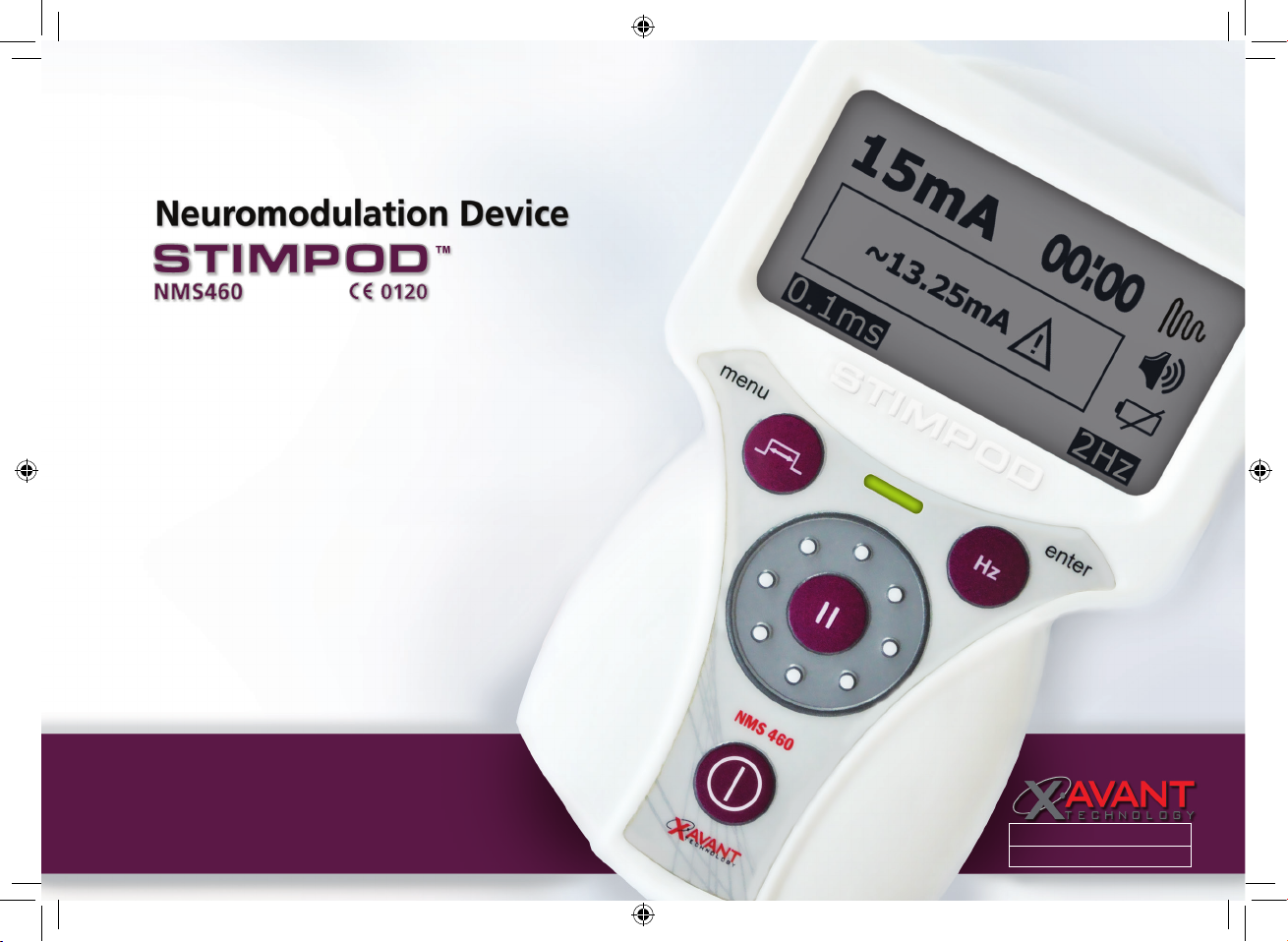
PRODUCT CODE: XT-46006-EN
XM400-31A04-07

i
Manufacturer
Xavant Technology Pty (Ltd)
Unit 102, The Tannery Industrial Park, 309 Derdepoort Rd
Silverton, Pretoria, South Africa, 0184
Tel: +27 (0) 12 743 5959
Fax: +27 (0) 86 547 0026
E-mail: support@xavant.com
Web: www.xavant.com
Legal Representative in the EU
Emergo Europe
Prinsessegracht 20
2514 AP The Hague
The Netherlands
Tel: +31 70 345 8570
Fax: +31 70 346 7299
Indications for use:
The STIMPOD NMS460 is a Transcutaneous Electrical Nerve Stimulation device
used for symptomatic relief and management of chronic intractable pain and/
or as an adjunctive treatment in the management of post-surgical pain, post
traumatic acute pain problems, as well as an adjunct for pain control due to
rehabilitation.
