB drive, send it over the network,
Onboard training videos
Minimizes operator learning curve
21 CFR Part 11
Regulatory compliance
Description 1.1
The 2900 Series Biochemistry Analyzer is a laboratory instrument intended for use in research, food-processing and
bioprocessing applicati ons . THE 2900 Series IS NOT FOR HUMAN MEDICAL DIAGNOSTIC USE OR FOR HUMAN
PERFORMANC E EVALUATION.
The 2900 Series can be set up to measure up to 6 different analytes in a sample. The total number of analytes depends
on the number of sample modules installed (up to two analytes per module) and the number of different buffers required
(up to three buffers). Available analytes are listed below.
Additional analytes are currently under development. For a current listing, please contact YSI Life Sciences Technical
Support (937 767-2769 or 800 659-8895 extension 2) or visit the YSI Life Sciences web site at www.ysi.com
/lifesciences.
User Features 1.2
Multiple units use much less bench space
of
or access it in a searchable database anytime
Models 1.3
• 2900D 2900 Biochemistry Analyzer with 1 biosensor module
• 2900M 2900 Online Monitor & Control System
• 2950D-0 2950 Biochemistry Analyzer with 1 biosensor module
• 2950D-1 2950 Biochemistry Analyzer with 2 biosensor modules
• 2950D-2 2950 Biochemistry Analyzer with 3 biosensor modules
• 2950D-3 2950 Biochemistry Analyzer with 2 biosensor modules and 1 ISE module
• 2950D-4 2950 Biochemistry Analyzer with 1 biosensor module and 1 ISE module
1
YSI does not currently offer calibration standards for this analyte.
5
Page 7

2900 Series General Specifications 1.4
Sample size ................................................. Adj us table f rom 10 to 50 m icr oliters ( as pirated volume), per module
Response time ............................................ Enzyme Sensors
• Sample results in 60 seconds ( average) for one module
• 100 seconds for 2 modules
• 135 seconds for 3 modules
• Complete sample-to-sample cycle in less than 3 minutes
(May vary with analyte and sample m atr ix.)
Ion Selective Electrodes (ISE)
• Sample results in 150 seconds (aver age) f or 2 modules
• 195 seconds for 3 modules
• Complete sample-to-sample cycle in less than 5 minutes
(May vary with analyte and sample m atr ix)
Output signals:
Serial .......................................................... USB and RS232
Power requirement ............................... 100–240 VAC ±10%
50–60 Hz ±5%
42 Watts nominal
Working environment:
Ambient temperature ................................ 15–35°C
Relative humidity ...................................... 10–75% (non-condensing)
Regulatory compliance .................................. ETL, CE, RoHS
61010-1 compliance:
• Pollution degree 2
• Installation category 2
• Altitude 2000m
• Atmosphere 75 KPa to 106 KPa
• Indoor use only
Instrument dimensions ................................ 14.0" wide x 20.5" deep x 15.75" h igh
35.6 cm x 52.1 cm x 40.0 cm
Instrument weight ........................................ 39 pounds
17.7 kilograms
2960 Online Monitor Specifications 1.5
1.5.1 Monitor
Size:
Monitor Cup ............................................... 0.75 x 0.75 x 0.88 inches
Pump Head ................................................ 2.0 x 2.2 x 0.85 inches
Sample Inlet Tubing ...................................... Silicone, 0.08 OD x 0.02 ID (inches)
Volume ....................................................... 5.1 microliters/inch
Inlet Channel Pump Tubing ........................... PharMed
, 0.13 OD x 0.035 ID (inches)
6
Page 8
Valve Tubing ................................................. 0.03 ID (inches)
Wasteline Tubing .......................................... Silicone, 0.16 OD x 0.10 ID (inches)
Nominal Flow Rate (inlet line) ....................... 100–2500 microliters/minute (± 8% @ ± 6 PSI)
1.5.2 Analog/Control
Full Scale Voltage ......................................... Selectable: +10.00 VDC or +5.00 VDC
Full Scale Concentration ............................... User selectable
Resolution ..................................................... 1:65,536 or 0.0015% FS,
0.153 mv on +10.00 VFS,
0.076 mv on +5.00 VFS
Maximum Gain Error ..................................... ±12 LSB
Linearity ......................................................... ±1 LSB
Minimum analog output Load Impedance ..... 2K Ohms
Logic output drive .......................................... 0 and 5 VDC nominal at 10 milliamps
Logic Input levels .......................................... < 0.8 VDC = logic 0,
...................................................................... > 2.0 VDC = logic 1
2. Safety
Important Safety Instructions 2.1
DO NOT PLUG THE INSTRUMENT IN AT THIS TIME. You should apply power only when directed to do so in the setup
instructions.
1. Use ONLY the line power cord supplied with the instrument. When directed to, connect the plug to a matching threepronged wall receptacle.
2. Use ONLY fuses of the type supplied. Replacement power cords and fuses can be obtained from YSI, or your Dealer
Representative.
3. Do NOT use an extension cord without protective grounding.
4. Do NOT remove rear cover. There are no user serviceable parts inside.
5. Repairs are to be performed only by trained and approved personnel.
6. This instrument must be connected to a protectively grounded (earthed) outlet.
7. The following notice is provided in compliance with IEC1010 Part 1 1990.
See Appendix for mains plug wiring and fusing instructions.
8. If the equipment is used in a manner not specified by YSI, the protection provided by the equipment may be impaired.
WARNING: For RS232 or USB connection, equipment should be EN/CSA/UL 61010 or EN/CSA/UL 60950
approved only.
9. The mains (power) switch is for functional purposes ONLY. To disconnect the instrument from the mains supply,
unplug the mains power cord from the back of the instrument.
10. Personal protective equipment (PPE) recommended—protective gloves and safety goggles or glasses.
7
Page 9
Warning indicates that misuse of the instrument could result in
Caution, consult accompanying documents. Caution indicates
Biological Risks
Chemical Irritant
Manufacturer
Authorized Representative in the European Union
Catalog number
Lot number
Date of manufacture
Use by Date
Temperature Limitation
REF
Explanation of Symbols 2.2
WARNING
AVERTISSEMENT
CAUTION
ATTENTION
2776
death or serious injury to a person.
Un avertissement indique qu'une mauvaise utilisation de l'instrument
peut entraîner la mort ou une blessure grave chez une personne.
that misuse of the instrument could result in mild or serious injury
to a person and/or damage to equipment.
Attention, consulter la documentation jointe. Cette mise en garde
indique qu'une mauvaise utilisation de l'instrument peut entraîner une
blessure légère ou grave chez une personne et/ou un endommagement
du matériel.
Risques biologiques
Irritant chimique
Fabricant
Représentant agréé dans l'Union européenne
Numéro de référence
15A100549
Numéro de lot
YEAR-MO
Date de fabrication
YEAR-MO
Date limite d'utilisation
Limite de température
8
Page 10

3. Getting Started
Remove tie strap
Unpacking 3.1
When you unpack your new 2900 Series for the first time, check the packing list to make sure you have received
everything listed. Note that reagents for the 2900 Series are not packaged in the same carton as the instrument. If there is
anything missing or damaged, call the dealer from whom you purchased the 2900 Series. If you do not know which of our
authorized dealers sold the system to you, call YSI Life Sciences Customer Service at 800 659-8895 or 937 767-7241,
and we'll be happy to help you.
1. After removing the instrument from the shipping box, tilt the display to the full upright position.
Figure 3.1
2. Grasp the hand hold in the right side cover of the instrument and pull up and out to remove the cover.
NOTE: Leave the cover off the instrument until you have aligned the sipper and installed the membranes as
described in the following sections.
3. Carefully cut the tie strap holding the sipper.
Figure 3.2
9
Page 11

Warranty Card 3.2
Cover
Sipper
Display/
Touch Panel
USB Port
Station 2
Bottle Rack
Station 1
Please complete the Warranty Card and return it to YSI. This will record your purchase of this instrument in our computer
system. Once your purchase is recorded, you will receive prompt, efficient service in the event any part of your 2900
Series should ever need repair.
What You Need 3.3
Several things are needed in order to analyze samples using the 2900 Series. The following list shows the basic items
required.
• 2900 Series Instrument (with AC Power Cord)
• Bottle Racks wi th Reagent Level Sensing (YSI 2936, 2938), or Bottles without Reagent Level Sensing (YSI
2934, 2937)
• YSI Buffer(s)
• YSI Calibrator Standard(s)
• YSI Linearity Standard(s )
• YSI Membrane(s)
• YSI ISE probes (2950D-3 and 2950D-4 models only)
• YSI 2960 Online Monitor (optional)
• YSI 2901 Printer (optional)
Major Components 3.4
Display
Figure 3.3
Graphical color LCD covered by a touc h s cr een
10
Page 12

USB ports
Sample Module
Sipper Pump
Waste Bottle
Buffer Bottle
Calibrator Bottles
The USB ports allow a flash drive to be con nected to the 2900
Series to download sample results or upgrade the ins trument’s
software. A USB port is located on the right s ide of the dis play. An
additional USB port is located on the rear of the ins trum ent . T he rear
port is used to connect the 2960 Online Monitor to the 2900 Series.
Sipper
Station 1
Station 2
Buffer Pumps
(not shown)
Calibrator Pumps
(not shown)
Can be raised, lowered, rotated, and moved horizontally to its
various positions.
The positions are: Calibrator W ells, Sam ple Module, Stations 1 an d
2, and Monitor Sample Cup.
The Sipper senses fluid level to control immersion depth and detect
errors.
Plate and rack holder accepts mos t standard plates /racks for batch
sampling of up to 96 samples.
Test tube holder for manual sampling.
Draw buffer from the buffer bottles, pump it through the Valves,
Sipper Pump and the Sipper, and flus h the Sample Module.
Draw the appropriate standard s olution f rom the C alibrator Bottles
and fill the Calibrator Wells.
Sipper pump
Sample modules
Figure 3.4
It retracts its piston to draw standar d f rom the Calibrator Wells or
sample from the sample stations.
It extends its piston to dispense standard or sample into the sample
module.
They are made of clear acrylic plastic . Sensor probes are screwed
into either side of the module.
11
Page 13

In biosensor modules, the imm obilized enz yme membranes are
mounted on O-rings which act as fluid s eals.
Power
Receptacle
RS232 Port
Power Switch
Fuse
Holder
Ethernet Port
USB Port
In ISE modules, an O-ring is pos itioned insid e a sleev e on the end of
the electrode.
A reference or auxiliary electrode is housed in the temperature probe
and positioned at the back of the Sam ple m odule.
Stir Bars
(not shown)
Buffer, Waste and
Calibrator Bottles
Power Switch
They are plastic encapsulat ed magnets. They are activated by
motors housed below the sample m odules. They provide thorough
mixing inside the sample modules.
Are conveniently located for m aintenanc e.
Fluid levels are monitored by sensors .
Operation is automatically halted when the B uf fer or Calibrator
Bottles are empty, or when the W aste bott les ar e full.
Rear Panel
Figure 3.5
The main power switch is an on/off rock er switch ( 0-off and I-on)
located on the back of the instrument
Fuse Holder
Power Receptacle
Ethernet Port
RS232 Serial Port
The fuse holder houses the power line fus e and ope n s up f or fuse
replacement.
One end of the power cord (supplied) plugs into t his rec eptac le,
while the other end plugs into a properl y grounded e l ect ric al outlet.
The instrument will automatically adj ust the voltage as needed.
It allows connection to a network or r outer via a RJ 45 Ethernet por t.
The RS232 connection is a standard D B9F c onnect or. It is used to
interface with the YSI 2901 Printer or a r em ote hos t.
4. Basic Setu p
The following list describes the basic steps necessary for sampling with the 2900 Series.
1. Install Bottle Racks
2. Connect Printer (optional)
3. Connect AC Power
12
Page 14

4. Connect 2960 Online Monitor (optional)
Buffer Bottle 1
Waste Bottle
Calibrator Bottle
CAL 1B
Waste 1
Buffer 1
CAL 1A
CAL 1B
Calibrator Bottle
5. Align Sipper
6. Configure Instrument Chemistries
7. Prepare and Install Buffer solution(s)
8. Install Calibrator Solution(s)
9. Install Membrane(s) and ISEs
10. Prime the Fluid System
11. Check Probe Currents
Install Bottle Racks 4.1
4.1.1 Right Side
1. Install the YSI 2938 Bottle Rack with Reagent Level Sensing onto the right side of the instrument by sliding the
slots in the tray over the pins on the side of the instrument.
If you are not using the reagent level sensing option, place the YSI 2934 Bottles on the right side of the
instrument.
2. Then remove the packing material holding the tubing to the right side of the instrument.
3. Next, connect bottle tubing and cables
a. Insert the large diameter waste tube into the hole in waste bottle 1.
b. Connect one end of a short cable marked to the threaded fitting on the waste bottle 1 cap and connect
the other end to the Waste 1 (top) fitting on the instrument.
c. Connect the tubing marked “B1” and one end of a short cable to the fittings on the buffer bottle 1 cap and
connect the other end of the cable to the Buffer 1 (2nd) fitting on the instrument.
d. Connect the tubing marked “C1B” and one end of a long cable to the fittings on the second calibrator
bottle cap and connect the other end of the cable to the CAL 1B (3rd) fitting on the instrument.
e. Connect the tubing marked “C1A” and one end of a long cable to the fittings on the first calibrator bottle
cap and connect the other end of the cable to the CAL 1A (bottom) fitting on the instrument.
CAL 1A
Figure 4.1
13
Page 15

