
XFT-2001E
Nerve and Muscle Stimulator (Trade name: Foot Drop System)
User Manual
Caution: Federal law restricts this device to sale by or on the order of a practitioner licensed by the law of the State in
which he/she practices to us e or order the use of the device.
1

Content
1. For Your Health and Safety ................................................................................................................................... 3
2. Overviews ............................................................................................................................................................ 10
3. Product Illustration ...............................................................................................................................................11
4. Operation Instruction ........................................................................................................................................... 13
5. Attentions ............................................................................................................................................................. 18
6. Care and Maintenance ......................................................................................................................................... 19
7. FAQ & Troubleshooting ...................................................................................................................................... 19
8. Product Configuration ......................................................................................................................................... 20
9. Product classification ........................................................................................................................................... 21
10. After-sales Service ............................................................................................................................................. 22
2
1. For Your Health and Safety
Declaration of conformity according to the applicable European directives and number of the
To avoid any danger or loss caused by inappropriate use, please read this manual carefully;
In the precautions, the hazards and losses caused by improper use are stated, and the safety precautions are divided
into three parts: “contraindications”, “warning” and “attention”;
Please keep this manual carefully.
List of Symbols
Contraindications, or will cause danger
Mandatory Abidance, or will c a us e acc ident / physical discomfort
Type BF Equipment
Use with Caution
Non-ionizing radiation
Date of manufacture
Manufacturer
Serial Number
notified body (0123)
European Authorize d Representative
Consult instructions for use
Please dispose of the device/battery/accessory/packing in accordance with the legal obligation in
your area
Contraindications
Do not use with electronic monitoring equipment, NMR-imaging, pace-maker, defibrillator and high-frequency
medical device.
Do not use near short-wave, microwave. (such as 1m)
Patients with heart disease, severe hypertension and skin disorder are forbidden to use this product.
Patients with epilepsy are forbidden to use this product.
Patients with active hemorrhage, acute purulent Inflammation, malignant neoplasms, thrombophlebitis, sepsis and
cardiopulmonary failure are forbidden to use this product.
Do not use this product for purpose other than treatment.
Do not apply this product to unconscious patients.
Do not disassemble, repair or rebuild this product.
3