Contraindications:
• Known neurological disorders.
• Do not use this device on patients who have a cardiac pacemaker, implanted de
fibrillator, or other implanted metallic or electronic device, because this may cause
electric shock, burns, electrical interference, or death.
• Do not use this device on patients whose pain syndromes are undiagnosed.
Warnings:
• Read the entire User Manual before attempting to use the device.
• Use of cables or accessories other than those supplied with the STIMPOD NMS460
may result in serious injury.
• Maintenance on this device may only be performed by the manufacturer or
persons explicitly authorized by the manufacturer.
• Do not use the STIMPOD NMS460 in close proximity to equipment that produces
strong electromagnetic fields, such as high frequency surgical equipment. The
cable leads could act as antennae and dangerous currents could be induced as
a result.
• The device should not be used adjacent to or stacked with other equipment and
that if adjacent or stacked use is necessary, the device should be observed to verify
normal operation in the configuration in which it will be used.
• The patient should avoid contact with metallic objects that are grounded, produce
an electrical conductive connection with other equipment and/or enable capacitive
coupling.
• The cables should be positioned in such a way that they do not contact either the
patient or other cables.
• Simultaneous connection of a patient to high frequency surgical ME equipment
and the STIMPOD NMS460 may result in burns and possible damage to the
stimulator.
• Operation in close proximity (e.g. 1m) to a shortwave or microwave therapy ME
equipment may produce instability in the stimulator output.
• Application of electrodes near the thorax may increase the risk of cardiac fibrillation.
• If battery acid has leaked into the device essential circuitry may have been
compromised. In the event of leakage the device must be returned to its
manufacturer for safety checks and possible repairs.
• Do not apply stimulation over the patient’s neck because this could cause severe
-
muscle spasms resulting in closure of the airway, difficulty in breathing, or adverse
effects on heart rhythm or blood pressure.
• Do not apply stimulation over open wounds or rashes, or over swollen, red,
infected, or inflamed areas or skin eruptions (e.g., phlebitis, thrombophlebitis,
varicose veins).
• Do not apply stimulation over, or in proximity to, cancerous lesions.
• Do not apply stimulation when the patient is in the bath or shower.
• Do not apply stimulation while the patient is sleeping.
• Do not apply the stimulation while the patient is driving, operating machinery,
or during any activity in which electrical stimulation can put the patient at risk of
injury.
• Do not apply stimulation when the leads are open circuited (e.g. when the
electrodes are held in the user’s hands).
• Do not remove electrodes when stimulation is being applied (to avoid unintended,
possibly hazardous, stimulation pathways).
ii
• Stimulation should not be applied across or through the head, directly on the
eyes, covering the mouth, on the front of the neck, (especially the carotid sinus),
or from electrodes placed on the chest and the upper back or crossing over the
heart.
• Use of accessories, transducers and cables other than those specified or provided
by the manufacturer of this equipment could result in increased electromagnetic
emissions or decreased electromagnetic immunity of this equipment and result in
improper operation.
• Do not apply stimulation over the menstruating or pregnant uterus.
Cautions:
• Remove elements which may adversely affect the connection between the ECG
electrodes and the skin, e.g., dirt, hair, oil.
• Ensure that ECG electrodes are not damaged or dried out.
• ECG electrodes may be used in the higher current ranges (> 10 mA).
• This product must be stored at room temperature.
• This product must be transported in the carry case provided.
• This product and all the accessories have been certified latex free.
• Inspect all parts for any damage or manipulation. Never use any damaged or
manipulated part!
• If an electrically conductive surface of the STIMPOD NMS460 device or its cables
are exposed, such electrically conductive surface may shock a person handling it.
Do not use such a device or accessory, please contact the manufacturer for repair.
• A sterile wipe should be used to disinfect the Nerve Mapping Probe prior to use.
• Keep this device out of reach of children.
• Use this device under the continued supervision of a licensed practitioner.
Precautions:
• Electrical nerve stimulators are not effective for pain of central origin, including
headache caused by central origin.
• Electrical nerve stimulators are not a substitute for pain medications and other
pain management therapies.
• Electrical nerve stimulator devices have no curative value.
• Electrical nerve stimulators are a symptomatic treatment and, as such, suppresses
the sensation of pain that would otherwise serve as a protective mechanism.
• Effectiveness is highly dependent upon patient selection by a practitioner qualified
in the management of pain patients.
• The long-term effects of electrical stimulation are unknown.