4.1.2 Left Side (2950 models only)
Buffer Bottle 2
Waste Bottle 3
Waste Bottle 2
Calibrator Bottle
Buffer Bottle 3
Calibrator Bottle
1. Install the YSI 2936 Bottle Rack with Reagent Level Sensing onto the left side of the instrument by sliding the
slots in the tray over the pins on the side of the instrument.
2. If you are not using the reagent level sensing option, place the YSI 2937 Bottles on the left side of the instrument.
3. Then remove the packing material holding the tubing to the left side of the instrument.
4. Next, connect bottle tubing and cables
a. Insert the large diameter waste tubing into the holes in waste bottles 2 and 3.
b. Connect one end of a long cable to the threaded fitting on the waste bottle 2 cap and connect the other
end to the W2 (top left) fitting on the instrument.
c. Connect one end of a long cable to the threaded fitting on the waste bottle 3 cap and connect the other
end to the W3 (top right) fitting on the instrument.
d. Connect the tubing marked “B2” and one end of a long cable to the fittings on the buffer 2 bottle cap and
connect the other end of the cable to the B2 (2
e. Connect the tubing marked “B3” and one end of a long cable to the fittings on the buffer 3 bottle cap and
connect the other end of the cable to the B3 (2
nd
from top on left) fitting on the instrument.
nd
from top on right) fitting on the instrument.
f. Connect the tubing marked “C2A” and one end of a short cable to the fittings on the calibrator 2A bottle
cap and connect the other end of the cable to the 2A (bottom left) fitting on the instrument.
g. Connect the tubing marked “C2B” and one end of a short cable to the fittings on the calibrator 2B bottle
cap and connect the other end of the cable to the 2B (3
rd
from top on left) fitting on the instrument.
h. Connect the tubing marked “C3A” and one end of a short cable to the fittings on the calibrator 3A bottle
cap and connect the other end of the cable to the 3A (bottom right) fitting on the instrument.
i. Connect the tubing marked “C3B” and one end of a short cable to the fittings on the calibrator 3B bottle
cap and connect the other end of the cable to the 3B (3
rd
from top on right) fitting on the instrument.
CAL 2A
CAL 3A
Figure 4.2
14
Page 16
Connect Printer 4.2
Connect the YSI 2901 Printer to the 2900 Series Biochemistry Analyzer using the data cable provided. The small RJ12
connector plugs into the bottom of the printer and the large DB9 connector plugs into the RS232 port on the back of the
analyzer. Refer to the instruction sheet included with the printer for details of printer operation.
Connect AC Power 4.3
1. Plug the power cord (included with the 2900 Series Analyzer) into the power receptacle on the back of the
instrument, then into a properly grounded electrical outlet provided with a 15 or 20 Amp circuit breaker. The
instrument will automatically adjust the voltage as needed.
If you are located outside the United States, see Appendix for Line Power Cord and Plug Wiring.
WARNING: Keep your hands clear of the sipper while the instrument is in operation.
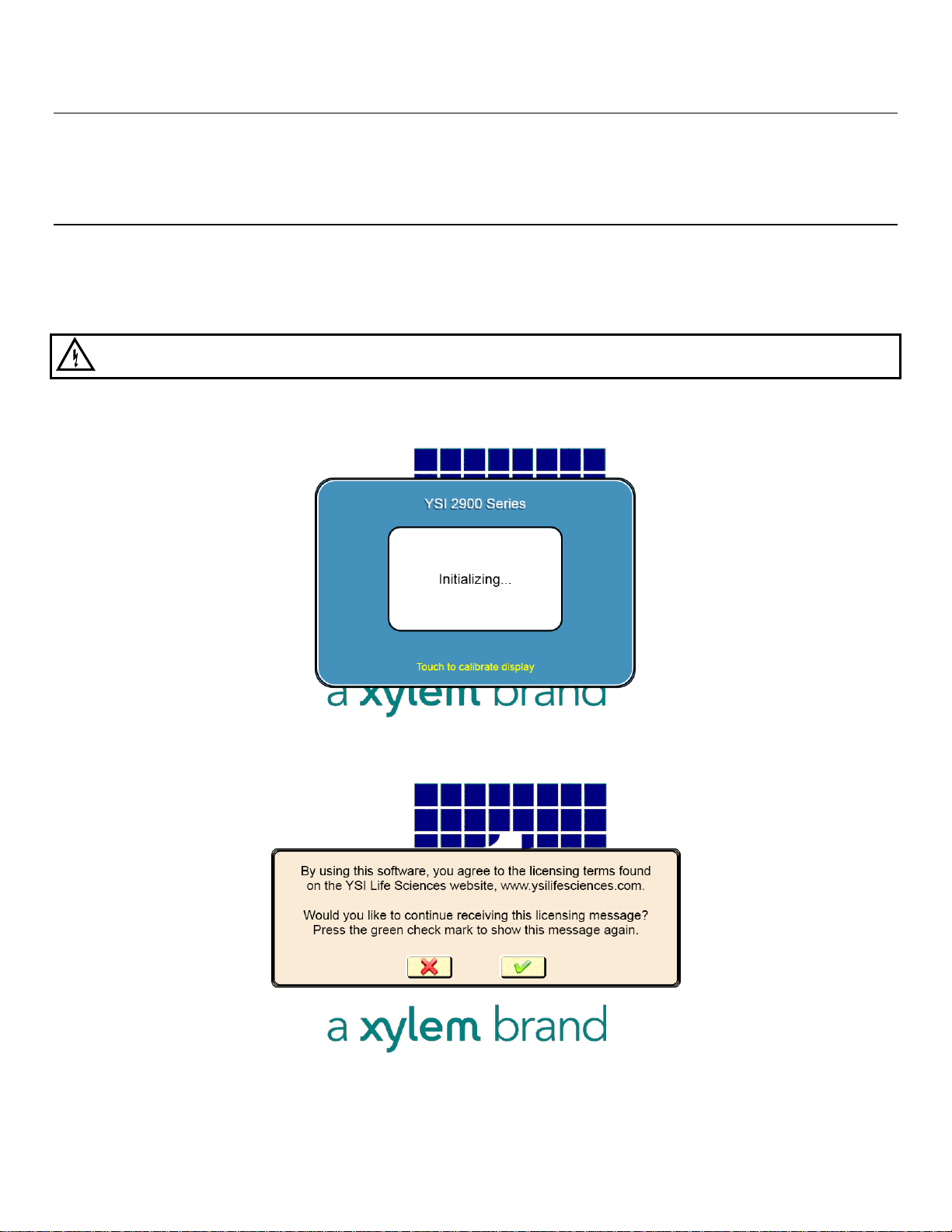
2. Turn the instrument on with the main power switch on the rear panel. After about 30 seconds, the Initializing
window should appear.
3. The first time the instrument is powered up, the software license window will appear. Touch [X] to prevent the
license screen from appearing each time the instrument is turned on.
4. Since the top cover of the instrument is removed, the Service screen will appear. Touch the Interlock [On] button
and change it to [Off] to disable the safety interlock, and then press to confirm.
15
Page 17
Connect 2960 Online Monitor 4.4
If you will be using the 2960 Online Monitor, see Section 6.1 for instructions on how to connect it.
Align Sipper 4.5
It is very important that the sipper be accurately adjusted.
WARNING: Keep your hands clear of the sipper while the instrument is in operation.
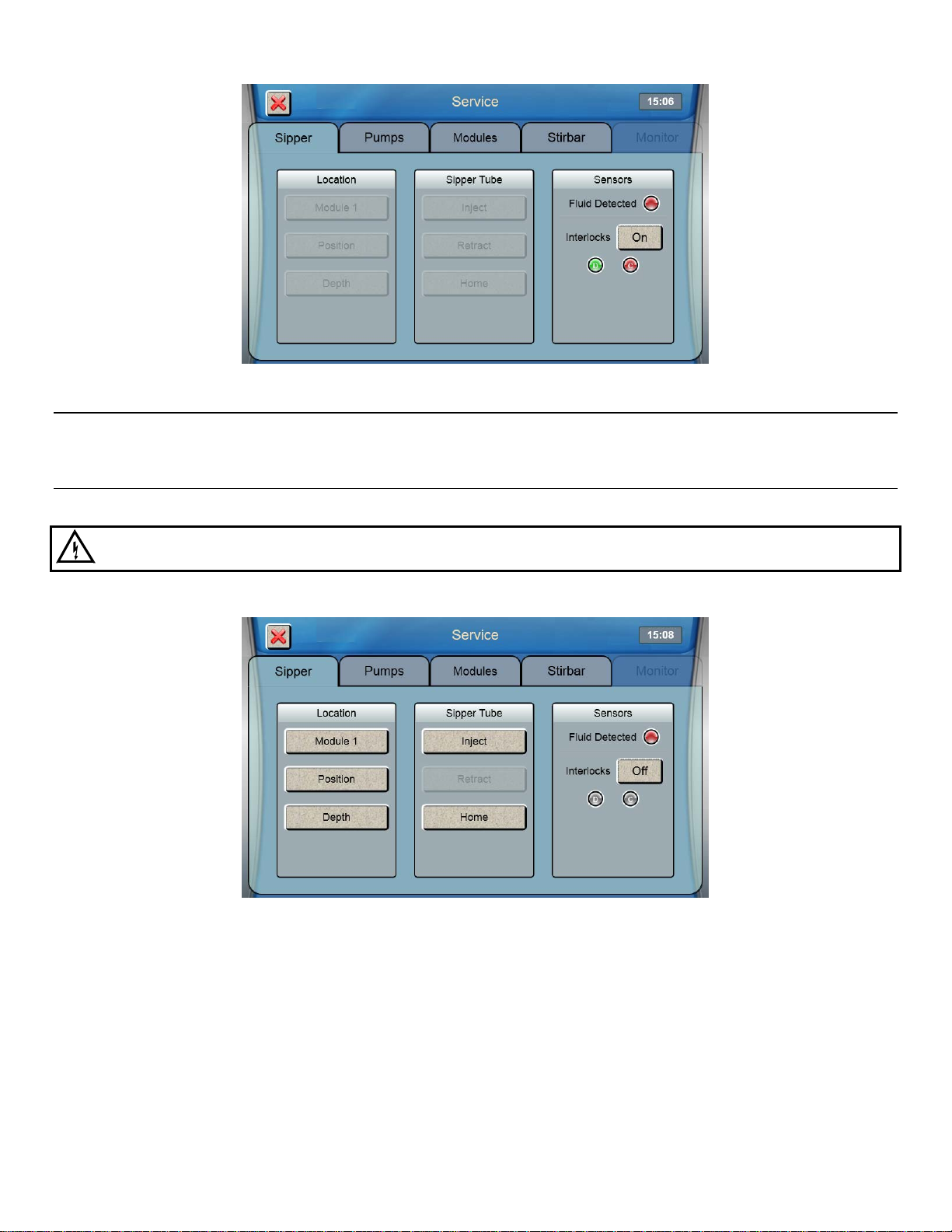
1. From the Sipper tab of the Service screen, touch [Module 1].
2. The Select Location screen will appear. Select [Module 1]. The sipper will move to sample module 1 and should
be centered above the cone shaped opening in the top of the module.
If the sipper does not move, make sure the packing material was removed.
16
Page 18
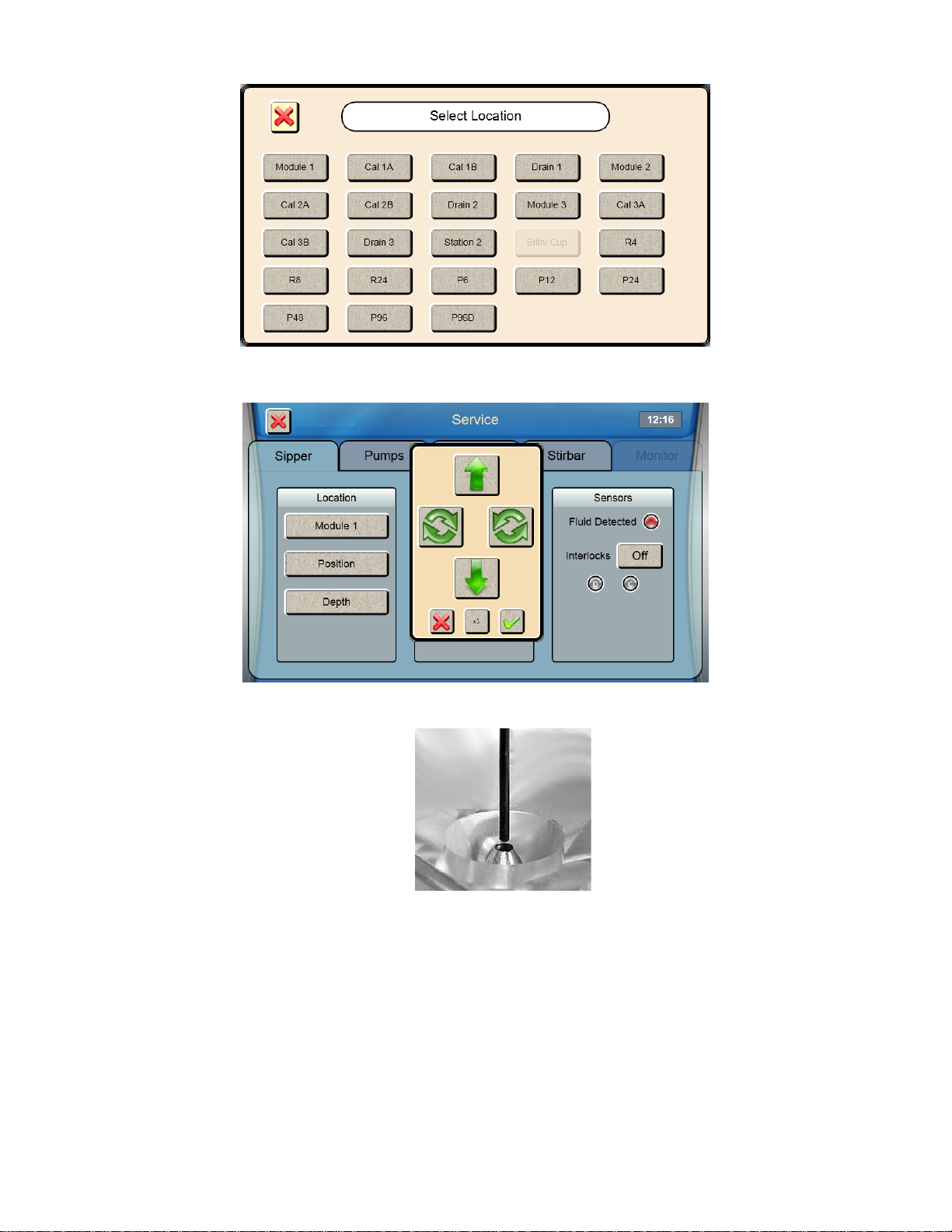
3. If the sipper is not centered, touch [Position] and use the arrow buttons to center the sipper.
4. Make certain the Sipper is centered, then touch at the bottom right of the adjustment window.
Sipper Adjustment Position
Figure 4.3
5. Touch to save the position and close the confirmation window.
6. To test the alignment of the sipper, Touch [Inject] to lower the sipper, then touch [Retract] to raise the sipper back
up.
If necessary, touch [Position] and repeat the adjustment.
17
Page 19
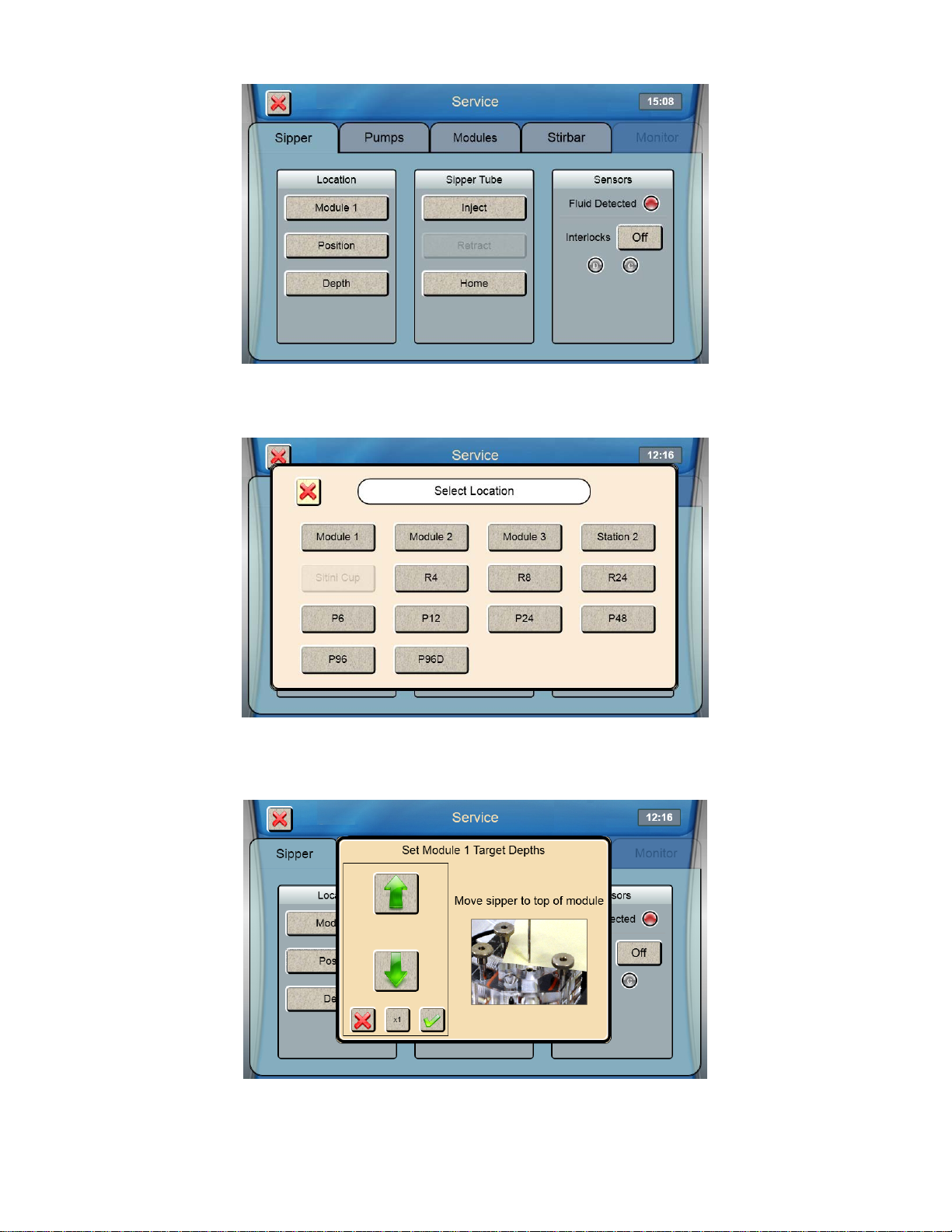
7. Once the sipper enters the sample module without hitting the cone, touch [Depth] to set the sipper depth. The
Select Location screen will appear.
8. Select [Module 1]. The tip of the sipper should be right at the top of the module. Use the arrow buttons to lower or
raise the sipper until the tip of the sipper is even with the top of the module.
9. Then touch at the bottom right of the adjustment window.
10. Touch to save the depth and close the confirmation window.
11. Check the sipper alignment at the Cal 1A, Cal 1B and Drain 1 locations and adjust the position if necessary.
18
Page 20