Do not touch the charging connector/battery and the patient simultaneously when charging/using.
Warning
The safety of usage durin g p regnancy or menstruati on has not been determined.
Electrode positioning and stimulation parameters’ setting should be conducted by professionals. If you keep
feeling pains or rash, ple a s e s t op us ing this product.
Please do not position the electrode in the area of malignant neoplasms, neck arteries (throat) or thrombus.
Be careful if the electrode positioning areas show following situa t ions:
– Bleeding trend caused by serious trauma;
– Muscle training might cause disorder of rehabilitation of a recent surgery;
– Electrode positioning areas are not sensitive enough.
Please use with caution when the arteries of used area show partial occlusion, when the patient has vascular
atrophy because of hemodialysis, or when the vascular system shows instability.
2
Please use with caution when the output current density exceeds 10mA/cm
(r.m.s).
Please use with caution if the used areas have structural deformity.
This product should be con ducted by doctors.
Patients should keep stable and not move while using this unit.
Patients should not move the electrode or be touched while using this unit.
Please stop using this product if the body shows any physical abnormality.
This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) This
device may not cause harmful interference, and (2) this device must accept any interference received, including
interference that may cause undesired operation.
FCC warning:
Any Changes or modifications not expressly approved by the party responsible for compliance could void the user's
authority to operate the equipment.
Note: This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to part
15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful interference in a
residential installation. This equipment generates uses and can radiate radio frequency energy and, if not installed and
used in accordance with the instructions, may cause harmful interference to radio communications. However, there is no
guarantee that interference will not occur in a particular installation. If this equipment does cause harmful interference
to radio or television reception, which can be determined by turning the equipment off and on, the user is encouraged to
try to correct the interference by one or more of the following measures:
-Reorient or relocate the receiving antenna.
-Increase the separation between the equipment and receiver.
-Connect the equipment into an outlet on a circuit different from that to which the receiver is connected.
-Consult the dealer or an experienced radio/TV technician for help.
The device has been evaluated to meet general RF exposure requirement. The device can be used in portable
exposure condition without restriction.
4
This equipment uses RF energy only for its internal function.
voltage power
Electromagnetic Compatibility (EMC)
This equipment generates, uses, and radiates radio frequency energy. The equipment may cause radio frequency
interference to other medical or non-medical devices and to radio communications.
If this equipment is found to cause interference, which can be determined by turning on and off the equipment, the
operator or qualified service personnel should take following actions:
Reorient or relocate the affected device;
Increase the distance between the equipment and the affected device;
Power the equipment by another source;
Consult the service engineer for further suggestions.
Caution: it is customer’s responsibility to assure that this equipment and vicinity equipment comply with the contents of
th
IEC 60601-1-2 4
Edition.
Caution: do not use any device that might send out RF signals, including cell phone, radio transceiver and radio control
products, which might cause operation parameters beyond the standards. Please shutdown these devices when you are
near the equipment. Operator has the responsibility to warn user or any others to comply with this rule.
Caution: manufacturer will not responsible for any unauthorized actions that cause interference.