• Since the effects of stimulation of the brain are unknown, stimulation should not
be applied across the head, and electrodes should not be placed on opposite sides
of the head.
• The safety of electrical stimulation during pregnancy has not been established.
• Some patients may experience skin irritation or hypersensitivity due to the
electrical stimulation or electrical conductive medium (gel).
• Patients with suspected or diagnosed epilepsy should follow precautions
recommended by their physicians.
• Use caution when patient has a tendency to bleed internally, such as following
an injury or fracture.
• Use caution following recent surgical procedures when stimulation may disrupt
the patient’s healing process.
• Use caution if stimulation is applied over areas of the skin that lack normal
sensation.
Adverse Reactions:
• Patients may experience skin irritation and burns beneath the stimulation
electrodes applied to the skin.
• Patients may experience headache and other painful sensation during or following
the application of electrical stimulation near the eyes and to the head and face.
• Patients should stop using the device and should consult with their physicians if
they experience adverse reactions from the device.
Warranty:
• The STIMPOD NMS460 carries a 12 Month Warranty against defects, provided
that the device was used in accordance with the operating instructions.
• The STIMPOD NMS460 enclosure should not be opened under any circumstances.
Opening the unit will void the warranty.
Note:
• The STIMPOD NMS460 is not intended for home use.
STIMPOD 460 conforms to the following standards:
• IEC 60601-1, IEC 60601-2-10
• IEC 60601-1-2: CISPR 11 Group1 class B; IEC 61000-4-2; IEC 61000-4-3
• ISO 13485, Directive 93-42-EEC, ISO 9001
iii

Guidance and manufacturers declaration – electromagnetic emissions– for all equipment and systems
The STIMPOD NMS460 is intended for use in electromagnetic environment specified below. The customer or user of the STIMPOD NMS460 should ensure that it is used in such an environment
Emission Test Compliance Electromagnetic Environment – Guidance
RF Emissions
CISPR 11
Group 2 – Class A The STIMPOD NMS460 must emit electromagnetic energy in order to per
form its intended function. Nearby electronic equipment may be affected.
The STIMPOD NMS460 is suitable for use in all establishments, other than do
mestic establishments and may be used in domestic establishments and those
directly connected to the public low voltage power supply network that supplies buildings used for domestic purposes, provided the following warning is
heeded:
WARNING: This equipment/system is intended to be used by healthcare professional only. This equipment/system may cause radio interference or disrupt
the operation of nearby equipment. It may be necessary to take mitigation
measures, such as re-orienting or re-locating the STIMPOD NMS460 or shielding the location
Guidance and manufacturers declaration – electromagnetic immunity- for all equipment and systems
The STIMPOD NMS460 is intended for use in the electromagnetic environment specified below. The customer or the user of the STIMPOD NMS460 should ensure that it is used in such an
environment
Immunity Test IEC 60601 test level Compliance level Electromagnetic environment - guidance
Electrostatic discharge (ESD)
IEC 61000-4-2
Power frequency (50/60 Hz)
magnetic field
IEC 61000-4-8
± 6 kV contact
± 15 kV air
30 A/m 50 Hz
± 6 kV contact
± 15 kV Air
30 A/m (Effective)
Floors should be wood, concrete or ceramic tile. If the floors are covered with
synthetic material, the relative humidity should be at least 30%
Power frequency magnetic fields should be at levels characteristic of a typical
location in a typical commercial or hospital environment.