12. Once you have aligned the sipper and properly set the depth, sipper alignment for Module 1 is complete. Touch
[X] at the top left of the screen to return to the main display.
Repeat this procedure for any additi on al modules installed on your 2950 analyzer.
After you have aligned the sipper with all installed modules, touch the Interlock button and change it back to [On]
to enable the safety interlocks.
Configure Instrument Chemistries 4.6
Before operating the 2900 Series, you must set the instrument parameters.
4.6.1 Assign Chemistries to Probes
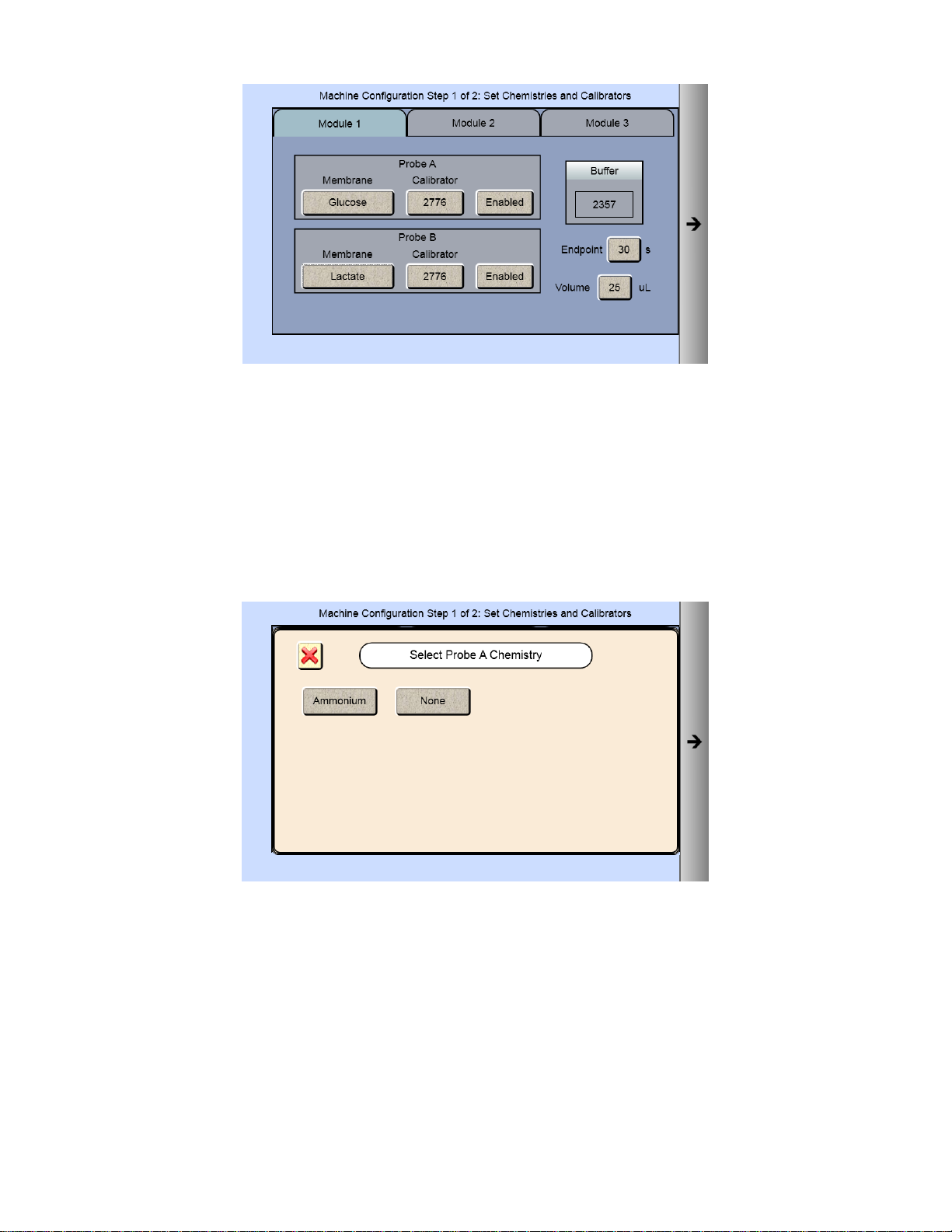
1. From the main display, touch
2. From the [Module 1] tab, touch the Probe A membrane button.
.
3. Select the chemistry you want to measure
19
Page 21

4. The Probe A Membrane button will now show the chemistry you have selected. The screen also indicates which
reagents should be installed.
5. To run a second chemistry in module 1, touch the Probe B membrane button. Only chemistries that can be run
simultaneously with your selected chemistry will be displayed.
6. Select the chemistry you want to measure. The Probe B Membrane button will now show the chemistry you have
selected.
20
Page 22
NOTE: The default Sample Volume and Endpoint for the chemistries are also displayed. Use the default settings unless
a particular application instruction specifies another value (see Section 8 Chemistry Setup for details).
7. Once you have selected one or two chemistries for the first module, proceed to the next installed module on your
instrument, and repeat the procedure.
NOTE: Changing chemistry assignments will change the calibrator values back to the default settings.
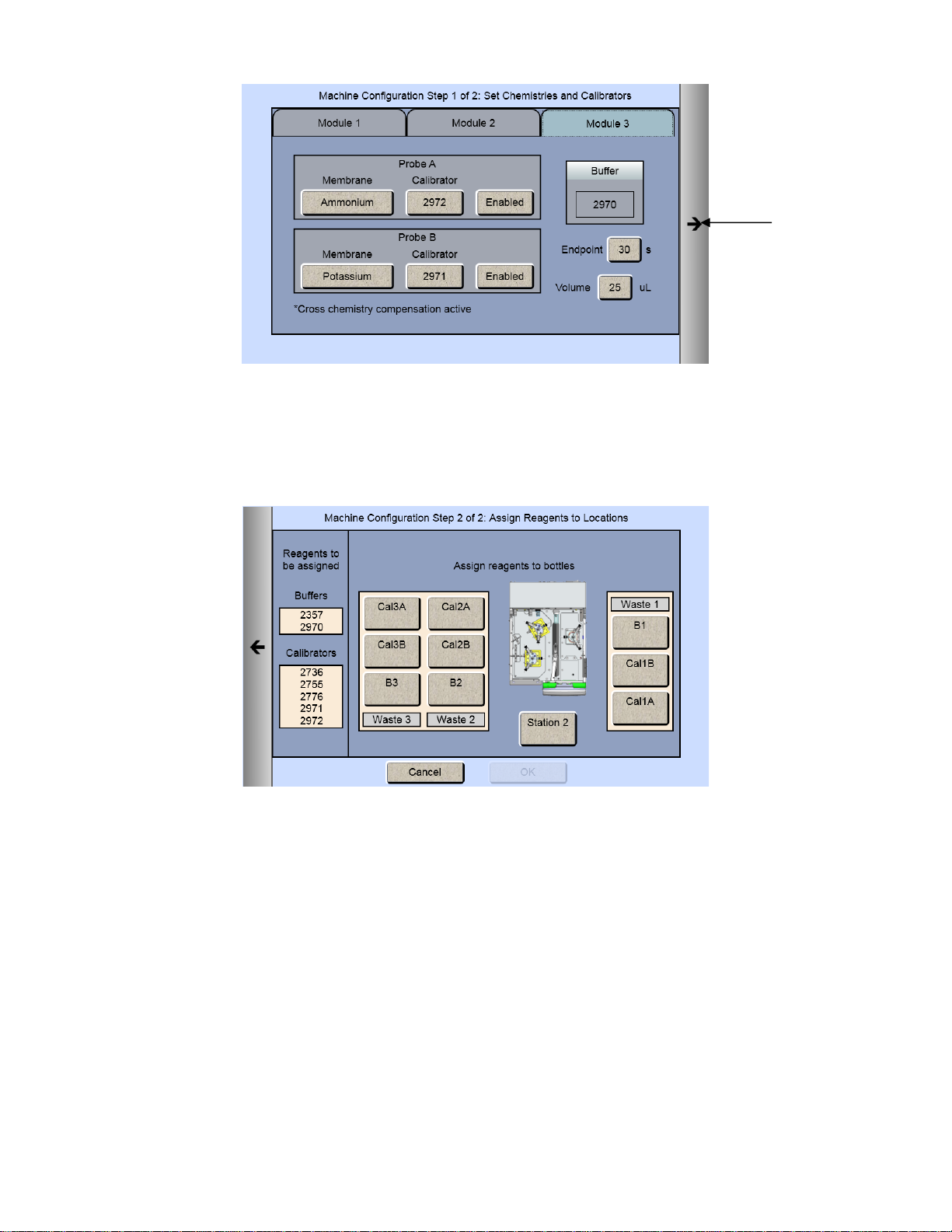
If your instrument has an ISE module:
1. From the [Module 3] tab, touch the Probe A membrane button and select Ammonium.
2. The Probe A Membrane button will now show the chemistry you have selected.
Potassium will automatically be selected for Probe B.
21
Page 23
Next Arrow
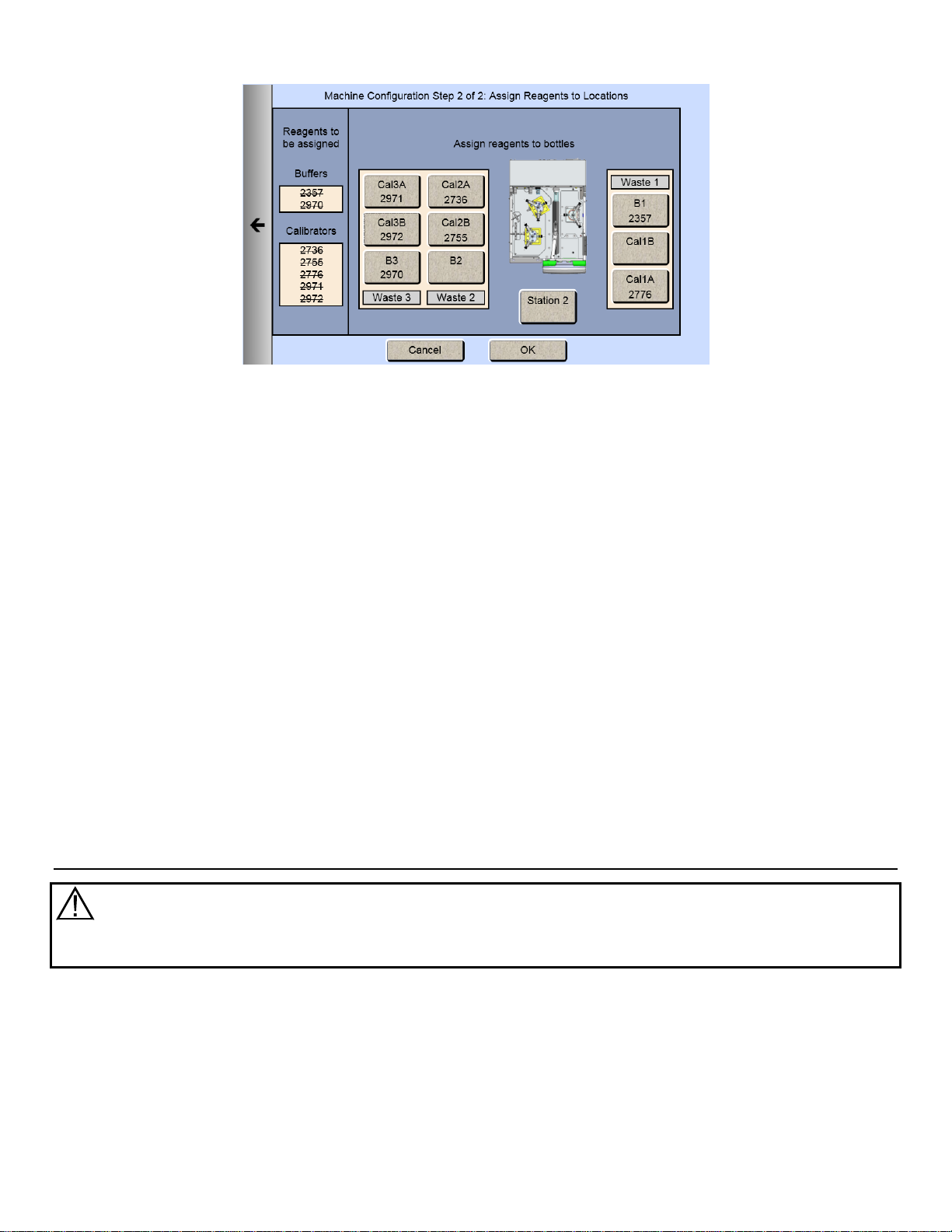
4.6.2 Assign Reagents
Once you have selected all the chemistries you want to measure, touch the Next arrow on the right of the screen. The
Assign Reagents screen will be displayed.
Reagents needed are listed on the left of the screen. Bottle positions are listed on the right. Each reagent must be
assigned to a bottle position.
4.6.2.1 Buffers
1. Touch the [B1] button to display the Select Buffer screen.
22
Page 24