Table 1
Guidance and manufacture’s declaration – electromagnetic emission
This equipment is intended for use in the electromagnetic environment specified below. User should assure that it is
used in such an environment.
Emissions test Compliance Electromagnetic environment - guidance
RF emissions
CISPR 11
Group 1
Its RF emissions are very low and are not likely to cause any
interference in nearby electronic.
RF emissions
CISPR 11
Harmonic emissions
Class B This equipment is suitable for domestic establishments and
those directly connected to the public low-
Class A
supply network.
IEC 61000-3-2
Voltage fluctuations/flicker
Complied
emissions
IEC 61000-3-3
5
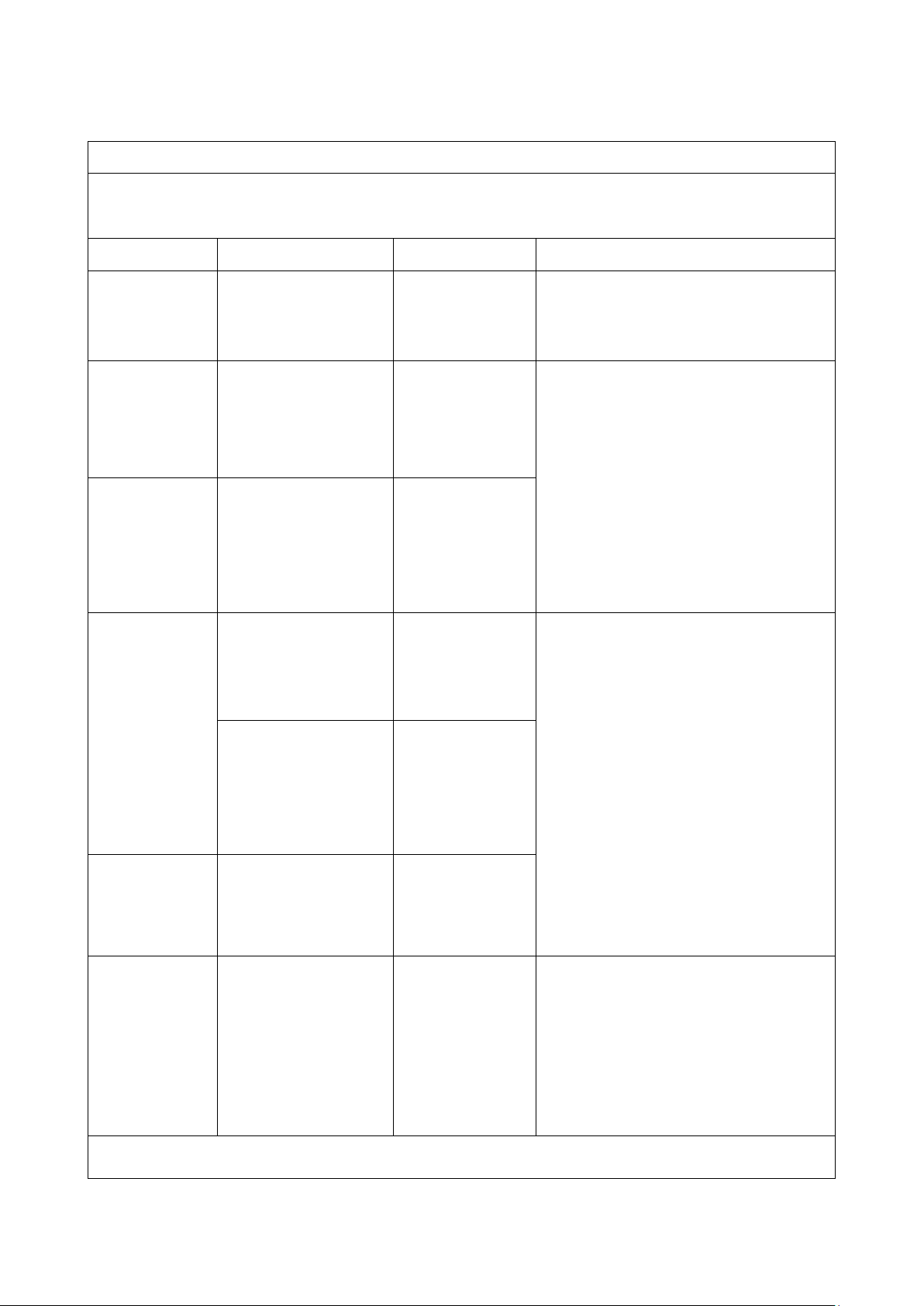
±0.5kV, ±1kV
±0.5kV, ±1kV
Voltage dips
0 % UT; 0.5 cycle
0 % UT; 0.5 cycle
At 0°, 45°, 90°,
135°, 180°, 225°,
0 % UT; 1 cycle
0 % UT; 1 cycle
Table 2
Guidance and manufacture’s declaration – electromagnetic immunity
This equipment is intended for use in the electromagnetic environment specified below. User should assure that it is
used in such an environment.
Immunity test IEC60601 test level Compliance level Electromagnetic environment - guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
Electrical fast
transients/bursts
(EFT)
IEC 61000-4-4
Surges
IEC 61000-4-5
IEC 61000-4-11
±8kV contact
±2kV, ±4kV, ±8kV,
±15kV air
±2kV
100kHz repetition
frequency
line-to-line
±0.5kV, ±1kV , ±2kV
line-to-ground
At 0°, 45°, 90°, 135°,
180°, 225°, 270° and
315°
±8kV contact
±2kV, ±4kV, ±8kV,
±15kV air
±2kV
100kHz repetition
frequency
line-to-line
±0.5kV, ±1kV,
±2kV
line-to-ground
270° and 315°
Floors should be wood, concrete or ceramic
tile. Humidity should be at least 30% if it is
synthetic materials.
Main power quality should be that of a
typical commercial or hospital environment.
Mains power quality should be typical
commercial or hospital environment. UPS
power is recommended if this device needs
to be used continuously.
Voltage
interruptions
IEC 61000-4-11
RATED power
frequency
magnetic fields
IEC 61000-4-8
Note: UT is the A.C. mains voltage prior to application of the test level.
and
70 % U
T; 25/30 cycles
Single phase: at 0°
T; 250/300 cycle 0% UT;
0% U
30A/m
50Hz or 60Hz
and
70 % U
T; 25/30
cycles
Single phase: at 0°
250/300 cycle
30A/m
50Hz or 60Hz
6
Power frequency
magnetic fields should be at levels
characteristic
of a typical location in a typical commercial
or
hospital environment.
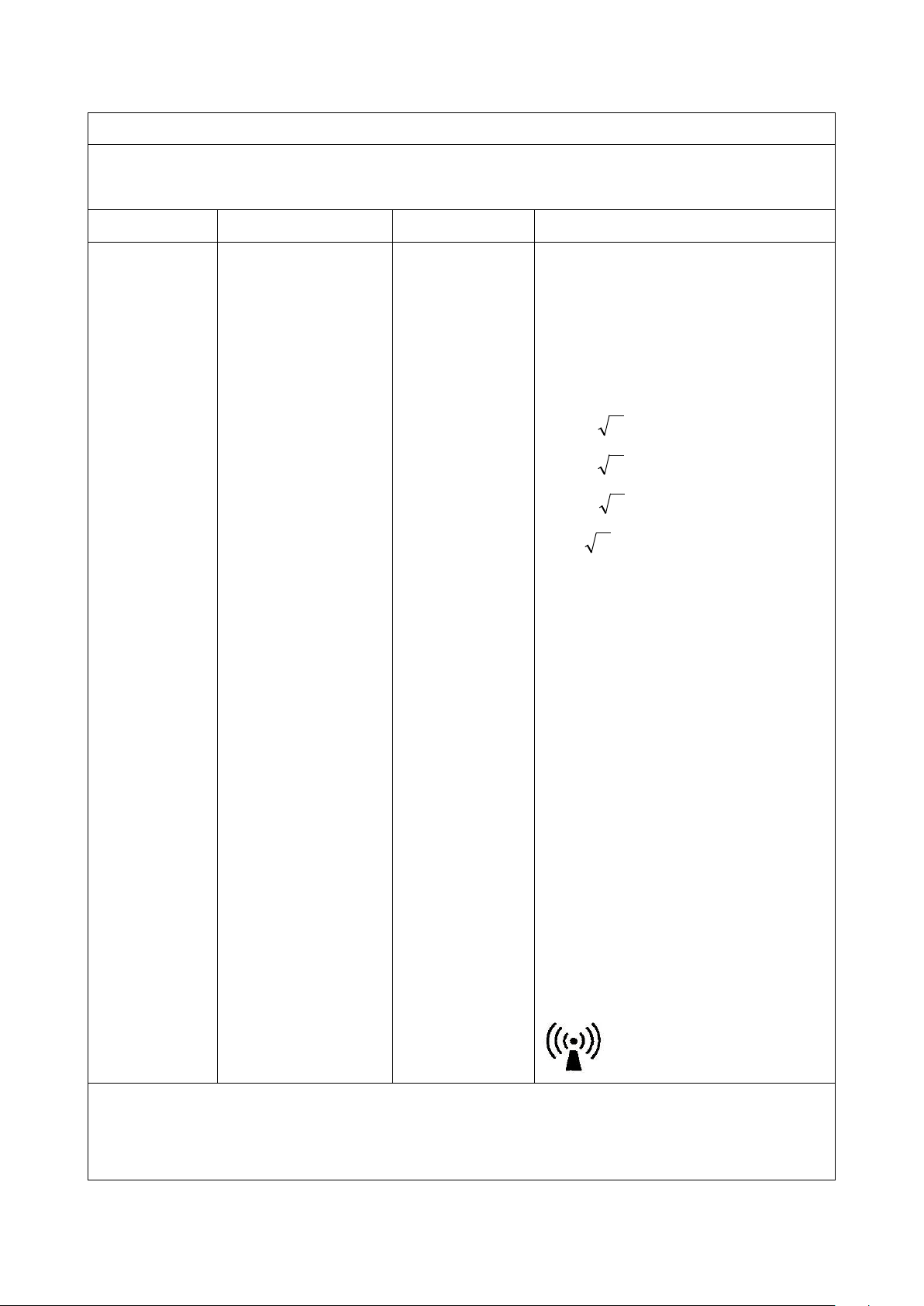
Table 3
Pd 2.1=
Pd 2.1=
Pd 3
.2=
EPd /6=
at RF wireless communications equipment
bands (Portable RF communications
ing peripherals such as
Guidance and manufacture’s declaration – electromagnetic immunity
This equipment should be used in the electromagnetic environment specified below. User should assure that it is used
in such an environment.
Immunity test IEC60601 test level Compliance level Electromagnetic environment - guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3Vrms
150 kHz to 80 MHz
6Vrms
in ISM and amateur
radio bands between 150
kHz and 80 MHz (a)
10 V/m
80MHz to 2.7GHz
3Vrms
6Vrms
10 V/m
Portable and mobile RF communications
equipment should be used no closer to any
parts than the recommended separation
distance that calculated from the equation
applicable to the frequency of the transmitter.
Recommended separation distance:
150 kHz to 80 MHz
80MHz to 800 MHz
800MHz to 2.7GHz
equipment (includ
antenna cables and external antennas) should
be used no closer than 30 cm (12 inches) to
any part of the device).
Where “P” is the maximum output power
Note1: At 80MHz and 800MHz, the higher frequency range applies.
Note2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
refection from structures, objects and people.
rating of the transmitter in watts according to
transmitter manufacturer and “d” is the
recommended separation distance in meters.
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site survey
(b), should be less than the compliance level
in each frequency range (c).
Interference may occur in the vicinity of
equipment marked with the following
symbol:
7