-
-
iv
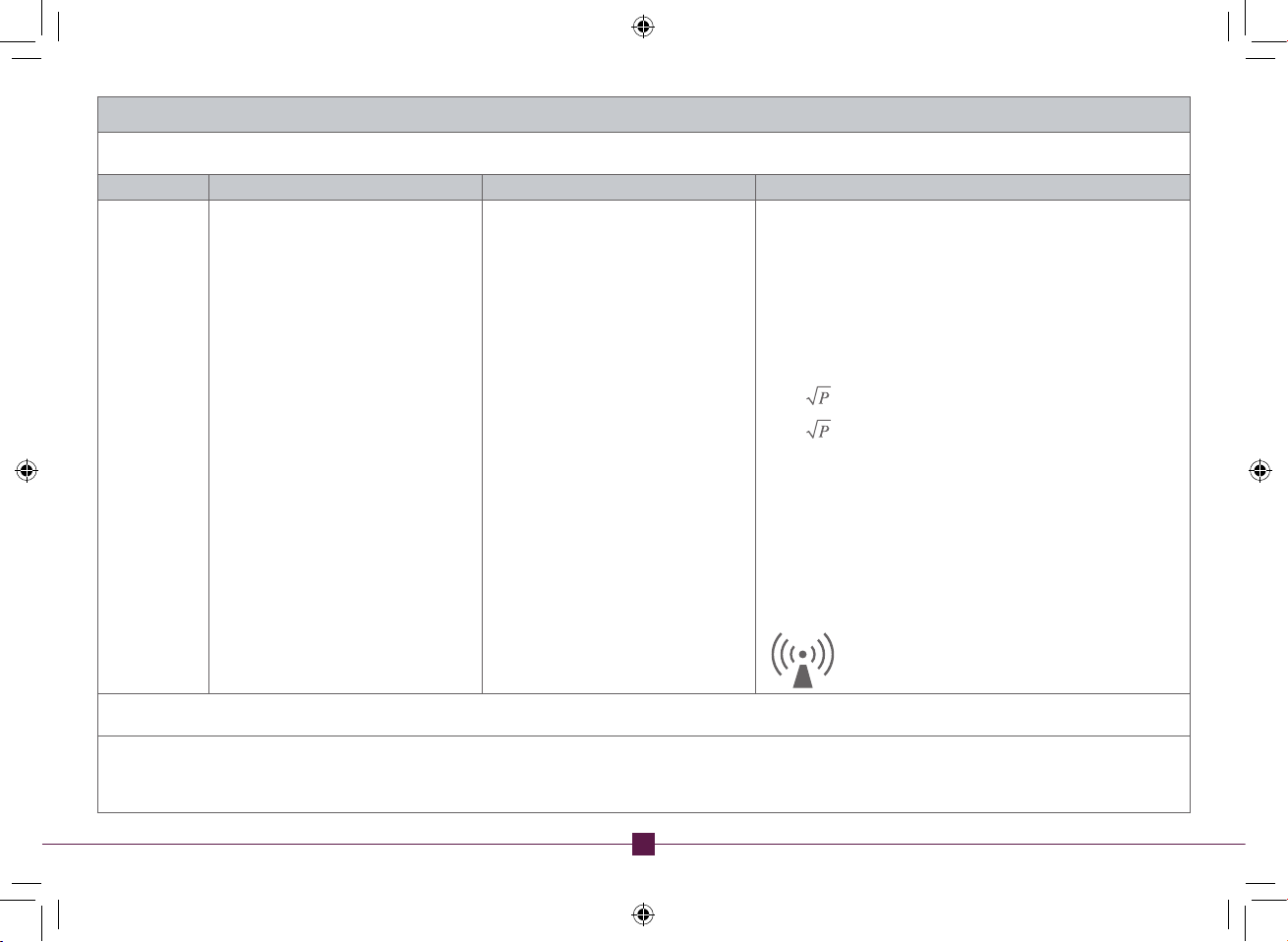
Guidance and manufacturer’s declaration – electromagnetic immunity
The STIMPOD NMS460 is intended for use in the electromagnetic environment specified below. The customer or the user of the STIMPOD NMS460 should assure that it is used in such an
environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment – guidance
Portable and mobile RF communications equipment should be used no
closer to any part of the STIMPOD
recommended separation distance calculated from the equation applicable
to the frequency of the transmitter.
NMS460
, including cables, than the
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3V at 0.15 - 80MHz and 6V at ISM
Frequency.
Home Healthcare: 3V at 0.15 - 80MHz, and
6V at ISM and Radio Amateur Frequency.
3 V/m (10V/m Home Healthcare) at
80 - 2,700MHz, AM Modulation. And
9-28V/m at 385-6000MHz, Pulse Mode and
other Modulation (upon Risk Analysis).
3V at 0.15 - 80MHz and 6V at ISM
Frequency.
Home Healthcare: 3V at 0.15 - 80MHz, and
6V at ISM and Radio Amateur Frequency.
3 V/m (10V/m Home Healthcare) at
80 - 2,700MHz, AM Modulation. And
9-28V/m at 385-6000MHz, Pulse Mode and
other Modulation (upon Risk Analysis).
Recommended separation distance
d = 1,2 80 MHz to 800 MHz
d = 2,3 800 MHz to 2,5 GHz
where P is the maximum output power rating of the transmitter in watts
(W) according to the transmitter manufacturer and d is the recommended
separation distance in metres (m).
Field strengths from fixed RF transmitters, as determined by an
electromagnetic site survey,
a
should be less than the compliance level in
each frequency range.
Interference may occur in the vicinity of equipment marked with the
following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV
broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be
considered. If the measured field strength in the location in which the STIMPOD NMS460 is used exceeds the applicable RF compliance level above, the STIMPOD NMS460 should
be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the STIMPOD NMS460.
v
 Loading...
Loading...