2. Pick your appropriate buffer and it will be assigned to the B1 button.
Note that the selected buffer is now marked out in the list of reagents needed.
3. For additional buffer assignment, please repeat process.
4.6.2.2 Calibrators
1. Touch the [Cal 1A] button. The Select Calibrator screen appears.
23
Page 25
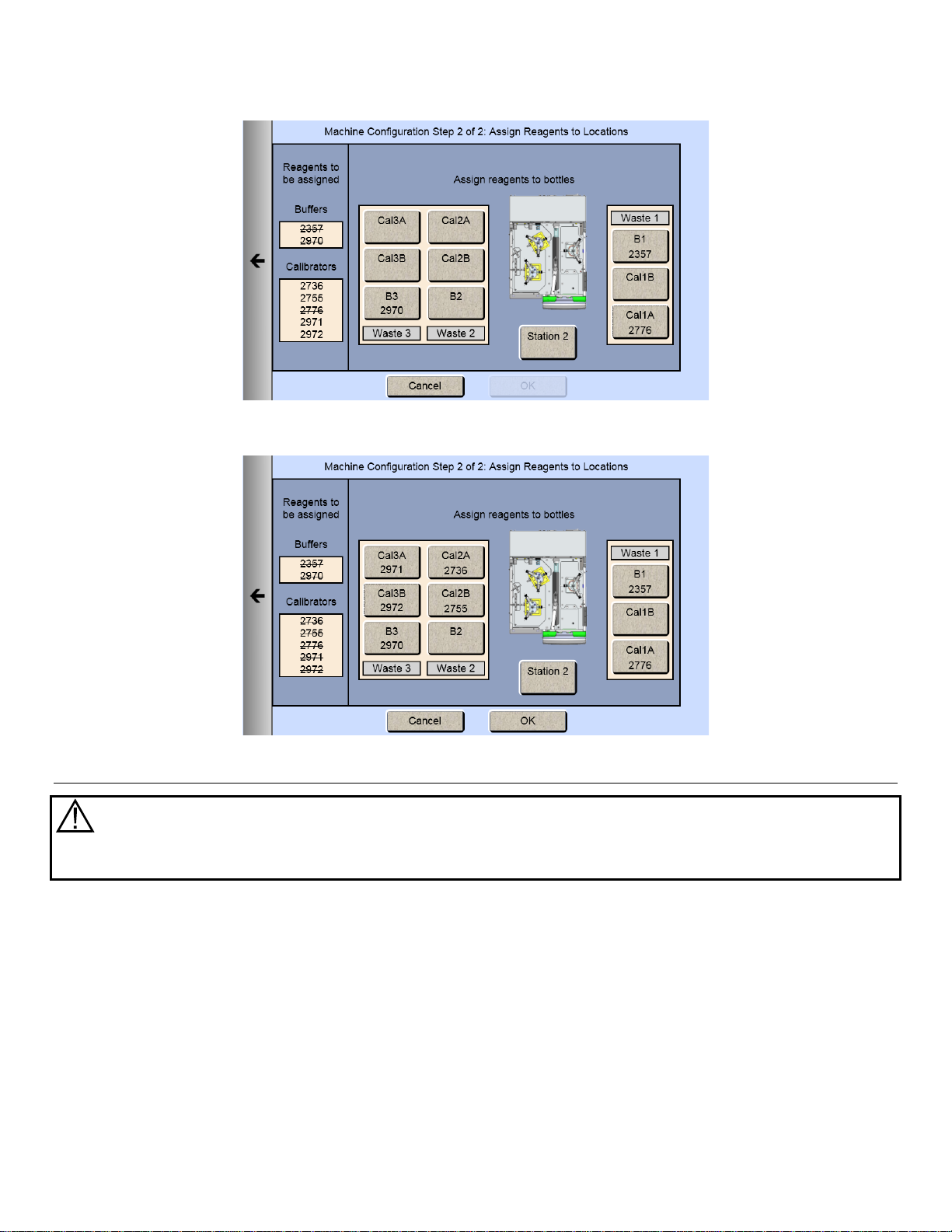
2. Pick the appropriate calibrator.
3. Select additional bottles until all reagents on the left side of the screen have been assigned and marked out.
Prepare and Install Buffer Solutions 4.7
Caution: To prevent possible damage due to an electrostatic discharge, do NOT touch the metal
tips of the connectors located at the ends of the bottle leads. Handle only the insulated section of
the connectors.
Prepare the system buffers and fill the buffer bottles as indicated on the display.
24
Page 26
4.7.1 Prepare Buffer
4.7.1.1 From powder concentrate:
1. Place about 500 mL of reagent water (distilled or deionized) into a 1000 mL flask or other clean container.
2. Add two packages of powder buffer concentrate and stir.
3. Add more reagent water until the total volume of solution is between 900 and 1000 mL.
4. Stir as necessary until the buffer chemicals have completely dissolved.
4.7.1.2 From liquid concentrate:
Mix the content of the bottle of liquid buffer concentrate with enough reagent water (distilled or deionized) to make
1000 mL.
4.7.2 Install Buffer Solution(s)
Unscrew and remove the lid from one of the buffer bottles.
IMPORTANT: When adding fresh buffer to the Buffer Supply Bottles, m ak e every effort to avoid contamination of
the lid and level sensor assemblies.
Pour the prepared buffer into the buffer bottle.
Install the bottle in the rack as indicated on the dis play.
Replace the bottle lid.
Install Calibrator Solution(s) 4.8
Caution: To prevent possible damage due to an electrostatic discharge, do NOT touch the metal
tips of the connectors located at the ends of the bottle leads. Handle only the insulated section of
the connectors.
25
Page 27

Probe B
Probe A
Unscrew and remove the lid from the empty calibrator bottle (Cal1A–Cal3B as indicated).
IMPORTANT: make every effort to avoid contamination of the lid and level sensor as s emblies.
Mark the date of installation on the label of the ne w bot tle of YSI calibrator soluti on.
Place the new bottle of calibrator in the bottle rack as indicated on the display.
Screw the lid and level sensor assem bl y onto it.
Repeat this process for any additional c alibrator bot tles ( Cal1A–Cal3B as indicated).
Install Membranes and ISEs 4.9
4.9.1 Enzyme Membranes
Each biosensor probe installed in your instrument is fitted with a protective "shipping membrane" which must be removed
and replaced with a new membrane. Make sure you install the correct membrane for each chemistry you are
measuring.
Enzyme membranes are color-coded for each type of chemistry. It is important that you install the specific membrane as
indicated on each probe (A or B).
.
Probe A is always on the left when looking in from the side of the instrument
Figure 4.4
26
Page 28
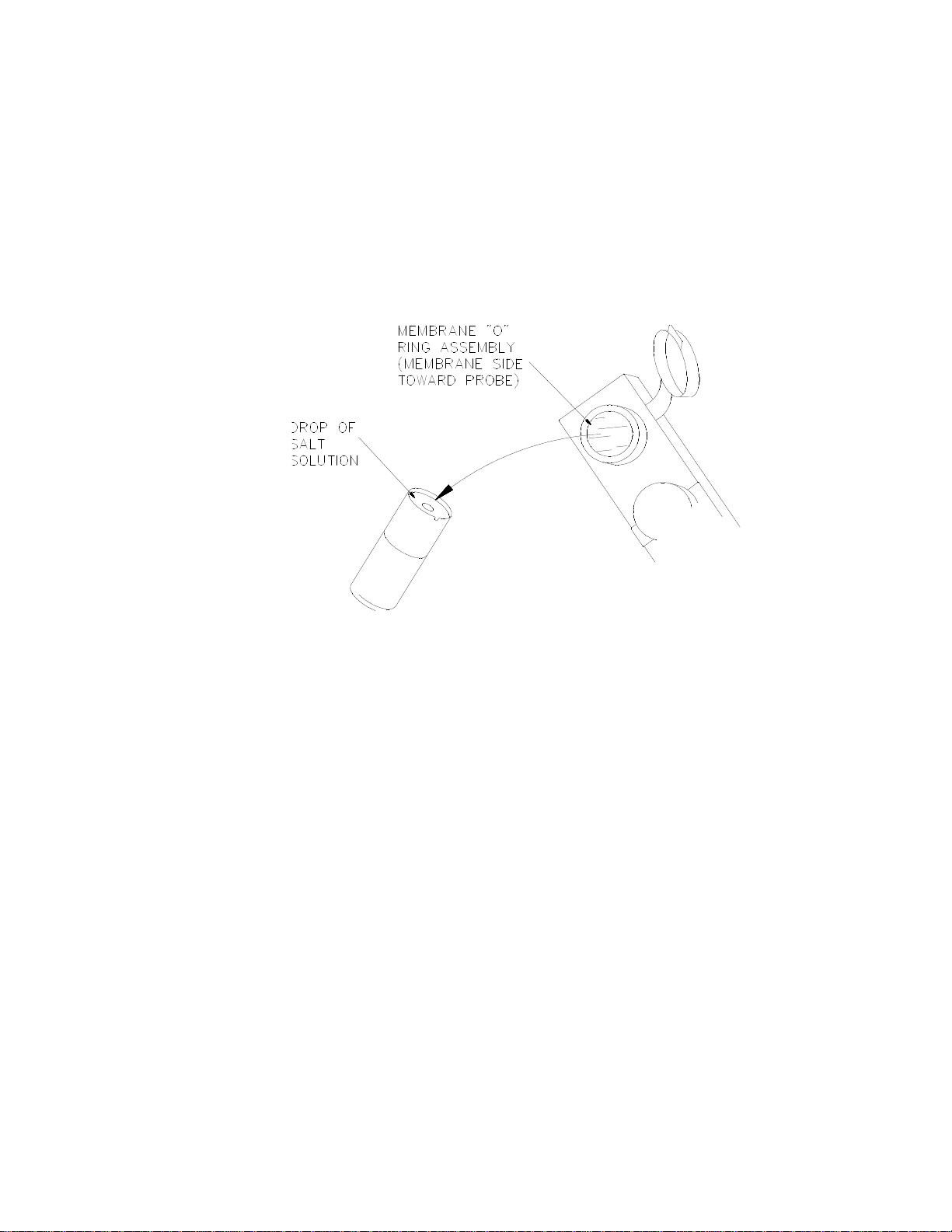
To install a membrane:
1. Make sure the top cover(s) are removed from the instrument.
2. Next, unscrew the appropriate enzyme probe retainer and gently pull the probe out of the module.
3. Remove the existing O-ring membrane assembly from the end of the enzyme probe. A lint free tissue or toothpick
or pipet tip may be needed to unseat the old membrane. Be careful not to scratch the enzyme probe face.
4. Examine the enzyme probe surface and remove any pieces of membrane that remained.
5. Open a cavity of the plastic membrane holder.
6. Rinse the membrane inside with a few drops of salt solution (YSI 2392).
7. Place one drop of salt solution on the enzyme probe face.
8. Using the plastic membrane holder, press the O-ring membrane assembly gently onto the probe face.
Figure 4.5
9. Wipe excess salt solution from the probe body.
10. Install a stir bar in the module.
11. Then return the enzyme probe to the module.
12. Finger tighten the probe retainer so that the O-ring seals the probe in place. Do not overtighten.
13. Return the membrane holder to the foil pouch and refrigerate it.
14. Note the expiration date on the membrane package
15. Repeat this procedure for the remaining enzyme probes.
You may want to maintain an instrument log book in which dates and lot numbers of reagents are recorded, along with
information from daily operational checks and other relevant information.
4.9.2 Ion Selective Electrodes
The 2900 Series is shipped without the Ion Selective Electrodes installed in module 3. To install an ISE:
1. First remove the packaging material and the cap from the ISE (save the cap for later use).
2. Slide a black probe retainer over each ISE cable (threaded end first).
3. Slide a white probe sleeve (notched end out) over the end of each ISE.
27
Page 29