a) The ISM (industrial, scientific and medical) bands between 0.15 MHz and 80 MHz are 6.765 MHz to6.795 MHz;
mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with
13.553 MHz to 13.567 MHz; 26.957 MHz to 27.283 MHz; and 40.66 MHz to 40.70 MHz. The amateur radio
bands between 0.15 MHz and 80 MHz are 1.8 MHz to 2.0 MHz, 3.5 MHz to 4.0 MHz, 5.3 MHz to 5.4 MHz, 7
MHz to 7.3 MHz, 10.1 MHz to 10.15 MHz, 14 MHz to 14.2 MHz, 18.07 MHz to 18.17 MHz,21.0 MHz to 21.4
MHz, 24.89 MHz to 24.99 MHz, 28.0 MHz to 29.7 MHz and 50.0 MHz to 54.0 MHz.
b) Field strengths from fixed transmitters, such as base stations for radio (cellular / cordless) telephones and land
accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey
should be considered. If the measured field strength in the location in which this is used exceeds the applicable RF
compliance level above, this should be observed to verify normal operation. If abnormal performance is observed,
additional measures may be necessary, such as re-orienting or relocating.
c) Field strengths should be le s s than 3V/m in the frequenc y r a nge of 150k~80MHz.
8

een portable and mobile RF communications equipment to prevent electromagnetic
Table 4 -Test specifications for ENCLOSURE PORT IMMUNITY to RF wireless communications equipment
Table 5
Recommended separation distance between portable and mobile RF communications equipment and the
Nerve and Muscle Stimulator
The device is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled.
The customer or the user of the device can help prevent electromagnetic interference by maintaining a minimum
distance between portable and mobile RF communications equipment (transmitters) and the Nerve and Muscle
Stimulator as recommended below, according to the maximum output power of the communications equipment.
This device can be used under the environment that radiated RF disturbances are controlled. User should maintain a
minimum distance betw
9