O-ring
Retainer
Potassium ISE
Reference Electrode
Ammonium ISE
Figure 4.6
Figure 4.7
4. Place an O-ring into the end of each sleeve, pushing the O-ring gently so that it is secured by the sleeve.
Figure 4.8
5. Install the reference electrode by screwing the probe retainer into the module (finger tighten only; do not
overtighten).
6. Install a stir bar in the module.
7. Secure the ammonium and potassium ISEs in place by screwing the probe retainers into the module (finger
tighten only; do not overtighten).
8. Connect the ISE cables to the matching connectors located at the rear of the instrument.
Figure 4.9
28
Page 30

Ammonium—NH4
Potassium—K
Reference—REF
Figure 4.10
9. Install the top covers on the instrument.
Prime the Fluid System 4.10
Please note that it may take from several minutes to more than an hour to initially stabilize the probes when setting up for
the first time.
To prime the fluid system:
1. From the Service screen, touch the [Pumps] tab.
2. Touch the button under B1 Pump to turn it on.
3. The instrument will prime the B1 buffer solution.
4. Once buffer flows from the end of the sipper, touch the button under B1 Pump to stop the pump.
5. Repeat this procedure to prime all other buffer bottles and calibrator bottles you have installed.
Prime all installed calibrator bottles daily to remove air bubbles from the tubing.
29
Page 31

Check Probe Currents 4.11
Flush Module 1
Enzyme Probe
Flush ISE Module 3
ISE Voltages
4.11.1 Biosensor Probes
1. From the [Modules] tab of the Service screen, touch the [Flush] button under Module 1 to flush the selected
sample module with buffer.
Currents
2. Observe the probe current values (baseline). They must be below 6 nA and stable.
3. Check to see if they are decreasing in value.
4. Check the sample module; it should be full of buffer.
5. If necessary, touch the [Flush] Button to flush the sample module again.
6. Flush any additional modules by touching the [Flush] button under the Module.
Please note that when the instrument is first powered up, it may take several hours for the baseline currents to drop below
6 nA.
4.11.2 ISE Probes
1. Touch the [Flush] button under Module 3 to flush the selected sample module with ISE buffer.
Note that the instrument will purge buffer to waste if the incorrect buffer is in the line.
2. Observe the ISE voltage values. They should be between -40 and 60mV and stable.
3. Check the module; it should be full of buffer.
4. If necessary, touch the [Flush] button to flush the sample module again.
Please note that when the instrument is first powered up, it may take an hour for the voltages to stabilize.
Once biosensor probe currents and ISE voltages are acceptable, touch the [X] button at the top left of the screen to exit to
the main display.
Enable 21 CFR Part 11 Mode 4.12
If you will be using the optional 21CFR Part 11 compliance features, enable 21 CFR Part 11 Mode (see Section 7.1.5.1 for
details).
5. Running the Instrument
Perform Daily Operational Checks 5.1
To ensure that your 2900 Series is operating properly, perform the Membrane Integrity and Linearity checks on a daily
basis before running samples.
30
Page 32

5.1.1 Enzyme Membrane Integrity Test
Use YSI 2363 Potassium Ferrocyanide (FCN) Standard to determine if your enzyme membranes are structurally intact.
1. Pour small amount of FCN standard (1000 mg/dL) in a test tube or multi-well plate
2. Press the Run icon to process it as a sample.
3. From the Run Batch tab, touch [New]
4. Choose from the selection of supported racks and plates.
5. We highly recommend renaming the rack/plate “Daily Checks” by touching its ID to indicate that it contains your
daily check batches.
6. After selecting your plate/rack, touch the [Edit] button
7. Touch the location of each sample for the first batch. Selected locations will be blue.
31
Page 33

8. Touch the [Batch] button.
9. Select only the chemistries that require the FCN test.
10. To change the Batch Name from the default value of TestBatch- #, touch the [TestBatch- #] button. The keypad
window will appear.
11. Type your new batch name and touch [DONE].
12. Touch
to save the batch.
32
Page 34

5.1.2 Linearity Test
13. Pour small amount of linearity standard in a test tube or multi-well plate.
14. Touch additional sample locations to create new batch for the linearity tests.
15. For the daily linearity checks, select only the chemistry that corresponds to the linearity standard in that sample
location.
16. Touch
17. Create additional batches for each linearity solution.
18. Touch [Close] when all batches are created.
19. Load the plate/rack in the sampling station 1.
20. Touch [Start] to run the FCN and the linearity standards as sa mples.
The analyzer will calibrate as required and run the batches.
to save the batch.
5.1.3 Results
21. Touch the Results tab
22. Select a sample location.
33
Page 35

Chemistry
Membrane
Calibration Standard
FCN Limit2
Ethanol
2786
2790 (2.00 g/L)
0.05 g/L
Ethanol-HC
7150
7151 (25.0 g/L)
0.10 g/L
Glucose
2365
2776 or 2747
0.05 g/L
L-Glutamate
2754
2755
0.06 g/L
L-Glutamine
2735
2736
0.06 g/L
L-Lactate
2329
2776
0.03 g/L
Methanol
2725
2726 (1.00 g/L)
0.05 g/L
Sucrose
2703
2780
0.10 g/L
Xylose
2761
2767
0.05 g/L
Select to show
Select a
sample
23. Touch a chemistry to show details
24. Listed in table 6-1 below are the recommended FCN limits.
a. Values less or equal to FCN limits indicate integral membranes
b. Values greater than FCN limits indicate membrane structural failure
c. If readings are high, recalibrate and repeat all the steps above to confirm.
d. If the reading is still out of tolerance, refer to the Troubleshooting Section.
details
Choline 2771 2772 0.02 g/L
2
If you are using units other than g/L for the FCN test, refer to 16 Appendix B – Concentration Unit Conversion for conversion values.
Table 6-1
34
Page 36

Chemistry
Calibration Std
Linearity Std
Acceptable Range (g/L)
25. See the list of acceptable valu es in table 6-2 below to interpret linearity readings.
a. Values that are ± 5% of specified tolerance limits indicate good membranes.
b. Values that are out of tolerance indicate an aging enzyme membrane.
c. If readings are out of tolerance limits, recalibrate and repeat all the steps above to confirm.
d. If the reading is still out of tolerance, refer to the Troubleshooting Section.
Ammonium3 2972 (0.50 g/L) 7179 (0.10 g/L) 0.095 to 0.105
Choline 2772 (0.175 g/L) 2773 (0.450 g/L) 0.43 to 0.47
Ethanol 2790 (2.00 g/L) 2790 (3.20 g/L) 3.04 to 3.36
Ethanol-HC 7151 (25.0 g/L) 7152 (40.0 g/L) 38.0 to 42.0
Glucose 2776 (2.50 g/L) 1531 (9.00 g/L) 8.55 to 9.45
L-Glutamate 2755 (0.73 g/L) 2756 (1.46 g/L) 1.39 to 1.53
L-Glutamine 2736 (0.73 g/L) 2737 (1. 17 g/L) 1.11 to 1.23
Glycerol 7141 (25.00 g/L) 7142 (40.00 g/L) 38.0 to 42.0
L-Lactate 2776 (0.50 g/L) 1530 (2.67 g/L) 2.54 to 2.80
Lactose 2783 (5.00 g/L) 2784 (25.00 g/L) 23.75 to 26.25
Methanol 2726 (1.00 g/L) 2726 (2.50 g/L) 2.38 to 2.63
Potassium
3
2971 (1.00 g/L) 7179 (0.20 g/L) 0.190 to 0.21 0
Sucrose 2780 (5.00 g/L) 2778 (25.00 g/L) 23.75 to 26.25
Xylose 2767 (20 g/L) 2768 (30 g/L) 25.5 to 34.5
Table 6-2
Sample Preparation 5.2
A variety of sample types can be analyzed with the 2900 Series. Generally, the only sample preparation that may be
required is dilution of the sample to bring the substrate concentration within the linear range of the instrument. (see
Section 8 Chemistry Setup for the working range of each chemistry).
Neither color nor turbidity interferes with measurements.
Small particles do not affect the reaction in the sample module that houses the probes, but samples with particles large
enough to clog the sipper should be avoided.
Run Batch 5.3
5.3.1 Create Batches
1. From the Run Batch tab of the Run screen, select a sample rack/plate and create batches for your samples.
3
Range for single linearity check. Refer to 8.2.14 Simultaneous Ammonium and Potassium for sample to s ample precision.
35
Page 37

Arrows
2. Touch [New] and choose from the selection of supported racks and plates. Alternatively, use the arrows to scroll
through the saved racks and plates until you find the type that you are using.
3. You may rename the rack/plate by touching its ID.
4. After selecting your plate or rack, touch the [Edit] button.
5. Touch the location of each sample for the first batch (selected locations are blue).
6. Touch [Batch].
7. Select the chemistries to run in this batch.
8. Enter any optional parameters, such as Batch Name (separate name for this batch), Dilution Factor, Units, MultiSample (multiples of each sample location in this batch), or Repeats (multiples of the entire batch).
Please note that the instrument does not auto m atically dilute samples.
9. If you diluted your samples:
a. Touch the Dilution Factor [1] button.
b. Enter your dilution factor then touch [OK].
10. To change the number of sample replicates:
a. Touch the Multi-Sample [1] button.
b. Enter the number of times each sample in the batch should be run, then touch [OK].
11. To repeat the entire batch:
a. Touch the Repeats [0] button
b. Enter the number of times the entire batch should be repeated.
12. Touch to save the batch.
13. You may also create one or more batches for samples. Alternatively, you may create a separate rack for sample
batches.
36
Page 38

14. Touch [Close] when all batches are created.
15. To save your plate configurations to a flash drive, touch [Export].
16. Select the plates you want to export, and then touch [Export].
17. Previously exported plates can be imported later using the [Import] button.
5.3.2 Load Samples
5.3.2.1 R24 and P6-P96 Racks/Plates
1. Open the front door of the instrument
2. Insert the plate/rack (end marked A1 first) into the instrument. Slide the front edge of the plate/rack in until it
stops.
Figure 5.1
3. Gently lower the rear of the plate/rack and push it down into position.
37
Page 39

Figure 5.2
R8 Tube Rack
5.3.2.2 R4 or R8 Tube Racks
1. Open the front door of the instrument.
2. Insert the R4 or R8 tube rack into the cavity just inside the front door.
3. Insert your sample tubes into the tube rack you installed.
Figure 5.3
38
Page 40

5.3.3 Start
Select module
Virtual Printer
Figure 5.4
Touch
The 2900 Series will calibrate as required and run the batches.
to run the current batches.
5.3.4 Status
1. Touch the Status tab.
2. Then select a module to view its status.
to view status
3. The virtual printer window displays details of previous samples and calibrations.
4. Use the arrow buttons to scroll the printer window.
Run Stat 5.4
A Stat sample at Station 2 runs without stopping a plate analysis or monitor session that is in progress.
NOTE: The Sipper is not designed to pierce septa.
39
Page 41

1. Touch the Run Stat tab
2. Place the sample in Station 2:
a. Insert your sample tube into the tube holder (Station 2) from below the spring clip
b. Slide it up all the way until it rests below the notch at the top.
The test tube holder accepts tubes sizes up to 16x100mm.
Any container other than this should be sampled manually by holding the sample at Station 2.
Figure 5.5
3. Touch [Configure] to setup the Stat sample.
4. Select the chemistries and units for the sample.
40
Page 42

5. Touch to return to the Run Stat screen.
Stat sample
6. Touch
7. The Stat sample results are displayed on the Run Stat tab.
If a Station 1 batch is in progress, the Stat sample will run as soon as the required module(s) is available.
to run the Stat sample at Station 2.
results
41
Page 43

Select a
Select to show
Results 5.5
1. Touch the Results tab.
2. Then select a sample position to display the results.
sample
3. Select a chemistry to display details.
details
6. Online Monitor and Control
The YSI 2960 Online Monitor and Control System allows “on-line” monitoring and control of sterile systems over long
periods of time without contamination. It also provides an alternative means of interfacing the 2900 Series with external
measurement/control systems.
The 2960 may draw sample from a process stream, bioreactor or other suitable source and deliver sample to the
analyzer. The system can operate unattended for days or weeks, provided sufficient reagent supply is considered.
The sample volume required for each analysis varies somewhat depending on the distance, flow rate and fluid interface
used, however, typically 1.5 milliliters is sufficient to purge the 2960 sample cup and deliver fresh sample.
The 2960 preserves sterility by filling the end of the sampling line with an antiseptic after every sample.
42
Page 44

2960
Online
Monitor
YSI 2900M Online Monitoring an d Control System
The analog output of the 2960 provides a voltage signal which is proportional to the concentration of the analyte. The
2960 provides this voltage output for up to two chemistries, two "handshake" signals, and a system status signal. In
addition, the user has the ability to adjust full scale analog output for each chemistry .
The 2960 also provides three discrete signal outputs (TTL logic level) to control external pumps which can be used to
replenish nutrients or optimize byproduct concentrations.
Figure 6.1
2960 Installation 6.1
1. Connect the small end of the supplied USB cable to the mini USB socket on the back of the 2960.
2. Connect the large end of the USB cable to the USB socket on the back of the 2900 Series.
43
Page 45

Power
Mini USB
Connection
Analog/Control
Remove
Connection
Connection
Rear panel of 2960 Online Monitor
Figure 6.2
3. Install the correct AC plug for your location onto the 2960 power supply, then plug the power supply into an AC
power outlet.
4. Connect the cable from the power supply to the power socket on the back of the 2960.
5. Using a 3/32” Allen hex wrench, remove the screw from the right rear of the 2900 Series deck as shown below.
Discard the screw.
screw
Figure 6.3
6. Using a 9/64” Allen hex wrench and the long hex screw provided with the 2960, install the Monitor Sample Cup on
the 2900 Series deck (see figure below).
44
Page 46

Monitor
Sample
To a waste
To bottom of
To side of
Cup
Figure 6.4
7. Connect the small sample tubing from the left side of the 2960 Online Monitor to the bottom of the Monitor Cup
you just installed inside the 2900 Series.
Monitor Cup
Monitor Cup
container
Figure 6.5
8. Connect the larger diameter waste tubing from the fitting on the left side of the 2960 Online Monitor to the waste
fitting on the side of the Monitor Sample Cup inside the 2900 Series.
9. Run the larger diameter waste tubing from the fitting on the right side of the 2960 Online Monitor to an appropriate
waste container.
45
Page 47

Align Sipper 6.2
Touch Sitini
Touch Sitini
1. From the Service menu, touch the Location button. The Select Location screen will appear.
Cup
2. Select [Sitini Cup]. The sipper will move to the Monitor Sample Cup and should be centered above the funnel
shaped opening in the top of the cup.
3. If the sipper is not centered, touch [Position] and use the arrow buttons to center the sipper.
4. Make certain the Sipper is centered, then touch at the bottom right of the adjustment window.
5. Touch [Depth] and select [Sitini Cup] to set the sipper depth.
Cup
6. Use the Up and Down Arrow buttons to adjust the sipper so the tip is flush with the top of the Monitor Sample
Cup.
7. Touch at the bottom right of the adjustment window to save the depth setting.
Sample Interface 6.3
When configured appropriately the 2960 automatically samples a bioreactor, process stream, or other suitable sample
source. Since color, turbidity, optical density and many other physical factors do not affect the YSI enzyme biosensor,
filtration and/or dilution may not be necessary and the 2960 may draw the sample directly. If cell loss is a concern, or if
high cell density is expected, a filtration device (e.g., tangential flow filter) which separates broth and cells may be
installed between the sample source and the 2960.
Using the additional tubing included with the 2960, connect the tubing from the outside slot of the 2960 valve to your
sample source—bioreactor, process stream, etc. Keep the tubing as short as possible to minimize purge time.
46
Page 48

From
source
From
Antiseptic
Sample
Figure 6.6
If you are using an antiseptic, connect the tubing from the inside slot of the 2960 valve to your antiseptic container. Typical
solutions might include 1% sodium hydroxide or 0.25% hypochlorite in reagent water. Even if you are not using an
antiseptic solution, the solenoid valve still switches to the antiseptic position and air or fluid is pumped through the
antiseptic line during sample aspiration. The antiseptic line must be kept free from obstructions. A dry 0.2 micron filter may
be connected to the antiseptic line to prevent contamination in the air from entering the line.
6.3.1 Sterilization
If your application requires aseptic monitoring, all tubing and connectors should be sterilized (autoclaved) prior to use. The
tubing, connectors and pump head should be assembled, the open ends of the tubing should be clamped off (two clamps
are provided) and the entire assembly (tubing with pump head) should be sterilized along with the bioreactor.
If the tubing is connected to the bioreactor after sterilization, a sterile connection must be made.
After sterilization the pump should be remounted onto the 2960 (see Section 9.6 2960 Maintenance for details). The
antiseptic cycle must be enabled and the antiseptic solution must be primed immediately after the tubing is reconnected.
Electrical Interface 6.4
6.4.1 Analog Outputs
The 2960 interface provides additional signals to aid in synchronizing the reading of the analog outputs. In addition to the
two analog outputs, three logical signals are provided. These "handshake signals" are nominally +5 volts for a logic 1 and
ground (0 volts) for a logic 0. The "READY" signals are output from the 2960 and are set to a logic 1 when the analog
output signal for that channel has been updated. This signal indicates to the host system that the analog voltage is "new"
and that it represents the most recent reading of the analyte concentration. The host system (the external system to which
the 2960 is connected) then can send a logic 0 to the "ACK" input of the 2960. This "ACK" (acknowledge) signal response
from the host resets the READY line of the 2960 to its low state before the next sample is ready.
The figure below shows the typical signal pattern that would occur during two sample update cycles. The READY signals
will reset themselves immediately prior to updating the analog output if not reset externally via the ACK\ signal input.
47
Page 49

Analog Output
Ready
Ack\
Figure 6.7
6.4.2 Pump Control Outputs
Three discrete outputs are provided on the 2960 which are intended to control external pumps. The means of control is on or off only, no intermediate states are supported. The three outputs are labeled Feed Pump A, Feed Pump B, and Filtrate Pump. All of these outputs are electrically identical and are designed as control signals only, i.e. they are incapable of driving any pumps directly. These signals must be buffered externally in a manner appropriate to the nature of the pumps being used. These output signals transition between +5 volts and 0 volts nominally. The logic of each, i.e. whether 5 volts turns on or off the external device, is selectable (See 6.5 Monitor Setup).
6.4.3 Auxiliary Connector / Signal List
On the back of the 2960 is the 15 pin "D" type connector where the signals emanate. The following table relates the
signals with the connector pin positions and cable wire colors.
2960 Signal Pin# Wire color
Ground 1 Black/White
Ground 2 Orange/Black
Ground 3 Blue/Black
Ground 4 Red/White
Ground 5 Black
Channel B Ready 6 White
Channel A Pump Control 7 Green/White
SysErr 8 Blue/White
Channel A Analog Output 9 Green/Black
Channel B Analog Output 10 Green
Channel B Pump Control 11 Red/Black
Filtrate Pump Control 12 Red
+12V Power Out 13 Blue
Ack\ 14 White/Black
Channel A Ready 15 Orange
Chassis Ground None Shield
Monitor Setup 6.5
1. From the Run menu, touch the [Monitor] tab.
2. Touch the [Configure] button
48
Page 50

3. When configuring the monitor for the first time, touch the [Edit] button to edit the default monitor configuration. To
add additional configurations in the future, touch [New] to create a new configuration.
6.5.1 Name
4. Touch the [Name] button and enter a name for this configuration.
49
Page 51

6.5.2 Flow Rate and Purge Time
Flow Rate
uL/min
Purge Time
Seconds
Flow Rate
uL/min
Purge Time
Seconds
100
900
1400
65
200
450
1500
60
300
300
1600
57
400
225
1700
53
500
180
1800
50
600
150
1900
48
700
129
2000
45
800
113
2100
43
900
101
2200
41
1000
90
2300
39
1100
82
2400
38
1200
75
2500
36
1300
69
The flow rate and purge time settings must be such that a sufficient volume of sample is pumped during each cycle to
completely purge the Monitor sample cup and system tubing. This is especially important when an antiseptic is used since
the antiseptic may damage the enzyme membrane if aspirated by the sipper.
The table below shows the flow rates and minimum time needed to purge the tubing lines from the solenoid valve to the
Monitor sample cup. These values are based upon a 6-fold volume turnover. Additional time ma y be necessar y to purge
the line from the sample source to the solenoid valve. The volume of the supplied sample tubing is 5.1uL per inch.
Table 7-1
5. Touch the [Purge Time] button and enter the required purge time.
If you are using a filter on your sample line, make sure your Flow Rate does not exceed the maximum rated flow rate of
your filter. If necessary, touch the [Flow Rate] button and enter the correct flow rate.
6.5.3 Antiseptic
If you are not using an antiseptic solution:
6. Touch the [Antiseptic Cycle] button.
50
Page 52

7. Touch the [On] button and change it to [Off] if you are not using and antiseptic solution.
8. Touch [Save] to save your change and return to the Edit Configuration screen.
6.5.4 Filtrate Pump
The filtrate pump control output from the 2960 is provided to control a user-supplied external pump for pumping the
sample from its point of origin to the 2960. The timing of this signal is the same as the purge pump of the 2960. This
option is normally not used since the 2960 pump can transport most samples to the Monitor sample cup. If an external
filtrate pump is used, the flow rate must be maintained at or above 580uL/minute in order to guarantee that the sipper
does not aspirate air during the sampling process.
The default filtrate pump output is Active High (+5V). Touch the [Filtrate Pump Active High] button to change it to Active
Low if your pump requires an Active Low signal (0V) to turn on.
6.5.5 Module
Set up a Module for the chemistries you want to monitor/control.
9. Touch the Module 1 [Edit] button.
51
Page 53

6.5.5.1 PreCal
10. Touch the PreCal [Off] button and change it to [On] to initiate an autocalibration at a predetermined interval before
each monitor sample.
11. Touch the PreCal time interval button [00:10:00] and enter the time in minutes before the monitor sample when an
autocalibration will be initiated.
When in the monitoring mode and when the 2900 Series is stabilized in terms of calibration drift, you may elect to disable
autocalibrations related to time and number of samples and use the PreCal option only.
6.5.5.2 Interval
12. Touch the Interval [00:30:00] button and enter the time interval between your monitor samples for Module 1.
6.5.5.3 Chemistry and Units
13. Touch the Probe A [Edit] button.
52
Page 54

14. Touch the Chemistry [None] button and select the chemistry you are monitoring.
15. Touch the Units [g/L] button and select the units for this chemistry.
6.5.5.4 Analog Output
16. Touch the DAC [None] button to enable the analog output for this probe.
17. Select the Analog output for this probe.
18. The Analog output can be set to 5 Volts or 10 Volts full scale. Touch the DAC [5V] button to change the Voltage to
[10V].
19. Touch the [10.00g/L] button to set the sample value that corresponds to the full scale Voltage.
53
Page 55

6.5.5.5 Control Type
Select Probe
20. See Section 6.8 Contr ol Se tup for setting up the control function.
21. Touch [Save] to save your Probe A changes and return to the Edit Chamber 1 screen.
22. To monitor an additional chemistry, touch the Probe B [Edit] button.
B
23. Enter the Chemistry and Units for Probe B. Analog output and Control can also be set for Probe B.
24. Touch [Save] to save your Probe B changes and return to the Edit Chamber 1 screen.
54
Page 56

25. Touch [Save] to save your Chamber 1 changes and return to the Edit Configuration screen.
26. Touch [Save] to save your Configuration and return to the Select Configuration screen.
27. Touch [Select] to choose the selected configuration and return to the Monitor screen.
55
Page 57

Start Monitor 6.6
1. Once you have setup and selected your monitor session, touch .
2. Enter a name for this specific run, then touch [DONE].
3. The monitor session will start.
The instrument will calibrate the required probe(s) if necessary, or if Precal is enabled, then run the first monitor sample.
56
Page 58

Once the sample has run, it will be displayed on the graph:
Sample
Zoom buttons
Reset
Units
Use zoom and reset to adjust the graph size.
Touch the Units “g/L” to change the displayed monitor units.
6.6.1 Manual Sample during Monitor Session
Results
A plate/rack or Stat sample may be run during a monitor session provided there is sufficient time between monitor
samples.
If you want to manually initiate a monitor sample (without waiting for the Next Sample Time to arrive)
1. Touch the [Start Manual Sample] button.
2. Select the Chamber that you want to start.
57
Page 59

This will initiate a monitor sample and reset the time until the next sample.
Stop Monitor 6.7
To stop the current monitor session, touch .
Control Setup 6.8
The 2960 offer two types of control, Proportional-Integral-Derivative (PID) control or Threshold. PID control attempts to
maintain the sample source at a user defined set point by adding an amount of feed stock proportional to the error.
Threshold simply triggers an alarm or feed pump when the measured sample value passes a user defined value.
The control algorithm incorporated into the 2960 is a variant of the PID algorithm used in many process controllers.
Because of the many factors which can affect control it is difficult, if not impossible, to accommodate all circumstances in
a single form of the PID control algorithm. Certain assumptions apply to the application of this control scheme of which the
user should be aware. Deviations from these assumed conditions will result in degraded regulation.
Assumptions:
1. Regulation is begun (enabled) when the analyte concentration is within ±10% of the set point.
2. The sampling interval is regular. That is, the period between samples (corrections) is constant. Random
calibration timing will degrade regulation performance if it affects sample timing.
3. Higher rates-of-change of the analyte are coupled with shorter sampling intervals to the maximum extent possible.
4. Maximum control pump on-time is kept less than the sampling interval.
NOTE: A sample performed at station 1 or 2 will not initiate regulation or affect analog output.
6.8.1 Control Type
1. From the Run menu [Monitor] tab, touch the [Configure] button.
58
Page 60

2. Touch [New] to create a new monitor configuration, or select an existing configuration and touch [Edit].
3. Touch the Module 1 [Edit] button.
4. Touch the [Edit] button for the probe you want to control.
59
Page 61

5. Touch the Control Type [None] button.
6. Select the type of control for this channel, [PID] or [Threshold], and follow the corresponding instructions in the
section below.
6.8.1.1 PID
For proportional control, select [PID] as the Control Type.
Setpoint
Touch the Setpoint [10.00g/L] button and enter the concentration at which the analyte will be regulated.
60
Page 62