interference. Following recommended distance is calculated according to the maximum output power of the
communication equipment.
150kHz -80MHz
P
d 2
.
1=
80MHz -800MHz
Pd 2.1=
800MHz -2.7GHz
P
d
3
.
2
=
Rated maximum output
power of transmitter (W)
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.79 3.79 7.27
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation distance “d” in
meters can be estimated using the equation applicable to the frequency of transmitter, where “P” is the maximum
output power rating of the transmitter in watts according to the transmitter manufacturer.
Note1: At 80M and 800MHz, the separation distance for the higher frequency range applies.
Note2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
refection from structures, objects and people.
Separation distance according to frequency of transmitter (m)
2. Overviews
2.1 Indication for Use
XFT-2001E Foot Drop System is intended to address the lack of ankle dorsiflexion in patients who have sustained
damage to upper motor neurons or pathways to the spinal cord. During the swing phase of walking, the XFT-2001E
electrically stimulates the appropriate muscles that cause ankle dor siflexion and may thus improve the ind ividual's gait.
Medical benefits of Functional Electrical Stimulation (FES) may include prevention/retardation of disuse atrophy,
increased local blood flow, muscle reeducation, and maintained or increased joint range of motion.
How does XFT-2001E Foot Drop System work?
When the leg swings to the angle of threshold, the electrical stimulation will be triggered.
2.3 Features and Innovation
Bluetooth 4.0, more stable and reliable data transmission, easy operation and setting.
Thin and light Stimulator & Cuff with patented design, easy and simple to wear.
Integrated stainless steel electrode, fully enclosed waterproof design, no need for additional consumables, easy to
clean and store.
Automatic shutdown function: low voltage or automatic shutdown after 30 minutes of standby.
Built-in rechargeable battery; one single charge for long-term use.
Electrical stimulation constant current output & low voltage alarm function.
It has two modes of walking and training, which can be selected according to the patient's state;
Equipped with APP management software; able to manage user information and store gait data. It is simple and
convenient to set the parameters of the host through the mobile phone.
10

2.4 Use Cycle
1st week
Walk for 15-60 minutes a day
every morning and evening, 15 minutes each time
2nd week
Walk for 1-4 hours a day
every morning and evening, 20 minutes each time
Power Adapter & charging cable
Adhere to the principle of g radual progress when usi ng XFT-2001E.
Cycle gait mode training mode
3rd week & later Walk for 4-8 hours a day every morning and evening, 20 minutes each time
Note: take off the cuff for 15 minutes after each use.
3. Product Illustration
3.1 Product Parts
XFT-2001E consists of the Stimulator, Power Adapter, charging cable, and APP software (optional).
3.1.1 Stimulator
3.1.2 Parts
Stimulator
11

3.2 Operation Panel
Switch/Mode composite button
intensity adjustment button
Cuff
3.2.1 Operation buttons
Electrical stimulation
intensity adjustment
button
OLED display
Electrical stimulation
This unit contains 3 buttons (1 switch / mode composite b utton, 2 electrical stimulation intensity adjustment buttons),
and 1 OLED display.
Switch/Mode composite button: Press and hold this button for 2 seconds to turn the unit on; after pow er on, the unit
display shows “XFT” LOGO for 2 seconds; tap this button to switch the working mode (gait mode and training mode
switching). When the unit is turned on, press and hold this button for 2 seconds to turn off the unit. In the working state,
tap this button to pause the electrical stimulation output.
Electrical stimulation intensity adjustment button: Tap this button to start electrical stimulation and increase or
decrease the electrical stimulation intensity; click the up button to increase the electrical stimulation, and click the down
button to decrease the electrical stimulation intensity.
OLED display: display various working states of the unit; such as gait mode, training mode, electrode loose, low
battery icon, electrical stimulation output icon, electrical stimulation intensity, etc.
3.2.2 Indicators
Power-on indication: Press and hold the switch/mode composite button for 2 seconds to turn the unit on; after
power-on, the unit display shows “XFT” LOGO for 2 seconds; tap this button to switch the working mode (gait
mode and training mode switching).
LOGO Gait mode Training Mode
Mode switching: After the unit is turned on or the unit is paused, press “ Switch/Mode Switch” to switch the
mode.
Gait mode Training Mode
Start/Pause: When the unit is in the pause state, press the “Electrical Stimulation Intensity Adjustment Button
” to activate the electrical stimulation intensity; press the up button to increase the electrical stimulation, and
press the down button to decrease the electrical stimulation. The display will show the corresponding stimulus
intensity.
12

Electrical stimulation output prompt: When the unit is in the state of electrical stimulation, the display will show
the lightning symbol, and the electrical stimulation will stop showing the electrical stimulation intensity. When the
gait mode is activated, there will be a “bee” sound prompt for each output of the electrical stimulation (the sound
can be muted by the APP software setting).
Lightning Symbol Electrical Stimulation Intensity
Load detection: When the electrode of the unit is in poor contact with the skin, the display screen will have an
exclamation point icon and an electrode loose icon alternately displayed. The unit automatically stops the electrical
stimulation output and has 3 “bee” prompts. After the electrode of the unit is in full contact with the skin of the leg,
press the electrical stimulation intensity adjustment button to continue the current mode.
Low voltage / charging prompt: When the unit is low, there will be a battery icon display (battery display zero
capacity) at low voltage and flash at 1 second interval. When the battery voltage is as low as the shutdown voltage,
the unit automatically shuts down. The dynamic charging icon is displayed while charging, and the full battery
icon is displayed when charging is completed.
Low battery, flash every 1 second Start charging Charging... Charging... Full Charge
Automatic screen saver/shutdown: When there is no change in the content of the display, the brightness of the
display will automatically reduce after 30 seconds, and enter the screen saver animation after 60 seconds; the
screen saver "XFT" LOGO in animation form will move to the right; when the unit is low voltage to the shutdown
voltage, automatic shutdown; the unit will automatically shut down in the standb y (no electrical stimulation output)
for 2 hours.
Screen Saver Icon
3.3 APP Software Description (optional)
Hardware requirements:
iPhone 5s and subsequent release models of the iPhone.
Software Environment:
System environment: IOS 8.x/9.x/10.x/11.x;
Development environment: Xcode 8.0 and above;
Security software: none;
Network requirement s : none .
4. Operation Instruction
4.1 How to wear G4?
This product can be used se p a ra tely or with APP software.
13