Error Direction
[Under] means that the user wishes to regulate to the set point in an environment where the analyte is being consumed
and as such tends to fall under the set point. In this circumstance the control algorithm will attempt to regulate by making
additions of correction feed stock (which contains the analyte) to the controlled volume.
[Over] error direction assumes that the correction applied by the controller will have the effect of diluting or removing
analyte to perform regulation. When [Over] is selected, enter the TPU manually.
Example: In the case of a fermentation where glucose is the controlled analyte the user should select [Under] error
direction since the organisms in the fermentation will consume glucose, thereby forcing the controller to add glucose via
feed stock additions.
Auto-Activation
Auto-Activation automatically starts control once the sample value is within a certain percentage of the setpoint to
minimize over/under correction. Using the default value, control will not start until the monitored sample value is within 5%
of the setpoint. To change this value, touch the Auto-Activation [5.00%] button and enter the new value.
When using Auto-Activation, make sure the monitored sample value is moving towards the setpoint value. If the monitored
sample value is moving further away from the setpoint value, control will need to be started manually.
TPU
Time-Per-Unit error reflects the amount of time that the correction pump must run to correct for an error in concentration
equal to the unit of measure (e.g. g/L, mg/L, etc.).
Touch the [TPU] button. Select [Automatic] to allow the 2960 to calculate the TPU or [Manual] to enter the TPU manually.
If your Error Direction is [Under], calculate the TPU manually.
Automatic TPU Calculation
61
Page 63

Touch the Average Reactor Volume [10.00] button and enter the average volume of your reactor.
Touch the Delivery Rate [10.00L/min] button and enter the flow rate of your feed pump.
Touch the Maximum Rate of Change [10.00g/L/min] button and enter the maximum rate of change expected during the
monitor session (maximum rate of consumption of the measured analyte during the run).
Touch the Feed Concentration [0.00g/L] button and enter the concentration of your feedstock solution. The minimum
value required is displayed in the [Min Feed Stock:] box.
62
Page 64

Manual TPU Calculation
Touch [Manual] to enter the TPU manually. Manual allows you to enter the TPU value that you calculated yourself.
Touch the TPU [10.00 sec/g/L] button and enter your manually calculated TPU value.
Touch [Save] to save changes and return to the Edit Probe screen.
63
Page 65

Feed Pump line
Touch the Feed Pump Line [None] button and select the 2960 feed pump output line that your external feed pump is
connected to.
The default feed pump output is Active High (+5V). Touch the Feed Pump Line [High] button to change it to [Low] if your
feed pump requires an Active Low signal (0V) to turn on.
Touch [Save] to save changes and return to the Edit Chamber 1 screen.
64
Page 66

Touch [Save] to save changes and return to the Edit Configuration screen.
Touch [Save] to save changes and return to the Select Configuration screen.
Touch [Select] to choose the selected Configuration and return to the Monitor screen.
6.8.1.2 Threshold
For simple threshold alarm, select [Threshold] as the Control Type.
65
Page 67

Touch the Setpoint [10.00g/L] button and enter the threshold value.
Set the Error Direction to trigger when the measured analyte value goes [Under] the threshold or [Over] the threshold.
Touch the Feed Pump Line [None] button and select the 2960 output line that your external alarm/pump is connected to.
The default output is Active High (+5V). Touch the Feed Pump Line [High] button to change it to [Low] if your alarm/pump
requires an Active Low signal (0V) to turn on.
Touch [Save] to save changes and return to the Edit Chamber 1 screen.
Touch [Save] to save changes and return to the Edit Configuration screen.
66
Page 68

Touch [Save] to save changes and return to the Select Configuration screen.
>SITINI ONLINE SAMPLER <
>Chamber 3: disabled
Print Monitor
Touch [Select] to choose the selected Configuration and return to the Monitor screen.
Print Monitor Configuration 6.9
Configuration
Touch the Print Monitor Configuration button to print the current monitor configuration. The configuration can then be
reviewed on the YSI 2901 Printer or from the Status tab:
> CONFIGURATION REPORT <
Config Name: GLUCOSE CON
TROL
Antiseptic Cycle:
00:00:30
Purge Time: 00:01:00
Flow Rate: 2000 uL/min
Filtrate Pump Active
Level: High
>Chamber 1
PreCal: 00:05:00
Sample Interval:
00:30:00
>Probe 1A
Chemistry: Glucose
Unit: g/L
DAC: None
Control Type: PID
Setpoint: 1.00 g/L
Auto Activation: 5%
TPU: 48.00
Error Direction:
Under
Feed Pump: None
>Probe 1B: disabled
>Chamber 2: disabled
67
Page 69

7. System Configuration
Touch Detailed
Settings 7.1
Touch [Settings] to display the global settings screen as shown below. Settings include System, Display, and Scheduler.
NOTE: If 21 CFR Part 11 mode is enabled, only an Administrator can access Settings.
7.1.1 System
Touch the [System] tab (if not already selected). The System tab is used to select the type of sample and calibration
reports, enable sipper fluid detection, and print the configuration.
7.1.1.1 Report Details
To change the Sample or Calibration Reports, touch the button below it to cycle through the selections—Detailed, Brief, or
None.
68
Page 70

7.1.1.2 Communication Port
Set the Communications Port to [Printer] when using the optional YSI 2901 Printer.
The RS232 Communications Port can also be used to connect the 2900 Series to a remote host. Set the Communications
Port to [Remote Commands] to enable the remote host to send commands to the 2900 Series.
7.1.1.3 Print Configuration
Touch the [Print Configuration] button to send the current instrument configuration to the printer.
7.1.1.4 Sipper options
Touch the Sensitivity [High] button and change it to [Low] for samples with high conductivity.
Touch the Fluid Detector [On] button and change it to [Off] to completely disable sipper fluid detection at all locations,
including the calibrator wells.
69
Page 71

Touch the Dynamic Fluid Depth [On] button and change it to [Off] to disable sipper fluid detection and use fixed depth at
sample stations.
Touch the “When no fluid detected” button and select the sample fluid detection mode y ou prefer:
[Error] Produce an error when no sample is detected (no sample result).
[Warn] Use fixed depth when no sample is detected (report a sample result).
7.1.2 Fluid Detection (Bottles)
From the Settings screen, touch the [Fluid Detection] tab.
70
Page 72

To change bottle Fluid Detection, touch the button for the bottle you wish to change. If the 2938 or 2936 Bottle Trays with
Reagent Level Sensing are not installed, turn off the bottle sensors. NOTE: When bottle sensors are off, check bottle
fluid levels regularly to prevent waste flui d from backing up into sample modules.
7.1.3 Display
From the Global Settings screen, touch the [Display] tab.
Adjust the display brightness or sound volume by touching [+] or [–].
7.1.3.1 Touch Screen Calibration
The touch screen is calibrated at the factory and should not require user calibration.
Touch the [Calibrate Display] button to enter touch screen calibration.
71
Page 73

OK
OK
Using a stylus, touch a nd h old the center of the red flashing
that appears at each corner of the display until
appears.
After you have held the in all four corners, the touch panel is calibrated.
Touch screen calibration can also be initiated by touching anywhere on the Initialization screen while the instrument is
powering up.
7.1.3.2 Clock Display Format
Touch the Clock display format button to select [12-hour] or [24-hour] clock format.
72
Page 74

7.1.4 Auto-Cal Settings
From the Configuration screen, touch the [Auto] tab to display the Auto-Cal and Auto-Flush settings.
Touch the [Calibration] button for a module to display the Calibration settings for that module.
The default Auto-calibration settings are shown above. You may alter any of these parameters to suit your application,
however, you may compromise precision and/or accuracy when doing so. YSI’s stated specifications are based on the
default settings. These selections are provided as part of the overall concept of the 2900 Series flexibility.
To change the value of a Calibration parameter, simply touch the value to open the numeric keypad. Enter the new value,
then touch [OK].
NOTE: To disable a Calibration parameter, touch the [ON] button and change it to [OFF].
With AutoCal set to [On], the instrument will keep the module calibrated while idle in the Run screen. If you only want the
instrument to calibrate while running samples/plates, change the AutoCal setting to [Off].
Touch [Save] to save your changes.
73
Page 75

Touch the [Set Auto-flush] button for a module to display the Auto-flush settings for that module.
Touch the Interval (hours) button and enter the number of hours between Auto-flushes.
Touch the Enabled [ON] button and change it to [OFF] to disable Auto-flush for this module.
Touch [Save] to save your changes.
7.1.5 Scheduler
From the Settings screen, touch the [Scheduler] tab.
74
Page 76

The 2900 Series can be set to automatically calibrate at a specific time of day, such as the start of each workday.
Touch the Scheduled calibration [OFF] button and change it to [ON]. Touch any days to select them. Days of the week
that the scheduler is enabled are green.
Touch the Hour button [9]:
Enter the Hour each day that the instrument should calibrate in 24 hour format (0–23), then touch [OK].
Touch the Minute button [00] and enter the Minutes. Touch [OK].
75
Page 77

7.1.5.1 Standby
Enable the Standby function by touching the [OFF] button next to “Standby” and changing it to [ON].
Touch the number of Minutes button [60] next to “Start after.” Enter the number of minutes that the instrument should
remain idle before enabling the screensaver, then touch [OK].
If 21 CFR Part 11 Mo de is enab le d, the screensaver locks the screen and a User name and Pin are required to unlock the
screen (see 7.1.6 21 CFR Part 11).
The maximum setting for number of minutes with 21 CFR Part 11 Mode enabled is 60.
After you have finished making your changes, touch the [X] button to return to the main display.
7.1.6 21 CFR Part 11
FDA 21 CFR Part 11, Electronic Records; Electronic Signatures, was established by the FDA to define the requirements
for submitting documentation in electronic form and the criteria for approved electronic signatures. Since YSI 2900 Series
analyzers generate electronic records, these systems must facilitate compliance with 21 CFR Part 11.
YSI 2900 Series analyzers feature the following functions to provide 21 CFR 11 compliance:
• Audit & event trails
• Secure user sign-on
• User level permissions
• Administrative configuration tools
From the Settings screen, touch the [21 CFR Part 11] tab.
To enable 21 CFR Part 11 mode, touch the [Enable 21 CFR Part 11 Mode] button.
76
Page 78

Touch the to enable 21 CFR Part 11 mode.
Enter a Name, User ID, and 8 character PIN to create at least one Admin account. Note that the PIN must be exactly 8
characters.
Touch [Save].
77
Page 79

7.1.6.1 Manage Users
Create User
Touch the [Create User] button to create additional accounts as required.
Leave the Admin box blank if you are creating a standard user. Touch the Admin box to mark it ONLY if you are
creating a new Administrator.
Note that standard users cannot access the Settings menu, Configure menu, the Sipper or Stirbar tabs under the Service
menu, or the Software tab under the Help menu (as shown below).
78
Page 80

Edit User
To edit an existing user, select the user.
Touch the [Edit User] button.
Change the Name, PIN, Active, or Admin fields as required. To disable a user, remove the X from the Active box.
After you have finished making your changes, touch [Save]. Touch the [X] button to return to the 21 CFR Part 11 screen.
7.1.6.2 View Audit Trail and Event Log
Audit Trail
Touch the [View History] button to display the audit trail.
79
Page 81

Use the scroll buttons to view events in the audit trail.
Event Log
Touch the [Event Log] tab to display the event log.
Use the scroll buttons to view events in the log.
Export
Plug a flash drive into the 2900 Series’ USB port, then touch the [Export] button to send the Audit Trail and Event Log to
the flash drive.
A folder named YSI\BiochemistryAnalyzer\21 CFR Part 11\ (followed by the Machine ID) will be created on the flash drive.
When you have finished exporting, touch the [X] button to return to the 21 CFR Part 11 screen.
80
Page 82

7.1.7 Date/Time
Date/Time
Hour
Date
To set the date/time, touch the time button on the main screen.
NOTE: If 21 CFR Part 11 mode is enabled, only an Administrator can change the date/time.
Touch the current date on the calendar to select it.
Touch the Hour button and enter the current hour in 24 hour format (0–23), then touch [OK].
Touch the Minute button and enter the current minute, then touch [OK].
When you have finished entering the date and time, touch [OK] to return to the main display.
81
Page 83

Service 7.2
Service
Touch [Service] to display the Service menu.
7.2.1 Sipper
See Section 4.5 Align Si pp er for details on aligning the sipper w ith each sample module.
If necessary, the sipper can also be aligned with Station 2, the different types of racks/plates used at Station 1, and the
calibrator we lls .
NOTE: If 21 CFR Part 11 mode is enabled, only an Administrator can access the Sipper tab.
7.2.1.1 Interlock
Touch the Interlock button and change the status to [Off] to disable the interlock switches on the front door and side panel
when aligning the sipper.
WARNING: Keep your hands clear of the sipper while the instrument is in operation.
Always turn the Interlock back [On] before operating the instrument!
82
Page 84

To align the sipper at other locations, touch the button under Location.
Location
Touch the button for your location, [Station 2] for example.
Touch the [Position] button and use the arrow buttons to align the sipper with the selected location.
83
Page 85

Touch at the bottom right of the adjustment window, then touch [YES] to save the position.
Touch [Inject] to lower the sipper and test the alignment, then touch [Retract] to raise the sipper back up. If necessary,
touch [Position] and repeat the adjustment.
7.2.1.2 Depth
Touch [Depth] and select the location. The sipper will move to the selected location.
Sample Modules: use the up and down arrows to set the tip of the sipper even with the top of the Module.
Sample Locations: use the up and down arrows to set the maximum depth the sipper will travel at that sample location.
84
Page 86

Touch at the bottom right of the adjustment window, then touch to confirm saving the position.
7.2.2 Pumps
From the Service menu, touch the [Pumps] tab.
7.2.2.1 Sipper Pump
Home
Touch the [Home] button to Home the sipper pump plunger. The pump plunger will extend fully, then retract slightly.
Aspirate
Touch the [Aspirate] button. The pump plunger will retract about half way as it does when it aspirates a sample. Note that
the actual distance depends on the sample size setting.
Dispense
Touch the [Dispense] button. The pump plunger will extend about half way as it does when it dispenses a sample into the
sample module.
When you have finished testing the sipper pump, touch the [X] button to return to the main display.
7.2.2.2 Buffer Pumps
Touch the [ON] button under a buffer pump. The selected buffer pump will run. Touch the [OFF] button to stop the pump.
When you have finished testing the buffer pumps, touch the [X] button to return to the main display.
7.2.2.3 Calibrator Pumps
Touch the [ON] button under a Cal Pump. The selected calibrator pump will run. Touch the [OFF] button under the same
pump to stop it.
NOTE: Prime all installed calibrator bottles daily to remove air bubbles from the tubing.
When you have finished testing the fluid pumps, touch the [X] button to return to the Main display.
85
Page 87

7.2.3 Modules
Calibrate Module 1
From the Service menu, touch the [Modules] tab.
The Modules tab displays the status of the probes, probe current for each enzyme probe, voltage for each ISE, and the
temperature. The probe current is expressed in nA (nanoamperes, 10
7.2.3.1 Flush
Touch the [Flush] button under Module 1 to flush the sample module with buffer. Observe the probe current values
(baseline). If they are above 6 nA, check to see if they are decreasing in value. Check the sample module; it should be full
of buffer. If necessary, touch the [Flush] key to flush the sample module again. Note that when the instrument is first
powered up, it may take several hours for the baseline currents to drop below 6 nA.
-9
amperes), a very low level of electrical current.
Touch the [Flush] button under Module 3 to flush the sample module with ISE buffer. Note that the instrument will purge
buffer to waste if the incorrect buffer is in the line.
Observe the ISE voltage values. They should be between -40 and 60mV and stable. When the instrument is first powered
up, it may take an hour for the voltages to stabilize.
7.2.3.2 Calibrate
The 2900 Series will automatically calibrate before running a sample batch. You may initiate manual calibration from the
[Modules] tab of the Service screen by touching the [Calibrate] button under each Module.
Calibration status is displayed on the screen.
86
Page 88

The instrument attempts to calibrate each active probe up to 5 times before aborting calibration. If calibration fails, see 11
Troubleshooting.
7.2.4 Stirbar
From the Service menu, touch the [Stirbar] tab.
NOTE: If 21 CFR Part 11 mode is enabled, only an Administrator can access the Stirbar tab.
87
Page 89

The StirbBar screen is used to adjust the speed of the stir bar. The stir bar has two operating speeds: stir speed, where
Increase
Speed
Decrease
Stir
Jump
the stir bar rotates smoothly in the sample module and jump speed, where the stir bar jumps to help clear air bubbles from
the module during the flush cycle.
Note: The sample module must be full of buffer when adjusting stir speed. To fill the module with buffer, see 7.2.3
Modules.
Touch the [Stir] button under Stirbar1. Verify the stir bar is rotating smoothly, but not jumping.
If the stir bar is jumping, use the Down Arrow Button to decrease the stir speed until the stir bar is spinning smoothly.
Touch the [Jump] button. The speed should increase to Jump speed and the stir bar should jump.
If necessary, increase the jump speed until the stir bar is jumping. If the stir bar won’t jump, even with the speed set to
maximum (100), replace it.
Touch the [Spin] button. Verify the stir bar is spinning smoothly and not jumping. If necessary, reduce the stir s peed unt il
the stir bar is not jumping.
Touch the [Off] button under the same stir bar to stop the stir bar.
For the 2950, repeat this process for the remaining modules (Stirbar2 and Stirbar3).
After you have adjusted the stir speed for all modules, touch the [X] button to return to the Main display.
88
Page 90

7.2.5 Monitor
From the Service menu, touch the [Monitor] tab.
Test Outputs
Touch the DAC A or DAC B [0.00V] button to test the analog output by setting the output to 0, 5 or 10VDC.
Touch the Ready, Feed Pump, Filtrate Pump, or SysErr [Off] button and change it to [On] to test the corresponding output.
Valve
Touch the Opened Valve [Antiseptic] button to change the state of the valve and change it to [Sample]
Prime Monitor Pump
Touch the Pump Sample [Off] button and change it to [On] to prime the monitor sample line. The pump will turn off
automatically.
If you are using an antiseptic solution, touch the Pump Antiseptic [Off] button and change it to [On] to prime the antiseptic
solution. The pump will turn off automatically.
NOTE: After sterilization, the antiseptic solution must be primed immediately after th e tubing is reconnected.
89
Page 91

Data 7.3
From the Main display, touch [Data].
7.3.1 Plate
Historical plates of sample data are displayed under the Pate tab. Touch the scroll buttons to page through plates.
Touch a specific plate to highlight it, then touch [View] to display the sample data for the selected plate.
Sample data for the first sample in the batch (A1) is displayed.
Touch any other sample location to display the sample data. Touch the sample result to show details.
90
Page 92

Touch [Close] to return to the Data screen.
Select to show
Select sample
Plate Name Filter
Touch the Name button and enter a plate name to filter the data.
details
Only plates that begin with that nam e will be displayed.
Export
Check the To Export box for each batch that you would like to export to a flash drive.
.
91
Page 93

Plug a flash drive into the 2900 Series’ USB port, then touch the [Export] button to send the selected sample results from
Plate Sequence Name
Batch Name
Local Completion Time
Completion State
Well Id
Chemistry Id
Probe Id
Concentration
Units
Endpoint
Sample Size
Initial Baseline
Plateau
Final Baseline
Net Plateau
Net Plateau Temp Adj
Cross Net Plateau
Cross Net Plateau Temp Adj
Plateau Slope
Temperature
Errors
P96-1 TestBatch-1 12/11/2013 10:31 Complete P96_A04 Glutamine Probe2B 0.10872 g/L 30 20 2.27 32.30 2.27 30.03 30.38 30.83 31.12 -0.022 24.55
P96-1 TestBatch-1 12/11/2013 10:31 Complete P96_A04 Glutamate Probe2A 1.43929 g/L 30 20 0.52 31.36 0.89 30.83 31.12 0.268 24.55
P96-1 TestBatch-1 12/11/2013 10:29 Complete P96_A05 Glucose Probe1A 4.60089 g/L 30 25 3.96 20.51 3.62 16.55 16.64 0.750 24.97
P96-1 TestBatch-1 12/11/2013 10:29 Complete P96_A05 Lactate Probe1B 0.80631 g/L 30 25 3.75 20.48 3.58 16.73 16.82 0.181 24.97
P96-1 TestBatch-1 12/11/2013 10:32 Complete P96_A05 Glutamine Probe2B 0 g/L 30 20 2.27 2.39 2.39 0.13 0.13 0.18 0.18 -0.422 24.46
P96-1 TestBatch-1 12/11/2013 10:32 Complete P96_A05 Glutamate Probe2A 0.00836 g/L 30 20 0.89 1.07 1.07 0.18 0.18 -0.165 24.46
R24-2 REACTOR 12 12/11/2013 9:58 Complete R24_A01 Glucose Probe1A 1.17388 g/L 30 25 3.39 7.61 3.60 4.22 4.25 0.158 24.97
R24-2 REACTOR 12 12/11/2013 9:58 Complete R24_A01 Lactate Probe1B 0.20807 g/L 30 25 3.28 7.59 3.39 4.32 4.34 -0.215 24.97
memory to the flash drive.
When you have finished exporting data, touch the [X] button to return to the Main screen.
A folder named YSI\BiochemistryAnalyzer will be created on the flash drive. Sample data files are copied to the Data
subfolder. The data file name will contain the instrument’s Mach ine ID along with the date and time.
Example Sample Data File:
7.3.2 Monitor
Touch the Monitor tab to display the list of monitor sessions.
Select a monitor session, then touch [View] to display the graph for the selected monitor session.
92
Page 94

Touch [View Data Points] to view the actual data.
Touch [Close] to return to the Data screen.
Name Filter
Touch the Name button and enter a monitor session name to filter the data.
Only sessions that begin with that name will be displayed.
Export
Check the To Export box for each session that you would like to export to a flash drive.
93
Page 95

.
Plug a flash drive into the 2900 Series’ USB port, then touch the [Export] button to send the selected sessions from
memory to the flash drive.
When you have finished exporting data, touch the [X] button to return to the Main screen.
A folder named YSI\BiochemistryAnalyzer will be created on the flash drive. Sample data files are copied to the Data
subfolder. The data file name will contain the instrument’s Mach ine ID along with the date and time.
7.3.3 Calibration
Touch the Calibration tab.
Plug a flash drive into the 2900 Series’ USB port. Select a date range, then press the [Export] button to send calibration
data to the flash drive.
When you have finished exporting data, touch the [X] button to return to the Main screen.
A folder named YSI\BiochemistryAnalyzer will be created on the flash drive. Calibration data files are copied to the Data
subfolder. The data file name will contain the instrument’s Mach ine ID along with the date and time.
Example Calibration File:
94
Page 96

Probe Id
Local Calibrated Time
Chemistry Id
Reagent Number
Concentration
Units
End Point
Sample Size
Initial Baseline
Plateau
Final Baseline
Net Plateau
Cross Net Plateau
Probe Slope
Temperature
Errors
1A 12/11/2013 10:43 Glucose 2776 2.5 g/L 30 25 3.240 14.478 3.275 11.239 NaN 0.144 25.20
1B 12/11/2013 10:43 Lactate 2776 0.5 g/L 30 25 3.276 14.372 3.303 11.096 NaN -0.172 25.20
2B 12/10/2013 16:11 Glutamine 2736 5 mmol/L 30 20 3.758 20.733 3.744 16.976 0.077 0.031 24.86
2A 12/10/2013 16:07 Glutamate 2755 5 mmol/L 30 20 4.245 20.151 4.250 15.906 14.238 0.514 24.83
1A 12/9/2013 11:48 Glucose 2776 2.5 g/L 30 25 3.057 12.100 3.103 9.043 NaN 1.167 25.12
1B 12/9/2013 11:48 Lactate 2776 0.5 g/L 30 25 3.067 13.495 3.134 10.428 NaN -0.145 25.12
2B 12/10/2013 16:11 Glutamine 2736 5 mmol/L 30 20 3.758 20.733 3.744 16.976 0.077 0.031 24.86
2A 12/10/2013 16:07 Glutamate 2755 5 mmol/L 30 20 4.245 20.151 4.250 15.906 14.238 0.514 24.83
1A 12/9/2013 11:48 Glucose 2776 2.5 g/L 30 25 3.057 12.100 3.103 9.043 NaN 1.167 25.12
1B 12/9/2013 11:48 Lactate 2776 0.5 g/L 30 25 3.067 13.495 3.134 10.428 NaN -0.145 25.12
2B 12/10/2013 16:11 Glutamine 2736 5 mmol/L 30 20 3.758 20.733 3.744 16.976 0.077 0.031 24.86
2A 12/10/2013 16:07 Glutamate 2755 5 mmol/L 30 20 4.245 20.151 4.250 15.906 14.238 0.514 24.83
Sitini Serial Number: 14B000024
Help 7.4
Touch the [Help] icon to display the Help selections as shown below.
7.4.1 About
Touch the [About] tab to display the About screen. This screen provides information about the instrument ID and serial
number, the current software (App) version, hardware, and IP address.
7.4.1.1 Machine ID
The default Machine ID is the instrument serial number. Touch the Machine ID button to enter a custom ID for this
instrument. Enter the new ID and touch [Done].
NOTE: The Machine ID is used as the folder name and file name for data files.
The 2900 Series software can be updated via a flash drive inserted into the USB port. See the YSI web site at
www.ysi.com for available updates. Install the update as shown below.
95
Page 97

7.4.2 Software
From the Help menu, touch the [Software] tab to display the Software screen.
NOTE: If 21 CFR Part 11 mode is enabled, only an Administrator can access the Software tab.
7.4.2.1 Export Configuration
Insert a flash drive and wait for the Flash Drive Inserted light to change to green.
Press [Export Config] to save the current instrument configuration to the flash drive.
NOTE: The configuration file can be imported at a later date to restore the instrument configuration.
7.4.2.2 Backup
Press [Backup] to backup the current instrument setup, database, log files, and 21 CFR 11 audit files to the flash drive.
7.4.2.3 Restore
Press [Restore] to restore a previous backup file for this analyzer from a flash drive.
7.4.2.4 Default
To default all instrument settings to the factory values, touch [Reset to Defaults].
NOTE: After resetting the instrumen t to d efault settings, the sipper must be realigned with ALL locations to
prevent sipper damage.
.
96
Page 98

7.4.2.5 Clear Database
NOTES: Make sure all sample data and audit logs have been exported before clearing the database!
The Clear Database function is not available when 21 CFR Part 11 Mode is enabled.
Press [Clear Database] to clear all saved sample and calibration data. Clearing old data from the database will reduce the
amount of time required to export and save sample data.
7.4.2.6 Update Software
From the Help screen, Software tab, insert the flash drive containing the software update in the instruments’ USB port.
After the Flash Drive Inserted light changes to green, touch the [Launch Updater] button.
The 2900 Series Updater will be displayed.
Touch the [Update Firmware] button to install the ne w firmware.
Touch the [Update Application] button to install the new software.
When the Installation Complete window is displayed, remove flash drive.
The instrument will restart.
NOTE: Updating the software will clear all sam p le data. After updating the software, the sipper must be aligned
with all positions.
7.4.3 FAQ
From the Help menu, touch the [FAQ] tab to display frequently asked questions and answers.
7.4.4 Training
From the Help menu, touch the [Training] tab.
97
Page 99

Connect headphones or speakers to the instruments audio port on the right side of the display.
Touch the button of the training video you wish to view.
While the video is playing, you may touch the screen to stop the video and return to the Training screen.
Ethernet Port 7.5
Connect one or more 2900 Series instruments to a LAN or router via the RJ45 Ethernet port.
7.5.1 LAN (Shared Network Connection)
Connect one or more 2900 Series instruments to a LAN via the RJ45 Ethernet port.
7.5.2 Router (Private Network Connection)
Connect one or more 2900 Series instruments to a router (DHCP server) via the RJ45 Ethernet port.
98
Page 100

7.5.3 Accessing Stored Data
Access the data stored in a 2900 Series connected to a LAN or router via ftp. See 7.4.1 About to view the instrument’s
current IP address.
If you are unable to connect via ftp, make sure the “Passive ftp” option is not checked under Advanced Internet Options
on your computer:
99
 Loading...
Loading...