4.1.2 Use separately
Please check if the Stimulator is fully charged before use. If necessary, please charge the unit; when charging, the main
unit display will indicate the icon.
During use, if you find that the electrical stimulation intensity is weak or the low-power icon appears on the main unit
display, please charge it in time; the main unit can be charged for about 3 hours, and it can be used for about 10 hours
after being fully charged; if the unit is not used, please shut down the host and storage it.
Note: Please use the charger supplied by our company to charge. When charging, please do not wear the unit.
4.1.2.1 Wear the Stimulator
Preparations: clean the covered area with a wet towel and keep the skin moist. If the hair is more, you can trim it.
Sit on a comfortable stool, relax the affected leg and bend naturally.
Place the Stimulator to correct position under the knee.
4.1.2.2 Power on and opera t e
Press and hold the switch/mode composite button for 2 seconds to turn the unit on; after power-on, the unit display
shows “XFT” LOGO for 2 seconds; tap this button to switch the working mode (gait mode and training mode
switching).
LOGO Gait mode Training Mode
When the Stimulator is in the pause state, press the “Electrical Stimulation Intensity Adjustment Button ” to
activate the electrical stimulation intensity; press the up button to increase the electrical stimulation, and press the
down button to decrease the electrical stimulation.
Note: In order to allow the skin area covered by the unit to be breathable and to prevent skin irritation and redness,
the unit should be suspended and removed at regular intervals to allow the skin to be fully breathable in the
process of using the product.
4.1.2.3 Power of f
When the Stimulator is turned on, press and hold the switch/mode composite button for 2 seconds to turn off it.
4.1.3 Use with APP
14

4.1.3.1 Install APP
1) First install the XFT-2001E foot drop APP on your mobile phone. Download the APP from APP Store, register an
account and remember your account and password when log in for the first time. Turn on the XFT-2001E Stimulator,
open the Bluetooth on your mobile phone and run the APP to connect the Stimulator.
Mobile phone hardware requirements: iPhone 5s and subsequent release models of the iPhone.
Mobile phone software environment: system environment: IOS 8.x/9.x/10.x/11.x;
development environment: Xcode 8.0 and above;
security software: none;
network requirement s : none .
APP interface: “Use” is used for each function and parameter setting; “Help” is for checking company news, product
usage guide, video and FAQ; “Me” is user and system related functions.
Log in Search Stimulator
4.1.3.2 Wear the Stimulator
Preparations: clean the covered area with a wet towel and keep the skin moist. If the hair is more, you can trim it.
Sit on a comfortable stool, relax the affected leg and bend naturally.
Place the Stimulator to correct position under the knee.
4.1.3.3 Power on and opera t e
Turn on
Press and hold the switch/mode composite button for 2 seconds to turn the unit on; after power-on, the unit display
shows “XFT” LOGO for 2 seconds; Turn on the phone Bluetooth and run the software to connect to the main unit.
15

Log in interface Search Stimulator List of Stimulators Successful connection
interface
Choose mode
Successful connection interface Choose one mode
Gait mode
– Start: The App sends the gait mode parameter and the walking start command to the host, receives the
host reply, and enters the walking working interface.
– Pause: The App sends a gait mode pause (stop) command to the host, receives a reply from the host, and
pauses the walk.
– Continue: The App sends the gait mode (start), at which time the cu rrent intensity giv en in the parameter
setting is the current intensity before the pause. After receiving the host reply, the walking work
continues.
– Stop: After long press for 2s, send the gait mode stop command to the host, receive the host reply, and the
gait mode stops.
– During the walking process, the number of steps of the host is sent, and the App will display
synchronously.
16

– Under the gait mode, user can choose among smart mode, normal mode, and manual mode. Click the
“smart mode” position to pop up the mode selection list and select one.
a. Smart mode: The host automatically calculates the tilt angle A of the electrical stimulation and the tilt
angle B of shutting down electrical stimulation according to the gait data of the first four steps of the
patient. The parameters that can be adjusted are the electrical stimulation intensity, frequency and pulse
width in the lower level interface of the “Parameter Settings”.
b. Normal mode: The host performs electrical stimulation according to the set parameters. The parameters
that can be adjusted are the electrical stimulation intensity, and the frequency, pulse width, tilt angle A, tilt
angle B, duration, delay time, ramp up and ramp down in the lower-level interface of the “Parameter
Setting”.
c. Manual mode: After starting this mode, the host performs electrical stimulation according to the
electrical stimulation button of the interface; press the button to start electrical stimulation, stop the
electrical stimulation by the release, and continue to hold the electrical stimulation for continuous output.
The parameters that can be adjusted are the electrical stimulation intensity and the frequency and pulse
width in the lower level interface of the “Parameter Settings”.
Training mode
– The training mode performs electrical stimulation based on a combination of parameters of the selected
training mode. The parameters combine 9 fixed combinations and one custom. The electrical stimulation
can be adjusted after the fixed mode starts, and other parameters cannot be adjusted. All parameters can
be adjusted under custom.
– Start: The App sends the training mode parameters and mode start command to the host, receives the host
reply, and enters the training work interface.
– Pause: The App sends a training mode pause (stop) command to the host, receives a host reply, and the
training is suspended.
– Continue: The App sends the training mode (start), at which point the current intensity given in the
parameter settings is the current strength before the pause. After receiving the host response, the training
work continues.
– Stop: After long press and stop for 2s, send the training mode stop command to the host, receive the host
reply, and the training mode stops.
1-9 Built-in 9 training modes with fixed parameters; custom parameters can be adjusted.
Evaluation mode
– Evaluation mode: Collect the angle data generated by the host during the patient's walk ing. The collectio n
duration is 60s by default and the maximum is 90s. The acquisition begins to draw an angle waveform
and displays the countdown of the acquisition time. Can stop in the middle. The acquisition is completed
to display the evaluation results.
– Start: The App sends an evaluation mode to start the command to the host, receives the host reply, and
enters the evaluation work interface.
17

– Stop: After long press and stop for 2s, send the evaluation mode stop command to the host, receive the
host 4 reply, and the evaluation mode stops.
– The evaluation mode stops calculating the reference parameters based on the collected data. The collected
data needs to meet certain conditions before the reference parameters can be calculated, otherwise the
prompts are re-acquired.
4.2.3 Shut down
When the instrument is turned on, press and hold the switch/mode composite button for 2 seconds to turn off the
instrument.
5. Attentions
5.1 Device Power Off
Force shut down: Press the shutdown button.
System shut down:
1) When the treatment duration set by the channel has ended, the functions stop.
2) When the battery voltage is low, the system will automatically shut down.
3) The host will automatically shut down when it is in standby (no electrical stimulation output) for 2 hours.
5.2 Troubleshooting
Malfunction indicator will show the following troubles:
5.2.1 Electrodes loose
The malfunction indicator flashes slowly (the frequency is 1 Hz). When the system detects that the electro de is off, it
indicator will display and the device will stop running. This trouble can be resolved by simply connecting the electrode
pads.
5.2.2 Low battery voltage
When the battery voltage is low, there will be a battery icon display (the battery display capacity is empty) at low
voltage and flash at 1 second intervals. When the battery voltage is as low as the shutdown voltage, the unit
automatically shuts down.
Low battery, flash every 1 second
5.3 Integrated electrode use precautions
The integrated electrode piece can be replaced and be used for long-term. Pay attention to clean the contact surface or
pay attention to clean the skin surface during use (users with more hair should pay attention to trimming, and
conductive adhesive can also be used to increase conductivity).
18

5.4
Skin Care
Before and after using the device, please examine the skin, it is normal that skin slightly turns red, which shows faster
blood flow.
Allergy Prevention Advice:
Do not position on the skin with makeup or oil.
Remove arm’s hair for better electrical conductivity. Electric razor or a pair of scissors is recommended.
If any skin irritation or allergy occurs, please stop using the stimulator immediately and follow doctor’s
instructions.
Do not position on allergic area.
6. Care and Maintenance
6.1 Device Storage
Please do not store it in the place of direct sunlight, high temperature, moist, dusty, or corrosive gas.
Please store it in the place where children cannot reach.
The user does not need to m aintain the hose device, please ask the seller or manufacturer.
Please use wet cloth with neutral detergent or alcohol to clean the surface of the Stimulator.
Please do not immerse the electronic components into to water.
Please do not throw, tread on, or heavy press the de vi c e.
6.2 Product Life
The product is designed to be used for 5 year, after which, please deal with product and battery according to the
provisions of the electronic products processing.
6.3 Use of Rechargeable Battery
Do not charge/discharge exceed the specified current.
Do not short the battery/battery pack. May cause permanent damage to the battery/battery.
Do not burn or destroy the battery/battery pack.
Do not expose the device to adverse conditions such as extreme temperatures, deep cycling and overcharging,
otherwise battery life may be reduced.
Store the battery/battery pack in a cool, dry place.
Stay away from children. If swallowed, contact doctor immediately.
Avoid sealing the battery compartment and ensure ventilation
※Rechargeable battery maintenance instructions
Periodic charge and discharge: If the unit is not used for a long time (when the battery is stored for more than 6 months),
it is recommended to do it once for the host.
7. FAQ & Troubleshooting
Q1. What should I do if the electrical stimulatio n intensity is weak?
A. -Adjust the placement position.
-Adjust the electrical stimulation intensity through the host or APP software.
-If the host is running low, please charge it in time.
-Wet the skin and increase the electrical conductivity between the electrode and the skin.
19

Q2. After turning on the training mode or gait mode on the unit, although the indicator light is on, there is no electrical
stimulation reaction.
A. -Check if the host is tied and close to the skin.
-Check if the current intensity set by the host is adjusted to the appropriate intensity.
-Add some water to the surface of the electrode sheet to increase the conductivity of the electrode sheet.
Q3. What should I do if the skin in the area covered by the electrode and the host band is severely red, stinging or
allergic?
A. Stop using it immediately. After observing for a period of time, if no abnormality is found, wa it until the skin is
completely improved before continuing to use the device. Remember to regularly ventilate the skin covered by the host.
Q4. The unit display battery icon is turned off after 1 second interval flashing.
A. This indicates that the host is running low and needs to be recharged. It takes about 3 hours for the unit to charge;
after the battery is fully charged, the unit can last for about 10 hours. When the battery is low, please charge it in time.
Q5. The host LED light flashes quickly, what should I do if the main unit display “ ”, “TBD Icon” flashes
alternately?
A. This indicator is a reminder that the electrode is off. Please check if the host is fastened. Or the skin is too dry and
needs to moisturize the skin. After re-fixing to the leg, press the main power stimulation adjustment button or press the
stimulation adjustment button in APP software to continue.
Q6. What happens when there is sporadic strong electrical stimulation?
A. -The surface of the electrode is not wet enough to sprinkle some water on the surface.
-Check if the skin in the area covered by the electrode is red or has a wound.
-Check if the cuff of the main unit is loose and the position of the electrode is accurate.
Q7. Why is there no electrical stimulation at the time of electrical stimulation?
A. Usually because the cuff position has been moved or the gait mode has changed. Please wear the cuff again or reset
the walking parameters.
Q8. Can I use oil or lotion on my legs?
A. No, please make sure the skin is clean before using the stimulator and fully wet the adhesive surface of integrated
electrode.
8. Product Configuration
8.1 Product specifications
Communication method: Bluetooth 4.0
Communication freque nc y : 2.4-2.4835GHz
8.2 Stimulator
Power Supply 3.7V rechargeable lithium battery or DC 5V, power adapter
Classification Type BF applied part, internally powered equipment
Shutdown current ≤10μA
Working current ≤120mA
20

Wave form Asymmetric biphasic balanced wave
Frequency
Pulse Width
16-50Hz (±10%), respectively in 16、20、25、33、40、50Hz
50-300μs (±10%), respectively in 100、150、200、250、300μs
Output intensity 0-90mA (±10% or ±2mA, whichever is greater, with 500Ω
load)
Dimension 419*103*13mm
8.3 Power Adapter
Dimension 71*41*31.5mm
Input AC100-240V, 50-60Hz, 0.3A
Output DC 5V, 1.2A
8.4 Working and storage environment
Working Conditions:
Temperature 5~40°C,
Relative humidity ≤80% (Non-condensing)
Atmospheric pressure 86~106KPa
Transport and Storage Conditions:
Temperature -20~55°C,
Relative humidity ≤93%(Non-condensing)
Atmospheric pressure 70~106KPa
Production date: see the label
Service life: 5 Years (battery is not included)
8.5 Accessories
Stimulator 1pc
Charging Cable 1pc
User manual 1pc
Power Adapter 1pc
9. Product classification
a) Classified by type of electric shock: intern a l power supply.
b) The application part is classified according to the degree of electric shock: BF type.
c) Classified by degree of protection against incoming liquid: IP66.
d) Classification according to the degree of safety when using flammable anesthetic gas mixed with air or flammable
anesthetic gas mixed with oxygen or nitrous oxide: no gas cylinder, non-AP and APG type equipment used in this
product.
e) Classified by operating mode: continuous operation.
f) Classified by voltage and frequency of the device: DC3.7V.
g) Whether the equipment has the application part of the protection against defibrillation discharge effect: This product
has no application part for the protection of defibrillation discharge effect, and there is a BF type application part
(referred to as a syringe, which is provided by the hospital) which is connected with the human body.
h) Whether the device has a signal output or input part: This product has no signal output or input part.
i) Permanent or non-permanent installation: This product is a non-permanent installation.
※Please handle this product in accordance with the national regulations on the handling of electronic products.
21

10. After-sales Service
1. The product is provided with a two-year warranty starting from the date of purchasing.
2. XFT will not provide free repair for the malfunctions caused by the following behaviors:
Disassemble or modify the product without authoriza t ion.
Accidentally blow or drop the product during use or transportation.
Lack of reasonable maintenance.
Operate not according to the instruction.
Repaired by unauthorized repair store.
3. When asking for warranty service, please take with the warranty card.
It is charged according to the stipulation of the repair service of the warranty.
Please contact XFT if you need warranty service.
Product Name: Nerve and Muscle Stimulator
Model: XFT-2001E
Date:2018/9/6
Doc. No.: XFT-2001E-A
Version: C
22
 Loading...
Loading...